Abstract
Objective:
Childhood epilepsy with centrotemporal spikes (CCECTS, formally benign epilepsy with centrotemporal spikes, BECTS) is a common childhood epilepsy syndrome characterized by psychiatric, behavioral, and cognitive abnormalities and self-limited seizures. Although CECTS is one of the most well-characterized electroclinical epilepsy syndromes, the natural history of neuropsychiatric outcomes is poorly understood. We report the psychiatric, behavioral, and cognitive profiles over the course of disease from a large, prospectively-enrolled, longitudinal cohort of children with CECTS. We further characterize the detailed seizure course and test the relationship between several proposed risk factors and neuropsychiatric and seizure outcomes in these children.
Methods:
Patients diagnosed with CECTS were enrolled as part of a community-based study and followed from diagnosis through disease resolution (16.0 +/− 3.1 yrs, N=60). 20 sibling controls were also recruited. We report the natural history of premorbid neuropsychiatric concerns, postmorbid neuropsychiatric diagnoses, long-term neuropsychological performance, seizure course, antiseizure medication (ASM) treatment response, and the relationship between duration seizure-free and remission. Age of onset and premorbid neuropsychiatric concerns were tested as predictors of seizure count, epilepsy duration, postmorbid neuropsychiatric diagnoses, and long-term neuropsychological performance. ASM treatment duration, seizure count, and epilepsy duration were tested as predictors of postmorbid neuropsychiatric diagnoses and long-term neuropsychological performance.
Results:
Children with CECTS had a high incidence of ADD/ADHD symptoms (18.3%) or learning difficulties (21.7%) before diagnosis. New or persistent ADHD (20%), mood disorders (23.6%), learning difficulties (14.5%), and behavioral disorders (7.3%) were common after CECTS diagnosis. At 9-year follow-up, performance on formal neuropsychological testing was comparable to population statistics and sibling controls. More than two-thirds of treated children experienced at least one seizure during treatment. Most children (61.7%) had entered terminal resolution after 12 months seizure-free. Among all children, for each month seizure-free, there was a 6–7% increase in the probability of achieving terminal remission (p<1e-10). The presence of a premorbid neurodevelopmental concern predicted a longer epilepsy duration (p=0.02), higher seizure count (p=0.02), and a postmorbid psychiatric or neurodevelopmental diagnosis (p=0.002). None of the tested features predicted long-term neuropsychological performance.
Significance:
Children are at high risk of neuropsychiatric symptoms along the course of the disease in CECTS, however, long-term cognitive performance is favorable. The majority of children had a seizure while being treated with ASMs, suggesting that CECTS is not as pharmacoresponsive as assumed or that treatment approaches are not optimized. Among treated and untreated children, future seizure-risk can be estimated from duration seizure-free. The presence of a premorbid neuropsychiatric concern predicted a more severe disease course in CECTS.
Keywords: rolandic, cognitive, mood, behavior, outcome, treatment
1.0. INTRODUCTION
Childhood epilepsy with centrotemporal spikes (CECTS, formally benign epilepsy with centrotemporal spikes, BECTS) is the most common focal childhood epilepsy syndrome, comprising approximately 10% of childhood-onset epilepsy cases [1–5]. CECTS is characterized by cognitive and behavioral abnormalities across multiple domains [6] and self-limited, stereotyped, predominantly nocturnal, hemi-facial motor seizures that can progress to from focal to bilateral tonic-clonic seizures. It is now established that children with CECTS have cognitive and behavioral abnormalities across multiple domains [6], with some suggesting that CECTS is a mild epileptic encephalopathy [7]. Long-term epilepsy outcome is favorable, as almost all children ultimately achieve remission by age 16 years [3, 8]. Long-term neuropsychological outcome remains largely unknown as formal assessments have not been evaluated [9].
Age of onset, seizure course, duration of epilepsy and antiseizure medication (ASM) treatment patterns are highly variable in CECTS. Children typically present during school-age years, though age of onset has been reported from age 3 months to 14 years [3, 8]. Approximately half of children will have five or fewer total seizures, approximately 10% will have greater than twenty seizures, and another 10% of children will have over 50 seizures spanning over the course of a decade [3, 4]. Sixty percent of practitioners favor treating CECTS patients with ASMs [10], though even among treated children, there is wide variability in treatment strategies and evidence is poor to guide duration of treatment [10–13].
Although CECTS is one of the most well-characterized electroclinical epilepsy syndromes, there is much controversy regarding whether specific clinical features predict disease severity. Expert opinion and retrospective studies have proposed that early age of onset [14–17] portends a more aggressive seizure course. However, a prospective study in 29 patients with CECTS did not find a relationship between age of onset and seizure count [3]. The presence of comorbid developmental disorders has been associated with increased epileptiform activity [14, 18], but the relationship with seizure severity is unclear [19, 20]. Additionally, retrospective studies have found that younger age of onset [21, 22], seizure count [23, 24], epilepsy duration [22, 23], and ASM treatment [22, 25], contribute to neuropsychological symptoms, though these findings are inconsistent [26]. Two prospective studies report opposite findings between age of onset and neurodevelopment deficits; one study of 61 children with CECTS reported early age of onset was associated with more language difficulties when tested an average of 2.2 years from onset [27], while another study of 58 children with CECTS found that later age of onset was associated with more symptoms of autism [28].
We characterized the neuropsychiatric profile of children at disease presentation through epilepsy resolution, the natural history of seizures, and treatment response from a large prospective US cohort of children with CECTS followed over the duration of disease. We then tested for a relationship between previously observed prognostic factors on seizure course and neuropsychiatric outcomes.
2.0. MATERIALS AND METHODS
2.1. Subject recruitment
All patients with a diagnosis of CECTS who were recruited into the longitudinal Connecticut Study of Epilepsy, and followed through the entire disease course, were selected for analysis. The methodology of data collection has been previously described [5, 29]. Briefly, children were recruited by 16 of 17 practicing child neurologists in the state of Connecticut from 1993–1997. Syndromes were characterized based on the 1989 ILAE Commission report supplemented by the common references at the time [30, 31]. Following epilepsy diagnostic criteria at the time, children were required to have had at least two unprovoked seizures on separate days. From this cohort, 65 children diagnosed with typical CECTS were identified. Of these, children who were followed for at least 10 years (n=2), were seizure-free for at least 5 years at study close (n=2), or both (n=56) were included. The mean duration of follow-up among the final 60 subjects included was 16.0 +/− 3.1 years.
