Abstract
Background
The estimated glomerular filtration rate (eGFR) is a biomarker not only for kidney function, but also for major clinical outcomes. We aimed to evaluate the patterns of mortality across the entire eGFR percentile spectrum using a population-based dataset.
Methods
We retrospectively reviewed the National Health Insurance Service (NHIS) database for people who received nationwide health check-ups from 2009 to 2012. Subjects who were ≥45 years old and had one or more serum creatinine values available were included in the study. The primary outcome was all-cause mortality as a function of eGFR percentile.
Results
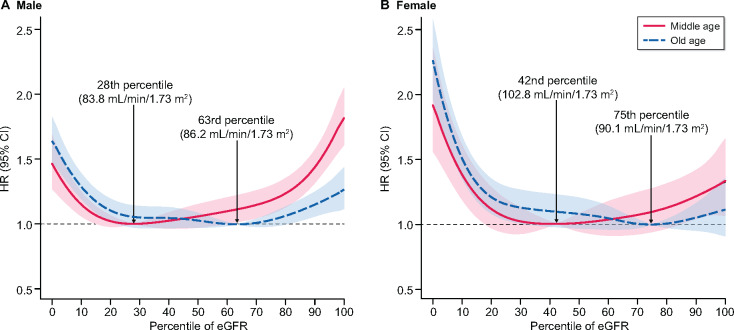
The middle-aged group (45–64 years) showed a U-shaped pattern of association between eGFR percentile and all-cause mortality. The minimum-mortality eGFR percentile was shifted upward in the elderly group (≥65 years). Specifically, the minimum-mortality eGFR percentiles were the 28th percentile (83.8 mL/min/1.73 m2) for middle-aged males, the 63rd percentile (86.2 mL/min/1.73 m2) for elderly males, the 42nd percentile (102.8 mL/min/1.73 m2) for middle-aged females and the 75th percentile (90.1 mL/min/1.73 m2) for elderly females. Diabetes and hypertension shifted the minimum-mortality eGFR percentile upward in the middle-aged group. This pattern was attenuated in the elderly group.
Conclusions
The eGFR percentile showing minimum mortality moves upward in the aged population as well as patients with diabetes and hypertension, which might reduce the clinical significance of hyperfiltration. Risk stratification for mortality should be approached differently according to the specific conditions of the patient group.
Keywords: all-cause mortality, estimated glomerular filtration rate, elderly
INTRODUCTION
The estimated glomerular filtration rate (eGFR) is not only the most frequently used biomarker representing kidney function, but also a prognostic biomarker for major clinical outcomes [1–3]. An eGFR <60 mL/min/1.73 m2, which occurs in <5% of the general population, is a well-known risk factor for all-cause mortality [3–5]. Recent studies have revealed that glomerular hyperfiltration, which is conventionally defined as an eGFR above the 95th percentile or >2 standard deviations above the mean [6], is also associated with an increased risk of all-cause mortality [7, 8]. Thus there are clearly high mortality risks at both ends of the eGFR spectrum. While the association between eGFR and mortality can change according to age, gender and various clinical situations, the eGFR criteria for risk assessment are defined as certain absolute or percentile values. This approach could either overestimate or underestimate the risk of major clinical outcomes, thereby limiting the value of eGFR as a prognostic biomarker in the general population.
While serum creatinine (sCr) is the most crucial variable for determining eGFR, age has a significant effect as well. Indeed, eGFR is known to decline by an average of 0.7–1.0 mL/min/1.73 m2 per year from middle age onward [9–11]. In addition to age, several medical conditions such as diabetes, hypertension, pregnancy and smoking increase eGFR at the nephron level, either physiologically or pathologically [12, 13]. Therefore it is crucial to assess the differences in mortality risk across the eGFR spectrum according to age, sex and presence of diabetes mellitus or hypertension. For this purpose, we aimed to evaluate the pattern of mortality across the entire eGFR percentile spectrum using a population-based dataset and determine the eGFR percentile with the minimal mortality rate in different clinical settings.
MATERIALS AND METHODS
Study subjects
Patients who underwent national health screening between January 2012 and December 2012 with a follow-up between January 2013 and December 2016 were included in the study. Of all eligible patients, those ≥45 years of age with one or more sCr value available were selected for the study. Subjects who had experienced dialysis, kidney transplantation, cerebrovascular disease or cardiovascular disease within 3 years before the health screening were excluded. The subject population ≥65 years of age was regarded as the elderly group and the rest were considered the middle-aged group.
Data source and acquisition
This study was performed using data from the Korean National Health Insurance Service (NHIS) and the Health Insurance Review and Assessment Service (HIRA) databases. In Korea, universal health insurance provides biannual health screening free of charge to all people ≥40 years of age. Health insurance coverage was ∼97% and the overall examination rate was >70% [14, 15]. We obtained information including demographic variables and laboratory results at the time of health examination from the NHIS database. Additionally, healthcare utilization information, diagnostic codes according to the International Classification of Diseases, 10th Revision (ICD-10), medical procedure history, prescription records and medical costs were retrieved from the HIRA database [16, 17].
