Abstract
Extracorporeal circulatory devices such as hemodialysis and extracorporeal membrane oxygenation can be lifesaving; however, they are also prone to pathologic events including device failure, venous and arterial thrombosis, hemorrhage, and an accelerated risk for atherosclerotic disease due to interactions between blood components and device surfaces of varying biocompatibility. While extracorporeal devices may be used acutely for limited periods of time (eg, extracorporeal membrane oxygenation, continuous venovenous hemofiltration, therapeutic apheresis), some patients require chronic use of these technologies (eg, intermittent hemodialysis and left ventricular assist devices). Given the substantial thrombotic risks associated with extracorporeal devices, multiple antiplatelet and anticoagulation strategies—including unfractionated heparin, low-molecular-weight heparin, citrate, direct thrombin inhibitors, and direct oral anticoagulants, have been used to mitigate the thrombotic milieu within the patient and device. In the following manuscript, we outline the current data on anticoagulation strategies for commonly used extracorporeal circulatory devices, highlighting the potential benefits and complications involved with each.
Keywords: anticoagulation, dialysis, ECMO, hemorrhage, thrombosis
1 |. BACKGROUND
Extracorporeal circuits like those used in extracorporeal membrane oxygenation (ECMO), left ventricular assist devices (LVAD), hemodialysis (HD), and therapeutic apheresis (TPE) are common in modern healthcare. The number of centers using ECMO has risen concurrently with the expansion of its clinical applications.1 In the United States, the incidence of end-stage renal disease (ESRD) has risen by roughly 20 000 cases per year since 2006.2 Over 63 percent of ESRD patients ultimately require HD, making it the most common form of renal replacement therapy.2 Therapeutic apheresis has been available since the early 1950s and, like ECMO, its use is expanding.
With the use of an extracorporeal circuit, blood is exposed to multiple non-biologic surfaces, leading to blood protein accumulation (protein fouling), upregulation of pro-inflammatory and procoagulant pathways through the activation of coagulation factor XII and platelet activation and subsequent dysfunction.3,4 Anticoagulation is therefore considered crucial to mitigate the risk of device associated thrombosis and device failure. Heparin, the most commonly used anticoagulant for both HD and ECMO, achieves its effect by enhancing the activity of antithrombin (AT), leading to the downregulation of thrombin and Factor Xa. This mechanism of action poses two primary risks: AT concentrations are frequently low in the patient populations using extracorporeal devices and the non-specific binding of heparin to acute phase reactants and apoptotic cell; additionally, AT may interfere with biologic functions outside of coagulation, including pro- and anti-inflammatory pathways.5–7
Unlike HD and ECMO, therapeutic apheresis and continuous renal replacement therapy commonly utilize citrate for circuit anticoagulation. Proper functioning of the coagulation cascade and platelet aggregation is dependent on ionized calcium functioning as a cofactor; citrate chelates ionized calcium, limiting the quantity available for clot formation. Due to the rapid physiologic metabolism of citrate to sodium bicarbonate, the effects of circuit-administered citrate remain largely local.8 Aside from avoiding the hemorrhagic risks associated with systemic anticoagulation, citrate has also been shown to extend the lifespan of circuits and may be better tolerated by patients with kidney disease.5 Finally, as patients with left ventricular assist devices (LVADs) are managed in the outpatient setting, warfarin is the preferred long-term anticoagulant. Warfarin exerts its anticoagulant effects by inhibiting the vitamin K dependent production of factors II, VII, IX, and X and requires routine monitoring as well as dietary compliance and avoidance of drug interactions to maintain therapeutic range.
All anticoagulation agents carry the potential for clinically relevant bleeding and other side effects. Heparin has many well-documented complications aside from bleeding, including heparin-induced thrombocytopenia (HIT) and associated thrombosis as well as long-term consequences like bone disease.9–12 Possible adverse outcomes with the use of citrate include symptomatic hypocalcemia, acid/base disorders, and cardiac arrhythmias.5 There is limited evidence in the setting of extracorporeal vascular devices to guide the optimal heparin formulation (unfractionated vs low-molecular-weight), the optimal dosing strategy, and the optimal anticoagulant monitoring strategies.13 Likewise, an evidence-based consensus for the use of citrate, direct thrombin inhibitors, or avoidance of anticoagulation remains elusive, despite data suggesting these may be safe and efficacious alternatives in certain settings.4,5,13 This paper will outline specific considerations for anticoagulation, synthesize and discuss various anticoagulation strategies used for extracorporeal circulatory devices, and evaluate the data on heparin alternatives.
2 |. SPECIFIC CONSIDERATIONS REGARDING ANTICOAGULATION STRATEGIES
2.1 |. Citrate
As previously discussed, the effects of citrate are largely local due to the drug’s metabolism. Blood is generally recalcified as it exits the device; however, small amounts of free citrate do enter the systemic circulation and can precipitate symptomatic hypocalcemia. The physical effects of hypocalcemia are generally mild, manifesting as perioral tingling or paresthesias in 12%−39% of patients, but may present more severely with palpitations, tetany, or other systemic effects in the setting of large volume infusions as with leukapheresis.4 To mitigate these risks, some centers prophylactically administer oral or parenteral calcium to patients. However, the utility of oral calcium as a prophylactic agent in the prevention of hypocalcemia is unclear, with data from one randomized controlled trial showing that 70% of patients were asymptomatic or mildly symptomatic without prophylactic treatment.14 While intravenous calcium may be more efficacious in the prevention of hypocalcemia, its use may lead to vasodilation, cardiac arrhythmias, and hemodynamic instability.15 Additionally, citrate’s hepatic metabolism to bicarbonate may precipitate metabolic alkalosis, further perturbing hemostasis especially in those with renal dysfunction.4
2.2 |. Heparin products
Unfractionated heparin (UFH) is commonly used in extracorporeal circuits including ECMO, HD, continuous renal replacement therapy, and certain types of apheresis due to its favorable pharmacokinetics. Aside from bleeding, one of the most significant adverse reactions seen with UFH is heparin-induced thrombocytopenia (HIT), which occurs in 0.8%−5% of patients. HIT is classified as type I (non-antibody-mediated reaction to heparin) or type II (antibody-mediated reaction against heparin-platelet factor 4 complex). HIT type II is more severe and leads to platelet activation and an increased risk of thrombosis.16 HIT is commonly associated with both venous and arterial thrombosis, leading to skin necrosis, limb gangrene, and organ ischemia.17 Other, less common side effects include potentially fatal anaphylactic reactions and an increased risk of osteoporosis when used chronically, especially in pregnancy.18–20
2.3 |. Heparin resistance
Heparin resistance occurs when standard doses of heparin do not result in the expected, a correlative rise in activated clotting time, an activated partial thromboplastin time (aPTT), or an anti-Xa levels.21 It affects an estimated 4%−26% of patients undergoing cardiopulmonary bypass, making it an important consideration in patients exposed to extracorporeal circuits.22 Antithrombin deficiency is believed to be the primary mechanism for heparin resistance and may be acquired or rarely congenital.23 The etiologies of acquired antithrombin deficiency are diverse and include reduced synthesis in the setting of hepatic dysfunction and increased clearance due to nephropathy among others.23 An additional consideration is that many patients undergoing ECMO and other extracorporeal therapies are predisposed to antithrombin deficiency due to their underlying illnesses.5–7,24
Clinically, heparin resistance may be addressed by the administration of higher doses of heparin, supplementation of antithrombin with fresh frozen plasma (FFP) or antithrombin concentrate, or switching to an alternative anticoagulant. Treatment with exogenous antithrombin is often favored, though there is a lack of randomized trials in this space.23,24 Given the lack of data, the true clinical consequences of heparin resistance are mostly unknown.
