Abstract
Aging is the greatest risk factor for most chronic diseases. The somatotropic axis is one of the most conserved biological pathways that regulates aging across species. 17α-Estradiol (17α-E2), a diastereomer of 17β-estradiol (17β-E2), was recently found to elicit health benefits, including improved insulin sensitivity and extend longevity exclusively in male mice. Given that 17β-E2 is known to modulate somatotropic signaling in females through actions in the pituitary and liver, we hypothesized that 17α-E2 may be modulating the somatotropic axis in males, thereby contributing to health benefits. Herein, we demonstrate that 17α-E2 increases hepatic insulin-like growth factor 1 (IGF1) production in male mice without inducing any changes in pulsatile growth hormone (GH) secretion. Using growth hormone receptor knockout (GHRKO) mice, we subsequently determined that the induction of hepatic IGF1 by 17α-E2 is dependent upon GH signaling in male mice, and that 17α-E2 elicits no effects on IGF1 production in female mice. We also determined that 17α-E2 failed to feminize the hepatic transcriptional profile in normal (N) male mice, as evidenced by a clear divergence between the sexes, regardless of treatment. Conversely, significant overlap in transcriptional profiles was observed between sexes in GHRKO mice, and this was unaffected by 17α-E2 treatment. Based on these findings, we propose that 17α-E2 acts as a pleiotropic pathway modulator in male mice by uncoupling IGF1 production from insulin sensitivity. In summary, 17α-E2 treatment upregulates IGF1 production in wild-type (and N) male mice in what appears to be a GH-dependent fashion, while no effects in female IGF1 production are observed following 17α-E2 treatment.
Keywords: 17α-Estradiol, Aging, Growth hormone, Insulin, Insulin-like growth factor 1
Aging and the onset of chronic diseases can be attenuated through interventional strategies that modulate growth and nutrient-sensing pathways (1). One of the more recently studied compounds found to elicit health benefits and extend longevity is 17α-estradiol (17α-E2). 17α-E2 is a diastereomer of 17β-estradiol (17β-E2) that is naturally present in both sexes (2). 17α-E2 extends longevity in a sex-specific manner (3). We have previously reported that 17α-E2 treatment reverses several conditions associated with advancing age in male mice, including visceral adiposity, ectopic lipid deposition, glucose intolerance, insulin resistance, chronic low-grade inflammation, and hepatocyte DNA damage in telomeric regions (4). These phenotypic alterations were found to be linked with the activation of AMPKα and suppression of mTORC1, which are consistent with beneficial outcomes with advancing age (5). Some effects of 17α-E2 are similar to that of calorie restriction (CR) because it reduces calorie intake by modulating hypothalamic anorexigenic pathways (4). 17α-E2 also elicits metabolic benefits in hyperphagic male mice, indicating that 17α-E2 improves metabolic parameters in the absence of significant reductions in calorie intake (6). Despite some similarities between health outcomes following 17α-E2 and CR treatments, we have also demonstrated that 17α-E2 treatment fails to modulate proteostasis similarly to CR in aged male mice (7), suggesting that 17α-E2 and CR may also differ in some of the ways in which they improve health and extend life span.
Decades of research has firmly established that the somatotropic axis is one of most conserved biological pathways that regulates life span and healthspan across species. In mammals, the biosynthesis of pituitary growth hormone (GH) and subsequent production of insulin-like growth factor 1 (IGF1) represent important mechanisms that modulate postnatal growth and development (8). While the inhibition of the GH/IGF1 axis by either GH deficiency or resistance (GH receptor ablation) significantly stunts growth rate and adult body size, it also significantly extends life span (1). Homozygous loss-of-function mutation at the Prop-1 locus in Ames dwarf (df/df) mice causes deficiency in GH, thyroid-stimulating hormone (TSH), and prolactin (PRL). Lack of GH production in these mice causes significant reductions in hepatic and circulating IGF1, yet despite these hormonal deficiencies df/df mice live up to 60% longer than their normal littermate controls (1). Similarly, the GH-resistant (GHR knockout [GHRKO]) mice, which we use in these studies, have normal levels of PRL and TSH, but also show robust reductions of hepatic and circulating of IGF1 (9). These mice also display reduced adult body size and significant life span and healthspan extension (9). Long-lived df/df and GHRKO mice are also characterized by hypersensitivity to insulin and maintain very low circulating insulin and glucose levels throughout their lifecycle (1). Both IGF1 and insulin are known to cross-react with each respective receptor, which complicates the understanding of detailed regulation and independent role insulin and IGF1 play in some physiological conditions (10). However, several studies evaluating long-lived mice suggest that insulin sensitivity and IGF1 production are coupled. For instance, insulin sensitivity in GHRKO mice cannot be improved by CR (11), suggesting that reduced activity of the GH/IGF1 axis and initiation of CR elicit health benefits through similar cellular mechanisms.
Several mammalian species exhibit differences in lifespan between the sexes and also frequently respond in divergent manners to interventional strategies aimed at curtailing diseases (12). As mentioned above, 17α-E2 improves life span in males, but not female, mice (3). Previous studies have linked sexual dimorphism in health parameters to differences in hepatic drug metabolism (13) and susceptibility to liver diseases (14). Interestingly, there are large differences in hepatic transcriptional profiles between male and female mice that essentially disappear in the absence of STAT5b signaling (15), a major mediator of GH signaling. Importantly, the pattern of pituitary GH secretion differs between males and females (16), which exerts significant effects on hepatic transcriptional activity, as demonstrated by ablating hepatic GH signaling which abolishes 90% of sex differences in hepatic gene expression (17). Moreover, persistent exposure to GH in male mice induces a feminization of hepatic transcriptional profiles (18).
