Abstract
Aging results in a chronic, proinflammatory state which can promote and exacerbate age-associated diseases. In contrast, physical activity in older adults improves whole body health, protects against disease, and reduces inflammation, but the elderly are less active making it difficult to disentangle the effects of aging from a sedentary lifestyle. To interrogate this interaction, we analyzed peripheral blood collected at rest and postexercise from 68 healthy younger and older donors that were either physically active aerobic exercisers or chronically sedentary. Subjects were profiled for 44 low-abundance cytokines, chemokines, and growth factors in peripheral blood. At rest, we found that regular physical activity had no impact on the age-related elevation in circulating IL-18, eotaxin, GRO, IL-8, IP-10, PDGF-AA, or RANTES. Similarly, there was no impact of physical activity on the age-related reduction in VEGF, EGF, or IL-12 (p70). However, older exercisers had lower resting plasma fractalkine, IL-3, IL-6, and TNF-α compared to sedentary older adults. In contrast to our resting characterization, blood responses following acute exercise produced more striking difference between groups. Physically active younger and older subjects increased over 50% of the analyzed factors in their blood which resulted in both unique and overlapping exercise signatures. However, sedentary individuals, particularly the elderly, had few detectable changes in response to exercise. Overall, we show that long-term physical activity has a limited effect on age-associated changes in basal cytokines and chemokines in the healthy elderly, yet physically active individuals exhibit a broader induction of factors postexercise irrespective of age.
Keywords: Growth factors, Human aging, Inflammation, Physical activity
Aging and its associated diseases such as diabetes, atherosclerosis, neurodegeneration, and arthritis are major contributors to global morbidity and mortality. As a result, health care costs are the greatest late in life (1) and are anticipated to balloon over the next several decades as the global elderly population expands (2). Circulating cytokines, chemokines, and growth factors are essential communication signals for the immune system and tissue maintenance; however, the secretion of these factors is altered over the human life span (3,4). Collectively, this results in chronic, low-grade inflammation and the extent of proinflammatory signaling is associated with mortality, several age-associated diseases as well as physical frailty (5,6). However, some circulating factors counteract inflammatory signaling (eg, IL-10) (7) or enhance tissue repair (eg, PDGF) (8). Thus, a comprehensive understanding of the humoral changes in the blood during aging and their reversibility is essential to successfully manage age-associated pathologies.
Exercise is a broadly anti-inflammatory intervention which improves physical function, lowers the risk of chronic disease, and reduces mortality (9). One hypothesis for why exercise benefits the health of elderly individuals is that it enables a more sensitive or potent immune response to infections, potentially as a result of its ability to lower baseline inflammation. For example, older adults have prolonged fever and inflammatory responses to pathogens compared to young individuals (10,11); however, the aerobically trained elderly have improved immune responses (12). Additionally, regular exercise is commonly associated with lower basal levels of inflammation as seen by reduced levels of IL-6, C-reactive protein, and TNF-α in the blood of physically active as compared to sedentary older individuals (13–15). However, most studies investigating aging, physical activity, and systemic signaling primarily focus on alterations in cytokines and chemokines in the basal, resting state. Exercise produces transient elevations in circulating factors which can counter inflammatory stress (16,17) and may contribute to a reversal of chronic, low-grade inflammation (18). Thus, a better understanding of postexercise responses may provide critical information regarding these beneficial anti-inflammatory processes. Prior studies involving aging and physical activity have typically characterized relatively few analytes in the blood, likely because of the high expense of this analysis in large subject cohorts. However, there are many cytokines and chemokines in circulation that are potentially modulated by aging and exercise and contribute to age-related pathologies. Thus, a broader characterization of the circulating factors impacted by aging and physical activity is needed.
To understand the influence of aerobic exercise on age-induced circulating factors, we analyzed a panel of 44 factors in the blood across 2 different contexts: (i) resting levels compared in young and old, sedentary and physically active exercisers and (ii) acute, postexercise changes from rest following a single bout of aerobic cycling exercise at the same relative intensity within these same age and physical activity groups.