Collected data
The baseline characteristics included age; sex; socio-economic status; history of smoking; anthropometric data such as height, weight and waist and hip circumference; and blood pressure. The laboratory variables consisted of sCr, serum glucose and the lipid profile. The sCr values were measured by traceable isotope dilution mass spectrometry (IDMS). We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation as follows: eGFR = 141 × minimum(sCr/κ, 1)α × maximum(Cr/κ, 1)−1.209 × 0.993age × 1.018 [if female], κ = 0.7 (female) or 0.9 (male), α = −0.329 (female) or −0.411 (male).
Study outcomes
The primary outcome was all-cause mortality as a function of eGFR. Two age groups were defined using a cut-off of 65 years and eGFR was divided into seven percentile categories: <5th, 5th–15th, 15th–40th, 40th–59th, 60th–84th, 85th–94th and ≥95th. The final outcome was the eGFR percentile at which the mortality risk, as defined by the hazard ratio (HR) for all-cause mortality, increased significantly. In addition, the following subgroups were compared: the diabetic and non-diabetic subgroups and the hypertensive and normotensive subgroups.
Statistical analysis
To compare baseline characteristics, we used the chi-squared test and the Kruskal–Wallis test. Continuous and categorical variables were presented as median values with interquartile ranges and numbers with percentages, respectively. The all-cause mortality risks were estimated by multivariate Cox regression analysis adjusted for variables including age, sex, body mass index (BMI), smoking status, drinking status, serum glucose, systolic blood pressure and total cholesterol. To allow the modeling of smooth non-linear effects, we used penalized splines implemented in the spline function of the R package ‘survival’ (R Foundation for Statistical Computing, Vienna, Austria). We performed the statistical analysis using SAS version 9.4 (SAS Institute, Cary, NC, USA) with a two-sided P-value <0.05 as the criterion for statistical significance.
Ethical considerations
This study was approved by the institutional review board (IRB) of Seoul National University Hospital (IRB no. E-1801-027-913). We obtained approval from the appropriate government organization to use data from the NHIS and HIRA. The study was conducted in accordance with the Declaration of Helsinki.
RESULTS
Study population
We reviewed the data of 1 285 208 subjects and 565 902 met the inclusion criteria (Figure 1). Among the seven eGFR strata, the highest stratum contained the highest percentages of current smoking, heavy drinking, low income and underweight. These variables, other than low income, showed a linear relationship between values and eGFR categories. Additionally, high-density lipoprotein cholesterol incrementally increased over ascending eGFR categories, while the opposite was true of low-density lipoprotein cholesterol (Table 1).
FIGURE 1:
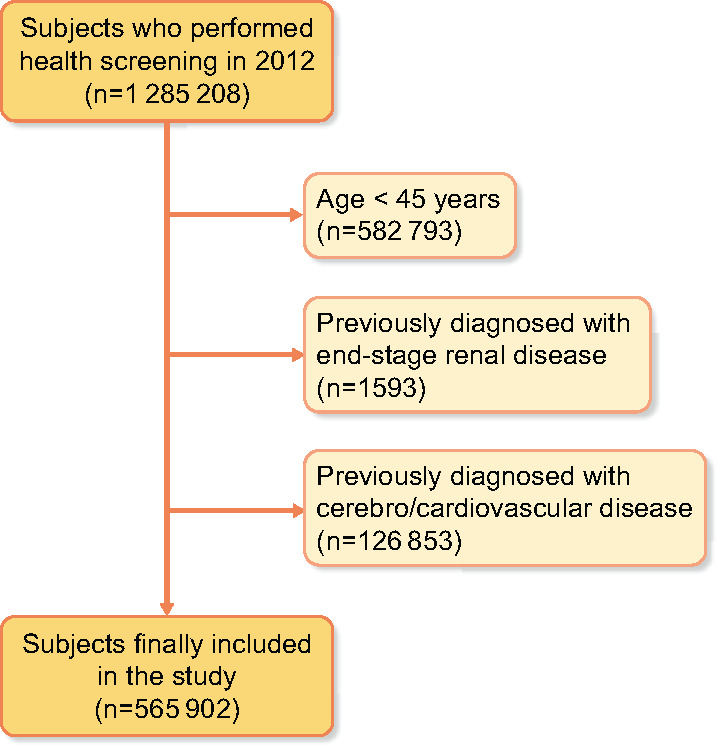
Flow diagram for the study populations.
Table 1.
