Abstract
Purpose of Review
This review highlights challenges associated with weight management in children and adolescents with type 1 diabetes (T1D). Our purpose is to propose potential solutions to improve weight outcomes in youth with T1D.
Recent Findings
A common barrier to weight management in T1D is reluctance to engage in exercise for fear of hypoglycemia. Healthcare practitioners generally provide limited guidance for insulin dosing and carbohydrate modifications to maintain stable glycemia during exercise. Adherence to dietary guidelines is associated with improved glycemia; however, youth struggle to meet recommendations. When psychosocial factors are addressed in combination with glucose trends, this often leads to successful T1D management. Newer medications also hold promise to potentially aid in glycemia and weight management, but further research is necessary.
Summary
Properly addressing physical activity, nutrition, pharmacotherapy, and psychosocial factors while emphasizing weight management may reduce the likelihood of obesity development and its perpetuation in this population.
Keywords: Type 1 diabetes, Youth, Obesity, Weight management, Exercise, Physical activity
Introduction
Children and adolescents with type 1 diabetes (T1D) have a high rate of obesity that is increasing globally at a rate of ~ 4% annually [1]. The prevalence of obesity among adolescents with T1D ranges from 13.1% in the Type 1 Diabetes Exchange Registry (aged 13–18 years) to 20.5% (aged 12 to 19 years) in the National Health and Nutrition Examination Survey (NHANES) [2, 3]. According to the NHANES, 22.1% of youth with T1D were overweight compared with 16.1% of youth without T1D [4]. Historically, obesity has been strongly associated with type 2 diabetes (T2D), but not with T1D. However, T1D itself does not provide protection against insulin resistance and obesity associated with the modern obesogenic environment [4]. In fact, a trend towards improvement in glucose control over the last decade and aspects of T1D management, such as hypoglycemia treatment and dietary counseling, may contribute to unwanted weight gain. In the diabetes population, obesity, hypertension, and suboptimal glycemic control are all contributing factors to cardiovascular disease (CVD) risk, and CVD is the most prevalent cause of mortality and morbidity in T1D [5].
In children and adolescents, regular physical activity has been shown to yield numerous benefits including improvements in mental and cognitive health [6], cardiovascular fitness [7], bone mineral density [8], blood lipid profiles, and body composition [9]. Similarly, youth with T1D also benefit from physical activity interventions [10–12]. However, for youth with T1D, physical activity often comes with the added challenge of maintaining blood glucose values in target range.
The aim of this review is to address some of the challenges associated with weight management in T1D, detail the benefits of exercise, and discuss nutrition, pharmacotherapy, and psychosocial interventions.
Benefits of Exercise in Youth
In the general pediatric population, children and adolescents who are physically active benefit from improved physical and mental health. Active youth have fewer cardiometabolic risk factors [13–15], better bone health [16] and muscular strength [17, 18], healthier weight status, and enhanced cognitive and behavioral health [19, 20]. Accordingly, the American Academy of Pediatrics has prioritized physical activity as an educational topic that should be discussed during each annual child health supervision examination starting at the age of 18 months and continuing throughout childhood and adolescence [21]. Children with T1D are at increased risk for CVD as are adults, and display signs of early atherosclerotic disease in childhood [22]. They also typically have reduced bone mineral density [23, 24], weigh more than their peers [4], and have increased rates of depression [25, 26]. Therefore, the beneficial effects of physical activity may be even more important in children and adolescents with T1D than children without T1D.
An improvement in cardiometabolic risk factors is the most recognized health benefit of being physically active. Children as young as 6 years of age demonstrate lower systolic and diastolic blood pressure and higher HDL-cholesterol with increased physical activity [27]. Most strikingly, physically active children without T1D have improved markers of insulin resistance including decreased plasma triglycerides and lowered plasma insulin levels [28]. Although the effect of physical activity during childhood on later cardiovascular morbidity and mortality in adulthood is unknown, several observational studies suggest that physical activity during childhood has a positive impact on adult cardiovascular health [29, 30]. Physical activity also results in improved bone mass and bone strength in children as young as the age of 3 in the general population and improves balance, reducing the risk of falls and fractures even into adulthood [16]. This improvement in bone mineral density is similar in children with T1D [31].
As mentioned, the benefit of physical activity in childhood and adolescence extends well beyond physical health as being active can decrease the risk for depression [32]. Physically active children also have improved attention, processing speed, memory, and cognition, resulting in improved academic performance [33, 34]. Finally, physical activity has been shown to decrease the potential for harmful social behaviors such as smoking, alcohol, and illicit drug use, even into young adulthood [35, 36]. Given the underlying risk factors that are present for CVD, poor bone health, and increased depression seen in T1D, exercise and regular physical activity are likely even more important in this population.
Challenges Related to Weight Management in Youth with T1D
It is well accepted that a healthy diet and an active lifestyle are the foundation of optimal weight management [37]. However, management of T1D can be at odds with these principles. While a healthy diet includes limiting simple carbohydrate intake, individuals with T1D often require simple carbohydrate administration to maintain euglycemia. These simple carbohydrates do not contain ideal nutritional content and are additional calories consumed. Historically, avoidance of nocturnal hypoglycemia required bedtime snacks, and while newer insulins’ pharmacokinetics and diabetes technology, such as continuous glucose monitoring (CGM) and automated insulin delivery [38], have decreased the risk of nocturnal hypoglycemia, many individuals with T1D are encouraged to consume carbohydrates to prevent hypoglycemia [37]. Fear of hypoglycemia is a commonly reported burden in youth with diabetes as well as their family members [39]. To avoid hypoglycemia, some youth with T1D will consume additional carbohydrates before recommended thresholds for hypoglycemia treatment are reached, resulting in unnecessary hyperglycemia and caloric intake. In the pediatric population, fear of hypoglycemia experienced by the parent/guardian on behalf of the child can also compound and exacerbate this behavior [39].
The current American Diabetes Association (ADA) and International Society for Pediatrics and Adolescent Diabetes guidelines recommend 60 min of daily moderate-to-vigorous activity for youth with T1D [40, 41]. However, youth with T1D generally do not meet this recommendation. Barriers to meet this recommendation are complex and centered around the competing demands of dual management of T1D and weight. Dysglycemia is the most common barrier to weight management reported as (1) fear of hypoglycemia preventing the initiation of rigorous activity; (2) out-of-target blood glucose values resulting in an inability to start exercise or in prematurely stopping exercise; (3) hypoglycemia during or after exercise requiring consumption of carbohydrates and calories, which remove the negative energy balance achieved by exercising; and (4) inadequate guidance from T1D providers on both the specific insulin and carbohydrate modifications to maintain euglycemia during exercise as well the over-reliance on pretreatment with carbohydrates before any activity [39, 42, 43]. As youth with T1D transition from childhood to adulthood, these issues identified in childhood regarding the dual management of T1D and weight rarely resolve and evolve to integrate other aspects of adulthood [43].
