Abstract
The immunoprevention of cancer and cancer recurrence is an important area of concern for the scientific community and society as a whole. Researchers have been working for decades to develop vaccines with the potential to alleviate these health care and economic burdens. So far, vaccines have made more progress in preventing cancer than in eliminating already established cancer. In particular, vaccines targeting oncogenic viruses, such as the human papillomavirus and the hepatitis B virus, are exceptional examples of successful prevention of virus-associated cancers, such as cervical cancer and hepatocellular carcinoma. Cancer-preventive vaccines targeting nonviral antigens, such as tumor-associated antigens and neoantigens, are also being extensively tested. Here, we review the currently approved preventive cancer vaccines; discuss the challenges in this field by covering ongoing preclinical and clinical human trials in various cancers; and address various issues related to maximizing cancer vaccine benefit.
Introduction
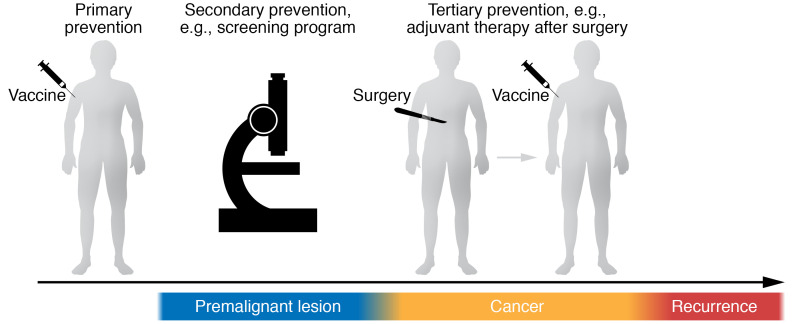
Globally, the number of new cancer cases is expected to reach nearly 22 million per year by 2030; the challenge of managing the escalating associated costs will be worldwide, not limited to high-income countries (1, 2). Thus, the development of cancer-preventive strategies is necessary to decrease human suffering and financial burden. Vaccines are a part of primary prevention to reduce premalignant and cancer occurrence, but also a part of tertiary prevention for cancer patients who have received curative treatment to reduce recurrence (secondary prevention involves tests, such as mammograms, to detect cancer at the earliest possible stage) (Figure 1). In 2019, the National Cancer Institute (NCI) budget included vaccines for cancer prevention as a priority area (3). The prevention component included identifying targets in precancerous lesions and developing early-intervention vaccines for known tumor antigens.
Figure 1. Model for prophylactic cancer vaccine.
Vaccines can work as primary prevention in healthy people to reduce premalignant and cancer occurrence and as tertiary prevention for cancer patients who received curative treatment to reduce recurrence.
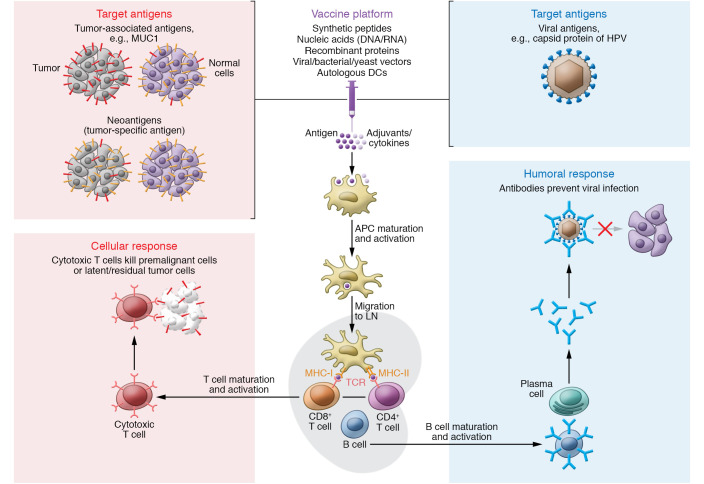
The basic concept of cancer prevention by vaccination involves harnessing our immune system to prevent cancer-causing viral infection through neutralizing the oncogenic virus to prevent uptake by target cells, or to attack premalignant and latent or residual cancer cells that are clinically inapparent (Figure 2). Currently, safe and effective licensed vaccines against human papillomavirus (HPV) (4, 5) and hepatitis B virus (HBV) (6) prevent virus-associated cervical cancer and hepatocellular carcinoma (HCC), respectively. Other oncogenic viruses, such as human T cell leukemia virus-1 (HTLV-1), Epstein-Barr virus (EBV), and polyoma virus, have recently seen renewed efforts related to vaccine development (7, 8), and also related to evaluating these vaccines’ impact on virus-associated malignancies (9, 10). Vaccines targeted toward well-known and personalized tumor antigens are also under investigation.
Figure 2. Basic mechanism of preventive cancer vaccine.
The vaccine’s antigen component is recognized and acquired by specialized antigen-presenting cells (APCs). Antigen-loaded APCs, such as dendritic cells (DCs), migrate to a draining lymph node (LN) to present the antigen via MHC class I and II to naive CD8+ or CD4+ T cells, respectively, with costimulatory molecules to induce T cell expansion or interaction with B cells. Eventually, cytotoxic T cells kill and eliminate the premalignant or clinically latent malignant cells expressing targeted antigen (cellular response, left), and plasma cells secrete antibodies that can neutralize oncogenic viruses by blocking virus–host cell interactions (humoral response, right).
Below, we review the current landscape of cancer vaccines that are approved and those under investigation. We also consider the role of vaccines in combination with other therapeutic components in attempts to maximize preventive effect.
Available prophylactic cancer vaccines targeting viral antigens
There are two types of licensed cancer prevention vaccines targeting oncogenic viruses: prophylactic vaccines for HPV and for HBV.
Prophylactic vaccines for HPV
HPV infection is one of the most common sexually transmitted diseases globally, and more than 70 million people are infected in the United States (11, 12). Over 200 HPV strains have been identified to date, and 14 of these (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) are known as high-risk/oncogenic HPV (hrHPV) (13–15). HPV oncoproteins E6 and E7 can inactivate tumor suppressor proteins p53 and pRb, respectively. Approximately 33,700 cancers are caused by HPV in the United States each year, including 12,900 oropharyngeal cancers, 10,800 cervical cancers, and 6000 anal cancers, comprising 5% of all human cancer diagnoses (16–19). HPV16 and HPV18 are the causative factors in most cases (73%–94%), while HPV16 and HPV18 along with five other hrHPV types (HPV types 31, 33, 45, 52, and 58) are responsible for up to 98% of HPV-related cases (19).
HPV-prophylactic vaccines against these hrHPV types are used to prevent persistent HPV infection and HPV-related cancers. HPV-prophylactic vaccines were developed to generate neutralizing antibodies against the virus-like particles derived from HPV’s capsid protein L1, which is well preserved in its genome (20). The Advisory Committee on Immunization Practices recommends routine HPV vaccination at age 11 to 12 years and catch-up HPV vaccination for all individuals through age 26. Catch-up vaccination is not recommended for adults over 26 years, as the benefit of HPV vaccination decreases in older age groups; however, the FDA approved the use of the vaccine in ages 9 to 45. The WHO guidelines recommend focusing HPV vaccination primarily on young girls, as females have ten times higher risk of HPV-related cancers than males, and high female vaccine coverage is expected to protect heterosexual males via herd immunity (21–23). Moreover, it was suggested that 12-year-old girls’ vaccination provides the best and most cost-effective solution against cervical cancer (24). The first commercial vaccines to prevent infection from HPV were Cervarix, a bivalent HPV vaccine protecting against HPV types 16 and 18, and Gardasil, a quadrivalent HPV vaccine targeting HPV types 16, 18, 6, and 11. In the United States, the 9-valent HPV vaccine (Gardasil 9) is now the most recent HPV vaccine, and it protects against oncogenic HPV types 16, 18, 31, 33, 45, 52, and 58 as well as non-oncogenic types 6 and 11 (25).
HPV vaccine efficacy was thoroughly investigated in several large randomized, placebo-controlled clinical trials with over 70,000 participants. The PATRICIA trial for Cervarix demonstrated 100% protection against HPV16 infection and 92.3% protection against HPV18 (26, 27). The FUTURE I/II trials for Gardasil prevented 98% of HPV16- and HPV18-related high-grade cervical lesions in participants not previously exposed to HPV16/18 (28–30). The Gardasil 9 vaccine study displayed equal efficacy to Gardasil in preventing diseases caused by HPV types 6, 11, 16, and 18, and 96% efficacy in preventing persistent infection and high-grade cervical, vulvar, and vaginal diseases related to HPV types 31, 33, 45, 52, and 58 (31). All three HPV vaccines are prepared from virus-like particles of the L1 protein.