17β-E2 is known to inhibit the GHR-JAK2-STAT5 signaling cascade and downstream transcriptional activity in liver by inducing suppressor of cytokine signaling (SOCS) 2 and SOCS3 expression (19). 17β-E2 can also directly affect pituitary GH secretion through estrogen receptor (ER) α and ERβ (20), which almost certainly contributes to the observed sex differences in GH secretion. Interestingly, 17β-E2 administration increases hepatic and plasma IGF1 in GHRKO, but not normal littermate, mice (21), thereby suggesting that estrogens may uncouple the insulin and IGF1 actions. If this is found to be true it could provide important insights into what role insulin and IGF1 play in differences in aging biology between the sexes. Given that 17β-E2 has been shown to modulate the GH/IGF1 signaling axis, coupled with our recent report demonstrating that 17α-E2 signals through ERα to elicit health benefits (22), we hypothesized that 17α-E2 improves health parameters in male mice by altering GH pulsatility, thereby feminizing the hepatic transcriptional profile. To test this hypothesis, we exposed aged male mice to 17α-E2 to measure GH secretion. We also evaluated circulating insulin and IGF1 and the sex-specific pattern of liver gene regulation prompted by 17α-E2 in male and female GHRKO mice.
Materials and Methods
Control and Experimental Diets
TestDiet, a division of Purina Mills (Richmond, IN), prepared all the diets for these studies. Study 1 utilized TestDiet 5LG6 (65.4% CHO, 22.4% PRO, 12.2% FAT) ± 17α-E2 (14.4 ppm; Steraloids). Study 2 utilized TestDiet 58YP (66.6% CHO, 20.4% PRO, 13.0% FAT) ± 17α-E2 (14.4 ppm).
Study 1, Animals
Eighteen-month-old male C57BL/6 were obtained from the National Institutes on Aging breeding colony and were individually housed at 22 ± 0.5 °C on a standard 12:12-hour light–dark cycle, with lights coming on at 07:00 each day. Unless otherwise noted, mice had ad libitum access to food and water throughout the experimental timeframe. Mice were handled extensively to acclimatize them to human interaction prior to performing blood draws for assessing GH pulsatility. Following acclimatization, mice were randomized by body mass, body composition, and fasting glucose to control (CON) or 17α-E2 treatment groups (n = 8/group). Prior to study initiation and following 15 weeks of treatment, all mice underwent tail-bleeding in the fed state to assess GH pulsatility as outlined below. At the conclusion of the 15-week treatment period, mice were anesthetized with isoflurane and euthanized by cervical dislocation prior to dissection. Blood was collected into ethylenediaminetetraacetic acid (EDTA)-lined tubes by cardiac puncture, and plasma was collected and frozen. Tissues were excised, weighed, flash frozen, and stored at −80 °C for future analyses. All procedures were approved by the appropriate Institutional Animal Care and Use Committees (IACUC).
Study 1, GH Pulsatility
Pulsatile GH release was measured. Briefly, tail-clip whole-blood collection (2 µL) began at 09:00 and was repeated every 10 minutes over a 6-hour period. Each collection of whole blood was transferred to 58 μL of 0.05% PBS-T. Samples were vortexed and placed on dry ice. After each blood collection, gentle pressure was applied to the tail wound to stem blood flow, and the animal was returned to the home cage. For repeated sampling, the surface of the original wound was disrupted and the tail wound was briefly immersed in physiological saline (0.9% sodium chloride) so any scab would soften and be easily removed. When necessary, gentle pressure was applied along the base to the tip of the tail to assist blood flow. Samples were then transferred to −80 °C for future evaluation by ELISA.
Study 2, Animals
GHRKO and normal (N; wild-type or heterozygous littermates) mice were bred and maintained at Southern Illinois University. Mice were group housed at 22 ± 0.5 °C on a standard 12:12-hour light–dark cycle, with lights coming on at 07:00 each day. Unless otherwise noted, mice had ad libitum access to food and water throughout the experimental timeframe. At 6 months of age, mice were randomized by mass into eight experimental groups (n = 8/group) including: Normal male, CON diet (N-CON-M); Normal male, 17α-E2 diet (N-17α-M); GHRKO male, CON diet (KO-CON-M); GHRKO male, 17α-E2 diet (KO-17α-M); Normal female, CON diet (N-CON-F); Normal female, 17α-E2 diet (N-17α-F); GHRKO female, CON diet (KO-CON-F); and GHRKO female, 17α-E2 diet (KO-17α-F). Body mass was assessed periodically throughout the study. At the conclusion of the 22-week treatment period, mice were anesthetized with isoflurane and euthanized by cervical dislocation prior to dissection. Blood was collected into EDTA-lined tubes by cardiac puncture, and plasma was collected and frozen. Tissues were excised, weighed, flash frozen, and stored at −80 °C for future analyses. All procedures were approved by the appropriate IACUC.
Insulin and IGF1 Assessments
Plasma insulin was evaluated using the Ultra-sensitive Rat Insulin ELISA Kit (Crystal Chem, Downers Grove, IL). Plasma IGF1 was evaluated using the Mouse/Rat IGF-I Quantikine ELISA Kit (R&D Systems, Minneapolis, MN). Tissue IGF1 was evaluated as follows. Liver, quadriceps, and white adipose tissue (WAT; ~100 mg) were homogenized in Cell Lysis Buffer (Cell Signaling, Danvers, MA) with protease inhibitors (Sigma-Aldrich) and total protein was quantified using BCA Protein Assay Reagent Kit (Pierce, Rockford, IL). Protein supernatant was then diluted 10× and analyzed using the ELISA described above. Supernatant protein concentrations were used to normalize across tissues.
Liver RNA Extraction
RNA was extracted from ~50 mg of frozen liver using RNeasy mini kits (Qiagen, Valencia, CA). RNA concentrations and integrities were determined using RNA ScreenTape assays and a 2200 Tapestation analyzer (Agilent Technologies, Santa Clara, CA). Only samples with RNA integrity numbers greater than 7 were sequenced. Extracted RNA was stored at −80 °C in preparation for sequencing. The whole transcriptome dataset is available on the Sequence Read Archive (SRA) at NCBI (accession #PRJNA643403).
