Abstract
Background
Orthostasis is a potent physiological stressor which adapts with age. The age-related accumulation of health deficits in multiple physiological systems may impair the physiological response to orthostasis and lead to negative health outcomes such as falls, depression, and cognitive decline. Research to date has focused on changes with orthostasis at prespecified intervals of time, without consideration for whole signal approaches.
Methods
One-dimensional statistical parametric mapping identified regions in time of significant association between variables of interest using a general linear model. Frailty index operationalized accumulated health and social deficits using 32-items from a computer-assisted interview. This study examined the association of frailty index on blood pressure, heart rate, and cerebral oxygenation during an orthostatic test in a sample of 2742 adults aged 50 or older from The Irish Longitudinal Study on Ageing.
Results
Frailty index was seen to be negatively associated with cerebral oxygenation changes from baseline over a period of 7 seconds (p = .036). Heart rate and systolic blood pressure were positively and negatively associated with frailty index over periods of 17 seconds (p = .001) and 10 seconds (p = .015), respectively.
Conclusions
Statistical parametric mapping demonstrated these significant regions of cerebral oxygenation during orthostasis provide indirect evidence of impaired autoregulation associated with frailty. Statistical parametric mapping also replicated prior relationships in heart rate and systolic blood pressure associated with a higher frailty index. These findings highlight the utility of 1-dimensional statistical parametric modeling in identifying significant regions of interest in physiological recordings.
Keywords: fNIRS, heart rate response, orthostatic hypotension
Background
Frailty is a state of increased vulnerability to stressors and considered a consequence of multisystem physiological dysregulation over a lifetime. Frailty can be considered as phenotypic (ie, robust, pre-frail, and frail), according to the number of criteria present among slowness, weakness, exhaustion, unintentional weight loss, and low physical activity (1). This phenotype is closely related to sarcopenia and impaired physical function (2). Alternatively, the frailty index (FI) considers the graded accumulation of health deficits (3) and is calculated as a ratio of deficits present in an individual from a predetermined list of 30 or more. Deficits encompass a broader range of age-related abnormalities (including symptoms, signs, diseases, disabilities) than those which define the phenotype.
Although dynamic responses underpin the state of increased vulnerability, that is, frailty, the characteristics of dynamic responses to physiological stress have not been widely examined (4). Orthostasis is the act of achieving upright posture and is a physiological stressor repeated many times each day and across the life span. It requires an integrated neurocardiovascular response to maintain adequate blood pressure (BP) and subsequently end-organ (eg, brain) perfusion (5). Hypotension in response to orthostasis has been considered as a final pathway of disordered physiology (6) and impairs cerebral function which commonly governs organ systems regulated via the autonomic nervous system. Prevalence of orthostatic hypotension increases with advancing age (7) and emerging evidence also suggests that orthostatic hypotension increases in frailty (8).
Impaired cerebral autoregulation is characterized by a mismatch between circulatory requirements of the brain and the ability of the body to generate and maintain brain blood flow requirements via neurocirculatory and humoral systems. Responses to physiological stress such as postural change are determined by neuronal and hormonal responses to peripheral hemodynamic change (9). Maintenance of consistent cerebral oxygenation is critical for appropriate responses to stressors. The absence of this control highlights the bidirectional interdependence of these systems, that is, cerebral function controls peripheral hemodynamics and peripheral hemodynamic function controls cerebral autoregulation and perfusion (10).
To date, investigation of orthostatic response has been predicated on peripheral physiological measures, without direct evidence of central dysfunction. Frailty is associated with orthostatic hypotension and more impairments in BP and heart rate (HR) recovery after standing (8,11,12). Heart rate variability is a marker of autonomic control and is attenuated in frail individuals (13). To our knowledge, continuous measurements of cerebral oxygenation coupled with peripheral hemodynamic changes have not been described before in frail individuals.
Statistical parametric mapping (SPM) is a form of vector-field analysis most commonly applied in the study of brain anatomy or functional brain activity (eg, functional MRI [fMRI]) (14). More recently this statistical approach has been validated for use in 1-dimensional data (15) and is appropriate for use in temporal conditions (eg, a measure which changes with time). One-dimensional SPM permits identification of regions significantly associated with the variable of interest. Traditional “zero-dimensional” statistical tests would be difficult to conduct at multiple time points (eg, 1-second intervals) given the need to adjust for multiple comparisons. One-dimensional SPM can utilize a general linear model and mitigates against type-1 and type-2 statistical errors (16) to assess this form of correlated data. Similar to fMRI, it is a method well-suited to identifying regions in a physiological trace for further investigation and future quantification of effect size.
The aim of this study was to apply 1-dimensional SPM in this novel context to examine the association of accumulated health deficits on BP, HR, and cerebral oxygenation during an orthostatic test in a large population sample of adults aged 50 or more from The Irish Longitudinal Study on Ageing (TILDA).
Method
Participants
The Irish Longitudinal Study on Ageing is a nationally representative prospective cohort study of community-dwelling adults aged 50 years and older residing in Ireland (17). It is designed using the Irish Geodirectory (a listing of all residential addresses in the Republic of Ireland) as a sampling frame. A random, clustered sample of addresses was chosen using the RANSAM system with residents aged ≥50 years and their spouses/partners (of any age) invited to participate in the study (n = 8175). Ethical approval for TILDA was granted by the Health Sciences Research Ethics Committee at Trinity College Dublin, Ireland. All participants provided written informed consent prior to assessment. Data were collected via computer-aided personal interviewing, self-completed questionnaire, and a center- or home-based physical health assessment.