Additionally, Gardasil showed 77.5% prevention of anal intraepithelial neoplasia among HPV-naive men (aged 16–26 years) who have sex with men (32). Cervarix also showed a 93% reduction in the prevalence of oral HPV16/18 infections after a 4-year vaccination period, suggesting that HPV vaccination could protect against the progression of oral cancers (33). As for the impact of the vaccines on cancer incidence, follow-up of two phase III trials of the Cervarix or Gardasil vaccine-trial cohorts from Finland was published as a cancer registry with more than 10 years of follow-up and over 3000 vaccinated women and 190,000 follow-up years (34). There were no cervical cancer cases or other HPV-related cancers (vulvar, vaginal, anal, or oropharyngeal cancers) among the vaccinated population, while there was incidence of 6.4 per 100,000 woman-years for cervical and 8.0 per 100,000 person-years for all HPV-associated cancers among the unvaccinated. Health economic models estimated that the number needed to vaccinate to prevent one case of anogenital warts, cervical intraepithelial neoplasia grade 2 or worse, or cervical cancer was 9, 22, and 202, respectively (35). Recently, Lei et al. used nationwide Swedish demographic and health registers to correlate Gardasil HPV vaccination with the invasive cervical cancer risk. An open population of 1,672,983 girls and women, aged 10 to 30 years, was evaluated for cervical cancer until age 31. Cervical cancer was diagnosed in 19 women who had received the Gardasil HPV vaccine and in 538 women who had not received it. Vaccinated women had a cumulative incidence of cervical cancer of 47 cases per 100,000 persons versus 94 cases per 100,000 persons among those unvaccinated. After adjustment for covariates, the incidence rate ratio was 0.12 (95% CI, 0.00–0.34) among women who had been vaccinated before the age of 17 years and 0.47 (95% CI, 0.27–0.75) among women who had been vaccinated at the age of 17 to 30 years (36). A simulation model predicted that cervical cancer could be eliminated (defined as 4 or fewer new cases per 100,000 women each year) by 2028 in Australia, and between 2038 and 2046 in the United States, if current vaccination rates and screening programs continue (37, 38). Importantly, a comparative modeling analysis predicted that if the WHO 90-70-90 triple-intervention strategy (combining intensive scaled-up HPV vaccination to 90% of the population, twice-lifetime cervical screening to 70%, and treatment of preinvasive lesions and invasive cancer to 90%) is achieved, the incidence of premature deaths due to cervical cancer would be reduced by 98.6% by 2120 in low-income and lower-middle-income countries (39).
Prophylactic vaccines for HBV
Chronic hepatitis B and hepatitis C are the most important causes of HCC and account for 80% of HCC cases globally (40, 41). Hepatitis B is caused by HBV. It is thought that HBV DNA contributes to carcinogenesis in multiple ways, including creating genomic instability caused by insertion to host genome, modulating the expression and activities of numerous genes, affecting DNA repair processes, and inhibiting apoptosis. Moreover, HBV can activate some nuclear transcription pathways, modulate transduction pathways, and inactivate or indirectly downregulate various tumor suppressors (e.g., p53) (42–45). In 2015, WHO estimated that approximately 3.6% of the world’s population (257 million) is chronically infected with HBV, defined as hepatitis B surface antigen–positive (HBsAg-positive) (46). More than 800,000 deaths occurred due to the spectrum of HBV-related illness, including HCC (46, 47). An estimated 800,000 new HCC cases occur each year; HCC is the fourth leading cause of cancer-related deaths overall worldwide, with chronic hepatitis B accounting for 18% (Europe) to 65% (China) of total cases (47). It is likely that HCC caused by HBV alone will result in 5 million deaths between 2015 and 2030 (45, 48).
Vaccination against HBV is the best protection against chronic HBV infection. The HBsAg contains multiple epitopes that elicit neutralizing antibodies, conferring protection from infection (49). Although HBV is classified into various serotypes, all HBV isolates share the same antigenic determinant; thus the hepatitis B vaccine is protective across subtypes (50). The first HBV vaccine, approved in the United States in 1981, was developed by purification of HBsAg from the plasma of asymptomatic HBsAg carriers who demonstrated high efficacy for preventing HBV infection (51–54). While the human plasma–derived hepatitis B vaccine is effective, its relatively high cost, as well as safety concerns associated with theoretical risks from HBV carriers who may be coinfected with HIV and other pathogens, limited its widespread use. Recombinant HBsAg synthesized in the yeast Saccharomyces cerevisiae had comparable quantity, quality, and specificity of anti-HBs response and similar protective efficacy compared with human plasma vaccine, leading to its licensing in the United States in 1986 (55–60). Since then, the recombinant vaccine has replaced the plasma HBV vaccine; all current HBV vaccines used worldwide are recombinant HBsAg, i.e., they are made from noninfectious parts of HBV using recombinant DNA technology. Engerix-B and Recombivax HB are approved in the United States for both pediatric and adult populations and are generally administered in three doses. Another two-dose hepatitis B vaccine, HEPLISAV-B, was licensed for adults in 2018 (5). It contains recombinant HBsAg combined with a TLR9 agonist adjuvant, stimulating B cells and plasmacytoid DCs by binding to TLR9 (61). Notably, HEPLISAV-B can provide a higher anti-HBs seroprotection rate (defined as anti-HBs titer >10 IU/L) compared with the conventional HBV vaccines (62–64). Commonly reported mild adverse events include injection site pain (3%–29%), erythema (3%), swelling (3%), and systemic adverse events, such as fever (1%–6%) and headache (3%) (65). HEPLISAV-B has a similar safety profile to Engerix-B (66).
There are two hepatitis B vaccination strategies: universal vaccination in all infants, and selective vaccination with concurrent use of HBV vaccine and hepatitis B immunoglobulin in HBV-exposed individuals, such as infants born to HBsAg-positive mothers (6). WHO recommends that all infants receive the vaccine against HBV as soon as possible after birth, preferably within 24 hours (67). In addition, children who did not receive the hepatitis B vaccine during infancy should receive a catch-up vaccination. Adults at risk of HBV exposure (e.g., health care workers, immunocompromised individuals, seronegative partners of those with chronic HBV infection) should be screened for markers of prior HBV infection and receive hepatitis B vaccine if seronegative (67, 68). It has been shown that both Recombivax HB and Engerix-B administered over three doses, and two doses of HEPLISAV-B, result in seroconversion rates of more than 90% in adults and seroprotection rates ranging from 88.5% to 95.8% in infants born to HBV-infected mothers (69–77). Because the response rate in healthy individuals is so high, routine post-vaccination testing in individuals receiving Recombivax HB and Engerix-B is not recommended. Moreover, studies demonstrated that the primary hepatitis B vaccination can provide long-term protective immunity, i.e., more than 30 years in children and adults (59).
The widespread use of the universal HBV vaccination program will likely contribute to the decline of chronic HBV infection and HCC. WHO estimated that the global prevalence of chronic HBV infection in children under 5 years of age was reduced from 4.7% in the pre-vaccine era to 1.3% in 2015 (67). Furthermore, HBsAg prevalence in the Western Pacific Region, where the birth-dose hepatitis B vaccine is routinely administered, dropped from 8.3% in the pre-vaccine era to 0.93% during 2002–2015 (78). As with HPV, historical comparison of immunized and unimmunized cohorts, at either the national or the community level, supports the hypothesis that HBV vaccination associates with a reduced HCC risk. One study with National Cancer Registry data from Taiwan, which adopted early universal HBV vaccination in children, showed that average annual HCC incidence in 6- to 14-year-olds declined from 0.70 (range, 0.65–0.78) per 100,000 children for the period from 1981 to 1986 (before widespread vaccination) to 0.36 (range, 0.23–0.48) between 1990 and 1994 (after initiation of widespread vaccination). The age-adjusted relative risk (RR) of HCC after as compared with before July 1990 was 0.33 (79). A long-term follow-up from this study added evidence of HBV vaccines preventing the occurrence of HCC; HCC incidence per 10,000 person-years was 0.92 in the unvaccinated cohort and 0.23 in the vaccinated birth cohorts (RR for HCC was 0.24) (80). The study of universal infant and catch-up plasma-derived HBsAg HBV vaccines for Native Alaskan people of the United States, initiated in 1984, revealed elimination of new HCC, with the incidence of HCC in individuals under 20 years decreased from 3 per 100,000 in 1984–1988 to zero in 1995–1999, and no cases have occurred since 1999 (81, 82). Moreover, studies from Korea and China have also indicated the efficacy of HBV vaccination programs to decrease HCC incidence (67, 83). Incomplete vaccination is one of the most crucial risk predictors for HCC (hazard ratio, 2.52; 95% CI, 1.25–5.05; P = 0.0094) (84).
