Summary
Parasites are by definition organisms that utilize resources from a host to support their existence, thus promoting their ability to establish long term infections and disease. Hence, sensing and acquiring nutrients for which the parasite and host compete is central to the parasitic mode of existence. Leishmania are flagellated kinetoplastid parasites that parasitize phagocytic cells, principally macrophages, of vertebrate hosts and the alimentary tract of sand fly vectors. Because nutritional supplies vary over time within both these hosts and are often restricted in availability, these parasites must sense a plethora of nutrients and respond accordingly. The flagellum has been recognized as an ‘antenna’ that plays a core role in sensing environmental conditions, and various flagellar proteins have been implicated in sensing roles. In addition, these parasites exhibit non-flagellar intracellular mechanisms of nutrient sensing, several of which have been explored. Nonetheless, mechanistic details of these sensory pathways are still sparse and represent a challenging frontier for further experimental exploration.
Introduction
Leishmania parasites experience a wide array of environmental conditions during their life cycle and must be able to sense and respond to the changing milieu to successfully navigate each step in this journey. These microorganisms live inside the phagolysosomal vesicles of vertebrate macrophages as disease causing non-motile amastigotes, and they live in the alimentary tract of sand fly vectors as flagellated motile forms, including variously developmentally distinct forms of promastigotes (Fig. 1 and Box 1). Parasitized macrophages are initially taken up by sand flies as part of a blood meal, and amastigotes are subsequently released from parasite-laden macrophages and transform into dividing promastigote forms. Promastigotes live within the peritrophic matrix of the sand fly, a glycan and proteoglycan rich composite that surrounds the blood meal and protects the insect midgut epithelium from deleterious components. Within the peritrophic matrix, the parasites are exposed to nutrients from the blood meal, including glucose, and are hence in a relatively nutrient rich environment. Following digestion of the blood meal, the parasites transform into nondividing nectomonads (Sunter & Gull, 2017) that adhere to the villi of the midgut by their flagella to avoid expulsion with the digested blood meal. They subsequently differentiate into long, highly-motile, dividing leptomonads that migrate to the foregut, and these forms differentiate into nondividing infectious metacyclic parasites with small bodies and long flagella, which are delivered to the mammalian host tissue following initiation of another blood meal by the parasitized sand fly. The latter three life cycle forms are probably exposed to relatively nutrient restricted environments, although they will have periodic access to sugars, such as sucrose and its digestion products glucose and fructose, following ingestion of plant sap or honeydew by the sand fly (Schlein, 1986). Finally, once metacyclic parasites invade mammalian macrophages, they are targeted to acidic phagolysosomal vesicles that are thought to be relatively rich in amino acids and fatty acids from digestion of proteins and complex lipids within this digestive organelle (Naderer & McConville, 2008), and parasites are also likely exposed to macromolecules prior to their digestion within the vacuole. In addition to these changes in nutrients, the parasites are also exposed to the change from neutral to acidic pH and from ambient temperature to the ~37° temperature of the mammalian host. Despite this apparently plentiful milieu, amastigotes enter a metabolic stringent state in which uptake and catabolism of sugars and amino acids is minimal and replication proceeds at a much slower rate than for promastigotes (McConville et al., 2015). During these developmental transitions, expression of many metabolic enzymes and transporters, or of their mRNAs, is regulated (Alcolea et al., 2018, Inbar et al., 2019), indicating that the parasites apparently sense and respond to these nutrient and metabolic alterations and that the ability to adapt to changes in nutrient composition is probably central to the success of the parasite in surviving pronounced alterations in environmental conditions.
Fig. 1.
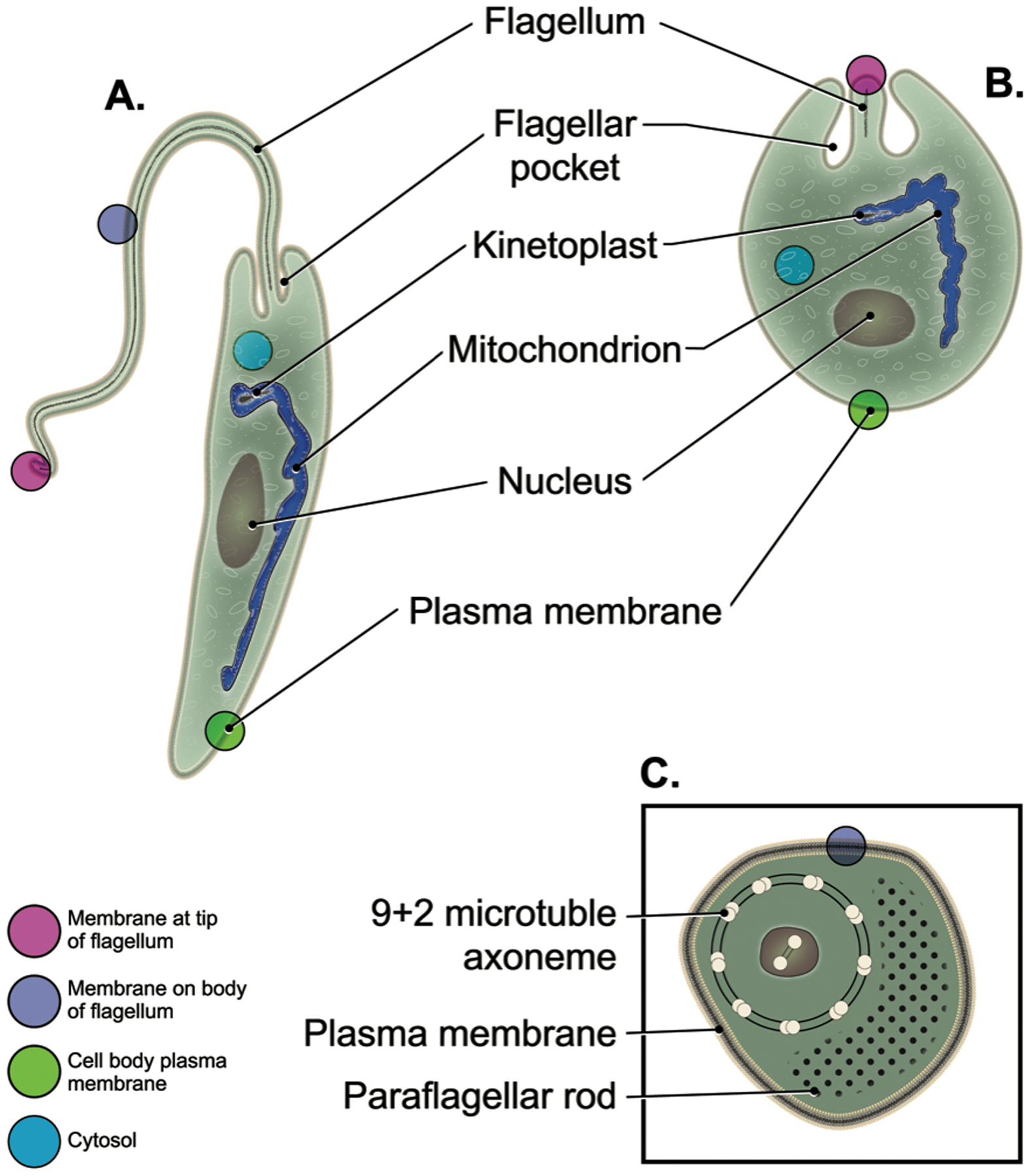
Leishmania parasites and locations of potential sensors. A. Insect stage promastigotes have an extended cell body and a long flagellum that exits from the flagellar pocket. B. Intracellular amastigotes have on oval-shaped cell body and a short flagellum that barely emerges from the flagellar pocket. In both life cycle stages, membrane bound sensors could be located at the distal tip of the flagellum (red sphere), along the length of the flagellum (purple sphere), or on the cell body membrane (green sphere), and other sensors could be cytosolic proteins (blue sphere). C. A cross section of the flagellum shows the surrounding flagellar membrane and an integral membrane protein (purple) representing a putative flagellar sensor. Other components of all images are as labeled. This figure was prepared by Laramie Studio, Portland, OR.
Box 1. Definitions of relevant terms.
Promastigotes, including various developmental forms such as procyclics, nectomonads, leptomonads, metacyclics.
Developmental forms of Leishmania parasites in the sand fly vector, listed here in order of their emergence following uptake of the blood meal. All of these promastigote forms have extended flagella and are motile. Metacyclics are the infectious forms that are delivered to the vertebrate host by a bite of the infected sand fly.
