Abstract
Background
The response to adrenocorticotropic hormone (ACTH) is poorly characterized in old-old adults and may provide insight into the physiologic response to stress.
Method
We performed a standard 250 µg ACTH stimulation test in a home-based substudy of 51 women aged 85–96 years enrolled in the Women’s Health and Aging Study II who were not taking corticosteroids. We examined the cortisol and dehydroepiandrosterone (DHEA) responses at 0, 30, 60, and 120 minutes, overall and by frailty status.
Results
The peak cortisol response to ACTH could not be determined, with the highest levels at the 120-minute time point. Pre- and post-ACTH stimulated cortisol levels did not differ by frailty status over this time frame, with no difference in the characteristics of the dose-response curves. Pre- and post-ACTH stimulated DHEA levels also did not differ by frailty status, though the dose-response curves suggested divergence after stimulation, with a more rapid DHEA response with increasing frailty.
Conclusions
Our data demonstrate a robust cortisol response to ACTH challenge testing, but inadequate negative feedback in old-old women, resulting in prolonged exposure to cortisol. Future studies should examine dynamic cortisol and DHEA responses in this age group, using a less potent ACTH stimulus and longer collection period.
Keywords: Cosyntropin, Adrenal, Stimulation
The circadian profile of the hypothalamic-pituitary-adrenal (HPA) axis changes with aging (1). Regulation of the HPA axis involves multiple inputs from endocrine, neural, and immune systems, as well as acute and chronic responses to environmental stress. In turn, cortisol is essential in gluconeogenesis, regulation of the inflammatory cascade, stress response, and survival. Even mild dysregulation of the stress system affects homeostasis of many physiologic processes, including endocrine, psychiatric, metabolic, cardiovascular, immune, and musculoskeletal systems.
It is generally agreed that aging is associated with an increase in 24-hour cortisol secretion and decline in adrenal androgen production (2,3). It has been hypothesized that chronic elevation of cortisol is due to an age-associated impairment of feedback to the HPA axis. Although basal levels of cortisol and dehydroepiandrosterone (DHEA) have been well characterized with respect to age, few studies have examined cortisol and DHEA responses to stressful stimuli by age (4,5), and none in the oldest-old.
In this study, we sought to determine the cortisol and DHEA response to adrenocorticotropic hormone (ACTH) stimulation in women in their 80s and 90s, looking in particular to baseline levels, peak response, and time to return to baseline. We selected ACTH stimulation as a standardized physiologic challenge to which we could assess the dynamic response overall and by frailty status. We hypothesized that, compared to nonfrail women, frail women would have similar baseline and peak cortisol levels and a more gradual cortisol return to baseline. For DHEA, we hypothesized similar baseline levels but a blunted DHEA response in frailty. We also hypothesized a less-coordinated cortisol and DHEA response in the frail, consistent with diminished synchronization of responses in frailty.
Method
Study participants were community-dwelling women who enrolled in 1994 in a longitudinal population-based study, the Women’s Health and Aging Study II (WHAS II) (6). Women ≥65 years old were recruited from a random sample selected from the Health Care Financing Adminstration’s Medicare Eligibility list for Baltimore, Maryland. WHAS II enrolled 436 women aged 70–79 representative of the two-thirds least disabled older women living in the community. All standardized evaluations, interviews, and physical examinations were conducted at the Johns Hopkins Functional Laboratory during seven study visits from 1994 to 2008. At the seventh study visit, subjects were invited to participate in a substudy in which ACTH stimulation tests were performed at the participant’s home. All participants in the home visit were ambulatory. The ACTH stimulation substudy visits occurred from November 2009 to May 2010. Women taking oral or injectable glucocorticoids or who had any previous allergic reaction to ACTH injection were excluded from the substudy. No women were taking inhaled corticosteroids. Information on topical corticosteroid use was not collected. The Johns Hopkins University Institutional Review Board approved the study, and all participants gave informed consent. A total of 51 participants participated.