Other antigens under development for cancer prevention
As with HPV and HBV, targeting other viral antigens could reduce the cancer burden. Hepatitis C virus (HCV), a single-stranded RNA virus, was classified in 1994 as an oncogenic virus, with 20% to 25% of HCC cases attributed to HCV infection (85, 86). The estimated global prevalence is 70 million to 170 million people worldwide, and there are 2 million new infections worldwide each year (87, 88). In 2016, the number of new HCV infections in the United States was 41,000 (89). Despite the advent of all-oral, interferon-sparing direct-acting antivirals (DAAs) to cure HCV infection, elimination of HCV and its related disease continues to face challenges, including reinfection due to insufficient immunity (10%–15% risk of HCV reinfection 5 years after successful antiviral treatment; ref. 90). Even after successful DAA treatment, HCC risk persists with an annual incidence of 1% in infected individuals (91), and it may become more prominent in patients displaying fibrosis and/or cirrhosis (92). Thus, a substantial number of the 2 million individuals infected annually with HCV worldwide will likely develop HCV-induced HCC over a period of 20 to 30 years. Moreover, 90% of people chronically infected with HCV worldwide are unaware of their infection, meaning that they could be an origin of infection and ultimately develop HCC in areas where screening systems are deficient. Consequently, a prophylactic vaccine is necessary for the global control of HCV. Models suggest that even a partially effective prophylactic HCV vaccine would decrease the prevalence of HCV infection (93, 94). However, barriers to development include limitations to HCV culture systems, virus diversity, and limited preclinical models, as well as an incomplete understanding of protective immune responses. Unfortunately, the HCV vaccine based on viral vectors expressing the nonstructural viral proteins failed to provide prophylactic properties compared with the placebo in a clinical trial (95, 96). Many studies are still under way to optimize the production, purification, and immunogenicity of HCV envelope proteins (97, 98). The presentation of these envelope proteins in a particulate form using ferritin-based nanoparticles may enhance the immunogenicity (99). Alternatively, a combined HBV/HCV vaccine could be produced by combination of the envelope proteins of both viruses, or by use of chimeric envelope particles (100, 101).
In 2009, the NCI listed a set of preferred tumor antigens that could be used in therapeutic vaccines based on accumulated knowledge (102). Although at that time, most therapeutic cancer vaccines had targeted tumor-associated antigens (TAAs), which are aberrantly expressed self-antigens in cancer cells, TAA-directed therapeutic cancer vaccines have generally had little success in clinical trials, despite detected immune responses (103, 104). Eventually, only one therapeutic vaccine based on a TAA was approved by the FDA: sipuleucel-T, an autologous DC vaccine targeting prostatic acid phosphatase (PAP), which is highly expressed in nearly all prostate cancer cells (95%). Sipuleucel-T was approved for the treatment of metastatic castration-resistant prostate cancer based on a 4.1-month increase in median survival (105, 106). Meanwhile, research on therapeutic vaccines provides a strong basis for developing prophylactic vaccines using the same list of candidate antigens.
Cancer Research UK launched a Grand Challenge in 2015 that includes a call for proposals for vaccines that work against nonviral cancers. Several studies in mice have shown the ability of tumor antigen–based vaccines to prevent cancer (107). Some examples of antigen-targeted vaccine platforms include shared mutations in driver genes, overexpressed immunogenic proteins, and modified shared antigens. Vaccination against mutant EGFR using a multipeptide vaccine decreased EGFR-driven lung carcinogenesis by 76.4% in an EGFR-mutant transgenic mouse model of human lung adenocarcinoma (108). The multivalent KRAS vaccine based on the peptides identified in a doxycycline-inducible KRAS G12D murine model can also inhibit more than 80% of KRAS-driven lung tumorigenesis, inducing predominantly Th1 T cell responses as opposed to eliciting Th2 responses (109). Moreover, vaccination focused on the common mutant H-ras epitope in 7,12-dimethylbenz(a)anthracene–induced (DMBA-induced) tumors, which are known to carry that mutation, worked in both preventive and therapeutic settings (110).
Over 80% of human cancers express a type I transmembrane glycoprotein, mucin 1 (MUC1; CD227), that can be a target for immune evasion mechanisms such as hypoglycosylation. The alteration attracts immature DCs to the tumor microenvironment but negatively affects their ability to stimulate Th1 T cell responses (111). The synthetic long peptide vaccine comprising immunogenic epitopes in MUC1 with a synthetic analog of double-stranded RNA (polyinosinic-polycytidylic acid stabilized with poly-l-lysine in carboxymethylcellulose [poly-ICLC]) was tested. Poly-ICLC activates DCs through signaling via TLR3 and cytoplasmic dsRNA sensors. The vaccine elicited high-titer IgG antibodies and a robust immune memory in almost half of the 39 evaluated subjects with a history of adenomatous polyps (112). Moreover, the vaccine-elicited antibodies had a range of affinities, similar to that reported for antiviral responses, and they specifically reacted with MUC1 on tumor, not normal, tissues (113). Clinical trials are under way to study the MUC1 peptide–poly-ICLC vaccine’s efficacy in preventing lung cancer in current and former smokers (114), as well as preventing recurrence of colon polyps in individuals with a history of an advanced adenoma, defined as being 1 cm or larger, having tubulovillous or villous histology, or having high-grade dysplasia (115).
Human epidermal growth factor receptor 2 (HER2) is a membrane tyrosine kinase, and aberrations of the HER2 gene commonly correspond with gain-of-function alterations leading to carcinogenesis. HER2 also represents one of the most studied TAAs. The decline of anti-HER2 responses was associated with progression from ductal carcinoma in situ (DCIS) to invasive breast cancer (116). Preoperative HER2-based vaccines in DCIS eliminated or decreased the size of DCIS and led to lymphocytic infiltration and loss of HER2 expression (117, 118). SOX2, an embryonic stem cell–associated antigen, is critical for self-renewal in embryonal stem cells, and dysregulation of SOX2 expression associates with a multitude of cancer types. The presence of a T cell response and preserved humoral responses against SOX2 emerged as an independent predictor of reduced risk for the progression of monoclonal gammopathy of undetermined significance (MGUS) to malignant myeloma (119). This finding also highlights the cancer-preventing potential of using a vaccine to boost preexisting immunity against the antigen observed in a precancerous setting. However, vaccination with TAA peptides has had limited clinical success, and the use of short peptides has been linked to immune tolerance. Overcoming immune tolerance to self-TAAs is another area of extensive research.
Challenges and perspectives for cancer vaccines
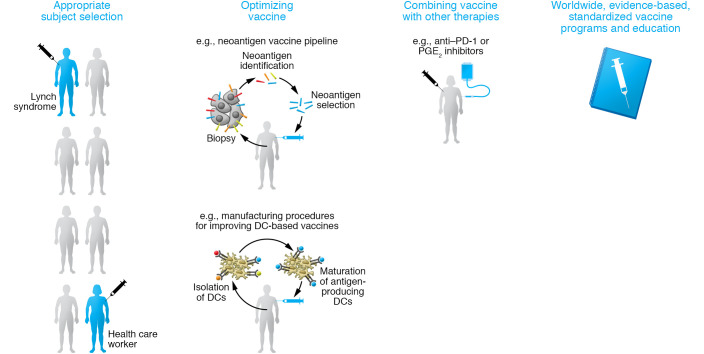
Next, we review more recent topics in the cancer vaccine field, including primary prevention for subjects harboring genetic risk for cancer, and the use of vaccines based on mutation-derived tumor antigens (neoantigens) as adjuvant therapy after curative treatment to reduce disease recurrence. Furthermore, we discuss the challenges of maximizing efficacy from the perspective of improving the vaccine itself (Figure 3).
Figure 3. Strategies for maximizing efficacy of cancer prevention by vaccine.
Strategies including appropriate selection of the subject who most likely benefits from the prophylactic cancer vaccines, optimization of the vaccine platform itself, combination of vaccine with other compounds, and spreading of vaccine based on proper understanding are essential to maximize the efficacy of vaccines in cancer prevention.
Targeting individuals with high genetic risk for cancer
Preventive measures to reduce cancer incidence in individuals with a high genetic risk include vaccination beyond screening practices, in which prophylactic surgery of potentially premalignant lesions is common. INO-5401 is a mixture of three synthetic DNA plasmids that target human telomerase reverse transcriptase (hTERT), Wilms’ tumor suppressor gene (WT-1), and prostate-specific membrane antigen (PSMA). It has been investigated as monotherapy and in combination with INO-9012, a synthetic DNA plasmid encoding IL-12, in carriers of germline mutations in the breast cancer 1 (BRCA1) or breast cancer 2 (BRCA2) genes, tumor suppressor genes for breast and ovarian cancers (120). These individuals have cumulative breast cancer and ovarian cancer risk to age 80 years of about 72% and 44%, respectively, for BRCA1, and 69% and 17%, respectively, for BRCA2 (121).