Materials and Methods
Subject Recruitment and Testing
Subjects were recruited from the greater Hamilton, Ontario region of Canada and are a subset of a research cohort previously described (19,20). Participants were permitted to be taking no more than 1 blood pressure medication and/or 1 lipid-lowering medication to be eligible for the study. Physically active subjects were those that completed 4 or more hours of aerobic exercise per week for the previous 10 years. Sedentary subjects were recruited that completed no more than 1 hour of moderate-to-vigorous exercise per week for the previous 10 years. An exception was made for those under the age of 25 years old, such that they had to have at least 5 years of aerobic training history. Young subjects were 20–45 years of age and old subjects were 64–86 years of age. These age limits were selected to avoid middle-aged adults, commonly identified as being in the range of 45–65 (21) and are nearly identical to age groups used in other aging cohorts (22). In a preliminary recruitment visit, sedentary elderly subjects underwent a walking treadmill stress test with EKG monitoring to screen for any unknown cardiac issues. On the day of the study, subjects arrived into the laboratory after an overnight fast, a catheter was inserted into the antecubital vein, and a resting blood sample was acquired. Subjects then underwent a series of anthropometric and functional tests as previously described (20). After a 5-minute break, all subjects performed a test to estimate aerobic capacity using an incrementally graded cycle ergometer test with indirect calorimetry. At the conclusion of this cycling test, maximum oxygen uptake (VO2 peak) was confirmed as an RER >1.1 and a plateau in VO2. The duration of the VO2peak test did not differ among the groups (young sedentary: 13.3 ± 2.2 minutes; young physically active: 12.0 ± 1.7 minutes; old sedentary: 11.9 ± 0.8 minutes; old physically active: 12.6 ± 1.8 minutes; mean ± SD, p ≥ .49 via analysis of variance [ANOVA]). After attaining VO2peak, subjects were permitted a 5-minute break. Participants then performed a 30-minute cycling session at a power setting that was 50% of the maximum attained at VO2peak and oxygen uptake was recorded during the final 10 minutes to assess exercise intensity. Immediately following the cessation of exercise, blood was again drawn for the postexercise blood sample. For blood sampling, venous blood was drawn into EDTA-treated tubes for plasma or allowed to clot for serum separation. A vial of whole, EDTA-treated blood was used for complete blood counts at the McMaster University Children’s Hospital Core Facility. All remaining blood samples were centrifuged within 10 minutes of sampling at 1500 × g for 5 minutes at room temperature. Separated serum or plasma from EDTA tubes was collected from the middle 50% of the upper layer lacking white and red blood cells using a clean, disposable bulb pipette into 1.5 mL Eppendorf tubes and immediately flash frozen in liquid nitrogen. Plasma and serum samples were stored at –80°C until analysis.
Cytokine and Chemokine Analysis
Plasma (EDTA) cytokines from the subjects were initially analyzed using a human 42-analyte multiplex ELISA assay (Millipore, Billerica, MA) using standard detection limits (3.2–10,000 pg/mL). The 42-plex analysis for each plasma sample included: sCD40L, EGF, Eotaxin/CCL11, FGF-2, Flt-3 ligand, Fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, sIL-2RA, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, MCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, TGF-α, TNF-α, TNF-β, and VEGF. However, due to the low abundance of some analytes, GM-CSF, IFN-γ, IL-10, IL-12 (p70), IL-13, IL-1β, IL-2, IL- 4, IL-5, IL-6, IL-7, IL-8, and TNF-α were re-assessed using high-sensitivity ELISAs (0.13–2000 pg/mL; Millipore, Billerica, MA, USA) in the same samples. Since the high-sensitivity 13-plex panel produced far fewer undetectable results, we only report these results combined with the lower-sensitivity analysis of the remaining factors. All multiplex ELISA analysis was performed in technical duplicate. Plasma IL-18 was assessed in duplicate by colorimetric ELISA (#7620, MBL International, Woburn, MA). Serum BDNF was assayed in duplicate by colorimetric ELISA (#DBD00, R&D Systems) using serum diluted 1:40. All samples that measured above or below the standard curve range were excluded from analysis and were considered not detectable. The number of samples from each analyte that were in the detectable range for analysis are provided in Supplementary Tables 1 and 2.
Statistical Analysis
All baseline anthropometric measurements, complete blood counts, and resting blood analyte data were analyzed using a 2-way ANOVA with age and activity group as factors. If significance (p < .05) was attained, a Tukey’s post hoc analysis was performed to identify specific differences between individual groups. Acute postexercise changes in blood analytes were compared to the respective resting values using a paired t test. The number of detectable samples from the blood cytokine and chemokine analysis at rest as well as their response to acute exercise were separately compared across all groups using a repeated measures ANOVA.
Results
Subject Characteristics
We initially sought to characterize the basal, resting concentration of cytokines, chemokines, and growth factors in the circulation altered by age and habitual aerobic exercise. We used fasting blood samples collected from a subset of young (20–45 years old) and old (64–86 years old) subjects who were either sedentary or highly physically active that we have previously described (19,20). We recruited subjects that were healthy irrespective of age or activity group and were free of chronic diseases. Descriptive characteristics of the subjects are provided in Table 1. As we have previously reported within the full cohort, young and old physically active subjects were of similar age to their respective sedentary group, but had lower body fat and a greater relative amount of lean mass (Table 1). Moreover, physically active subjects engaged in far more moderate-to-vigorous exercise per week, had greater aerobic capacity (VO2peak), and exhibited higher cycling power output at VO2peak (Table 1).
Table 1.
