Abstract
Background
Children and adolescents undergoing allogeneic hematopoietic cell transplantation (HCT) are at high risk for invasive fungal disease (IFD).
Methods
This multicenter, randomized, open-label trial planned to enroll 560 children and adolescents (3 months to <21 years) undergoing allogeneic HCT between April 2013 and September 2016. Eligible patients were randomly assigned to antifungal prophylaxis with caspofungin or a center-specific comparator triazole (fluconazole or voriconazole). Prophylaxis was administered from day 0 of HCT to day 42 or discharge. The primary outcome was proven or probable IFD at day 42 as adjudicated by blinded central review. Exploratory analysis stratified this evaluation by comparator triazole.
Results
A planned futility analysis demonstrated a low rate of IFD in the comparator triazole arm, so the trial was closed early. A total of 290 eligible patients, with a median age of 9.5 years (range 0.3–20.7), were randomized to caspofungin (n = 144) or a triazole (n = 146; fluconazole, n = 100; voriconazole, n = 46). The day 42 cumulative incidence of proven or probable IFD was 1.4% (95% confidence interval [CI], 0.3%–5.4%) in the caspofungin group vs 1.4% (95% CI, 0.4%–5.5%) in the triazole group (P = .99, log-rank test). When stratified by specific triazole, there was no significant difference in proven or probable IFD at day 42 between caspofungin vs fluconazole (1.0%, 95% CI, 0.1%–6.9%, P = .78) or caspofungin vs voriconazole (2.3%, 95% CI, 0.3%–15.1%, P = .69).
Conclusions
In pediatric HCT patients, prophylaxis with caspofungin did not significantly reduce the cumulative incidence of early proven or probable IFD compared with triazoles. Future efforts to decrease IFD-related morbidity and mortality should focus on later periods of risk.
Trial Registration
Keywords: caspofungin, fluconazole, pediatric, transplant, voriconazole
In the current era, the incidence of invasive fungal disease (IFD) early after pediatric allogeneic hematopoietic cell transplantation (HCT) is low. Caspofungin and fluconazole prophylaxis is associated with similar rates of IFD early after pediatric allogeneic HCT.
Pediatric allogeneic hematopoietic cell transplant (HCT) recipients are at risk for invasive fungal disease (IFD), predominantly caused by Candida and Aspergillus species. The reported 1-year incidence of IFD in the pediatric HCT population is 7%–20%, with a 52%–83% case fatality [1–5]. The efficacy of fluconazole to prevent IFD was established in 2 placebo-controlled trials inclusive of patients greater than 12 years of age [6, 7]. An additional advantage of fluconazole prophylaxis included less acute gastrointestinal graft-vs-host disease (GVHD) compared with placebo [8]. Despite the lack of a pediatric-specific trial, pediatric guidelines reference adult trials in supporting a recommendation of fluconazole as the primary prophylactic agent [9, 10].
Fluconazole’s effectiveness is limited by its lack of activity against Aspergillus spp. and resistant Candida spp., such as C. krusei and C. glabrata. Alternatives to fluconazole include voriconazole and echinocandins that have broader activity. However, voriconazole failed to show superiority to fluconazole in a trial that included 51 children [11] and has significant drug interactions. The echinocandins, including caspofungin, represent a potentially better prophylactic option, given broad activity against Candida spp. and Aspergillus spp., few drug interactions, and a reassuring safety profile [12–14].
The primary objective was to determine if prophylaxis with caspofungin would be associated with a lower incidence of proven or probable IFD during the first 42 days following allogeneic HCT compared with prophylaxis with a triazole.
PATIENTS AND METHODS
Study Design
ACCL1131 was a randomized, open-label trial of caspofungin vs either fluconazole or voriconazole for the prevention of IFD in pediatric allogeneic HCT recipients conducted by the Children’s Oncology Group (COG) and approved by the National Cancer Institute’s Central Institution Review Board (IRB) and IRBs at each participating institution. Similar to a previous adult pragmatic trial, centers were required to declare their institutional standard-of-care comparator triazole (fluconazole or voriconazole) prior to enrollment of patients [15]. The protocol and statistical analytic plan are available (Supplementary 1). Patients or their guardians provided written informed consent and assent (if appropriate) prior to enrollment. The trial was registered at www.clinicaltrials.gov as NCT01503515.
Patients
Eligible patients were above or equal to 3 months of age (≥2 years at voriconazole centers) to younger than 21 years of age undergoing allogeneic HCT. Patients with any donor, stem cell source, and indication for HCT were permitted. Recipients of a matched sibling HCT were excluded prior to July 2014, then added via an amendment after data suggesting that their risk of IFD was similar to other allogeneic recipients [4]. Patients were required to have adequate performance level (Eastern Cooperative Oncology Group 0–2), renal function (glomerular filtration rate ≥70 mL/min/1.73 m2), and liver function (total bilirubin <2.5 mg/dL and transaminases <5× the upper limit of normal). Patients were excluded if they had a proven or probable invasive mold disease within 90 days of enrollment or were still undergoing treatment for a prior IFD. Patients with an elevated galactomannan (GM) level (≥0.5 index) within 30 days of enrollment were required to have undergone an evaluation (including chest computerized tomography [CT]) to exclude invasive aspergillosis.
Randomization and Blinding
Patients were randomized 1:1 to caspofungin (intervention group) or triazole (comparator group) prophylaxis in block sizes of four. The allocation sequence, generated by the COG trial management system, and block size were concealed to all investigators, clinicians, and patients. Randomization was stratified by the center choice of azole, age (≥1 to <12 years or ≥12 to <21 years), and type of HCT (umbilical cord blood [UCB] vs non-UCB with ex vivo T-cell depletion vs non-UCB with pharmacologic GVHD prophylaxis).