3 |. CONTINUOUS EXTRACORPOREAL CIRCULATORY DEVICES
3.1 |. Extracorporeal membrane oxygenation
Despite the increasing use of ECMO and Extracorporeal Life Support Organization’s (ELSO) guidelines recommending anticoagulant type and dose, no randomized controlled trials have directly studied the efficacy of various anticoagulation strategies in this setting. Achieving a balance between the risk of thrombotic and bleeding events is both critical and difficult in ECMO (Figure 1). Aside from the pro-thrombotic and pro-inflammatory complications inherent in extracorporeal devices, continuous device such as ECMO can lead to accelerated proteolysis of von Willebrand factor, platelet dysfunction, and consumption of coagulation factors leading to a pro-hemorrhagic phenotype. The favorable pharmacokinetic profile (including rapid onset and reversibility), cost-effectiveness, and availability of UFH have led to its ubiquitous use as the anticoagulant of choice in ECMO.25 As of 2019, ELSO recommends the following: a 50–100 unit/kg bolus dose of UFH at the time of cannulation followed by the initiation of a UFH infusion at 7.5–20 units/kg/h.26 They make no recommendations regarding anticoagulation monitoring, however, deferring instead to individual institutional policy. Institutional approaches have considerable variability, as noted in a large, international survey regarding anticoagulation practices in the setting of ECMO.10 Across 54 centers, the only consistent practice was the use of UFH as the primary anticoagulant (45/47 responders).10 Our review of the literature revealed that institutional frameworks for anticoagulant therapy, in the setting of ECMO, may be broadly classified into three categories: those using continuous heparin, those using heparin-sparing or anticoagulant-free approaches, and those using heparin alternatives (Table 1). While the data are limited to single-center case series and small, retrospective reviews, these studies still provide valuable information about what, if any, differences exist between various approaches as far as thrombotic and bleeding complications.
FIGURE 1.
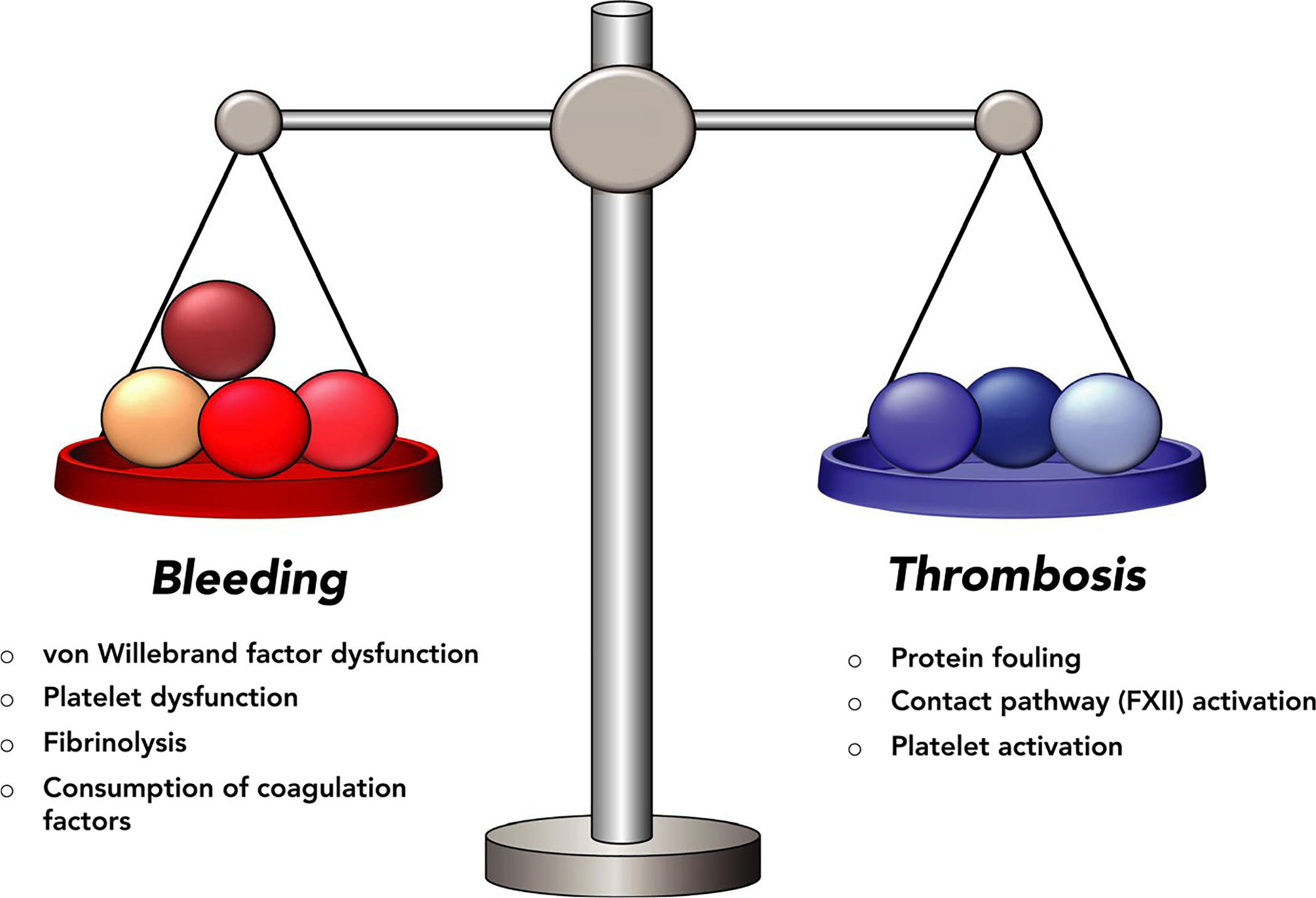
The precarious balance between thrombosis and bleeding in extracorporeal devices
TABLE 1.
Sample of various anticoagulation protocols in ECMO
| Author, year | Country | Study type | Number of patients | Anticoagulant approach; study aim | Anticoagulant dose | AC target | Notes |
|---|---|---|---|---|---|---|---|
| Coleman et al, 2019101 | USA | Retrospective chart review | 123 (72 preprotocol, 51 postprotocol) | Continuous heparin; aPTT and TEG monitoring | UNFH at 7.0 units/kg/h | aPTT: 60–80 s TEG: 2–4× baseline× | No significant differences in bleeding or thrombosis between groups |
| Panigada et al, 2018102 | Italy | Multicenter, randomized control trial | 42 (21 aPTT, 21 TEG) | Continuous heparin; aPTT vs TEG monitoring | Loading bolus of UNFH at 70 or 50 units/kg, then 18 units/kg/h | aPTT ratio: 1.5–2.0; TEG: 16–24 min | No significant differences in bleeding or thrombosis, significantly more dose titrations in TEG group |
| Mazzeffi et al, 2019103 | USA | Retrospective cohort study | 121 (50 ACT, 71 aPTT) | Continuous heparin; ACT vs aPTT monitoring | Not specified | ACT: 180–200; aPTT: 60–80 s | No significant differences in bleeding or thrombosis, 30% less transfusion in aPTT group |
| Carter et al, 2019104 | USA | Retrospective chart review | 40 (17 preprotocol, 23 postprotocol) | Heparin sparing; full therapeutic anticoagulation vs sparing protocol | Bolus at surgeon’s discretion, no heparin or low-dose continuous | ACT: 140–180 | No significant differences in bleeding or thrombosis between groups |
| Chung et al, 2017105 | S. Korea | Retrospective chart review | 55 (29 interrupted heparin, 26 continuous) | Heparin sparing; interrupted vs continuous heparin | Bolus at 100 units/kg, then 10 units/kg/h or no heparin (≥3 d) | ACT: 170–230 | No significant differences in bleeding, thrombosis or weaning between groups |
| Murphree et al, 201936 | USA | Systematic review | 154 | Anticoagulant free; safety of anticoagulant-free ECMO | N/A | N/A | No significant difference in rates of bleeding, thrombosis compared to ECMO w/anticoagulation |
| Muellenbach et al, 201237 | Germany | Case series | 3 | Anticoagulant free; safety of anticoagulant-free ECMO | N/A | N/A | No adverse bleeding or thrombotic events |
| Robba et al, 201738 | UK | Systematic review | 31 (13 w/out anticoagulation) | Anticoagulant free; safety of anticoagulant-free ECMO | N/A | N/A | 2 thrombotic events, no bleeding or deaths |
| Beiderlinden et al, 200730 | Germany | Retrospective chart review | 9 | Argatroban; determine required dose, anticoagulation profile in HIT | Initial dose of 0.2 μg/kg/min; maintenance 0.15 μg/kg/min | aPTT: 50–60 s | 1 bleeding event, no thrombosis |
| Cornell et al, 200731 | USA | Case series | 5 | Argatroban; alternative anticoagulation in possible HIT | 0.2–3.5 μg/kg/min | ACT: 210–230 | 3 bleeding events, no adverse drug reactions |
| Kim et al, 201829 | S. Korea | Retrospective chart review | 10 | Argatroban; alternative anticoagulation in possible HIT | Average dose: 0.1 μg/kg/min | aPTT 1.5× upper limit normal | No adverse bleeding or thrombotic events |
| Young et al, 200427 | USA | In vitro, sham circuits | N/A | Argatroban; argatroban vs heparin | 1.0 mg bolus, then titrated to ACT goal | ACT: 180–220 s | No clot formation, decreased thrombin formation and less prolonged aPTT in argatroban circuit |
| Sanfilippo et al, 201732 | Multiple | Systematic review | 8 publications 5 case reports | Bivalirudin; review of literature | Range: 0.1 mg/kg/h without loading dose to 0.5 mg/kg/h after loading dose | ACT range: 180–220 | No consensus, further research needed |
| Netley et al, 201833 | USA | Retrospective chart review | 11 | Bivalirudin; feasibility of Bivalirudin for anticoagulation | 2.5 mcg/kg/min | aPTT Ranges: 40–50 s, 60–70 s, 60–80 s | Within 14 h 73% of patients at AC goal, 100% at 24 h |
| Macielak et al, 201934 | USA | Retrospective chart review | 153 (10 bivalirudin, 43 heparin to bivalirudin) | Bivalirudin; comparison of heparin, heparin-bivalirudin and bivalirudin protocols | Heparin: bolus of 50–100 U/kg, then 12 U/kg/h Bivalirudin: 0.01–0.1 mg/kg/h | Heparin: aPTT 75–90 s Bivalirudin aPTT 60–80 s | No significant differences in thrombosis or bleeding between groups |
| Berei et al, 201835 | USA | Retrospective chart review | 72 (44 bivalirudin) | Bivalirudin; comparison of heparin vs bivalirudin | Heparin: bolus 80 mg/kg, then 8–12 units/kg/h Bivalirudin: 0.04 mg/kg/h | aPTT 65–90 s (high intensity); 45–60 s (low intensity) | No differences between groups in thrombotic events, bleeding, 30-d mortality, time in therapeutic range or neurological events |
3.1.1 |. Direct thrombin inhibitors in ECMO
Direct thrombin inhibitors (DTI) such as argatroban and bivalirudin are appealing alternatives because they exert their anticoagulant effects independently of antithrombin, have lower-risk side effect profiles, and may require fewer circuit exchanges compared to heparin.27
Argatroban is a synthetic direct thrombin inhibitor that reversibly binds to the active site of thrombin; it is fast-acting, reaching steady state in 1–3 hours, and has a short half-life.28 Argatroban’s predictable pharmacokinetics reduce the risk of exposure to sub- or supratherapeutic levels, theoretically translating to a reduced risk of thrombosis and bleeding.27 However, few studies describe the use of argatroban in the setting of ECMO. Those that do exist are case series and retrospective reviews with 10 or fewer patients; the limited data seem to support the safety and efficacy of argatroban in ECMO.27,29–31
Bivalirudin, a hirudin analog, binds both the active and fibrin-binding sites of thrombin and, like argatroban, has a short half-life (25 minutes).28 Again, the literature on its efficacy in ECMO is limited to smaller (<50 patients) retrospective reviews. The information available, however, does appear to support its feasibility as a heparin alternative, especially in cases of HIT and heparin resistance.32–35