RNA Sequencing
Six samples from each experimental group were randomly selected for RNA sequencing (N = 48). Library preparation and sequencing was performed at Beijing Novogene Co., Ltd. using the Illumina HiSeq 2500 instrument. The mapping of sequencing reads to the mouse transcriptome (Illumina iGenomes annotation for UCSC mm10, http://support.illumina.com/sequencing/sequencing_software/igenome.html) was performed using HiSat2 (23). The number of reads aligned to its corresponding gene was calculated by HTSeq 0.6.1 (24). Genes with an average FPKM lower than 1/100 000th of the total aligned reads in more than 50% of the samples were eliminated from further analyses. Statistical analyses for differentially expressed mRNAs were performed using the software R (3.2.2) and the Bioconductor package EdgeR using the HTSeq output count. Read counts were normalized for library depth, and pairwise comparisons, measuring fold change (FC), uncorrected p-values from the negative binomial distribution, and adjusted p-values (false discovery rate [FDR]) were obtained. Principal component analysis (PCA) was also performed using R to observe sample distribution in a two-dimensional plot and eliminate outliers. Unsupervised hierarchical clustering was performed also using the DESeq package to observe sample clustering. Genes with a FDR <0.05 and FC >2.0 were considered upregulated; and with FDR <0.05 and FC <0.5 were considered downregulated. mRNAs were further processed for pathway analysis using the Generally Applicable Gene-set Enrichment (GAGE), which uses log-based FCs as per gene statistics, and Pathview packages in R (25). p-Values lower than .05 were considered significant for pathways and GO Terms analysis.
Statistical Analysis
Analyses of differences between groups for Study 1 were performed by Student’s t test (plasma, liver, muscle, WAT IGF1). For plasma GH, the area under the curve was calculated and then compared by Student’s t test. For Study 2, two-way analysis of variance (ANOVA) was used to compare the effect of genotype, treatment, and potential interactions on plasma insulin and IGF1 with a Tukey post hoc test to compare individual means. GraphPad Prism Software, version 8.3.1, was used for statistical analysis. Values are presented as mean ± SEM and were considered significantly different when p <.05.
Results
17α-E2 Increases Hepatic IGF1 Production in Male Mice Without Altering GH Secretion
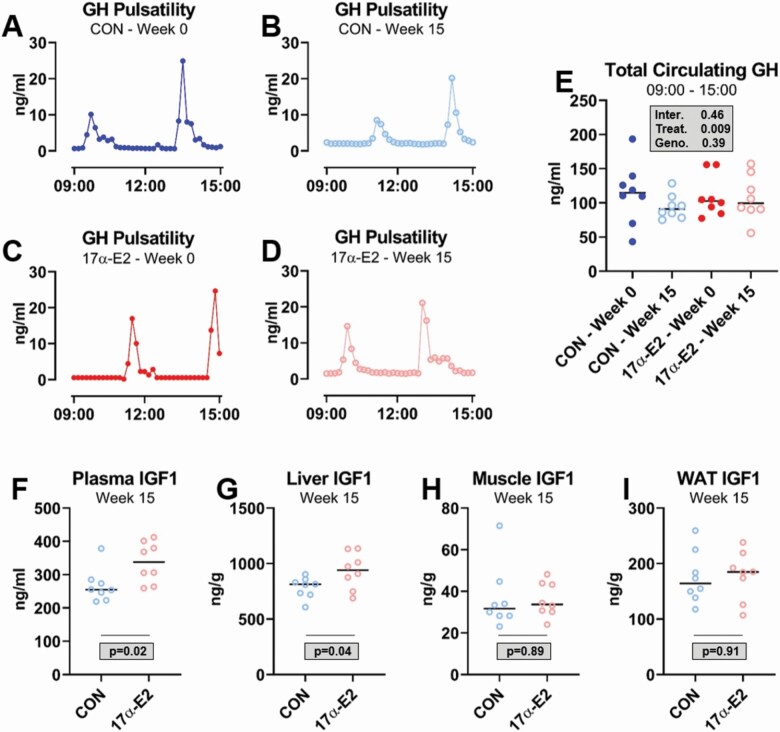
It is well established that GH pulsatility patterns differ between the sexes and this contributes to divergent transcriptional profiles in liver. In addition, there is also emerging evidence that the age-related detriments of IGF1 signaling may be greater in females, whereas males appear less affected (26). Given that 17β-E2 is known to alter the GH/IGF1 axis, we sought to determine if 17α-E2 would modulate GH pulsatility and tissue-specific IGF1 production. We found that GH pulse patterns were nearly identical between CON and 17α-E2-treated animals at baseline and following 15 weeks of treatment (Figure 1A–D and Supplementary Figures 1–4). Total GH secretion during the testing period was also not different between treatment groups or timepoints (Figure 1E), indicating that 17α-E2 almost certainly does not alter GH pulsatility in male mice. Surprisingly, despite the lack of differences in GH secretion following 17α-E2 treatment, circulating and hepatic IGF1 concentrations were increased by 17α-E2 in these mice (Figure 1F and G). We also found that muscle and WAT IGF1 production were unaffected by 17α-E2 (Figure 1H and I), suggesting that any increase in circulating IGF1 induced by 17α-E2 was likely being produced in the liver. These observations were contrary to our initial hypothesis, which led to additional studies in GHRKO mice aimed at unraveling how 17α-E2 may alter the GH/IGF1 axis.
Figure 1.
17α-E2 increases hepatic IGF1 production in male mice without altering GH secretion. Representative GH pulse patterns over a 6-h period in (A) male C57BL6/n control (CON) mice at baseline, (B) male C57BL6/n mice at baseline prior to 17α-E2 treatment, (C) male C57BL6/n CON mice following the 15-wk treatment period, and (D) male C57BL6/n mice following 15 wk of 17α-E2 treatment. (E) Total GH secretion during the 6-h testing period and (F) plasma, (G) liver, (H) muscle (quadriceps), and white adipose tissue (WAT; epididymal) IGF1 protein concentrations in C57BL6/n CON and 17α-E2-treated mice following a 15-wk intervention. All data are presented as mean ± SEM and were analyzed by Student’s t test. n = 8/group.