The study was open label; the known elevation of calcineurin inhibitors with concomitant fluconazole [16] or voriconazole [17] made blinding of the study drugs considered infeasible. However, the primary endpoint was adjudicated by a blinded central review committee (see later).
Study Treatments
Assigned prophylaxis was initiated within 24 hours of HCT day 0 and continued until day 42 or hospital discharge, whichever occurred sooner. Following the discontinuation of assigned prophylaxis, patients resumed local standard-of-care prophylaxis. Study drugs could be held for up to 7 days at physician discretion for possible toxicities, empiric therapy for fever and neutropenia, or presumed IFD. If the study drug could not be re-started within 7 days, the patient was removed from protocol therapy but was followed for IFD until the criteria to discontinue IFD monitoring (see later) were met.
Caspofungin was administered as a loading 70 mg/m2 (maximum 70 mg) intravenous infusion over 1 hour, followed by a 50 mg/m2 (maximum 50 mg) daily. Caspofungin was supplied by Merck and Company Inc., USA. Fluconazole was administered at a dose of 12 mg/kg once per day (maximum 400 mg/day) in patients younger than 18 years, and 6 mg/kg/day (maximum 400 mg/day) in patients between 18 and younger than 21 years, either by intravenous infusion over 1–2 hours or by mouth. Voriconazole was administered with a 1- to 2-hour intravenous loading dose of 6–9 mg/kg every 12 hours for 2 doses, followed by maintenance dosing of 4–8 mg/kg given over 1–2 hours intravenously, or 200–350 mg orally every 12 hours, with exact doses per kilogram according to both age and weight [18]. Subsequent doses of voriconazole were allowed to be dose-adjusted based on trough concentrations, when obtained per local practice, but this was not required by the study. Additional details regarding study drug dosing, including modifications for toxicity, are described in Supplementary 1.
Patients who developed fever lasting 3–5 days despite broad-spectrum antibiotic therapy were recommended to stop the assigned protocol therapy and start empiric antifungal therapy with a lipid formulation of amphotericin B. If empiric antifungal therapy was discontinued, patients were to resume the study drug. The study did not dictate the institutional approach to diagnosing IFD, and the local diagnosis of IFD did not influence the study-directed central adjudication of IFD. Patients with institutionally diagnosed IFD continued to be monitored centrally by study investigators until they met the study criteria (see later) to discontinue IFD monitoring.
Covariates collected were duration of protocol prophylaxis by day 42, absolute neutrophil count (ANC) recovery at day 42, days to ANC recovery (≥500 cells/ul), and days of exposure to systemic corticosteroids (methylprednisolone, prednisone, and/or hydrocortisone).
Monitoring for IFD began on the day of prophylaxis initiation and ended when the patient met the criterion for discontinuation of IFD monitoring (day 100, death, or lost-to-follow-up). An ancillary research study to evaluate the diagnostic properties of beta-d-glucan (BDG) was conducted within this study cohort, but the results (to be reported separately) were not disclosed to treating clinicians or central reviewers and did not inform the institutional or study-directed central adjudication of IFD.
Endpoints
The primary endpoint was the cumulative incidence of proven or probable IFD at day 42 post-HCT according to the European Organisation for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria [19]. An a priori rule agreed upon by the review committee dictated that “dense, well-circumscribed lesion(s)” in the lungs must be greater than or equal to 0.5 cm in size.
Central review of all IFD outcomes was conducted by 3 reviewers blinded to allocation. Central reviews were informed by clinically available source documents, comprising: pathology, autopsy, radiology (CT and magnetic resonance imaging), ophthalmology, bronchoscopy reports, and mycology results, including culture, molecular testing, serologies, and antigen assays (such as GM and BDG). Central reviewers viewed the source documents via webinar and made an IFD consensus designation by applying the EORTC/MSG criteria. Disagreements were resolved by consensus. For patients designated to have IFD, date, site of infection, and causative pathogen (if identified) were documented. If GM or BDG was used to meet probable IFD mycology criteria, then the pathogen was reported as Aspergillus not-otherwise-specified (NOS) and fungus NOS, respectively. If histopathology alone was used to meet proven IFD mycology criteria, then the pathogen was reported as mold NOS.
Exploratory endpoints included the cumulative incidence of proven or probable IFD at day 100, fungal-free survival (FFS) at days 42 and 100, and acute GVHD incidence and severity. FFS was defined as the absence of proven or probable IFD, or death. Acute GVHD was classified by standard criteria [20], and severity was defined by the incidence of grade II–IV GVHD and grade III–IV GVHD.
Post hoc outcomes included the cumulative incidence of proven, probable, or possible IFD by days 42 and 100, overall survival (OS) by day 100, and treatment-related mortality (TRM) by day 100. TRM was defined as death occurring in the absence of progressive underlying disease [21].
Nonhematological grade 4 or greater adverse events were reported using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Statistical Analysis
Eligible patients were analyzed according to their randomization group irrespective of adherence to protocol therapy. Ineligible patients were excluded. The planned sample size of 560 patients assumed the cumulative incidence of proven or probable IFD would be 2% with caspofungin and 7% with triazole at day 42, assuming 80% power at a 2-sided log-rank test with an alpha level of 0.05. Power calculation assumed an exponential time-to-IFD model and a 5% exponential loss-to-follow-up during the at-risk period.