This analysis included participants who had completed the center-based health assessment and computer-aided personal interviewing (n = 5364) at Wave 3 (March 2014 to April 2016). Individuals without valid continuous recordings during active stand were then excluded (leaving n = 2820). Further exclusions were made for those younger than 50 years (n = 30); Mini-Mental State Examination (MMSE) less than 24, diagnosis of Alzheimer’s disease or dementia (n = 38) and those with a diagnosis of Parkinson’s disease, as this condition may cause autonomic nervous system impairment (n = 11).
Frailty
Frailty was operationalized as an FI encompassing 32 self-reported deficits which cover multiple dimensions of health in older adults (18,19). Deficits included within the FI calculation are provided in Supplementary Table 1. Dichotomous variables are considered in their original form where 0 = absent and 1 = present. Categorical variables are considered as 5 fractions of the all possible responses (range: 0, 0.25, 0.5, 0.75, 1; where 0 is no deficits and 1 is all deficits). In practice the calculation is considered valid where up to 20% of the measures are absent. In these instances, the denominator is altered from the total number of measure (ie, 32) to the number of measures with complete responses. For visualization purposes, participants were classified as robust, pre-frail (cutoff: >0.10), and frail (cutoff: >0.25) as per previous studies (19). Frailty index was considered as a continuous variable in all statistical analyses.
Active Stand Protocol
In TILDA, the majority of participants attending the health center assessment during the third wave of data collection (2014–2015) completed an orthostatic test. This is an instrumented assessment described as an “active stand” and has been reported on in this sample extensively elsewhere (20,21). Participants were asked to stand as quickly as possible following a period of supine rest for a duration of 10 minutes. Participants with mobility difficulties were assisted by a research nurse if required. Continuous noninvasive beat-to-beat BP was recorded using a Finometer MIDI device (Finapres Medical Systems BV, Amsterdam, The Netherlands) at a sampling rate of 200 Hz. Data recording was initiated during supine resting and extended for 180 seconds after the participant had achieved orthostasis. Baseline values are calculated using data in 60- to 30-second period prior to orthostasis. The beginning of the orthostatic test, estimated using an integrated height sensor, was set to time zero for each participant. Beat-to-beat systolic BP (SBP) and HR were recorded. Nadir is the term employed in describing the initial drop in BP following orthostatic change.
Near-Infrared Spectroscopy
Real-time cerebral oxygenation was measured during the active stand using a continuous wave Artinis Portalite near-infrared spectroscopy system (Artinis Medical Systems, BV, Zetten, The Netherlands). Implementation of this technique has been described extensively in other settings (22) and also within TILDA active stand protocol (23). Briefly, an optical sensor on the patient’s forehead (over the left frontal lobe) measures changes in concentrations of oxyhemoglobin (O2Hb) and deoxyhemoglobin (HHb) continuously at a rate of 50 Hz. This is possible as a consequence of human tissue demonstrating relative permeability to light in the near-infrared spectrum. Tissue saturation index (TSI) was calculated from values of O2Hb and HHb (eqn 1) and considered as the primary measure of cerebral oxygenation in this study. Tissue saturation index is a commonly used value for cerebral oxygenation, representing the ratio of oxygenated hemoglobin to the sum of oxygenated and deoxygenated hemoglobin detected.
| (1) |
Statistical Analysis
Descriptive statistics were performed using STATA 15.0 (StataCorp, College Station, TX) to demonstrate demographic and clinical characteristics of the cohort. Temporal analyses of peripheral and central physiological measures were conducted within Python 3.6 (Python Software Foundation, https://www.python.org/) using the bundled Iterated Design Learning Environment. Open-source package SPM1d 0.4 (http://www.spm1d.org/) (24) was used for the analyses and is dependent primarily on SPM8 (https://www.fil.ion.ucl.ac.uk/spm/), originally described by Friston et al (14).
All data were examined as changes from baseline values established in a supine position prior to standing. Heart rate, SBP, and cerebral oxygenation data were resampled to 1 Hz, following which moving average and median filters were applied. The initial 30-second window after standing of data collected was analyzed using a “region-of-interest” approach (25). This a priori decision was justified given that the majority of participants normalize to their baseline values of cardiovascular function within this time frame (21). This facilitates a more accurate estimation of the true within-region association. Inclusion of the resting period prior to and long after orthostasis in the SPM analysis would not be informative regarding the outcomes, as the cardiovascular curves of these resting periods are inherently flat in nature. The region-of-interest chosen was justified given the extensive literature to date which examines the orthostatic response (ie, increase in BP [or HR], followed by an initial orthostatic drop and a final recovery to baseline).
The alpha level was set at 0.05 for all analysis and specific p values are reported for each significant region of the curve analyzed. Univariate regression considered the association of higher FI on dependent variables. Multivariate regressions used the same model with the addition of covariates sex, age, and an age-squared term (to account for potentially nonlinear association with age). Residuals were inspected visually for normality of distribution, which was found to be the case. PP and QQ plots were also produced to confirm this which gave a linear fit of R2 = 0.97. Sex was adjusted for given the well-described differences evident in frailty (26) and age was included to isolate the association attributed to the accumulation of deficits. One-dimensional SPM analysis returns regions of significance in the form of clusters. These are contiguous values over which the curve is determined to be not consistent with random sampling (ie, 2 clusters would indicate that 2 regions of contiguous values were significantly associated with the independent variable). Temporal range, reported in the results tables, is the period following standing (in seconds) over which significant associations with FI were identified. Extent is analogous with duration of this significant region of the curve and reported as seconds in our analysis.