Amastigotes.
Disease causing stages of the Leishmania parasite that live within phagolysosomal vacuoles of vertebrate macrophages. Amastigotes have a short flagellum that barely emerges from the flagellar pocket, and these parasites are non-motile.
Sand fly.
Hematophagous insects, including the genera Phlebotomus and Lutzomyia, which serve as vectors for Leishmania parasites. Parasites initially develop within the insect midgut after ingestion of the parasitized blood meal, but developmental forms of the parasite migrate forward to the mouthparts, where they are delivered to another vertebrate host during acquisition of a second blood meal.
Flagellar membrane.
The component of the surface membrane that covers the flagellum and has a distinct protein and lipid composition from the rest of the plasma membrane. This membrane emerges from the flagellar pocket membrane.
Flagellar pocket.
An invagination of the surface membrane at the point where the flagellum emerges from the cell body. The flagellar pocket consists of a membrane, which is contiguous with both the plasma membrane that surrounds the cell body and the flagellar membrane, and it also encompasses a lumen that is in contact with the extracellular space.
Flagellum attachment zone, FAZ.
This complex structure was first identified in T. brucei, where it mediates attachment of the flagellum to the cell body along most of its length. It was subsequently identified in L. mexicana, where is constitutes a discrete adhesion between the base of the flagellar membrane and the flagellar pocket membrane. The polytopic membrane protein FAZ5 is a constituent of the flagellar pocket membrane component of the FAZ, whereas the FLA1 binding protein and the Ca2+ channel encoded by LmxM.33.0480 are flagellar membrane components of the FAZ.
Transceptor.
A protein that is related in structure to a family of membrane transporters but acts as a signal transduction receptor for a bound ligand. Some transceptors have retained ligand transport activity as well as signaling capacity, whereas others do not transport ligands but exhibit only sensory signaling function.
Aquaporins, AQPs.
Channel proteins that mediate uptake of water, glycerol, and various other small organic solutes.
Adenylate cyclase, AC.
A membrane bound enzyme that synthesizes cAMP from ATP. In most eukaryotes, adenylate cyclases have 12 transmembrane segments and two intracellular loops containing catalytic domains. AC activity is typically controlled by binding of ligands to upstream G-protein coupled receptors (GPRCs) that activate various heterotrimeric G-proteins, and these activated G-proteins then activate or inhibit AC activity. In kinetoplastid parasites, adenylate cyclases have an intracellular catalytic domain that is conserved in sequence to those of other eukaryotes, but they only have a single transmembrane domain and a large extracellular domain that may bind unknown ligands.
cAMP phosphodiesterase, PDE.
An enzyme that cleaves cAMP to AMP. These enzymes play important roles in controlling the intracellular level of the second messenger cAMP.
Decades of work on eukaryotes from yeast to humans have revealed common patterns by which cells sense their internal and external status. Broadly, mechanisms can be divided into sensors that reside internally, often within the cytosol, or externally, exposed at the surface of the plasma membrane (Efeyan et al., 2015). One example of a well-studied internal sensor is the GCN2 kinase, which binds to and is activated by uncharged tRNAs, resulting in phosphorylation of the translation initiation factor eIF2α and arrest of translation. Examples of external nutrient sensors (Holsbeeks et al., 2004) include i) some G-protein coupled receptors, ii) carriers that have dual functions as nutrient receptors and transporters, and iii) carrier-like proteins that have lost transport capacity but retain sensing capability. Both classes ii and iii are referred to as ‘transceptors’. One remarkable outcome of the initial genome sequencing projects for Trypanosoma brucei, Leishmania major, and Trypanosoma cruzi (El-Sayed et al., 2005), was the observation that the genomes of these parasites were completely lacking in many of the well-known surface receptor classes common in other eukaryotes, including G-protein coupled receptors and receptor tyrosine kinases. This unanticipated result suggests that these parasites must rely upon intracellular sensors, less conventional plasma membrane sensors such as transceptors, or both, perhaps to a greater extent than most other eukaryotes. Another feature of sensing in kinetoplastid protists that has come to the fore is the recognition that eukaryotic cilia and flagella have central roles as sensory organelles (Wheway et al., 2018), mediating such processes through sensory proteins located, often selectively, in the ciliary/flagellar membrane. Since Leishmania and other kinetoplastid protists are flagellated throughout their life cycles, the flagellum presents itself as a likely organelle for mediating sensing of nutrients and other environmental cues (Fig. 1).
Another notable distinction between kinetoplastid parasites and other eukaryotes is that the genome organization of these protists largely precludes transcriptional regulation of gene expression. Thus, genes are organized in large polycistronic arrays that are transcribed from a single upstream initiation site, requiring that differential regulation must occur at post-transcriptional levels such as mRNA stability, translation, protein stability, post-translational modifications, etc. (Clayton, 2019). In contrast, nutrient sensing in many other eukaryotes often entails transcriptional control of the differentially affected genes. Thus, both the initial nutrient sensors and the mechanisms of response to nutritional availability will often be distinct in these parasites.
Flagellar membrane proteins implicated in sensing
Several studies in recent years have identified flagellar membrane proteins that may be involved in sensing nutrients or other molecular components of the extracellular milieu. One of the first of these to be identified in Leishmania was a flagellar glucose/hexose transporter, GT1. Unlike other glucose transporters (GTs), GT1 is selectively targeted to the flagellar membrane (Piper et al., 1995) (Fig. 2A), suggesting by its unique location that it might be involved in glucose sensing. L. mexicana promastigotes in which the GT1 gene had been knocked out by targeted gene replacement achieved a cell density that was higher than wild type or add-back parasites, but instead of entering stationary phase like their wild type counterparts, they underwent a catastrophic loss of viability in dense cultures that had depleted glucose from the medium (Rodriguez-Contreras et al., 2015). Addition of supplemental glucose to the medium greatly blunted the reduction in cell density and allowed the Δgt1 null mutants to survive at stationary phase similarly to wild type parasites. These results suggest that GT1 may sense external glucose and send a signal that allows successful transition from logarithmic to stationary phase when glucose is depleted. How such a sensory pathway may work is still unclear, but searching for potential partners that interact with GT1 could identify components of a signal transduction system. Indeed, among the first transceptors identified were the Snf3 and Rgt2 glucose transporter-like proteins from Saccharomyces cerevisiae that do not transport glucose but rather bind to and sense this nutrient. These proteins associate with other partner proteins involved in activating signal transduction cascades that control transcription of bona fide glucose transporter genes (Holsbeeks et al., 2004). Of potential significance, the L. major TOR kinase, TOR3, is essential for survival of promastigotes when they are starved for glucose (Madeira da Silva & Beverley, 2010) but not when they are starved for amino acids, and a TOR3 signaling pathway might thus be linked to glucose sensing through GT1.
Fig. 2.
