Abstract
Background
Baloxavir marboxil has demonstrated safety and efficacy in treating adult and adolescent outpatients with acute influenza (CAPSTONE-1 trial). Here, we report a subgroup analysis of outcomes in adolescents from the trial.
Methods
CAPSTONE-1 was a randomized, double-blind, placebo-controlled study. Eligible adolescent outpatients (aged 12-17 years of age) were randomized in a ratio of 2:1 to a single dose of baloxavir 40/80 mg if less than/greater than or equal to 80 kg or placebo. The main outcomes were the time to alleviation of symptoms (TTAS), duration of infectious virus detection, and incidence of adverse events (AEs).
Results
Among 117 adolescent patients, 90 (77%) comprised the intent-to-treat infected population (63 baloxavir and 27 placebo; 88.9% A(H3N2)). The median TTAS was 38.6 hours shorter (95% confidence interval: −2.6, 68.4) in the baloxavir group compared with placebo (median TTAS, 54.1 hours vs 92.7 hours, P = .0055). The median time to sustained cessation of infectious virus detection was 72.0 hours for baloxavir compared with 120.0 hours for placebo recipients (P < .0001). Treatment-emergent PA/I38X-substituted viruses were detected in 5 of the 51 (9.8%) baloxavir recipients. In the safety population (76 baloxavir and 41 placebo), AEs were less common in baloxavir than placebo recipients (17.1% vs 34.1%; P = .0421). In the baloxavir group, no AEs except for diarrhea were reported in 2 or more patients.
Conclusions
Baloxavir demonstrated clinical and virologic efficacy in the otherwise healthy adolescents with acute influenza compared with placebo. There were no safety concerns identified. These results were similar to the adult population in CAPSTONE-1 and support baloxavir as a treatment option in adolescents.
Keywords: adolescent, baloxavir, cap-dependent endonuclease, influenza
Single-dose baloxavir marboxil was clinically and virologically effective in otherwise healthy adolescent influenza patients (12-17 years) in CAPSTONE-1 randomized, double-blind, placebo-controlled study. This analysis supports the use of baloxavir marboxil in an adolescent population with uncomplicated influenza.
Influenza is a significant cause of morbidity in pediatric and adolescent patient populations. The Centers for Disease Control and Prevention estimates that over 5 consecutive influenza seasons from 2014–2015 influenza illnesses occurred in US patients at rates of 11 028 to 18 448 and 7705 to 14 300 per 100 000 patients aged 0–4 years and 5–17 years, respectively [1]. Patients aged 0–17 years were estimated to have accounted for 11.3 million symptomatic illnesses (31.8% of total), 6.4 million medical visits (38.8% of total), 46 340 hospitalizations (9.5% of total), and 477 deaths (1.4% of total) in 2018–2019 influenza season [1]. An analysis of UK data collected between 2006 and 2011 found that infection rates were typically highest in children aged 5–15 years in seasonal and pandemic influenza A [2], and influenza has been associated with 16% of respiratory hospitalizations in children 5–17 years old worldwide [3].
Current treatment options that are used in pediatric and adolescent patients are neuraminidase inhibitors, particularly oseltamivir, but data in adolescents (age 12-17 years) are limited. Systematic meta-analysis demonstrated treatment with oseltamivir led to a reduction in the duration of influenza illness in adolescents (age 12-17 years) [4]. Oseltamivir appears to have better clinical efficacy in reducing the duration of influenza symptoms and fever in influenza A, compared with influenza B, in children [5, 6]. Oseltamivir therapy has been associated with the emergence of resistant variants, including global circulation of oseltamivir-resistant seasonal A(H1N1) in 2008–2009. Clearly, there is an unmet need for new agents for the treatment of influenza for use in the pediatric and adolescent populations.
Baloxavir marboxil (hereafter, baloxavir) is a selective cap-dependent endonuclease inhibitor, which shows in vitro and in vivo antiviral activity against influenza A and B viruses including pandemic and avian strains [7–9]. Single-dose baloxavir has demonstrated safety and efficacy in a trial of 1064 otherwise healthy adult and adolescent patients with acute influenza (CAPSTONE-1) [10] and was approved for the treatment of uncomplicated influenza in Japan and the United States in 2018. In CAPSTONE-1, baloxavir rapidly reduced infectious virus titers and significantly reduced the time to alleviation of symptoms (TTAS, primary outcome) compared with placebo without apparent safety concerns [10]. However, amino acid substitutions at position 38 of polymerase acidic protein (PA/I38X) were detected in 9.7% of those with paired sequence data [10, 11].