Procedure
The study was completed at the participant’s home. All participants were fasting and all tests were initiated between 10 and 11 AM. An intravenous catheter was inserted and a serum sample collected (Time 0), followed by intravenous administration of 250 μg Cosyntropin (synthetic ACTH) over 1–2 minutes. Additional serum samples were collected at 30, 60, and 120 minutes. During the collection period, participants were at rest. All samples were processed on site in a centrifuge, transported on ice, and stored at −80°C for batched analysis. Cortisol was measured using a radioimmunoassay kit from Diagnostic Systems Laboratory, Inc. Assay sensitivity was 0.11 μg/dL, and inter-assay and intra-assay coefficients of variation were 4.65% and 6.3%, respectively. DHEA was measured using a radioimmunoassay kit from Rocky Mountain Diagnostics. Assay sensitivity was 0.02 ng/mL, and inter-assay and intra-assay coefficients of variation were 4.31% and 5.53%, respectively.
Outcomes
Frailty status was assessed from data at the seventh study visit using the standardized definition developed by Fried and colleagues (7) in the Cardiovascular Health Study and validated by Bandeen-Roche and colleagues (6) in the WHAS studies. Five criteria were used: shrinking (body mass index < 18.5 kg/m2 or 5% annual weight loss [BMI]), weakness (grip strength equivalent to lowest 20% in Cardiovascular Health Study, by gender and BMI), poor endurance (self-reported exhaustion), slowness (walking speed equivalent to lowest 20% in Cardiovascular Health Study, by height) and low activity (activity level in kilocalories per week equivalent to the lowest 20% in Cardiovascular Health Study). Those with zero criteria were categorized as nonfrail, those with one to two criteria as prefrail, and those with more than three criteria as frail.
Baseline characteristics were compared by frailty status using the chi-squared test for binary outcomes and Student’s t tests for continuous outcomes. Median values (±interquartile range) at 0, 30, 60, 90, or 120 minutes were compared. Integration of the cortisol and DHEA curves (ie, area under the curve) was calculated by the standard trapezoid method using participants with measurements at all timepoints. The three frailty groups were treated as separate categorical groups in analyses.
Results
The mean age of study participants was 87.4 years (range 85–96 years) and 17% were non-white (Table 1). There were 14 nonfrail, 32 prefrail, and five frail participants. When categorized by the number of frailty criteria, 14 participants had zero; 21 had one; 11 had two; and five had three criteria. None had four or five criteria.
Table 1.
Demographic and Clinical Characteristics of Women by Frailty Status, WHAS II
| All (n = 51) | Nonfrail (n = 14) | Prefrail (n = 32) | Frail (n = 5) | p Value | |
|---|---|---|---|---|---|
| Age (y) | 86.4 (81–93) | 86.5 (84–93) | 86.3 (81–92) | 86.0 (84–88) | .84 |
| White (%) | 83.0 | 85.7 | 84.8 | 60 | .38 |
| Education (y) | 13.1 | 13.8 | 13.0 | 12.4 | .88 |
| BMI (kg/m2) | 27.0 (17.7–37.3) | 24.6 (20.3–29.5) | 27.8 (17.7–37.3) | 28.8 (23.5–36.4) | .28 |
| Cortisol (μg/dL) | |||||
| Pre-ACTH | 13.4 (7.5–25.9) | 13.0 (7.5–22.9) | 13.4 (8.2–25.9) | 14.6 (10.9–21.5) | .77 |
| 30 min | 27.4 (18.1–42.3) | 26.2 (18.3–34.6) | 27.8 (18.7–39.5) | 28.6 (18.1–42.3) | .51 |
| 60 min | 32.5 (17.7–51.2) | 31.3 (17.7–39.5) | 33.3 (23.3–51.2) | 31.6 (22.8–41.1) | .77 |
| 120 min | 39.3 (23.1–70.9) | 37.7 (23.1–43.3) | 40.2 (29.2–70.9) | 38.6 (35.6–43.5) | .73 |
| Integrated area | 30.1 (18.4–45.1) | 28.8 (18.4–35.5) | 30.9 (22.6–45.2) | 29.5 (22.6–45.2) | .73 |
| DHEA (µg/dL) | |||||
| Pre-ACTH | 1.9 (0.4–5.6) | 1.8 (0.6–3.7) | 2.0 (0.4–5.6) | 1.9 (1.2–3.2) | .86 |
| 30 min | 5.2 (0.7–13.7) | 4.4 (1.2–7.6) | 5.4 (0.7–11.8) | 6.4 (2.1–13.7) | .81 |
| 60 min | 6.2 (0.9–14.8) | 5.2 (1.5–8.1) | 6.5 (0.9–14.8) | 7.3 (2.6–13.6) | .85 |
| 120 min | 7.1 (0.9–15.1) | 6.5 (3.9–9.6) | 7.3 (0.9–15.1) | 7.1 (2.8–13.7) | .85 |
| Integrated area | 5.6 (0.8–12.6) | 4.8 (1.1–7.6) | 5.8 (0.8–12.6) | 6.3 (2.4–12.2) | .86 |
Note: Mean values and ranges are reported. ACTH = Adrenocorticotropin hormone; DHEA = dehydroepiandrosterone; WHAS II = Women’s Health and Aging Study II.