3.1.2 |. Anticoagulant-free ECMO
Forgoing anticoagulation entirely in the setting of ECMO may reduce the risk of bleeding in high-risk populations, specifically those that have relative contraindications to ECMO such as intracranial hemorrhage (ICH) or other recent or ongoing bleeding.36–39 A systematic review by our group examining outcomes of anticoagulant-free ECMO (96 patients with vv-ECMO; 58 patients with va-ECMO) found no significant differences in the frequency of thrombosis and bleeding, even ICH, when compared to the historical rates seen in ECMO run with systemic anticoagulation, though this review was limited by small, non-randomized studies.36 The feasibility of anticoagulant-free ECMO was further supported by another systematic review published by our group that reported thrombosis and bleeding events in patients on anticoagulant-free ECMO to be comparable to that of patients undergoing conventionally anticoagulated ECMO.39 Similarly, another systematic review focused on vv-ECMO for acute respiratory distress syndrome in trauma patients found that of the 13 patients who underwent heparin-free ECMO, only two experienced thrombotic complications, leading the authors to suggest that heparin-free ECMO should be considered in patients with an increased risk of bleeding.38
Surface modifications broadly classified as surface passivation, biomimetic, and endothelialization are techniques used to mitigate the bio-incompatibility of ECMO circuitry and may facilitate anticoagulant-free ECMO.40,41 A large, systematic review evaluating the effect of heparin-bonded circuits (HBC) on multiple clinical outcomes determined that HBCs did significantly reduce the incidence of blood transfusion, duration of ventilation, and ICU length of stay compared to non-HBCs.42 Similarly, a systematic review and meta-analysis evaluating the efficacy of biocompatible surfaces for cardiopulmonary bypass found that patients exposed to more biocompatible surfaces had reduced rates of atrial fibrillation and decreased ICU length of stay as compared to patients treated with non-biocompatible surfaces.43 These studies suggest that the use of biocompatible surfaces may mitigate the pro-inflammatory effects of circuit exposure as well as reduce platelet activation and adhesion, potentially reducing the risk of adverse bleeding and thrombotic events without systemic anticoagulation.43
3.2 |. Left ventricular assist devices (LVAD)
The prevalence of heart failure in the United Sates is increasing; it is estimated that more than 870 000 new cases will be diagnosed each year.44 Heart transplantation remains the best therapy for end-stage heart failure; however, few patients are deemed adequate candidates for transplant, which has led to the development of mechanical therapies to augment cardiac function. Left ventricular assist devices have become an established therapeutic option for end-stage heart failure, providing hemodynamic circulatory support in settings such as cardiogenic shock, advanced heart failure, or cardiopulmonary arrest. They can be used as a bridge to cardiac transplants or a “destination therapy” in patients ineligible for heart transplant.45,46
3.2.1 |. Thrombotic and hemorrhagic complications
Despite technological and medical advancements, LVADs are associated with high rates of both thrombosis and hemorrhage which are major sources of morbidity and mortality.47 Gastrointestinal (GI) bleeding is the most characteristic bleeding event associated with implanted VADs, occurring in approximately 23% of LVAD recipients.48 Another study revealed that the cumulative risk of GI bleeding at one year was 21% and increased to 31% after five years.49 Aside from GI bleeding, intracranial bleeding is a risk with the HeartMate II destination therapy trial showing an 11% risk of hemorrhagic stroke within the first two years after LVAD placement.50
Despite evolving practices in anticoagulation and antithrombotic therapy, thromboembolic events are still common in patients with LVADs.44 Data from the REMATCH study showed the following relative incidence rates for adverse events in LVADS compared to medical therapy: non-neurologic bleeding (9.47), peripheral embolic events (3.92), myocardial infarction (0.65), and neurologic dysfunction (eg, stroke, TIA, toxic/metabolic encephalopathy) (4.35). Pump thrombosis is another adverse outcome commonly requiring device exchange and occurred in 4% of patients implanted with the HeartWare LVAD.51
Continuous-flow LVADs (CF-LVAD) produce less thrombosis and bleeding than pulsatile flow LVADs. As reported in the Heartmate II trial, CF-LVAD showed improved 2-year survival, free from disabling stroke and device failure when compared to pulsatile flow LVADs (46% vs 11%).50 The trial also reported fewer bleeding events, hemorrhagic strokes, and ischemic strokes in continuous-flow LVADs.50 However, continuous VADs are not without risk; the non-physiologic flow offered by continuous VADs is associated with impaired platelet function, acquired von Willebrand disease, and an increased prevalence of GI arteriovenous malformations/angiodysplasia due to the minimal pulse pressure.52 In a retrospective analysis of 101 patients with the Heartmate II (CF-LVAD), it was noted a significantly higher incidence of GI bleeding (22.8% vs 10%) when compared to an incidence of pulsatile flow LVADS.53–55
3.2.2 |. Anticoagulation strategies in LVAD
Current guidelines for VADs recommend dual anticoagulation and antiplatelet therapy to prevent thrombosis and device failure.56 Warfarin (target INR 2–3) and aspirin (ASA) taken in combination have become the standard practice, as they are thought to offer the optimal bleeding-thrombosis risk profile.57,58 A single-center retrospective study of HeartMate II patients compared several antithrombotic regimens (ASA 81 mg, ASA 81 mg + dipyridamole 75 mg and ASA 325 mg) with warfarin and found that hemorrhagic events occurred most frequently in the ASA 325 mg group, followed by the ASA 81 mg + dipyridamole 75 mg group, and then the ASA 81 mg group. The authors stated that high dose ASA and dipyridamole produced no change in thrombotic event rates but increased bleeding risk, and they concluded that ASA 81 mg was the ideal antithrombotic therapy.59
Nevertheless, the need for dual antithrombotic therapy remains controversial and approaches vary dramatically in the literature due, in part, to the acquired pro-hemorrhagic phenotype imparted by VADs. The European TRACE study attempted to evaluate the incidence of bleeding and thrombosis in patients on the reduced antithrombotic (RT) protocol of vitamin K antagonist (VKA) alone, without the use of antiplatelet agents. Outcomes were compared with established data previously published on the HM2, which showed a similar rate of hemorrhagic stroke, ischemic stroke, and pump thrombosis, but a lower rate of overall bleeding compared to standard dual agent antithrombotic therapy. After 2 years of sole VKA therapy, the freedom from the following events was observed: bleeding (81%), hemorrhagic stroke (96%), ischemic stroke (94%), and pump thrombosis (94%).60,61
Warfarin has well-known barriers to effectiveness, including the challenge of maintaining a consistent INR within a narrow therapeutic range, requiring consistent dietary habits, carefully assessing for drug-drug interactions, and frequent laboratory monitoring. Thus, even in well-controlled trials, only 55%−60% of patients maintain their INR with regular frequency within the therapeutic range.62 Because of this issue, a retrospective, single-center review of 118 patients who underwent CF-LVAD or HVAD implantation between 2013 and 2014 sought to determine the safety of enoxaparin as a bridging agent in LVAD patients with subtherapeutic INRs. The authors determined that patients being bridged with enoxaparin had a statistically significant increase in major bleeding events during the bridging period compared to the enoxaparin free group; thus, they concluded that enoxaparin bridging in LVAD patients with subtherapeutic INRs is not without risk. It was suspected that this increased bleeding risk was partially due to the acquired von Willebrand’s factor deficiency that inherent in LVAD patients due to the device shear stress properties.63