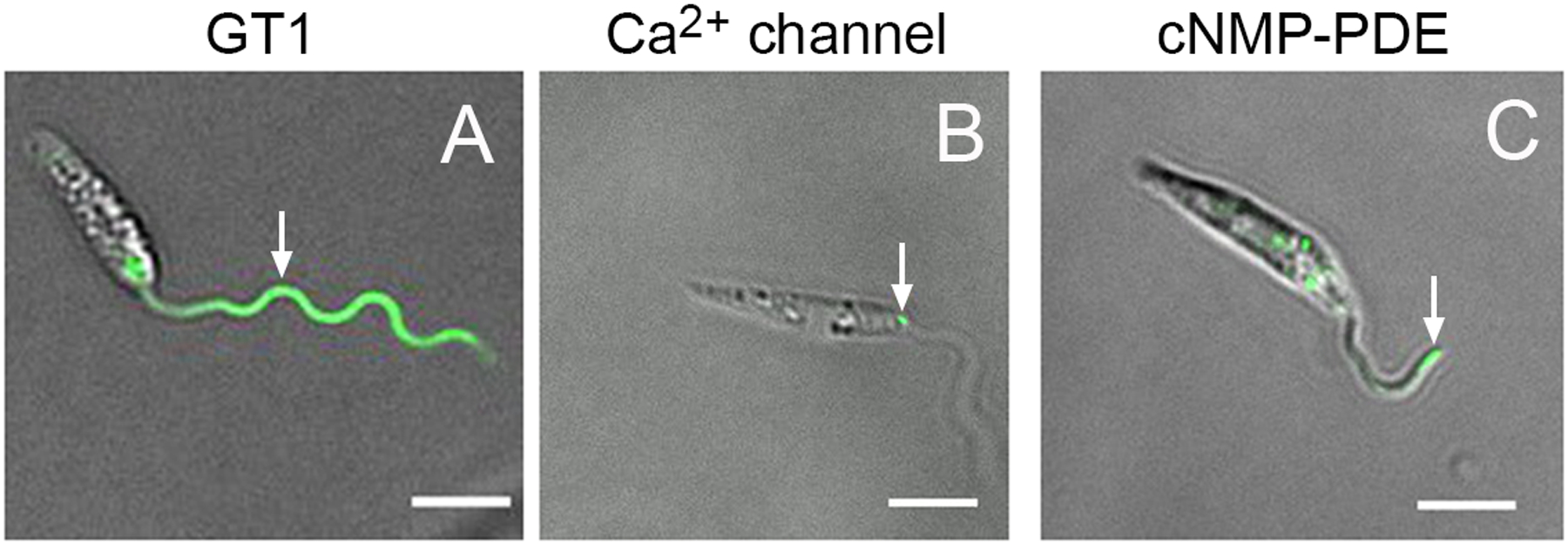
Localization of three proteins with potential roles in signaling to different components of the flagellar membrane in L. mexicana promastigotes. A) The flagellar glucose transporter GT1, tagged at the C-terminus with GFP, localizes along the length of the flagellum (white arrow). B) The putative Ca2+ channel encoded by LmxM.33.0480, fused at its C-terminus to mNeonGreen, is restricted to the flagellum attachment zone (white arrow), a localized adhesion between the flagellar membrane and the flagellar pocket membrane near the base of the flagellum. C) A cNMP-PDE encoded by LmxM.08_29.2440, fused at its C-terminus to mNeonGreen, localizes to the flagellum with a concentration at the distal tip (white arrow) in many parasites. All images are of live parasites imaged in CyGel and represent superpositions of fluorescence and differential interference microscopy. Scale bars are 5 μm.
A flagellar aquaglyceroporin, AQP1, was identified in L. major and shown to be involved in uptake of water, various small organic solutes, antimonials and arsenicals, and cellular volume regulation (Figarella et al., 2007). Promastigotes overexpressing wild type AQP1 migrated more rapidly up an osmotic gradient than those not overexpressing this protein or expressing point mutants that were inactive in transport. Hence, this flagellar channel is directly involved in osmotaxis, thus likely playing a sensory role. Leishmania parasites experience osmotic gradients while in the sand fly, and it is possible that AQP1 plays a role in migration of the parasites within the insect vector. In addition, amastigotes face osmotic stress within the mammalian host, and since AQP1 is also expressed in that life cycle stage in the flagellar pocket and contractile vacuole/spongiosome complex, this channel may play important physiological roles in both the vector and the mammalian host. It has been demonstrated that phosphorylation of AQP1 by MAPK2 both decreases AQP1 turnover and leads to its redistribution across the entire plasma membrane (Mandal et al., 2012). L. major MAPK2 knockout mutants recover from hypo-osmotic stress more slowly than wild type parasites. Whether phosphorylation via MAPK2 is part of the molecular mechanism that links AQP1 to osmotaxis has not been investigated.
Arginine is a critical nutrient for Leishmania parasites, being a precursor for the synthesis of essential polyamines (Roberts & Axel, 1982) and for protein biosynthesis. Amastigotes must compete with the macrophage to salvage Arg from the macrophage phagolysosome, as i) the host cell uses Arg as a precursor for the synthesis of nitric oxide (NO), a cytotoxic effector employed to kill the parasite within the phagolysosome; ii) in Leishmania parasites, Arg is the biosynthetic precursor for trypanothione, the principal reducing agent employed to protect against oxidative damage, including effectors such as NO. L. donovani parasites have evolved a system to sense Arg deprivation, which they experience when they enter macrophages (Goldman-Pinkovich et al., 2016). Specifically, one isoform of the AAP3 Arg permease (Shaked-Mishan et al., 2006), AAP3.2, is upregulated, at both the mRNA and protein level, when either promastigotes or amastigotes are deprived for Arg (Goldman-Pinkovich et al., 2016). This upregulation of AAP3.2 increases the Arg transport capacity of the parasites and presumably promotes its survival and its ability to compete for Arg with the host cell. In addition to upregulating expression of AAP3.2, RNA Seq demonstrated the coordinate upregulation of six other mRNAs, five of which encoded other permeases. The authors designated this coordinate regulation as the Arginine Deprivation Response (ADR) due to the initiating Arg deprivation signal, but it is not clear how upregulating the other permeases promotes survival during Arg deprivation. Notably, this ADR was dependent upon the kinase MPK2, implicating a probable phosphorylation cascade in the sensory response.
Subsequently, the AAP3.2 gene was knocked out by CRISPR-Cas9-mediated gene replacement to test the role of the regulatory response (Goldman-Pinkovitch, bioRxiv, 2019, https://doi.org/10.1101/751610). These knockouts were generated by inserting a linker into the ORF that introduced stop codons, leading to translational termination, but the mutant AAP3.2 mRNA was still expressed and its response to Arg starvation monitored. Basal Arg transport was retained by the intact adjacent AAP3.1 gene, which is not regulated by Arg deprivation. Amastigotes of the knockout line were impaired in their ability to grow inside THP-1 macrophages in vitro at a physiological extracellular Arg concentration of 0.1 mM but could be rescued by supplementing the medium with 1.5 mM Arg. Furthermore, two AAP3.2 null mutants were strongly impaired in their ability to infect the livers of BALB/c mice, confirming that the ADR is critical for robust virulence in L. donovani.
AAP3.2 is located in the flagellar membrane as well as in intracellular organelles called glycosomes that enclose multiple metabolic and purine salvage enzymes (Goldman-Pinkovich et al., 2016). Hence, one might postulate that AAP3.2 is a flagellar-associated arginine transceptor that both senses and transports Arg and induces the ADR. Studies with Arg analogs (Pawar et al., 2019) indicate that some substrate analogs that inhibit transport activity do not inhibit Arg sensing or the ADR and that the unmodified amidino group on Arg is essential for sensing but not for transport. These results suggest that the sensory and transport binding sites are distinct; however, they leave open the question of whether these two sites are on AAP3.2 or on different proteins. Furthermore, translational termination knockouts of AAP3.2 were still able to regulate the level of the mutant AAP3.2 mRNA, which encoded the truncated protein, and of two other mRNAs that are induced by ADR. Hence, AAP3.2 cannot be a sensor that is essential for ADR. A separate study on AAP3 proteins from L. amazonensis (Castilho-Martins et al., 2011) has suggested that the parasites may sense both external and internal Arg levels. Hence, it is possible that although ADR regulates expression of the AAP3.2 transporter, the sensor is an intracellular protein.
Proteomic studies of isolated flagella from both Trypanosoma brucei (Subota et al., 2014) and L. mexicana (Beneke et al., 2019) have identified other candidate flagellar membrane proteins of interest. In T. brucei bloodstream forms, a putative Ca2+ channel, FS179, is located in the flagellar membrane component (Sanchez et al., 2016) of the flagellum attachment zone (FAZ), the adhesive structure that attaches the flagellum along most of its length to the cell body. Since fluxes in Ca2+ often mediate sensory responses (Sanders et al., 2002), it is possible that FS179 plays some role in flagellar-mediated sensing. The ortholog of FS179 in L. mexicana, LmxM.33.0480, is located in the FAZ (Fig. 2B), which in Leishmania parasites constitutes a discrete spot-like adhesion between the flagellar membrane, near its exit from the flagellar pocket, and the flagellar pocket membrane (Wheeler et al., 2016). Similarly, several transporters and a cNMP-phosphodiesterase (Beneke et al., 2019, Kelly et al., 2020a) are also components of the flagellar membrane, suggesting that some of these proteins could have roles in flagellar sensing. Future studies examining each of these flagellar membrane components will be necessary to determine whether any of them has a sensory role.
cAMP signaling and the flagellum
cAMP is a virtually universal second messenger involved in transmitting information from the exterior to the interior of cells. The role of cAMP and the proteins that mediate its synthesis, adenylate cyclases or ACs, and turnover, phosphodiesterases or PDEs, have been most extensively studied in T. brucei (Makin & Gluenz, 2015, Saha et al., 2020, Salmon, 2018), and this work provides a paradigm for exploration of the roles of Leishmania flagella in this central signaling pathway. While cAMP signaling has not been explicitly connected with nutrient sensing in Leishmania, it is reviewed here as a pathway that is likely involved in environmental sensing in these parasites and for which some molecular components are known. ACs in mammals and many other eukaryotes are integral membrane proteins with 12 transmembrane domains (TMDs) and two conserved AC catalytic domains (Khannpnavar et al., 2020). These ACs are typically regulated by G protein coupled receptors (GPCRs) with 7 TMDs and heterotrimeric G proteins that transmit signals from the GPCRs to the target ACs. In contrast, the genomes of the TriTryp parasites, T. brucei, T. cruzi, and Leishmania species, do not encode any recognizable GPCRs or heterotrimeric G-proteins (El-Sayed et al., 2005). However, these parasites express a variety (~65 in T. brucei and 6 in L. mexicana) of single TMD proteins with single conserved AC catalytic domains oriented on the cytosolic side of the plasma membrane and a large hydrophilic extracellular domain. This structure is similar to some mammalian ligand-activated guanylate cyclases (Pandey, 2014) and suggests that the parasite proteins may undergo similar activation by binding of currently unknown ligands to the extracellular domain.