17α-E2 Fails to Increase Circulating IGF1 in Male GHRKO, or Female, Mice
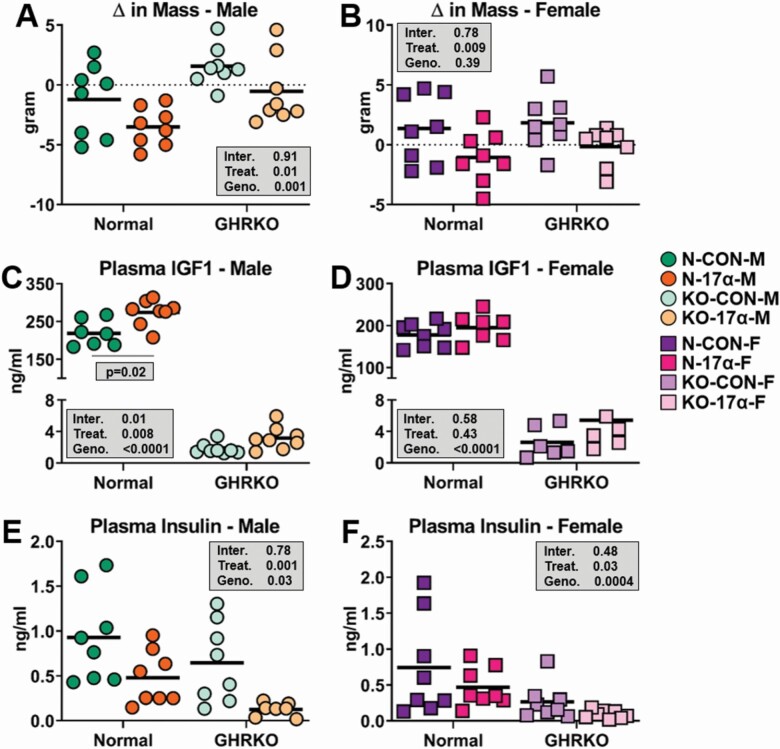
As previously reported, 17α-E2 reduced body mass more robustly in N male mice as compared to female mice (Figure 2A and B). 17α-E2 also induced greater losses of mass in N mice than that observed in GHRKO (Figure 2A and B), which is not surprising given the small size of GHRKO mice. In alignment with the results from Study 1, 17α-E2 increased circulating IGF1 in N male mice, but this effect was absent in GHRKO mice receiving 17α-E2 (Figure 2C). This finding suggests that GH signaling is involved in 17α-E2-mediated increases in hepatic IGF1 production, although the results from Study 1 indicate that this is occurring independent of changes in GH pulsatility. 17α-E2 failed to modulate circulating IGF1 in female mice of either genotype in our study (Figure 2D), which is contrary to a previous report showing that long-term 17α-E2 treatment increased circulating IGF1 in both sexes (27). As expected, 17α-E2 also reduced circulating insulin in N male mice (Figure 2E), thereby indicating that 17α-E2 “uncouples” the connections between circulating insulin and IGF1 in male mice. Unexpectedly, we also observed a mild treatment effect of 17α-E2 on circulating insulin levels in female mice. Given the sexually dimorphic responsiveness in IGF1 production following 17α-E2 treatment, we wanted to determine if the hepatic transcriptome was being differentially affected between the sexes by 17α-E2.
Figure 2.
17α-E2 uncouples insulin and IGF1 production in male mice. (A, B) Change in mass from baseline in male and female normal (N) and GHRKO following a 22-wk intervention period. (C, D) Plasma IGF1 in male and female N and GHRKO following a 22-wk intervention period. (E, F) Plasma insulin in male and female N and GHRKO following a 22-wk intervention period. All data are presented as mean ± SEM and were analyzed by two-way ANOVA. n = 6–8/group.
GHR Ablation Alters the Sex-Specific Hepatic Transcriptome Responses to 17α-E2 Treatment
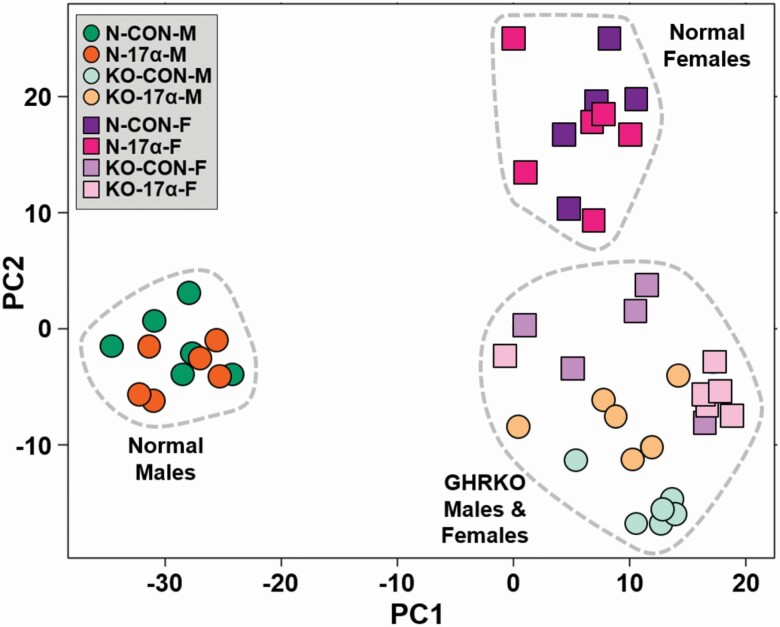
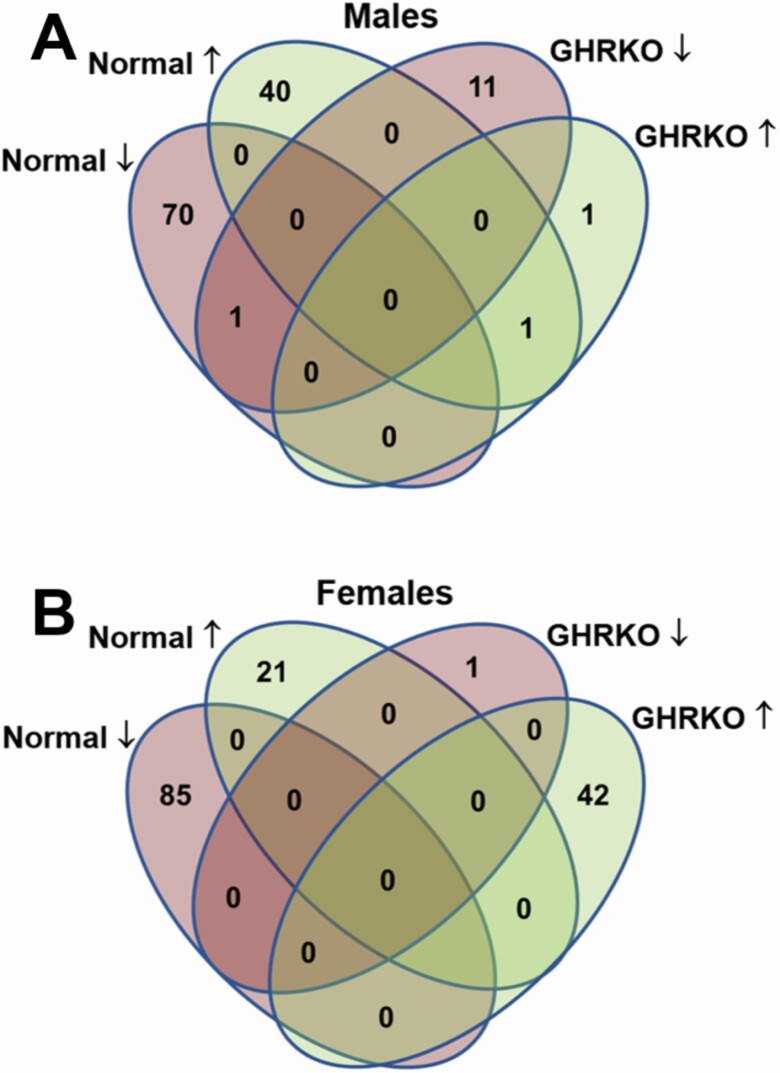
On average 39 933 632.15 ± 423 256.43 reads were obtained after processing the liver samples. From these, an average of 95.6% ± 0.1% of the reads aligned to the mouse mm10 genome. After removing transcripts with very low reads, 14 357 transcripts were detected and analyzed. The PCA analyses of the 500 most variable genes are represented in Figure 3. To our surprise, 17α-E2 treatment did not significantly shift the expression profile of hepatic genes of either sex in this study. There is a clear distinction between the expression profiles of N male and N female mice, regardless of treatment. Conversely, both male and female GHRKO mice of both treatments clustered together closely, indicating similar expression profiles. In N male mice, 112 genes were differentially expressed following treatment with 17α-E2, whereas 106 were found to be differentially expressed in N female mice receiving 17α-E2 (Figure 4). In GHRKO mice, only 13 genes in males and 43 genes in females were found to be differentially expressed following 17α-E2 treatment. There were very few commonly regulated genes among GHRKO and N mice (Figure 4). Several genes that have