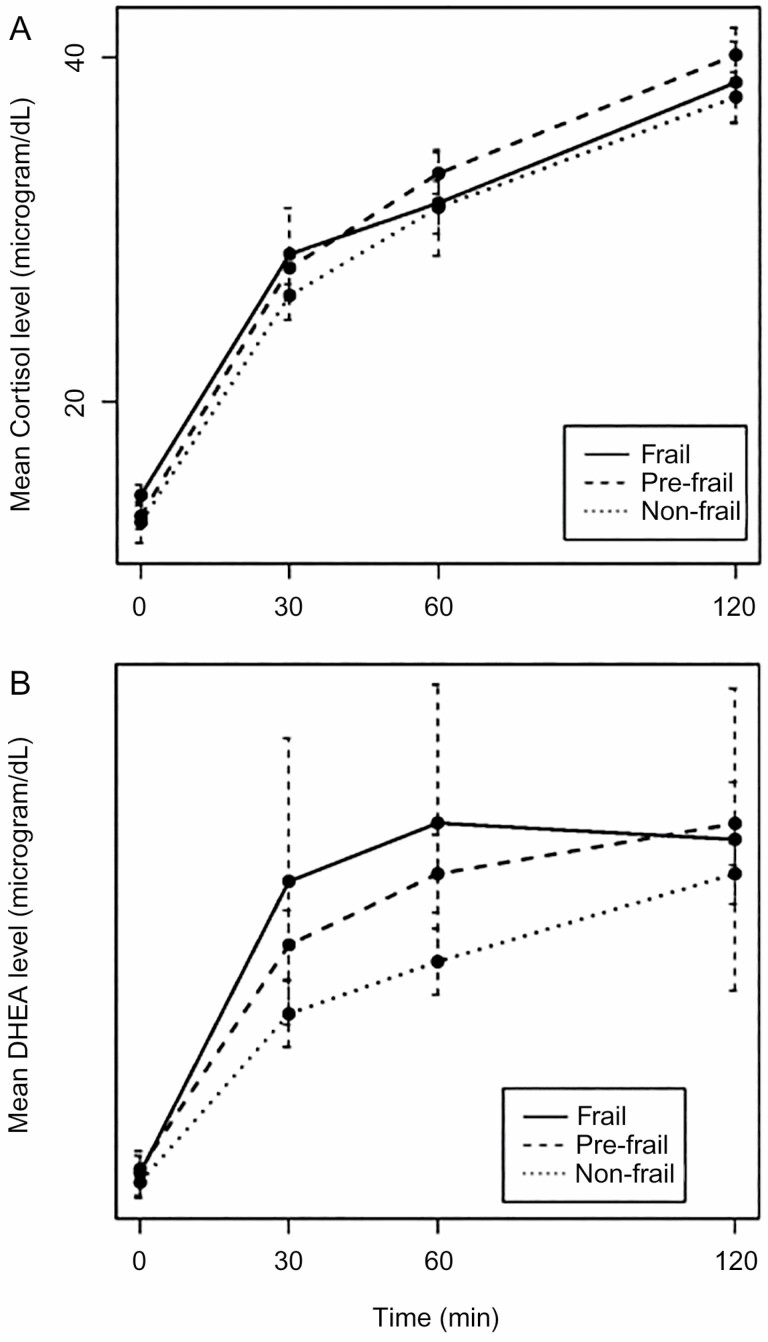
No participants had adrenal insufficiency, defined as stimulated 30- and 60-minute cortisol less than 18 μg/dL. However, the timing of the peak cortisol response to ACTH stimulation could not be determined during the 120-minute sampling time, with the mean cortisol levels continuing to trend upwards in all groups at this time point (Figure 1). In addition, DHEA levels were low at baseline in all participants.