Heterozygous pathogenic germline mutations in one of the DNA mismatch repair (MMR) genes (MutL homolog 1 [MLH1], MutS homolog 2 [MSH2], MutS homolog 6 [MSH6], postmeiotic segregation 2 [PMS2]) or the loss of expression of MSH2 due to deletion in the EPCAM gene cause Lynch syndrome (LS), an autosomal dominant cancer syndrome associated with hereditary nonpolyposis colorectal cancer (CRC), endometrial carcinoma, and other malignancies. The estimated population frequency is at least 1 in 226 of the population carrying mutations in MMR genes (122–124). The total prevalence of LS among 450 CRCs diagnosed under age 50 years was 8.2% (125). Also, LS accounted for 1.8% of all endometrial cancer cases and 9% of endometrial cancer cases in women diagnosed younger than age 50 (126, 127). Patients with LS have a high mutation rate from coding microsatellite instability–induced (MSI-induced) shifts of the translational reading frame resulting in indels and giving rise to numerous neoepitopes (128, 129). A phase I/IIa trial with three frameshift peptide (FSP) neoantigens demonstrated safety and immunogenicity with robust cellular and humoral immune responses in all vaccinated patients (130). A preliminary study of the LS mouse model showed that vaccination with peptides encoding intestinal cancer FSP neoantigens (coding microsatellite mutations in the genes Nacad, Maz, Xirp1, and Senp6) promoted anti-neoantigen immunity, reduced intestinal tumorigenicity, and prolonged overall survival (131). Roudko, Bozkus, and colleagues recently revealed the widespread occurrence and strong immunogenicity of tumor-specific antigens that originated from indel mutations shared among patients with MSI-high endometrial, colorectal, and stomach cancer and are distinctly unlike self- and viral antigen. The findings could lead to the development of a universal off-the-shelf cancer vaccine for patients with MSI-high cancer as well as LS (132). Although not yet widely studied, targeting of individuals with other syndromes associated with genetic predisposition to cancer (e.g., Li-Fraumeni syndrome, neurofibromatosis, and Von Hippel–Lindau disease) is an area of increasing interest in the immuno-oncology field (133–135). Furthermore, the treatment of precancerous lesions (e.g., oral leukoplakia, Barrett’s esophagus, intraepithelial neoplasia, intraductal papillary mucinous neoplasms) constitutes an additional setting in which prophylactic cancer vaccination may play an important role. A peptide vaccine against the HPV16 oncoproteins E6 and E7 in women with HPV16-positive, high-grade vulvar intraepithelial neoplasia demonstrated immunological and clinical responses, including complete regression of the lesions in 47% of patients (136).
Cancer vaccines as adjuvant therapies after curative treatment
Although curative treatment represented by surgery is the primary option for clinically nonmetastatic localized tumors, residual cancer cells may lead to tumor recurrence. Cancer vaccines have been tested in the adjuvant setting after curative treatment to decrease the risk of post-treatment recurrence (tertiary prevention). The highly immunogenic melanoma cancer-testis antigen NY-ESO-1 was incorporated into adjuvant vaccine trials for patients with resected melanoma. It showed tumor-specific immunogenic responses and decreased risk of recurrence and death (137–139). Several studies have tested one to seven RAS peptides as monotherapy in the setting of adjuvant therapy in resected pancreas cancer; 58% to 100% of patients mounted an immune response, and five of these patients survived more than 5 years (140). Furthermore, a KRAS-targeting vaccine combining TG01 (a mixture of seven synthetic RAS peptides representing the seven most common codon 12 and 13 oncogenic mutations in KRAS) with human GM-CSF was evaluated in combination with standard postoperative chemotherapy (gemcitabine) as adjuvant therapy for patients with resected pancreatic cancer. The clinical efficacy endpoints compared favorably with published data for adjuvant gemcitabine (141).
Seviprotimut-L, an allogeneic, polyvalent, alum-adjuvanted vaccine derived from three human melanoma cell lines, was similarly tested in a phase III trial in stage IIB–III melanoma patients. Although the trial was not positive in the intent-to-treat population, patients with stage IIB/IIC (particularly those younger than 60 and with ulcerated primary stage IIB/IIC melanoma) had longer recurrence-free survival (142). Notably, DC vaccines loaded with autologous tumor lysates were used in a cohort of completely resected liver metastasis of colon adenocarcinoma; this cohort showed fewer and later relapses in the vaccine arm, suggesting that the strategy might play a role in more disseminated disease (143). In addition, recent data show that the post-treatment adjuvant HPV vaccine’s effectiveness contributes to decreased risk of recurrence of HPV-associated lesions, such as cervical and anal intraepithelial neoplasia (144–146).
Mutation-derived tumor antigens, due to somatic mutations, can be unique to a patient’s tumor and are also one of the key targets for vaccine therapy (neoantigen vaccine) (147–149). Tumor tissue resected by curative surgery enables the identification of neoantigens using next-generation sequencing and computational pipelines, enabling the use of the personalized neoantigen vaccine. Vaccines tested in melanoma and glioblastoma were feasible and immunogenic, and might contribute to the immunological elimination of residual cancer as well as the favorable response of anti–PD-1 therapy even after disease recurrence (150–153). Various trials have been conducted in the adjuvant setting after curative treatment across cancer types (Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI146956DS1).
The combination of vaccine and other compounds
To enhance immune-preventive efficacy, the combination of a cancer vaccine with other compounds has been tested. An NSAID, naproxen, which is expected to have chemopreventive efficacy in preventing CRC in LS patients through an immune interaction in colorectal mucosa (154), increased T cell immunity against neoantigens and prolonged survival compared with FSP vaccine alone (131).
With the success of immune checkpoint inhibitors, especially PD-1/PD-L1 inhibitors, in subsets of patients with advanced cancer (155), there are some hypotheses that these therapies can also reduce cancer risk. In a mouse model, anti–PD-1 treatment markedly prevented the development and progression of carcinogen-induced oral premalignant lesions, increased effector T cells, and increased apoptosis of lesion cells (156). Mancuso et al. described an individual with Muir-Torre syndrome (a variant of LS) with a 19-year history of 136 neoplastic lesions, showing that anti–PD-1 (pembrolizumab) inhibited the development of a new neoplasm for 22 months following treatment (157). The addition of PD-1 inhibition to vaccines against several types of antigens has shown potential benefit in different tumor types. Those antigens include HPV (e.g., GX-188E, a therapeutic DNA vaccine targeting HPV16/18) (158), immunomodulatory enzymes and molecules (e.g., O102/IO103, a peptide vaccine containing the immunomodulatory enzyme indoleamine 2,3-dioxygenase [IDO] and a PD-L1–derived long peptide) (159), and neoantigens (e.g., NEO-PV-01, a personalized neoantigen vaccine) (160). These combinations are shifting to the adjuvant setting, i.e., vaccination after surgery to reduce the postoperative recurrence. One trial will evaluate atezolizumab every 3 weeks plus up to ten doses of personalized neoantigen–based vaccination (PGV001) plus poly-ICLC in 15 patients with urothelial cancer (adjuvant or metastatic) (NCT03359239) (161). In 2019, a randomized phase II study with mRNA-4157, an mRNA-based personalized cancer vaccine targeting 20 TAAs specifically expressed by the patients’ cancer cells, in combination with pembrolizumab was initiated for melanoma patients with complete resection who have a high risk of recurrence (NCT03897881/KEYNOTE-942) (162). This trial includes a control arm with pembrolizumab alone and aims to recruit 150 patients by December 2021 (Supplemental Table 1).
Other factors for effective cancer prevention by vaccine
Vaccine platforms.
In addition to appropriately selecting subjects, efforts to optimize vaccines (163–165) include the design of effective vaccine adjuvants, the optimization of adjuvant formulations, and the use of antigen delivery systems (166, 167).
In 2016, BioNTech reported that adjusting the net charge of lipid carriers optimized the expression of mRNA in DCs in vivo following intravenously administered RNA-lipoplexes (RNA-LPXs) (168). The LPX protects RNA from extracellular ribonucleases and mediates efficient RNA uptake and expression of the encoded antigen by DCs and macrophages in lymphoid compartments. These universally applicable techniques were also used in the development of coronavirus disease 2019 (COVID-19) vaccine trials. BNT162b1, a lipid nanoparticle–formulated nucleoside-modified mRNA that encodes the SARS-CoV-2 spike protein, induced robust and specific antibody, as well as favorable Th1 T cell responses (169). An intravenously administered RNA-LPX vaccine encoding four nonmutated TAAs (NY-ESO-1, melanoma-associated antigen A3 [MAGE-A3], tyrosinase, and transmembrane phosphatase with tensin homology [TPTE]) alone or in combination with PD-1 inhibition mediated durable objective responses with an induction of strong T cell immunity against those antigens in checkpoint inhibitor–experienced patients with unresectable melanoma (170). Our group recently reported a regimen of fms-like tyrosine kinase 3 ligand (Flt3L; a drug known as CDX-301), poly-ICLC, and a vaccine comprising anti–DEC-205–NY-ESO-1, a fusion antibody targeting CD205 linked to NY-ESO-1. Sixty high-risk melanoma patients were randomized to receive the vaccine, with or without CDX-301, in a postoperative setting. Results of this phase II randomized double-blind trial showed that adding Flt3L to the treatment strategy effectively increased DC populations and increased T cell responses compared with the control arm (139).