Physical Characteristics of the Study Participants
| Young | Old | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Variable | Sedentary | Physically Active | Sedentary | Physically Active | Age Effect | Group Effect | Age × Group |
| N | 15 | 16 | 23 | 14 | |||
| Sex (M/F) | 5/10 | 8/8 | 14/9 | 10/4 | |||
| Age (years) | 29.6 ± 7.0 | 31.5 ± 8.0 | 71.8 ± 7.1 | 71.7 ± 5.6 | p < .001 | NS | NS |
| Height (cm) | 167 ± 11 | 176 ± 7 | 170 ± 8 | 172 ± 8 | NS | p = .013 | NS |
| Weight (kg) | 68.5 ± 18.3 | 70.1 ± 8.9 | 80.1 ± 12.5a | 68.1 ± 10.1b | NS | NS | p = .02 |
| Body fat (%) | 35.4 ± 8.1 | 20.8 ± 8.7 | 36.5 ± 15.8 | 25.3 ± 6.7 | NS | p < .001 | NS |
| Body fat (kg) | 24.8 ± 10.9 | 14.5 ± 6.0 | 30.6 ± 16.1 | 17.1 ± 4.5 | NS | p < .001 | NS |
| Lean mass (%) | 64.6 ± 8.1 | 79.3 ± 8.7 | 63.5 ± 15.8 | 74.7 ± 6.7 | NS | p < .001 | NS |
| Lean mass (kg) | 43.7 ± 10.6 | 55.7 ± 9.9a | 49.5 ± 9.2 | 51.0 ± 10.0 | NS | p = .005 | p = .025 |
| Cycling VO2 max (mL/kg/min) | 33.8 ± 7.9 | 57.3 ± 8.4a,b | 26.7 ± 6.5a | 36.6 ± 9.3b,c | p < .001 | p < .001 | p = .05 |
| Power at VO2 max (W) | 144 ± 41 | 319 ± 67a,b | 125 ± 41 | 175 ± 52b,c | p < .001 | p < .001 | p = .005 |
| Peak power relative to body mass (W/kg) | 2.2 ± 0.5 | 4.6 ± 0.8 | 1.6 ± 0.5 | 2.6 ± 0.6 | p < .001 | p < .001 | NS |
| Recent moderate-to-vigorous exercise volume (h/wk) | 0.03 ± 0.09 | 8.8 ± 3.5 | 0.01 ± 0.05 | 6.0 ± 3.5 | NS | p < .001 | NS |
Notes: NS = nonsignificant. Data are mean ± SD. Statistically significant results (p < .05) are bolded.
aSignificantly different (p < .05) from Sedentary Young. bSignificantly different (p < .05) from the Sedentary Old. cSignificantly different (p < .05) from Physically Active Young.
Circulating Blood Cell Populations
To establish the baseline hematological properties of our subjects which might influence the abundance of cytokines and chemokines in the circulation, a complete blood count (CBC) analysis was performed on freshly collected venous blood samples. All CBC results were within the normal, healthy range, indicating no overt signs of infection, anemia, or other confounding clinical issues. While there was no effect of age or activity group on platelets, older adults had lower relative lymphocytes and monocytes (Table 2). Additionally, absolute leukocytes were reduced in physically active individuals irrespective of age and absolute lymphocytes were reduced by both aging and group factors (Table 2). Thus, in the setting of healthy aging, habitual exercise causes relatively modest changes in some white blood cell subsets.
Table 2.
Complete Blood Count Results of the Study Participants
| Young | Old | Statistics | ||||
|---|---|---|---|---|---|---|
| Blood Cell Parameters | Sedentary | Physically Active | Sedentary | Physically Active | Age Effect | Group Effect |
| White blood cells (×109/L) | ||||||
| Leukocytes | 5.9 ± 0.4 | 5.2 ± 0.3 | 5.8 ± 0.3 | 4.6 ± 0.3 | NS | p = .009 |
| Lymphocytes | 2.1 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 | p = .0004 | p = .002 |
| Monocytes | 0.4 ± 0.04 | 0.4 ± 0.04 | 0.5 ± 0.03 | 0.4 ± 0.03 | NS | p = .079 |
| Neutrophils | 3.3 ± 0.4 | 3.2 ± 0.3 | 3.5 ± 0.3 | 2.7 ± 0.2 | NS | NS |
| Basophils | 0 | 0 | 0 | 0 | NS | NS |
| Eosinophils | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.1 ± 0.02 | 0.1 ± 0.02 | NS | NS |
| White blood cells (relative) | ||||||
| Lymphocytes | 0.38 ± 0.02 | 0.30 ± 0.02 | 0.27 ± 0.02 | 0.29 ± 0.02 | p = .022 | NS |
| Monocytes | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | p = .016 | NS |
| Neutrophils | 0.53 ± 0.02 | 0.59 ± 0.03 | 0.60 ± 0.02 | 0.59 ± 0.02 | p = .096 | NS |
| Basophils | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | NS | NS |
| Eosinophils | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | p = .062 | NS |
Note: NS = nonsignificant (p > .05). Data are mean ± SEM. Statistically significant results (p < .05) are bolded. There were no significant age by group statistical interactions.