A subsequent double-blind, randomized, placebo- and oseltamivir-controlled trial (CAPSTONE-2) demonstrated that baloxavir reduced the incidence of influenza complications in high-risk outpatients with influenza A or B virus compared with placebo and was more effective than oseltamivir both clinically and virologically in patients infected with influenza B virus [12]. While this study included a limited number (42 patients) of adolescent patients with comorbidities, primarily asthma, the findings enabled the Food and Drug Administration to extend baloxavir’s approval for treatment of high-risk outpatients with influenza [13].
Here, we present data on the clinical and virologic effects, pharmacokinetics, and tolerability of baloxavir in the adolescent subpopulation from the CAPSTONE-1 trial.
MATERIALS AND METHODS
CAPSTONE-1 was a phase 3, double-blind, placebo- and active comparator-controlled, randomized trial conducted in Japan and the United States between December 2016 and March 2017 (ClinicalTrials.gov: NCT02954354). The methods, including sample collection, virologic analyses, and primary results of the trial, have been reported previously [10]. CAPSTONE-1 was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation–Good Clinical Practice guidelines. All participants provided written informed consent or assent as appropriate for adolescents.
Eligible patients (aged ≥12 and ≤19 years) were randomized in a ratio of 2:1 to receive either a single dose of baloxavir (40 or 80 mg depending on body weight) or matching placebo on day 1 only [10]. The patients received the assigned study drug without regard to meals. Since oseltamivir was to be avoided in adolescents except high-risk patients (per the Japanese labeling restriction existing at that time, since removed), subjects who were 12 to 19 years of age were not randomized to oseltamivir, unlike older participants [10]. Acetaminophen was permitted as a rescue therapy only for the relief of fever or pain at a dose of 3000 mg/day or less.
Outcomes
The primary endpoint was the TTAS [10], defined as the time from the start of treatment to the time when all 7 influenza-related symptoms were rated by the patients as absent or mild for at least 21.5 hours. Secondary endpoint included the time to resolution of fever, measured with a standardized electronic thermometer and incidence of influenza-related complications (hospitalization, sinusitis, otitis media, bronchitis, or pneumonia). The virology outcomes included the change from baseline in infectious virus and viral ribonucleic acid (RNA) titers, the times to first cessation and to sustained cessation of infectious virus detection (time between initiation of study treatment and sustained negativity), and the frequency of amino acid substitutions at position PA/I38 in patients who had paired baseline and follow-up reverse transcription-polymerase chain reactions (RT-PCR)-positive samples evaluable for Sanger sequencing.
The plasma concentrations of baloxavir acid, the active form of baloxavir, were determined using samples taken on days 2 and 5, and when possible on days 1 (0.5-4 hours postdose), 3, and 15 per subject. The plasma concentrations at 24 hours postdose (C24), the maximum plasma concentration (Cmax), and the area under the plasma concentration-time curve (AUC) were estimated using the Bayesian approach based on the population pharmacokinetic parameters of baloxavir acid [14].
Statistical Analysis
The intent-to-treat infected (ITTI) population consisted of the patients who received the study drug with a confirmed diagnosis of influenza based on the results of RT-PCR on day 1. Of ITTI population, adolescent patients aged 12 to 17 years of age were included in the target analysis population of these subgroup analyses. All analyses were performed using SAS System Release 9.2 or 9.4 (SAS Institute, Inc, Cary, NC) [15, 16]. The primary endpoint, TTAS, was compared between the baloxavir and the placebo groups in the ITTI population using the stratified Peto-Prentice’s generalized Wilcoxon test (the stratified generalized Wilcoxon test) with the composite symptom score at baseline (≤11 or ≥12) and the region (Japan/Asia or the Rest of the world) as the stratification factors.
The Kaplan-Meier curves were plotted for each treatment group, and the median TTAS and its 95% confidence interval (CI) were calculated. The Brookmeyer and Crowley method was used for the CIs of median TTAS. In addition, the treatment group difference in median time was calculated. The time to alleviation of each symptom (nasal congestion, cough, fatigue, sore throat, headache, feverishness or chills, and muscle or joint pain), time to resolution of fever, time to first cessation of viral shedding, and time to sustained cessation of viral shedding were analyzed using the same method used for the primary endpoint.