The first such kinetoplastid AC studied was ESAG4 (Paindavoine et al., 1992), encoded by an expression site-associated gene (ESAG) and localized to the flagellar membrane of BF trypanosomes. RNAi knockdown of ESAG4 RNA, and of two of its non-expression site encoded paralogs, generated cytokinesis defects and impaired parasitemia in mice (Salmon et al., 2012a). Studies using expression of a dominant-negative mutant of ESAG4 suggested that active ESAG4 blunts the innate immune response of a murine host by reducing expression of tumor necrosis factor-α (TNF−α) (Salmon et al., 2012b). The model proposed is that phagocytosis of trypanosomes results in ESAG4 being delivered to phagocytes, following lysis of the ingested trypanosomes, thus increasing the intracellular cAMP levels in these host cells. It is further suggested that the augmented cAMP reduces expression of TNF-α at the transcriptional level in those phagocytes and thus promotes parasite survival. In addition several related ACs (AC1, AC2, AC4, and AC6) have been studied in procyclic form (PF) trypanosomes, where they are localized either along the length of the flagellum or concentrated at its distal tip (Saada et al., 2014), and RNAi-mediated knockdown of several of these ACs resulted in increased ‘social motility’ (SOMO) in which parasites showed increased concerted movement (or a hypersocial phenotype in which large streams of parasites migrate in concert and form increased numbers of macroscopic projections on agarose plates) compared to uninduced PFs (Lopez et al., 2015). These results implied that cAMP is involved in regulating SOMO and may play important roles in parasite social motility in vivo as well.
Two PDEs in African trypanosomes, PDEB1 and PDEB2, are associated with the paraflagellar rod component of the flagellar cytoskeleton (Oberholzer et al., 2007). RNAi-mediated knockdown of both proteins simultaneously produced a strong cytokinesis defect in BF trypanosomes and resulted in parasite death in vitro and avirulence in mice. A null mutant of PDEB1 was subsequently shown to be defective for social motility as PFs, being unable to form projecting streams when PFs were plated on agarose (Shaw et al., 2019). Of particular interest, this null mutant was also unable to migrate out of the peritrophic matrix that surrounds PFs in the insect midgut early after infection of the tsetse fly, and it could not complete further development within the insect. Overall, these studies show that cAMP signaling plays central roles in both social motility in vitro and migration of parasites within the tsetse fly and hence is essential for transmission of the parasite by its insect vector.
This body of work in African trypanosomes suggests that interrogation of cAMP signaling in Leishmania flagella is likely to uncover critical biological functions potentially linked to flagellum sensing of the extracellular environment. Five clustered genes encoding receptor-like ACs were identified some years ago in L. donovani, and two of these proteins, RAC-A and RAC-B, were functionally expressed in Xenopus oocytes (Sanchez et al., 1995). RAC-A exhibited AC activity, RAC-B did not, but co-expression of RAC-B with RAC-A decreased the AC activity of the latter, suggesting that the two proteins may form heterodimers. The genome of L. mexicana predicts the existence of 6 such ACs (LmxM.17.0190, 17.0191, 17.0200, 17.0236, 17.0237, and 36.3180). Notably, a recently published flagellar proteome from L. mexicana promastigotes (Beneke et al., 2019) found at least one AC, LmxM36.3180, to be enriched in the detergent solubilized fraction from purified flagella, thus suggesting that it is an FM protein (Kelly et al., 2020a). Of interest, a non-flagellar cytosolic protein (LmxM.28.0090) has also been identified as a heme-containing AC that is activated by O2 (Sen Santara et al., 2013). Gene knockdown and overexpression indicate that this O2-activated AC is critical for parasite survival under conditions of oxidative stress induced by hypoxia.
Additionally, a putative cyclic nucleotide phosphodiesterase (PDE), LmxM.08_29.2440, was also enriched in the detergent extracted flagellar proteome, and it is predicted to have a single TMD near its C-terminus. Tagging and localization of this protein confirmed that it is present in the flagellum and is concentrated toward the distal tip in many promastigotes (Kelly et al., 2020b) (Fig. 2C). This protein also appears to localize to intracellular vesicles, so whether its biologically relevant activity is exclusively at the flagellum or also elsewhere is currently uncertain. Several PDEs have been studied in L. major (Johner et al., 2006) and L. donovani (Bhattacharya et al., 2009), but to our knowledge, none of these other PDEs is flagellar in localization.
In summary, a number of components of cAMP signaling in Leishmania promastigotes appear to be FM proteins. Given the precedent from T. brucei, investigation of the biological functions of these proteins throughout the parasite life cycle, for instance by gene knockout approaches, has the potential to uncover critical roles for cAMP signaling in the flagellum, and we believe such studies should be a high priority. One long standing challenge in both trypanosomes and Leishmania is to identify potential ligands for receptor-like ACs and to link such molecules to possible host- and vector-parasite interactions. Given the absence of clues to the identities of such putative ligands and the possibility that they could represent unusual or non-abundant components of the tsetse fly, sand fly or macrophage, it is perhaps not surprising that the resolution of this puzzle has eluded molecular parasitologist to date.
Iron sensing in Leishmania
In addition to the role of the flagellar membrane in sensing the environment, other environmental components, including nutrients, are sensed intracellularly. A notable example involves parasite sensing of the critical micronutrient iron, which Leishmania parasites require for many conserved metabolic pathways, including electron transport and for activity of the iron-containing superoxide dismutase (FeSOD) that converts superoxide to H2O2 and is a major defense against oxidative damage (Mittra et al., 2013). Iron may be acquired either as the metal ion or from external heme, but excessive levels of either substituent are toxic. Hence, these parasites have elaborated sensory mechanisms to increase import of iron and heme under limiting conditions while maintaining their intracellular concentrations at non-toxic levels.
Within the acidic parasitophorous vacuole, where amastigote replication takes place, or within the sand fly, free iron is present in the oxidized and typically protein bound ferric form (Fe3+), but Leishmania parasites import water soluble reduced ferrous iron (Fe2+). This oxidation state of iron is first generated at the parasite plasma membrane by the membrane-spanning ferric reductase LFR1 (Flannery et al., 2011), which reduces Fe3+ to Fe2+ at the cell surface in an NADPH-dependent process, and then imported into the parasite by the ZIP family iron transporter LIT1 (Huynh et al., 2006) (Fig. 3). Once in the parasite cytosol, iron can be translocated into the mitochondrion by the inner mitochondrial membrane transporter, MIT1, a homolog of mitoferrin from yeast and mammals (Mittra et al., 2016), where it can associate with FeSOD and other iron containing proteins. Heme, which cannot be synthesized by Leishmania parasites (Chang & Chang, 1985), is imported by two dedicated import proteins, the heme permease LHR1, which is related to the Caenorhabditis elegans heme transporter HRG-4 (Huynh et al., 2012), and the major facilitator superfamily protein FLVCRb (Cabello-Donayre et al., 2016). Since conserved cytosolic iron storage proteins have not been identified in Leishmania parasites, it is thought that these cells need to regulate the level of intracellular iron through balancing import and export. The major facilitator family protein LIR1 exports iron from the cytoplasm (Laranjeira-Silva et al., 2018), and thus protects parasites from iron overload toxicity.
Fig. 3.