Youth with T1D are subject to the same obesogenic environment that has resulted in the staggering rates of obesity for all youth globally. In the general pediatric population, increased calorie consumption, consumption of sugar-sweetened beverages, large portion sizes, increased sedentary activities, and maladaptive coping strategies (such as eating to suppress negative emotions or due to boredom) are postulated mechanisms in the rise in pediatric obesity [44, 45]. Unique to youth with T1D, exogenous insulin use and intensive glucose control are both associated with weight gain [37]. Finally, psychosocial factors, such as depression, low social support, poor quality of life, or negative body image, are associated with higher rates of overweight and obesity in youth with T1D, particularly in girls [46]. In addition, youth with T1D have higher rates of disordered eating and can omit insulin to limit calorie absorption with significant consequences which further complicates the approach of weight management in T1D [37, 47].
Possible Solutions for Weight Management in Youth with T1D
Exercise and Physical Activity
In children and adolescents, exercise has been shown to increase lean body mass, decrease visceral adiposity, and increase insulin sensitivity [48]. Exercise also leads to a significant reduction in mortality rates, but it should also be noted that exercise alone may not be the optimal weight loss strategy [48]. Community-based intervention programs focusing on nutrition and increasing physical activity can lead to weight loss in overweight and obese youth [49]. In addition, combined exercise and dietary interventions are effective in reducing metabolic risks in overweight and obese children, at least in the short term [50]. However, nearly 80% of school-aged adolescents do not meet the recommended 60 min of daily physical activity [51] and youth with T1D often engage in even fewer minutes of activity [52–54] and energy expenditure [55] compared with their peers without T1D. Consensus guidelines on exercise have shown that for youth with T1D, physical activity and exercise can also cause drastic disturbances to glycemic control and increase the burden of diabetes management. Aerobic activities, such as brisk walking, cycling, and swimming, tend to increase the likelihood of hypoglycemia, and subsequently, supplemental carbohydrates are needed to either reduce or treat hypoglycemia [40]. On the other hand, anaerobic activities (e.g. sprinting, weight-lifting, etc.) under basal insulin conditions may reduce the risk of hypoglycemia during activity [40, 56]. The type and intensity of activity are important considerations for blood glucose control, as Yardley et al. [57] suggest performing resistance before aerobic exercise may improve glucose stability during activity. Providing guidance and advice around safe exercise strategies can also be challenging for healthcare providers. In particular, the importance of using relevant questions and often starting with the “patient’s exercise goal” as the primary focus for an effective consultation is an effective method to structure exercise consultations in youth with T1D [58]. By better understanding some of the challenges associated with weight management in children and adolescents with T1D, such as the fear of hypoglycemia as a primary barrier to physical activity, we can begin to target possible weight management solutions.
Although the literature suggests strategies for reducing the risk of hypoglycemia by reducing basal insulin rates by 50–80% set 60–90 min before exercise [60, 61], this requires significant preplanning and is often not a practical approach (Table 1). In fact, a recent study in adolescents found that many do not adjust their insulin dosing in preparation for exercise [62]. Therefore, for unplanned or spontaneous activity, a common occurrence for youth, one of the most effective strategies for reducing the risk of hypoglycemia is ingesting supplemental carbohydrates [40]. Although effective for reducing hypoglycemia risk, the added carbohydrate feeding is not optimal for weight management or weight loss. When prescribing physical activity, youth should consider bolus insulin dose reductions as a more effective approach to targeting weight loss versus carbohydrate ingestion for gaining muscle mass [63]. For example, a 25–75% bolus insulin reduction at the meal prior to exercise, a regular bolus of insulin pre-exercise with additional carbohydrates during exercising, or consuming carbohydrates without insulin before exercise may all protect against exercise-induced hypoglycemia [40]. However, the consumption of carbohydrates before exercise results in an overall increase in calorie consumption, often resulting in a positive energy balance and weight gain [59]. An added challenge in T1D management includes the increased risk of late-onset or nocturnal hypoglycemia due to increases in insulin sensitivity and delayed replenishment of liver and muscle glycogen following exercise [64, 65]. For these reasons, current exercise consensus statements and experts suggest a 20% basal insulin reduction for 6 h overnight after exercise to reduce the risk of nocturnal hypoglycemia [40, 66, 67]. Table 1 outlines a series of strategies to aid in reducing the risk of hypoglycemia during and post-exercise in youth with T1D.
Table 1.
Strategies to reduce risk of hypoglycemia during and post-exercise in youth with T1D
| Condition | Recommendations |
|---|---|
| Exercise intensity | If no insulin dose adjustment made, consider: -High-intensity, anaerobic exercise (e.g. short sprints) -Mixed activity (combined aerobic and anaerobic) -Short duration aerobic exercise (< 45 min) |
| Basal insulin adjustment | Pre-exercise: -50–80% basal reduction set 60–90 min pre-exercise -Basal suspension at exercise onset (for short duration activity < 45 min) Post-exercise: -Basal reduction by 20% for 6 h overnight |
| Bolus insulin adjustment | Pre-exercise: Meal bolus reductions pre-exercise (if within 1–3 h of exercise): For short duration activity: -25–50% bolus reduction For long duration activity: -50–75% bolus reduction Post-exercise: -Up to 50% bolus reduction |
| Closed loop strategies | Pre-exercise: -Set exercise target 1–2 h pre-exercise until end of exercise Post-exercise: -Exercise target may also be necessary for several hours in recovery or overnight if at high risk of hypoglycemia post-exercise (e.g. following prolonged aerobic exercise) |
| CGM strategies | -Check alerts and trend arrows -Set higher “low glucose limit” to allow more proactive alerts -Add “follower” to increase safety* |
| Nutrition | Pre-exercise (CHO suggestions for stabilizing glycemia): Below target (< 126 mg/dL): -10–30 g CHO before exercise Target range (126–180 mg/dL): -No CHO necessary Above target (> 180 mg/dL): -No CHO necessary Post-exercise: -CHO fueling post-exercise important for glycogen replenishment and prevention of late-onset hypoglycemia |
| Circulating insulin | -Aim to exercise with little-to-no active or “on-board” insulin before exercise -Ideally, plan exercise immediately following meal bolus or > 3 h following meal |
Impaired awareness of hypoglycemia is another risk factor for severe hypoglycemia that affects ~ 25% of patients with T1D [68]. Interestingly, a recent proof-of-concept study proposed that patients with impaired awareness of hypoglycemia can acutely restore counter-regulatory hormonal responses to hypoglycemia with a bout of high-intensity training [69]. It is speculated that impaired hypoglycemia awareness develops through habituation, often described as a reduced response to a stimulus following repeated exposure [69]. The high-intensity training was introduced as a single stress stimulus, also referred to as a dishabituating stimulus and a subsequent increase in heart rate, epinephrine, and lactate. Although this data is preliminary, the introduction of high-intensity training may offer a novel and promising avenue for restoring hypoglycemia awareness in individuals with T1D and, ultimately, decrease the risk of severe hypoglycemia.