Descriptive demographics of study populations stratified by eGFRs
| Variables | Group 1 (n = 22 130) |
Group 2 (n = 58 422) |
Group 3 (n = 138 942) |
Group 4 (n = 113 245) |
Group 5 (n = 140 424) |
Group 6 (n = 57 329) |
Group 7 (n = 34 097) |
P-value |
|---|---|---|---|---|---|---|---|---|
| Age (years), n (%) | ||||||||
| 45–49 | 4844 (21.9) | 12 976 (22.2) | 28 895 (20.8) | 26 964 (23.8) | 31 210 (22.2) | 11 655 (20.3) | 7222 (21.2) | <0.0001 |
| 50–54 | 4815 (21.8) | 13 463 (23.0) | 36 581 (26.3) | 27 165 (24.0) | 43 052 (30.7) | 15 100 (26.3) | 12 534 (36.8) | |
| 55–59 | 4873 (22.0) | 12 415 (21.3) | 26 571 (19.1) | 20 172 (17.8) | 19 405 (13.8) | 8864 (15.5) | 2235 (6.6) | |
| 60–64 | 2343 (10.6) | 7569 (13.0) | 20 108 (14.5) | 13 816 (12.2) | 22 551 (16.1) | 10 475 (18.3) | 6693 (19.6) | |
| 65–69 | 2580 (11.7) | 5410 (9.3) | 9763 (7.0) | 10 579 (9.3) | 9217 (6.6) | 3350 (5.8) | 1293 (3.8) | |
| 70–74 | 1357 (6.1) | 3715 (6.4) | 10 791 (7.8) | 9467 (8.4) | 9965 (7.1) | 6166 (10.8) | 3238 (9.5) | |
| ≥75 | 1318 (6.0) | 2874 (4.9) | 6233 (4.5) | 5082 (4.5) | 5024 (3.6) | 1719 (3.0) | 882 (2.6) | |
| Sex (male), n (%) | 11 488 (51.9) | 29 920 (51.2) | 72 199 (52.0) | 55 790 (49.3) | 73 579 (52.4) | 29 205 (50.9) | 17 458 (51.2) | <0.0001 |
| Smoking, n (%) | ||||||||
| None | 14 161 (64.0) | 37 619 (64.4) | 87 813 (63.2) | 72 690 (64.2) | 86 112 (61.3) | 35 285 (61.6) | 20 832 (61.1) | <0.0001 |
| Ex-smoker | 4330 (19.6) | 10 955 (18.8) | 26 621 (19.2) | 20 183 (17.8) | 25 780 (18.4) | 9703 (16.9) | 5354 (15.7) | |
| Current smoker | 3639 (16.4) | 9848 (16.9) | 24 508 (17.6) | 20 372 (18.0) | 28 532 (20.3) | 12 341 (21.5) | 7911 (23.2) | |
| Drinking, n (%) | ||||||||
| None | 14 161 (64.0) | 37 619 (64.4) | 87 813 (63.2) | 72 690 (64.2) | 86 112 (61.3) | 35 285 (61.6) | 20 832 (61.1) | <0.0001 |
| Moderate | 4330 (19.6) | 10 955 (18.8) | 26 621 (19.2) | 20 183 (17.8) | 25 780 (18.4) | 9703 (16.9) | 5354 (15.7) | |
| Heavy | 3639 (16.4) | 9848 (16.9) | 24 508 (17.6) | 20 372 (18.0) | 28 532 (20.3) | 12 341 (21.5) | 7911 (23.2) | |
| Physical activity, n (%) | 5036 (22.8) | 13 627 (23.3) | 32 648 (23.5) | 25 897 (22.9) | 32 042 (22.8) | 12 774 (22.3) | 7072 (20.7) | <0.0001 |
| Low income, n (%) | 4843 (21.9) | 12 143 (20.8) | 28 129 (20.3) | 23 195 (20.5) | 27 630 (19.7) | 11 730 (20.5) | 7473 (21.9) | <0.0001 |
| BMI, n (%) | ||||||||
| <18.5 | 368 (1.7) | 1008 (1.7) | 2664 (1.9) | 2658 (2.4) | 3364 (2.4) | 1662 (2.9) | 1249 (3.7) | <0.0001 |
| 18.5–22.9 | 6636 (30.0) | 19 017 (32.6) | 47 897 (34.5) | 41 275 (36.5) | 53 454 (38.1) | 22 597 (39.4) | 14 020 (41.1) | |
| 23.0–24.9 | 5962 (26.9) | 16 084 (27.5) | 38 898 (28.0) | 31 260 (27.6) | 38 282 (27.3) | 15 193 (26.5) | 8699 (25.5) | |
| 25.0–29.9 | 8116 (36.7) | 20 114 (34.4) | 45 034 (32.4) | 34 641 (30.6) | 41 260 (29.4) | 16 110 (28.1) | 9063 (26.6) | |
| ≥30 | 1048 (4.7) | 2199 (3.8) | 4449 (3.2) | 3411 (3.0) | 4064 (2.9) | 1767 (3.1) | 1066 (3.1) | |
| Waist circumference (cm) | 82.3 ± 8.9 | 81.9 ± 8.7 | 81.6 ± 8.5 | 81.2 ± 8.5 | 81.1 ± 8.5 | 81.0 ± 8.5 | 80.5 ± 8.7 | <0.0001 |
| SBP (mmHg) | 125.6 ± 15.7 | 124.6 ± 15.1 | 124.1 ± 14.9 | 123.7 ± 14.9 | 123.6 ± 14.9 | 124.1 ± 15.0 | 123.9 ± 15.3 | <0.0001 |