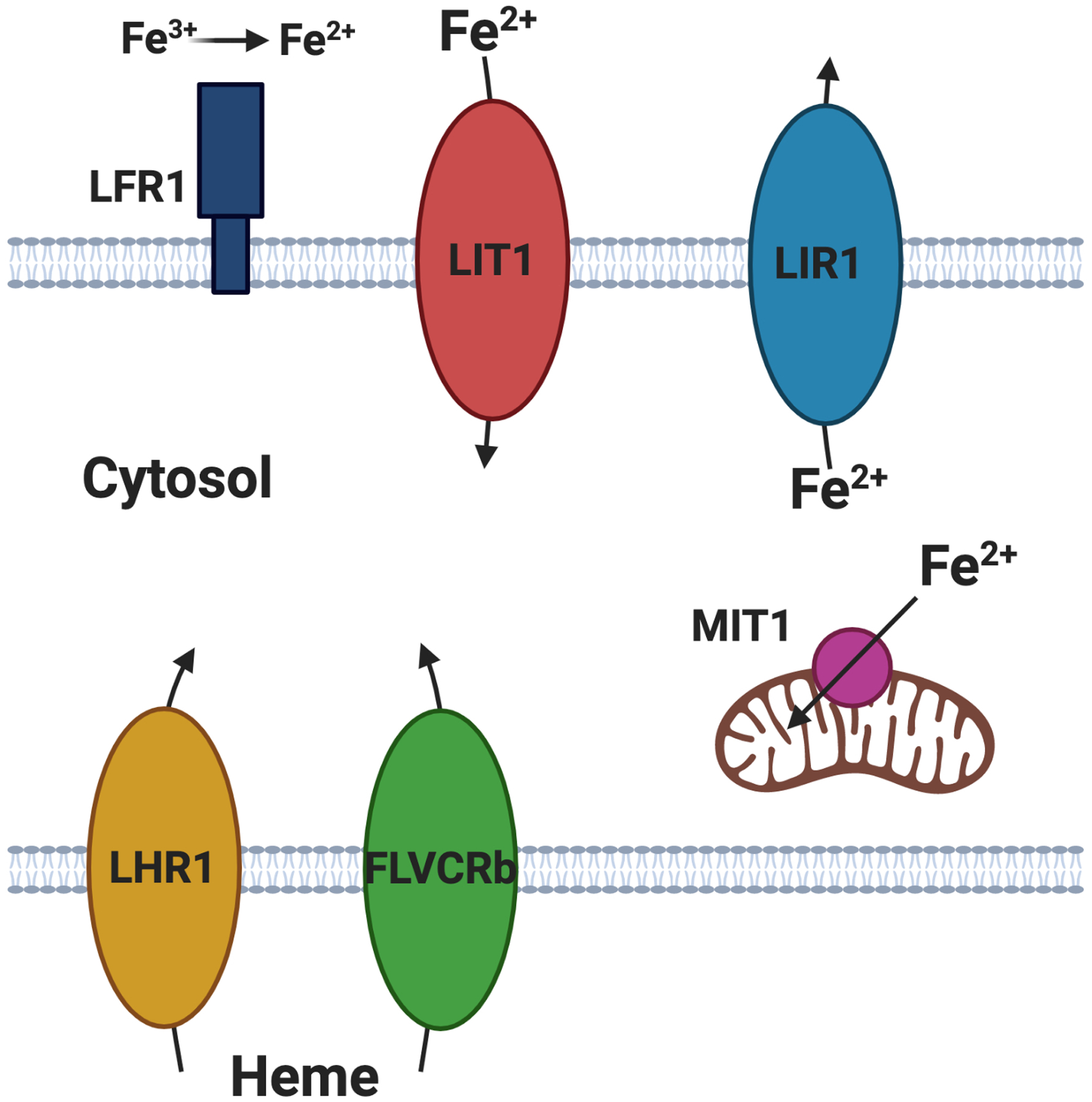
Import and export of iron and heme in Leishmania amazonensis. Lipid bilayers representing the plasma membrane are indicated at the top and bottom with the cytosol in between, and the mitochondrion is indicated in brown. LFR1 is an extracellularly oriented iron reductase that converts Fe3+ to Fe2+ at the parasite surface, generating the substrate for the iron permeases. Transporters for import (LIT1) or export (LIR1) of Fe2+ across the plasma membrane, for import of Fe2+ across the mitochondrial inner membrane (MIT1), and for import of heme across the plasma membrane (LHR1 and FLVCRb) are indicated as either ovals or a circle of different colors. Arrows indicate the direction of transport. Figure created with BioRender.com.
These pathways of iron regulation are particularly important for amastigotes growing within host cells in the iron-poor parasitophorous vacuole. Null mutants of LFR1, LIT1, and LIR1 all show severe growth defects within host cells and attenuated virulence in a mouse model of cutaneous leishmaniasis (Flannery et al., 2011, Huynh et al., 2006, Laranjeira-Silva et al., 2018). The requirement for LFR1 and LIT1 can be complemented by supplementation with iron; when host cells were preloaded with high levels of ferritin, amastigote growth was restored to Δlfr1 and Δlit1 parasites. In contrast, iron chelation enhanced the replication of Δlir1 intracellular amastigotes relative to wild-type parasites (Sarkar et al., 2019), confirming that this permease protects parasites against accumulation of toxic levels of iron. Both of the heme import proteins, LHR1 and FLVCRb, and the mitochondrial iron import protein MIT1, are essential for the growth of promastigotes, and deletion of even one allele of these genes reduced virulence of amastigotes in vivo (Cabello-Donayre et al., 2016, Huynh et al., 2012, Mittra et al., 2016).
The levels of these iron transport proteins change in response to iron availability, indicating that parasites sense and respond to iron. The mRNA encoding LFR1, the ferric reductase, is upregulated in response to iron deprivation, and cell lysates made from iron-deprived cells show increased levels of reductase activity (Flannery et al., 2011). Similarly, expression of LIT1 mRNA is induced when parasites are grown in iron-depleted medium (Mittra et al., 2013). In contrast, the iron export protein LIR1, but not its mRNA, is induced by addition of FeSO4 to the medium and decreased by iron deprivation, consistent with its activity being required under conditions of excess iron (Laranjeira-Silva et al., 2018). The parasite response to iron levels extends beyond the proteins directly involved with import and export. Iron starvation of promastigotes induces differentiation to amastigotes (Mittra et al., 2013). Paradoxically, this response appears to be mediated by induction of LIT1 in response to iron deprivation, resulting in increased intracellular Fe2+, enhanced FeSOD activity, and increased levels of H2O2, which acts as an inducer of amastigote differentiation. This differentiation pathway requires LFR1, LIT1, and MIT1 (Flannery et al., 2011, Mittra et al., 2013, Mittra et al., 2016), and can also be induced by the addition of exogenous H2O2.
The mechanisms of parasite iron sensing and subsequent regulation of iron import and export proteins are largely obscure, and this area represents a frontier for future research. However, it seems likely that iron sensing is an intracellular process, because Fe2+ is produced at the cell surface and then rapidly imported, and Fe3+ is not soluble but is bound to extracellular proteins. The differentiation of promastigotes to amastigotes upon iron depletion is also probably dependent upon increased intracellular iron, resulting from higher levels of LIT1, and subsequent import of Fe2+ into mitochondria by MIT1, where it increases the level of the differentiation inducer H2O2 (Mittra et al., 2013). While downstream components of nutrient sensing pathways in Leishmania are in general poorly understood, the FeSOD is one such component in the iron sensing pathway, being activated by this micronutrient and inducing promastigote to amastigote differentiation by production of H2O2.
Purine Sensing in Leishmania.
A well characterized example of intracellular nutrient sensing in Leishmania involves monitoring the intracellular purine nucleotide pool as part of the purine stress response. Purines are essential macromolecules that i) form the building blocks for DNA, RNA, and various cofactors, ii) act as the principal molecules for storing and transferring energy in all cells, and iii) function as important intracellular and extracellular signaling molecules. Like all known parasitic protists, Leishmania are incapable of de novo purine synthesis and must salvage these critical nutrients from their hosts (see (Boitz et al., 2012) for a detailed review). An assortment of secreted and cell-surface nucleases, nucleotidases, and phosphatases convert nucleic acids (RNA and DNA) or nucleotides from the host milieu into substrates for uptake by purine nucleoside and nucleobase transporters. A complex network of intracellular purine salvage enzymes shunt the internalized purine nucleosides and nucleobases into the adenine and guanine nucleotide pools. Because adenine and guanine nucleotides can be readily interconverted, any nucleobase (adenine, guanine, hypoxanthine, xanthine) or nucleoside (adenosine, guanosine, inosine, xanthosine) can serve as the sole purine source for the parasites.