Primary Outcome Analysis
The primary analysis compared time from day 0 post-HCT to proven or probable IFD by day 42 post-HCT. Patients without an event were censored when they met the criteria for discontinuation of IFD monitoring or day 42, whichever occurred first. The 42-day cumulative incidence and its 95% confidence interval (CI) were described and compared between groups using the log-rank test.
Exploratory Outcome Analyses
A similar analytic approach used for the primary outcome was also applied for the cumulative incidence of proven or probable IFD at day 100. These analyses at days 42 and 100 were also performed for comparisons between caspofungin vs individual triazole (fluconazole or voriconazole). The incidence and severity of acute GVHD were compared by Chi-square test.
Post hoc Analyses
The estimated cumulative incidence of proven, probable, or possible IFD by days 42 and 100, OS by day 100, and TRM by day 100 were compared between groups using the log-rank test.
Interim Analysis
Formal interim monitoring analyses for efficacy and futility on the primary outcome were planned to be performed after one-third and two-thirds of patients completed study treatment and IFD observation, with monitoring boundary based on Lan–Demets’ method with the second power spending function of αt2 [22]. The futility analysis mandated that if the 95% 1-sided CI for the observed incidence of IFD during the first 42 days following HCT in patients randomized to the comparator group did not contain the assumed rate of 7%, the study would be referred to the Data Safety and Monitoring Committee (DSMC) for consideration of the closure.
All tests of significance were 2-sided and P < .05 was considered statistically significant. Analyses were conducted using the SAS statistical program (SAS-PC, version 9.4; SAS Institute Inc., Cary, NC).
RESULTS
Study Population
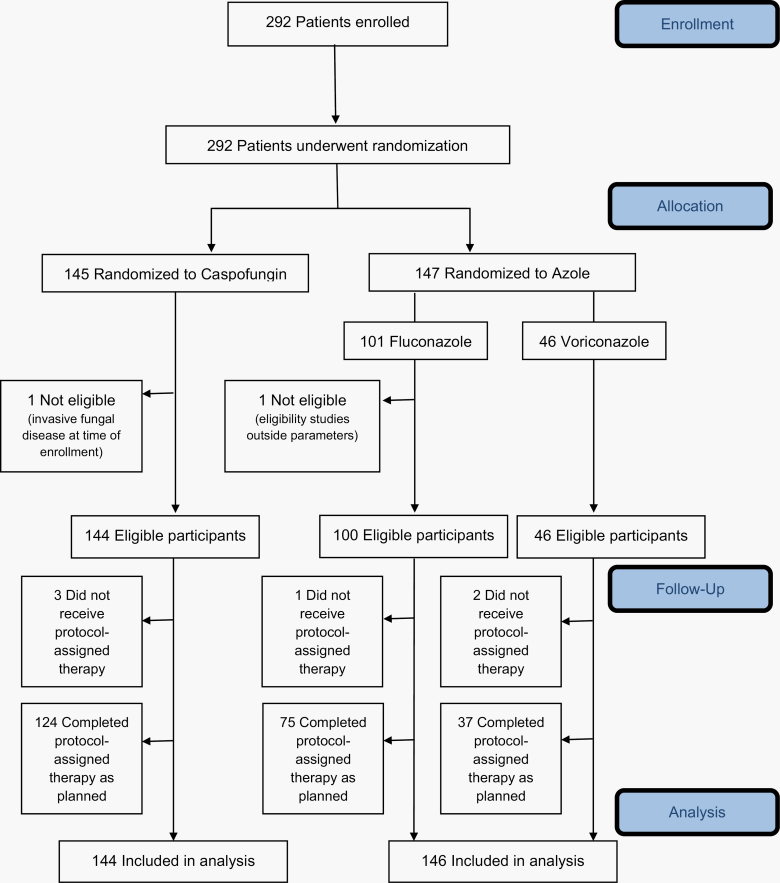
Between April 2013 and September 2016, 292 patients (caspofungin [n = 145] and triazole [n = 147]) were enrolled at 31 institutions (Figure 1). Two patients were ineligible, resulting in 290 patients (caspofungin [n = 144] and triazole [n = 146] with 100 fluconazole and 46 voriconazole) included in the analysis. Baseline patient, disease (Table 1), and transplant (Table 2) characteristics were similar across treatment groups. Table 3 presents post-randomization factors that might affect IFD incidence by the treatment group. No major differences were observed.
Figure 1.
Study participation and flow through the trial (CONSORT diagram).
Table 1.