Head-to-head clinical trials to investigate the optimal vaccine platform are still lacking. Also, the optimal vaccination route for cancer vaccines to elicit robust immunological and clinical responses is unknown. Historically, cancer vaccines were investigated as subcutaneous and intradermal injections. However, mucosal vaccines are gaining momentum in the scientific community, and since gastroenteric pathogens, e.g., Helicobacter pylori, cause chronic infections that can lead to peptic ulcers and gastric cancer, such vaccines would likely have an impact on the prevalence of certain cancers (171).
Antigen selection.
Advances in antigen selection are also proceeding rapidly. For example, advances in computational analysis (bioinformatics pipelines) have improved identification of epitopes for patient-specific neoantigen vaccines. Continued improvements include precise HLA haplotyping, appropriate somatic variant calling, and gene and transcript expression, as well as a reliable assessment of HLA-based agretopicity, a value defined by the ratio of mutant to wild-type peptide binding affinity. Recent mass spectrometry approaches using nano–ultra-performance liquid chromatography coupled to high-resolution mass spectrometry (nUPLC–MS/MS) and algorithm interpretations that assign mass spectra to amino acids can isolate and characterize MHC-bound peptides (172). Also recently, mass spectrometry–based proteogenomics approaches were shown to identify shared and tumor-specific non-canonical HLA peptides (173). Escalating these types of analyses to clinical samples will be a tremendous advancement. These algorithms must be clinically validated by ongoing neoantigen trials to refine their accuracy. Furthermore, Wells et al. recently reported that a global consortium wherein each participant predicted immunogenic epitopes from shared tumor sequencing data was able to develop a model of tumor epitope immunogenicity that filtered out 98% of non-immunogenic peptides with a precision above 0.70. This data resource available among the research community enables the identification of parameters underlying effective antitumor immunity (174).
Vaccination strategies.
Although there are effective vaccines for preventing cancer, there are many factors that influence the efficacy of vaccination strategies. A recently reported randomized trial to estimate whether targeted educational interventions can increase HPV vaccine acceptability and knowledge among young women showed that 51.7% of participants in the educational video arm were willing to accept the HPV vaccine compared with 33.3% and 28.2% of participants in the educational handout and control arms, respectively (P < 0.01). Both interventions were reported as helpful in learning (97.7% vs. 92.9%, P = 0.15), but the educational video was more likely to be helpful in deciding on vaccination (86.2% vs. 70.2%, P < 0.01) (175). Moreover, 6-month increases in HPV vaccination coverage were larger for patients in clinics that received announcement training versus those in control clinics (5.4% difference; 95% CI, 1.1%–9.7% (176). These results suggest that educational interventions strongly influence HPV vaccine coverage. More broadly, there is a worldwide need for standardized vaccination strategies and educational programs, but also for rigorous data interpretation, especially regarding adverse events. In general, HPV national programs cover about 30% of the global target population, with low full-dose coverage in many regions (177). Most low- and middle-income countries remain unprotected; only about 1% of adolescent females in low-income countries received a full HPV vaccine course (177). The implementation of an organized vaccine program is urgently needed for public health intervention in these countries. Moreover, governmental policy had a substantial effect on vaccine coverage. In Japan, the HPV vaccine stopped being recommended after a cloud of suspicion surrounding adverse events (e.g., complex regional pain syndrome [ref. 178], demyelinating diseases [ref. 179]) in 2013 (180). The vaccination rate reverted from nearly 70% to almost zero for girls born in 2000 and after, as they refused to be vaccinated. Despite the absence of evidence of a link with HPV vaccination in subsequent domestic surveys (181, 182), the policy remains unchanged. Simms et al. reported an additional 24,600–27,300 cases and 5000–5700 deaths over the lifetime of cohorts born between 1994 and 2007 due to negligible coverage since 2013 (183). Another recent modeling study estimated that the increase in future annual cervical cancer cases and related deaths attributable to this policy decision would exceed 3500 and 1000, respectively, for individuals born in the early 2000s (184). Actions to further strong evidence-based policy are essential to avoid preventable cancer suffering and related deaths.
Conclusion
The impact of preventive cancer vaccines against viral antigens is linked to the dramatic drop in the incidence of corresponding virus-associated cancers. Revolutionary work is also being done in the field of nonviral antigen–targeting vaccines aimed at preventing cancer development or recurrence. Still, there is room to optimize the vaccine platforms themselves. This includes research about their immunogenicity and side-by-side comparisons to ascertain their effects and to select the population and setting most likely to benefit from a certain strategy, as well as development of suitable combinations with other compounds. Meanwhile, continuous efforts are necessary to maximize the benefit of the vaccine both at the level of the individual and across the general population. Once these goals are achieved, cancer immunoprevention by vaccination will no longer be a theoretical concept but rather will be the standard of long-term cancer treatment.
Supplementary Material
Acknowledgments
This work was funded by the Melanoma Research Alliance, NIH grant R01CA180913, and The Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine at Mount Sinai. We thank Leonardo Imatani and Emily Mongeau for excellent administrative assistance.
Version 1. 05/03/2021
Electronic publication
Footnotes
Conflict of interest: AM has received speaker or consultant fees from AbbVie, Almirall, Novartis, Bristol Myers Squibb, Pfizer, and Roche. AM is employed by Novartis; the work described in this publication was completed prior to his employment at Novartis. NB is an extramural member of the Parker Institute for Cancer Immunotherapy, receives research funds from Regeneron, Harbor Biomedical, and Dragonfly Therapeutics, and is on the advisory boards of Neon Therapeutics, Novartis, Avidea, Boehringer Ingelheim, Rome Therapeutics, Roswell Park Comprehensive Cancer Center, BreakBio, Carisma Therapeutics, CureVac, Genotwin, BioNTech, Gilead Therapeutics, Tempest Therapeutics, and the Cancer Research Institute.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(9):e146956.https://doi.org/10.1172/JCI146956.
Contributor Information
Tomohiro Enokida, Email: tomohiro.enokida@gmail.com.
Alvaro Moreira, Email: alvaro.moreira@mssm.edu.
Nina Bhardwaj, Email: Nina.Bhardwaj@mssm.edu.
References
- 1.Bray F, et al. Long-term realism and cost-effectiveness: primary prevention in combatting cancer and associated inequalities worldwide. J Natl Cancer Inst. 2015;107(12):djv273. doi: 10.1093/jnci/djv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bray F, Soerjomataram I. The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. In Gelband H, et al., eds. Cancer: Disease Control Priorities. Vol 3, 3rd ed. 2015:23–44. [PubMed] [Google Scholar]
- 3. National Cancer Institute. About the Annual Plan and Budget Proposal. https://www.cancer.gov/about-nci/budget/about-annual-plan#:~:text=About%20the%20Annual%20Plan%20and,most%20rapid%20progress%20against%20cancer Accessed March 17, 2021.