| DBP (mmHg) | 77.3 ± 10.4 | 77.3 ± 10.0 | 76.9 ± 9.9 | 76.5 ± 9.9 | 76.6 ± 10.0 | 76.6 ± 10.2 | 76.5 ± 10.5 | <0.0001 |
| Glucose (mg/dL) | 102.1 ± 28.4 | 100.1 ± 23.8 | 99.5 ± 22.8 | 99.1 ± 22.8 | 99.3 ± 23.5 | 99.6 ± 24.8 | 100.0 ± 25.9 | <0.0001 |
| Total cholesterol (mg/dL) | 198.8 ± 39.6 | 201.3 ± 37.6 | 200.5 ± 36.9 | 199.5 ± 36.4 | 198.5 ± 36.3 | 197.0 ± 36.2 | 195.2 ± 36.7 | <0.0001 |
| Triglycerides (mg/dL), median (IQR) | 125.8 (124.9–126.7) | 119.4 (118.9–120.0) | 116.9 (116.6–117.3) | 114.7 (114.4–115.1) | 114.1 (113.8–114.4) | 112.1 (111.6–112.6) | 111.4 (110.8–112.1) | <0.0001 |
| HDL (mg/dL) | 51.8 ± 13.8 | 53.2 ± 13.7 | 53.6 ± 13.6 | 54.1 ± 13.8 | 54.3 ± 14.1 | 54.6 ± 14.2 | 55.0 ± 14.6 | <0.0001 |
| LDL (mg/dL) | 117.6 ± 35.9 | 120.8 ± 34.5 | 120.2 ± 34.0 | 119.3 ± 33.6 | 118.1 ± 33.4 | 116.6 ± 33.3 | 114.5 ± 34.0 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 50.6 ± 17.8 | 68.9 ± 7.2 | 81.0 ± 7.8 | 93.2 ± 7.5 | 99.2 ± 6.1 | 103.6 ± 6.3 | 110.9 ± 7.5 | <0.0001 |
| CCI score | 1.3 ± 1.6 | 0.9 ± 1.3 | 0.9 ± 1.2 | 0.9 ± 1.2 | 0.8 ± 1.2 | 0.9 ± 1.3 | 0.9 ± 1.2 | <0.0001 |
Values are presented as mean ± SD unless stated otherwise.
SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CCI, Charlson Comorbidity Index.
Glomerular filtration rate trends associated with mortality
When the 40th–59th percentile of eGFR was regarded as a reference, the lowest range of the eGFR percentile showed a significantly increased risk for all-cause mortality in all subjects. Male subjects with the highest range of eGFR percentile showed significantly increased risk for all-cause mortality regardless of age, but this statistical significance was attenuated in the female group (Table 2). All-cause mortality across the entire eGFR percentile showed a U-shaped curve (Figure 2). At low eGFR percentiles, both males and females had a significantly increased risk of mortality regardless of age. The minimum-mortality eGFR percentiles were the 28th percentile (83.8 mL/min/1.73 m2) for middle-aged males, the 63rd percentile (86.2 mL/min/1.73 m2) for elderly males, the 42nd percentile (102.8 mL/min/1.73 m2) for middle-aged females and the 75th percentile (90.1 mL/min/1.73 m2) for elderly females. Finally, mortality risk significantly increased above and below the range from the 16th to 62nd percentile (79.5–97.9 mL/min/1.73 m2) and from the 18th to 86th percentile (82.1–110.4 mL/min/1.73 m2) in middle-aged males and females, respectively. For the older males, mortality risk significantly increased above and below the range from the 23rd to 81st percentile (56.77–96.29 mL/min/1.73 m2), while there was only a lower limit (33rd percentile, 73.37 mL/min/1.73 m2) below which mortality risk was significantly increased in older females. Since the minimum-mortality eGFR percentiles were lower in the middle-aged group than in the elderly group in both sexes, the upward slope on the right side of the curve, showing mortality rates at the high eGFR percentile levels, was steeper in the middle-aged group than in the elderly group. The eGFR values for each percentile by age group and sex are described in Supplementary data (Supplementary data, Tables S1-1, S1-2 and S1-3).
Table 2.