The frequency of treatment-emergent variant viruses with PA/I38 substitutions conferring reduced susceptibility to baloxavir was calculated. The patients in the baloxavir group were divided into those with and without postdose detection of PA/I38X-substituted viruses for comparison of the TTAS, time to resolution of fever, the time to first cessation of viral shedding, and time to sustained cessation of viral shedding. The clinical or virological outcomes by each patient with PA/I38X substitutions were reported since the number of the evaluated patients with PA/I38X-substituted viruses was extremely limited.
The safety population consisted of all randomized patients who received at least 1 dose of the study drug. The number of patients with adverse events (AEs) and the number of AEs were reported for each treatment group. Fisher’s exact test was used to compare the incidence of AEs between treatment groups.
The adolescent subgroup analyses of the primary endpoint and AEs were prespecified in the statistical analysis plan. The adolescent subgroup analyses for other than primary endpoint and AEs were not prespecified in the statistical analysis plan but are presented in this manuscript.
RESULTS
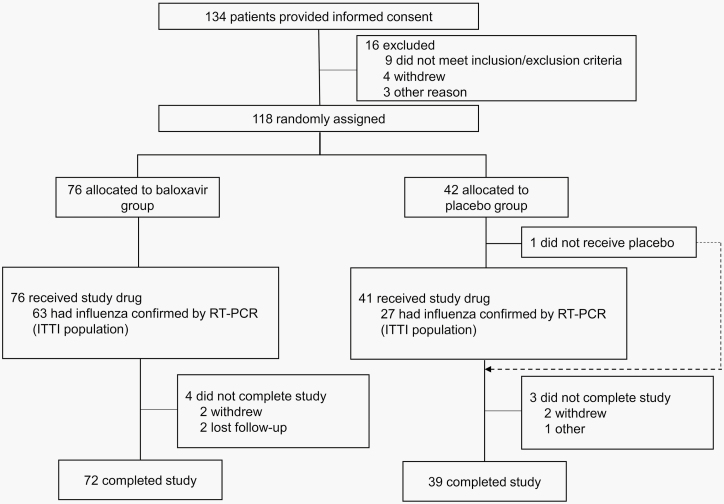
Overall, 117 adolescent patients (aged 12-17 years) received study drug treatment (Figure 1, Supplementary Table 1), of whom 90 (77%) had an RT-PCR-confirmed diagnosis of influenza virus infection and were included in the ITTI population (63 baloxavir and 27 placebo). In the ITTI population, 88.9% of patients were infected with influenza A(H3N2) virus, 51.1% of patients received the study drug within 24 hours after symptom onset, 58.9% of patients were enrolled in Japan, and 18.9% had been given influenza vaccine in the current season (Table 1).
Figure 1.
Patient disposition. When dual enrollment is occurred, the patient was counted only once. Abbreviation: ITTI, intent-to-treat infected.
Table 1.
Baseline Demographics and Clinical Characteristics in Adolescent Subpopulation of ITTI Population
| Baloxavir (N = 63) n (%) | Placebo (N = 27) n (%) | ||
|---|---|---|---|
| Age (years) | Mean | 14.7 | 14.4 |
| SD | 1.6 | 1.7 | |
| Weight (kg) | Mean | 60.44 | 60.06 |
| SD | 16.13 | 15.14 | |
| ≥80 | 8 (12.7) | 2 (7.4) | |
| BMI (kg/m2) | Mean | 22.15 | 21.93 |
| SD | 4.93 | 4.77 | |
| Sex | Male | 35 (55.6) | 17 (63.0) |
| Region | Japan | 35 (55.6) | 18 (66.7) |
| United States | 28 (44.4) | 9 (33.3) | |
| Smoking | Yes | 0 | 0 |
| Composite symptom scores at baseline | Mean | 13.5 | 13.8 |
| SD | 3.4 | 4.1 | |
| Body temperature (°C) at baseline | Mean | 38.65 | 38.36 |
| SD | 0.47 | 0.57 | |
| Time to treatment from flu onset (hours) | ≥0 to ≤12 | 12 (19.0) | 8 (29.6) |
| >12 to ≤24 | 21 (33.3) | 5 (18.5) | |
| >24 to ≤36 | 25 (39.7) | 11 (40.7) | |
| >36 to ≤48 | 5 (7.9) | 3 (11.1) | |
| Influenza virus subtype based on RT-PCR | A(H1N1)pdm09 | 0 | 0 |
| A(H3N2) | 55 (87.3) | 25 (92.6) | |
| B | 5 (7.9) | 2 (7.4) | |
| Mixed infection | 1 (1.6) | 0 | |
| A, uncertain subtype | 2 (3.2) | 0 | |
| Negative | 0 | 0 | |
| Influenza vaccination | Yes | 11 (17.5) | 6 (22.2) |
Abbreviations: BMI, body mass index; RT-PCR, reverse transcription-polymerase chain reaction; SD, standard deviation.