Demographic Baseline Characteristics by Study Group
| Characteristic | Caspofungin (n = 144) | Combined Triazole (n = 146) | Fluconazole (n = 100) | Voriconazole (n = 46) |
|---|---|---|---|---|
| Age in years, median (range) | 10.0 (0.3–20.5) | 9.4 (0.3–20.7) | 8.7 (0.3–20.0) | 11.9 (2.2–20.7) |
| Sex (n, %) | ||||
| Male | 89 (61.8) | 90 (61.6) | 65 (65.0) | 25 (54.3) |
| Female | 55 (38.2) | 56 (38.4) | 35 (35.0) | 21 (45.7) |
| Race (n, %) | ||||
| White | 109 (75.7) | 97 (66.4) | 63 (63.0) | 34 (73.9) |
| Black | 11 (7.6) | 22 (15.1) | 13 (13.0) | 9 (19.6) |
| Asian | 9 (6.3) | 6 (4.1) | 5 (5.0) | 1 (2.2) |
| Other | 4 (2.8) | 2 (1.4) | 2 (2.0) | 0 (0) |
| Unknown | 11 (7.6) | 19 (13) | 17 (17.0) | 2 (4.3) |
| Ethnicity (n/N, %) | ||||
| Hispanic | 26/138 (18.8) | 26/133 (19.5) | 16/92 (17.4) | 10/41 (24.4) |
| ECOG performance score | ||||
| 0 (fully active) | 96 (66.7) | 103 (70.5) | 74 (74.0) | 29 (63.0) |
| 1 (restricted strenuous) | 38 (26.4) | 41 (28.1) | 24 (24.0) | 17 (37.0) |
| 2 (ambulatory) | 10 (6.9) | 2 (1.4) | 2 (2.0) | 0 (0) |
| Disease (n, %) | ||||
| Malignancy | 99 (68.8) | 106 (72.6) | 67 (67.0) | 39 (84.8) |
| ALL | 44 (30.6) | 47 (32.2) | 30 (30.0) | 17 (37.0) |
| AML/MDS | 30 (20.8) | 45 (30.8) | 29 (29.0) | 16 (34.8) |
| Other leukemiaa | 11 (7.6) | 8 (5.5) | 6 (6.0) | 2 (4.3) |
| Other malignancyb | 14 (9.7) | 6 (4.1) | 2 (2.0) | 4 (8.7) |
| Nonmalignancy | 45 (31.2) | 40 (27.4) | 33 (33.0) | 7 (15.2) |
| Primary immunodeficiency | 14 (9.7) | 10 (6.8) | 7 (7.0) | 3 (6.5) |
| Bone marrow failure | 10 (6.9) | 12 (8.2) | 8 (8.0) | 4 (8.7) |
| Hemoglobinopathy | 10 (6.9) | 8 (5.5) | 8 (8.0) | 0 (0) |
| Metabolic syndrome | 11 (7.6) | 9 (6.2) | 9 (9.0) | 0 (0) |
| Missing | 0 (0) | 1 (0.7) | 1 (1.0) | 0 (0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; JMML, juvenile myelomonocytic leukemia.
aIncludes CML, JMML, leukemia not-otherwise-specified, and T-cell large granular lymphocytic leukemia.
bIncludes Hodgkin lymphoma, non-Hodgkin lymphoma, and solid tumors.
Table 2.
Transplant Baseline Characteristics by Study Group
| Characteristic | Caspofungin (n = 144) | Combined Triazole (n = 146) | Fluconazole (n = 100) | Voriconazole (n = 46) |
|---|---|---|---|---|
| Donor type (n, %) | ||||
| Matched related | 31 (21.5) | 25 (17.1) | 15 (15.0) | 10 (21.7) |
| Mismatched related | 11 (7.6) | 9 (6.2) | 6 (6.0) | 3 (6.5) |
| Unrelated | 102 (70.8) | 112 (76.7) | 79 (79.0) | 33 (71.8) |
| Graft source | ||||
| Bone marrowa | 77 (53.5) | 83 (56.8) | 57 (57.0) | 26 (56.6) |
| Peripheral blood | 39 (27.1) | 35 (24) | 25 (25.0) | 10 (21.7) |
| Umbilical cord blood | 28 (19.4) | 28 (19.2) | 18 (18.0) | 10 (21.7) |
| Donor HLA Match | ||||
| BM/PBSC 8/8 | 73 (50.7) | 70 (47.9) | 49 (49.0) | 21 (45.7) |
| BM/PBSC <8/8 | 17 (11.8) | 27 (18.5) | 17 (17.0) | 10 (21.7) |
| UCB no known mismatch (6/6 or 8/8) | 7 (4.9) | 5 (3.4) | 3 (3.0) | 2 (4.3) |
| UCB at least 1 mismatch | 16 (11.1) | 20 (13.7) | 12 (12.0) | 8 (17.4) |
| Unknown | 31 (21.5) | 24 (16.4) | 19 (19.0) | 5 (10.9) |
| Conditioning Regimen | ||||
| TBI-Based | 58 (40.3) | 58 (39.7) | 35 (35.0) | 23 (50.0) |
| Non-TBI Based | 86 (59.7) | 88 (60.3) | 65 (65.0) | 23 (50.0) |
| Serotherapy | ||||
| None | 56 (38.9) | 55 (37.7) | 32 (32.0) | 23 (50.0) |
| Antithymocyte Globulin | 62 (43.1) | 65 (44.5) | 50 (50.0) | 15 (32.6) |
| Alemtuzumabb | 26 (18.1) | 26 (17.8) | 18 (18.0) | 8 (17.4) |
| GVHD Prophylaxis | ||||
| CNI + 2 agents | 18 (12.5) | 14 (9.6) | 8 (8.0) | 6 (13.0) |
| CNI + methotrexate | 67 (46.5) | 69 (47.3) | 50 (50.0) | 19 (41.3) |
| CNI + other | 18 (12.5) | 27 (18.5) | 17 (17.0) | 10 (21.8) |
| CNI alone | 14 (9.7) | 13 (8.9) | 8 (8.0) | 5 (10.9) |
| Other | 19 (13.2) | 16 (11.0) | 10 (10.0) | 6 (13.0) |
| None (ex vivo T-cell depletion) | 8 (5.6) | 7 (4.8) | 7 (7.0) | 0 (0) |
Abbreviations: HLA, human leukocyte antigen; BM, bone marrow; PBSC, peripheral blood stem cells; UCB, umbilical cord blood; TBI, total-body irradiation; GVHD, graft-vs-host disease; CNI, calcineurin inhibitor.
aIncludes 4 patients who received both bone marrow and cord blood.
bIncludes 2 patients who received both antithymocyte globulin and alemtuzumab.
Table 3.