As discussed above, the latter three Leishmania life cycle forms in the sand fly vector likely encounter host environments depleted of nutrients, including purines. While purine restriction does not, by itself, trigger differentiation into the infectious metacyclic form, purine depletion is required for efficient metacyclogenesis, both in the sand fly and in vitro (Serafim et al., 2012). To survive indeterminate periods of purine scarcity within the host, Leishmania have evolved a robust and coordinated stress response pathway. Purine-starved Leishmania promastigotes exit the cell cycle, entering a quiescent-like state in which they can persist for >90 days in purine-free culture medium (Martin et al., 2014). This quiescent-like state is accompanied by dramatic remodeling of the transcriptome and proteome, elongation of the cell body, increased motility, and stress granule formation (Carter et al., 2010, Martin et al., 2016, Martin et al., 2014, Shrivastava et al., 2019a, Shrivastava et al., 2019b). The earliest manifestations of the purine stress response are directed at increasing the acquisition of extracellular purines by upregulating expression of purine salvage enzymes and transporters (Carter et al., 2010, Martin et al., 2014, Ortiz et al., 2010). Regulation of a purine nucleobase transporter, NT3, by purine starvation has been studied in L. donovani and shown to be dependent upon a stem-loop regulatory element within the 3’-untranslated region of the mRNA that controls both mRNA translation and stability (Licon & Yates, 2020). Later changes are focused on the establishment of the quiescent-like state, including downregulation of DNA, RNA, and protein synthesis, and upregulation of general stress response pathways, especially those protecting against oxidative stress (Martin et al., 2014).
Adenine nucleotide levels are substantially reduced in purine-starved promastigotes, while guanine nucleotide levels are mostly unaffected at early timepoints, and actually increase over time (Martin et al., 2016). A direct role for alterations of the adenine nucleotide pool in purine sensing was demonstrated using mutants in the purine salvage pathway incapable of interconverting adenine and guanine nucleotides (Martin et al., 2016). The adenine and guanine nucleotide pools were independently depleted by culturing the mutants in medium with specific purine nucleobases that could be incorporated into only one of the two nucleotide branches. Mutants starved for adenine nucleotides, but not guanine nucleotides, responded identically to purine-starved wild type promastigotes, increasing the expression of a suite of known purine-responsive genes, and persisting in a quiescent-like state for at least a month. Normal induction of the purine stress response in the presence of extracellular purines renders direct sensing of extracellular purine availability via a membrane-associated purine receptor unlikely. In contrast, mutants starved for guanine nucleotides, but not adenine nucleotides, failed to upregulate a subset of purine-responsive genes, exhibited an incomplete cell cycle arrest, and experienced a precipitous drop in viability after ten days. This suggested that purine availability is sensed by monitoring the adenine nucleotide pool, and that a reduction in the adenine nucleotide pool is required to instigate the genetic program for long term survival of purine starvation.
The mechanisms of purine sensing and signaling are likely complex and have thus far remained elusive. For example, some purine stress-responsive genes were upregulated normally in mutants starved exclusively for guanine nucleotides, while the expression of others was unchanged, intimating the existence of at least two independent purine sensing/signaling pathways (Martin et al., 2016). An obvious candidate purine sensor and signaling molecule in Leishmania is the 5’ AMP-activated protein kinase (AMPK), which is highly conserved in eukaryotes and modulates cellular energy status by sensing reductions in the intracellular ATP-to-ADP or ATP-to-AMP ratio (Lin & Hardie, 2018). The substantial reduction in the ATP-to-AMP ratio following five days of purine starvation, but not in earlier timepoints, suggests the possibility that AMPK signaling may play a role in the adaptation to chronic purine starvation (Martin et al., 2016). The potential role of AMPK in the purine stress response, the identification of other purine sensors and signaling pathways, and determining if the purine stress response is operative in amastigotes within host macrophages represent compelling topics for future research.
Summary and Perspectives
Sensing of nutrients is critical for survival of Leishmania parasites. Thus, parasites that are null mutants for the flagellar GT1 permease grow to higher maximal cell density in culture than wild type cells but are not able to enter stationary phase and subsequently die with rapid kinetics (Rodriguez-Contreras et al., 2015). This growth expansion followed by catastrophe is reminiscent of similar phenotypes reported for Δlit1 null mutants (Mittra et al., 2013) and LMIT1/Δlmit1 (Mittra et al., 2016) heterozygous null mutants that are deficient for iron uptake. Parasites are also critically dependent on their ability to sense intracellular purine availability. When the Δgmpr/Δimpdh dual null mutant of L. donovani was cultured in the presence of hypoxanthine as the sole purine, conditions that allowed continued synthesis of adenine nucleotides but not of guanine nucleotides, parasites died (Martin et al., 2016). Lethality ensued because the parasites did not experience a reduction in intracellular adenine nucleotides, the critical signal for sensing purine starvation, even though they were undergoing guanine nucleoside starvation. Failure to sense purine depletion prevented these mutant promastigotes from mounting the protective response exhibited by purine-starved wild type parasites, which induces entry into a palliative quiescent state. Thus, continuing studies mapping mechanisms of nutrient sensing will elucidate processes that are critical for the survival of parasites experiencing hostile environments within their hosts.
There are various other nutrients not covered above that are critical for Leishmania parasites, and exploring whether and how their availability is sensed would be of considerable interest. A number of amino acids, including arginine, histidine, phenylalanine, serine, tyrosine, threonine, valine, leucine, lysine, and proline, are considered essential for these parasites (Krasner and Flory, 1971). Heme is an essential nutrient that is not synthesized by these parasites and is transported by the LHR1 transporter (Renberg et al., 2015). Similarly, folates and unconjugated pteridines are not synthesized by Leishmania parasites and are salvaged by a variety of transporters (Vickers & Beverley, 2011). Zn2+ is another micronutrient that is required by Leishmania parasites, but its intracellular level must be controlled to avoid zinc-overload toxicity (Carvalho et al., 2015). This metal ion and others are transported by the ZIP3 permease, and the expression of this transporter is regulated, at the mRNA level, by the availability of Zn+2 thus ensuring zinc homeostasis. Additionally, the mRNA for a phosphate transporter from L infantum is upregulated ~2-fold by restriction for phosphate (Russo-Abrahao et al., 2013), while starvation for either phosphate or purine upregulates expression of a surface membrane expressed 3’-nucleotidase that cleaves extracellular purine nucleotides and provides both purine nucleosides and phosphate for uptake by the parasite (Sacci et al., 1990). Hence, there are a variety of uptake systems that are likely to respond to sensing of their cognate nutrients that remain to be explored.
A major gap in our knowledge for sensing of nutrients or other environmental cues in Leishmania is the paucity of information on the cascades that transmit alterations in nutrient composition to phenotypic changes in the parasite. Recent progress in dissecting two sensory pathways in African trypanosomes provides instructive examples that may be relevant to their phylogenetic cousins. Differentiation of bloodstream resident stumpy form (SF) trypanosomes into procyclic form (PF) parasites within the gut of the tsetse fly vector is promoted by the metabolites citrate and cis-aconitate (Szoor et al., 2020). Work form the Matthews laboratory has identified two carboxylate transporters expressed in stumpy forms, PAD1 and PAD2, that import these differentiation inducers and hence allow their intracellular sensing. Furthermore, two protein phosphatases that interact with each other, TbPTP1 and TbPIP39, have been implicated as regulators of SF to PF differentiation, and two protein kinases, TbNRKA and TbNRKB, also play a role in differentiation. Of considerable interest, TbPIP39 binds citrate and inhibits its activation of TbPTP1, which in turn increases the level of phosphorylated TbPIP39, thus linking the differentiation inducing metabolite to the downstream signaling components. In the bloodstream, dividing long slender (LS) trypanosomes employ a quorum sensing mechanism that induces differentiation into nondividing SFs at high parasite density (Rojas & Matthews, 2019), developmental forms that are, as indicated above, primed for differentiation into insect stage PFs once they enter a tsetse fly. The long sought stumpy inducing factor, SIF, has been identified recently as oiligopeptides that are probably generated from host proteins as LS parasites lyse and release intracellular proteases. Sensing increasing levels of these oligopeptides requires their internalization by an unusual oligopeptide transporter, GPR89, and a genome-wide RNAi screen has implicated several protein kinases and phosphatases in this signaling pathway as well. Thus, protein phosphorylation seems to play a central role in both SF to PF and LS to SF differentiation, as it does in many eukaryotic signaling pathways. Identification of the molecular components of nutrient sensing pathways in Leishmania parasites, possibly by phosphoproteomic and genetic approaches, awaits similar advances.