Parent’s written informed consent and child’s assent were obtained at entry to the study, and when children reached age, the majority of young adult consents were also obtained. This study was approved by Institutional Review Boards at Massachusetts General Hospital and Lurie Children’s Hospital of Chicago. The original study was conducted under the auspices of the Yale Human Investigations Committee.
2.2. Data Collection
Early seizure course and the presence of neuropsychiatric concerns before epilepsy diagnosis were documented at enrollment. Parents were asked about school performance including learning problems and diagnosis of attention deficit disorder (ADD) or attention deficit hyperactivity disorder (ADHD) or suggestive symptoms. Specifically, parents were asked whether the child has been described using any of the following terms: 1) held back in school; 2) immature, developmentally delayed, maturation lag, or slow to develop; 3) slow learner; 4) autistic; 5) aphasic, or language difficulty; 6) learning disabled; 7) minimal brain dysfunction; 8) brain damage; 9) having ADD; 10) hyperkinetic or hyperactive. Subjects who answered yes or maybe to at any of questions 1–8 were grouped as having concerns for learning difficulties. Subjects who answered yes or maybe to questions 9–10 were grouped as having concerns for ADD/ADHD. Information about ongoing seizure course, treatment, and new neuropsychiatric diagnoses were collected using structured parent interviews with parents or young adult participants every 3–4 months and periodic medical record review over the duration of follow-up. Specifically, parents were asked if their child had received any of the following diagnoses: 1) developmental delay; 2) language problem; 3) learning disability; 4) ADHD; 5) depression; 6) anxiety; 7) obsessive compulsive disorder; 8) schizophrenia; 9) conduct disorder; 10) oppositional disorder. Subjects who answered yes to questions 1–3 were grouped as having a learning difficulty. Subjects who answered yes to questions 5–8 were group as having a mood disorder. Subjects who answered yes to questions 9–10 were grouped as having a behavioral disorder. Original phone notes were reviewed to verify diagnoses, seizure count, seizure dates, ASM treatments and duration. The reports from all clinical EEGs obtained over the course of follow up were reviewed for the presence and location of epileptiform activity, slowing, or other background abnormalities. The reports from all clinical MRIs obtained over the course of follow up were reviewed for the presence of abnormalities. In addition, 39 subjects and 20 sibling controls underwent formal neuropsychological testing at 9 years of follow up including the WISC III/WAIS III, Test of Nonverbal Intelligence, Connors Continuous Performance Test, and the Wide Range Achievement Tests (WRAT) in reading, spelling, and arithmetic.
2.3. Statistical Analysis
2.3.1. Cohort characteristics
Cohort clinical, EEG, imaging characteristics, and pre- and post-morbid neuropsychiatric symptoms were reported using descriptive statistics.
2.3.2. Long term neuropsychological outcome
For subjects who underwent formal neuropsychological testing at 8–9 years after initial enrollment, performance on each subtest was compared to the population mean and standard score using a one sample t-test. We further tested these subtest results in 20 case-control pairs with participants’ siblings.
2.3.3. Clinical predictors of postmorbid neuropsychiatric diagnoses
Age at first seizure, epilepsy duration (here defined as years from first to last seizure), total seizure count, duration of ASM treatment, and the presence of any premorbid comorbidities were examined as predictors in simple logistic regression models with the presence of any neuropsychiatric diagnoses as well as at least one new neuropsychiatric diagnosis since epilepsy diagnosis as the dependent variables.
2.3.4. Clinical predictors of long-term neuropsychological outcome
Age at first seizure, the presence of a pre-morbid neuropsychiatric diagnosis, epilepsy duration, total seizure count, and duration of ASM treatment were examined in simple linear regression models with the performance on WISC III/WAIS III, Test of Nonverbal Intelligence, Connors Continuous Performance Test, and the Wide Range Achievement Tests (WRAT) in reading, spelling, and arithmetic as the dependent variable.
2.3.5. Seizure course
To model the distribution of ages at first and last seizure, we fit a kernel density plot to the histograms for all subjects, and for subjects separated by whether or not they received ASM treatment. Here, we included children in the treated group if they were ever treated with an ASM for a total duration of greater than one month. Observed duration of epilepsy was the time from first to last observed seizure.
2.3.6. Clinical predictors of seizure course
Age at first seizure and the presence of any premorbid concern were examined as predictors in simple linear regression models with (i) duration of epilepsy and (ii) seizure count as the dependent variables. To evaluate the impact of specific premorbid concerns, we also examined the concern for learning difficulties or concern for ADHD separately as predictors.
2.3.7. Duration seizure free and seizure risk
To model the relationship between duration seizure-free and probability of achieving terminal remission, we first computed the inter-seizure interval for each subject. To determine the minimum duration seizure-free required before no further seizures occurred (e.g. terminal remission), we identified the longest inter-seizure interval for each subject and added one month. We then determined the proportion of subjects that ever had a subsequent seizure after an elapsed duration seizure-free, measured in one month increments, beginning at 0 months and ending at 127 months. By contrast, those that never had a subsequent seizure had entered terminal remission. To characterize the shape of this relationship, we fit a generalized linear model (log link, Poisson distribution transformed for proportion data).
2.3.8. Correction for multiple comparisons
To address concerns that may arise from multiple comparisons, for each analysis above we used the Benjamini-Hochberg procedure to control the false discovery rate (FDR), with q=0.05 [32]
3.0. RESULTS
3.1. Clinical characteristics
Children with CECTS had a slight male predominance (35/60, 58.3%). Half (30/60) of children had a family history of epilepsy, though only 4/60 (6.7%) patients had a first degree relative with epilepsy. No child had a history of neonatal seizures, and 7/60 (11.7%) had at least 1 febrile seizure prior to diagnosis of CECTS. Clinical characteristics of the cohort are included in Table 1.
Table 1.