Post Randomization Factors Stratified by Group
| Factor | Caspofungin (n = 144) | Combined Triazole (n = 146) | Fluconazole (n = 100) | Voriconazole (n = 46) |
|---|---|---|---|---|
| Median days on protocol prophylaxis (range) | 29.5 (0–44) | 28.5 (0–45) | 30 (0–44) | 23 (0–45) |
| Patients receiving other antifungal therapy during protocol-assigned therapy period, n (%) | 24 (16.7%) | 33 (22.6%) | 27 (27%) | 6 (13%) |
| ANC recovery at day 42 (95% CI) | 97.9% (92.7%–99.5%) | 93.8% (89.0%–96.9%) | 94% (86.2%–97.5%) | 93.1% (83.2%–98.2%) |
| Median days to ANC recovery from HCT (IQR)a | 18 (15, 22) | 17 (14, 23) | 18.5 (16, 25) | 14 (11, 18) |
| Patients receiving systemic corticosteroids (through day 100 or end of IFD monitoring), n (%) | 91 (63.2%) | 95 (65.1%) | 63 (63%) | 32 (69.6%) |
| Percent of days on systemic corticosteroids (through day 100 or end of IFD monitoring) (IQR)b | 21% (6, 51) | 38% (11, 60) | 24% (6, 53) | 49% (20, 70) |
Abbreviations: IFD, invasive fungal disease; CI, confidence interval; ANC, absolute neutrophil count; HCT, hematopoietic cell transplant; IQR, interquartile range.
aFor those patients who recovered by Day 42.
bFor those patients who received systemic corticosteroids.
The first planned interim futility analysis based on June 30, 2016 data demonstrated the 95% 1-sided CI for observed incidence of IFD did not contain the assumed rate of 7% in the comparator group, resulting in DSMC recommendation for study closure to new enrollments. All enrolled patients completed study treatments and observation. The final analysis was based upon June 30, 2019 data.
Primary Outcome
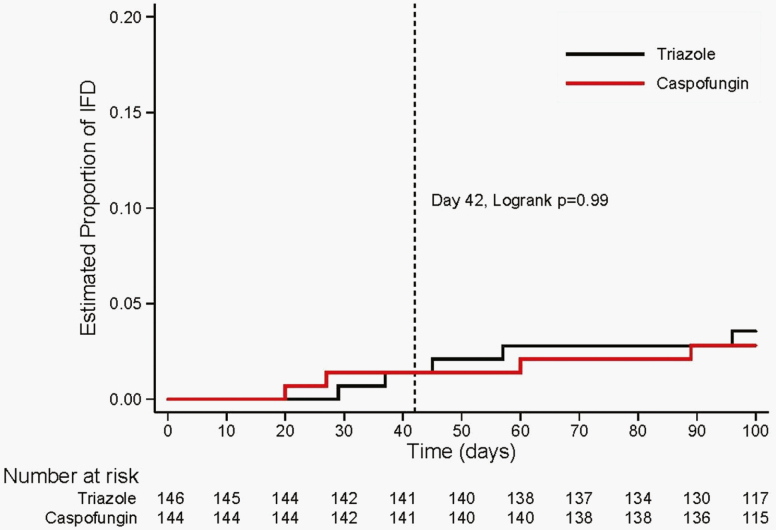
The estimated cumulative incidence of proven or probable IFD at 42 days post-HCT was 1.4% (95% CI, 0.3%–5.4%) in the caspofungin group and 1.4% (95% CI, 0.4%–5.5%) in the triazole group (P = .99) (Table 4 and Figure 2). The 4 proven or probable IFD events (2 in the caspofungin group and 2 in the triazole group) consisted of 1 Candida spp. and 3 other fungi (fungus NOS, n = 1; mold NOS, n = 2; Table 5).
Table 4.
Cumulative Incidence of Invasive Fungal Disease and Other Outcomes by Main Study Group
| Study Outcome | Caspofungin (n = 144) | Combined Triazole or Specific Triazole for Subgroup Analysis (n = 146) | P-value | ||
|---|---|---|---|---|---|
| Events | Probability (95% CI) | Events | Probability (95% CI) | ||
| Primary outcome | |||||
| Proven or probable IFD at day 42 | 2 | 1.4 (0.3–5.4) | 2 | 1.4 (0.4–5.5) | .99 |
| Exploratory outcomes | |||||
| Proven or probable IFD at day 100 | 4 | 2.8 (1.1–7.3) | 5 | 3.6 (1.5–8.4) | .73 |
| Proven or probable IFD at day 42 | |||||
| Caspofungin vs. fluconazole | 2 | 1.4 (0.3–5.4) | 1 | 1.0 (0.1–6.9) | .78 |
| Caspofungin vs. voriconazole | 2 | 1.4 (0.3–5.4) | 1 | 2.3 (0.3–15.1) | .69 |
| Proven or probable IFD at day 100 stratified by triazole | |||||
| Caspofungin vs. fluconazole | 4 | 2.8 (1.1–7.3) | 4 | 4.2 (1.6–10.7) | .59 |
| Caspofungin vs. voriconazole | 4 | 2.8 (1.1–7.3) | 1 | 2.3 (0.3–15.1) | .85 |
| Fungal-free survival | |||||
| At day 42 | 3 | 97.9 (93.7–99.3) | 3 | 97.9 (93.7–99.3) | .99 |
| At day 100 | 8 | 94.3 (88.9–97.1) | 13 | 90.8 (84.8–94.6) | .26 |
| Proportion with acute GVHD | |||||
| Grade I-IV | 58 | 40.3 (32.3–48.3) | 64 | 43.8 (35.8–51.9) | .54 |
| Grade II-IV | 38 | 26.4 (19.2–33.6) | 43 | 29.5 (22.1–36.9) | .56 |
| Grade III-IV | 17 | 11.8 (6.5–17.1) | 25 | 17.1 (11.0–23.2) | .2 |
| Post hoc outcomes | |||||
| Proven, probable, or possible IFD | |||||
| At day 42 | 4 | 2.8 (1.1–7.3) | 6 | 4.2 (1.9–9.1) | .52 |
| At day 100 | 7 | 4.9 (2.4–10.1) | 9 | 6.3 (3.3–11.8) | .59 |
| Overall survival at day 100 | 4 | 97.2 (92.7–98.9) | 8 | 94.4 (89.2–97.2) | .25 |
| Treatment-related mortality at day 100 | 2 | 1.4 (0.3–5.4) | 4 | 2.8 (1.1–7.3) | .41 |
Abbreviations: CI, Confidence Interval; IFD, Invasive Fungal Disease; GVHD, Graft-vs-Host Disease.