Results
This analysis was conducted with a sample of 45.9% female participants, with a mean age of 64.4 years (±7.7 years). Calculated values of FI for this sample ranged from 0 to 0.51 based on 32-items representing a range of health deficits, with a mean of 0.1 (±0.08). According to Rockwood classifications based on FI (where >0.10 indicates pre-frailty and >0.25 indicates frailty); the majority of this sample were Robust (60.5%) or Pre-Frail (32.6%). Frail participants with the highest accumulation of health deficits represented 6.9% of the sample analyzed. Baseline summary statistics of HR, SBP, and TSI (measured using functional near-infrared spectroscopy) are provided in Table 1. All reported findings are expressed in changes from this calculated baseline.
Table 1.
Participant Characteristics (n = 2742)
| Robust (n = 1659) | Pre-Frail (n = 894) | Frail (n = 189) | |
|---|---|---|---|
| Age (years) | 62.6 ± 7.0 | 66.7 ± 7.9 | 68.4 ± 8.1 |
| Sex (% female) | 48.8% | 42.1% | 38.0% |
| Baseline heart rate (BPM) | 64.9 ± 9.9 | 65.4 ± 9.8 | 67.1 ± 10.9 |
| Baseline systolic blood pressure (mmHg) | 139.9 ± 20.5 | 142.5 ± 22.2 | 144.0 ± 22.7 |
| Baseline tissue saturation index (%) | 72.7 ± 4.9 | 72.4 ± 5.0 | 72.5 ± 4.7 |
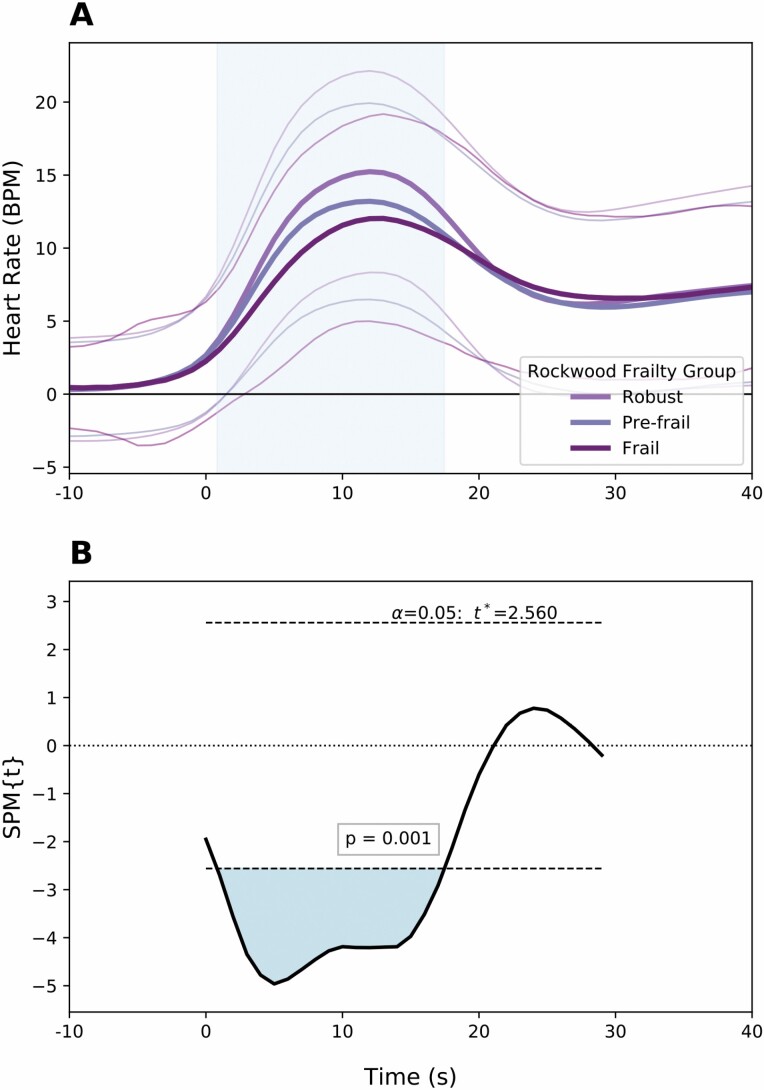
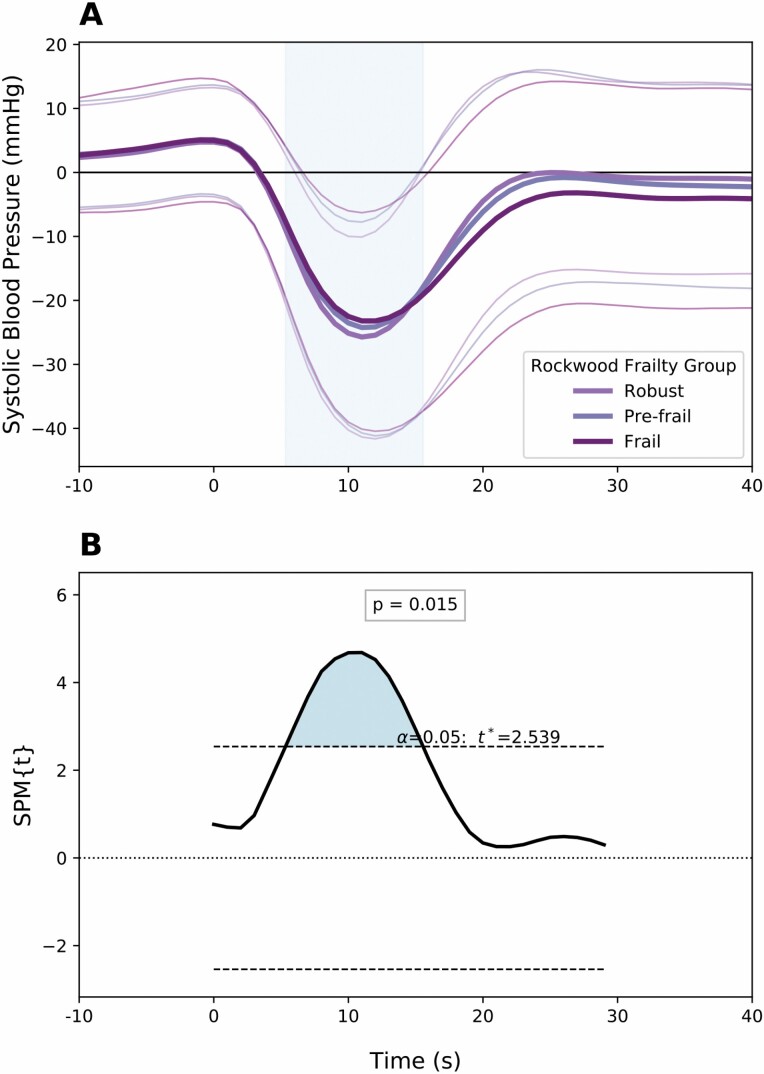
Using SPM, FI was seen to have a significant association with regions of HR, SBP, and cerebral oxygenation recordings when adjusting for demographic confounders (Table 2). Using multivariate regression, the association with HR was significant (p = .001) over a period up to 17 seconds after standing. Participants with higher FI demonstrated curves with lower HR recovery over this period (Figure 1). There was a significant contralateral association with BP for those with a high FI (p = .015). Higher SBP values were recorded over the period from 5 to 15 seconds after standing (Figure 2). This corresponds to lower magnitude nadir in peripheral pressures after achieving standing position.
Table 2.
Relationship Between Frailty Index and Hemodynamic Function Within 30 Seconds of Standing Estimated by Statistical Parametric Mapping
