Abstract
During JADPRO Live Virtual 2020, Cissimol Joseph, APRN, AOCNP®, and Prachee Singh, PA-C, MS, highlighted the clinical features and diagnostic workup of soft-tissue sarcoma and presented on a multidisciplinary approach to treatment planning.
A rare tumor constituting less than 1% of all cancer types, sarcoma presents unique challenges for both patients and health-care providers throughout the treatment course.
During JADPRO Live Virtual 2020, Cissimol Joseph, APRN, AOCNP®, and Prachee Singh, PA-C, MS, of The University of Texas MD Anderson Cancer Center, described the clinical features and diagnostic workup of soft-tissue sarcoma and how to navigate the complexity of treatment plans for this disease.
CLINICAL PRESENTATION
As Ms. Singh explained, sarcomas can present with or without symptoms. Pain is present in approximately one third of the patient population, but in the asymptomatic population, it could be discovered as an incidental finding during injury, surgery, or workup for another condition. Due in part to these factors, 70% of patients have at least a 4-month delay from the time of evaluation to diagnosis. Soft-tissue sarcomas most commonly affect the extremities (40% of all cases) but can form almost anywhere in the body.
Ms. Singh listed a number of risk factors associated with soft-tissue sarcoma, including chronic lymphedema, immune suppression (from either HIV, autoimmune conditions, or other cancer subtypes such as chronic lymphocytic leukemia), and radiation exposure. Sarcoma is also associated with genetic syndromes such as Li Fraumeni syndrome and neurofibromatosis. Sarcoma represents 25% of all tumors in TP53 mutation carriers.
DIAGNOSTIC EVALUATION
In general, workup for sarcoma begins with a biopsy, said Ms. Joseph, and core biopsy is preferred over excisional biopsy due to its low incidence of complications and high diagnostic accuracy. Next, imaging is used to complete staging by evaluating for metastatic site(s), including organ involvement, and the modality is chosen based on site of disease and histology. MRI is used for extremities, head/neck, and pelvis, while CT is used for retroperitoneum and abdomen. PET-CT is sometimes used to define grading based on the activity of lesions.
“It is better to review the pathology again at the tertiary center to confirm that an accurate diagnosis has been made,” said Ms. Joseph. “There are some instances that diagnosis might change after review.”
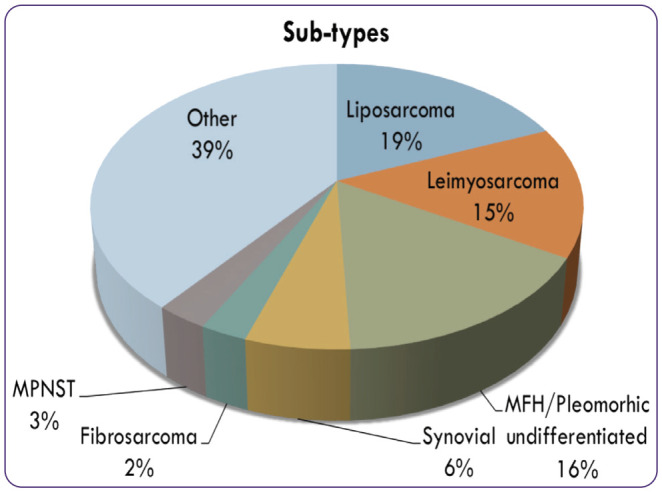
With over 70 to 80 different subtypes, sarcoma is a heterogeneous disease, and each subtype is different in its natural history and management, said Ms. Joseph, who noted that sarcoma can range from low grade to highly aggressive (Figure 1). The most common sarcomas are liposarcoma, leiomyosarcoma, and undifferentiated pleomorphic sarcoma, which constitute half of all diagnoses.
Figure 1.
Classification of sarcomas. Courtesy of Dr. Somaiah, MD Anderson Cancer Center.
As Ms. Joseph explained, the subtypes of sarcoma are generally named based on the type of the connective tissue from which they arise. Liposarcoma arises from adipocytic tissue, for example, while leiomyosarcoma arises from smooth muscle.
In some subtypes like synovial sarcoma, clinicians look for translocations such as SS18. With a gastrointestinal stromal tumor (GIST), on the other hand, patients are evaluated for the driving mutation, such as KIT or PDGFRA. All of this helps with accurate diagnosis and management, said Ms. Joseph.
Diagnostic staging is based on histologic grade and cell of origin. The most widely used staging system is the AJCC classification, which uses tumor size, lymph node involvement, depth of invasion, presence or absence of distant metastasis, and histology grading, but there are separate criteria for soft-tissue sarcomas arising in different locations. The most common tests performed on the tumor specimens to get the details of the histology are immunohistochemical staining, fluorescence in situ hybridization (FISH), and reverse transcription-polymerase chain reaction (RT-PCR).
TREATMENT PLANNING
Because of the rarity of the disease, a multidisciplinary approach is essential and often involves medical oncology, surgery, plastic surgery, or radiation.
“Early incorporation of these services actually improves the overall survival and clinical outcome,” said Ms. Joseph. “A multidisciplinary approach also optimizes the treatment planning, minimizes the duplication of the diagnostic studies, and reduces the time to treatment initiation.”