Baseline Blood Values of Cytokines, Chemokines, and Growth Factors
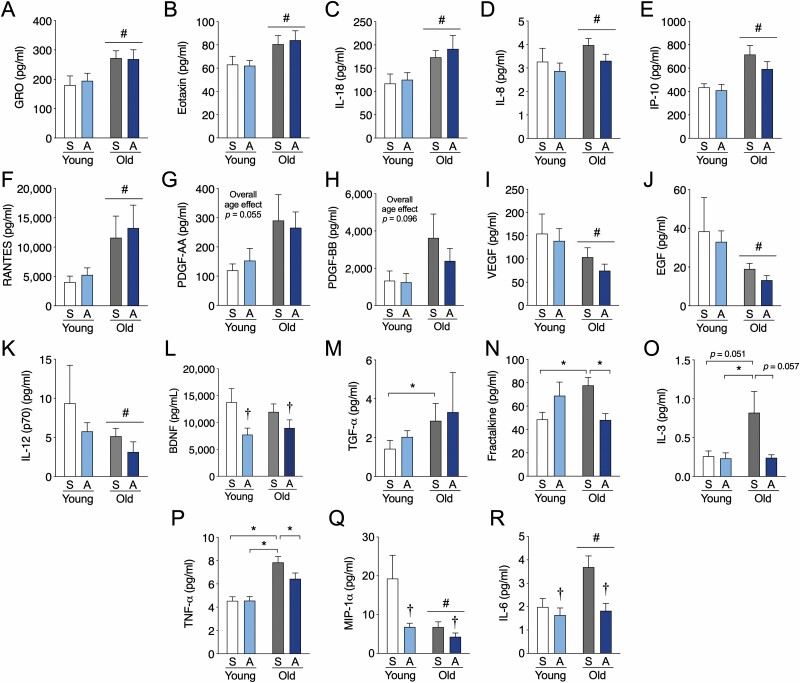
To broadly assess the impact of aging and long-term aerobic exercise on resting levels of circulating factors, we characterized the basal abundance of 44 proteins in previously collected plasma and serum samples. The proteins included: sCD40L, EGF, Eotaxin/CCL11, FGF-2, Flt-3 ligand, Fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-1α, IL-1β, IL-1RA, IL-2, sIL-2RA, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10, MCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, RANTES, TGF-α, TNF-α, TNF-β, VEGF, IL-18, and BDNF. As the cytokine/chemokine panel analysis is prone to relatively high numbers of undetectable samples, we have provided group mean ± SD and the number of detectable samples in Supplementary Table 1. When analyzing all factors at baseline, the young physically active group had a significantly higher fraction of detectable samples compared to the young sedentary (p = .006) and old sedentary (p = .039) group and tended to be greater than the old physically active group (p = .054). We found that several factors were significantly higher in older individuals at rest independent of physical activity group including, GRO, eotaxin, IL-18, IL-8, IP-10, RANTES, PDGF-AA, and PDGF-BB (trend, p = .096) (Figure 1A–H). Conversely, VEGF, EGF, and IL-12 (p70) were lower in older individuals independent of physical activity group (Figure 1I–K) and BDNF was overall lower in physically active individuals, independent of aging effects (Figure 1L). Several interesting age by activity group interactive effects were also evident in our analysis. TGF-α levels were higher in old sedentary subjects versus young sedentary subjects (Figure 1M). There was a protective influence of long-term physical activity against the effects of sedentary aging on plasma fractalkine, IL-3, and TNF-α, whereby levels were higher in older versus younger sedentary individuals but lower in older physically active participants compared to older sedentary subjects (Figure 1N–P). We also found 2 circulating factors that exhibited both age effects and activity group effects without an interactive effect. MIP-1α was lower in both physically active and older individuals (Figure 1Q) and IL-6 was generally lower in physically active individuals, but higher with age (Figure 1R). Thus, only a small number of resting circulating factors that are altered by aging are abrogated by long-term aerobic exercise adherence.
Figure 1.
Aerobic exercise training partially modifies the aging profile of resting humoral factors to mimic that of young individuals. (A–H) Circulating factors in peripheral blood elevated in older adults or (I–K) reduced in older adults irrespective of exercise history compared to younger individuals. (L) Factors that are lower in physically active individuals irrespective of age. (M–P) Factors with interactive effects in younger and older sedentary and physically active individuals. (Q–R) Resting analytes displaying a significant effect of both age and physical activity. Data are mean ± SEM. A = physically active; S = sedentary. *Significantly different (p < .05) from the indicated group as determined from an age by physical activity group interaction. †Significant overall effect (p < .05) of physical activity. #Significant overall effect (p < .05) of aging.