| Clusters | Association of Frailty Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate Regression | Multivariate Regression | ||||||||
| Temporal Range (s) | Duration (s) | t Value | p Value | Temporal Range (s) | Duration (s) | t Value | p Value | ||
| Heart rate | 1 | 0.9–19.3 | 18.5 | −2.56 | .0005 | 0.8–17.5 | 16.6 | −2.56 | 0.0011 |
| Systolic blood pressure | 2 | 4.7–13.2 | 8.5 | 2.54 | .0218 | 5.3–15.6 | 10.2 | 2.54 | 0.0149 |
| 18.4–24.4 | 6.0 | −2.54 | .0328 | ||||||
| Tissue saturation index | 1 | 17.7–29.0 | 11.3 | −2.48 | .0199 | 20.1–26.9 | 6.8 | −2.47 | 0.0363 |
Notes: The alpha level was set at 0.05 for all analysis and specific p values are reported for each significant region of the curve analyzed. Univariate regression considered the association of higher frailty index on dependent variables. Multivariate regressions used the same model with the addition of covariates sex, age, and an age-squared term (to account for potentially nonlinear association with age). Temporal range is consistent with the period following standing (in seconds) over which significant differences between curves were identified. Extent is analogous with duration of the significant region of the curve.
Figure 1.
(A) Mean ± SD values of change from baseline in heart rate within 30 seconds of orthostasis. Separate traces are provided for frailty phenotypes (derived from values of frailty index). (B) Statistical parametric map demonstrating the consecutive values (in seconds) of heart rate change from baseline for which frailty index is significantly associated. Notation spm{t} refers to the t-statistic and the threshold for significance is provided within the figure.
Figure 2.
(A) Mean ± SD values of change from baseline in systolic blood pressure within 30 seconds of orthostasis. Separate traces are provided for frailty phenotypes (derived from values of frailty index). (B) Statistical parametric map demonstrating the consecutive values (in seconds) of systolic blood pressure change from baseline for which frailty index is significantly associated. Notation spm{t} refers to the t-statistic and the threshold for significance is provided within the figure.