Pooled estimates of the adjusted HRs for all-cause mortality in different eGFR percentiles according to age and sex
| Groups | Range of eGFR percentiles | Patients, n | Deaths, n | Person- years | Incidence rate (per 1000 person-year) | Model 1, HR (95% CI) |
Model 2, HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Male, age 45–64 years | <5 | 11 917 | 276 | 63 740.6 | 4.33 | 1.43 (1.25–1.64) | 1.48 (1.29–1.7) |
| 5–14 | 22 188 | 306 | 118 791.5 | 2.58 | 0.85 (0.75–0.97) | 0.91 (0.8–1.04) | |
| 15–39 | 57 668 | 855 | 308 838.9 | 2.77 | 0.92 (0.83–1.01) | 0.95 (0.86–1.05) | |
| 40–59 | 49 468 | 800 | 264 813.8 | 3.02 | 1(Ref.) | 1 (Ref.) | |
| 60–84 | 57 012 | 968 | 305 291.8 | 3.17 | 1.05 (0.96–1.15) | 1 (0.91–1.1) | |
| 85–94 | 24 014 | 493 | 128 315.5 | 3.84 | 1.27 (1.14–1.42) | 1.14 (1.02–1.27) | |
| ≥95 | 14 329 | 396 | 76 378.8 | 5.18 | 1.72 (1.52–1.94) | 1.44 (1.28–1.63) | |
| Male, age ≥65 yeras | <5 | 2629 | 378 | 13 624.6 | 27.74 | 1.62 (1.44–1.83) | 1.66 (1.47–1.87) |
| 5–14 | 4321 | 537 | 22 578.0 | 23.78 | 1.39 (1.25–1.54) | 1.45 (1.3–1.61) | |
| 15–39 | 14 258 | 1334 | 75 240.1 | 17.73 | 1.03 (0.95–1.12) | 1.06 (0.97–1.15) | |
| 40–59 | 9912 | 900 | 52 409.4 | 17.17 | 1 (Ref.) | 1 (Ref.) | |
| 60–84 | 13 979 | 1367 | 73 862.6 | 18.51 | 1.08 (0.99–1.17) | 1.02 (0.94–1.11) | |
| 85–94 | 4815 | 486 | 25 447.4 | 19.10 | 1.11 (0.99–1.24) | 1.03 (0.92–1.15) | |
| ≥95 | 3129 | 351 | 16 510.4 | 21.26 | 1.23 (1.09–1.39) | 1.09 (0.97–1.24) | |
| Female, age 45–64 years | <5 | 10 429 | 126 | 56 352.0 | 2.24 | 2.04 (1.65–2.53) | 1.98 (1.6–2.45) |
| 5–14 | 21 839 | 171 | 117 487.9 | 1.46 | 1.33 (1.1–1.62) | 1.32 (1.09–1.61) | |
| 15–39 | 53 183 | 329 | 286 875.8 | 1.15 | 1.05 (0.89–1.24) | 1.04 (0.89–1.23) | |
| 40–59 | 42 584 | 251 | 229 645.6 | 1.09 | 1 (Ref.) | 1 (Ref.) | |
| 60–84 | 54 101 | 340 | 291 793.4 | 1.17 | 1.07 (0.91–1.25) | 1.06 (0.9–1.25) | |
| 85–94 | 25 061 | 189 | 135 003.6 | 1.40 | 1.28 (1.06–1.55) | 1.26 (1.04–1.52) | |
| ≥95 | 10 773 | 74 | 58 103.4 | 1.27 | 1.16 (0.9–1.51) | 1.13 (0.87–1.47) | |
| Female, age ≥65 years | <5 | 2626 | 278 | 13 954.8 | 19.92 | 2.40 (2.08–2.77) | 2.38 (2.06–2.75) |
| 5–14 | 6007 | 389 | 32 349.8 | 12.02 | 1.44 (1.27–1.64) | 1.46 (1.28–1.67) | |
| 15–39 | 12 973 | 732 | 70 199.6 | 10.43 | 1.25 (1.12–1.4) | 1.24 (1.11–1.39) | |
| 40–59 | 11 951 | 542 | 64 931.0 | 8.35 | 1 (Ref.) | 1 (Ref.) | |
| 60–84 | 14 756 | 652 | 80 283.3 | 8.12 | 0.97 (0.87–1.09) | 0.96 (0.85–1.07) | |
| 85–94 | 5727 | 196 | 31 236.9 | 6.27 | 0.75 (0.64–0.88) | 0.74 (0.63–0.87) | |
| ≥95 | 2940 | 148 | 15 988.8 | 9.26 | 1.11 (0.92–1.33) | 1.06 (0.88–1.27) |
Model 1: non-adjusted.
Model 2: adjusted for age, sex, BMI, systolic blood pressure, smoking status, drinking status, serum glucose and total cholesterol.
CI, confidence interval; Ref.: reference.
FIGURE 2:
All-cause mortality according to eGFR percentile at different ages in (A) males and (B) females. The red line represents the middle-aged group and the blue dotted line represents the elderly group. The minimum-mortality eGFR percentile is indicated by the upward dotted line labeled with the specific value in each group. Adjusted variables were age, sex, BMI, systolic blood pressure, smoking status, drinking status, serum glucose and total cholesterol.