The Response of Circulating Factors to Acute Exercise
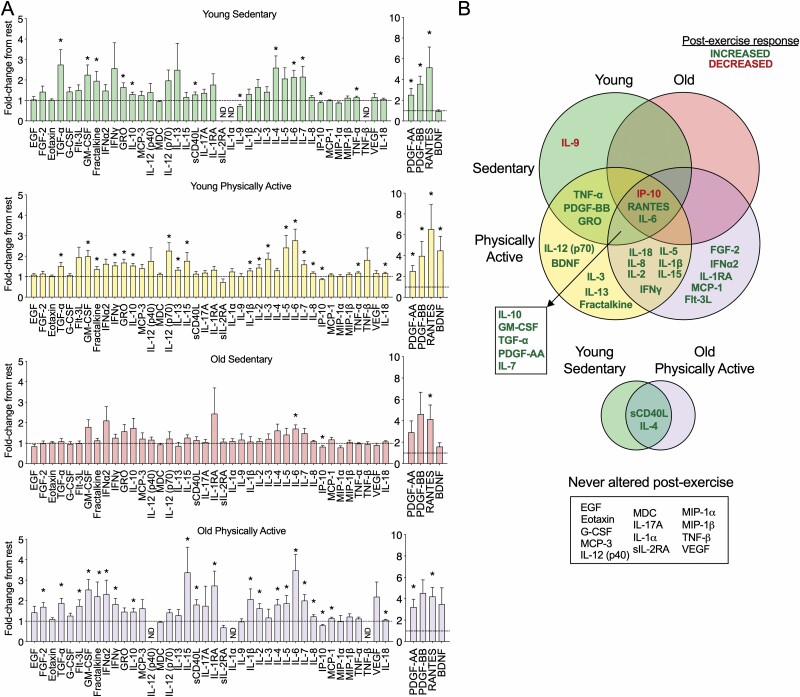
We were surprised at the modest associations between physical function and resting circulating factors in our cohort. However, we recruited disease-free subjects to enable more explicit activity and aging comparisons, whereas previous studies showing stronger relationships of inflammation and frailty were more inclusive of the broader community-dwelling elderly population without exclusions for pre-existing medical conditions (23,24). Thus, we reasoned that the response to acute exercise might be more informative in terms of distinguishing healthy physically active versus sedentary individuals. To address this, we determined which cytokines and chemokines of our panel of 44 were altered in the circulation immediately postexercise in each of our 4 subject groups. In order to be able to perform all of our blood sampling and exercise testing in a single study visit, we opted to combine a maximal graded cycling test and 30-minute cycling session. We used an acute exercise protocol which started with a graded cycling test so that we could ascertain peak aerobic capacity of the subjects (~10–15 minutes), followed by a 5-minute break and then 30 more minutes of cycling at 50% of VO2 peak cycling power. Notably, the 30-minute exercise period resulted in a similar fraction of peak oxygen uptake (VO2peak) during the final 5–10 minutes of exercise in all groups (young sedentary: 76.1% ± 6.6; young physically active: 74.9% ± 4.8; old sedentary: 77.4% ± 8.5; old physically active: 75.1% ± 5.8; mean ± SD, p ≥ .39 for all comparisons via ANOVA). Venous blood samples were then acquired within 3 minutes of the cessation of exercise from the same young sedentary, young physically active, and old physically active participants where we assessed resting alterations. In the old sedentary group, we only analyzed acute exercise responses from the subset of participants (n = 12; 7 male/5 female). Group mean ± SD and the number of detectable samples in the acute exercise analyses are provided in Supplementary Table 2. Similar to the resting comparisons, the young physically active group had a significantly higher fraction of detectable samples compared to the young sedentary group (p = .011) and there was a trend to be greater relative to the old physically active (p = .070) group. We found that acute exercise produced a wide variety of alterations among the 4 groups, but all except 2 factors were acutely increased, rather than decreased, postexercise compared to rest (Figure 2A and B). Only 3 factors were altered in a similar fashion in all 4 groups, including a reduction in IP-10 and an increase in IL-6 and RANTES. Many factors were similarly altered by acute exercise in 2 or more groups and relatively few changes were unique to a particular group. Overall, while there were many cytokines and chemokines altered postexercise in the young sedentary (14 factors), young physically active (23 factors), and old physically active participants (22 factors), there were minimal changes in the older sedentary group (3 factors). Also notable were several factors that were never altered by acute exercise from rest, including EGF, Eotaxin, G-CSF, MCP-3, IL-12 (p40), MDC, IL-17A, IL-1α, sIL-2RA, MIP-1α, MIP-1β, TNF-β, and VEGF (Figure 2A and B). It is noteworthy that some factors not significantly altered by exercise had relatively few samples in the detectable range (EGF, MCP-3, IL-12 (p40), IL-1α, sIL-2RA, MIP-1α, TNF- β, see Supplementary Table 2); however, other analytes were detectable in most samples (Eotaxin, G-CSF, MDC, IL-17A, MIP-1β, VEGF). Overall, this suggests that sedentary individuals, particularly the sedentary elderly, are less able to induce transient elevations in a wide variety of cytokines and chemokines that appear to be “primed” by exercise training.
Figure 2.
Sedentary aging is characterized by relatively few alterations in circulating postexercise cytokines and chemokines. (A) Factors significantly altered by exercise compared to rest in the young sedentary, young physically active, old sedentary and old physically active subjects. (B) A venn diagram comparison of plasma humoral factors that are significantly altered by acute aerobic exercise in young and old subjects that are sedentary or physically active. Green indicates an increase from baseline and red indicates a decrease. Overlap of the circles indicates a response common to 2 or more groups. Data in (A) are mean ± SEM. *Significantly different (p < .05) from respective resting sample. ND = not detectable in 3 or more subject samples.
Discussion
In the current study, we found significant effects of both aging and habitual aerobic exercise on circulating cytokines and chemokines using a comprehensive analysis of 44 known factors in peripheral blood. Despite sedentary older adults exhibiting higher adiposity, lower muscle power, and lower aerobic capacity compared to older physically active participants, we observed relatively few aging-induced changes in resting blood cell populations or circulating chemokines and cytokines that were impacted by habitual exercise (Figure 1). We expect that this is because our subjects were quite healthy as the presence of several risk factors for cardiovascular disease (3) or diabetes (25) can impact the extent of inflammation. In contrast, following acute exercise, we found stark differences in blood profiles between age and activity groups (Figure 2), suggesting that long-term exercise training preserves the ability to mobilize circulating factors in the elderly.