Clinical characteristics of CECTS cohort.
| Cohort Feature | n (%) |
|---|---|
| Male | 35 (58.3) |
| Abnormal Imaging | 2* (3.3) |
| EEG Slowing | 6 (10) |
| Extrarolandic Spike-Wave | 4 (6.7) |
| Generalized Spike-Wave | 2 (3.4) |
| 1st Degree Family History | 4 (6.7) |
| ≥ 2nd Degree Family History | 30 (50.0) |
| Febrile Seizures | 7 (12.0) |
| Treated with anticonvulsant (ever; first) | 42 (70.0) |
| carbamazepine | 39 (92.9); 34 (80.9) |
| phenytoin | 10 (23.8); 6 (14.3) |
| valproic acid | 4 (9.5); 2 (4.8) |
| gabapentin | 2 (4.8) |
| oxcarbazepine | 1 (2.4) |
| carbamazepine | 1 (2.4) |
| topiramate | 1 (2.4) |
| felbamate | 1 (2.4) |
| phenobarbital | 1 (2.4) |
| lamotrigine | 1 (2.4) |
n=1 hippocampal asymmetry, n=1 benign cyst
3.1.1. EEG and imaging characteristics
Subjects had on average 2.55 EEGs (median 2, range 1–9). All subjects had centrotemporal spikes observed on EEG. 4/60 (6.7%) subjects had spikes reported in other lobes (2 frontotemporal, 1 occipital, 1 parietal). 2/60 (3.3%) subjects were noted to have generalized spike and wave activity. 6/60 (10%) participants were reported as having slowing on EEG, 3 of which were recorded within 1 week (range 0–5 days) of the last clinical seizure (1 focal slowing, 1 generalized slowing, 1 not specified). 3 subjects were reported to have slowing on EEG collected greater than 1 week (range 9 days to 2.5 months) after the last clinical seizure (1 focal slowing, 2 generalized slowing). Of the children who underwent neuroimaging, including research imaging offered 9 years after initial epilepsy diagnosis (n=55), one child had an incidental finding of a benign cyst, and one child was noted to have hippocampal asymmetry.
3.2. Neuropsychiatric profile and outcomes
3.2.1. Premorbid neuropsychiatric symptoms
At initial presentation, 17/60 children (28.3%) had a neuropsychiatric concern (Figure 1A). 11/60 children (18.3%) had concerns for ADD/ADHD. 13/60 children (21.7%) had a learning difficulty. No child was diagnosed with autism or brain damage prior to CECTS diagnosis.
Figure 1.
There is a high burden of neuropsychiatric and behavioral diagnoses in CECTS. (A) Premorbid symptoms of learning disorders and ADHD. (B) Postmorbid diagnoses of learning disorders, ADHD, psychiatric disorders, and behavioral disorders. (C) Scores on formal neuropsychological testing after 9 years. The blue dashed line represents population mean scores on each test. The black bar and diamond in the middle of the box and whisker plot represent the median and mean respectively. The first 10 scores are standardized to a mean of 100 and standard deviation of 15. The last 4 scores are standardized to a mean of 50 and standard deviation of 10. FSIQ=Full Scale IQ; VIQ=Verbal IQ; PIQ=Performance IQ; Verbal comp=Verbal comprehension subtest; Organize=Perceptual Organization subtest; Distract=Freedom from Distractibility subtest; Process Speed=Processing Speed; WRAT-R=Wide Range Achievement Test (WRAT)-Reading; WRAT-S=WRAT-Spelling; WRAT-A=WRAT-Arithmetic; TONI=Test of Nonverbal Intelligence; CPT-Om=Continuous Performance Test (CPT)-Omissions; CPT-Com=CPT Commissions; CPT-RT=CPT Reaction Time
3.2.2. Postmorbid neuropsychiatric diagnoses
Longitudinal neuropsychiatric follow up was available in 55 children. 19/55 children (34.5%) reported a neuropsychiatric diagnosis after CECTS diagnosis, of whom 17/55 (30.9%) had at least one new diagnosis without a related premorbid concern (Figure 1B). Mood disorders were the most common postmorbid neuropsychiatric diagnosis, present in 13/55 (23.6%) children, including depression in 12/55 (21.8%) and anxiety in 3/55 (5.4%). 11/55 children (20%) had a diagnosis of ADHD after diagnosis with CECTS, 4 of whom had no premorbid concerns. 8/55 children (14.5%) were diagnosed with a learning difficulty after CECTS diagnosis, half of whom (4/8) had no premorbid learning concerns.
3.2.3. Long term neuropsychological outcome
Among the 39 subjects who underwent formal neuropsychological testing 9 years after study enrollment, subjects performed above population average across several domains (Figure 1C). The mean Full Scale Intelligence Quotient (IQ) was 106.1 (range 62–144, p=0.03), mean verbal IQ was 106.9 (range 71–151, p=0.015), and mean performance IQ was 105.5 (68–155, p=0.065). Verbal comprehension (109.1, p=0.002), perceptual organization scores (107.2, p=0.02) and freedom from distractibility scores (104.1, p=0.12) were above average. Subjects performed higher than the population mean in reading (104.5, p=0.02) and spelling (104.0, p=0.05) and average on arithmetic (100.7, p=0.8). Subjects performed average on the Test of Nonverbal Intelligence, CPT omissions and reactions (50.0, p=0.93; 52.6, p=0.5, 49.7, p=0.82) and below average on CPT commissions (45.2, p=0.009); we note that for each of these tests the population mean is 50. After correction for multiple comparisons (see Methods, Statistical Analysis), only verbal comprehension remained significantly different than the population mean.
In 20 case and sibling pairs, there were no significant differences in performance on any subtest (p >0.08 for all tests).
3.2.4. Predictors of postmorbid neuropsychiatric diagnosis
There was no relationship between age of onset (p=0.91; p=0.85), duration of ASM exposure (p=0.28; p=0.39), epilepsy duration (p=0.08; p=0.63), or seizure count (p=0.09; p=0.11) and the presence of any postmorbid neuropsychiatric diagnosis or a new neuropsychiatric diagnosis respectively. The presence of a premorbid neuropsychiatric concern predicted the presence of a postmorbid neuropsychiatric diagnosis (p=0.002) as well as the development of a new neuropsychiatric diagnosis (p=0.02).
3.2.5. Predictors of long-term neuropsychological performance
There was no relationship between age of onset, epilepsy duration, seizure count, nor ASM treatment duration and performance on any of the neuropsychological subtests measured at 9 years after diagnosis (p>0.06 for all tests, 56 tests measured, see Supplementary Table 1).