Direct oral anticoagulants (DOACs) have been compared against warfarin in several settings including stroke prevention in atrial fibrillation, and DOACs have shown a greater reduction in occurrences of stroke and systemic embolization, with decreased risks of bleeding, rivaroxaban notwithstanding.64,65 DOACs also offer the benefit of fewer drug interactions compared to warfarin, as well as avoiding INR testing and dose adjustments. However, enthusiasm for the use of DOACs for mechanical devices was tempered by the RE-ALIGN trial, which compared dabigatran to warfarin in mechanical heart valves. The trial was stopped early due to significantly more thromboembolic events and bleeding in the dabigatran arm: 5% vs 0%, MI: 3% vs 0%, transient ischemic attack (TIA): 2% vs 2%, any bleeding 45% vs 10% and major bleeding 7% vs 2%.66
Similarly, a small, randomized, open-label, single-center study specifically in patients with HeartWare VADs compared Dabigatran + ASA with VKA + ASA for long-term anticoagulation. The study was also stopped early due to a substantially higher incidence of thrombosis in the dabigatran + ASA arm compared to warfarin + ASA (50% vs 13%).67
A separate study compared outcomes in 7 patients with the Heartmate II LVADs who had suffered adverse outcomes with acenocoumarol (VKA) and were transitioned to dabigatran.68 This study utilized events/patient-year to assess risk of event occurrence over an extended period of observation and showed similar rates of thromboembolic events between dabigatran and acenocoumarol with regards to stroke (0.094 vs 0 events/patient-year) and device thromboses (0.053 vs 0.258 events/patient-year).68 Dabigatran also yielded a similar rate of bleeding events (0.82 vs 0.22 events/patient-year) and life-threatening bleeds (0.27 vs 0.18 bleeds/patient-years).68 The majority of data regarding DOAC use in HM2 or other LVAD therapy has been conducted in smaller samples, usually as a second line anticoagulant after failure of VKA therapy.
To summarize, for patients with end-stage heart failure refractory to medical therapy, ventricular assist devices offer both a bridge to transplant and prolonged survival in non-transplant candidates. However, the rate of adverse events in patients with these devices is high, with potential risk for bleeding and thrombotic complications. The current data suggest that the combination of warfarin, with an INR goal of 2–3, and a low-dose antiplatelet agent (ASA 81 mg), offers an acceptable balance between the risks of thromboembolic events and clinically relevant bleeding in patients with VADs.
4 |. INTERMITTENT EXTRACORPOREAL MEDICAL DEVICES
4.1 |. Hemodialysis
The incidence of ESRD has risen by an estimated 20 000 cases per year since 2006, and the prevalence is expected to increase 29%−68% between 2015 and 2030.2,69 Despite the increasing demand for HD, a standardized protocol for anticoagulation during HD does not exist in the United States. Rather, like anticoagulation strategies in ECMO, the regimen is left to clinician or institutional preference. Anticoagulation with UFH is currently the most commonly employed strategy in the United States. Alternatives to heparin include low-molecular-weight heparin (LMWH), which is increasingly used in Europe, as well as direct thrombin inhibitors, regional citrate anticoagulation (RCA), and anticoagulant-free methods.
4.1.1 |. Unfractionated heparin
As previously discussed, UFH has multiple advantageous characteristics that have led to its ubiquitous use. Patients undergoing HD typically receive a loading dose of UFH followed by a continuous infusion which is stopped before completion of the dialysis session. Table 2 details a small selection of suggested UFH dosing regimens.
TABLE 2.
A sample of suggested protocols from different authors/institutions/countries for unfractionated heparin use in long-term intermittent hemodialysis
| Author | Institution/country | Loading dose | Maintenance dose | Bolus dose regimen | Adjustments to dosing | Monitoring |
|---|---|---|---|---|---|---|
| Davenport et al, 201171 | Royal Free Hospital, University College London Medical School/UK | 1500–2000 IU (15–25 IU/kg) | 1000–1500 IU (10–20 IU/kg) | 4000 IU. Check ACT after 3 min; if <180% baseline, then increase subsequent bolus. Check ACT after 2 h; if ACT < 150%, bolus another 2000 IU | Reduced if < 50 kg and increased if > 90 kg | N/A |
| Shen and Winkelmayer, 201274 | Stanford University School of Medicine/US | 25 IU/kg | Continuous infusion of 1000 IU/h, to be stopped 30–60 min before the end of dialysis session | N/A | If patient requires > 15 min to clot at needle puncture sites, decrease maintenance dose by 500 IU/h and eventually eliminate if prolonged bleeding does not improve with subsequently reduced doses. If circuit clots during run, increase maintenance dose by 500 IU/h | N/A |
| Ashby et al, 201970 | Various institutions of the NHS Trust; teaching and community hospitals/UK | 1000–2000 IU | 500–1500 IU/h; discontinue ~ 30 min before end of dialysis session | N/A | N/A | aPTTr or ACT, aiming for ~150% of predialysis or center normal values |
Some heparin dosing strategies suggest actively monitoring anticoagulation during HD using laboratory or point-of-care testing. For instance, one approach involves obtaining an activated partial thromboplastin time (aPTT) during dialysis to achieve a goal of 150% of predialysis aPTT.70 Another protocol involves checking a bedside activated clotting time (ACT) after 3 minutes of HD and increasing subsequent bolus doses if <180% of baseline.71 The utility of these monitoring strategies has long been disputed. As early as 1979, it was demonstrated that frequent (as often as every 30 minutes) aPTT monitoring throughout HD helped lower heparin requirements in routine dialysis patients.72 A more recent study observed that aPTT did not correlate with the degree of dialyzer clotting.73 Moreover, a significant issue with laboratory monitoring is the associated high cost due to increased technician involvement. Thus, most US dialysis units rely on clinical signs of under- and over-anticoagulation by observing for visual clots in the circuit and prolonged bleeding at the venipuncture site after the dialysis session.74
Patients undergoing HD are at an increased risk for bleeding events due to UFH exposure, uremic platelet dysfunction, and other comorbidities. A study in Japan reported that patients undergoing maintenance HD had a higher incidence of cerebral hemorrhage (8.7 per 1000 patient-years) compared to the general population, with a 3-month mortality rate of 71.4% in those who suffered a cerebral hemorrhage.75 Another study found no significant difference in mortality from cerebrovascular hemorrhage between HD patients using antiplatelet/anticoagulant medications and those not using such agents.76
Similarly, patients receiving HD are at an increased risk for upper gastrointestinal bleeding (UGIB) compared to the general population.77 However, no robust studies demonstrate an increased risk of UGIB with heparin use in HD. Although the study did not measure or analyze UFH use separately, a retrospective cohort study using data from the US Renal Data System reported no association between antiplatelet/anticoagulant use at baseline in ESRD patients and risk of UGIB.78 Current understanding of the bleeding risk associated with heparin use during HD is limited by the lack of studies directly analyzing this relationship, but the aforementioned studies suggest the relative safety profile of heparin products in patients receiving HD.