While our analysis of healthy older adults may constrain our ability to detect the influence of aging to some degree, we did observe aging effects on cytokines and chemokines that were consistent with prior studies, including age-related elevations in eotaxin (26), IL-18 (27), IP-10 (27), IL-8 (28), and RANTES (29). Additionally, previous research has shown age-associated reductions in circulating VEGF (30) and EGF (31). Interestingly, no previous studies have shown a reduction in IL-12 (p70) with increasing age; however, reduced IL-12 (p70) has been found in elderly individuals with chronic illness when compared to elderly subjects in good health (32). Similarly, no previous studies have revealed higher baseline blood levels of GRO (CXCL1), a neutrophil chemoattractant molecule (33), in older versus younger adults. It is also notable that highly physically active elderly individuals were not protected against many of the changes in cytokines and chemokines in old age, suggesting that their greater skeletal muscle and cardiovascular function has a limited impact on several canonical pathways of aging. However, exercise did protect against aging-induced basal elevations in several proinflammatory cytokines such as IL-3, IL-6, and TNF-α, consistent with previous work (14,34,35). Habitual exercise also had a protective effect on the age-related increase in plasma fractalkine (CX3CL1) levels, which is the first report of this age and exercise interaction. Fractalkine attracts monocytes and T cells and promotes leukocyte adhesion to endothelial cells in cardiovascular disease progression (36), so lower fractalkine levels in habitual exercisers may partially underlie the reduced incidence of atherosclerosis in exercise trained individuals (37).
We also unexpectedly found that resting levels of the neural growth factor BDNF were lower in physically active subjects overall. However, acute exercise increases BDNF in the blood of physically active individuals and thus may compensate for basal reductions in signaling. This is broadly consistent with the concept that pulsatile signaling of such factors is physiologically adaptive, while a chronic low-grade elevation is deleterious. This concept is strengthened by the wide-ranging increase in cytokines and chemokines postexercise in young sedentary, young physically active, and old physically active subjects that was nearly absent in old sedentary subjects. Thus, the postexercise rise in many cytokines and chemokines may predominate over resting levels as a primary mode of signaling in younger or more physically active individuals.
Our acute aerobic exercise protocol was tailored so that it required an intense effort but also remained achievable for both young and old sedentary individuals. To contextualize our findings of a differential postexercise response in circulating cytokines and chemokines based on age and physical activity history, it is important to consider whether this exercise session was an intense enough stimulus. Subjects all cycled at approximately 75% of maximum aerobic capacity, which is relatively intense exercise, particularly for sedentary individuals. Since IL-6, TNF-α, and IL-1β are among the most frequently reported postexercise circulating factors in young individuals (38), these factors can provide some context of the magnitude of the exercise response between studies. The significant postexercise increase in IL-6 in the present study (young sedentary: 2.1-fold; young physically active: 2.8-fold) appears most similar to studies employing moderate-intensity exercise (50%–70% VO2max, range of 1.3- to 4.2-fold (39,40)) rather than high-intensity exercise (>70% VO2max (40,41)). In contrast, the elevation in postexercise TNF-α found in our study (young sedentary and young physically active: 1.2-fold) is typically only elevated after high-intensity (range of 1.3- to 2-fold; (41–43)) and no changes are found after moderate-intensity exercise (39,44). Similarly, the postexercise increase in IL-1β we observe (young physically active: 1.3-fold) is typically only found after high-intensity exercise (1.1- to 1.5-fold; (41,43,45). Thus, we reason that our exercise response was comparable to prior high-intensity protocols. However, since the limited fitness of the sedentary individuals in the current study that constrained the exercise intensity, our exercise responses generally did not reach the levels of cytokine and chemokine induction found in studies using highly trained athletes (43,46).
An overarching question concerns why the cytokine and chemokine responses following exercise were more dynamic in the physically active versus sedentary elderly? There was not a substantive difference in immune cell populations at rest (Table 2) nor were there fat or lean mass differences in older versus younger sedentary subjects (Table 1) that might explain a reduced secretion of molecules. Physically fit elderly subjects have a more sensitive immune response to various types of pathogen stimuli (12), but the triggers for this type of immune signaling are unlikely to occur during exercise. The most likely explanation is that sedentary older adults have the lowest aerobic capacity and muscle function (20), so the absence of cytokine and chemokine induction is potentially related to the force of muscle contractions. Many cytokines and chemokines are secreted directly from muscle or increased in the blood by exercise (47,48). Even the well-established muscle-derived “myokine” IL-6, which increased after acute exercise in all 4 groups, was less altered in old sedentary versus old physically active subjects (mean of 1.7-fold and 3.3-fold, respectively). Thus, there may be a threshold of muscle power required to induce many bloodborne factors after exercise which would be negatively impacted by aging, obesity, or inactivity. Perhaps this is why frailty has been frequently linked to morbidity and mortality (49,50) as there is insufficient muscle power and/or mass to produce the physiological cytokine and chemokine cues for immune, metabolic, and endocrine signaling. An intriguing concept is whether increases in chronic, low-grade inflammation result in part from this reduced signaling during regular physical activity.