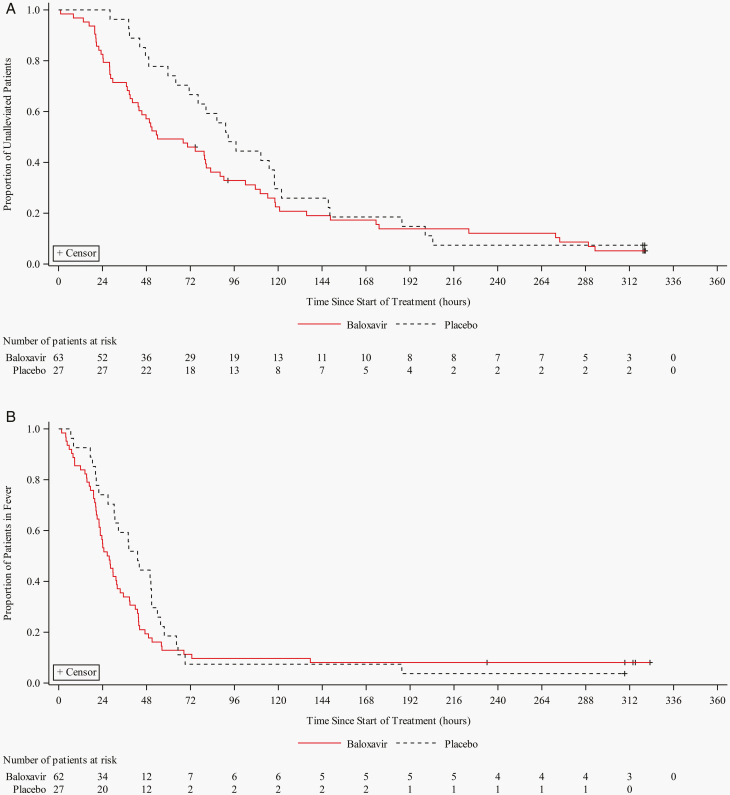
The median TTAS was 38.6 hours shorter in the baloxavir group compared with the placebo group (54.1 hours vs 92.7 hours, P = .0055) (Figure 2A) [10]. For 6 influenza-related symptoms analyzed separately, the difference of the median time to alleviation ranged from 1.2 to 38.7 hours shorter in the baloxavir group vs the placebo group, except for headache, which lasted 3.2 hours longer in the baloxavir group (Supplementary Table 2). The median time to resolution of fever was 27.1 hours (95% CI: 22.0, 32.0) in the baloxavir group compared with 43.1 hours (95% CI: 26.9, 50.8) in the placebo group (Figure 2B). The proportion of acetaminophen use was 11.1% in the baloxavir group compared with 14.8% in the placebo group. Otitis media was developed in 2 placebo recipients. Bronchitis was developed in 2 placebo recipients, 1 received an oral antibiotic, and 1 baloxavir recipient. No hospitalizations occurred.
Figure 2.
Time to alleviation of symptoms (TTAS) (A) and time to resolution of fever (B) in the adolescent subpopulation of ITTI population. TTAS was defined as the time between the initiation of the study treatment and the time when the patient’s self-assessed 7 influenza-related symptoms become absent or mild for at least 21.5 hours. Time to resolution of fever was defined as the time between the initiation of the study treatment and the time when the patient’s self-measured axillary temperature becomes less than 37°C for at least 12 hours. Abbreviation: ITTI, intent-to-treat infected.