Acknowledgements
We thank Kat Schmidt and Jess Hatfield for collecting some of the fluorescence microscopy images in Fig. 2 and Jeffrey Nolz for a critical reading of the manuscript.
Funding information
Work in the authors’ laboratories was supported by grants R01AI121160 (SML) and R03AI137636 (PAY).
Footnotes
Conflict of interest
The authors declare no financial conflict of interest.
REFERENCES
- Alcolea PJ, Alonso A, Baugh L, Paisie C, Ramasamy G, Sekar A, Sur A, Jimenez M, Molina R, Larraga V, and Myler PJ (2018) RNA-seq analysis reveals differences in transcript abundance between cultured and sand fly-derived Leishmania infantum promastigotes. Parasitol Int 67: 476–480. [DOI] [PubMed] [Google Scholar]
- Beneke T, Demay F, Hookway E, Ashman N, Jeffery H, Smith J, Valli J, Becvar T, Myskova J, Lestinova T, Shafiq S, Sadlova J, Volf P, Wheeler RJ, and Gluenz E (2019) Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog 15: e1007828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Biswas A, and Das PK (2009) Role of a differentially expressed cAMP phosphodiesterase in regulating the induction of resistance against oxidative damage in Leishmania donovani. Free Radic Biol Med 47: 1494–1506. [DOI] [PubMed] [Google Scholar]
- Boitz JM, Ullman B, Jardim A, and Carter NS (2012) Purine salvage in Leishmania: complex or simple by design? Trends Parasitol 28: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello-Donayre M, Malagarie-Cazenave S, Campos-Salinas J, Galvez FJ, Rodriguez-Martinez A, Pineda-Molina E, Orrego LM, Martinez-Garcia M, Sanchez-Canete MP, Estevez AM, and Perez-Victoria JM (2016) Trypanosomatid parasites rescue heme from endocytosed hemoglobin through lysosomal HRG transporters. Mol Microbiol 101: 895–908. [DOI] [PubMed] [Google Scholar]
- Carter NS, Yates PA, Gessford SK, Galagan SR, Landfear SM, and Ullman B (2010) Adaptive responses to purine starvation in Leishmania donovani. Mol Microbiol 78: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho S, Barreira da Silva R, Shawki A, Castro H, Lamy M, Eide D, Costa V, Mackenzie B, and Tomas AM (2015) LiZIP3 is a cellular zinc transporter that mediates the tightly regulated import of zinc in Leishmania infantum parasites. Mol Microbiol 96: 581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho-Martins EA, Laranjeira da Silva MF, dos Santos MG, Muxel SM, and Floeter-Winter LM (2011) Axenic Leishmania amazonensis promastigotes sense both the external and internal arginine pool distinctly regulating the two transporter-coding genes. PLoS One 6: e27818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CS, and Chang KP (1985) Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol Biochem Parasitol 16: 267–276. [DOI] [PubMed] [Google Scholar]
- Clayton C (2019) Regulation of gene expression in trypanosomatids: living with polycistronic transcription. Open biology 9: 190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Comb WC, and Sabatini DM (2015) Nutrient-sensing mechanisms and pathways. Nature 517: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed NM, Myler PJ, Blandin G, Berriman M, Crabtree J, Aggarwal G, Caler E, Renauld H, Worthey EA, Hertz-Fowler C, Ghedin E, Peacock C, Bartholomeu DC, Haas BJ, Tran AN, Wortman JR, Alsmark UC, Angiuoli S, Anupama A, Badger J, Bringaud F, Cadag E, Carlton JM, Cerqueira GC, Creasy T, Delcher AL, Djikeng A, Embley TM, Hauser C, Ivens AC, Kummerfeld SK, Pereira-Leal JB, Nilsson D, Peterson J, Salzberg SL, Shallom J, Silva JC, Sundaram J, Westenberger S, White O, Melville SE, Donelson JE, Andersson B, Stuart KD, and Hall N (2005) Comparative genomics of trypanosomatid parasitic protozoa. Science 309: 404–409. [DOI] [PubMed] [Google Scholar]
- Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, and Mukhopadhyay R (2007) Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol 65: 1006–1017. [DOI] [PubMed] [Google Scholar]
- Flannery AR, Huynh C, Mittra B, Mortara RA, and Andrews NW (2011) LFR1 ferric iron reductase of Leishmania amazonensis is essential for the generation of infective parasite forms. J Biol Chem 286: 23266–23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Pinkovich A, Balno C, Strasser R, Zeituni-Molad M, Bendelak K, Rentsch D, Ephros M, Wiese M, Jardim A, Myler PJ, and Zilberstein D (2016) An arginine deprivation response pathway Is induced in Leishmania during macrophage invasion. PLoS Pathog 12: e1005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsbeeks I, Lagatie O, Van Nuland A, Van de Velde S, and Thevelein JM (2004) The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem Sci 29: 556–564. [DOI] [PubMed] [Google Scholar]
- Huynh C, Sacks DL, and Andrews NW (2006) A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med 203: 2363–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh C, Yuan X, Miguel DC, Renberg RL, Protchenko O, Philpott CC, Hamza I, and Andrews NW (2012) Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog 8: e1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar E, Shaik J, Iantorno SA, Romano A, Nzelu CO, Owens K, Sanders MJ, Dobson D, Cotton JA, Grigg ME, Beverley SM, and Sacks D (2019) Whole genome sequencing of experimental hybrids supports meiosis-like sexual recombination in Leishmania. PLoS Genet 15: e1008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johner A, Kunz S, Linder M, Shakur Y, and Seebeck T (2006) Cyclic nucleotide specific phosphodiesterases of Leishmania major. BMC Microbiol 6: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FD, Sanchez MA, and Landfear SM (2020a) Touching the Surface: Diverse roles for the flagellar membrane in kinetoplastid parasites. Microbiol Mol Biol Rev 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FD, Tran KD, Hatfield J, Schmidt K, Sanchez MA, and Landfear SM (2020b) A cytoskeletal protein complex is essential for division of intracellular amastigotes of Leishmania mexicana. J Biol Chem 295: 13106–13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khannpnavar B, Mehta V, Qi C, and Korkhov V (2020) Structure and function of adenylyl cyclases, key enzymes in cellular signaling. Curr Opin Struct Biol 63: 34–41. [DOI] [PubMed] [Google Scholar]
- Laranjeira-Silva MF, Wang W, Samuel TK, Maeda FY, Michailowsky V, Hamza I, Liu Z, and Andrews NW (2018) A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog 14: e1007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licon MH, and Yates PA (2020) Purine-responsive expression of the Leishmania donovani NT3 purine nucleobase transporter is mediated by a conserved RNA stem-loop. J Biol Chem 295: 8449–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, and Hardie DG (2018) AMPK: Sensing glucose as well as cellular energy status. Cell Metab 27: 299–313. [DOI] [PubMed] [Google Scholar]
- Lopez MA, Saada EA, and Hill KL (2015) Insect stage-specific adenylate cyclases regulate social motility in African trypanosomes. Eukaryot Cell 14: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira da Silva L, and Beverley SM (2010) Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc Natl Acad Sci U S A 107: 11965–11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin L, and Gluenz E (2015) cAMP signalling in trypanosomatids: role in pathogenesis and as a drug target. Trends Parasitol 31: 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal G, Sharma M, Kruse M, Sander-Juelch C, Munro LA, Wang Y, Vilg JV, Tamas MJ, Bhattacharjee H, Wiese M, and Mukhopadhyay R (2012) Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol Microbiol 85: 1204–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Yates PA, Boitz JM, Koop DR, Fulwiler AL, Cassera MB, Ullman B, and Carter NS (2016) A role for adenine nucleotides in the sensing mechanism to purine starvation in Leishmania donovani. Mol Microbiol 101: 299–313. [DOI] [PubMed] [Google Scholar]
- Martin JL, Yates PA, Soysa R, Alfaro JF, Yang F, Burnum-Johnson KE, Petyuk VA, Weitz KK, Camp DG 2nd, Smith RD, Wilmarth PA, David LL, Ramasamy G, Myler PJ, and Carter NS (2014) Metabolic reprogramming during purine stress in the protozoan pathogen Leishmania donovani. PLoS Pathog 10: e1003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Saunders EC, Kloehn J, and Dagley MJ (2015) Leishmania carbon metabolism in the macrophage phagolysosome- feast or famine? F1000Res 4: 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra B, Cortez M, Haydock A, Ramasamy G, Myler PJ, and Andrews NW (2013) Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J Exp Med 210: 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra B, Laranjeira-Silva MF, Perrone Bezerra de Menezes J, Jensen J, Michailowsky V, and Andrews NW (2016) A trypanosomatid iron transporter that regulates mitochondrial function Is required for Leishmania amazonensis virulence. PLoS Pathog 12: e1005340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderer T, and McConville MJ (2008) The Leishmania-macrophage interaction: a metabolic perspective. Cell Microbiol 10: 301–308. [DOI] [PubMed] [Google Scholar]
