Abstract
Background
Older cancer survivors are at risk of the development or worsening of both age- and treatment-related morbidity. Sedentary behavior increases the risk of or exacerbates these chronic conditions. Light-intensity physical activity (LPA) is more common in older adults and is associated with better health and well-being. Thus, replacing sedentary time with LPA may provide a more successful strategy to reduce sedentary time and increase physical activity.
Objective
This study primarily aims to evaluate the feasibility, acceptability, and preliminary efficacy of a home-based mobile health (mHealth) intervention to interrupt and replace sedentary time with LPA (standing and stepping). The secondary objective of this study is to examine changes in objective measures of physical activity, physical performance, and self-reported quality of life.
Methods
Overall, 54 cancer survivors (aged 60-84 years) were randomized in a 1:1:1 allocation to the tech support intervention group, tech support plus health coaching intervention group, or waitlist control group. Intervention participants received a Jawbone UP2 activity monitor for use with their smartphone app for 13 weeks. Tech support and health coaching were provided via 5 telephone calls during the 13-week intervention. Sedentary behavior and physical activity were objectively measured using an activPAL monitor for 7 days before and after the intervention.
Results
Participants included survivors of breast cancer (21/54, 39%), prostate cancer (16/54, 30%), and a variety of other cancer types; a mean of 4.4 years (SD 1.6) had passed since their cancer diagnosis. Participants, on average, were 70 years old (SD 4.8), 55% (30/54) female, 24% (13/54) Hispanic, and 81% (44/54) overweight or obese. Malfunction of the Jawbone trackers occurred in one-third of the intervention group, resulting in enrollment stopping at 54 rather than the initial goal of 60 participants. Despite these technical issues, the retention in the intervention was high (47/54, 87%). Adherence was high for wearing the tracker (29/29, 100%) and checking the app daily (28/29, 96%) but low for specific aspects related to the sedentary features of the tracker and app (21%-25%). The acceptability of the intervention was moderately high (81%). There were no significant between-group differences in total sedentary time, number of breaks, or number of prolonged sedentary bouts. There were no significant between-group differences in physical activity. The only significant within-group change occurred within the health coaching group, which increased by 1675 daily steps (95% CI 444-2906; P=.009). This increase was caused by moderate-intensity stepping rather than light-intensity stepping (+15.2 minutes per day; 95% CI 4.1-26.2; P=.008).
Conclusions
A home-based mHealth program to disrupt and replace sedentary time with stepping was feasible among and acceptable to older cancer survivors. Future studies are needed to evaluate the optimal approach for replacing sedentary behavior with standing and/or physical activity in this population.
Trial Registration
ClinicalTrials.gov NCT03632694; https://clinicaltrials.gov/ct2/show/NCT03632694
Keywords: light-intensity physical activity, physical activity, sedentary behavior, mobile health, cancer survivors, consumer wearable, activity monitor, mobile phone
Introduction
Background
By 2030, there will be 22.1 million cancer survivors living in the United States, and two-thirds of them will be more than 65 years old [1]. Older cancer survivors are faced with both age- and treatment-related morbidity that increase their risk of physical function impairment and other comorbidities, including cardiovascular disease, diabetes mellitus, and osteoporosis [2-5]. These comorbidities further increase the risk of functional limitations. Compared with individuals without a history of cancer, cancer survivors have a 2- to 5-fold increased risk of having one or more functional limitations [5]. These chronic conditions are associated with diminished quality of life (QoL), premature death, and substantial financial costs [6-11]. Physical inactivity and sedentary behavior (too much sitting, which is distinct from too little exercise [12]) can increase the risk of or exacerbate these chronic conditions [13-19].
Recent research suggests that sedentary behavior has molecular and physiological effects distinct from a lack of exercise [20,21]. Sedentary behavior is defined as any waking behavior (ie, not sleep) characterized by minimal energy expenditure (≤1.5 metabolic equivalents [METs]) while in a sitting, lying, or reclining position [22]. Sedentary behavior is associated with an increased risk of cardiovascular disease [23,24], premature all-cause mortality [23,25-27], greater fatigue [28,29], and decreased physical function [11,29,30]. Furthermore, how sedentary time is accumulated throughout the day is important, as frequent short breaks in sedentary time can attenuate the negative physiological response associated with prolonged, uninterrupted periods of inactivity [31-34].