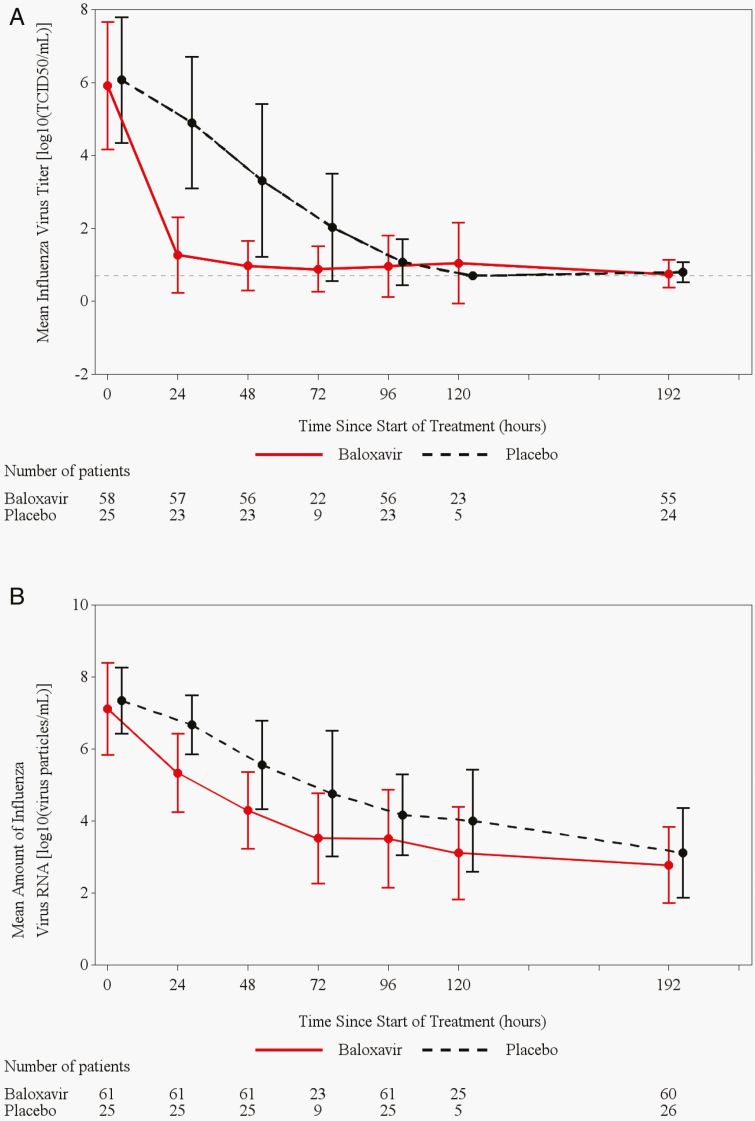
The median time to first cessation of viral shedding was shorter in the baloxavir group (24.0 hours, 95% CI: 24.0, 48.0) than in the placebo group (96.0 hours, 95% CI: 96.0, 168.0, P < .0001) (Table 2) (Supplementary Figure 1). The median time to sustained cessation of viral shedding was 72.0 hours (95% CI: 48.0, 96.0) for the baloxavir recipients compared with 120.0 hours (95% CI: 96.0, 168.0, P = .0014) for placebo recipients (Table 2) (Supplementary Figure 2). The change from baseline in infectious virus titer was faster and of greater magnitude in the baloxavir group than in the placebo group. Baloxavir reduced the mean virus titer to 1.27 log10tissue culture infectious dose (TCID)50/mL by 1 day after dosing (24 hours) and 0.98 log10TCID50/mL by 2 days (48 hours) afterward, whereas the mean virus titer in placebo recipients was 4.90 and 3.31 log10TCID50/mL, respectively, on these days (Figure 3A). The reductions in viral RNA loads were greater with baloxavir than with placebo (Figure 3B).
Table 2.
Time to Cessation of Infectious Viral Shedding by Virus Titer in Adolescent Subpopulation of ITTI Population
| Baloxavir (N = 58) | Placebo (N = 25) | |
|---|---|---|
| Time to first cessation of viral shedding | ||
| Median (hours) | 24.0 | 96.0 |
| 95% confidence interval (hours) | 24.0, 48.0 | 96.0, 168.0 |
| Difference (vs Placebo) (hours) | −72.0 | — |
| P-value by stratified generalized Wilcoxon test vs Placeboa | <.0001 | — |
| Time to sustained cessation of viral shedding | ||
| Median (hours) | 72.0 | 120.0 |
| 95% confidence interval (hours) | 48.0, 96.0 | 96.0, 168.0 |
| Difference (vs Placebo) (hours) | −48.0 | — |
| P-value by stratified generalized Wilcoxon test vs Placeboa | .0014 | — |
Patients whose virus titer has not reached cessation by the last observation time point were treated as censored data at that time point. Patients with a positive virus titer on day 1 whose time to cessation of viral shedding by virus titer was not missing were included in this analysis.
aStratification factors: region and composite symptom scores at baseline.
Figure 3.