We also recognize that there are several limitations to the current study. Given the cross-sectional design of this initial set of resting comparisons, factors other than habitual activity including diet, genetics, or lifestyle could contribute to our findings. Additionally, we have only examined the time period immediately postexercise, which may not overlap with the peak secretion and expression of specific cytokines and chemokines. Future studies should analyze a broader time-course of bloodborne postexercise factors. Additionally, our sample sizes are relatively small, particularly for several analytes with generally poor detectability (ie sIL-2Rα, TNF-β) limiting our ability to detect differences between groups. However, while there was increased detectability of samples in the young physically active group, this was not true in the old physically active group and thus cannot explain our observation that both young and old physically active subjects experienced a more widespread induction of cytokines and chemokines in the blood after exercise. It is possible that an underlying reason for greater sample detectability in young physically active blood samples is due to a generally richer diversity of factors in their circulation, but this requires further investigation. Overall, these data help to further elaborate upon the bloodborne signaling changes that occur in response to exercise and aging.
Supplementary Material
Acknowledgments
The authors wish to thank the study participants for their invaluable contributions to our research as well as D. Reid for her assistance with the study logistics.
Funding
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and MitoCanada to M.A.T.
Conflict of Interest
M.A.T. is the CEO and CSO of Exerkine Corporation. M.A.T. and J.D.C. both hold a patent regarding the use of interleukin-15 for skin and muscle disorders. L.G.M. report no conflict of interest.
Author Contributions
J.D.C. and M.A.T. applied for funding for the study as well as conceived and designed the study. J.D.C., L.G.M., and M.A.T. performed the study procedures and experiments. J.D.C. and L.G.M. analyzed and interpreted the results. J.D.C. wrote the manuscript and L.G.M. and M.A.T. reviewed the manuscript.
References
- 1. Lassman D, Hartman M, Washington B, Andrews K, Catlin A. US health spending trends by age and gender: selected years 2002–10. Health Affair. 2014;33(5):815–822. 10.1377/hlthaff.2013.1224 [DOI] [PubMed] [Google Scholar]
- 2. Dall TM, Gallo PD, Chakrabarti R, West T, Semilla AP, Storm MV. An aging population and growing disease burden will require alarge and specialized health care workforce by 2025. Health Affair. 2013;32(11):2013–2020. 10.1377/hlthaff.2013.0714 [DOI] [PubMed] [Google Scholar]
- 3. Ferrucci L, Corsi A, Lauretani F, et al. . The origins of age-related proinflammatory state. Blood. 2005;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Furman D, Campisi J, Verdin E, et al. . Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771 [DOI] [PubMed] [Google Scholar]
- 8. Pierce GF, Mustoe TA, Lingelbach J, et al. . Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109(1):429–440. doi: 10.1083/jcb.109.1.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. 2008;168(15):1638–1646. doi: 10.1001/archinte.168.15.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krabbe KS, Bruunsgaard H, Hansen CM, et al. . Ageing is associated with a prolonged fever response in human endotoxemia. Clin Diagn Lab Immunol. 2001;8(2):333–338. doi: 10.1128/CDLI.8.2.333-338.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruunsgaard H, Skinhøj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis. 1999;180(2):551–554. doi: 10.1086/314873 [DOI] [PubMed] [Google Scholar]
- 12. Sellami M, Gasmi M, Denham J, et al. . Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:2187. doi: 10.3389/fimmu.2018.02187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153(3):242–250. doi: 10.1093/aje/153.3.242 [DOI] [PubMed] [Google Scholar]
- 14. Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE; MacArthur Studies of Successful Aging . The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51(8):1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x [DOI] [PubMed] [Google Scholar]
- 15. Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105(15):1785–1790. doi: 10.1161/hc1502.107117 [DOI] [PubMed] [Google Scholar]
- 16. Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje [DOI] [PubMed] [Google Scholar]
- 17. Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285(2):E433–E437. doi: 10.1152/ajpendo.00074.2003 [DOI] [PubMed] [Google Scholar]
- 18. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041 [DOI] [PubMed] [Google Scholar]
- 19. Crane JD, MacNeil LG, Lally JS, et al. . Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14(4):625–634. doi: 10.1111/acel.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crane JD, Macneil LG, Tarnopolsky MA. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci. 2013;68(6):631–638. doi: 10.1093/gerona/gls237 [DOI] [PubMed] [Google Scholar]
- 21. Weiss EP, Albert SG, Reeds DN, et al. . Effects of matched weight loss from calorie restriction, exercise, or both on cardiovascular disease risk factors: a randomized intervention trial. Am J Clin Nutr. 2016;104(3):576–586. doi: 10.3945/ajcn.116.131391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talbot LA, Musiol RJ, Witham EK, Metter EJ. Falls in young, middle-aged and older community dwelling adults: perceived cause, environmental factors and injury. BMC Public Health. 2005;5(1):86. doi: 10.1186/1471-2458-5-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L; InCHIANTI Investigators . Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60(6):760–767. doi: 10.1093/gerona/60.6.760 [DOI] [PubMed] [Google Scholar]