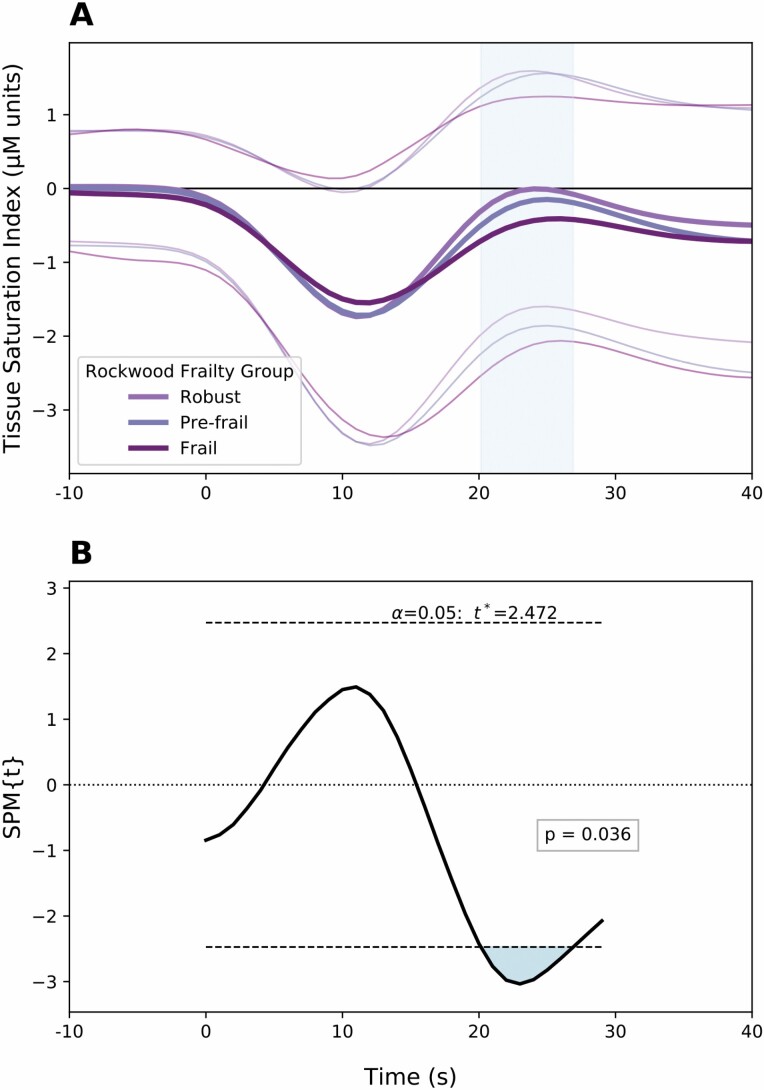
In comparison to associations noted with HR and peripheral BP, cerebral oxygenation was seen to be associated with FI at a later time point (Figure 3). Significant differences were observed between 20 and 26 seconds after standing (p = .036). Higher FI was associated with a reduced cerebral oxygenation (t = −2.771). This region is described as following the initial orthostatic nadir, where oxygenation appears to trend toward recovery to baseline. No significant difference in cerebral oxygenation was observed for participants with higher FI during the initial nadir, in contrast to BP and HR.
Figure 3.
(A) Mean ± SD values of change from baseline in cerebral oxygenation within 30 seconds of orthostasis. Separate traces are provided for frailty phenotypes (derived from values of frailty index). (B) Statistical parametric map demonstrating the consecutive values (in seconds) of cerebral oxygenation change from baseline for which frailty index is significantly associated. Notation spm{t} refers to the t-statistic and the threshold for significance is provided within the figure.
Significant associations for covariates (age and sex) within the multivariate regression were noted across HR, SBP, and cerebral oxygenation. The duration and limits of these periods are detailed within the Supplementary Material. Supplementary Figures 1–3 demonstrating statistical parametric maps with contrast vectors for age and sex are also provided for each peripheral and central hemodynamic variable.
Conclusions
For the first time, we demonstrate that frailty is associated with differences in cerebral oxygenation following orthostasis. Uniquely we utilized an application of vector-field analysis, SPM, which detailed differences in neurocardiovascular responses to orthostasis.
Our group has previously reported an association between frailty phenotype and failure of stabilization of BP after orthostasis using beat-to-beat BP recordings and a linear spline approach within a mixed-associations model in the TILDA cohort (11). This built upon a prior univariate analysis in a convenience sample of older adults which emphasizes the importance of a beat-to-beat approach to BP measurement (12). The use of novel SPM analysis in this study is a major strength and provides evidence for further utilization of this technique in the field. Notably, the approach utilizes all the temporal data recorded from a recording device such as a Finometer. The significant regions of cerebral perfusion, BP, and HR change following orthostasis are identified as potentially subtle. With regards cerebral perfusion, a standard analysis utilizing only prespecified arbitrary time points (eg, 5, 10, 20 seconds post orthostasis) would have potentially missed the signal identified using 1-dimensional SPM.
In line with previous studies, we also demonstrated differences in peripheral hemodynamic parameters (ie, BP, HR) in the setting of orthostasis and frailty. Frailty index demonstrated an incremental slowing of HR in response to orthostasis. This slowing effect was confined to the initial period following standing. These differences may represent a progressive deconditioning and/or autonomic decoupling of neurocardiovascular integration. In keeping with frailty being a predictor of mortality (27), early impairment in the vector of HR changes during orthostasis (within the same temporal window—10 to 20 seconds) is a risk factor for cardiovascular disease and for all-cause mortality (28,29).
A novel finding is that there was a significant relationship between FI and cerebral oxygenation. A greater FI significantly decreased the TSI during the period 20–27 seconds following postural change. The timing and duration of this deficit is significant given that there is evidence to suggest that some latency exists in the ability of the cerebral blood flow to respond to peripheral changes (eg, those brought about by orthostasis) (30). Ní Bhuachalla et al propose that this neurocardiovascular instability may precipitate oxidative stress and a cascade-like effect, contributing to potential end-organ damage (31). Similarly, Canney et al reported a relationship between kidney function and impaired orthostatic BP control (32). These pathways may in turn contribute to impaired homeostasis of adequate end-organ oxygenation and predispose to further insult.