Observed infectious virus titer (A) and observed infectious viral RNA titers (B) (mean ± SD) in the adolescent subpopulation of ITTI population. Patients with positive for influenza virus titer at baseline were included in this analysis. Abbreviations: ITTI, intent-to-treat infected; TCID50, 50% tissue culture infectious dose; SD, standard deviation.
Treatment-emergent PA/I38X-substituted viruses were observed in 5 (9.8%) of 51 baloxavir recipients who had paired baseline and follow-up RT-PCR-positive samples evaluable for Sanger sequencing. Those with substituted viruses included 4 with A(H3N2) infection and 1 coinfection with A(H3N2) and type B (Supplementary Table 3). Time to sustained cessation of viral shedding in those with PA/I38X-substituted virus detection (range: 144.0-192.0 hours) was longer than the median time in patients without PA/I38X-substituted viruses (72.0 hours) or the placebo group (120.0 hours) (Supplementary Table 4). The TTAS of 4 patients with PA/I38X-substituted viruses (range: 20.3-88.3 hours) was shorter than the median TTAS of the placebo group (92.7 hours), whereas the TTAS of 1 patient (110.2 hours) was longer. The time of resolution of fever of the 4 patients with PA/I38X-substituted viruses (range: 14.3 to 31.3 hours) was shorter than the median time in the placebo group (43.1 hours), whereas fever of 1 baloxavir patient with PA/I38X-substituted virus lasted until 47.1 hours.
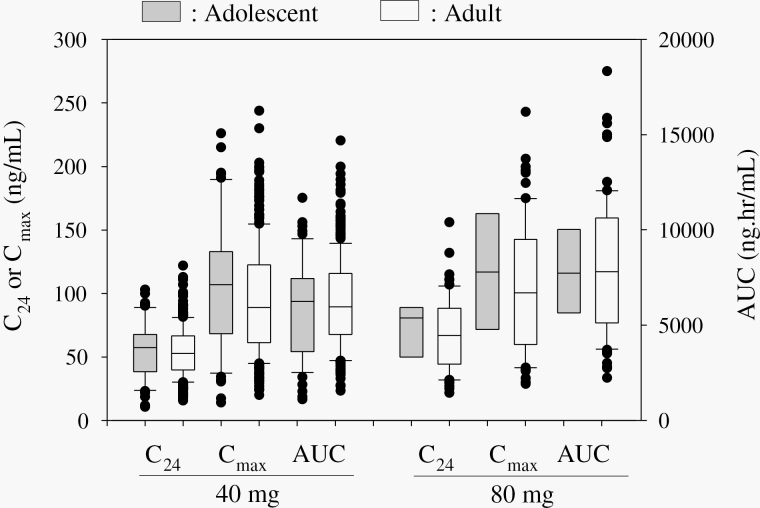
For the pharmacokinetic analysis, the C24, Cmax, and AUC of baloxavir acid were compared with the findings in adult patients who participated in CAPSTONE-1 (Figure 4). In those receiving the 40 mg dose, the mean (standard deviation [SD]) C24, Cmax, and AUC of baloxavir acid in adolescent patients (n = 55) were 55.9 (22.8) ng/mL, 105 (50.7) ng/mL, and 5929 (2592) ng·hr/mL, respectively, compared with 54.2 (19.1) ng/ml, 94.8 (42.9) ng/mL, and 6201 (2382) ng·hr/mL, respectively, for adults (n = 313). In those receiving the 80 mg dose, the corresponding mean (SD) C24, Cmax, and AUC were 72.0 (21.8) ng/mL, 118 (45.4) ng/mL, and 7623 (2537) ng·hr/mL, respectively, in adolescents (n = 8) compared with 68.3 (28.4), 106 (51.2) ng/mL, and 8053 (3492) ng·hr/mL, respectively, for adults (n = 70).
Figure 4.
Comparison of pharmacokinetic parameters in adolescents (12-17) and adults (18-64) patients in CAPSTONE-1 study.
AEs were less common in the baloxavir recipients than placebo recipients (17.1% baloxavir vs 34.1% placebo; P = .0421). In the baloxavir recipients, no AEs except for diarrhea were reported in 2 or more patients. Also, AEs considered by the blinded investigators to be treatment-related were numerically fewer in the baloxavir than in placebo recipients (3.9% vs 9.8%; P = .2380) (Table 3). No death or serious AEs were reported in either the baloxavir or placebo recipients.
Table 3.