Figure 1.
Cortisol and DHEA response to ACTH stimulation test by frailty status. Mean ± SE (error bars) for (A) cortisol and (B) DHEA values at 0, 30, 60, 120 min after 250 μg ACTH.
Pre- and post-ACTH stimulated cortisol levels did not differ by frailty status (Table 1), with no difference in the characteristics of the dose-response curves (Figure 1). Pre- and post-ACTH stimulated DHEA levels were not statistically significantly different by frailty status (Table 1), though the dose-response curves suggested divergence after stimulation, with a more rapid DHEA response to ACTH by frailty status (Figure 1). There was no significant trend in either cortisol area under the curve or DHEA area under the curve versus number of frailty criteria.
Discussion
Although the diurnal pattern of cortisol and DHEA in aging has been previously evaluated, our study is the first, to the best of our knowledge, to examine the ACTH response in anyone over the age of 80 years, and to examine its relationship to frailty. Our study demonstrates similar pre-ACTH stimulation levels of cortisol and DHEA between frail, prefrail, and nonfrail participants. Our findings after ACTH stimulation show a prolonged cortisol response to a strong physiologic stimulus in oldest-old women, with inadequate feedback to dampen the initial vigorous response. These data add to existing data on higher diurnal exposure to cortisol with increasing age and may show that the chronically primed adrenal gland has an exaggerated response to an ACTH stimulus. Additional possibilities include diminished metabolism of ACTH or cortisol clearance. In keeping with the concept of adrenal priming, all of the women showed an increase in DHEA levels after ACTH stimulation, and there appeared to be a greater initial DHEA response to stimulation with increasing degree of frailty, though this was not statistically significant.
There is a general consensus that the diurnal pattern of cortisol secretion changes with age. When comparing healthy young to healthy old participants, studies have found that in older participants, there was elevated evening plasma or salivary cortisol levels (8,9) and decreased diurnal variability (8,10) of cortisol. In these studies, the mean age of older participants varied between 60 to 70 years.
There is increasing evidence that the diurnal patterns are also different in frailty, consistent with more severe HPA axis dysregulation. In a previous analysis of 214 WHAS II participants who were aged 80–90 years old, frailty burden was independently associated with higher evening salivary cortisol, greater 24-hour mean cortisol, and smaller diurnal amplitude of cortisol (11). Each additional frailty criterion contributed a 12% increase in evening salivary cortisol and 7% increase in 24-hour mean cortisol level. An analysis from the KORA study confirmed these findings, with less diurnal variation in salivary cortisol with frailty (12). A smaller study of 69 institutionalized residents (mean age 77) found elevated morning and evening salivary cortisol levels in frail residents, with frailty load significantly associated with the morning cortisol level (13). Taken together, these studies suggest a greater exposure to elevated levels of cortisol and loss of fine-tuning of the HPA axis in frailty.
Previous studies comparing stressor response (psychosocial or pharmacological) in healthy young to healthy old subjects have shown variable results. Several studies found that older subjects showed a greater response to stress than young, demonstrated by a greater increase in cortisol levels in response to the stressor (4,14–16). Conversely, one study found no change in peak response to the hypothalamic hormone corticotropin-releasing hormone in men aged 66–78 years, though a longer recovery period, with 80% higher post-stimulation minimum cortisol levels in these older men (17). Our study is consistent with both a large increase in cortisol levels in response to 250 μg ACTH (mean cortisol change from baseline to 120 minutes of 26 μg/dL) and a recovery period that was too long to be captured during the 120-minute time frame. It is possible that we would have found differences by frailty status if we had extended our sampling period. Although peak cortisol levels generally occur before 120 minutes, they have been found as late as 120 to 150 minutes in some healthy young people after receiving 250 μg ACTH (18,19). Alternatively, the 250 μg ACTH dose used in our study is designed to produce maximal cortisol response, such as would be found under extreme physiologic stress. With chronic physiologic exposure to endogenous ACTH over the course of the day, it is likely that the adrenal glands of older women are primed for a vigorous response to this dose of ACTH. A lower dose of ACTH may have been sufficient to elicit differential responses by frailty, if present, over a shorter time frame.