3.3. Seizure course, impact of treatment, and resolution
3.3.1. Overall seizure course
There was a bimodal trend in the distribution of age at first and last seizure, with peaks in the age at first seizure near 5 and 8 years, and peaks in the age of last seizure near 5 and 11 years (Figure 2). The average ages of first and last seizure were 7.2 yr (range 3.1–10.6 yr) and 10.0 yr (range 4.3–22.3 yr), respectively. The median duration of epilepsy among all participants was 2.0 yr (range 0.003–16.9 yr), with a median of eight seizures (mode 2, range 2–264) per child. A quarter of participants (15/60) experienced no further seizures after the first two unprovoked seizures required for enrollment in the study. Twelve of 60 children (20%) had 3–5 seizures, 23/60 (38.3%) had 6–20 seizures, and 10/60 (16.7%) children had greater than 20 seizures. One child had a single unprovoked seizure during young adulthood, after 8 years of seizure-freedom.
Figure 2.
Age of onset and termination of disease in CECTS. (A) Kernel density estimates of age of first and last seizure in all subjects. (B) Empirical histograms of age of first and last seizure in all subjects. (C) Kernel density estimates of age of first and last seizure in untreated children, and (D) treated children. Age at medication end is also shown.
3.3.2. Antiseizure medication treatment
More than two-thirds of children (42/60, 70%) were treated for at least one month with an antiseizure medication, the majority of whom were treated with carbamazepine (n=39). Children were treated for a median of 2.13 years (IQR 1.41–2.71 yrs) after their last seizure before ASM were stopped. Of the 42 children who received treatment, 29 (69%) continued to have seizures while on ASM, all of whom had multiple seizures during treatment (median 9 seizures, IQR 3–13). 16/29 children (55.2%) were trialed on more than one ASM. Two children failed treatment with two ASMs, meeting criteria for refractory epilepsy [33, 34]; one of whom received concurrent polytherapy with two ASMs.
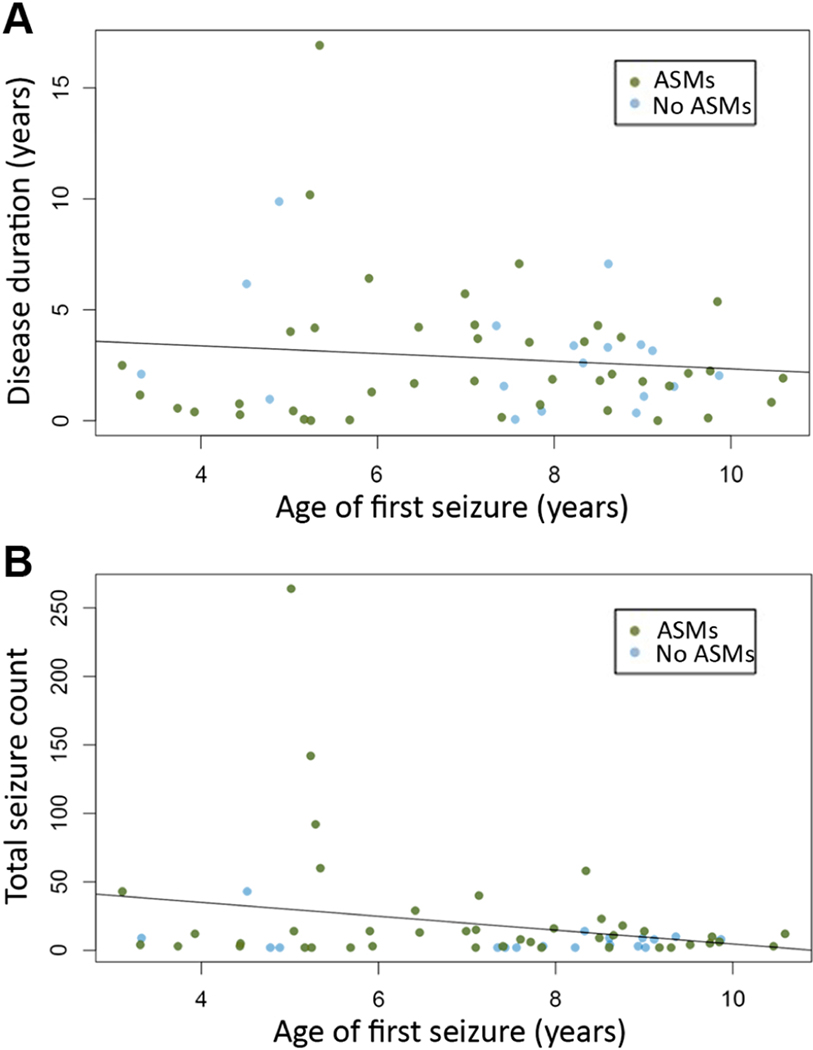
3.3.3. Clinical predictors of seizure course
Neither age at first seizure (p=0.37, Figure 3A), nor treatment duration (p=0.16) predicted epilepsy duration. There was a trend toward younger age of onset correlating with increased seizure count, but the strength of this relationship was weak (p=0.05, r2=0.05 Figure 3B). Total seizure count tended to be moderate in children with age of onset before 4 years (median 9, range 3 – 43), and visual review suggests that the trend observed was driven by a single outlier with age of onset at 5 years.
Figure 3.
Age of onset does not predict disease course. (A) Scatter plot of age of onset versus duration of disease. (B) Scatter plot of age of onset versus total number of seizures.
Having an existing neuropsychiatric concern prior to CECTS diagnosis was associated with a longer disease duration (p=0.02) and greater total number of seizures (p=0.02). Specific concerns for ADHD predicted a greater total number of seizures (p=0.01). Specific concerns for learning difficulties predicted a longer disease duration (p=0.01) and a greater total number of seizures (p=0.03).