There is a growing quantitative data to support impaired physiological response to stressors which define frailty. Brain, endocrine, inflammatory, immune and metabolic and oxidative stress dysfunction have been identified (33). Wang et al highlight the difficulties in attributing causation in these instances given the cross-sectional nature of the majority of studies. Associations with frailty have been demonstrated in a variety of intrinsic physiological processes including diurnal cortisol variation (34) and muscle energetics, metabolism and repair (35). Kalyani et al provide an example of impaired stressor response in frailty by exploring the performance in older women during oral glucose tolerance test (36). Dysregulation in these frail individuals is interpreted as physiological vulnerability and decreased reserve. Specifically related to hemodynamics our group reported impaired peripheral hemodynamic compensation following orthostasis in the TILDA study at baseline (11). The inability of cerebral autoregulation to account for these changes and maintain consistent tissue oxygenation as reported in this current study provides further evidence of decrement in homeostatic response in more frail individuals. This is proposed as a further indicator of health in aging, adding to the broad collection of such markers already in use (37).
Exploration of cerebral blood flow or oxygenation in frailty has been limited to date. The cardinal study by Lutski et al identified a differences in cerebral hemodynamics for patients with Fried frailty (38). Using breath-holding index and transcranial Doppler to measure resting readings of cerebrovascular reactivity (divided into tertiles), an association was identified with incident frailty phenotype at 2-year follow-up. The analysis was confined to individuals with cardiovascular disease. This analysis in TILDA presents results of a cross-sectional nature (FI was quantified at the same time point as the health assessment) and a larger and more generalizable population. While breath-holding index utilized by Lutski may be considered a similar mild stressor (the key component in the definition of frailty), this TILDA analysis uses measurement of organ oxygenation directly as opposed to blood flow through a feeding artery (for which alternative compensatory mechanisms may play a significant role). There is limited evidence to date comparing the interplay between cerebral oxygenation, frailty, and white matter hyperintensity burden. Given that FI and white matter hyperintensity burden are correlated (39), the results presented here suggest scope for white matter hyperintensity to be an underlying process, or consequence of, impaired cerebral oxygenation.
This study demonstrates the advantage of a 1-dimensional approach to analysis of physiological signals over traditional methods, a technique usually applied to biomechanical analysis but of which the prerequisites are also satisfied by hemodynamic trace measurements. Given the robustness of SPM analysis stemming from MRI research over the past 3 decades, this group suggests that adoption of 1-dimensional SPM may be of benefit to this field of research. One-dimensional SPM facilitates hypothesis-driven research using mathematical models which are well-developed and extensively validated (40). Statistical parametric mapping is reported to mitigate against regional conflation and a priori assumption of time points of interest (41). In this data set, previous analysis of continuously measured BP demonstrated moderate associations with frailty. This study has demonstrated that associations are distributed in a temporal manner which would not have been readily identifiable at discrete time points. Furthermore, these differences are shown to be consistent across a single time period and not isolated at the time points previously analyzed (ie, 10, 20, 30 seconds, etc.) (11).
Strengths of this analysis include the large population from which the data are collected. The impact of accumulated deficits (FI) on cerebral oxygenation has not been examined in such a large cohort to date. This analysis contributes significantly to the body of literature on frailty and neurocardiovascular instability. Similarly, this is the largest study to date which implements 1-dimensional SPM analysis. Statistical parametric mapping use to date has been confined to smaller samples with limited ability to adjust for confounding variables. The combination of our large sample and detailed data on comorbidities allows for some generalizability of the results to the population from which they are sampled, namely older community-dwelling adults.
This study is limited by measurement of frontal cerebral oxygenation without any objective measure of total brain area perfusion. However, literature on appropriate analysis of near-infrared spectroscopy recordings notes that there is good correlation between these 2 functions (42). Near-infrared spectroscopy is noted to have good correlation with transcranial Doppler, a methodology used widely in the measurement of cerebral hypotension (43). The Python implementation of the SPM toolbox does not enable survey settings to be applied to regression models. Therefore, we were unable to make use of the health assessment weighting and information on sampling strata within TILDA. Interpretations of the above findings should consider that participants who attended health center assessment were healthier than participants who did not. Effect sizes were not calculated in this analysis. Furthermore, the participants in TILDA present with relatively low accumulated deficits with 92.8% of participants being classified as Robust or Pre-Frail. A broader analysis of hospitalized or patients attending clinic may demonstrate more pronounced changes in peripheral and central cardiovascular function. Not examined in this study, a 1-dimensional SPM analysis of frailty phenotype response to orthostasis would further elaborate on differences between the 2 cohorts. Use of 3-level variables within the SPM regression toolbox was not validated at the time of analysis and therefore this research question was not explored. While not possible to date, integration of all neurocardiovascular data recorded during an activity such as orthostasis may provide a comprehensive overview of the systematic response to an external stressor. Future studies should consider this approach and the potential role of 1-dimensional SPM to achieve robust results.