- 4.Markowitz LE, et al. Human papillomavirus vaccine introduction — the first five years. Vaccine. 2012;30(suppl 5):F139–F148. doi: 10.1016/j.vaccine.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 5.A two-dose hepatitis B vaccine for adults (Heplisav-B) JAMA. 2018;60(1539):17–18. doi: 10.1001/jama.2018.1097. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Yu H. Prevention strategies of mother-to-child transmission of hepatitis B virus (HBV) infection. Pediatr Investig. 2020;4(2):133–137. doi: 10.1002/ped4.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zyl DG, et al. Progress in EBV vaccines. Front Oncol. 2019;9:104. doi: 10.3389/fonc.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam S, et al. Predicting and designing epitope ensemble vaccines against HTLV-1. J Integr Bioinform. 2020;16(4):20180051. doi: 10.1515/jib-2018-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rühl J, et al. Vaccination against the Epstein-Barr virus. Cell Mol Life Sci. 2020;77(21):4315–4324. doi: 10.1007/s00018-020-03538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabachnick-Cherny S, et al. Polyomavirus-driven Merkel cell carcinoma: prospects for therapeutic vaccine development. Mol Carcinog. 2020;59(7):807–821. doi: 10.1002/mc.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaisamrarn U, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PLoS One. 2013;8(11):e79260. doi: 10.1371/journal.pone.0079260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meites E, et al. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. International Agency for Research on Cancer. Chemical Agents and Related Occupations. WHO; 2012. [Google Scholar]
- 14.Muñoz N, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 15.Bihl MP, et al. Human papillomavirus (HPV) detection in cytologic specimens: similarities and differences of available methodology. Appl Immunohistochem Mol Morphol. 2017;25(3):184–189. doi: 10.1097/PAI.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: a review. World J Gastrointest Surg. 2016;8(1):41–51. doi: 10.4240/wjgs.v8.i1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brianti P, et al. Review of HPV-related diseases and cancers. New Microbiol. 2017;40(2):80–85. [PubMed] [Google Scholar]
- 18.Näsman A, et al. A global epidemic increase of an HPV-induced tonsil and tongue base cancer — potential benefit from a pan-gender use of HPV vaccine. J Intern Med. 2020;287(2):134–152. doi: 10.1111/joim.13010. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention. Cancers Associated With Human Papillomavirus, United States—2011–2015. USCS data brief, no. 4. CDC; 2018. [Google Scholar]
- 20.Buck CB, et al. The papillomavirus major capsid protein L1. Virology. 2013;445(1–2):169–174. doi: 10.1016/j.virol.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization Human papillomavirus vaccines: WHO position paper. Wkly Epidemiol Rec. 2017;92(19):241–268. doi: 10.1016/j.vaccine.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 22.Bogaards JA, et al. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ. 2015;350:h2016. doi: 10.1136/bmj.h2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Comprehensive Cervical Cancer Control: A Guide to Essential Practice. WHO; 2014. [PubMed] [Google Scholar]
- 24.Taira AV, et al. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004;10(11):1915–1923. doi: 10.3201/eid1011.040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuzick J. Gardasil 9 joins the fight against cervix cancer. Expert Rev Vaccines. 2015;14(8):1047–1049. doi: 10.1586/14760584.2015.1051470. [DOI] [PubMed] [Google Scholar]
- 26.Paavonen J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 27.Lehtinen M, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 28.Garland SM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 29.Muñoz N, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on All HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 30.FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 31.Joura EA, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 32.Palefsky JM, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 33.Herrero R, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7):e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luostarinen T, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186–2187. doi: 10.1002/ijc.31231. [DOI] [PubMed] [Google Scholar]
- 35. Chesson HW. Overview of Health Economic Models for HPV Vaccination of Mid-Adult. CDC; 2019. [Google Scholar]
- 36.Lei J, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 37.Hall MT, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4(1):e19–e27. doi: 10.1016/S2468-2667(18)30183-X. [DOI] [PubMed] [Google Scholar]
- 38.Burger EA, et al. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):e213–e222. doi: 10.1016/S2468-2667(20)30006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Canfell K, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seeger C, et al. Hepadnaviruses. In: Knipe DM, Howley PM, eds. Fields Virology. 6th ed. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 43.Zoulim F, et al. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994;68(3):2026–2030. doi: 10.1128/JVI.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 suppl):S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 45.Yang JD, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. World Health Organization. Hepatitis B. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Updated July 27, 2020. Accessed March 18, 2021.
- 47.Schweitzer A, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. WHO; 2016. [Google Scholar]
- 49.Gerin JL, et al. Chemically synthesized peptides of hepatitis B surface antigen duplicate the d/y specificities and induce subtype-specific antibodies in chimpanzees. Proc Natl Acad Sci U S A. 1983;80(8):2365–2369. doi: 10.1073/pnas.80.8.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szmuness W, et al. Hepatitis B vaccine in medical staff of hemodialysis units. N Engl J Med. 1982;307(24):1481–1486. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- 51.Szmuness W, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- 52.Stevens CE, et al. Hepatitis B vaccine: immune responses in haemodialysis patients. Lancet. 1980;2(8206):1211–1213. doi: 10.1016/s0140-6736(80)92477-0. [DOI] [PubMed] [Google Scholar]
- 53.Maupas P, et al. Efficacy of hepatitis B vaccine in prevention of early HBsAg carrier state in children. Controlled trial in an endemic area (Senegal) Lancet. 1981;1(8215):289–292. doi: 10.1016/s0140-6736(81)91908-5. [DOI] [PubMed] [Google Scholar]
- 54.Krugman S. The newly licensed hepatitis B vaccine. Characteristics and indications for use. JAMA. 1982;247(14):2012–2015. [PubMed] [Google Scholar]
- 55.Valenzuela P, et al. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298(5872):347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 56.Scolnick EM, et al. Clinical evaluation in healthy adults of a hepatitis B vaccine made by recombinant DNA. JAMA. 1984;251(21):2812–2815. [PubMed] [Google Scholar]
- 57.Jilg W, et al. Clinical evaluation of a recombinant hepatitis B vaccine. Lancet. 1984;2(8413):1174–1175. doi: 10.1016/s0140-6736(84)92740-5. [DOI] [PubMed] [Google Scholar]
- 58.Davidson M, Krugman S. Immunogenicity of recombinant yeast hepatitis B vaccine. Lancet. 1985;63(10):1021–1051. doi: 10.1016/S0140-6736(85)92000-8. [DOI] [PubMed] [Google Scholar]
- 59.Bruce MG, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 60.Yeoh EK, et al. Efficacy and safety of recombinant hepatitis B vaccine in infants born to HBsAg-positive mothers. J Infect. 1986;13(suppl A):15–18. doi: 10.1016/s0163-4453(86)92608-3. [DOI] [PubMed] [Google Scholar]
- 61.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 62.Sablan BP, et al. Demonstration of safety and enhanced seroprotection against hepatitis b with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine. 2012;30(16):2689–2696. doi: 10.1016/j.vaccine.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Heyward WL, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31(46):5300–5355. doi: 10.1016/j.vaccine.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 64.Janssen JM, et al. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18-70 years of age. Vaccine. 2015;33(31):3614–3618. doi: 10.1016/j.vaccine.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 65. World Health Organization. Information Sheet. Observed Rate Of Vaccine Reactions: Hepatitis B Vaccine. https://www.who.int/initiatives/the-global-vaccine-safety-initiative/tools-and-methods/reaction-rates-information-sheets. Updated May 13, 2020. Accessed September 17, 2020.
- 66.Hyer R, et al. Safety of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant in adults. Vaccine. 2018;36(19):2604–2611. doi: 10.1016/j.vaccine.2018.03.067. [DOI] [PubMed] [Google Scholar]
- 67.Wu QJ, et al. Cancer incidence among adolescents and young adults in urban Shanghai, 1973–2005. PLoS One. 2012;7(8):e42607. doi: 10.1371/journal.pone.0042607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abara WE, et al. Hepatitis B vaccination, screening and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794–804. doi: 10.7326/M17-1106. [DOI] [PubMed] [Google Scholar]
- 69.Zahradnik JM, et al. Hepatitis B vaccine: immune responses in children from families with an HBsAg carrier. Vaccine. 1985;3(5):407–413. doi: 10.1016/0264-410X(85)90132-X. [DOI] [PubMed] [Google Scholar]
- 70.Perrin J, et al. Hepatitis B immunization of newborns according to a two dose protocol. Vaccine. 1986;4(4):241–244. doi: 10.1016/0264-410X(86)90137-4. [DOI] [PubMed] [Google Scholar]
- 71.Giammanco G, et al. Immune response to simultaneous administration of a recombinant DNA hepatitis B vaccine and multiple compulsory vaccines in infancy. Vaccine. 1991;9(10):747–750. doi: 10.1016/0264-410X(91)90291-D. [DOI] [PubMed] [Google Scholar]
- 72.Koff RS. Immunogenicity of hepatitis B vaccines: implications of immune memory. Vaccine. 2002;20(31–32):3695–3701. doi: 10.1016/s0264-410x(02)00405-x. [DOI] [PubMed] [Google Scholar]
- 73.Hu Y, et al. Influence of maternal antibody against hepatitis B surface antigen on active immune response to hepatitis B vaccine in infants. Vaccine. 2008;26(48):6064–6067. doi: 10.1016/j.vaccine.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 74.Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50(4):805–816. doi: 10.1016/j.jhep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Huang LM, et al. Hepatitis B virus infection, its sequelae, and prevention by vaccination. Curr Opin Immunol. 2011;23(2):237–243. doi: 10.1016/j.coi.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 76.Halperin SA, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18-55 years of age. Vaccine. 2012;30(15):2556–2563. doi: 10.1016/j.vaccine.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 77.Huang H, et al. Comparison of antibody response to hepatitis B vaccination in infants with positive or negative maternal hepatitis B e antigen (HBeAg) in cord blood: implication for the role of HBeAg as an immunotolerogen. Hum Vaccin Immunother. 2019;15(9):2183–2186. doi: 10.1080/21645515.2019.1575712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wiesen E, et al. Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990-2014. Vaccine. 2016;34(25):2855–2862. doi: 10.1016/j.vaccine.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 79.Chang MH, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336(26):1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 80.Chang MH, et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology. 2016;151(3):472–480. doi: 10.1053/j.gastro.2016.05.048. [DOI] [PubMed] [Google Scholar]
- 81.Harpaz R, et al. Elimination of new chronic hepatitis B virus infections: results of the Alaska immunization program. J Infect Dis. 2000;181(2):413–418. doi: 10.1086/315259. [DOI] [PubMed] [Google Scholar]
- 82.McMahon BJ, et al. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54(3):801–807. doi: 10.1002/hep.24442. [DOI] [PubMed] [Google Scholar]
- 83.Gwack J, et al. Hepatitis B vaccination and liver cancer mortality reduction in Korean children and adolescents. Asian Pac J Cancer Prev. 2011;12(9):2205–2208. [PubMed] [Google Scholar]
- 84.Chien YC, et al. Incomplete hepatitis B immunization, maternal carrier status, and increased risk of liver diseases: a 20-year cohort study of 3.8 million vaccinees. Hepatology. 2014;60(1):125–132. doi: 10.1002/hep.27048. [DOI] [PubMed] [Google Scholar]
- 85.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3(2):47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balsano C, Alisi A. Hepatitis C virus (HCV): an RNA virus with a pro-oncogenic potential. Dig Liver Dis. 2007;39(suppl 1):S46–S51. doi: 10.1016/s1590-8658(07)80010-7. [DOI] [PubMed] [Google Scholar]
- 87.Blach S, et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 88. World Health Organization. Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-C Accessed November 12, 2020.