previously been linked to hepatic steatosis were found to be differentially expressed following 17α-E2 treatment in this study. In male mice only, 17α-E2 upregulated Cyp17a1, Abcb1a, and Ace2, all of which have effects on hepatic lipid metabolism (Supplementary Tables 1–4). Scd3 and Tff3, regulators of lipogenesis, were also found to be upregulated in N mice of both sexes. A KEGG pathway analysis revealed that 17α-E2 treatment downregulated both steroid and terpenoid biosynthesis in both N and GHRKO male mice (Supplementary Tables 5–8). Interestingly, both the steroid and terpenoid biosynthesis pathways are involved in sterol, cholesterol, and steroid synthesis the liver (28). Similar KEGG pathway analyses in female mice revealed that 8 pathways were regulated by 17α-E2 treatment in N females and 13 pathways in GHRKO females. Most notable were the observations that 17α-E2 upregulated the bile secretion pathway in N males, which further suggests that 17α-E2 reduces liver steatosis by increasing cholesterol excretion from liver (Supplementary Table 5). Additionally, 17α-E2 downregulated xenobiotic and drug metabolism by cytochrome P450 enzymes in N females, whereas peroxisome and fatty acids metabolism were upregulated by 17α-E2 in GHRKO females.
Figure 3.
17α-E2 fails to feminize the global transcriptional profile in male mice. PCA plot of hepatic transcriptional profiles by RNA sequencing analyses in male and female normal (N) and GHRKO mice following a 22-wk intervention period. Notably, N mice clustered by sex regardless of treatment, whereas GHRKO clustered closely together regardless of treatment or sex.
Figure 4.
17α-E2 elicits far greater transcriptional responses in Normal than GHRKO mice. Venn diagrams showing the number of differentially expressed hepatic genes in (A) male and (B) female normal (N) and GHRKO mice following a 22-wk intervention period.
Discussion
In the current studies, we observed that 17α-E2 treatment increased IGF1 production in wild-type (and N) male mice. Surprisingly, these increases occurred in the absence of changes in GH pulsatility, yet were blunted by GHR ablation. These data suggest that 17α-E2 modulates IGF1 production by direct actions in the liver and that GH signal transduction may be at least partially involved. This observation is contrary to a previous report showing that 17β-E2 increases IGF1 production in GHRKO, but not N, mice (21), suggesting that 17α-E2 and 17β-E2 may differentially regulate IGF1 expression in the liver. Previous work has established that ERα can modulate IGF1 gene expression (29), and we recently showed that the global ablation of ERα prevents nearly all the metabolic benefits of 17α-E2 in the liver of male mice (22). Moreover, dietary amino acids can regulate the transcriptional activity of hepatic ERα through an mTOR-dependent mechanism, thereby increasing hepatic IGF1 expression and circulating IGF1. CR and the ablation of ERα in the liver were found to decrease circulating IGF1 in these studies (30). In alignment with these observations, a recent report established that 17α-E2 increases the abundance of several amino acids in the liver of male mice (31). Collectively, these observations suggest that 17α-E2 likely modulates hepatic IGF1 production through several mechanisms.