Among cancer survivors, less than 2% of waking hours are spent in moderate-to-vigorous physical activity (MVPA), up to 70% of waking hours are spent in sedentary activities, and the remaining time is spent in light-intensity physical activity (LPA) [35]. LPAs are associated with better physical health [36,37], including better physical function [37-40], reduced risk of incident disability [39,41], and better emotional well-being [36,40,42,43], independent of MVPA. The association between LPA and health outcomes is either only apparent or appears stronger in older adults and adults who are less physically active or have impaired lower extremity function [41,44-47]. Thus, disrupting and replacing sedentary time with LPA, rather than MVPA, are likely a more feasible approach to reducing sedentary behavior in older cancer survivors.
Behavior change interventions based on theory are generally more effective than atheoretical approaches [48-50]. Recent reviews suggest that goal setting, feedback, self-monitoring, problem solving, and social support are the most promising behavioral change techniques for interventions designed to reduce sedentary behavior [51-53]. Unlike simple pedometers, consumer wearable activity trackers include multiple behavior change techniques [54,55]. The ability to provide feedback in real time is particularly salient for sedentary behavior, as it is a largely subconscious behavior [51]. Furthermore, wearable activity trackers are readily available and low cost and, if effective, represent a scalable option for expanding the reach to a large number of cancer survivors, including in rural areas.
Given the deleterious effects of sedentary behavior on health, including cardiovascular disease and diabetes mellitus, conditions that are commonly observed in older cancer survivors, or for which they are at an elevated risk [56], the role of sedentary behavior in cancer survivorship has been identified as a research priority [35,57]. However, to date, few interventions have been designed to reduce sedentary time among cancer survivors [51]. Recently, several mobile health (mHealth) pilot or feasibility interventions have evaluated text messaging or wearable activity trackers as an intervention tool to decrease sedentary behavior in breast, prostate, and colorectal cancer survivors [58-60]. These interventions encouraged standing and stepping to replace sedentary behavior, with a primary focus on moderate-intensity activity. Preliminary results suggest that mHealth interventions are feasible and acceptable in this population and have the potential to replace sedentary behavior with physical activity, at least in the short term. However, additional research is needed to further evaluate effective strategies to reduce sedentary time by either replacing it with standing, stepping, or both.
Objectives
The purpose of this study is to examine the feasibility, acceptability, and preliminary efficacy of an mHealth intervention for disrupting (frequent breaks) and replacing sedentary time with intermittent bouts of LPA (standing and stepping). The 13-week intervention used the Jawbone UP2 activity monitor and associated smartphone app to promote awareness and enable self-monitoring of both physical activity and inactivity. We evaluated 2 versions of the mHealth intervention: a low-touch approach providing only tech support and a higher resource approach that included health coaching in addition to the tech support. This would allow us to determine whether a low-cost, consumer-based technology (wearable activity tracker plus smartphone app) is effective in meeting the goals or whether health coaching is needed to cover additional behavior change techniques not provided in the wearable activity tracker. Our primary objective is to determine the feasibility and acceptability of the 2 versions of the mHealth intervention by assessing recruitment, retention, and adherence rates; monitoring adverse events; and evaluating satisfaction with the program. In addition, we examined the preliminary efficacy of the intervention on changes in objective measures of daily total sedentary time and the number of breaks in sedentary time. Our secondary objective is to explore changes in objective measures of physical activity, physical performance, and self-reported QoL.
Methods
Study Design
This study was a 3-arm pilot randomized controlled trial (RCT). Older cancer survivors were randomized in a 1:1:1 allocation to the tech support intervention group, the tech support plus health coaching intervention group, or a modified waitlist control group. The intervention used a consumer wearable activity tracker (Jawbone UP2 wristband) that was paired with a smartphone app to promote awareness and enable self-monitoring of both inactivity (band gently vibrates after a specified time of inactivity) and physical activity (eg, steps per day). We evaluated 2 versions of the intervention: a low-touch approach providing only tech support and a higher resource approach that included health coaching in addition to the tech support. Each intervention group was compared with the waitlist control group. Recruitment for the trial began in June 2016, and data collection was completed in July 2017.