- 89.Chhatwal J, Sussman NL. Universal screening for hepatitis C: an important step in virus elimination. Clin Gastroenterol Hepatol. 2019;17(5):835–837. doi: 10.1016/j.cgh.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Simmons B, et al. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(6):683–694. doi: 10.1093/cid/civ948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanwal F, et al. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 92.Hamdane N, et al. HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology. 2019;156(8):2313–2329. doi: 10.1053/j.gastro.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stone J, et al. The potential impact of a hepatitis C vaccine for people who inject drugs: is a vaccine needed in the age of direct-acting antivirals? PLoS One. 2016;11(5):e0156213. doi: 10.1371/journal.pone.0156213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Echevarria D, et al. Modeling indicates efficient vaccine-based interventions for the elimination of hepatitis C virus among persons who inject drugs in Metropolitan Chicago. Vaccine. 2019;37(19):2608–2616. doi: 10.1016/j.vaccine.2019.02.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Swadling L, et al. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6(261):261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. National Institute of Allergy and Infectious Diseases. Trial Evaluating Experimental Hepatitis C Vaccine Concludes. NIH; 2019. [Google Scholar]
- 97.Logan M, et al. Native folding of a recombinant gpE1/gpE2 heterodimer vaccine antigen from a precursor protein fused with Fc IgG. J Virol. 2017;91(1):JVI.01552-16. doi: 10.1128/JVI.01552-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vietheer PT, et al. The core domain of hepatitis C virus glycoprotein E2 generates potent cross-neutralizing antibodies in guinea pigs. Hepatology. 2017;65(4):1117–1131. doi: 10.1002/hep.28989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan Y, et al. A nanoparticle-based hepatitis C virus vaccine with enhanced potency. J Infect Dis. 2020;221(8):1304–1314. doi: 10.1093/infdis/jiz228. [DOI] [PubMed] [Google Scholar]
- 100.Beaumont E, et al. Chimeric hepatitis B virus/hepatitis C virus envelope proteins elicit broadly neutralizing antibodies and constitute a potential bivalent prophylactic vaccine. Hepatology. 2013;57(4):1303–1313. doi: 10.1002/hep.26132. [DOI] [PubMed] [Google Scholar]
- 101.Ortiz E, et al. Effectiveness of interventions for hepatitis B and C: a systematic review of vaccination, screening, health promotion and linkage to care within higher income countries. J Community Health. 2020;45(10):201–218. doi: 10.1007/s10900-019-00699-6. [DOI] [PubMed] [Google Scholar]
- 102.Cheever MA, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan AC, et al. A quantitative analysis of therapeutic cancer vaccines in phase 2 or phase 3 trial. J Immunother Cancer. 2015;3:48. doi: 10.1186/s40425-015-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janowitz T, et al. Cross-sectional and longitudinal analysis of cancer vaccination trials registered on the US Clinical Trials Database demonstrates paucity of immunological trial endpoints and decline in registration since 2008. Drug Des Devel Ther. 2014;8:1539–1553. doi: 10.2147/DDDT.S65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 106.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–2526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 107.Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18(3):183–194. doi: 10.1038/nri.2017.140. [DOI] [PubMed] [Google Scholar]
- 108.Ebben JD, et al. Epidermal growth factor receptor derived peptide vaccination to prevent lung adenocarcinoma formation: an in vivo study in a murine model of EGFR mutant lung cancer. Mol Carcinog. 2016;55(11):1517–1525. doi: 10.1002/mc.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan J, et al. Immunoprevention of KRAS-driven lung adenocarcinoma by a multipeptide vaccine. Oncotarget. 2017;8(47):82689-82699. doi: 10.18632/oncotarget.19831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nasti TH, et al. Immunoprevention of chemical carcinogenesis through early recognition of oncogene mutations. J Immunol. 2015;194(6):2683–2695. doi: 10.4049/jimmunol.1402125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carlos CA, et al. Human tumor antigen MUC1 is chemotactic for immature dendritic cells and elicits maturation but does not promote Th1 type immunity. J Immunol. 2005;175(3):1628–1635. doi: 10.4049/jimmunol.175.3.1628. [DOI] [PubMed] [Google Scholar]
- 112.Kimura T, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res. 2013;6(1):18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lohmueller JJ, et al. Antibodies elicited by the first non-viral prophylactic cancer vaccine show tumor-specificity and immunotherapeutic potential. Sci Rep. 2016;6:31740. doi: 10.1038/srep31740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Finn OJ, et al. Abstract CT222: A pilot study of a MUC1 vaccine in current and former smokers at high risk for lung cancer. Cancer Res. 2019;79(13):CT222 [Google Scholar]
- 115.Finn OJ, et al. Abstract CT236: Randomized, double-blind, placebo-controlled trial of preventative MUC1 vaccine in patients with newly diagnosed advanced adenomas: results from one-year booster. Cancer Res. 2019;79(13):CT236 [Google Scholar]
- 116.Gil Del Alcazar CR, et al. Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov. 2017;7(10):1098–1115. doi: 10.1158/2159-8290.CD-17-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Czerniecki BJ, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 118.Sharma A, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer. 2012;118(17):4354–4362. doi: 10.1002/cncr.26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dhodapkar MV, et al. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood. 2015;126(22):2475–2478. doi: 10.1182/blood-2015-03-632919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. ClinicalTrials.gov. INO 5401 Vaccination in BRCA1/2 Mutation Carriers. https://ClinicalTrials.gov/show/NCT04367675 Updated April 29, 2020. Accessed November 12, 2020.