Figure 2.
Cumulative incidence of proven/probable invasive fungal disease.
Table 5.
Organisms Causing Invasive Fungal Disease by Study Group
| Caspofungin (n = 144) | Combined Triazole (n = 146) | Fluconazole (n = 100) | Voriconazole (n = 46) | |
|---|---|---|---|---|
| By day 42 | ||||
| Proven | 2 | 1 | 1 | 0 |
| Candida glabrata | 1 | 0 | 0 | 0 |
| Aspergillus spp. | 0 | 0 | 0 | 0 |
| Other fungia | 1 | 1 | 1 | 0 |
| Probable | 0 | 1 | 0 | 1 |
| Candida spp. | 0 | 0 | 0 | 0 |
| Aspergillus spp. | 0 | 0 | 0 | 0 |
| Other fungib | 0 | 1 | 0 | 1 |
| Day 43–100 | ||||
| Proven | 1 | 1 | 1 | 0 |
| Candida lusitaniae | 0 | 1 | 1 | 0 |
| Aspergillus spp. | 0 | 0 | 0 | 0 |
| Rhizopus spp. | 1 | 0 | 0 | 0 |
| Probable | 1 | 2 | 2 | 0 |
| Candida spp. | 0 | 0 | 0 | 0 |
| Aspergillus spp.c | 1 | 1 | 1 | 0 |
| Other fungib | 0 | 1 | 1 | 0 |
Abbreviation: NOS, not otherwise specified.
aMold NOS (caspofungin arm) and mold NOS (fluconazole arm).
bFungus NOS.
c Aspergillus NOS.
Exploratory Outcomes
The estimated cumulative incidence of proven or probable IFD at day 100 was 2.8% (95% CI, 1.1%–7.3%) in the caspofungin group and 3.6% (95% CI, 1.5%–8.4%) in the triazole group (P = .73). Between days 43 and 100 post-HCT, an additional 5 proven or probable IFD events occurred (2 in the caspofungin group and 3 in the triazole group) that consisted of 1 Candida spp., 2 Aspergillus spp. and 2 other fungi (Rhizopus spp., n = 1; fungus NOS, n = 1; Table 5). By day 100 post-HCT, among the 9 cases of proven or probable IFD, 22% were caused by Candida spp., 22% by Aspergillus spp., and 56% by other fungi, though the latter group may have included non-identified Aspergillus spp.
When stratified by comparator group, the estimated cumulative incidence of proven or probable IFD at day 42 was 1.4% (95% CI, 0.3%–5.4%) in the caspofungin group and 1.0% (95% CI, 0.1%–6.9%) in the fluconazole group (P = .78), and at day 100 was 2.8% (95% CI, 1.1%–7.3%) in the caspofungin group and 4.2% (95% CI, 1.6%–10.7%) in the fluconazole group (P = .59). The estimated cumulative incidence of proven or probable IFD at day 42 was 1.4% (95% CI, 0.3%–5.4%) in the caspofungin group and 2.3% (95% CI, 0.3%–15.1%) in the voriconazole group (P = .69), and at day 100 was 2.8% (95% CI, 1.1%–7.3%) in the caspofungin group and 2.3% (95% CI, 0.3%–15.1%) in the voriconazole group (P = .85). Table 4 also presents data demonstrating that FFS at days 42 and 100, as well as the proportion of patients with acute GVHD, were similar by randomized group.
Post Hoc Outcomes
When possible cases of IFD were considered, there were no significant differences between the caspofungin and triazole groups at day 42 (P = .52) and day 100 (P = .59) post-HCT. The estimated 100-day post-HCT OS was 97.2% (95% CI, 92.7%–98.9%) and 94.4% (95% CI, 89.2%–97.2%) for the caspofungin and triazole groups, respectively (P = .25). There was no significant difference in 100-day TRM. During the period of protocol-assigned therapy, at least one grade 4–5 toxicity was reported in 12.1% and 14% of caspofungin and triazole groups, respectively (Supplementary 2), with most events determined by the treating physician to be unrelated to study drug.
DISCUSSION
In this multicenter, randomized trial in children and adolescents, antifungal prophylaxis with caspofungin did not significantly reduce proven or probable IFD at day 42 post-allogeneic HCT compared with triazoles (fluconazole or voriconazole). There were no significant differences in proven or probable IFD at day 100, incidence or severity of acute GVHD, or FFS at day 42 or 100.