Previous studies have shown that there are large differences in hepatic transcriptional profiles between male and female mice, which essentially disappears when circulating GH is abolished (17). Our liver transcriptome analyses also revealed a clear distinction between N male and female mice, with surprisingly modest effects of 17α-E2 on the global transcriptional profiles of either sex in these studies. As alluded to above, this sexually dimorphic profile was completely absent in GHRKO mice, as evidenced by GHRKO mice of both sexes and treatments displaying similar transcriptomes. This observation clearly demonstrates that the phenotypic response to GHR ablation far exceeds any response elicited by 17α-E2. Surprisingly, 17α-E2 treatment in N mice failed to elicit a convergence of the transcriptional profiles between the sexes, indicating that 17α-E2 failed to feminize the global transcriptome in male livers. It remains unclear if a greater effect would be observed in older mice (>18 months) with greater morbidity. Future studies will be needed to determine if 17α-E2 elicits more robust effects in male mice under challenged conditions (eg, obesity or old age).
Historically, negative modulation of the GH/IGF1 axis increases healthspan and/or life span as demonstrated in models such as the GHRKO and GH-deficient Ames Dwarf mice (1). These long-lived models are characterized by a simultaneous decrease in plasma levels of GH, IGF1, and insulin (1). In a rare exception to this example, 17α-E2 treatment increases plasma IGF1 in wild-type (and N) male mice while also significantly reducing body mass and insulin (4,6,27). We have also firmly established that 17α-E2 improves systemic and tissue-specific insulin sensitivity in male mice and rats (4,22). Although 17α-E2 failed to alter plasma IGF1 in male GHRKO or female mice of either genotype, plasma insulin was reduced by 17α-E2 treatment in these groups. This indicates that 17α-E2 uncouples IGF1 production from insulin sensitivity in male mice, and more importantly suggests that IGF1 expression and activity is not a primary determinant in male longevity since 17α-E2 significantly extends lifespan in male mice (3). These data provide insight into the relationship between the role insulin and IGF1 play in aging biology between the sexes. Previous work has demonstrated that inhibiting IGF1 signaling can extend life span only in females (26), and may point to why 17α-E2 treatment did not work in females in this and previous studies (27,32). Other studies also suggest that early-life IGF1 deficiency can improve life span in females only (33), further emphasizing this sex-specific effect. Although it is surmised that 17α-E2 treatment exerts at least part of this its beneficial effects on longevity through reductions in calorie intake (6), CR reduces plasma IGF1 and insulin (34), similarly to GH-deficient mice. Therefore, it seems reasonable to surmise that 17α-E2 likely alters healthspan and longevity through mechanisms that are generally more dissimilar, than similar, to those of CR or GH/IGF1 axis inhibition.
When looking for specific transcriptional links to how 17α-E2 treatment may be divergent from GH/IGF1 axis inhibition, we found that genes and pathways regulated by 17α-E2 treatment in N and GHRKO mice do not generally overlap. 17α-E2 treatment regulated almost 10 times more genes in the liver of N male than GHRKO male mice. In females, this difference was smaller, with twice as many genes regulated in N compared to GHRKO. This alone can point to some gender-specific regulation that blunts positive effects of 17α-E2 treatment in females. As mentioned above, sexual dimorphism is often dependent on differences in hepatic drug metabolism (13) and susceptibility to liver diseases (14). We did observe that in both N and GHRKO male mice, the terpenoid and steroid biosynthesis pathways were downregulated by 17α-E2 treatment. Terpenoid and steroid biosynthesis pathways are the primary mechanism responsible for sterol, cholesterol, and steroid biosynthesis in the liver (28). Interestingly, both of these pathways are upregulated in mice with diet-induced hepatic steatosis (35), indicating that a suppressive effect by 17α-E2 treatment could be indicative of preventing or reversing liver damage. Importantly, we have previously shown that 17α-E2 does indeed reduce hepatic steatosis, hepatic fibrosis, and hepatocyte DNA damage in male mice (4,22). Interestingly, the terpenoid and steroid biosynthesis pathways were unchanged by 17α-E2 in female mice, providing further support for the idea that 17α-E2 is predominantly effective in males.
The liver plays a critical role in modulating systemic metabolic homeostasis and conditions such as obesity and advancing age promote a variety of liver conditions, including steatosis, fibrosis, and insulin resistance (36), all of which are associated with hallmarks of aging (37). ERα has been shown to beneficially modulate the expression and activity of genes that regulate hepatic lipid metabolism (38), thereby curtailing liver steatosis (39). As alluded to above, we have previously shown that 17α-E2 improves several pathological-related parameters in the liver in an ERα-dependent manner (22). In the current study, we also discovered that 17α-E2 regulated myriad of genes related to hepatic lipid metabolism. These included Abcb1a, Scd3, Tff3, and Ace2. Abcb1a was upregulated 4-fold exclusively in N male mice treated with 17α-E2. The ABC transporter family transport lipids and sterols across membranes and maintain lipid homeostasis. Previous studies have shown that Abcb1a is upregulated by CR (40). Interestingly, Abcb1a null-mice display increased body mass, hepatic steatosis, and hyperinsulinemia (41) predominately in male mice, which again supports the idea of sexually divergent phenotypes in liver diseases and responsiveness to 17α-E2. Ace2 was also upregulated exclusively in N male mice receiving 17α-E2. Others have shown that Ace2 is protective against liver fibrosis in models of chronic liver injury (42). Scd3 was strongly regulated in males and to a lesser extent in females treated with 17α-E2. Scd3 synthesizes palmitoleate (16:1n-7) from saturated fatty acid precursors. Studies suggest that palmitoleate can reduce hepatic de novo lipogenesis and improve systemic insulin sensitivity (43). Tff3 was highly upregulated in both N males and females treated with 17α-E2. Previous reports indicate that restoring Tff3 expression in the liver of obese mice attenuates hepatic steatosis. These observations provide additional evidence that 17α-E2 has profound effects on liver pathophysiology, which could lead to future clinical studies aimed at alleviating fatty liver disease and potentially liver fibrosis.