The utilization of consumer-based physical activity trackers as part of short-term interventions has been shown to potentially increase levels of physical activity in a wide-range of populations [70]. In the pediatric population, an additional challenge in utilizing physical activity trackers may be poor compliance with accelerometers, although many studies fail to report compliance entirely [71]. At Stanford, we are engaged in a pilot study with the aim to incorporate consumer-based physical activity trackers to collect activity levels in newly diagnosed youth with T1D (Fig. 1). Physical activity trackers will provide us with insight into patient activity patterns, trends, and behaviors and determine whether differences exist between more compared with less active youth with T1D. We will utilize patient-generated physical activity data to assess sedentary and active behaviors by evaluating the proportion of youth who use their device, sync their data, and have data extracted and aligned to CGM data to assess key characteristics such as wear time, active hours, sedentary hours, and variation in activity during the observed study period. We also plan to develop and implement education materials and training modules regarding safe exercise strategies in newly diagnosed patients with T1D and their families over telehealth. Ultimately, the goal of this research is to have a translatable program that is readily accessible, scalable, and can be shared with newly diagnosed youth and families.
Fig. 1.
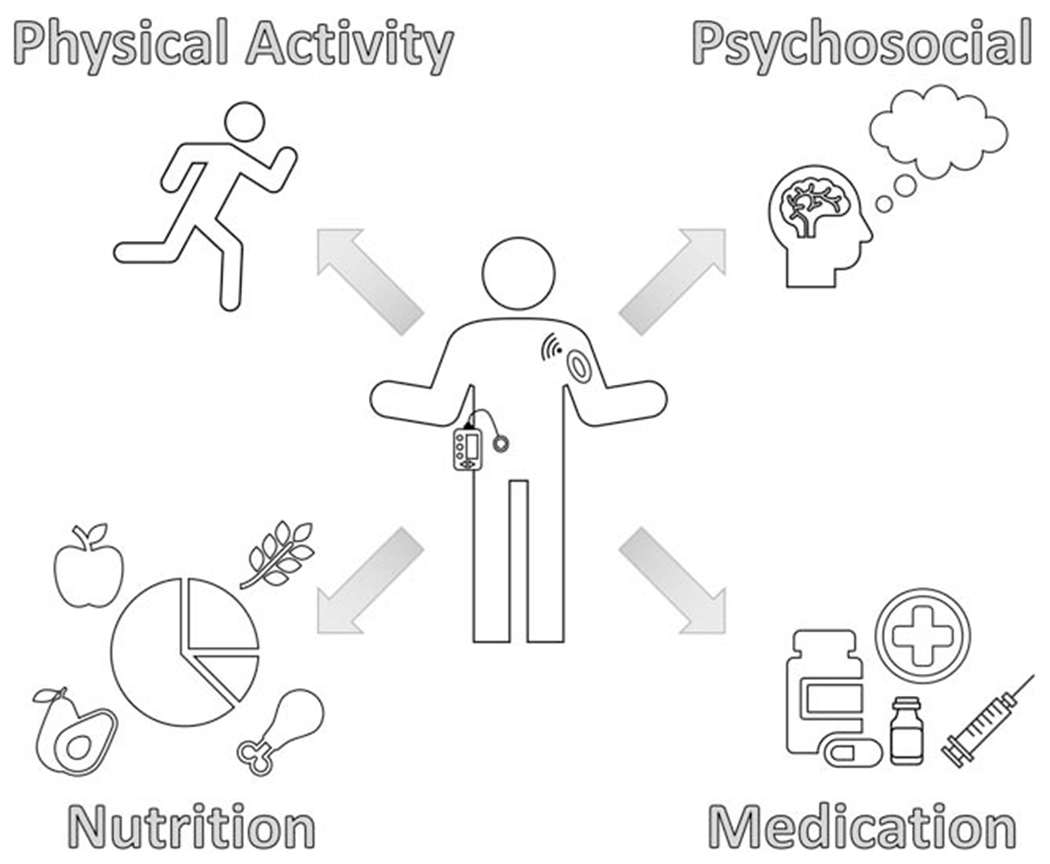
Possible solutions for weight management in youth with T1D
Diabetes Technology
Some advantages in the use of continuous subcutaneous insulin infusion (CSII) include convenience, use of multiple basal insulin rates, ease of adjusting insulin doses, flexibility, and freedom [72]. Ultimately, CSII therapy has demonstrated sustained long-term improvements in blood glucose control in children and adolescents with T1D and reductions in severe hypoglycemia [73]. Real-time CGM technology allows for the assessment of frequent sensor glucose values, glucose directional rate of change, and current trends [74]. The ADA’s Standards of Medical Care in Diabetes recommend CGM use in all children and adolescents with T1D [75]. In numerous studies, CGM use has been shown to improve glycemic control and quality of life in children with T1D using either multiple daily injections or CSII [76–79]. A recent publication also shows that CGM initiation in newly diagnosed youth with T1D is feasible and accepted by youth [80]. Further advancements in diabetes technology over the last decade, particularly with automated insulin delivery and hybrid closed loop systems, have led to improvements in glucose time-in-range (TIR) and reduced time spent in hypoglycemia, even during physical activity, for youth with T1D [81–83]. While automated insulin delivery systems are likely advantageous in promoting exercise by reducing the time spent in hypoglycemia, what effect automated insulin delivery systems might have on weight is unclear. For example, the MiniMed™ 670G pivotal trial reported a 1.4 kg weight gain in adults with T1D on this hybrid closed loop system over a 3-month period (p < 0.001) whereas, adolescents in the same trial gained 1.0 kg (p = 0.065) [84].
Nutrition
A number of studies have shown safety and efficacy of weight-loss diets [85, 86], but fewer studies have looked at nutrition-based interventions specifically in T1D. At this time, national and international guidelines recommend eating a varied diet, providing intensive education on coupling insulin delivery with carbohydrate ingestion, monitoring growth, and limiting high-sugar food and drinks [41, 87]. For youth with T1D, close adherence to dietary guidelines is correlated with improved glycemic control [88]. However, youth with T1D often struggle with adherence to daily recommendations for fruit, vegetable, and whole grain intake [89]. Early studies suggest that the order and timing of macronutrient consumption may play a role. For example, consuming vegetables or protein before carbohydrate results in a lower postprandial glycemic surge [90].