Association between GFRs and mortality according to diabetes
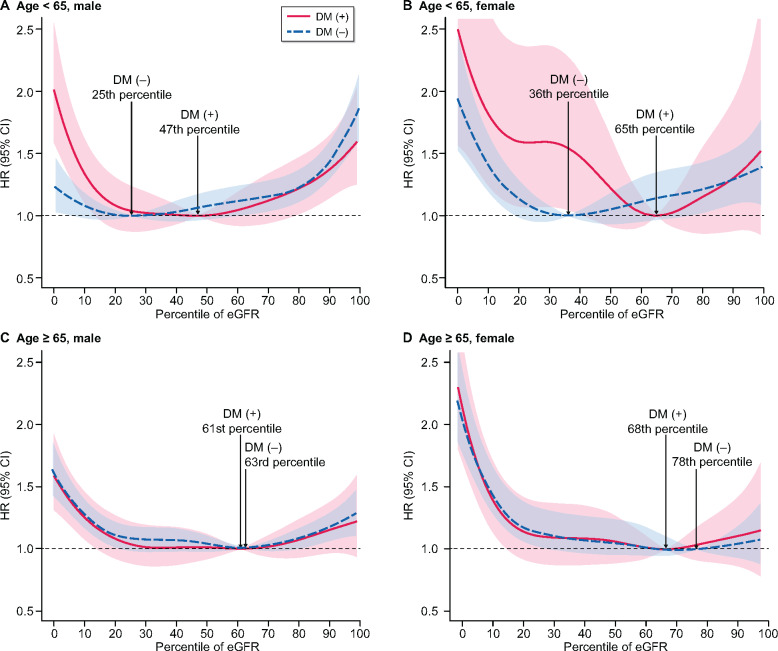
The minimal-mortality eGFR percentiles varied in the middle-aged group depending on whether diabetes was present. Subjects with diabetes had minimum mortality at the 47th and 65th percentiles, which corresponded to an eGFR of 88.1–90.5 and 94.5–107.4 mL/min/1.73 m2 in middle-aged males and females, respectively. Meanwhile, subjects without diabetes had minimum mortality at the 25th and 36th percentiles (77.0–88.0 and 80.4–102.1 mL/min/1.73 m2) in middle-aged males and females, respectively. In contrast, there was little difference in the minimum-mortality eGFR percentile between the diabetes [61st and 68th percentiles (78.2–91.8 and 81.1–95.3 mL/min/1.73 m2) in males and females] and non-diabetes groups [63rd and 78th percentiles (78.7–91.9 and 82.2–98.0 mL/min/1.73 m2) in males and females] in the elderly group (Figure 3). The number of patients included in the different eGFR percentiles is described in Supplementary data, Table S2.
FIGURE 3:
All-cause mortality according to eGFR percentile among patients with and without diabetes in (A) the middle-aged male, (B) the middle-aged female, (C) the older-aged male and (D) the older-aged female groups. The red line represents the diabetic group and the blue dotted line represents the non-diabetic group. The minimum-mortality eGFR percentile is indicated by the upward dotted line labeled with the specific value in each group. Adjusted variables were age, sex, BMI, systolic blood pressure, smoking status, drinking status, serum glucose and total cholesterol.
Association between glomerular filtration rates and mortality according to hypertension
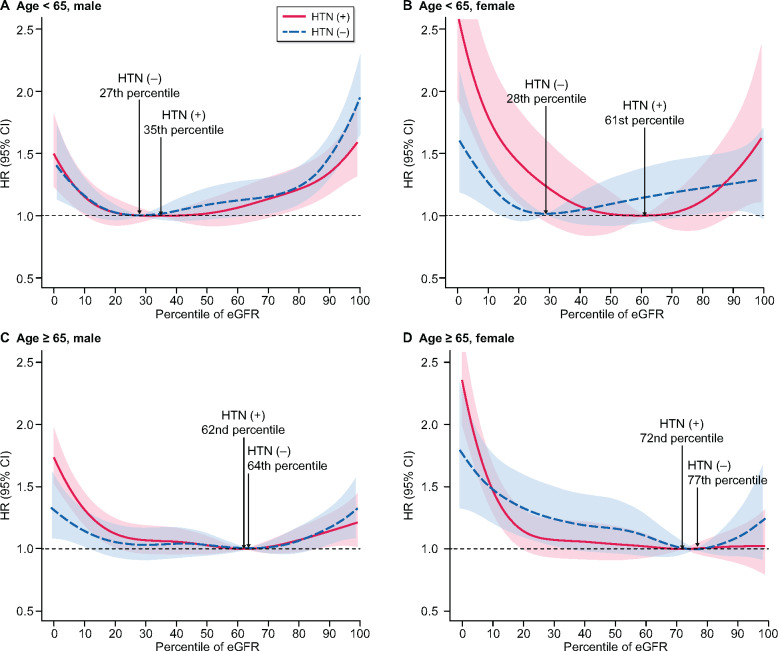
The middle-aged males showed a U-shaped relationship between eGFR and all-cause mortality regardless of the presence of hypertension. In middle-aged subjects, the presence of hypertension moved the minimum-mortality eGFR percentile to the right. Also, the difference between the hypertension [61st percentile (94.0–105.0 mL/min/1.73 m2)] and non-hypertension group [28th percentile (78.7–88.7 mL/min/1.73 m2)] was prominent in middle-aged females. The difference in the minimum-mortality eGFR percentile between hypertensive and normotensive subjects was attenuated in the elderly group (Figure 4).