Another potential risk associated with chronic heparin use, UFH and LMWH alike, in HD is HIT. A national survey of more than 13 000 UK patients receiving HD reported that the incidence of HIT type II was 0.26%, which is lower than that reported within the general medical population.79 Of the patients who developed HIT type II, 14% experienced complications including petechial rash, pulmonary embolism, retroperitoneal hematoma, and deep vein thrombosis.79 Another review reported similar findings, strengthening the idea that the risk of HIT in dialysis patients is lower than that of other studied populations. Anti-heparin-platelet factor 4 (anti-HPF4) antibodies were detected in 1.1%−12% of patients receiving dialysis, and only a small portion of these patients experienced thrombocytopenia (0%−3.8%) or thrombosis (0$−3.8%).80 Some studies have suggested that the mere presence of anti-HPF4 antibodies in HD patients receiving heparin is associated with increased morbidity and mortality.81,82 However, this complex relationship is not yet thoroughly understood.
4.1.2 |. Low-molecular-weight heparin
In many settings, LMWH has become the preferred agent for the treatment and prevention of thromboembolic events given its efficacy, predictability, and convenience. Current data suggest that LMWH can be a safe and effective UFH alternative during HD.83,84 The two most commonly used LMWH agents include enoxaparin (initial dose of 0.5–0.8 mg/kg) and tinzaparin (initial dose of 2500–4500 IU), delivered prior to the start of dialysis.85 A retrospective single-center study demonstrated no significant difference in bleeding rates between HD patients treated with UFH vs LMWH.83 In this study, the incidence of a major bleed in patients receiving UFH vs LMWH was 1.33% and 1.92%, respectively, and the incidence of clinically significant bleeding was 3.33% and 3.96%, respectively.83 No significant difference in bleed-free survival between UFH and LMWH was observed (OR 0.904).83 A meta-analysis of 11 randomized controlled trials also found when compared to UFH, LMWH did not significantly affect the number of bleeding events or extracorporeal hemodialysis circuit thromboses.84
4.1.3 |. Direct thrombin inhibitors
Of the currently available DTIs, argatroban is the best-described agent in HD. Given its high cost relative to heparin, its use has been mostly limited to patients prior history of HIT. However, the available data suggest that it may also be a safe and effective heparin alternative. A review of 253 patients showed that argatroban infusion doses of 0.02–9.3 μg/kg/min during hemodialysis, with aPTT target of at least 1.5 times the baseline or ACT target of at least 140 seconds, were safe and well-tolerated in patients on HD.86 Another study reported the successful use of bivalirudin for prevention of circuit thrombosis during HD in patients who were positive for anti-HPF4 antibodies without significant bleeding or adverse events.87
4.1.4 |. Citrate
Regional citrate anticoagulation during HD and anticoagulant-free HD have also been suggested as potential substitutes to heparin in patients with high bleeding risk. A prospective study of 1009 consecutive citrate-anticoagulated high-flux HD treatments performed in 59 patients demonstrated the safety and efficacy of anticoagulation using regional citrate.88 During the 2-year study period, only 2 adverse events (oral paresthesia due to hypocalcemia and nausea/vertigo due to metabolic acidosis) were reported and both were attributed to handling errors early in the study course; 99.6% of HD treatments were completed successfully, and the degree of anticoagulation, measured using a semi-quantitative clotting score, was encouraging.88 Unfortunately, this method is cost-prohibitive due to frequent calcium level checks and calcium replacement fluid adjustments that necessitate increased technician involvement. Although strategies for automation of regional citrate anticoagulation (RCA) have been proposed, it does not yet appear to have been implemented in practice.89
4.1.5 |. Anticoagulant-free HD
In anticoagulant-free HD, the extracorporeal circuit may be intermittently flushed with 100–200 mL of saline every 15–60 minutes to prevent thrombosis.74 A retrospective analysis of 1043 HD treatments in 365 patients showed that anticoagulant-free HD was associated with higher rates of circuit thrombosis (9.1% vs 2.4%) and circuit change due to clotting (7.3% vs 0.8%).90 However, a separate study found that anticoagulant-free HD had no significant association with all-cause mortality or bleeding compared to HD with heparin.91 These studies suggest that anticoagulant-free HD is associated with increased risk of circuit clotting causing interrupted treatment, without proven safety or survival benefit in high-risk patients.
4.1.6 |. Continuous renal replacement therapy
Many critically ill patients suffering from acute kidney injury require renal replacement therapy in the acute setting. If these patients can-not tolerate intermittent hemodialysis, continuous renal replacement therapy (CRRT) is used, in the form of continuous venovenous hemodialysis (CVVH) or continuous venovenous hemodiafiltration (CVVHDF). Anticoagulation during CRRT is important in prolonging the filter lifespan and reducing interruptions to renal replacement therapy. During CRRT, the two best-studied methods of anticoagulation are UFH and RCA.