Summary of Adverse Events in Adolescent Subpopulation of Safety Population
| Baloxavir (N = 76) n (%) | Placebo (N = 41) n (%) | |
|---|---|---|
| Any adverse events | 13 (17.1) | 14 (34.1) |
| Adverse events reported in >= 2 patients in any group | ||
| Bronchitis | 1 (1.3) | 2 (4.9) |
| Otitis media | 0 | 2 (4.9) |
| Nightmare | 0 | 2 (4.9) |
| Headache | 1 (1.3) | 2 (4.9) |
| Diarrhea | 3 (3.9) | 2 (4.9) |
| Increase in Alanine transaminase level | 1 (1.3) | 2 (4.9) |
| Any treatment-related adverse events | 3 (3.9) | 4 (9.8) |
| Leukopenia | 0 | 1 (2.4) |
| Nightmare | 0 | 2 (4.9) |
| Abnormal behavior | 0 | 1 (2.4) |
| Dysgeusia | 1 (1.3) | 0 |
| Parosmia | 1 (1.3) | 0 |
| Headache | 0 | 1 (2.4) |
| Cough | 1 (1.3) | 0 |
| Abdominal pain upper | 1 (1.3) | 0 |
| Diarrhea | 1 (1.3) | 1 (2.4) |
| Flatulence | 1 (1.3) | 0 |
| Serious adverse event | 0 | 0 |
DISCUSSION
Baloxavir demonstrated clinical and virological efficacy compared with placebo in otherwise healthy adolescents with acute uncomplicated influenza. There were no safety concerns identified, and the overall proportion with any AEs was lower in the baloxavir group. The exposures of baloxavir acid in adolescents were similar to those in adults. This small subset analysis is not large enough to draw definitive conclusions, but these clinical, virologic, pharmacokinetic, and safety outcomes were comparable to those found in adults aged 18–64 years old (data not shown), therefore supporting the use of baloxavir as a promising treatment option in adolescents.
Baloxavir showed a strong antiviral effect, which suggests the potential for risk reduction of virus transmission by baloxavir treatment. Children and adolescents play a major role in influenza transmission in the household. Our ferret study has shown a possibility of interrupting onward transmission with baloxavir treatment [17], and a clinical trial in a household-based transmission (ClinicalTrials.gov: NCT03969212) is underway to determine to what extent treatment can reduce the risk of transmission to close contacts.
PA/I38X-substituted viruses were detected in 9.8% of patients, which is comparable to the adults study population (9.7%, 31 of 319) and lower than that in a pediatric population (age < 12 years) participating in an open-label study of baloxavir conducted during the same influenza season (23.4%) [18]. Both adult and pediatric studies found that low baseline neutralizing antibody titers were associated with risk of variant emergence [11, 18], and the observed frequency in our adolescent population suggests that influenza immunity of adolescent population may have matured, probably due to past vaccination and natural influenza infections. In the small number of patients with the emergence of PA/I38X-substituted viruses, no obvious impact on illness recovery was observed but the detection of infectious virus was prolonged as reported previously in young children and adults [11, 18].
Sporadic cases of human-to-human transmission of baloxavir-resistant influenza A viruses have been reported in Japan [19], and Japanese National Resistance Surveillance reported that 3 A(H1N1)pdm09 and 5 A(H3N2) baloxavir-resistant viruses with PA/I38 substitutions were detected from patients without baloxavir treatment among 395 samples of A(H1N1)pdm09 and 424 samples of A(H3N2), respectively, during the 2018–2019 season [20]. Human-to-human transmission of such variants is an important area of study, and continuous resistance monitoring is warranted.
CONCLUSIONS
Single-dose baloxavir was clinically and virologically effective in otherwise healthy adolescents with acute influenza treated within 48 hours of the symptom onset. The emergence of PA/I38X amino acid substitutions associated with reduced drug susceptibility was observed in similar proportions between adolescents and adults. The safety of baloxavir was comparable to placebo, and no adverse drug reactions were identified. Our findings indicate that baloxavir is a valuable treatment option in adolescents with acute uncomplicated influenza.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
Notes
Acknowledgments. The authors would like to thank all study participants and investigators who participated in the clinical study. We thank Jennifer Garas and Benjamin Georgiades in Shionogi Inc. and Satoshi Kojima in Shionogi & Co., Ltd. for assistance in preparing the manuscript.
Authors contributions. All authors participated in the interpretation of study results and in the drafting, critical revision, and approval of the final version of the manuscript. S. P., F. G. H., K. K., T. I., T. S., K. T., and T. U. were involved in the study design and/or data collection. K. K. conducted the statistical analysis.