Eligibility
Eligibility criteria for the feasibility study included (1) men and women aged 60 years and older (reduced from 65 years to increase the number of participants who own a smartphone); (2) those who were diagnosed as having an invasive, local or regionally staged cancer within the past 7 years (time frame increased the likelihood that address and phone number in cancer registry were still current) and completed primary treatment (surgery, radiation, and chemotherapy); (3) those who owned a smartphone capable of running the Jawbone UP2 smartphone app; (4) those who were willing to be randomized to any of the 3 study arms, attend 2 clinic visits, and wear activity monitors; (5) those who were able to read, speak, and understand English; (6) those who were living independently and were capable of walking 3 blocks (approximately 1/4 mile or 1300 steps) without an assistive device (eg, cane and walker); (7) self-reported sedentary time (during waking hours) of ≥6 hours/day (Longitudinal Aging Study Amsterdam Sedentary Behavior Questionnaire: hours and minutes in a day spent in 10 activities, on average, during a weekday [61]); (8) those who were not currently participating in a program to decrease sedentary time or increase physical activity and not currently using a fitness tracker; (9) those who had no paid employment or volunteer position for more than 20 hours per week (to avoid potential confounding by occupational activity/inactivity); (10) those who had no severe impairments (in seeing or hearing) or preexisting medical limitations for engaging in daily LPA (eg, severe orthopedic conditions, pending hip/knee replacement, dementia, and oxygen dependent); (11) those who had residence within 60 miles of the research clinic (to reduce travel burden and improve retention and compliance); and (12) those who had a wrist size of 14 cm to 20 cm to wear the Jawbone UP2 activity wristband during the intervention. Individuals who met the physical activity guidelines (150 minutes per week of MVPA) [17,62] were eligible because sedentary behavior is a risk factor for morbidity and mortality independent of MVPA.
Recruitment
The population-based New Mexico Tumor Registry, a founding member of the Surveillance, Epidemiology, and End Results Program [63], was used as the primary source for identifying potential study participants. Additional sources included posting flyers at selected locations, including senior centers and libraries. After identifying potentially eligible study participants, the New Mexico Tumor Registry mailed a letter that introduced the study and gave potentially eligible participants the opportunity to decline further contact. Contact information for individuals not refusing further contact was provided to the study team after a 3-week waiting period. Potential participants were then mailed a letter explaining the study and a consent form. One week later, the staff telephoned to discuss the study, answer questions, begin the consent process, and verify eligibility. Up to 3 attempts (later expanded to 4) were made to reach individuals who had a valid telephone number. A written informed consent for the interested and eligible participants was obtained during the baseline clinic visit.
Randomization
After a 1-week run-in period, a member of the research team opened the next sequentially numbered sealed envelope (created by a biostatistician) to reveal the randomization status. Participants were block randomized with equal allocation to 3 arms (tech support, tech support plus health coaching, or modified waitlist control) according to obesity status (BMI <30 vs ≥30 kg/m2).
mHealth Intervention
Theoretical Framework
The theoretical framework used to guide this intervention was the social cognitive theory [64,65]. The intervention primarily targeted the theoretical constructs of knowledge, behavioral skills, behavioral capability, and self-efficacy. Wearable activity trackers, such as Jawbone, include a number of behavioral change techniques associated with decreasing sedentary behavior and increasing physical activity (eg, goal setting, graded tasks, and self-monitoring) [54,55]. However, some of the key techniques are missing and were supplemented with educational materials and technology support. Additional behavior change techniques were provided by the health coaches for the health coaching intervention, such as the identification of barriers and problem solving. Health coaches also provided encouragement and support and encouraged positive support from family and friends. A list of the behavior change techniques, theoretical constructs, and examples of strategies to promote behavior change in this mHealth intervention is presented in Table 1.
Table 1.
Behavior change techniques and strategies to promote behavior change via educational materials, the Jawbone tracker and app, or tech support coaching or health coaching.