In recent years, low-carbohydrate (< 100 g carbohydrate per day) and ketogenic (< 55 g carbohydrate per day) diets have become increasingly popular weight-loss strategies in the general population [91]. While the efficacy of low-carbohydrate diets is more established in adults with T2D with studies demonstrating improvements in HbA1c [92] and reduced glucose variability [93], data are again limited in youth with T1D. When considering low-carbohydrate diets for youth with T1D, numerous issues have been raised to urge caution including the concern of increased frequency of hypoglycemia [94], inadequate carbohydrate intake to support normal pediatric growth, adverse effects on CVD risk factors, and the theoretical risk of increased diabetic ketoacidosis (DKA). Pediatric studies of low-carbohydrate diets have demonstrated a mean decrease in height standard deviation score (SDS) in youth with T1D who use a low-carbohydrate diet [95, 96]. For example, in a study of 34 youth who chose a very low-carbohydrate diet, there was a statistically significant decrease in height from SDS 0.41 ± 1.27 to 0.20 ± 1.02 (p = 0.05) [96]. Additionally, the increase in hypoglycemia and a lack of response to glucagon are hypothesized to occur in low-carbohydrate diets because of the decreased carbohydrate intake, thereby impairing glycogen storage in the liver [97]. Finally, in the most extreme low-carbohydrate diet, such as a ketogenic diet, production of ketones can increase the risk of DKA [98, 99]. Further studies are needed to evaluate the efficacy and safety of lower carbohydrate diets in youth with T1D with specific care to evaluate the effect on growth in the pediatric population.
Psychosocial Approach
Data are limited on the optimal approach to pediatric obesity in the setting of T1D, particularly with respect to the role of psychosocial strategies as one aspect of the comprehensive treatment of weight and T1D. Modeling after effective treatment of pediatric obesity and pediatric T1D, successful dual management of weight and T1D should address psychosocial factors and a thoughtful clinical approach [50]. Psychosocial factors, such as depression, poor family dynamics, low self-esteem, bullying, and poor body image, are associated with higher rates of overweight and obesity in the pediatric population at large. Strategies for treatment of pediatric obesity include (1) motivational interviewing, a patient-centered counseling method; (2) improving coping mechanisms to avoid emotion driven eating; (3) behavioral interventions targeting the community, school, parent, or whole family; and (4) discussion of barriers to healthy eating and physical activity [44, 100].
The literature is overwhelmingly consistent: for successful T1D management, psychosocial factors must be addressed in consort with glucose trends [101, 102]. When general psychosocial factors (such as depression, anxiety, family dynamics, and school support) or T1D-specific psychosocial factors (such as diabetes distress, coping with a chronic disease diagnosis, T1D-related family dynamics, and fear of hypoglycemia) are addressed, youth with T1D experience improvements in quality of life, glycemic outcomes, T1D behaviors, and self-efficacy.
The strong relationship of psychosocial factors with pediatric obesity and pediatric T1D separately can inform a model for the dual management of T1D and weight by addressing fear of hypoglycemia, psychological conditions, and family and school dynamics through the lens of motivational interviewing. First, addressing fear of hypoglycemia through blood glucose awareness and cognitive behavioral therapy [103] and addressing psychosocial barriers to diabetes technology uptake are important strategies. The use of diabetes technologies, such as CGM, insulin pumps, and hybrid closed loop pumps, has demonstrated lower rates of hypoglycemia [41, 75, 104, 105]. Second, screening for depression, diabetes distress, anxiety, negative body image, or disordered eating offers context to the discussion of weight and T1D management and importantly avoid exacerbating or contributing to psychopathology. Third, youth are strongly influenced by their community, school, peers, and family in general and in the context of weight and T1D management. For example, understanding and modifying family behavior is an important aspect in the ability to create sustainable change in youth. Therefore, addressing factors in a youth’s environment is an important aspect of addressing weight and T1D (Fig. 1).
Pharmacotherapy
Intensive insulin therapy has been shown to slow the progression of diabetes complications and improve overall glycemic control [106]. However, studies have also shown that weight gain commonly occurs with intensive insulin therapy and is a contributing factor to obesity in individuals with T1D [106, 107]. This weight gain is likely due to the additional calorie intake required to counter hypoglycemia, increase total calorie intake with euglycemia, non-physiologic subcutaneous delivery of insulin in T1D, and the growth properties inherent to insulin [108, 109]. Therefore, adjunctive therapies to insulin are increasing in popularity in adults, but have not been extensively evaluated in the pediatric population.
Sodium Glucose Co-Transporter 2 Inhibitors
Sodium glucose co-transporter 2 (SGLT2) inhibitors are a relatively new class of oral anti-hyperglycemic, glucose-lowering medication, commonly prescribed for patients with T2D [110]. Although currently off-label in the USA for individuals with T1D, the utility of SGLT2 inhibitors as adjunctive therapy in T1D has sparked much discussion relating to the associated benefits versus risks. When added to insulin therapy, SGLT2 inhibitors have shown benefits including a moderate lowering of HbA1c [110], cardiac and renal protective effects [111], weight loss, and reduction in the total daily insulin dose [112]. The current controversy around SGLT2 inhibitor adjunctive use in T1D is the increased risk of euglycemic DKA [110]. More research is needed on this class of medications to better understand the long-term effects of SGLT2 inhibitors in T1D and whether DKA risk can be mitigated with extensive surveillance measures.
In a proof-of-concept, randomized, placebo-controlled study, Biester et al. [113] examined single-dose effects of an SGLT2 inhibitor, in adolescents with T1D, and found a significant reduction in mean insulin dose. A follow-up study examined the effects of SGLT2 inhibitors on glucose TIR between 70 and 180 mg/dL with the use of a closed loop system in adolescents with T1D [114]. The findings revealed that again in an acute setting (24 h), a significantly increased postprandial TIR was apparent in the SGLT2 inhibitor and closed loop group versus closed loop technology alone. Currently, published literature on SGLT2 inhibitor is predominately focused on adults with T1D, although studies are inherently moving towards studying the pediatric T1D population as this may be a promising avenue for weight loss and improved glycemic control, particularly in youth that are overweight.
Glucagon-Like Peptide-1 Receptor Agonists
Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced by the gut that enhances insulin secretion [115]. Notably, the use of GLP-1 receptor agonists (GLP-1 RA) such as liraglutide and exenatide has become increasingly popular in T2D management and has been shown to reduce appetite and gastric emptying and, in turn, improve insulin sensitivity, increase weight loss, and lower blood pressure and HbA1c [116]. In adults, higher dosages of liraglutide have also been approved as a treatment for obesity [117]. Similar to SGLT2 inhibitors, GLP-1 RA were initially approved in T2D, and although not currently approved for use in individuals with T1D, there is a growing interest in the safety and efficacy of this medication in conjunction with insulin in individuals with T1D.
In a 3-month observational study, the use of once-weekly GLP-1 RA added on to usual insulin therapy in individuals with T1D was found to have improvements in overall glucose control and body weight [118]. Similarly, the implementation of GLP-1 RA in adolescents with T1D also found that adjunctive GLP-1 RA therapy delayed gastric emptying and reduced postprandial glucose excursions [119]. In addition, a recent meta-analysis on GLP-1 RA in individuals with T1D also revealed significant improvements in glucose control, reduced total daily insulin dose, and weight loss, without increases in severe hypoglycemia [120]. Although not currently approved by the FDA for use in T1D, GLP-1 RA use has elicited promising results; however, future research is needed with a greater emphasis on long-term interventions specifically on overweight adolescents with T1D as a potential avenue for weight management.