3.3.4. Duration seizure free and seizure risk
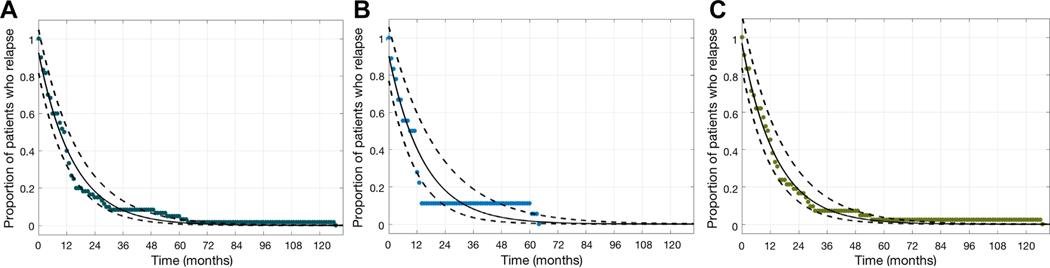
Duration seizure free was a reliable predictor of terminal remission. Most children (37/60, 61.7%) had entered terminal resolution after 12 months seizure-free. After 24 months seizure-free, 9/60 (15.0%) children had entered terminal resolution. Using a generalized linear model to describe this data, among all patients, for each one-month increase in duration of time seizure-free, there was a 6.5% (95% confidence interval [6.1%, 7.0%], p<1e-10, Figure 4A) reduction in the risk of ever having a subsequent seizure. Analyzing only patients that never received treatment with ASMs did not qualitatively change the results of the model; there was a 6.5% reduction in the risk of ever having a subsequent seizure (95% confidence interval [5.6%, 7.4%], p<1e-10, Figure 4B) for each month seizure-free. A similar relationship was observed among patients that were treated with ASMs (6.7%, 95% confidence interval [6.1%, 7.2%], p<1e-10, Figure 4C).
Figure 4:
The number of patients with a seizure decreases with time. The number of patients at each month (asterisks) and model fit (solid curve is mean estimate, and dashed curve is 95% confidence interval) decreases similarly for (A) all patients, (B) patients not treated with ASMs and (C) patients treated with ASMs. Among all children, after 9.1 months seizure-free, there is a 50% chance of having a subsequent seizure. After 15.1 months seizure-free, there is a 33% chance of having a subsequent seizure. After 2 years seizure-free, there is an 18.3% chance of having a subsequent seizure. After 3 years seizure-free, there is an 8.1% chance of having a subsequent seizure.
4.0. DISCUSSION
Utilizing data from a large, prospectively-enrolled, longitudinal cohort of children with CECTS, we characterize the natural history of cognitive, behavioral, and psychiatric symptoms over the course of disease. We further characterize the detailed seizure course and test the relationship between several proposed risk factors and neuropsychiatric and seizure outcomes in CECTS. Our findings support the growing recognition that children with CECTS are at high risk of neuropsychiatric comorbidities. These data also provide strong evidence that despite these significant comorbidities, long-term cognitive performance is not affected.
The high prevalence of cognitive, behavioral, and psychiatric comorbidities reported in this cohort is in line with recent recognition that neuropsychiatric symptoms are common in CECTS [6, 24, 35]. Here, over a quarter of children (28.3%) had neuropsychiatric concerns at diagnosis and over a third of children (34.5%) had concerns over the course of follow up. Given the prevalence of neuropsychiatric symptoms coincident with the presentation of epilepsy and abundant spikes during NREM sleep, CECTS is considered a mild epileptic encephalopathy [36] with genetic and clinical overlap with both Landau Kleffner syndrome (LKS) and Encephalopathy with Continuous Spike and Wave activity (CSWS) [37, 38]. The severity of symptoms can vary in CECTS, where 6.6% of children meet criteria for a more severe epileptic encephalopathy, either LKS or CSWS [36]. Although broadly reduced scores across all domains on neuropsychological testing have been reported in children with CECTS when tested early in the disease [6, 9, 22, 23, 26, 27, 35, 39–41] the duration of these cognitive symptoms is poorly characterized. Here, we found that when tested 9 years after enrollment, children with CECTS performed average to above average in all domains tested. Although our sample was incomplete (20 out of a possible 60 case-control pairs received neuropsychological testing), we were able to control for socioeconomic status and parental education using sibling controls, and our findings are consistent with prior, smaller, observational cohorts [42, 43]. These results suggest that the cognitive symptoms in this epileptic encephalopathy are mild enough to enable complete recovery after disease resolution. Future studies are required to help clarify the time course of the cognitive comorbidities observed in this disease and whether they are related to the epilepsy or a shared pathophysiological process underlies both.
Mood and behavioral disorders were present in nearly a third of children (30.9%) with CECTS. Of note, data on mood and behavioral disorders in this cohort were collected via parent report and children were not formally screened for these disorders. Therefore, the rate of disorders such as depression may actually be higher than reported here. Although remission from these diagnoses was not evaluated here, we note that in a 30-year longitudinal follow up study of 32 patients with CECTS, 34% reported at least one visit with a mental health professional during the follow-up period, 10/32 (31.3%) had a behavioral disorder and 3/32 (9.4%) had a psychiatric diagnosis [4], suggesting that these comorbidities can be long lasting. Due to the high frequency of mood and behavior disorders in children with CECTS, clinicians should screen for these comorbidities when evaluating children with CECTS so that appropriate supports and treatments may be offered.
Early age of onset [14–17, 21, 22, 27], ASM side effects [22, 25], uncontrolled seizures [23, 24], and duration of disease [22, 23] have been implicated as potential contributors to increased epilepsy duration, total seizure count and neuropsychiatric outcomes, though these relationships have not been consistently observed [3, 26, 28]. We did not find a relationship between any of these variables and epilepsy or neuropsychiatric disease severity. We note that we included children with CECTS diagnosed according to the 1989 ILAE criteria [30] and thus children with initial seizure onset under 3 years of age were not identified. As very early onset epilepsy has separately been implicated as a risk factor for developmental outcome [44, 45], prognostic information regarding very young children with clinical features otherwise consistent with CECTS remains uncertain. We did find a relationship between a premorbid neuropsychiatric concern and epilepsy severity and risk of a post morbid neuropsychiatric diagnosis, suggesting that preexisting symptoms may indicate a more severe disease. This finding provides support for a common mechanism underlying both the neuropsychiatric deficits and epilepsy in this disorder.
Similar to a prior prospective study [3], we found a much higher seizure burden than reported in prior retrospective studies [8]; here, more than half of children experience greater than five seizures, and nearly 10% of children have more than 50 seizures. Although CECTS is characterized as a pharmacoresponsive disease [5, 36], seizures were not typically responsive to treatment in this cohort. Currently, no class I or class II evidence for ASM treatment currently exists to support medication efficacy in CECTS [10, 46, 47] and clinical observations are confounded by spontaneous remission [48]. These findings suggest that either CECTS is not as responsive to medication as assumed, or that current medication approaches are not optimal, either due to drug side effects, drug choices or doses selected. We note that only two children met criteria for drug-resistant epilepsy [5], suggesting that ASM drug levels were not optimized or that patients were not compliant. Future clinical trials are needed to better understand and optimize ASM treatment in this disease.