In conclusion, this study demonstrates a dose-dependent relationship between the burden of health deficits and cerebral oxygenation in older adults. This finding provides further evidence to support progressive physiological dysregulation with maladaptive aging, which has previously been shown to be linked with mortality and poor health outcomes (44). Evidence that physiological stressors in more frail individuals brings about relative oxygenation deficits could support a feed-forward process, whereby frailer individuals deteriorate in aging at a more rapid rate than their healthy peers. We propose that further investigations into the role of white matter hyperintensities in such a process may be insightful. In addition to specific decrements in oxygenation, this analysis also provides support for the definition of frailty, whereby individuals express impaired ability to maintain homeostasis when faced with a mild physiological stressor (3). Results reported above for differences in HR, BP, and cerebral oxygenation response demonstrate that physiological regulation in these individuals is potentially a global phenomenon. This highlights the importance of assessing dynamic system response in frail individuals (45). Further exploration of deranged physiological systems in frail individuals may highlight further pathways of maladaptive aging.
Supplementary Material
Acknowledgments
We would like to thank all participants in TILDA for their continued dedication of time to research.
Funding
Financial support for TILDA is provided by the Irish Department of Health, the Atlantic Philanthropies, and Irish Life. Funders have no involvement in the study design, collection, analysis, and interpretation of data. R.R.-O. is supported by Science Foundation Ireland grant number 18/FRL/6188.
Conflict of Interest
None declared.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2. Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc. 2014;15(12):853–859. doi: 10.1016/j.jamda.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 3. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 4. Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997;26(4):315–318. doi: 10.1093/ageing/26.4.315 [DOI] [PubMed] [Google Scholar]
- 5. van Wijnen VK, Hove DT, Finucane C, et al. Hemodynamic mechanisms underlying initial orthostatic hypotension, delayed recovery and orthostatic hypotension. J Am Med Dir Assoc. 2018;19(9):786–792. doi: 10.1016/j.jamda.2018.05.031 [DOI] [PubMed] [Google Scholar]
- 6. Wieling W, Schatz IJ. The consensus statement on the definition of orthostatic hypotension: a revisit after 13 years. J Hypertens. 2009;27(5):935–938. doi: 10.1097/HJH.0b013e32832b1145 [DOI] [PubMed] [Google Scholar]
- 7. Low PA. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18(suppl 1):8–13. doi: 10.1007/s10286-007-1001-3 [DOI] [PubMed] [Google Scholar]
- 8. Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people: orthostatic BP responses and frailty in elders. J Am Geriatr Soc. 2011;59(4):655–665. doi: 10.1111/j.1532-5415.2011.03352.x [DOI] [PubMed] [Google Scholar]
- 9. Tan CO, Taylor JA. Integrative physiological and computational approaches to understand autonomic control of cerebral autoregulation: autonomic control of cerebral autoregulation. Exp Physiol. 2014;99(1):3–15. doi: 10.1113/expphysiol.2013.072355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183 [DOI] [PubMed] [Google Scholar]
- 11. O’Connell MD, Savva GM, Finucane C, Romero-Ortuno R, Fan CW, Kenny RA. Impairments in hemodynamic responses to orthostasis associated with frailty: results from The Irish Longitudinal Study on Ageing (TILDA): frailty and orthostatic blood pressure. J Am Geriatr Soc. 2018;66(8):1475–1483. doi: 10.1111/jgs.15327 [DOI] [PubMed] [Google Scholar]
- 12. Romero-Ortuno R, Cogan L, O’Shea D, Lawlor BA, Kenny RA. Orthostatic haemodynamics may be impaired in frailty. Age Ageing. 2011;40(5):576–583. doi: 10.1093/ageing/afr076 [DOI] [PubMed] [Google Scholar]
- 13. Varadhan R, Chaves PH, Lipsitz LA, et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci. 2009;64(6):682–687. doi: 10.1093/gerona/glp013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2(4):189–210. doi: 10.1002/hbm.460020402 [DOI] [Google Scholar]
- 15. Pataky TC. rft1d: smooth one-dimensional random field upcrossing probabilities in Python. J Stat Softw. 2016;71(7). doi: 10.18637/jss.v071.i07 [DOI] [Google Scholar]
- 16. Pataky TC, Vanrenterghem J, Robinson MA. The probability of false positives in zero-dimensional analyses of one-dimensional kinematic, force and EMG trajectories. J Biomech. 2016;49(9):1468–1476. doi: 10.1016/j.jbiomech.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 17. Donoghue OA, McGarrigle CA, Foley M, Fagan A, Meaney J, Kenny RA. Cohort profile update: The Irish Longitudinal Study on Ageing (TILDA). Int J Epidemiol. 2018;47(5):1398–1398l. doi: 10.1093/ije/dyy163 [DOI] [PubMed] [Google Scholar]
- 18. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Theou O, O’Connell MDL, King-Kallimanis BL, O’Halloran AM, Rockwood K, Kenny RA. Measuring frailty using self-report and test-based health measures. Age Ageing. 2015;44(3):471–477. doi: 10.1093/ageing/afv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cronin H, O’Regan C, Finucane C, Kearney P, Kenny RA. Health and aging: development of the Irish Longitudinal Study on Ageing health assessment. J Am Geriatr Soc. 2013;61(suppl 2):S269–S278. doi: 10.1111/jgs.12197 [DOI] [PubMed] [Google Scholar]