Conclusions
Rates of overweight and obesity in youth with T1D, particularly in girls, continue to escalate and are more apparent with increasing age [121]. In order to address this growing concern, it is important to better understand the challenges and burdens associated with weight management in youth with T1D. In addition to the low rates of daily physical activity in this population, challenges with effective glucose control during exercise are the most common barrier to weight management in youth with T1D [39, 42, 43].
For healthcare providers, clinicians, and diabetes care team members caring for youth with T1D, it is important to increase education and provide additional support to address the fear of hypoglycemia, psychological conditions, and family and school dynamics to facilitate the dual management of weight and T1D [43]. By using a structured approach to exercise consultation and increasing education around diabetes management strategies for safe exercise and insulin modification, healthcare practitioners can help both families and youth with T1D achieve their exercise goals [58]. In addition to increased education on exercise, dietary interventions, and increasing physical activity, adding adjunctive therapy in combination with insulin in overweight youth with T1D may improve weight management and glycemic control and merits further investigation. Currently, a challenge in the literature is the lack of specific weight management and evidence-based guidelines for youth with T1D [37]. In summary, the foundation of optimal weight management includes both a healthy diet and active lifestyle. However, general and T1D-specific psychosocial factors must be addressed in addition to providing guidance and advice around safe exercise strategies for youth with T1D in order to reduce the barriers to successful weight management in this population.
Footnotes
Conflict of Interest DPZ has received speaking honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet. AA is supported by the Maternal Child Health Research Institute at Stanford University and is an Ernest and Amelia Gallo Endowed Postdoctoral Fellow. KMS is a consultant for Dexcom and has had research support from NIH, JDRF, and the Helmsley Charitable Trust. DMM has had research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust, and his institution has had research support from Medtronic Diabetes, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. DMM has also consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, and Insulet.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Minges KE, Whittemore R, Grey M. Overweight and obesity in youth with type 1 diabetes. Annu Rev Nurs Res. 2013;31:47–69. 10.1891/0739-6686.31.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: the T1D exchange clinic registry. Diabetes Res Clin Pract. 2017;126:68–78. 10.1016/j.diabres.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315:2292–9. 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the search for diabetes in youth study. Pediatr Diabetes. 2010;11:4–11. [DOI] [PubMed] [Google Scholar]
- 5.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789. 10.1155/2013/653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeley TJH, Fox KR. The impact of physical activity and fitness on academic achievement and cognitive performance in children. Int J Sport Exerc Psychol. 1999;2:198–214. [Google Scholar]
- 7.Kemper HC, Twisk JW, Koppes LL, Wv M, Post GB. A 15-year physical activity pattern is positively related to aerobic fitness in young males and females (13-27 years). Eur J Appl Physiol. 2001;84:395–402. [DOI] [PubMed] [Google Scholar]
- 8.Burrows M. Exercise and bone mineral accrual in children and adolescents. J Sports Sci Med. 2007;6:305–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Baran J, Weres A, Czenczek-Lewandowska E, Wyszynska J, Luszczki E, Deren K, et al. Blood lipid profile and body composition in a pediatric population with different levels of physical activity. Lipids Health Dis. 2018;17:171. 10.1186/s12944-018-0817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quirk H, Blake H, Tennyson R, Randell TL, Glazebrook C. Physical activity interventions in children and young people with type 1 diabetes mellitus: a systematic review with meta-analysis. Diabet Med. 2014;31:1163–73. 10.1111/dme.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMillan F, Kirk A, Mutrie N, Matthews L, Robertson K, Saunders DH. A systematic review of physical activity and sedentary behavior intervention studies in youth with type 1 diabetes: study characteristics, intervention design, and efficacy. Pediatr Diabetes. 2014;15:175–89. 10.1111/pedi.12060. [DOI] [PubMed] [Google Scholar]
- 12.Pivovarov JA, Taplin CE, Riddell MC. Current perspectives on physical activity and exercise for youth with diabetes. Pediatr Diabetes. 2015;16:242–55. 10.1111/pedi.12272. [DOI] [PubMed] [Google Scholar]
- 13.Andersen LB, Riddoch C, Kriemler S, Hills AP. Physical activity and cardiovascular risk factors in children. Br J Sports Med. 2011;45:871–6. 10.1136/bjsports-2011-090333. [DOI] [PubMed] [Google Scholar]
- 14.Tarp J, Child A, White T, Westgate K, Bugge A, Grontved A, et al. Physical activity intensity, bout-duration, and cardiometabolic risk markers in children and adolescents. Int J Obes. 2018;42:1639–50. 10.1038/s41366-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann SD, Angadi SS. Children’s physical activity and sedentary time and cardiometabolic risk factors. Clin J Sport Med. 2013;23:408–9. 10.1097/01.jsm.0000433154.58936.a8. [DOI] [PubMed] [Google Scholar]
- 16.Fritz J, Coster ME, Nilsson JA, Rosengren BE, Dencker M, Karlsson MK. The associations of physical activity with fracture risk: a 7-year prospective controlled intervention study in 3534 children. Osteoporos Int. 2016;27:915–22. 10.1007/s00198-015-3311-y. [DOI] [PubMed] [Google Scholar]
- 17.Detter F, Nilsson JA, Karlsson C, Dencker M, Rosengren BE, Karlsson MK. A 3-year school-based exercise intervention improves muscle strength - a prospective controlled population-based study in 223 children. BMC Musculoskelet Disord. 2014;15:353. 10.1186/1471-2474-15-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritz J, Coster ME, Stenevi-Lundgren S, Nilsson JA, Dencker M, Rosengren BE, et al. A 5-year exercise program in children improves muscle strength without affecting fracture risk. Eur J Appl Physiol. 2016;116:707–15. 10.1007/s00421-015-3310-x. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Bueno C, Pesce C, Cavero-Redondo I, Sanchez-Lopez M, Martinez-Hortelano JA, Martinez-Vizcaino V. The effect of physical activity interventions on children’s cognition and meta-cognition: a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56:729–38. 10.1016/j.jaac.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Eime RM, Young JA, Harvey JT, Charity MJ, Payne WR. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act. 2013;10:98. 10.1186/1479-5868-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagan JF, Shaw JS, PM D. Bright futures: guidelines for health supervision of infants, children, and adolescents. 4th ed: American Academy of Pediatrics; 2017. [Google Scholar]