- 24. Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52(4):M201–M208. doi: 10.1093/gerona/52a.4.m201 [DOI] [PubMed] [Google Scholar]
- 25. Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693 [DOI] [PubMed] [Google Scholar]
- 26. Villeda SA, Luo J, Mosher KI, et al. . The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miles EA, Rees D, Banerjee T, et al. . Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis. 2008;196(1):298–305. doi: 10.1016/j.atherosclerosis.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 28. Krishnan VV, Ravindran R, Wun T, Luciw PA, Khan IH, Janatpour K. Multiplexed measurements of immunomodulator levels in peripheral blood of healthy subjects: effects of analytical variables based on anticoagulants, age, and gender. Cytometry B Clin Cytom. 2014;86(6):426–435. doi: 10.1002/cyto.b.21147 [DOI] [PubMed] [Google Scholar]
- 29. Mansfield AS, Nevala WK, Dronca RS, Leontovich AA, Shuster L, Markovic SN. Normal ageing is associated with an increase in Th2 cells, MCP-1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF-AA between sexes. Clin Exp Immunol. 2012;170(2):186–193. doi: 10.1111/j.1365-2249.2012.04644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian J, Lei XX, Xuan L, Tang JB, Cheng B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets. 2019;30(6):773–792. doi: 10.1080/09537104.2018.1514110 [DOI] [PubMed] [Google Scholar]
- 31. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–266. doi: 10.1111/sji.12413 [DOI] [PubMed] [Google Scholar]
- 32. Yu Y, Singh H, Kwon K, et al. . Protein signatures from blood plasma and urine suggest changes in vascular function and IL-12 signaling in elderly with a history of chronic diseases compared with an age-matched healthy cohort. GeroScience. 2020;141:1–14. 10.1007/s11357-020-00269-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zlotnik A, Yoshie O, Nomiyama H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol. 2006;7(12):243. doi: 10.1186/gb-2006-7-12-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colbert LH, Visser M, Simonsick EM, et al. . Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52(7):1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x [DOI] [PubMed] [Google Scholar]
- 35. Arai MH, Duarte AJ, Natale VM. The effects of long-term endurance training on the immune and endocrine systems of elderly men: the role of cytokines and anabolic hormones. Immun Ageing. 2006;3(1):9. doi: 10.1186/1742-4933-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White GE, Greaves DR. Fractalkine: a survivor’s guide. Arterioscleros Thromb Vasc Biol. 2012;32(3):589–594. 10.1161/atvbaha.111.237412 [DOI] [PubMed] [Google Scholar]
- 37. Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587(Pt 23):5541–5549. doi: 10.1113/jphysiol.2009.178822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cerqueira É, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise—a systematic review. Front Physiol. 2019;10:1550. doi: 10.3389/fphys.2019.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brenner IK, Natale VM, Vasiliou P, Moldoveanu AI, Shek PN, Shephard RJ. Impact of three different types of exercise on components of the inflammatory response. Eur J Appl Physiol Occup Physiol. 1999;80(5):452–460. doi: 10.1007/s004210050617 [DOI] [PubMed] [Google Scholar]
- 40. Wadley AJ, Chen Y-W, Lip GYH, Fisher JP, Aldred S. Low volume–high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J Sport Sci. 2015;34(1):1–9. 10.1080/02640414.2015.1035666 [DOI] [PubMed] [Google Scholar]
- 41. Nieman DC, Konrad M, Henson DA, Kennerly K, Shanely RA, Wallner-Liebmann SJ. Variance in the acute inflammatory response to prolonged cycling is linked to exercise intensity. J Interferon Cytokine Res. 2012;32(1):12–17. doi: 10.1089/jir.2011.0038 [DOI] [PubMed] [Google Scholar]
- 42. Ulven SM, Foss SS, Skjølsvik AM, et al. . An acute bout of exercise modulate the inflammatory response in peripheral blood mononuclear cells in healthy young men. Arch Physiol Biochem. 2015;121(2):41–49. doi: 10.3109/13813455.2014.1003566 [DOI] [PubMed] [Google Scholar]
- 43. Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol. 1998;513(Pt 3):889–894. doi: 10.1111/j.1469-7793.1998.889ba.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marklund P, Mattsson CM, Wåhlin-Larsson B, et al. . Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol (1985). 2013;114(1):66–72. doi: 10.1152/japplphysiol.01538.2011 [DOI] [PubMed] [Google Scholar]
- 45. Mucci P, Durand F, Lebel B, Bousquet J, Préfaut C. Interleukins 1-beta, -8, and histamine increases in highly trained, exercising athletes. Medicine Sci Sports Exerc. 2000;32(6):1094–1100. 10.1097/00005768-200006000-00009 [DOI] [PubMed] [Google Scholar]
- 46. de Gonzalo-Calvo D, Dávalos A, Montero A, et al. . Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol. 2015;119(2):124–134. 10.1152/japplphysiol.00077.2015 [DOI] [PubMed] [Google Scholar]
- 47. Whitham M, Parker BL, Friedrichsen M, et al. . Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27(1):237–251.e4. doi: 10.1016/j.cmet.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 48. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 49. Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;41(2):141–149. doi: 10.1016/j.archger.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 50. Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–736. doi: 10.1016/j.arr.2012.03.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.