| Behavior change technique | Theoretical construct | Examples of strategies | TSa group | HCb group | |||||
|
|
|
|
EMc | JBd | TS | EM | JB | HC | |
| Information on consequences of behavior | Knowledge | Educational materials on harms of physical inactivity and sedentary behavior; also discussed with health coach | ✓e |
|
|
✓ |
|
✓ | |
| Goal setting (behavior) | Behavioral skills; self-efficacy | Set weekly short-term and long-term step goals; tech support for changing goal settings on app; idle alert goal (every 30 min) and step goal (graded increase in steps) |
|
✓ | ✓ |
|
✓ | ✓ | |
| Barrier identification and problem solving | Barrier self-regulatory efficacy | Work with health coach to assess barriers and identify solutions to breaking up sedentary time and getting more steps throughout the day |
|
|
|
|
|
✓ | |
| Set graded tasks | Self-efficacy | Encourage incremental and achievable sedentary (breaks) and step goals |
|
✓ |
|
|
✓ |
|
|
| Review of behavioral goals | Behavioral skills | Using Jawbone app to review daily progress and weekly patterns for longest idle time and steps |
|
✓ |
|
|
✓ |
|
|
| Generalization of a target behavior | Behavioral capability | Educational materials with suggestions for breaking up sedentary time in different ways and locations; additional support from health coach | ✓ |
|
|
✓ |
|
✓ | |
| Self-monitoring of behavior | Behavioral skills | Using Jawbone app to review daily progress and weekly patterns and provide immediate feedback (idle alert and longest idle time) |
|
✓ |
|
|
✓ |
|
|
| Feedback on behavior | Behavioral skills | Jawbone tracker and app provide immediate feedback; health coach to discuss whether goals were met |
|
✓ |
|
|
✓ | ✓ | |
| Information on where and when to perform behavior | Behavioral capability | Education materials to suggest tips for disrupting SBf; Jawbone idle alert to prompt when to stand up and move | ✓ | ✓ |
|
✓ | ✓ |
|
|
| Instructions on how to perform the behavior | Self-efficacy; behavioral skills | Print materials and coaching provide instructions on setting up and using the Jawbone tracker and app | ✓ |
|
✓ | ✓ |
|
✓ | |
| Social support | Social support | Health coach provides support and encouragement; provide information and suggestions when asked; encourage enlisting positive support from family members and friends to take more steps throughout the day |
|
|
|
|
|
✓ | |
| Use prompts/cues; prompt practice | Cues to action | Jawbone idle alert will prompt user to disrupt sitting with standing or stepping; Jawbone alerts will prompt more steps to reach daily goal |
|
✓ |
|
|
✓ |
|
|
aTS: tech support.
bHC: health coaching.
cEM: educational material.
dJB: Jawbone tracker and app.
ePrimary source for the behavior change technique.
fSB: sedentary behavior.
Components of the Intervention
The mHealth intervention consisted of educational materials; a Jawbone (in)activity tracker; a free, commercially available smartphone app; and support via 5 telephone calls. The only difference between the 2 intervention groups was the level of telephone support. One group received only support related to the use of technology (tracker and app, tech support group), whereas the other group received additional health coaching to meet the study goals (tech support plus health coaching group).
Educational Materials
Upon randomization, both intervention groups received brief educational materials by mail. These materials explained the negative consequences of sedentary behavior, especially prolonged periods of sitting, and included suggestions for how to disrupt and replace sedentary time with LPA. Examples of suggestions provided included walking around the house during television commercial breaks, standing while talking on the telephone, and parking the car further away from the entrance [66]. The summary graph representing the most active and least active days from the week-long collection of objectively measured sedentary time, standing, and stepping (output from the activPAL3 monitor) was mailed to study participants (for later discussion with their coach; Multimedia Appendix 1). The waitlist control group received educational materials at the postintervention follow-up when they received their activity tracker and smartphone app.
Jawbone UP2 Activity Tracker
Upon randomization to either of the 2 intervention groups, participants were mailed the Jawbone UP2 activity wristband and provided detailed instructions for installing the free, commercially available app on their smartphone and for using the wristband with the app. At the time the study was designed (2015), this was one of the few consumer wearable activity trackers that had the ability to alert the wearer after a specified time of inactivity. For the Jawbone monitor, this feature was known as an idle alert, which notified the user of inactivity via a gentle vibration of the wristband (eg, users select time in increments of 15 minutes). The assigned coach telephoned participants to assist with the installation and setup of the activity tracker and smartphone app.
The goal was to decrease daily total sedentary time and increase the number of breaks in sedentary time by replacing/disrupting sedentary time with intermittent bouts of LPA (standing and stepping). The key message for the activity prescription was to “sit less, stand more, and move more, throughout the day, every day.” This message was included in the educational materials and was repeated during each of the 5 support telephone calls. Participants were encouraged to stand up and move at least once every 30 minutes. To encourage more movement than standing, participants were provided with a graduated steps per day goal of adding 3000 steps per day above their baseline level by week 9 (schedule in Figure 1). This target represents approximately 40 extra minutes of leisurely paced walking [67] and is associated with health benefits [36,68]. When combined with 20 minutes of standing, this would result in replacing 1 hour of sedentary time with 1 hour of LPA per day. A minimum intensity and a minimum bout duration for stepping were not provided, thus allowing the participant to self-select how to accumulate their extra daily steps.
Figure 1.
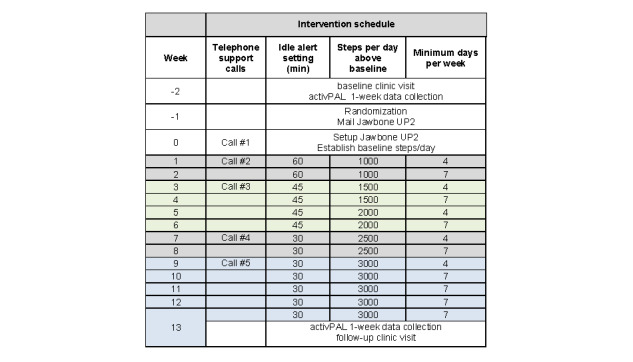
Weekly schedule for the tech support and health coaching intervention groups.