FIGURE 4:
All-cause mortality according to eGFR percentile among patients with and without hypertension in (A) the middle-aged male, (B) the middle-aged female, (C) the older-aged male and (D) the older-aged female groups. The red line represents the hypertensive group and the blue dotted line represents the normotensive group. The minimum-mortality eGFR percentile is indicated by the upward dotted line labeled with the specific value in each group. Adjusted variables were age, sex, BMI, systolic blood pressure, smoking status, drinking status, serum glucose and total cholesterol
DISCUSSION
All-cause mortality was found to be a U-shaped function of eGFR percentile. The minimum-mortality eGFR percentile varied depending on age, sex and comorbidities. The prognostic impact of higher eGFR percentiles was higher in the middle-aged group than in the elderly group, since the former group had a lower minimum-mortality eGFR percentile than the latter group. Old age, diabetes and hypertension increase the prognostic impact of lower eGFR by moving the minimum-mortality eGFR percentile upward on the eGFR percentile spectrum. This study is the first investigation to identify the minimum-mortality eGFR percentile, which increases in the aged population and subjects with comorbidities, using a nationwide population-based dataset.
In this study, the elderly group had a higher minimum-mortality eGFR percentile than the middle-aged group. As a result, the proportion of subjects above the minimum-mortality eGFR percentile was lower in the elderly group than in the middle-aged group. For this reason, the effect of glomerular hyperfiltration on mortality was not statistically significant in the elderly group. On the contrary, the minimum-mortality eGFR percentile moved downward in the middle-aged group, therefore a high eGFR percentile, indicating glomerular hyperfiltration, should be taken as a warning sign in this group.
The minimum-mortality eGFR percentile was higher in females than in males. Additionally, the difference in eGFR percentile between the middle-aged and elderly groups was more distinct in females than in males. However, the sex gap was attenuated in the elderly group, possibly due to a difference in age-related changes in muscle mass between males and females [18, 19]. Males show incremental decreases in skeletal muscle mass with age. Although skeletal muscle mass is significantly lower in females than in males for a whole range of ages, female muscle mass does not change in the aging process and the sex gap in skeletal muscle mass incrementally decreases with age [18]. For this reason, we suggest that the sex-specific difference in the gap between middle-aged and elderly subjects’ minimum-mortality eGFR percentiles was related to disparities of muscle mass. Furthermore, for females, the decreased number of subjects above the minimum-mortality eGFR percentile caused glomerular hyperfiltration to be less clinically significant than in males, especially in the elderly group.
Subjects with diabetes or hypertension also had higher minimum-mortality eGFR percentiles than those without comorbidities, especially in the middle-aged group. Both diseases are well-known contributors to the development of chronic kidney disease (CKD). Given the increasing proportion of people with low eGFRs among patients with comorbidities, the minimum-mortality eGFR percentile tended to move upward. In this regard, the clinical significance of lower eGFR was prominent, meaning that physicians need to focus on patients with low eGFRs to reduce risk in these populations. Aging is also a relative risk factor for decreased eGFR, therefore the impact of the disease on eGFR was attenuated in the elderly group.
CKD is defined in terms of stages based on specific eGFR ranges, but extremely high eGFR, representing glomerular hyperfiltration, is not clearly defined by eGFR. Most research considering glomerular hyperfiltration was conducted with a definition as the 95th percentile after adjusting for age and sex [6]. However, the impact of glomerular hyperfiltration on all-cause mortality disappeared in the female group [20]. Having already moved towards a higher eGFR percentile for minimum mortality could attenuate the clinical impact of glomerular hyperfiltration, which was defined by percentile in females.
The lowest point of the U-shaped graph of all-cause mortality was moved to the right (higher eGFR) in the elderly group and the female group, but the usual pattern was maintained. Therefore we suggest that the clinically meaningful range could be differentially determined depending on the specific condition by not only the percentile of the eGFR, but also the absolute eGFR value. Considering that the eGFR value showing minimum risk for mortality does not change, unlike the eGFR percentile, a healthy eGFR could be defined as ∼90 mL/min/1.73 m2 regardless of age and sex. Nevertheless, evaluating the minimum-mortality percentile across the entire eGFR percentile spectrum could suggest the comparative impact of the eGFR percentile for all but the extreme groups, which are already regarded as disease populations.