In contrast to the findings in males, N female mice treated with 17α-E2 displayed a strong downregulation of cytochrome P450 pathways, providing additional evidence of sexually divergent responsiveness to 17α-E2. The cytochrome P450 superfamily of enzymes catalyze the metabolism of several molecules, ranging from lipids and steroidal hormones to xenobiotics (44). In mice, there is evidence of sex-specific expression of hepatic cytochrome P450 enzymes and drug metabolism (13). There is also evidence in humans suggesting that these sex-specific profiles play a role in adverse drug reactions that only occur in a single sex (45). Therefore, our observation that 17α-E2 downregulates cytochrome P450 enzymes may provide insight into a lack of 17α-E2-mediated benefits in female mice. Conversely, Cyp17a1, a member of the cytochrome P450 superfamily, was upregulated 8-fold in N males, with no significant effect being observed in any other group. Interestingly, following translation, CYP17A1 enzymatically converts pregnenolone to dehydroepiandrosterone (DHEA) (46). DHEA is an abundantly circulating sex hormone precursor that is known to decrease with advancing age (47) and is positively associated with metabolic homeostasis (48). Of particular importance, fasting has been shown to increase Cyp17a1 expression and hepatic DHEA concentrations (49,50), indicating that 17α-E2 and fasting may elicit similar cellular responses that are important for longevity in males. Future studies will be needed to determine if 17α-E2 treatment alters systemic and/or local concentrations of DHEA.
There are a few notable limitations to the current study. First, in Study 1, we did not perform GH pulsatility assessments in female mice. This prevented us from comparing GH secretion profiles across sexes. However, given that we failed to observed differences in GH secretion between treated and untreated males, we speculate that the additional female mice in this experiment would not have provide any additional insight. Second, Study 2 utilized mice that were relatively young (6 months) and treated them for a relatively short period of time (5 months). Given that the mice were lean and healthy, this may have limited our ability to observe a greater effect of 17α-E2 between treated and untreated mice. Future studies should undoubtedly consider the use of older, and/or challenged (eg, obese) mice. Lastly, our library sequencing depth in Study 2 could have been greater, which would have allowed us to detect more genes and potential differences in lowly expressed genes and splice variants.
In summary, we show that 17α-E2 treatment upregulates IGF1 production in wild-type (and N) male mice, in what appears to be a GH-dependent fashion. 17α-E2 did not alter IGF1 production in female mice. These observations occurred in conjunction with modest overall effects on the liver transcriptome, with the exception of a few very specific pathways in involved in terpenoid and steroid biosynthesis and cytochrome P450 metabolism, which display sex-specific regulation. Most notable were the observed changes in genes and related pathways known to improve liver pathophysiology, which likely promote lipid oxidation and DHEA formation. These studies indicate that 17α-E2 uncouples IGF1 production from insulin sensitivity in male mice. More importantly, it suggests that IGF1 reduction is not necessary to improve male longevity since 17α-E2 significantly extends lifespan in male mice despite increasing IGF1.
Supplementary Material
Funding
This work was supported by the National Institutes of Health (R00 AG51661 to M.B.S. and R56 AG061414, R03 AG059846, and R15 AG059190 to M.M.M.) and American Diabetes Association (1-19-IBS-126 to A.B.).
Conflict of Interest
None declared.
Author Contributions
S.S. and A.S. prepared the manuscript; S.S., A.S., Y.F., S.M., J.D., R.S., A.K.P., F.J.S., and M.B.S. performed experiments and data analysis; J.G., J.J.K., A.B., S.S., M.M.M., and M.B.S. designed the study, interpreted the results, and edited the final manuscript.
References
- 1. Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411 [DOI] [PubMed] [Google Scholar]
- 2. Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann NY Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009 [DOI] [PubMed] [Google Scholar]
- 3. Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stout MB, Steyn FJ, Jurczak MJ, et al. 17Alpha-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci. 2017;72:3–15. doi: 10.1093/gerona/glv309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 6. Steyn FJ, Ngo ST, Chen VP, et al. 17Alpha-estradiol acts through hypothalamic pro-opiomelanocortin expressing neurons to reduce feeding behavior. Aging Cell. 2018;17. doi: 10.1111/acel.12703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller BF, Pharaoh GA, Hamilton KL, et al. Short-term calorie restriction and 17Alpha-estradiol administration elicit divergent effects on proteostatic processes and protein content in metabolically active tissues. J Gerontol A Biol Sci Med Sci. 2020;75(5):849–857. doi: 10.1093/gerona/glz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yakar S, Adamo ML. Insulin-like growth factor 1 physiology: lessons from mouse models. Endocrinol Metab Clin North Am. 2012;41:231–247, v. doi: 10.1016/j.ecl.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374 [DOI] [PubMed] [Google Scholar]
- 10. Siddle K. Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol. 2011;47:R1–10. doi: 10.1530/JME-11-0022 [DOI] [PubMed] [Google Scholar]
- 11. Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. doi: 10.1371/journal.pone.0004567 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Austad SN, Bartke A. Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology. 2015;62:40–46. doi: 10.1159/000381472 [DOI] [PubMed] [Google Scholar]
- 13. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buzzetti E, Parikh PM, Gerussi A, Tsochatzis E. Gender differences in liver disease and the drug-dose gender gap. Pharmacol Res. 2017;120:97–108. doi: 10.1016/j.phrs.2017.03.014 [DOI] [PubMed] [Google Scholar]
- 15. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489 [DOI] [PubMed] [Google Scholar]