- 22.Zhang Y, Zhang H, Li P. Cardiovascular risk factors in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2019;32:699–705. 10.1515/jpem-2018-0382. [DOI] [PubMed] [Google Scholar]
- 23.Gunczler P, Lanes R, Paz-Martinez V, Martins R, Esaa S, Colmenares V, et al. Decreased lumbar spine bone mass and low bone turnover in children and adolescents with insulin dependent diabetes mellitus followed longitudinally. J Pediatr Endocrinol Metab. 1998;11:413–9. 10.1515/jpem.1998.11.3.413. [DOI] [PubMed] [Google Scholar]
- 24.Pan H, Wu N, Yang T, He W. Association between bone mineral density and type 1 diabetes mellitus: a meta-analysis of cross-sectional studies. Diabetes Metab Res Rev. 2014;30:531–42. 10.1002/dmrr.2508. [DOI] [PubMed] [Google Scholar]
- 25.Buchberger B, Huppertz H, Krabbe L, Lux B, Mattivi JT, Siafarikas A. Symptoms of depression and anxiety in youth with type 1 diabetes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2016;70:70–84. 10.1016/j.psyneuen.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Caruso NC, Radovanovic B, Kennedy JD, Couper J, Kohler M, Kavanagh PS, et al. Sleep, executive functioning and behaviour in children and adolescents with type 1 diabetes. Sleep Med. 2014;15:1490–9. 10.1016/j.sleep.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Vaisto J, Eloranta AM, Viitasalo A, Tompuri T, Lintu N, Karjalainen P, et al. Physical activity and sedentary behaviour in relation to cardiometabolic risk in children: cross-sectional findings from the physical activity and nutrition in children (panic) study. Int J Behav Nutr Phys Act. 2014;11:55. 10.1186/1479-5868-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Physical Activity Guidelines Advisory Committee. 2018 Physical activity guidelines advisory committee scientific report. Washington, DC: Department of Health and Human Services; 2018. [Google Scholar]
- 29.Juonala M, Viikari JS, Kahonen M, Taittonen L, Laitinen T, Hutri-Kahonen N, et al. Life-time risk factors and progression of carotid atherosclerosis in young adults: the cardiovascular risk in young finns study. Eur Heart J. 2010;31:1745–51. 10.1093/eurheartj/ehq141. [DOI] [PubMed] [Google Scholar]
- 30.Ried-Larsen M, Grontved A, Kristensen PL, Froberg K, Andersen LB. Moderate-and-vigorous physical activity from adolescence to adulthood and subclinical atherosclerosis in adulthood: prospective observations from the European Youth Heart Study. Br J Sports Med. 2015;49:107–12. 10.1136/bjsports-2013-092409. [DOI] [PubMed] [Google Scholar]
- 31.Maggio AB, Rizzoli RR, Marchand LM, Ferrari S, Beghetti M, Farpour-Lambert NJ. Physical activity increases bone mineral density in children with type 1 diabetes. Med Sci Sports Exerc. 2012;44:1206–11. [DOI] [PubMed] [Google Scholar]
- 32.Korczak DJ, Madigan S, Colasanto M. Children’s physical activity and depression: a meta-analysis. Pediatrics. 2017;139. 10.1542/peds.2016-2266. [DOI] [PubMed] [Google Scholar]
- 33.Van Dusen DP, Kelder SH, Kohl HW 3rd, Ranjit N, Perry CL. Associations of physical fitness and academic performance among schoolchildren. J Sch Health. 2011;81:733–40. 10.1111/j.1746-1561.2011.00652.x. [DOI] [PubMed] [Google Scholar]
- 34.Karlsson M, Rosengren B. Physical activity and academic achievements. Acta Paediatr. 2020;109:14–6. 10.1111/apa.15052. [DOI] [PubMed] [Google Scholar]
- 35.Korhonen T, Kujala UM, Rose RJ, Kaprio J. Physical activity in adolescence as a predictor of alcohol and illicit drug use in early adulthood: a longitudinal population-based twin study. Twin Res Hum Genet. 2009;12:261–8. 10.1375/twin.12.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali MM, Amialchuk A, Heller LR. The influence of physical activity on cigarette smoking among adolescents: evidence from add health. Nicotine Tob Res. 2015;17:539–45. 10.1093/ntr/ntu171. [DOI] [PubMed] [Google Scholar]
- 37.Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev. 2018;39:629–63. 10.1210/er.2017-00191. [DOI] [PubMed] [Google Scholar]
- 38.Zaharieva DP, Messer L, Paldus B, O’Neal DN, Maahs D, Riddell MC. Glucose control during physical activity and exercise using closed loop technology in type 1 diabetes. Can J Diabetes. 2020; 10.1016/j.jcjd.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep. 2016;16:77. 10.1007/s11892-016-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adolfsson P, Riddell MC, Taplin CE, Davis EA, Fournier PA, Annan F, et al. Ispad clinical practice consensus guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):205–26. 10.1111/pedi.12755. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S163–82. 10.2337/dc20-S013. [DOI] [PubMed] [Google Scholar]
- 42.Kahkoska AR, Watts ME, Driscoll KA, Bishop FK, Mihas P, Thomas J, et al. Understanding antagonism and synergism: a qualitative assessment of weight management in youth with type 1 diabetes mellitus. Obes Med. 2018;9:21–31. 10.1016/j.obmed.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Addala A, Igudesman D, Kahkoska AR, Muntis FR, Souris KJ, Whitaker KJ, et al. The interplay of type 1 diabetes and weight management: a qualitative study exploring thematic progression from adolescence to young adulthood. Pediatr Diabetes. 2019;20: 974–85. 10.1111/pedi.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92:251–65. 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Rajjo T, Mohammed K, Alsawas M, Ahmed AT, Farah W, Asi N, et al. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab. 2017;102:763–75. 10.1210/jc.2016-2574. [DOI] [PubMed] [Google Scholar]
- 46.Driscoll KA, Corbin KD, Maahs DM, Pratley R, Bishop FK, Kahkoska A, et al. Biopsychosocial aspects of weight management in type 1 diabetes: a review and next steps. Curr Diab Rep. 2017;17:58. 10.1007/s11892-017-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forney KJ, Buchman-Schmitt JM, Keel PK, Frank GK. The medical complications associated with purging. Int J Eat Disord. 2016;49:249–59. 10.1002/eat.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulbul S Exercise in the treatment of childhood obesity. Turk Pediatri Ars. 2020;55:2–10. 10.14744/TurkPediatriArs.2019.60430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu F, Marchand S, Corcoran C, DiBiasio H, Clough R, Dyer CS, et al. A community-based nutrition and physical activity intervention for children who are overweight or obese and their caregivers. J Obes. 2017;2017:2746595. 10.1155/2017/2746595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ho M, Garnett SP, Baur LA, Burrows T, Stewart L, Neve M, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167:759–68. 10.1001/jamapediatrics.2013.1453. [DOI] [PubMed] [Google Scholar]
- 51.Guthold R, Stevens GA, Riley LM, Bull FC. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1.6 million participants. Lancet Child Adolesc Health. 2020;4:23–35. 10.1016/S2352-464219)30323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundberg F, Forsander G, Fasth A, Ekelund U. Children younger than 7 years with type 1 diabetes are less physically active than healthy controls. Acta Paediatr. 2012;101:1164–9. 10.1111/j.1651-2227.2012.02803.x. [DOI] [PubMed] [Google Scholar]