Financial support. This work was supported by Shionogi & Co., Ltd., manufacturer/licensee of baloxavir marboxil.
Role of the sponsor. Shionogi & Co., Ltd. was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Potential conflicts of interest. M. K. and K. T. hold stock in Shionogi. F. G. H. receives travel support from Shionogi and Roche, personal fees from WHO and the University of Alabama Antiviral Drug Discovery and Development Consortium, donations to the Ford Haitian Orphanage and School from Cidara, resTORbio, Seqirus, and Shionogi for consulting, payments to the University of Virginia from GlaxoSmithKline, Celltrion Healthcare, and Vaccitech for Data Safety Monitoring Board work, has served as an unpaid consultant to following companies (CoCrystal, Farmak, FujiFilm/Toyama, Gilead Sciences GlaxoSmithKline, Janssen, MedImmune, Regeneron, Roche/Genentech), and as an unpaid member of the Scientific Advisory Board for SAB Biotherapeutics, Vir, and Visterra that are developing investigational therapeutics for influenza. K. K., T. I., M. K., T. S., K. T., and T. U. are employees of Shionogi & Co., Ltd. S. P. is an employee of Shionogi Inc.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States. Accessed May 18, 2020. https://www.cdc.gov/flu/about/burden/2018–2019.html
- 2. Hayward CA, Fragaszy BE, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the flu watch cohort study. Lancet Respir Med 2014; 2:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lafond KE, Nair H, Rasooly MH, et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med 2016; 13:e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malosh RE, Martin ET, Heikkinen T, et al. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66:1492–500. [DOI] [PubMed] [Google Scholar]
- 5. Lee N, Hui DS, Zuo Z, et al. A prospective intervention study on higher-dose oseltamivir treatment in adults hospitalized with influenza A and B infections. Clin Infect Dis 2013; 57:1511–9. [DOI] [PubMed] [Google Scholar]
- 6. Sugaya N, Mitamura K, Yamazaki M, et al. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis 2007; 44:197–202. [DOI] [PubMed] [Google Scholar]
- 7. Noshi T, Kitano M, Taniguchi K, et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res 2018; 160:109–17. [DOI] [PubMed] [Google Scholar]
- 8. Taniguchi K, Ando Y, Nobori H, et al. Inhibition of avian-origin influenza A(H7N9) virus by the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Sci Rep 2019; 9:3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukao K, Ando Y, Noshi T, et al. Baloxavir marboxil, a novel cap-dependent endonuclease inhibitor potently suppresses influenza virus replication and represents therapeutic effects in both immunocompetent and immunocompromised mouse models. PLoS One 2019; 14:e0217307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayden FG, Sugaya N, Hirotsu N, et al. ; Baloxavir Marboxil Investigators Group. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 11. Uehara T, Hayden FG, Kawaguchi K, et al. Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated Influenza. J Infect Dis 2020; 221:346–55. [DOI] [PubMed] [Google Scholar]
- 12. Ison GM, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis 2020; 20:1204–1214. [DOI] [PubMed] [Google Scholar]
- 13. Food and Drug Administration Center for Drug Evaluation and Research. Approval Package for: APPLICATION NUMBER: 210854Orig1s001. Accessed October 26, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/210854Orig1s001.pdf
- 14. Koshimichi H, Tsuda Y, Ishibashi T, Wajima T. Population pharmacokinetic and exposure-response analyses of baloxavir marboxil in adults and adolescents including patients with influenza. J Pharm Sci 2019; 108:1896–904. [DOI] [PubMed] [Google Scholar]
- 15. SAS [computer program]. Version 9.2. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 16. SAS [computer program]. Version 9.4. Cary, NC: SAS Institute Inc; 2016:11 [Google Scholar]
- 17. Lee LYY, Zhou J, Frise R, et al. Baloxavir treatment of ferrets infected with influenza A(H1N1)pdm09 virus reduces onward transmission. PLoS Pathog 2020; 16:e1008395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirotsu N, Sakaguchi H, Sato C, et al. Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis 2019; 71:971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takashita E, Ichikawa M, Morita H, et al. Human-to-human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir, Japan, February 2019. Emerg Infect Dis 2019; 25:2108–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Institute of Infectious Disease. Antiviral resistance surveillance in Japan. Accessed May 26, 2020.https://www.niid.go.jp/niid/en/influ-resist-e/9515-flu-r-e20200430.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.