The majority of studies and meta-analyses comparing heparin and RCA have suggested that RCA is superior in prolonging the filter circuit lifespan and in reducing the risk of bleeding complications. Liu et al found no significant difference in mortality between critically ill patients receiving CRRT with regional citrate vs heparin anticoagulation, but found regional citrate to be superior in prolonging circuit lifespan by a mean of 15.69 hours and lowering the risk of bleeding.92
Typical RCA dosing during CRRT ranges from 3 to 5 mmol/L blood, though lower doses have been suggested. A recent prospective study found that a lower initial citrate dose of 2.5 mmol/L resulted in a lower rate of citrate-related complications with no difference in anticoagulation efficacy.93 In this study, the odds of hypocalcemia were 4.67 times higher in patients receiving 3.0 mmol/L of citrate compared to those receiving 2.5 mmol/L, while the rate of circuit clotting was similar (9.9% vs 9.2% in 3.0 vs 2.5 mmol/L dose, respectively). A separate meta-analysis showed RCA in CRRT is safe among liver failure patients, an important consideration given that many critically ill patients requiring CRRT also have hepatic dysfunction.94
One of the primary drawbacks of RCA is the increased cost. A cost comparison study of critically ill patients found that the median cost of anticoagulation in the citrate group was 317.91 Australian dollars ($211.16 US) higher per patient per day compared to the heparin group with a significant portion of this increased cost being due to more frequent blood gas draws to monitor systemic and postfilter calcium levels.95 However, a study examining six different blood gas instruments in patients receiving RCA in CRRT showed that there is a significant discrepancy in the different instruments in postfilter calcium levels, which determine the change in citrate flow as recommended by the manufacturer. Given this, they do not recommend using postfilter calcium levels to manage RCA but instead to follow the ionized calcium levels to make adjustments.96
Avenues for improvements in anticoagulation may include the addition of LMWH to RCA. In a randomized controlled trial of 53 patients, mean filter runtime among patients receiving CVVH was significantly longer in a group receiving RCA plus low-dose dalteparin (40.4 ± 30.9 hours) compared to those receiving RCA only (21.2 ± 13.5 hours) and dalteparin only (25.1 ± 24.0 hours), without any difference in the rate of premature clotting or new onset of bleeding.97 Another retrospective study published in March 2020 in patients receiving CRRT while on ECMO showed that RCA in addition to LMWH resulted in lower incidence in circuit clotting (11% vs 38%).98
4.2 |. Therapeutic apheresis
Therapeutic apheresis has become an increasingly common treatment modality for a variety of medical conditions. Apheresis encompasses a range of procedures in which blood flows through an extracorporeal circuit and is separated into components prior to recirculation. Two of the more commonly used modalities are double filtration plasmapheresis, in which substances are filtrated out from the plasma, and therapeutic plasma exchange, in which the patient’s plasma is removed and replaced with donor plasma or another type of replacement fluid. The American Society for Apheresis recently published guidelines on 84 diseases and their indications for apheresis, including the current levels of evidence.99
Citrate is the predominant anticoagulant in apheresis although, in certain situations such as large volume leukapheresis and photopheresis, unfractionated heparin may be used. The administration of citrate is dependent on the total blood volume, citrate infusion rate, and ratio of whole blood to citrate (WB:AC), which are all calculated by the apheresis instruments. Depending on the apheresis instrument manufacturer and type of apheresis, there are different anticoagulant and WB:AC ratios are recommended.4
5 |. CONCLUSION
Extracorporeal circulatory devices, including ECMO, LVAD, HD, and therapeutic apheresis are exciting therapeutic technologies that are being employed with increasing frequency in both inpatient and outpatient settings. The engineering of these various devices necessitates the exposure of blood products to non-biologic circuitry, making anticoagulation a critical factor in the prevention of pathologic thrombosis. However, anticoagulant agents are not benign and their individual risk-benefit profiles must be taken into consideration. For continuous extracorporeal circuits, systemic anticoagulation is the mainstay due to the time course and the increased blood flow. However, there is promising evidence, as detailed by a limited number of case reports, that biocompatible circuitry may allow anticoagulant-free interventions in the future. Intermittent extracorporeal circuits have seen broader success with regional anticoagulation strategies, due in part, to their time course and the decreased blood flow handled in the setting of HD, CRRT, and apheresis compared to ECMO.
Despite the ubiquitous use of extracorporeal devices and the risks associated with anticoagulation, there is a scarcity of evidence with respect to anticoagulant agent, dose, route of administration, and monitoring schema across all devices. While there have been several small studies that have attempted to evaluate the safety and efficacy of various strategies, at this time, institutions are utilizing a variety of independently developed approaches due to a lack of evidence-based guidelines.
The continued study of various anticoagulation strategies will serve to greatly benefit the field and enable the eventual implementation of evidence-based guidelines. Emerging preclinical data suggest that novel agents targeting factor XII may offer improved efficacy and safety profiles; however, data in humans with medical devices are not yet available.100 Likewise, advancement in our understanding of the blood-device interface and the development of increasingly biocompatible device materials will be equally critical to the field. Thus, this subject remains a great area of further interest that warrants additional study.
Funding information
Foundation for the National Institutes of Health, Grant/Award Number: R01 HL101972
Footnotes
CONFLICT OF INTEREST
Dr Shatzel reports receiving consulting fees from Aronora Inc. The remaining authors have nothing to disclose.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(USRDS) USRDS. 2018 USRDS ANNUAL DATA REPORT; 2018;2. [Google Scholar]
- 3.Mulder MMG, Fawzy I, Lancé MD. ECMO and anticoagulation: a comprehensive review. Netherlands J Crit Care. 2018;26:6–13. [Google Scholar]
- 4.Lee G, Arepally GM. Anticoagulation techniques in apheresis: from heparin to citrate and beyond. J Clin Apher. 2012;27(3):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oudemans-van Straaten HM, Kellum JA, Bellomo R. Clinical review: anticoagulation for continuous renal replacement therapy – heparin or citrate? Crit Care. 2010;15(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panigada M, Artoni A, Passamonti SM, et al. Hemostasis changes during veno-venous extracorporeal membrane oxygenation for respiratory support in adults. Minerva Anestesiol. 2016;82:170–179. [PubMed] [Google Scholar]
- 7.Malfertheiner MV, Philipp A, Lubnow M, et al. Hemostatic changes during extracorporeal membrane oxygenation: a prospective randomized clinical trial comparing three different extracorporeal membrane oxygenation systems. Crit Care Med. 2016;44(4):747–754. [DOI] [PubMed] [Google Scholar]
- 8.Passero BA, Zappone P, Lee HE, Novak C, Maceira EL, Naber M. Citrate versus heparin for apheresis catheter locks: an efficacy analysis. J Clin Apher. 2015;30(1):22–27. [DOI] [PubMed] [Google Scholar]
- 9.Oudemans-van Straaten HM, van Schilfgaarde M, Molenaar PJ, Wester JP, Leyte A. Hemostasis during low molecular weight heparin anticoagulation for continuous venovenous hemofiltration: a randomized cross-over trial comparing two hemofiltration rates. Crit Care. 2009;13(6):R193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esper SA, Welsby IJ, Subramaniam K, et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang. 2017;112(5):443–452. [DOI] [PubMed] [Google Scholar]
- 11.Matchar DB, Mark DB. Strategies for incorporating resource allocation and economic considerations: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):132S–140S. [DOI] [PubMed] [Google Scholar]
- 12.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355(8):809–817. [DOI] [PubMed] [Google Scholar]
- 13.Kessler M, Moureau F, Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474–489. [DOI] [PubMed] [Google Scholar]
- 14.Bolan CD, Wesley RA, Yau YY, et al. Randomized placebo-controlled study of oral calcium carbonate administration in plateletpheresis: I. Associations with donor symptoms. Transfusion. 2003;43(10):1403–1413. [DOI] [PubMed] [Google Scholar]
- 15.Buchta C, Macher M, Bieglmayer C, Hocker P, Dettke M. Reduction of adverse citrate reactions during autologous large-volume PBPC apheresis by continuous infusion of calcium-gluconate. Transfusion. 2003;43(11):1615–1621. [DOI] [PubMed] [Google Scholar]
- 16.Farley S, Cummings C, Heuser W, et al. Prevalence and overtesting of true heparin-induced thrombocytopenia in a 591-bed tertiary care, Teaching Hospital. J Intensive Care Med. 2019;34(6):464–471. [DOI] [PubMed] [Google Scholar]
- 17.Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1 Suppl):64S–94S. [DOI] [PubMed] [Google Scholar]
- 18.Barbour LA, Kick SD, Steiner JF, et al. prospective study of heparin-induced osteoporosis in pregnancy using bone densitometry. Am J Obstet Gynecol. 1994;170:862–869. [DOI] [PubMed] [Google Scholar]
- 19.Dahlman TC, Sjöberg HE, Ringertz H. Bone mineral density during long-term prophylaxis with heparin in pregnancy. Am J Obstet Gynecol. 1994;170:1315–1320. [DOI] [PubMed] [Google Scholar]
- 20.Galambosi P, Hiilesmaa V, Ulander VM, Laitinen L, Tiitinen A, Kaaja R. Prolonged low-molecular-weight heparin use during pregnancy and subsequent bone mineral density. Thromb Res. 2016;143:122–126. [DOI] [PubMed] [Google Scholar]
- 21.Durrani J, Malik F, Ali N, Jafri SIM. To be or not to be a case of heparin resistance. J Community Hosp Intern Med Perspect. 2018;8(3):145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawatsu S, Sasaki K, Sakatsume KO, et al. Predictors of heparin resistance before cardiovascular operations in adults. Ann Thorac Surg. 2018;105(5):1316–1321. [DOI] [PubMed] [Google Scholar]
- 23.Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg. 2013;116(6):1210–1222. [DOI] [PubMed] [Google Scholar]
- 24.Chlebowski MM, Baltagi S, Carlson M, Levy JH, Spinella PC. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. 2020;24(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burstein B, Wieruszewski PM, Zhao YJ, Smischney N. Anticoagulation with direct thrombin inhibitors during extracorporeal membrane oxygenation. World J Crit Care Med. 2019;8(6):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(ELSO) TELSO. ELSO Anticoagulation Guideline; 2014.