The prevalence of ASM treatment in CECTS is highly variable, ranging from 29–86%; our cohort (70% treated with ASM) represents treatment strategies from the turn of the century, and recent studies suggest a trend favoring increased treatment [1, 3, 49, 50, 51]. With recently reported cases of SUDEP in CECTS [52], it is probable that the percent of families and practitioners who elect treatment will continue to increase. To avoid unnecessary exposure to the side effects of ASMs, guidelines on when to stop treatment are needed. Current practice typically relies on waiting for a variable duration seizure-free to occur before initiating ASM taper [53, 54], although prior work supporting a relationship between seizure risk and duration seizure-free has focused on mixed epilepsy populations with low temporal resolution [5, 55–57]. Historically, most practitioners have counseled to treat CECTS for 1–2 years seizure-free [53, 54], though cases of ASM treatment for over 25 years have been reported [4]. In our cohort, the median duration of treatment was 25.5 months after the last seizure; in the prospective Dutch Study of Childhood Epilepsy, the mean duration of ASM treatment in CECTS was over 32 months [3]. Based on our model, after 25 months seizure free, ~82% of children will have entered remission. The ongoing seizure risk may be too low to justify continuing ASM treatment this long for some families, while the risk of seizure may still be too high at 25 months for others. The detailed data on the relationship between duration seizure-free and seizure risk provided here will enable parents and providers to select a time point for ASM taper based on risk-benefits and personal thresholds for medication exposure and seizure risk. This model can also inform future clinical trials of ASM efficacy based on the natural history of remission.
We provide results from a large, prospective cohort of children with CECTS, though some limitations exist. This cohort enrolled children only after a second unprovoked seizure, consistent with criteria during the 1990s; recent guidelines for diagnosis of CECTS are not as stringent [58]. When using more liberal diagnostic criteria, as many as 15% of children with CECTS do not report a second seizure [8]. Thus, our cohort excluded children with the mildest seizure course. Furthermore, our results rely largely on parent report and not every child underwent formal psychiatric and neuropsychological evaluation. Thus, while subtle neuropsychiatric symptoms may have been missed, our results likely reflect the most clinically prominent concerns.
5.0. CONCLUSION
Here we report clinical characteristics and risk factors for seizure and neuropsychological outcomes in a large prospective cohort of children with CECTS. We find that neuropsychiatric comorbidities are common at diagnosis and across the course of disease. However, cognitive symptoms do not persist after disease resolution. Premorbid neuropsychiatric concerns are a predictor of epilepsy severity and a postmorbid neuropsychiatric diagnosis. Seizure burden and poor response to treatment are higher than previously assumed. We confirm that duration seizure-free is a reliable predictor of the likelihood of terminal remission. This work helps to further characterize the features of this common childhood disease, clarify clinical predictors of risk, and identify the need for clinical trials to determine best treatment practices to optimize seizure control in children who are treated with ASMs.
Supplementary Material
HIGHLIGHTS.
Children with CECTS are at high risk of neuropsychiatric symptoms
Premorbid neuropsychiatric concern predicts comorbidities and worse seizure course
Long term neuropsychological outcome is favorable in CECTS
Most children continue to have seizures during antiseizure medication treatment
Duration seizure-free reliably predicts likelihood of terminal remission in CECTS
Acknowledgments
Funding: This work was supported by NINDS R37-NS31146 (ATB) and NINDS K23-NS092923 (CJC).
Dr. Berg reports the following disclosures: Research Funding from the Pediatric Epilepsy Research Foundation, NIH, and the Dravet Syndrome Foundation. Consultant for West Pharmaceuticals. Speaking Honoraria from Sun Pharmaceuticals and Biomarin.
Dr. Chu reports the following disclosures: Consultancies with Biogen, SleepMed, and Alliance Family of Companies. She also receives research support from an NIH-funded grant, NIH K23 NS092923
Ms. Ross, Ms. Stoyell, and Dr. Kramer report nothing to disclose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Astradsson A, Olafsson E, Ludvigsson P, Bjorgvinsson H, Hauser WA. Rolandic Epilepsy: An Incidence Study in Iceland. Epilepsia. 1998;39(8):884–6. doi: 10.1111/j.1528-1157.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- [2].Larsson K, Eeg-Olofsson O. A population based study of epilepsy in children from a Swedish county. Eur J Paediatr Neuro. 2006;10(3):107–13. doi: 10.1016/j.ejpn.2006.02.005. [DOI] [PubMed] [Google Scholar]
- [3].Callenbach PMC, Bouma PAD, Geerts AT, Arts WFM, Stroink H, Peeters EAJ, et al. Long term outcome of benign childhood epilepsy with centrotemporal spikes: Dutch Study of Epilepsy in Childhood. Seizure. 2010;19(8):501–6. doi: 10.1016/j.seizure.2010.07.007. [DOI] [PubMed] [Google Scholar]
- [4].Camfield CS, Camfield PR. Rolandic epilepsy has little effect on adult life 30 years later: A population-based study. Neurology. 2014;82(13):1162–6. doi: 10.1212/wnl.0000000000000267. [DOI] [PubMed] [Google Scholar]
- [5].Berg AT, Rychlik K. The course of childhood-onset epilepsy over the first two decades: a prospective, longitudinal study. Epilepsia. 2015;56(1):40–8. Epub 2014/11/28. doi: 10.1111/epi.12862. [DOI] [PubMed] [Google Scholar]
- [6].Wickens S, Bowden SC, D’Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia. 2017;58(10):1673–85. doi: 10.1111/epi.13865. [DOI] [PubMed] [Google Scholar]