- 53.Czenczek-Lewandowska E, Leszczak J, Weres A, Baran J, Wyszynska J, Grzegorczyk J, et al. Sedentary behaviors in children and adolescents with type 1 diabetes, depending on the insulin therapy used. Medicine (Baltimore). 2019;98:e15625. 10.1097/MD.0000000000015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kummer S, Stahl-Pehe A, Castillo K, Bachle C, Graf C, Strassburger K, et al. Health behaviour in children and adolescents with type 1 diabetes compared to a representative reference population. PLoS One. 2014;9:e112083. 10.1371/journal.pone.0112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fintini D, Di Giacinto B, Brufani C, Cafiero G, Patera PI, Turchetta A, et al. Impaired energy expenditure despite normal cardiovascular capacity in children with type 1 diabetes. Horm Res Paediatr. 2012;78:1–7. 10.1159/000339465. [DOI] [PubMed] [Google Scholar]
- 56.Riddell MC, Perkins BA. Type 1 diabetes and vigorous exercise: applications of exercise physiology to patient management. Can J Diabetes. 2006;30:63–71. 10.1016/S1499-2671(06)01010-0. [DOI] [Google Scholar]
- 57.Yardley JE, Kenny GP, Perkins BA, Riddell MC, Malcolm J, Boulay P, et al. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care. 2012;35:669–75. 10.2337/dc11-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chetty T, Shetty V, Fournier PA, Adolfsson P, Jones TW, Davis EA. Exercise management for young people with type 1 diabetes: a structured approach to the exercise consultation. Front Endocrinol (Lausanne). 2019;10:326. 10.3389/fendo.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill JO, Wyatt HR, Peters JC. The importance of energy balance. Eur Endocrinol. 2013;9:111–5. 10.17925/EE.2013.09.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McAuley SA, Horsburgh JC, Ward GM, La Gerche A, Gooley JL, Jenkins AJ, et al. Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia. 2016;59:1636–44. 10.1007/s00125-016-3981-9. [DOI] [PubMed] [Google Scholar]
- 61.Zaharieva D, McGaugh S, Pooni R, Vienneau T, Ly T, Riddell M. Improved open-loop glucose control with basal insulin reduction 90 minutes before aerobic exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Care. 2019;42:824–31. [DOI] [PubMed] [Google Scholar]
- 62.Neyman A, Woerner S, Russ M, Yarbrough A, DiMeglio LA. Strategies that adolescents with type 1 diabetes use in relation to exercise. Clin Diabetes. 2020; 10.2337/cd19-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moser O, Eckstein ML, West DJ, Goswami N, Sourij H, Hofmann P. Type 1 diabetes and physical exercise: moving (forward) as an adjuvant therapy. Curr Pharm Des. 2020. 10.2174/1381612826666200108113002. [DOI] [PubMed] [Google Scholar]
- 64.Scott SN, Anderson L, Morton JP, Wagenmakers AJM, Riddell MC. Carbohydrate restriction in type 1 diabetes: a realistic therapy for improved glycaemic control and athletic performance? Nutrients. 2019;11. 10.3390/nu11051022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maran A, Pavan P, Bonsembiante B, Brugin E, Ermolao A, Avogaro A, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010;12:763–8. 10.1089/dia.2010.0038. [DOI] [PubMed] [Google Scholar]
- 66.Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5:377–90. 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 67.Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo-Scharer R. Preventing post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr. 2010;157:784–8. 10.1016/j.jpeds.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2008;25:501–4. 10.1111/j.1464-5491.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 69.Farrell CM, McNeilly AD, Fournier P, Jones T, Hapca SM, West D, et al. A randomised controlled study of high intensity exercise as a dishabituating stimulus to improve hypoglycaemia awareness in people with type 1 diabetes: a proof-of-concept study. Diabetologia. 2020;63:853–63. 10.1007/s00125-019-05076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brickwood KJ, Watson G, O’Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019;7:e11819. 10.2196/11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bohm B, Karwiese SD, Bohm H, Oberhoffer R. Effects of mobile health including wearable activity trackers to increase physical activity outcomes among healthy children and adolescents: systematic review. JMIR Mhealth Uhealth. 2019;7:e8298. 10.2196/mhealth.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maahs DM, Horton LA, Chase HP. The use of insulin pumps in youth with type 1 diabetes. Diabetes Technol Ther. 2010;12(Suppl 1):S59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. 2013;56:2392–400. 10.1007/s00125-013-3007-9. [DOI] [PubMed] [Google Scholar]
- 74.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40. 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes-2020. Diabetes Care. 2020;43: S77–88. 10.2337/dc20-S007. [DOI] [PubMed] [Google Scholar]
- 76.Battelino T, Conget I, Olsen B, Schütz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155–62. 10.1007/s00125-012-2708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the juvenile diabetes research foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17–22. 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riveline JP, Schaepelynck P, Chaillous L, Renard E, Sola-Gazagnes A, Penfornis A, et al. Assessment of patient-led or physician-driven continuous glucose monitoring in patients with poorly controlled type 1 diabetes using basal-bolus insulin regimens: a 1-year multicenter study. Diabetes Care. 2012;35:965–71. 10.2337/dc11-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong JC, Foster NC, Maahs DM, Raghinaru D, Bergenstal RM, Ahmann AJ, et al. Real-time continuous glucose monitoring among participants in the T1D exchange clinic registry. Diabetes Care. 2014;37:2702–9. 10.2337/dc14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care. 2020;43:e3–4. 10.2337/dc19-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekhlaspour L, Forlenza GP, Chernavvsky D, Maahs DM, Wadwa RP, Deboer MD, et al. Closed loop control in adolescents and children during winter sports: use of the tandem control-iq ap system. Pediatr Diabetes. 2019;20:759–68. 10.1111/pedi.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dovc K, Macedoni M, Bratina N, Lepej D, Nimri R, Atlas E, et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: a randomised controlled crossover trial. Diabetologia. 2017;60:2157–67. 10.1007/s00125-017-4395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Breton MD, Chernavvsky DR, Forlenza GP, DeBoer MD, Robic J, Wadwa RP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40:1644–50. 10.2337/dc17-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19: 155–63. 10.1089/dia.2016.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 86.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 87.Smart CE, Annan F, Higgins LA, Jelleryd E, Lopez M, Acerini CL. ISPAD clinical practice consensus guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):136–54. 10.1111/pedi.12738. [DOI] [PubMed] [Google Scholar]