- 27.Young G, Yonekawa KE, Nakagawa P, Nugent DJ. Argatroban as an alternative to heparin in extracorporeal membrane oxygenation circuits. Perfusion. 2004;19(5):283–288. [DOI] [PubMed] [Google Scholar]
- 28.Kelton JG, Arnold DM, Bates SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med. 2013;368(8):737–744. [DOI] [PubMed] [Google Scholar]
- 29.Kim YS, Lee H, Yang J-H, et al. Use of argatroban for extracorporeal life support in patients with nonheparin-induced thrombocytopenia: analysis of 10 consecutive patients. Medicine (Baltimore). 2018;97(47):e13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beiderlinden M, Treschan T, Gorlinger K, Peters J. Argatroban in extracorporeal membrane oxygenation. Artif Organs. 2007;31(6):461–465. [DOI] [PubMed] [Google Scholar]
- 31.Cornell T, Wyrick P, Fleming G, et al. A case series describing the use of argatroban in patients on extracorporeal circulation. ASAIO J. 2007;53(4):460–463. [DOI] [PubMed] [Google Scholar]
- 32.Sanfilippo F, Asmussen S, Maybauer DM, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. 2017;32(5):312–319. [DOI] [PubMed] [Google Scholar]
- 33.Netley J, Roy J, Greenlee J, Hart S, Todt M, Statz B. Bivalirudin anticoagulation dosing protocol for extracorporeal membrane oxygenation: a retrospective review. J Extra Corpor Technol. 2018;50(3):161–166. [PMC free article] [PubMed] [Google Scholar]
- 34.Macielak S, Burcham P, Whitson B, Abdel-Rasoul M, Rozycki A. Impact of anticoagulation strategy and agents on extracorporeal membrane oxygenation therapy. Perfusion. 2019;34(8):671–678. [DOI] [PubMed] [Google Scholar]
- 35.Berei TJ, Lillyblad MP, Wilson KJ, Garberich RF, Hryniewicz KM. Evaluation of systemic heparin versus bivalirudin in adult patients supported by extracorporeal membrane oxygenation. ASAIO J. 2018;64(5):623–629. [DOI] [PubMed] [Google Scholar]
- 36.Murphree CR, Shatzel JJ, Olson SR. Bleeding and thrombotic outcomes in anticoagulant free extracorporeal membrane oxygenation (ECMO) in adults: a systematic review. Blood. 2019;134(Suppl 1):2436. [Google Scholar]
- 37.Muellenbach RM, Kredel M, Kunze E, et al. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. 2012;72(5):1444–1447. [DOI] [PubMed] [Google Scholar]
- 38.Robba C, Ortu A, Bilotta F, et al. Extracorporeal membrane oxygenation for adult respiratory distress syndrome in trauma patients: a case series and systematic literature review. J Trauma Acute Care Surg. 2017;82(1):165–173. [DOI] [PubMed] [Google Scholar]
- 39.Olson SR, Murphree CR, Zonies D, et al. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO). Without anticoagulation: a systematic review. ASAIO J. 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eytan D, Bitterman Y, Annich GM. VV extracorporeal life support for the Third Millennium: will we need anticoagulation? J Thorac Dis. 2018;10(Suppl 5):S698–S706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ontaneda A, Annich GM. Novel surfaces in extracorporeal membrane oxygenation circuits. Front Med (Lausanne). 2018;5:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangoush O, Purkayastha S, Hajyahia S, et al. Heparin-bonded circuits versus nonheparin-bonded circuits: an evaluation of their effect on clinical outcomes. Eur J Cardiothorac Surg. 2007;31(6): 1058–1069. [DOI] [PubMed] [Google Scholar]
- 43.Ranucci M, Balduini A, Ditta A, Boncilli A, Brozzi S. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann Thorac Surg. 2009;87(4):1311–1319. [DOI] [PubMed] [Google Scholar]
- 44.Hilal T, Mudd J, DeLoughery TG. Hemostatic complications associated with ventricular assist devices. Res Pract Thromb Haemost. 2019;3(4):589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47(5):320–332. [DOI] [PubMed] [Google Scholar]
- 46.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. [DOI] [PubMed] [Google Scholar]
- 47.Stehlik J, Johnson SA, Selzman CH. Gold standard in anticoagulation assessment of left ventricular assist device patients?: how about bronze. JACC Heart Fail. 2015;3(4):323–326. [DOI] [PubMed] [Google Scholar]
- 48.Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous-flow left ventricular assist devices: a systematic review and meta-analysis. Gastrointest Endosc. 2014;80(3):435–446 e431. [DOI] [PubMed] [Google Scholar]
- 49.Stulak JM, Davis ME, Haglund N, et al. Adverse events in contemporary continuous-flow left ventricular assist devices: a multi-institutional comparison shows significant differences. J Thorac Cardiovasc Surg. 2016;151(1):177–189. [DOI] [PubMed] [Google Scholar]
- 50.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361(23):2241–2251. [DOI] [PubMed] [Google Scholar]
- 51.Najjar SS, Slaughter MS, Pagani FD, et al. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33(1):23–34. [DOI] [PubMed] [Google Scholar]
- 52.Miceli A, Glauber M. The use of new anticoagulant drugs in ventricular assist devices: another brick in the wall? J Thorac Cardiovasc Surg. 2016;151(4):e83–e84. [DOI] [PubMed] [Google Scholar]
- 53.Crow S, John R, Boyle A, et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;137(1):208–215. [DOI] [PubMed] [Google Scholar]
- 54.Guha A, Eshelbrenner CL, Richards DM, Monsour HP Jr. Gastrointestinal bleeding after continuous-flow left ventricular device implantation: review of pathophysiology and management. Methodist Debakey Cardiovasc J. 2015;11(1):24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aggarwal A, Pant R, Kumar S, et al. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012;93(5):1534–1540. [DOI] [PubMed] [Google Scholar]
- 56.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–187. [DOI] [PubMed] [Google Scholar]
- 57.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29(4 Suppl):S1–S39. [DOI] [PubMed] [Google Scholar]
- 58.Sage W, Gottiparthy A, Lincoln P, Tsui SSL, Pettit SJ. Improving anticoagulation of patients with an implantable left ventricular assist device. BMJ Open Qual. 2018;7(4):e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saeed O, Shah A, Kargoli F, et al. Antiplatelet therapy and adverse hematologic events during heart mate II support. Circ Heart Fail. 2016;9(1):e002296. [DOI] [PubMed] [Google Scholar]
- 60.Netuka I, Litzler P-Y, Berchtold-Herz M, et al. Outcomes in HeartMate II patients with no antiplatelet therapy: 2-year results from the European TRACE study. Ann Thorac Surg. 2017;103(4):1262–1268. [DOI] [PubMed] [Google Scholar]
- 61.Litzler PY, Smail H, Barbay V, et al. Is anti-platelet therapy needed in continuous flow left ventricular assist device patients? A single-centre experience. Eur J Cardiothorac Surg. 2014;45(1):55–59; discussion 59–60. [DOI] [PubMed] [Google Scholar]
- 62.Wartak SA, Bartholomew JR. Dabigatran: will it change clinical practice? Cleve Clin J Med. 2011;78(10):657–664. [DOI] [PubMed] [Google Scholar]
- 63.Bhatia A, Juricek C, Sarswat N, et al. Increased risk of bleeding in left ventricular assist device patients treated with enoxaparin as bridge to therapeutic international normalized ratio. ASAIO J. 2018;64(2):140–146. [DOI] [PubMed] [Google Scholar]
- 64.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962. [DOI] [PubMed] [Google Scholar]
- 65.van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320–328. [DOI] [PubMed] [Google Scholar]
- 66.Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. [DOI] [PubMed] [Google Scholar]
- 67.Andreas M, Moayedifar R, Wieselthaler G, et al. Increased thromboembolic events with Dabigatran compared with vitamin K antagonism in left ventricular assist device patients: a randomized controlled pilot trial. Circ Heart Fail. 2017;10(5):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terrovitis JV, Ntalianis A, Kapelios CJ, et al. Dabigatran etexilate as second-line therapy in patients with a left ventricular assist device. Hellenic J Cardiol. 2015;56(1):20–25. [PubMed] [Google Scholar]