This was the first multicenter pediatric-focused trial of antifungal prophylaxis for HCT recipients. It was designed to test whether caspofungin, a newer antifungal agent, would be superior to the gold standard of triazoles in the early post-HCT period. While it is recognized that many patients develop IFD later post-HCT during the treatment for GVHD, reducing IFD in the early post-HCT period was of interest due to high case of fatality rates. The trial was closed early because of a low event rate in the comparator group. Previous observational cohort studies described incidences of 9%–21% in pediatric allogeneic HCT recipients by 1-year post-HCT IFD, with median times to IFD diagnosis of 25–34 days post-HCT [1, 4, 23, 24]. In this randomized trial, the cumulative incidence of IFD by day 42 was less than 2% in pediatric and adolescent patients receiving either fluconazole or voriconazole prophylaxis. There are several possible explanations for the low event rate in our comparator arm. First, it is possible that contemporary infection control and supportive care practices have lowered the IFD risk early post-HCT, as suggested by recent reports [5]. Specifically, an increasing number of HCT patients, such as those with acute myeloid leukemia (AML), receive antifungal prophylaxis in the pre-HCT period, which may abrogate their IFD risk early post-HCT [25, 26]. We captured IFD events out to days 42 and 100 post-HCT but may have missed a later peak in IFD during the treatment for GVHD. Second, previous reports may have overestimated IFD events, possibly due to lack of application of the EORTC/MSG criteria to define IFD events, or inclusion of patients with a history of IFD pre-HCT. Third, we may have missed IFD events, as we relied upon centers performing the tests required to diagnose an IFD rather than mandating a specific diagnostic approach. However, this practice is in alignment with most observational studies and we systematically collected primary clinical source data for all patients in a manner similar to a COG trial conducted in patients with AML [26]. Fourth, despite the suggestion that matched sibling HCT recipients have similar IFD risk [4], their inclusion may have lowered the IFD incidence. Fifth, fluconazole centers may have elected to not enroll patients whom they clinically considered to be high risk for IFD in order to administer alternate antifungal prophylaxis. The COG infrastructure does not capture information on screened, but not enrolled, patients, so that the impact of this concern cannot be quantified. Finally, the inclusion of voriconazole in the comparator group may have reduced the IFD rate, although this concern was not supported by stratified analysis of the comparator group.
The strengths of this study include its multicenter design, randomized nature, and utilization of a blinded central review. While the data from this trial do not inform the superiority of a specific antifungal prophylaxis agent in the immediate post-HCT period, they do offer insights into the fungal pathogens that breakthrough different antifungal prophylaxis regimens. Only 44% of the proven or probable IFD events were definitely invasive candidiasis or aspergillosis (though some cases of aspergillosis may have lacked sufficient data to categorize as such). This suggests that while current prophylactic strategies are generally effective for the prevention of IFD, additional development of diagnostic tools for identifying non-Aspergillus molds may be needed [27]. Future efforts to prevent IFD early after pediatric and adolescent HCT should be focused on interventions during later periods after HCT when patients are being treated for GVHD, as well as the creation, validation, and implementation of risk-stratification tools, including potential genetic determinants of risk for IFD development [28].
This study has several limitations. First, there was potential for differential cessation of assigned prophylaxis, though the duration of randomized therapy was similar between allocated groups. Second, diagnostic testing was dictated by participating centers. Differential clinician testing for an IFD may have limited the overall incidence of IFD and altered the incidence by treatment group [29]. Study interpretation is also limited by early termination due to a planned interim analysis that suggested futility. Importantly, we did not evaluate prophylaxis or describe IFD rates through the period of immunosuppression for GVHD; this is an important knowledge gap.
In conclusion, in pediatric patients, prophylaxis with caspofungin did not significantly reduce the cumulative incidence of proven or probable IFD compared with triazoles in the first 100 days following allogeneic HCT. As IFD is an important cause of morbidity and mortality following pediatric allogeneic HCT, future efforts should focus on later periods of risk as well as identification of individuals at increased risk for development of this complication.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Authors contributions. C. C. D., B. T . F., H. D., D. V., M. L. N., L. C., M. S., T. E. Z., and L. S. contributed to the study design, patient recruitment, data collection, and data analysis. A. J. E., S. A., and W. J. S. served on the blinded central review committee. C. C. D. wrote the manuscript; all authors commented on and revised the manuscript.
Acknowledgments. The authors would like to thank Mark Sorenson, Mary Bancroft, Bayrta Prosvirova, Tanya Shannon, Sarah Vargas, Linda Li, Alison M. Friedmann, Sabah-E-Noor Servaes, and Andrea Sheehan for their assistance with this study.
Disclaimer.. H. D. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The National Cancer Institute was the study sponsor and reviewed and approved the protocol and integrated study. Apart from the DSMB, the study sponsor did not have a role in the conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Financial support. This work was supported by research funding from the Children’s Oncology Group, the .National Cancer Institute at the .National Institutes of Health (award numbers U10CA 095861, U10CA 180899, and UG1CA 189955), and the ..St. Baldrick’s Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health Division of Cancer Prevention. Caspofungin and funds for centralized shipping of drugs were provided by Merck, Inc. via an investigator-initiated study program.
Potential conflicts of interest. C. C. D. served on scientific advisory boards for Alexion Inc., Jazz Pharmaceuticals, and Atara Bio. B. F.’s institution receives funding from Merck and Pfizer for research studies not related to this project. B. F. Also serves as a data safety monitoring board for Astellas. T. E. Z. provides consultant services for T2 Biosystems and Nabriva Therapeutics. The remaining authors declare no competing financial interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability
Individual-level de-identified datasets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use.