- [7].Carvill GL, Regan BM, Yendle SC, O’Roak BJ, Lozovaya N, Bruneau N, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013;45(9):1073–6. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bouma PAD, Bovenkerk AC, Westendorp RGJ, Brouwer OF. The course of benign partial epilepsy of childhood with centrotemporal spikes: A meta-analysis. Neurology. 1997;48(2):430–7. doi: 10.1212/wnl.48.2.430. [DOI] [PubMed] [Google Scholar]
- [9].Vannest J, Tenney JR, Gelineau-Morel R, Maloney T, Glauser TA. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2015;45:85–91. doi: 10.1016/j.yebeh.2015.01.041. [DOI] [PubMed] [Google Scholar]
- [10].Mellish LC, Dunkley C, Ferrie CD, Pal DK. Antiepileptic drug treatment of rolandic epilepsy and Panayiotopoulos syndrome: clinical practice survey and clinical trial feasibility. Arch Dis Child. 2015;100(1):62–7. Epub 2014/09/08. doi: 10.1136/archdischild-2013-304211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tan HJ, Singh J, Gupta R, de Goede C. Comparison of antiepileptic drugs, no treatment, or placebo for children with benign epilepsy with centro temporal spikes. Cochrane Db Syst Rev. 2014. doi: 10.1002/14651858.cd006779.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oguni H. Treatment of benign focal epilepsies in children: When and how should be treated? Brain Dev. 2011;33(3):207–12. doi: 10.1016/j.braindev.2010.10.024. [DOI] [PubMed] [Google Scholar]
- [13].Shields W, Snead O 3rd. Benign epilepsy with centrotemporal spikes. Epilepsia. 2009;50:10–5. doi: 10.1111/j.1528-1167.2009.02229.x. [DOI] [PubMed] [Google Scholar]
- [14].Hughes JR. Benign epilepsy of childhood with centrotemporal spikes (CECTS): To treat or not to treat, that is the question. Epilepsy Behav. 2010;19(3):197–203. doi: 10.1016/j.yebeh.2010.07.018. [DOI] [PubMed] [Google Scholar]
- [15].Kramer U, Zelnik N, Lerman-Sagie T, Shahar E. Benign Childhood Epilepsy With Centrotemporal Spikes: Clinical Characteristics and Identification of Patients at Risk for Multiple Seizures. J Child Neurol. 2002;17(1):17–9. doi: 10.1177/088307380201700104. [DOI] [PubMed] [Google Scholar]
- [16].You SJ, Kim DS, Ko TS. Benign childhood epilepsy with centro-temporal spikes (BCECTS): early onset of seizures is associated with poorer response to initial treatment. Epileptic Disord. 2006;8(4):285–8. doi: 10.1684/epd.2006.0049 [DOI] [PubMed] [Google Scholar]
- [17].Loiseau P, Duché B, Cordova S, Dartigues JF, Cohadon S. Prognosis of Benign Childhood Epilepsy with Centrotemporal Spikes: A Follow-Up Study of 168 Patients. Epilepsia. 1988;29(3):229–35. doi: 10.1111/j.1528-1157.1988.tb03711.x. [DOI] [PubMed] [Google Scholar]
- [18].Besag FMC. Cognitive and Behavioral Outcomes of Epileptic Syndromes: Implications for Education and Clinical Practice. Epilepsia. 2006;47(s2):119–25. doi: 10.1111/j.1528-1167.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- [19].Yung AW, Park YD, Cohen MJ, Garrison TN. Cognitive and behavioral problems in children with centrotemporal spikes. Pediatr Neurol. 2000;23(5):391–5. doi: 10.1016/S0887-8994(00)00220-4 [DOI] [PubMed] [Google Scholar]
- [20].Weglage J, Demsky A, Pietsch M, Kurlemann G. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev Med Child Neurol. 1997;39(10):646–51. doi: 10.1111/j.1469-8749.1997.tb07357.x [DOI] [PubMed] [Google Scholar]
- [21].Piccinelli P, Borgatti R, Aldini A, Bindelli D, Ferri M, Perna S, et al. Academic performance in children with rolandic epilepsy. Dev Med Child Neurol. 2008;50(5):353–6. doi: 10.1111/j.1469-8749.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- [22].Samaitienė R, Norkūnienė J, Jurkevičienė G, Grikinienė J. Behavioral Problems in Children with Benign Childhood Epilepsy With Centrotemporal Spikes Treated and Untreated with Antiepileptic Drugs. Medicina. 2012;48(7):50. doi: 10.3390/medicina48070050. [DOI] [PubMed] [Google Scholar]
- [23].Papavasiliou A, Mattheou D, Bazigou H, Kotsalis C, Paraskevoulakos E. Written language skills in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2005;6(1):50–8. doi: 10.1016/j.yebeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- [24].Liu X, Han Q. Depression and anxiety in children with benign childhood epilepsy with centrotemporal spikes (BCECTS). BMC Pediatrics. 2016;16(1). doi: 10.1186/s12887-016-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Filippini M, Boni A, Giannotta M, Gobbi G. Neuropsychological development in children belonging to CECTS spectrum: Long-term effect of epileptiform activity. Epilepsy Behav. 2013;28(3):504–11. doi: 10.1016/j.yebeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- [26].Goldberg-Stern H, Gonen OM, Sadeh M, Kivity S, Shuper A, Inbar D. Neuropsychological aspects of benign childhood epilepsy with centrotemporal spikes. Seizure. 2010;19(1):12–6. doi: 10.1016/j.seizure.2009.10.004. [DOI] [PubMed] [Google Scholar]
- [27].Jurkevičienė G, Endzinienė M, Laukienė I, Šaferis V, Rastenytė D, Plioplys S, et al. Association of language dysfunction and age of onset of benign epilepsy with centrotemporal spikes in children. Eur J Paediatr Neuro. 2012;16(6):653–61. doi: 10.1016/j.ejpn.2012.03.011. [DOI] [PubMed] [Google Scholar]
- [28].Bektaş G, Tekin U, Yıldız EP, Aydınlı N, Çalışkan M, Özmen M. Autism spectrum disorder and attention-deficit/hyperactivity disorder-related symptoms in benign childhood epilepsy with centrotemporal spikes: A prospective case-control study. Epilepsy Behav. 2019;95:61–4. doi: 10.1016/j.yebeh.2019.03.044. [DOI] [PubMed] [Google Scholar]
- [29].Berg AT, Rychlik K, Levy SR, Testa FM. Complete remission of childhood-onset epilepsy: stability and prediction over two decades. Brain. 2014;137(12):3213–22. doi: 10.1093/brain/awu294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].ILAECommission. Proposal for Revised Classification of Epilepsies and Epileptic Syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30(4):389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- [31].Roger J. Epileptic syndromes in infancy, childhood, and adolescence. 2nd ed. London: J. Libbey; 1992. xiv, 418 p. p. [Google Scholar]