- 69.McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM. Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol. 2019;30(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashby D, Borman N, Burton J, et al. Renal association clinical practice guideline on haemodialysis. BMC Nephrol. 2019;20(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davenport A Optimization of heparin anticoagulation for hemodialysis. Hemodial Int. 2011;15(Suppl 1):S43–S48. [DOI] [PubMed] [Google Scholar]
- 72.Shapiro WB, Faubert PF, Porush JG, Chou SY. Low-dose heparin in routine hemodialysis monitored by activated partial thromboplastin time. Artif Organs. 1979;3(1):73–77. [DOI] [PubMed] [Google Scholar]
- 73.Shinoda T, Arakura H, Katakura M, Shirota T, Nakagawa S. Usefulness of thrombelastography for dosage monitoring of low molecular weight heparin and unfractionated heparin during hemodialysis. Artif Organs. 1990;14(6):413–415. [DOI] [PubMed] [Google Scholar]
- 74.Shen JI, Winkelmayer WC. Use and safety of unfractionated heparin for anticoagulation during maintenance hemodialysis. Am J Kidney Dis. 2012;60(3):473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T. Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis. 1998;31(6):991–996. [DOI] [PubMed] [Google Scholar]
- 76.Sakamoto N, Ishikawa E, Aoki K, Uemae Y, Komatsu Y, Matsumura A. Clinical outcomes of intracerebral hemorrhage in hemodialysis patients. World Neurosurg. 2014;81(3):538–542. [DOI] [PubMed] [Google Scholar]
- 77.Kuo CC, Kuo HW, Lee IM, Lee CT, Yang CY. The risk of upper gastrointestinal bleeding in patients treated with hemodialysis: a population-based cohort study. BMC Nephrol. 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64(4):1455–1461. [DOI] [PubMed] [Google Scholar]
- 79.Hutchison CA, Dasgupta I. National survey of heparin-induced thrombocytopenia in the haemodialysis population of the UK population. Nephrol Dial Transplant. 2007;22(6):1680–1684. [DOI] [PubMed] [Google Scholar]
- 80.Chang JJ, Parikh CR. When heparin causes thrombosis: significance, recognition, and management of heparin-induced thrombocytopenia in dialysis patients. Semin Dial. 2006;19(4):297–304. [DOI] [PubMed] [Google Scholar]
- 81.de la Vega LP, Miller RS, Benda MM, et al. Association of heparin-dependent antibodies and adverse outcomes in hemodialysis patients: a population-based study. Mayo Clin Proc. 2005;80(8): 995–1000. [DOI] [PubMed] [Google Scholar]
- 82.Mureebe L, Coats RD, Silliman WR, Shuster TA, Nichols WK, Silver D. Heparin-associated antiplatelet antibodies increase morbidity and mortality in hemodialysis patients. Surgery. 2004;136(4):848–853. [DOI] [PubMed] [Google Scholar]
- 83.Nadarajah L, Fan S, Forbes S, Ashman N. Major bleeding in hemodialysis patients using unfractionated or low molecular weight heparin: a single-center study. Clin Nephrol. 2015;84(5):274–279. [DOI] [PubMed] [Google Scholar]
- 84.Lim W, Cook DJ, Crowther MA. Safety and efficacy of low molecular weight heparins for hemodialysis in patients with end-stage renal failure: a meta-analysis of randomized trials. J Am Soc Nephrol. 2004;15(12):3192–3206. [DOI] [PubMed] [Google Scholar]
- 85.Davenport A Review article: low-molecular-weight heparin as an alternative anticoagulant to unfractionated heparin for routine outpatient haemodialysis treatments. Nephrology. 2009;14(5):455–461. [DOI] [PubMed] [Google Scholar]
- 86.Hursting MJ, Murray PT. Argatroban anticoagulation in renal dysfunction: a literature analysis. Nephron Clin Pract. 2008;109(2):c80–c94. [DOI] [PubMed] [Google Scholar]
- 87.Al-Ali FS, Elsayed M, Khalifa S, et al. Successful use of a bivalirudin treatment protocol to prevent extracorporeal thrombosis in ambulatory hemodialysis patients with heparin-induced thrombocytopenia. Hemodial Int. 2016;20(2):204–207. [DOI] [PubMed] [Google Scholar]
- 88.Apsner R, Buchmayer H, Gruber D, Sunder-Plassmann G. Citrate for long-term hemodialysis: prospective study of 1,009 consecutive high-flux treatments in 59 patients. Am J Kidney Dis. 2005;45(3):557–564. [DOI] [PubMed] [Google Scholar]
- 89.Szamosfalvi B, Frinak S, Yee J. Automated regional citrate anticoagulation: technological barriers and possible solutions. Blood Purif. 2010;29(2):204–209. [DOI] [PubMed] [Google Scholar]
- 90.Liang E, Rodriguez M, Mueller M, Abramowitz MK, Mokrzycki MH. Outcomes associated with a heparin-free hemodialysis protocol and review of the literature. J Clin Nephrol Ren Care. 2016;2(010). [Google Scholar]
- 91.Shen JI, Mitani AA, Chang TI, Winkelmayer WC. Use and safety of heparin-free maintenance hemodialysis in the USA. Nephrol Dial Transplant. 2013;28(6):1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poh CB, Tan PC, Kam JW, et al. Regional citrate anticoagulation for continuous renal replacement therapy – a safe and effective low-dose protocol. Nephrology. 2020;25(4):305–313. [DOI] [PubMed] [Google Scholar]
- 94.Zhang W, Bai M, Yu Y, et al. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care. 2019;23(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dissanayake CU, Bharat CI, Roberts BL, Anstey MH. A cost comparison of regional citrate versus low-dose systemic heparin anticoagulation in continuous renal replacement therapy. Anaesth Intensive Care. 2019;47(3):281–287. [DOI] [PubMed] [Google Scholar]
- 96.Schwarzer P, Kuhn S-O, Stracke S, et al. Discrepant post filter ionized calcium concentrations by common blood gas analyzers in CRRT using regional citrate anticoagulation. Crit Care. 2015;19(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu B, Zhang K, Xu B, Ji D, Liu Z, Gong D. Randomized controlled trial to evaluate regional citrate anticoagulation plus low-dose of dalteparin in continuous veno-venous hemofiltration. Blood Purif. 2015;39(4):306–312. [DOI] [PubMed] [Google Scholar]
- 98.Giani M, Scaravilli V, Stefanini F, et al. Continuous renal replacement therapy in venovenous extracorporeal membrane oxygenation: a retrospective study on regional citrate anticoagulation. ASAIO J. 2020;66(3):332–338. [DOI] [PubMed] [Google Scholar]
- 99.Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American Society for Apheresis: The Eighth Special Issue. J Clin Apheresis. 2019;34(3):171–354. [DOI] [PubMed] [Google Scholar]
- 100.DeLoughery EP, Olson SR, Puy C, McCarty OJT, Shatzel JJ. The safety and efficacy of novel agents targeting factors XI and XII in early phase human trials. Semin Thromb Hemost. 2019;45(5):502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Colman E, Yin EB, Laine G, et al. Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature. J Thorac Dis. 2019;11(8):3325–3335. 10.21037/jtd.2019.08.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Panigada M, Iapichino GE, Brioni M, et al. Thromboelastography-based anticoagulation management during extracorporeal membrane oxygenation: a safety and feasibility pilot study. Ann Intensive Care. 2018;8(1). 10.1186/s13613-017-0352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mazzeffi MA, Tanaka K, Roberts A, et al. Bleeding, Thrombosis, and Transfusion With Two Heparin Anticoagulation Protocols in Venoarterial ECMO Patients. J Cardiothorac Vasc Anesth. 2019; 33(5):1216–1220. 10.1053/j.jvca.2018.07.045 [DOI] [PubMed] [Google Scholar]
- 104.Carter KT, Kutcher ME, Shake JG, et al. Heparin-Sparing Anticoagulation Strategies Are Viable Options for Patients on Veno-Venous ECMO. J Surg Res. 2019;243:399–409. 10.1016/j.jss.2019.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung YS, Cho DY, Sohn DS, et al. Is Stopping Heparin Safe in Patients on Extracorporeal Membrane Oxygenation Treatment? ASAIO J. 2017;63(1):32–36. 10.1097/mat.0000000000000442 [DOI] [PubMed] [Google Scholar]