References
- 1. Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2005; 36:621–9. [DOI] [PubMed] [Google Scholar]
- 2. Hovi L, Saarinen-Pihkala UM, Vettenranta K, Saxen H. Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant 2000; 26:999–1004. [DOI] [PubMed] [Google Scholar]
- 3. Mikulska M, Raiola AM, Bruno B, et al. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant 2009; 44:361–70. [DOI] [PubMed] [Google Scholar]
- 4. Hol JA, Wolfs TF, Bierings MB, et al. Predictors of invasive fungal infection in pediatric allogeneic hematopoietic SCT recipients. Bone Marrow Transplant 2014; 49:95–101. [DOI] [PubMed] [Google Scholar]
- 5. Linke C, Ehlert K, Ahlmann M, et al. Epidemiology, utilisation of healthcare resources and outcome of invasive fungal diseases following paediatric allogeneic haematopoietic stem cell transplantation. Mycoses 2020; 63:172–80. [DOI] [PubMed] [Google Scholar]
- 6. Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med 1992; 326:845–51. [DOI] [PubMed] [Google Scholar]
- 7. Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation–a prospective, randomized, double-blind study. J Infect Dis 1995; 171:1545–52. [DOI] [PubMed] [Google Scholar]
- 8. Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 2000; 96:2055–61. [PubMed] [Google Scholar]
- 9. Tomblyn M, Chiller T, Einsele H, et al. ; Center for International Blood and Marrow Research; National Marrow Donor program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention . Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Science M, Robinson PD, MacDonald T, et al. Guideline for primary antifungal prophylaxis for pediatric patients with cancer or hematopoietic stem cell transplant recipients. Pediatr Blood Cancer 2014; 61:393–400. [DOI] [PubMed] [Google Scholar]
- 11. Wingard JR, Carter SL, Walsh TJ, et al. ; Blood and Marrow Transplant Clinical Trials Network . Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood 2010; 116:5111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zaoutis T, Lehrnbecher T, Groll AH, et al. Safety experience with caspofungin in pediatric patients. Pediatr Infect Dis J 2009; 28:1132–5. [DOI] [PubMed] [Google Scholar]
- 13. Koo A, Sung L, Allen U, et al. Efficacy and safety of caspofungin for the empiric management of fever in neutropenic children. Pediatr Infect Dis J 2007; 26:854–6. [DOI] [PubMed] [Google Scholar]
- 14. van Burik JA, Ratanatharathorn V, Stepan DE, et al. ; National Institute of Allergy and Infectious Diseases Mycoses Study Group . Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39:1407–16. [DOI] [PubMed] [Google Scholar]
- 15. Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007; 356:348–59. [DOI] [PubMed] [Google Scholar]
- 16. Osowski CL, Dix SP, Lin LS, et al. Evaluation of the drug interaction between intravenous high-dose fluconazole and cyclosporine or tacrolimus in bone marrow transplant patients. Transplantation 1996; 61:1268–72. [DOI] [PubMed] [Google Scholar]
- 17. Trifilio SM, Scheetz MH, Pi J, Mehta J. Tacrolimus use in adult allogeneic stem cell transplant recipients receiving voriconazole: preemptive dose modification and therapeutic drug monitoring. Bone Marrow Transplant 2010; 45:1352–6. [DOI] [PubMed] [Google Scholar]
- 18. Friberg LE, Ravva P, Karlsson MO, Liu P. Integrated population pharmacokinetic analysis of voriconazole in children, adolescents, and adults. Antimicrob Agents Chemother 2012; 56:3032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Pauw B, Walsh TJ, Donnelly JP, et al. ; European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15:825–8. [PubMed] [Google Scholar]
- 21. Alexander S, Pole JD, Gibson P, et al. ; International Pediatric Oncology Mortality Classification Group . Classification of treatment-related mortality in children with cancer: a systematic assessment. Lancet Oncol 2015; 16:e604–10. [DOI] [PubMed] [Google Scholar]
- 22. Kim K, DeMets D. Design and analysis of group sequential tests based on the type I error spending rate function. Biometrika 1987; 74:149–54. [Google Scholar]
- 23. Benjamin DK Jr, Miller WC, Bayliff S, et al. Infections diagnosed in the first year after pediatric stem cell transplantation. Pediatr Infect Dis J 2002; 21:227–34. [DOI] [PubMed] [Google Scholar]
- 24. Girmenia C, Raiola AM, Piciocchi A, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant 2014; 20:872–80. [DOI] [PubMed] [Google Scholar]
- 25. Fisher BT, Kavcic M, Li Y, et al. Antifungal prophylaxis associated with decreased induction mortality rates and resources utilized in children with new-onset acute myeloid leukemia. Clin Infect Dis 2014; 58:502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fisher BT, Zaoutis T, Dvorak CC, et al. Effect of caspofungin vs fluconazole prophylaxis on invasive fungal disease among children and young adults with acute myeloid leukemia: a randomized clinical trial. JAMA 2019; 322:1673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steinbach A, Cornely OA, Wisplinghoff H, et al. Mould-reactive T cells for the diagnosis of invasive mould infection–a prospective study. Mycoses 2019; 62:562–9. [DOI] [PubMed] [Google Scholar]
- 28. Fisher CE, Hohl TM, Fan W, et al. Validation of single nucleotide polymorphisms in invasive aspergillosis following hematopoietic cell transplantation. Blood 2017; 129:2693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ceesay MM, Desai SR, Berry L, et al. A comprehensive diagnostic approach using galactomannan, targeted β-d-glucan, baseline computerized tomography and biopsy yields a significant burden of invasive fungal disease in at risk haematology patients. Br J Haematol 2015; 168:219–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual-level de-identified datasets that would be sufficient to reproduce results provided in a publication containing the primary study analysis can be requested from the NCTN/NCORP Data Archive at https://nctn-data-archive.nci.nih.gov/. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use.