This study was conducted with data from a large population-based cohort. We strictly excluded subjects with previous major comorbidities. In addition, we exclusively used eGFR based on the IDMS-traceable Cr value. However, there are several limitations to be discussed. First, the study design was retrospective in nature and its generalizability was limited in that it drew its subjects from only a single country. Second, we performed the analysis using only one measurement eGFR. Third, we could not adjust for the levels of urinary albumin excretion, which is independently associated with mortality. Fourth, we could not adjust for the history of medications that potentially affect the eGFR of the subjects. Fifth, we excluded subjects with a history of cerebrovascular or cardiovascular disease, which potentially limits the generalizability of the study results. In addition, the short duration of follow-up hinders the evaluation of outcomes.
The eGFR was closely related to all-cause mortality. This relationship changed in response to several conditions, such as age, sex and presence of comorbidities. Overall, low eGFR significantly increased the risk of all-cause mortality irrespective of various conditions. However, the minimum-mortality eGFR percentile increased in the elderly group and females. In contrast, the middle-aged group showed a significant impact of higher eGFR on all-cause mortality, with a U-shaped pattern, regardless of comorbidities. Thus, while it is mainly low eGFR that needs to be focused on in elderly people and those with comorbidities, high eGFR is of greater clinical interest than low eGFR in middle-aged people. These distinct approaches could suggest helpful guidelines for clinicians to evaluate and manage deterioration of renal function.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
This study was funded by the Seoul National University Hospital (3020180050). The funders had no role in the study design, data collection and analysis or decision to submit for publication.
AUTHORS’ CONTRIBUTIONS
Y.K. and D.K.K. contributed to the research idea and study design. S.L., Y.L. and Sehoon P. were responsible for the data acquisition. Sanghyun P. and K.H. contributed to the data analysis and interpretation. Sanghyun P. performed the statistical analysis. Y.C.K., S.S.H., H.L., J.P.L., K.W.J., C.S.L. and Y.S.K. were responsible for supervision. J.H.P., W.Y.P., K.J. and S.H. critically revised the manuscript. K.H. and D.K.K. are the guarantors of the paper. Each author contributed important intellectual content during manuscript drafting or revision and is accountable for the overall work by ensuring that any issues are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Antman EM, Anbe DT, Armstrong PW. et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation 2004; 110: 588–636 [DOI] [PubMed] [Google Scholar]
- 2. Sarnak MJ, Levey AS, Schoolwerth AC. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Circulation 2003; 108: 2154–2169 [DOI] [PubMed] [Google Scholar]
- 3. Matsushita K, Selvin E, Bash LD. et al. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 2009; 20: 2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turin TC, Coresh J, Tonelli M. et al. Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int 2013; 83: 684–691 [DOI] [PubMed] [Google Scholar]
- 5. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cachat F, Combescure C, Cauderay M. et al. A systematic review of glomerular hyperfiltration assessment and definition in the medical literature. Clin J Am Soc Nephrol 2015; 10: 382–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park M, Yoon E, Lim YH. et al. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 2015; 26: 1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Altay S, Onat A, Ozpamuk-Karadeniz F. et al. Renal “hyperfiltrators” are at elevated risk of death and chronic diseases. BMC Nephrol 2014; 15: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdelhafiz AH, Brown SH, Bello A. et al. Chronic kidney disease in older people: physiology, pathology or both? Nephron Clin Pract 2010; 116: c19–c24 [DOI] [PubMed] [Google Scholar]
- 10. Lindeman RD, Tobin J, Shock NW.. Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 1985; 33: 278–285 [DOI] [PubMed] [Google Scholar]
- 11. Pottel H, Delanaye P, Weekers L. et al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 2017; 10: 545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma A, Mucino MJ, Ronco C.. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 2014; 127: 94–100 [DOI] [PubMed] [Google Scholar]
- 13. Helal I, Fick-Brosnahan GM, Reed-Gitomer B. et al. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol 2012; 8: 293–300 [DOI] [PubMed] [Google Scholar]
- 14. Shin DW, Lee JW, Jung JH. et al. Disparities in cervical cancer screening among women with disabilities: a national database study in South Korea. J Clin Oncol 2018; 36: 2778–2786 [DOI] [PubMed] [Google Scholar]
- 15. Seong SC, Kim YY, Park SK. et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017; 7: e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheol Seong S, Kim YY, Khang YH. et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol 2017; 46: 799–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park S, Lee S, Kim Y. et al. Risk of cancer in pre-dialysis chronic kidney disease: a nationwide population-based study with a matched control group. Kidney Res Clin Pract 2019; 38: 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shimokata H, Ando F, Yuki A. et al. Age-related changes in skeletal muscle mass among community-dwelling Japanese: a 12-year longitudinal study. Geriatr Gerontol Int 2014; 14: 85–92 [DOI] [PubMed] [Google Scholar]
- 19. Serra-Prat M, Papiol M, Vico J. et al. Factors associated with poor muscle mass and strength in a community-dwelling elderly population: a cross-sectional study. J Gerontol Geriatr Res 2017; 6: 418 [Google Scholar]
- 20. Yoo KD, Yoon HJ, Hwang SS. et al. Different association between renal hyperfiltration and mortality by sex. Nephrology (Carlton) 2017; 22: 804–810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.