It has been well established that adrenal androgens, in particularly DHEA and DHEA-S, decrease with age (1) and in the old frail (20). Our study confirmed this, with all participants demonstrating low DHEA levels at baseline. Additionally, a study comparing older, postmenopausal women (age range 55–68 years) showed that maximal DHEA response to 250 μg ACTH stimulation was significantly lower in older women compared to younger women (15). It is intriguing that our study suggested increased DHEA responsivity to ACTH stimulation in the frail group, albeit with limited power to confirm this effect. This is consistent with a generalized hyperresponsivity to ACTH stimulation in the frail, due to priming of the adrenal gland from chronic stimulation (8), as well as generalized dysregulation of physiologic responses commonly found in frail women (21). The dose of ACTH could have been too high to elicit subtle differences in cortisol responses to ACTH, but appropriate to observe a difference in DHEA response in the frail. This is also suggested by Parker et al., who found that cortisol secretion was approximately two times more sensitive to ACTH than DHEA was (15). However, historical comparison data are not available to evaluate whether the DHEA response in our study was prolonged.
The strengths of this study include the inclusion of very old participants, with the mean age of 87 years. Our study also incorporated a non-stressful setting at the participant’s home, where they were at rest throughout the sampling interval. In addition, the 250 μg ACTH stimulation test is the standard clinical test for adrenal insufficiency, allowing generalizability of our findings to the clinical setting and also allowing for a standardized challenge. However, this produced a profound cortisol response, which likely affected our results. Additional limitations include the small number of study participants, particularly those with frailty; the low severity of frailty in those who were frail; lack of a concurrent comparator group of younger women; no measurement of ACTH levels to evaluate for possible prolongation of ACTH clearance; lack of assessment of major depressive disorder or recent infection; and inclusion of only women, which limits generalizability to older men. We did not measure DHEA-S, which has a longer half-life and larger pool of distribution than DHEA, or cortisol binding globulin levels.
Frailty is a clinical syndrome characterized by decreased reserve and resistance to stressors, resulting in vulnerability to adverse outcomes (7). The dynamic response to stressors is thought to be impaired in frailty, and may be impaired at an early stage of the pathogenesis of frailty. There is likely to be heterogeneity in the degree of HPA axis impairment that is not exclusively attributable to age, as previously demonstrated in studies of diurnal cortisol variation in WHAS II (11). Although our study did not show a difference in response to challenge by frailty status, the changes to HPA axis may be better explored with a less potent dose of ACTH and/or longer sampling time. Our study results suggested a possible exaggeration of DHEA in response to ACTH, which would be better assessed with a larger sample size and longer sampling time.
Summary
This is the first study to look at ACTH stimulation responses in old-old people. The results suggest a vigorous cortisol response under clinically standard 250 μg doses, which may be too potent to distinguish differences in cortisol response in the old frail, though this may be an appropriate dose for observing DHEA response to stimulus. More investigation is needed of this important axis in this age group. Future studies should examine dynamic cortisol and DHEA responses in this age group, using a less-potent stimulus and more sustained collection period.
Funding
This work was supported by the National Institutes of Health (R37 AG19905, UL1 RR025005) and the Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center Clinical Research Units.
Conflict of Interest
None reported.