- 88.Patton SR, Dolan LM, Powers SW. Dietary adherence and associated glycemic control in families of young children with type 1 diabetes. J Am Diet Assoc. 2007;107:46–52. 10.1016/j.jada.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 89.Patton SR. Adherence to diet in youth with type 1 diabetes. J Am Diet Assoc. 2011;111:550–5. 10.1016/j.jada.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faber EM, van Kampen PM, Clement-de Boers A, Houdijk E, van der Kaay DCM. The influence of food order on postprandial glucose levels in children with type 1 diabetes. Pediatr Diabetes. 2018;19:809–15. 10.1111/pedi.12640. [DOI] [PubMed] [Google Scholar]
- 91.Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients. 2019;11. 10.3390/nu11050962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krebs JD, Parry Strong A, Cresswell P, Reynolds AN, Hanna A, Haeusler S. A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac J Clin Nutr. 2016;25:78–84. 10.6133/apjcn.2016.25.1.11. [DOI] [PubMed] [Google Scholar]
- 93.Eiswirth M, Clark E, Diamond M. Low carbohydrate diet and improved glycaemic control in a patient with type one diabetes. Endocrinol Diabetes Metab Case Rep. 2018;2018:18–0002. 10.1530/EDM-18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leow ZZX, Guelfi KJ, Davis EA, Jones TW, Fournier PA. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet Med. 2018. 10.1111/dme.13663. [DOI] [PubMed] [Google Scholar]
- 95.de Bock M, Lobley K, Anderson D, Davis E, Donaghue K, Pappas M, et al. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: an illustrative case series. Pediatr Diabetes. 2018;19:129–37. 10.1111/pedi.12527. [DOI] [PubMed] [Google Scholar]
- 96.Lennerz BS, Barton A, Bernstein RK, Dikeman RD, Diulus C, Hallberg S, et al. Management of type 1 diabetes with a very low-carbohydrate diet. Pediatrics. 2018;141. 10.1542/peds.2017-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ranjan A, Schmidt S, Damm-Frydenberg C, Steineck I, Clausen TR, Holst JJ, et al. Low-carbohydrate diet impairs the effect of glucagon in the treatment of insulin-induced mild hypoglycemia: a randomized crossover study. Diabetes Care. 2017;40:132–5. 10.2337/dc16-1472. [DOI] [PubMed] [Google Scholar]
- 98.Chen TY, Smith W, Rosenstock JL, Lessnau KD. A life-threatening complication of Atkins diet. Lancet. 2006;367:958. 10.1016/S0140-6736(06)68394-3. [DOI] [PubMed] [Google Scholar]
- 99.Chalasani S, Fischer J. South beach diet associated ketoacidosis: a case report. J Med Case Rep. 2008;2:45. 10.1186/1752-1947-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:709–57. 10.1210/jc.2016-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.American Diabetes Association. Chapter 5: facilitating behavior change and well-being to improve health outcomes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S48–65. 10.2337/dc20-S005. [DOI] [PubMed] [Google Scholar]
- 102.Delamater AM, de Wit M, McDarby V, Malik JA, Hilliard ME, Northam E, et al. Ispad clinical practice consensus guidelines 2018: Psychological care of children and adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19(Suppl 27):237–49. 10.1111/pedi.12736. [DOI] [PubMed] [Google Scholar]
- 103.Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder-Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns. 2007;68:10–5. 10.1016/j.pec.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 104.Muller L, Habif S, Leas S, Aronoff-Spencer E. Reducing hypoglycemia in the real world: a retrospective analysis of predictive low-glucose suspend technology in an ambulatory insulin-dependent cohort. Diabetes Technol Ther. 2019;21:478–84. 10.1089/dia.2019.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sherr JL, Tauschmann M, Battelino T, de Bock M, Forlenza G, Roman R, et al. ISPAD clinical practice consensus guidelines 2018: Diabetes technologies. Pediatr Diabetes. 2018;19(Suppl 27):302–25. 10.1111/pedi.12731. [DOI] [PubMed] [Google Scholar]
- 106.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 107.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care. 2016;39:686–93. 10.2337/dc15-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matteucci E, Giampietro O, Covolan V, Giustarini D, Fanti P, Rossi R. Insulin administration: present strategies and future directions for a noninvasive (possibly more physiological) delivery. Drug Des Devel Ther. 2015;9:3109–18. 10.2147/DDDT.S79322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Edgerton DS, Lautz M, Scott M, Everett CA, Stettler KM, Neal DW, et al. Insulin’s direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116:521–7. 10.1172/JCI27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylor SI, Blau JE, Rother KI, Beitelshees AL. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: balancing benefits and risks. Lancet Diabetes Endocrinol. 2019;7:949–58. 10.1016/S2213-8587(19)30154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cherney DZ, Perkins BA. Sodium-glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes. 2014;38:356–63. 10.1016/j.jcjd.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377:2337–48. 10.1056/NEJMoa1708337. [DOI] [PubMed] [Google Scholar]
- 113.Biester T, Aschemeier B, Fath M, Frey M, Scheerer MF, Kordonouri O, et al. Effects of dapagliflozin on insulin-requirement, glucose excretion and ss-hydroxybutyrate levels are not related to baseline hba1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017;19:1635–9. 10.1111/dom.12975. [DOI] [PubMed] [Google Scholar]
- 114.Biester T, Nieswandt A, Biester S, Remus K, Muller I, Atlas E, et al. Adjunctive therapy with dapagliflozin improves full closed loop post prandial glycaemic control in type 1 diabetic young adults - The DAPADream International Diabetes Federation 2017 Congress. 2017: Abu Dhabi. [Google Scholar]
- 115.Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22:277–82. 10.1097/MED.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 116.Hinnen D Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30:202–10. 10.2337/ds16-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 118.Traina AN, Lull ME, Hui AC, Zahorian TM, Lyons-Patterson J. Once-weekly exenatide as adjunct treatment of type 1 diabetes mellitus in patients receiving continuous subcutaneous insulin infusion therapy. Can J Diabetes. 2014;38:269–72. 10.1016/j.jcjd.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 119.Raman VS, Mason KJ, Rodriguez LM, Hassan K, Yu X, Bomgaars L, et al. The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care. 2010;33:1294–6. 10.2337/dc09-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu L, Shao Z, Xia Y, Qin J, Xiao Y, Zhou Z, et al. Incretin-based therapies for patients with type 1 diabetes: a meta-analysis. Endocr Connect. 2019;8:277–88. 10.1530/EC-18-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marlow AL, Rowe CW, Anderson D, Wynne K, King BR, Howley P, et al. Young children, adolescent girls and women with type 1 diabetes are more overweight and obese than reference populations, and this is associated with increased cardiovascular risk factors. Diabet Med. 2019;36:1487–93. 10.1111/dme.14133. [DOI] [PubMed] [Google Scholar]