- 21. Finucane C, O’Connell MD, Fan CW, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130(20):1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831 [DOI] [PubMed] [Google Scholar]
- 22. Mirelman A, Maidan I, Bernad-Elazari H, et al. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014;11:85. doi: 10.1186/1743-0003-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Briggs R, Carey D, Claffey P, et al. The association between frontal lobe perfusion and depressive symptoms in later life. Br J Psychiatry. 2019;214(4):1–7. doi: 10.1192/bjp.2018.288 [DOI] [PubMed] [Google Scholar]
- 24. Pataky TC. One-dimensional statistical parametric mapping in Python. Comput Methods Biomech Biomed Engin. 2012;15(3):295–301. doi: 10.1080/10255842.2010.527837 [DOI] [PubMed] [Google Scholar]
- 25. Pataky TC, Robinson MA, Vanrenterghem J. Region-of-interest analyses of one-dimensional biomechanical trajectories: bridging 0D and 1D theory, augmenting statistical power. PeerJ. 2016;4:e2652. doi: 10.7717/peerj.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hubbard RE. Sex differences in frailty. In: Theou O, Rockwood K, eds. Interdisciplinary Topics in Gerontology and Geriatrics. Vol 41. S. Karger AG; 2015:41–53. doi: 10.1159/000381161 [DOI] [PubMed] [Google Scholar]
- 27. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61(9):1537–1551. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 28. McCrory C, Berkman L, Nolan H, O’Leary N, Foley M, Kenny RA. Speed of heart rate recovery in response to orthostatic challenge: a strong risk marker of mortality. Circ Res. 2016;119(5):666–675. doi: 10.1161/CIRCRESAHA.116.308577 [DOI] [PubMed] [Google Scholar]
- 29. Romero-Ortuno R, O’Connell MD, Finucane C, Fan CW, Kenny RA. Higher orthostatic heart rate predicts mortality: the Irish Longitudinal Study on Ageing (TILDA). Aging Clin Exp Res. 2015;27(2):239–242. doi: 10.1007/s40520-014-0261-8 [DOI] [PubMed] [Google Scholar]
- 30. Tan CO. Defining the characteristic relationship between arterial pressure and cerebral flow. J Appl Physiol (1985). 2012;113(8):1194–1200. doi: 10.1152/japplphysiol.00783.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ní Bhuachalla B, McGarrigle CA, Kenny RA. Neurocardiovascular instability may modulate end-organ damage: a review of this hypothesis investigating the eye and manifestations of NCVI. Med Hypotheses. 2015;85(5):594–602. doi: 10.1016/j.mehy.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 32. Canney M, O’Connell MDL, Sexton DJ, et al. Graded association between kidney function and impaired orthostatic blood pressure stabilization in older adults. J Am Heart Assoc. 2017;6(5). doi: 10.1161/JAHA.117.005661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J, Maxwell CA, Yu F. Biological processes and biomarkers related to frailty in older adults: a state-of-the-science literature review. Biol Res Nurs. 2019;21(1):80–106. doi: 10.1177/1099800418798047 [DOI] [PubMed] [Google Scholar]
- 34. Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63(2):190–195. doi: 10.1093/gerona/63.2.190 [DOI] [PubMed] [Google Scholar]
- 35. Varadhan R, Russ DW, Gabr RE, et al. Relationship of physical frailty to phosphocreatine recovery in muscle after mild exercise stress in the oldest-old women. J Frailty Aging. 2019;8(4):162–168. doi: 10.14283/jfa.2019.21 [DOI] [PubMed] [Google Scholar]
- 36. Kalyani RR, Varadhan R, Weiss CO, Fried LP, Cappola AR. Frailty status and altered glucose-insulin dynamics. J Gerontol A Biol Sci Med Sci. 2012;67(12):1300–1306. doi: 10.1093/gerona/glr141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112(30):E4104–4110. doi: 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lutski M, Haratz S, Weinstein G, Goldbourt U, Tanne D. Impaired cerebral hemodynamics and frailty in patients with cardiovascular disease. J Gerontol A Biol Sci Med Sci. 2018;73(12):1714–1721. doi: 10.1093/gerona/glx253 [DOI] [PubMed] [Google Scholar]
- 39. Siejka TP, Srikanth VK, Hubbard RE, et al. Frailty and cerebral small vessel disease: a cross-sectional analysis of the Tasmanian Study of Cognition and Gait (TASCOG). J Gerontol A Biol Sci Med Sci. 2018;73(2):255–260. doi: 10.1093/gerona/glx145 [DOI] [PubMed] [Google Scholar]
- 40. Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. NeuroImage. 1995;2(3):173–181. doi: 10.1006/nimg.1995.1023 [DOI] [PubMed] [Google Scholar]
- 41. Pataky TC. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J Biomech. 2010;43(10):1976–1982. doi: 10.1016/j.jbiomech.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 42. Tak S, Ye JC. Statistical analysis of fNIRS data: a comprehensive review. Neuroimage. 2014;85:72–91. doi: 10.1016/j.neuroimage.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 43. Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke. 1998;29(1):104–111. doi: 10.1161/01.str.29.1.104 [DOI] [PubMed] [Google Scholar]
- 44. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation: frailty prevalence and outcome. J Am Geriatr Soc. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 45. Varadhan R, Walston JD, Bandeen-Roche K. Can a link be found between physical resilience and frailty in older adults by studying dynamical systems? J Am Geriatr Soc. 2018;66(8):1455–1458. doi: 10.1111/jgs.15409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.