- [32].Benjamini Y, Hochberg Y. Controlling The False Discovery Rate - A Practical And Powerful Approach To Multiple Testing. J. Royal Statist. Soc. 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- [33].Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi. 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- [34].Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13:277–282. doi. 10.1111/j.1468-1331.2006.01215.x[35] [DOI] [PubMed] [Google Scholar]
- [35].Tovia E, Goldberg-Stern H, Ben Zeev B, Heyman E, Watemberg N, Fattal-Valevski A, et al. The prevalence of atypical presentations and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2011;52(8):1483–8. doi: 10.1111/j.1528-1167.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- [36].Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21. Epub 2017/03/08. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee YJ, Hwang SK, Kwon S. The Clinical Spectrum of Benign Epilepsy with Centro-Temporal Spikes: a Challenge in Categorization and Predictability. J Epilepsy Res. 2017;7(1):1–6. Epub 2017/06/30. doi: 10.14581/jer.17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gobbi G, Boni A, Filippini M. The spectrum of idiopathic Rolandic epilepsy syndromes and idiopathic occipital epilepsies: from the benign to the disabling. Epilepsia. 2006;47 Suppl 2:62–6. doi: 10.1111/j.1528-1167.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- [39].Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: Systematic review. Epilepsia. 2008;49(9):1570–80. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- [40].Smith AB, Kavros PM, Clarke T, Dorta NJ, Tremont G, Pal DK. A neurocognitive endophenotype associated with rolandic epilepsy. Epilepsia. 2012;53(4):705–11. Epub 2012/01/05. doi: 10.1111/j.1528-1167.2011.03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia-Ramos C, Jackson DC, Lin JJ, Dabbs K, Jones JE, Hsu DA, et al. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015;56(10):1615–22. Epub 2015/09/04. doi: 10.1111/epi.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].D’Alessandro P, Piccirilli M, Tiacci C, Ibba A, Maiotti M, Sciarma T, et al. Neuropsychological features of benign partial epilepsy in children. Ital J Neurol Sci. 1990;11(3):265–9. doi: 10.1007/bf02333856. [DOI] [PubMed] [Google Scholar]
- [43].Völkl-Kernstock S, Bauch-Prater S, Ponocny-Seliger E, Feucht M. Speech and school performance in children with benign partial epilepsy with centro-temporal spikes (BCECTS). Seizure. 2009;18(5):320–6. doi: 10.1016/j.seizure.2008.11.011. [DOI] [PubMed] [Google Scholar]
- [44].Vasconcellos E, Wyllie E, Sullivan S, Stanford L, Bulacio J, Kotagal P, et al. Mental retardation in pediatric candidates for epilepsy surgery: the role of early seizure onset. Epilepsia. 2001;42(2):268–74. doi: 10.1046/j.1528-1157.2001.12200.x [DOI] [PubMed] [Google Scholar]
- [45].Chu-Shore CJ, Major P, Camposano S, Muzykewicz D, Thiele EA. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51(7):1236–41. Epub 2009/12/22. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE Treatment Guidelines: Evidence-based Analysis of Antiepileptic Drug Efficacy and Effectiveness as Initial Monotherapy for Epileptic Seizures and Syndromes. Epilepsia. 2006;47(7):1094–120. doi: 10.1111/j.1528-1167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- [47].Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–63. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- [48].Xie W, Ross EE, Kramer MA, Eden UT, Chu CJ. Timing matters: Impact of anticonvulsant drug treatment and spikes on seizure risk in benign epilepsy with centrotemporal spikes. Epilepsia Open. 2018;3(3):409–17. doi: 10.1002/epi4.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Berg AT, Levy SR, Testa FM, Shinnar S. Treatment of Newly Diagnosed Pediatric Epilepsy. Arch Pediat Adol Med. 1999;153(12):1267. doi: 10.1001/archpedi.153.12.1267. [DOI] [PubMed] [Google Scholar]
- [50].Ma CKL, Chan KY. Benign childhood epilepsy with centrotemporal spikes: a study of 50 Chinese children. Brain Dev. 2003;25(6):390–5. doi: 10.1016/s0387-7604(03)00003-2. [DOI] [PubMed] [Google Scholar]
- [51].Peters JM, Camfield CS, Camfield PR. Population study of benign rolandic epilepsy:: Is treatment needed? Neurology. 2001;57(3):537–9. doi: 10.1212/wnl.57.3.537. [DOI] [PubMed] [Google Scholar]
- [52].Doumlele K, Friedman D, Buchhalter J, Donner EJ, Louik J, Devinsky O. Sudden Unexpected Death in Epilepsy Among Patients With Benign Childhood Epilepsy With Centrotemporal Spikes. JAMA Neurology. 2017;74(6):645. doi: 10.1001/jamaneurol.2016.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bourgeois BFD. Drug Treatment of Benign Focal Epilepsies of Childhood. Epilepsia. 2000;41(8):1057–8. doi: 10.1111/j.1528-1157.2000.tb00297.x. [DOI] [PubMed] [Google Scholar]
- [54].Willmore LJ. Treatment of Benign Epilepsy Syndromes Throughout Life. Epilepsia. 2001;42(s8):6–9. doi: 10.1046/j.1528-1157.2001.08005.x. [DOI] [PubMed] [Google Scholar]
- [55].Krauss GL, Krumholz A, Carter RC, Li G, Kaplan P. Risk factors for seizure-related motor vehicle crashes in patients with epilepsy. Neurology. 1999;52(7):1324-. doi: 10.1212/wnl.52.7.1324. [DOI] [PubMed] [Google Scholar]
- [56].Arts WFM. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127(8):1774–84. doi: 10.1093/brain/awh200. [DOI] [PubMed] [Google Scholar]
- [57].Berg AT, Lin J, Ebrahimi N, Testa FM, Levy SR, Shinnar S. Modeling remission and relapse in pediatric epilepsy: application of a Markov process. Epilepsy Res. 2004;60(1):31–40. doi: 10.1016/j.eplepsyres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- [58].Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.