- 16. Adams JM, Otero-Corchon V, Hammond GL, Veldhuis JD, Qi N, Low MJ. Somatostatin is essential for the sexual dimorphism of GH secretion, corticosteroid-binding globulin production, and corticosterone levels in mice. Endocrinology. 2015;156:1052–1065. doi: 10.1210/en.2014-1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wauthier V, Sugathan A, Meyer RD, Dombkowski AA, Waxman DJ. Intrinsic sex differences in the early growth hormone responsiveness of sex-specific genes in mouse liver. Mol Endocrinol. 2010;24:667–678. doi: 10.1210/me.2009-0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lau-Corona D, Suvorov A, Waxman DJ. Feminization of male mouse liver by persistent growth hormone stimulation: activation of sex-biased transcriptional networks and dynamic changes in chromatin states. Mol Cell Biol. 2017;37. doi: 10.1128/MCB.00301-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernández-Pérez L, Guerra B, Díaz-Chico JC, Flores-Morales A. Estrogens regulate the hepatic effects of growth hormone, a hormonal interplay with multiple fates. Front Endocrinol (Lausanne). 2013;4:66. doi: 10.3389/fendo.2013.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Avtanski D, Novaira HJ, Wu S, et al. Both estrogen receptor alpha and beta stimulate pituitary GH gene expression. Mol Endocrinol. 2014;28:40–52. doi: 10.1210/me.2013-1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venken K, Schuit F, Van Lommel L, et al. Growth without growth hormone receptor: estradiol is a major growth hormone-independent regulator of hepatic IGF-I synthesis. J Bone Miner Res. 2005;20:2138–2149. doi: 10.1359/JBMR.050811 [DOI] [PubMed] [Google Scholar]
- 22. Mann SN, Hadad N, Nelson-Holte M, et al. Health benefits attributed to 17α-estradiol, a lifespan-extending compound, are mediated through estrogen receptor α. BioRxiv. 2020. doi: 10.1101/2020.06.02.130674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830–1831. doi: 10.1093/bioinformatics/btt285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao K, Quipildor GF, Tabrizian T, et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat Commun. 2018;9:2394. doi: 10.1038/s41467-018-04805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garratt M, Bower B, Garcia GG, Miller RA. Sex differences in lifespan extension with acarbose and 17-α estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell. 2017;16:1256–1266. doi: 10.1111/acel.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanson JR. Biological chemistry .1. Terpenoids and steroids. Ann Rep Progr Chem B. 1979;76:363–381. doi: 10.1039/oc9797600363 [DOI] [Google Scholar]
- 29. Fournier B, Gutzwiller S, Dittmar T, Matthias G, Steenbergh P, Matthias P. Estrogen receptor (ER)-alpha, but not ER-beta, mediates regulation of the insulin-like growth factor I gene by antiestrogens. J Biol Chem. 2001;276:35444–35449. doi: 10.1074/jbc.M105418200 [DOI] [PubMed] [Google Scholar]
- 30. Della Torre S, Rando G, Meda C, et al. Amino acid-dependent activation of liver estrogen receptor alpha integrates metabolic and reproductive functions via IGF-1. Cell Metab. 2011;13:205–214. doi: 10.1016/j.cmet.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 31. Garratt M, Lagerborg KA, Tsai YM, et al. Male lifespan extension with 17-alpha estradiol is linked to a sex-specific metabolomic response modulated by gonadal hormones in mice. Aging Cell. 2018;17(4):e12786. doi: 10.1111/acel.12786. Epub 2018 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garratt M, Leander D, Pifer K, et al. 17-alpha estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019;18(2):e12920. doi: 10.1111/acel.12920. Epub 2019 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashpole NM, Logan S, Yabluchanskiy A, et al. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 35. Nishikawa S, Sugimoto J, Okada M, Sakairi T, Takagi S. Gene expression in livers of BALB/C and C57BL/6J mice fed a high-fat diet. Toxicol Pathol. 2012;40:71–82. doi: 10.1177/0192623311422078 [DOI] [PubMed] [Google Scholar]
- 36. Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda). 2017;32:9–19. doi: 10.1152/physiol.00012.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunt NJ, Kang SWS, Lockwood GP, Le Couteur DG, Cogger VC. Hallmarks of aging in the liver. Comput Struct Biotechnol J. 2019;17:1151–1161. doi: 10.1016/j.csbj.2019.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang H, Liu Y, Wang L, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345–357. doi: 10.1194/jlr.M028969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Lu Y, Wang E, et al. Hepatic estrogen receptor α improves hepatosteatosis through upregulation of small heterodimer partner. J Hepatol. 2015;63:183–190. doi: 10.1016/j.jhep.2015.02.029 [DOI] [PubMed] [Google Scholar]
- 40. Renaud HJ, Cui JY, Lu H, et al. Effect of diet on expression of genes involved in lipid metabolism, oxidative stress, and inflammation in mouse liver-insights into mechanisms of hepatic steatosis. PLoS ONE. 2014;9:e88584. doi: 10.1371/journal.pone.0088584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foucaud-Vignault M, Soayfane Z, Ménez C, et al. P-glycoprotein dysfunction contributes to hepatic steatosis and obesity in mice. PLoS ONE. 2011;6:e23614. doi: 10.1371/journal.pone.0023614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osterreicher CH, Taura K, De Minicis S, et al. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929–938. doi: 10.1002/hep.23104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McDonnell AM, Dang CH. Basic review of the cytochrome p450 system. J Adv Pract Oncol. 2013;4:263–268. doi: 10.6004/jadpro.2013.4.4.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson GD. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health (Larchmt). 2005;14:19–29. doi: 10.1089/jwh.2005.14.19 [DOI] [PubMed] [Google Scholar]
- 46. Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. doi: 10.1159/000134751 [DOI] [PubMed] [Google Scholar]
- 47. Vermeulen A. Dehydroepiandrosterone sulfate and aging. Ann N Y Acad Sci. 1995;774:121–127. doi: 10.1111/j.1749-6632.1995.tb17376.x [DOI] [PubMed] [Google Scholar]
- 48. Han DH, Hansen PA, Chen MM, Holloszy JO. DHEA treatment reduces fat accumulation and protects against insulin resistance in male rats. J Gerontol A Biol Sci Med Sci. 1998;53:B19–B24. doi: 10.1093/gerona/53a.1.b19 [DOI] [PubMed] [Google Scholar]
- 49. Bauer M, Hamm AC, Bonaus M, et al. Starvation response in mouse liver shows strong correlation with life-span-prolonging processes. Physiol Genomics. 2004;17:230–244. doi: 10.1152/physiolgenomics.00203.2003 [DOI] [PubMed] [Google Scholar]
- 50. Grasfeder LL, Gaillard S, Hammes SR, et al. Fasting-induced hepatic production of DHEA is regulated by PGC-1alpha, ERRalpha, and HNF4alpha. Mol Endocrinol. 2009;23:1171–1182. doi: 10.1210/me.2009-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.