If possible, evaluation and management of soft-tissue sarcomas should be carried out in a center with expertise. Five-year survival for stage I sarcoma is 90%, but it is only 10% to 20% for metastatic cases.
If the tumor is less than 5 centimeters without metastatic disease, surgical resection is the general approach, and depending upon the surgical margin, radiation may be used.
If the tumor is more than 5 cm without metastatic disease, the general approach is neoadjuvant chemotherapy for tumor shrinkage, with or without radiation, followed by surgery.
In metastatic disease, chemotherapy is always the approach, said Ms. Joseph. In special cases, however, providers are able to incorporate surgery and radiation for local control.
The types of systemic treatment include the following: chemotherapy, oral therapies such as tyrosine kinase inhibitors and vascular endothelial growth receptor inhibitors, antibodies such as denosumab, immunotherapy, and chimeric antigen receptor (CAR) T-cell therapies for limited use on clinical trial for specific subtypes.
There are different types of chemotherapy regimens (either single agent or in combination) used to treat soft-tissue sarcoma, but the most common and active regimens are doxorubicin (anthracycline; 60–75 mg/m2) and ifosfamide (alkylating agent; 10–14 g/m2). According to Ms. Joseph, there is a high variation of treatment recommendations based on the specific subtypes and patient factors.
ASCO UPDATES
Standard of care for soft-tissue sarcoma has remained relatively unchanged for the past few decades. This is partly due to the fact that sarcomas are so heterogeneous in histology and in mutation type, and because it’s a rare cancer, the sample size in each subtype is limited.
“For these reasons, it may be difficult to obtain funding from the FDA or pharmaceutical companies to run trials to get approval for therapies in this orphan disease since it affects a relatively small population,” said Ms. Singh.
Nevertheless, the FDA has recently approved four clinically relevant medications. Larotrectinib (Vitrakvi) was approved for use in sarcomas with the NTRK fusion, and tazemetostat (Tazverik), an EZH2 inhibitor, was approved for epithelioid sarcomas with EZH2 mutation.
In addition, two agents were approved for GIST: avapritinib (Ayvakit) and ripretinib (Qinlock). Avapritinib is recommended in the NCCN Guidelines as primary/first-line treatment for unresectable or metastatic GIST with PDGFRA exon 18 mutation. It’s also recommended as a postoperative treatment for persistent gross residual disease in patients with PDGFRA D842V mutation.
Ripretinib, a novel, switch-control kinase inhibitor, is indicated for the treatment of adult patients with advanced GIST who have received prior treatment with three or more kinase inhibitors, including imatinib (Gleevec).
UNIQUE CHALLENGES
Sarcoma patients also experience a number of special challenges throughout their treatment journey. For one, there are a limited number of sarcoma specialized centers as well as sarcoma pathologists.
“Sarcoma diagnoses are challenging to analyze even at sarcoma centers, and in certain cases, the diagnosis changes,” said Ms. Singh. “If patients want to be treated at one of these specialized centers, they may have financial barriers in seeking treatment or even evaluation at a sarcoma center.”
“This is not to say that patients cannot be treated in a nonspecialized center or by community oncologists,” Ms. Singh continued, “but some providers may have concerns in administering certain regimens safely due to the greater risks for neutropenic fever or cardiotoxicity, for example.”
Sarcoma patients may also face challenges due to limited staffing, clinic hours, or accessibility to hospitals that are familiar with treating complications associated with sarcoma therapies. According to Ms. Singh, it is essential to have lab monitoring and symptom assessment followed closely. In addition, the sarcoma centers need to be aware of any outside emergent imaging or changes in dosage or administration of therapy by the local facility.
“Patients need integration of family and social support during these times because they are very vulnerable and not well enough for self-care,” said Ms. Singh, who noted that many patients undergo multiple modalities of treatment, including surgery, radiation, and chemotherapy, and endure a treatment course that often has no clear endpoint. “Having someone observe changes in the patient, who may be overwhelmed, could lead to less interruption or early abandonment of treatment.”
“It’s also critical to help set up the outlook that treatment is not a sprint but actually a marathon,” she added. Patients need motivation to remain adherent to their regimens, “especially those who have predicted good outcome,” said Ms. Singh.
Patients whose treatments have failed multiple lines of therapy, on the other hand, must deal with end-of-life issues such as guilt, and ultimately, the discussion must turn to the difference between extension and quality of life.
“This is true for other cancer types, but in sarcoma, these issues are more apparent, more frequent, and more intense,” said Ms. Singh.
Lastly, genetic testing for both patients and biological family members is a key discussion point. For example, sarcoma is considered a core cancer in Li Fraumeni syndrome, and patients affected by this syndrome have an increased lifetime cancer risk up to 90%.
“There are many points of consideration for referring a sarcoma patient to a geneticist, including the age of onset, multiple primary malignancies, or family history of core cancers or childhood cancers,” said Ms. Singh. “However, it isn’t automatically recommended for all sarcoma patients.”
Footnotes
Ms. Joseph has served on the speakers bureau for Deciphera. Ms. Singh had no conflicts of interest to disclose.