References
- 1. Yiallouris A, Tsioutis C, Agapidaki E, et al. . Adrenal aging and its implications on stress responsiveness in humans. Front Endocrinol (Lausanne). 2019;10:54. doi: 10.3389/fendo.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yen SS, Laughlin GA. Aging and the adrenal cortex. Exp Gerontol. 1998;33:897–910. doi: 10.1016/s0531-5565(98)00046-1 [DOI] [PubMed] [Google Scholar]
- 3. Moffat SD, An Y, Resnick SM, Diamond MP, Ferrucci L. Longitudinal change in cortisol levels across the adult life span. J Gerontol A Biol Sci Med Sci. 2020;75:394–400. doi: 10.1093/gerona/gly279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 5. Giordano R, Di Vito L, Lanfranco F, et al. . Elderly subjects show severe impairment of dehydroepiandrosterone sulphate and reduced sensitivity of cortisol and aldosterone response to the stimulatory effect of ACTH(1-24). Clin Endocrinol (Oxf). 2001;55:259–265. doi: 10.1046/j.1365-2265.2001.01317.x [DOI] [PubMed] [Google Scholar]
- 6. Bandeen-Roche K, Xue QL, Ferrucci L, et al. . Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 8. Deuschle M, Gotthardt U, Schweiger U, et al. . With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61:2239–2246. doi: 10.1016/s0024-3205(97)00926-0 [DOI] [PubMed] [Google Scholar]
- 9. Raff H, Raff JL, Duthie EH, et al. . Elevated salivary cortisol in the evening in healthy elderly men and women: correlation with bone mineral density. J Gerontol A Biol Sci Med Sci. 1999;54:M479–M483. doi: 10.1093/gerona/54.9.m479 [DOI] [PubMed] [Google Scholar]
- 10. Kumari M, Badrick E, Sacker A, Kirschbaum C, Marmot M, Chandola T. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. 2010;35:1091–1099. doi: 10.1016/j.psyneuen.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 11. Varadhan R, Walston J, Cappola AR, Carlson MC, Wand GS, Fried LP. Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol A Biol Sci Med Sci. 2008;63:190–195. doi: 10.1093/gerona/63.2.190 [DOI] [PubMed] [Google Scholar]
- 12. Johar H, Emeny RT, Bidlingmaier M, et al. . Blunted diurnal cortisol pattern is associated with frailty: a cross-sectional study of 745 participants aged 65 to 90 years. J Clin Endocrinol Metab. 2014;99:E464–E468. doi: 10.1210/jc.2013-3079 [DOI] [PubMed] [Google Scholar]
- 13. Holanda CM, Guerra RO, Nóbrega PV, Costa HF, Piuvezam MR, Maciel ÁC. Salivary cortisol and frailty syndrome in elderly residents of long-stay institutions: a cross-sectional study. Arch Gerontol Geriatr. 2012;54:e146–e151. doi: 10.1016/j.archger.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 14. Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009 [DOI] [PubMed] [Google Scholar]
- 15. Parker CR Jr, Slayden SM, Azziz R, et al. . Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85:48–54. doi: 10.1210/jcem.85.1.6265 [DOI] [PubMed] [Google Scholar]
- 16. Vermeulen A, Deslypere JP, Schelfhout W, Verdonck L, Rubens R. Adrenocortical function in old age: response to acute adrenocorticotropin stimulation. J Clin Endocrinol Metab. 1982;54:187–191. doi: 10.1210/jcem-54-1-187 [DOI] [PubMed] [Google Scholar]
- 17. Waltman C, Blackman MR, Chrousos GP, Riemann C, Harman SM. Spontaneous and glucocorticoid-inhibited adrenocorticotropic hormone and cortisol secretion are similar in healthy young and old men. J Clin Endocrinol Metab. 1991;73:495–502. doi: 10.1210/jcem-73-3-495 [DOI] [PubMed] [Google Scholar]
- 18. Amsterdam JD, Maislin G, Abelman E, Berwish N, Winokur A. Adrenocortical responsiveness to the ACTH stimulation test in depressed patients and healthy volunteers. J Affect Disord. 1986;11:265–274. doi: 10.1016/0165-0327(86)90078-9 [DOI] [PubMed] [Google Scholar]
- 19. Rubin RT, Miller TH, Rhodes ME, Czambel RK. Adrenal cortical responses to low- and high-dose ACTH(1-24) administration in major depressives vs. matched controls. Psychiatry Res. 2006;143:43–50. doi: 10.1016/j.psychres.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 20. Cappola AR, Xue QL, Fried LP. Multiple hormonal deficiencies in anabolic hormones are found in frail older women: the Women’s Health and Aging studies. J Gerontol A Biol Sci Med Sci. 2009;64:243–248. doi: 10.1093/gerona/gln026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen AA, Legault V, Li Q, Fried LP, Ferrucci L. Men sustain higher dysregulation levels than women without becoming frail. J Gerontol A Biol Sci Med Sci. 2018;73:175–184. doi: 10.1093/gerona/glx146 [DOI] [PMC free article] [PubMed] [Google Scholar]