- Oberholzer M, Marti G, Baresic M, Kunz S, Hemphill A, and Seebeck T (2007) The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J 21: 720–731. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Valdes R, Sanchez MA, Hayenga J, Elya C, Detke S, and Landfear SM (2010) Purine restriction induces pronounced translational upregulation of the NT1 adenosine/pyrimidine nucleoside transporter in Leishmania major. Mol Microbiol 78: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux J, Dinsart C, Huet G, and Pays E (1992) A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol. Cell. Biol 12: 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KN (2014) Guanylyl cyclase/natriuretic peptide receptor-A signaling antagonizes phosphoinositide hydrolysis, Ca(2+) release, and activation of protein kinase C. Front Mol Neurosci 7: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar H, Puri M, Fischer Weinberger R, Madhubala R, and Zilberstein D (2019) The arginine sensing and transport binding sites are distinct in the human pathogen Leishmania. PLoS Negl Trop Dis 13: e0007304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Xu X, Russell DG, Little BM, and Landfear SM (1995) Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J. Cell. Biol 128: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renberg RL, Yuan X, Samuel TK, Miguel DC, Hamza I, Andrews NW, and Flannery AR (2015) The Heme Transport Capacity of LHR1 Determines the Extent of Virulence in Leishmania amazonensis. PLoS Negl Trop Dis 9: e0003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM, and Axel R (1982) Gene amplification and gene correction in somatic cells. Cell 29: 109–119. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras D, Aslan H, Feng X, Tran K, Yates PA, Kamhawi S, and Landfear S (2015) Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: a potential glucose sensor. FASEB J 29: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas F, and Matthews KR (2019) Quorum sensing in African trypanosomes. Curr Opin Microbiol 52: 124–129. [DOI] [PubMed] [Google Scholar]
- Russo-Abrahao T, Alves-Bezerra M, Majerowicz D, Freitas-Mesquita AL, Dick CF, Gondim KC, and Meyer-Fernandes JR (2013) Transport of inorganic phosphate in Leishmania infantum and compensatory regulation at low inorganic phosphate concentration. Biochim Biophys Acta 1830: 2683–2689. [DOI] [PubMed] [Google Scholar]
- Saada EA, Kabututu ZP, Lopez M, Shimogawa MM, Langousis G, Oberholzer M, Riestra A, Jonsson ZO, Wohlschlegel JA, and Hill KL (2014) Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the Trypanosoma brucei Flagellar membrane. Eukaryot Cell 13: 1064–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacci JB Jr., Campbell TA, and Gottlieb M (1990) Leishmania donovani: regulated changes in the level of expression of the surface 3’-nucleotidase/nuclease. Exp Parasitol 71: 158–168. [DOI] [PubMed] [Google Scholar]
- Saha A, Bhattacharjee A, Vij A, Das PK, Bhattacharya A, and Biswas A (2020) Evaluation of Modulators of cAMP-Response in Terms of Their Impact on Cell Cycle and Mitochondrial Activity of Leishmania donovani. Front Pharmacol 11: 782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D (2018) Adenylate cyclases of Trypanosoma brucei, environmental sensors and controllers of host innate immune response. Pathogens 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D, Bachmaier S, Krumbholz C, Kador M, Gossmann JA, Uzureau P, Pays E, and Boshart M (2012a) Cytokinesis of Trypanosoma brucei bloodstream forms depends on expression of adenylyl cyclases of the ESAG4 or ESAG4-like subfamily. Mol Microbiol 84: 225–242. [DOI] [PubMed] [Google Scholar]
- Salmon D, Vanwalleghem G, Morias Y, Denoeud J, Krumbholz C, Lhomme F, Bachmaier S, Kador M, Gossmann J, Dias FB, De Muylder G, Uzureau P, Magez S, Moser M, De Baetselier P, Van Den Abbeele J, Beschin A, Boshart M, and Pays E (2012b) Adenylate cyclases of Trypanosoma brucei inhibit the innate immune response of the host. Science 337: 463–466. [DOI] [PubMed] [Google Scholar]
- Sanchez MA, Tran KD, Valli J, Hobbs S, Johnson E, Gluenz E, and Landfear SM (2016) KHARON Is an essential cytoskeletal protein involved in the trafficking of fagellar membrane proteins and cell division in African trypanosomes. J Biol Chem 291: 19760–19773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MA, Zeoli D, Klamo EM, Kavanaugh MP, and Landfear SM (1995) A family of putative receptor-adenylate cyclases from Leishmania donovani. J. Biol. Chem 270: 17551–17558. [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, and Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14 Suppl: S401–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Andrews NW, and Laranjeira-Silva MF (2019) Intracellular iron availability modulates the requirement for Leishmania Iron Regulator 1 (LIR1) during macrophage infections. Int J Parasitol 49: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlein Y (1986) Sandfly diet and Leishmania. Parasitol. Today 2: 175–177. [DOI] [PubMed] [Google Scholar]
- Sen Santara S, Roy J, Mukherjee S, Bose M, Saha R, and Adak S (2013) Globin-coupled heme containing oxygen sensor soluble adenylate cyclase in Leishmania prevents cell death during hypoxia. Proc Natl Acad Sci U S A 110: 16790–16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafim TD, ., Figueiredo AB, Costa PA, Marques-da-Silva EA, Goncalves R, de Moura SA, Gontijo NF, da Silva SM, Michalick MS, Meyer-Fernandes JR, de Carvalho RP, Uliana SR, Fietto JL, and Afonso LC (2012) Leishmania metacyclogenesis is promoted in the absence of purines. PLoS Negl Trop Dis 6: e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked-Mishan P, Suter-Grotemeyer M, Yoel-Almagor T, Holland N, Zilberstein D, and Rentsch D (2006) A novel high-affinity arginine transporter from the human parasitic protozoan Leishmania donovani. Mol Microbiol 60: 30–38. [DOI] [PubMed] [Google Scholar]
- Shaw S, DeMarco SF, Rehmann R, Wenzler T, Florini F, Roditi I, and Hill KL (2019) Flagellar cAMP signaling controls trypanosome progression through host tissues. Nature communications 10: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava R, Drory-Retwitzer M, and Shapira M (2019a) Nutritional stress targets LeishIF4E-3 to storage granules that contain RNA and ribosome components in Leishmania. PLoS Negl Trop Dis 13: e0007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava R, Tupperwar N, Drory-Retwitzer M, and Shapira M (2019b) Deletion of a Single LeishIF4E-3 Allele by the CRISPR-Cas9 System Alters Cell Morphology and Infectivity of Leishmania. mSphere 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subota I, Julkowska D, Vincensini L, Reeg N, Buisson J, Blisnick T, Huet D, Perrot S, Santi-Rocca J, Duchateau M, Hourdel V, Rousselle JC, Cayet N, Namane A, Chamot-Rooke J, and Bastin P (2014) Proteomic analysis of intact flagella of procyclic Trypanosoma brucei cells identifies novel flagellar proteins with unique sub-localization and dynamics. Mol Cell Proteomics 13: 1769–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter J, and Gull K (2017) Shape, form, function and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open biology 7: 170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoor B, Silvester E, and Matthews KR (2020) A Leap Into the Unknown - Early Events in African Trypanosome Transmission. Trends Parasitol 36: 266–278. [DOI] [PubMed] [Google Scholar]
- Vickers TJ, and Beverley SM (2011) Folate metabolic pathways in Leishmania. Essays Biochem 51: 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RJ, Sunter JD, and Gull K (2016) Flagellar pocket restructuring through the Leishmania life cycle involves a discrete flagellum attachment zone. J Cell Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheway G, Nazlamova L, and Hancock JT (2018) Signaling through the primary cilium. Front Cell Dev Biol 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]