- 121.Kuchenbaecker KB, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 122.Win AK, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(3):404–412. doi: 10.1158/1055-9965.EPI-16-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hampel H, de la Chapelle A. How do we approach the goal of identifying everybody with Lynch syndrome? Fam Cancer. 2013;12(2):313–317. doi: 10.1007/s10689-013-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haraldsdottir S, et al. Comprehensive population-wide analysis of Lynch syndrome in Iceland reveals founder mutations in MSH6 and PMS2. Nat Commun. 2017;8:14755. doi: 10.1038/ncomms14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pearlman R, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 2017;3(4):464–471. doi: 10.1001/jamaoncol.2016.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hampel H, et al. Comment on: Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res. 2007;67(19):9603. doi: 10.1158/0008-5472.CAN-07-2308. [DOI] [PubMed] [Google Scholar]
- 127.Lu KH, et al. Prospective determination of prevalence of Lynch syndrome in young women with endometrial cancer. J Clin Oncol. 2007;25(33):5158–5164. doi: 10.1200/JCO.2007.10.8597. [DOI] [PubMed] [Google Scholar]
- 128.von Knebel Doeberitz M, Kloor M. Towards a vaccine to prevent cancer in Lynch syndrome patients. Fam Cancer. 2013;12(2):307–312. doi: 10.1007/s10689-013-9662-7. [DOI] [PubMed] [Google Scholar]
- 129.Kloor M, von Knebel Doeberitz M. The immune biology of microsatellite-unstable cancer. Trends Cancer Res. 2016;2(3):121–133. doi: 10.1016/j.trecan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Kloor M, et al. Vaccination of MSI-H colorectal cancer patients with frameshift peptide antigens: a phase I/IIa clinical trial. J Clin Orthod. 2015;33(15):3020–3020. doi: 10.1200/jco.2015.33.15_suppl.3020. [DOI] [Google Scholar]
- 131.Gelincik O, et al. Abstract 2732: Frameshift neoantigen vaccination prevent Lynch syndrome mouse model intestinal cancer. Cancer Res. 2019;79(13):2732–2732. [Google Scholar]
- 132.Roudko V, et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell. 2020;183(6):1634–1649. doi: 10.1016/j.cell.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tamura R, et al. A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2. Nat Commun. 2019;10(1):5758. doi: 10.1038/s41467-019-13640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moreira A, et al. Effective anti-programmed death-1 therapy in a SUFU-mutated patient with gorlin-goltz syndrome. Br J Dermatol. 2018;179(3):747–749. doi: 10.1111/bjd.16607. [DOI] [PubMed] [Google Scholar]
- 135.Chen L, et al. CAR T-cell therapy for a relapsed/refractory acute B-cell lymphoblastic lymphoma patient in the context of Li-Fraumeni syndrome. J Immunother Cancer. 2020;8(1):e000364. doi: 10.1136/jitc-2019-000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kenter GG, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 137.Lattanzi M, et al. Adjuvant NY-ESO-1 vaccine immunotherapy in high-risk resected melanoma: a retrospective cohort analysis. J Immunother Cancer. 2018;6(1):38. doi: 10.1186/s40425-018-0345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pavlick A, et al. Combined vaccination with NY-ESO-1 protein, poly-ICLC, and montanide improves humoral and cellular immune responses in high-risk melanoma patients. Cancer Immunol Res. 2020;8(1):70–80. doi: 10.1158/2326-6066.CIR-19-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhardwaj N, et al. Flt3 ligand augments immune responses to anti-DEC-205-NY-ESO-1 vaccine through expansion of dendritic cell subsets. Nat Cancer. 2020;1(12):1–14. doi: 10.1038/s43018-020-00143-y. [DOI] [PubMed] [Google Scholar]
- 140.Wedén S, et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer. 2011;128(5):1120–1128. doi: 10.1002/ijc.25449. [DOI] [PubMed] [Google Scholar]
- 141.Palmer DH, et al. TG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas (CT TG01-01): a single-arm, phase 1/2 trial. Br J Cancer. 2020;122(7):971–977. doi: 10.1038/s41416-020-0752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Slingluff CL, et al. Final analysis of relapse-free survival in a multicenter, double-blind, placebo-controlled trial of seviprotimut-L polyvalent melanoma vaccine after resection of high-risk melanoma. J Clin Oncol. 2020;38(15 suppl):10017–10017. doi: 10.1200/JCO.2020.38.15_suppl.10017. [DOI] [Google Scholar]
- 143.Rodriguez J, et al. A randomized phase II clinical trial of dendritic cell vaccination following complete resection of colon cancer liver metastasis. J Immunother Cancer. 2018;6(1):96. doi: 10.1186/s40425-018-0405-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dion GR, et al. Adjuvant human papillomavirus vaccination for secondary prevention: a systematic review. JAMA Otolaryngol Head Neck Surg. 2017;143(6):614–622. doi: 10.1001/jamaoto.2016.4736. [DOI] [PubMed] [Google Scholar]
- 145.Deshmukh AA, et al. Adjuvant HPV vaccination for anal cancer prevention in HIV-positive men who have sex with men: the time is now. Vaccine. 2017;35(38):5102–5109. doi: 10.1016/j.vaccine.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lichter K, et al. Adjuvant human papillomavirus vaccine to reduce recurrent cervical dysplasia in unvaccinated women: a systematic review and meta-analysis. Obstet Gynecol. 2020;135(5):1070–1083. doi: 10.1097/AOG.0000000000003833. [DOI] [PubMed] [Google Scholar]
- 147.Carreno BM, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 149.Hu Z, et al. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sahin U, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 151.Keskin DB, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565(7738):234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hilf N, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565(7738):240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 153.Ott PA, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547(7662):217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reyes-Uribe L, et al. Naproxen chemoprevention promotes immune activation in Lynch syndrome colorectal mucosa. Gut. 2020;70(3):555–556. doi: 10.1136/gutjnl-2020-320946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oliveira LJC, et al. Spectrum and clinical activity of PD-1/PD-L1 inhibitors: regulatory approval and under development. Curr Oncol Rep. 2020;22(7):70. doi: 10.1007/s11912-020-00928-5. [DOI] [PubMed] [Google Scholar]
- 156.Wang J, et al. PD-1 blockade prevents the development and progression of carcinogen-induced oral premalignant lesions. Cancer Prev Res. 2017;10(12):684–693. doi: 10.1158/1940-6207.CAPR-17-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mancuso JG, et al. Cancer immunoprevention: a case report raising the possibility of “immuno-interception.”. Cancer Prev Res. 2020;13(4):351–356. doi: 10.1158/1940-6207.CAPR-19-0528. [DOI] [PubMed] [Google Scholar]
- 158.Youn JW, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020;21(12):1653–1660. doi: 10.1016/S1470-2045(20)30486-1. [DOI] [PubMed] [Google Scholar]
- 159.Svane IM, et al. LBA48 Clinical efficacy and immunity of combination therapy with nivolumab and IDO/PD-L1 peptide vaccine in patients with metastatic melanoma: a phase I/II trial. Ann Oncol. 2020;31(4):S1176. doi: 10.1016/annonc/annonc325. [DOI] [Google Scholar]
- 160.Ott PA, et al. A phase Ib trial of personalized neoantigen therapy plus anti-PD-1 in patients with advanced melanoma, non-small cell lung cancer, or bladder cancer. Cell. 2020;183(2):347–362. doi: 10.1016/j.cell.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 161. Galsky M. Atezolizumab Given in Combination With a Personalized Vaccine in Patients With Urothelial Cancer. https://clinicaltrials.gov/ct2/show/NCT03359239 Updated March 4, 2021. Accessed November 12, 2020.
- 162. NIH. An Efficacy Study of Adjuvant Treatment With the Personalized Cancer Vaccine mRNA-4157 and Pembrolizumab in Patients With High-Risk Melanoma (KEYNOTE-942). https://clinicaltrials.gov/ct2/show/NCT03897881 Updated February 6, 2020. Accessed November 12, 2020.
- 163.Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10(1):5408. doi: 10.1038/s41467-019-13368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pearson FE, et al. Human CLEC9A antibodies deliver Wilms’ tumor 1 (WT1) antigen to CD141+ dendritic cells to activate naïve and memory WT1-specific CD8+ T cells. Clin Transl Immunology. 2020;9(6):e1141. doi: 10.1002/cti2.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Santos PM, Butterfield LH. Dendritic cell–based cancer vaccines. J Immunol. 2018;200(2):443–449. doi: 10.4049/jimmunol.1701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shi S, et al. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37(24):3167–3178. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 167.Lee SH, et al. DNA vaccines, electroporation and their applications in cancer treatment. Hum Vaccin Immunother. 2015;11(8):1889–1900. doi: 10.1080/21645515.2015.1035502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kranz LM, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 169.Sahin U, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 170.Sahin U, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 171.Sjökvist Ottsjö L, et al. Induction of mucosal immune responses against helicobacter pylori infection after sublingual and intragastric route of immunization. Immunology. 2017;150(2):172–183. doi: 10.1111/imm.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Purcell AW, et al. Mass spectrometry–based identification of MHC-bound peptides for immunopeptidomics. Nat Protoc. 2019;14(6):1687. doi: 10.1038/s41596-019-0133-y. [DOI] [PubMed] [Google Scholar]
- 173.Chong C, et al. Integrated proteogenomic deep sequencing and analytics accurately identify non-canonical peptides in tumor immunopeptidomes. Nat Commun. 2020;11(1):1293. doi: 10.1038/s41467-020-14968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wells DK, et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell. 2020;183(3):818–834. doi: 10.1016/j.cell.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cory L, et al. Effects of educational interventions on human papillomavirus vaccine acceptability: a randomized controlled trial. Obstet Gynecol. 2019;134(2):376–384. doi: 10.1097/AOG.0000000000003379. [DOI] [PubMed] [Google Scholar]
- 176.Brewer NT, et al. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139(1):e20161764. doi: 10.1542/peds.2016-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bruni L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 178. European Medicines Agency. Review Concludes Evidence Does Not Support That HPV Vaccines Cause CRPS or POTS. EMA; 2015. Accessed March 19, 2021. https://www.ema.europa.eu/en/news/review-concludes-evidence-does-not-support-hpv-vaccines-cause-crps-pots.
- 179.Scheller NM, et al. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA. 2015;313(1):54–61. doi: 10.1001/jama.2014.16946. [DOI] [PubMed] [Google Scholar]
- 180. World Health Organization. Global Advisory Committee on Vaccine Safety Statement on Safety of HPV Vaccines. WHO; 2015. [Google Scholar]
- 181.Okita T, et al. The controversy on HPV vaccination in Japan: criticism of the ethical validity of the arguments for the suspension of the proactive recommendation. Health Policy. 2020;124(2):199–204. doi: 10.1016/j.healthpol.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 182.Suzuki S. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: results of the Nagoya study. Papillomavirus Res. 2018;5:96–103. doi: 10.1016/j.pvr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Simms KT, et al. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020;5(4):E223–E234. doi: 10.1016/S2468-2667(20)30010-4. [DOI] [PubMed] [Google Scholar]
- 184.Yagi A, et al. Potential for cervical cancer incidence and death resulting from Japan’s current policy of prolonged suspension of its governmental recommendation of the HPV vaccine. Sci Rep. 2020;10(1):15945. doi: 10.1038/s41598-020-73106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.