The participants were instructed to wear the Jawbone during waking hours and were encouraged to track their activity at least once a day by viewing their results on the app. A commercially available app was used without any modifications by the research team. The app included a daily summary of total steps, total and longest active time, and longest idle time (longest time spent sedentary). To promote gradual and sustained change in LPA, participants were asked to increase the number of steps per day (above their individual baseline level), during weeks 1 to 9, and then work to maintain their goal during weeks 10 to 13 (Figure 1). Similarly, the idle alert setting began at 1 hour, decreased to 45 minutes, and then every 30 minutes. Participants in both intervention groups received guidance from their coaches on how to change the settings in their app.
Tech Support and Health Coaching Calls
The coaches were graduate students who received study-specific training, including 4 practice calls with staff members before calls to study participants. One coach was assigned to each intervention group participant based on their type of phone, for example, iPhone vs android or other mobile operating system. Phone scripts were used to guide the coaches to deliver only tech support versus tech support plus health coaching. During the first telephone call (week 0; Figure 1), coaches helped the participants to set up their Jawbone monitor. During the second telephone call (week 1), each coach reviewed the activPAL3 baseline summary data (total and percentage of time spent sedentary, standing, and stepping for best and worst days) with the participant and discussed the importance of reducing sedentary time, especially prolonged periods of inactivity. Additional telephone calls (15-20 minutes) were made during weeks 3, 7, and 9 to verify completion or to assist participants with changing the steps per day goal and idle alert setting on their app (if needed). Tech support coaches provided support related only to the technology (Jawbone UP2 activity tracker and/or smartphone app), including troubleshooting technical issues. In contrast, health coaches provided additional support to help their participants identify a list of LPAs to replace/disrupt sedentary time and to achieve the ≥3000 steps per day goal, review the importance of goal setting and self-monitoring, and help troubleshoot problems and find solutions to meet their goals.
Problems With Jawbone UP2 Monitors
During the intervention, the Jawbone UP2 wristbands started to fail (ie, losing settings, losing connection with app, and not syncing data), affecting 13 of 36 intervention group participants. New Jawbone UP2 wristbands were purchased by the study team through other sources (Amazon website), but many of these wristbands also failed. We were able to buy and test UP2 wristbands to replace the failed units for the intervention group participants. Given these major issues and lack of support from Jawbone, waitlist control participants enrolled later in the study were provided with a Fitbit Alta (Fitbit Inc) at the end of the 13-week study. This product was similar to the Jawbone UP2 in that it provided an inactivity alert (reminder to move every hour) and allowed the user to set a step goal and track their steps.
Waitlist Control Group
Upon completion of the study, the control group received a shortened version of the intervention, that is, education materials, tracker, and smartphone app, and instructions for use to track their activity/inactivity. During the postintervention clinic visit, a study team member helped the participant to install the app on their smartphone; pair the tracker to their phone; and select settings for the idle alert and step goal. Each participant in this group was also offered up to 2 telephone calls with one of the coaches to receive tech support or other support to meet their personal goals for reducing sedentary behavior and increasing their activity via steps.
Procedures
Baseline Assessment
Pre- and postintervention clinic visits were conducted at the University of New Mexico Clinical and Translational Science Center. Assessments were conducted primarily by study team members not involved in intervention delivery; however, occasionally, there was overlap owing to limited resources. The baseline assessment included obtaining written informed consent, simple anthropometric measurements (height and weight), and objective physical function measures (physical tests of lower extremity function and mobility). At the end of the visit, study participants were instructed on how to attach the activPAL3 research-grade activity monitor and then observed to verify correct placement. Participants were instructed to wear the activPAL3 monitor for 24 hours/day for 1 week and on how to remove and return (via self-addressed stamped mailer) the monitor to study staff at the end of that week.
Follow-Up Assessment
At the end of the intervention, the activPAL3 research-grade monitor, attachment supplies, and instructions were mailed to all participants to collect 1 week of sedentary behavior and physical activity data. The project manager called to review the instructions for use and answer any questions. Additional postintervention outcome measures were collected at the clinic visit at the end of week 13. Participants received US $50 gift cards to complete the baseline and follow-up assessments and to help cover the costs of accessing the app on their smartphone. In addition, participants were allowed to keep the Jawbone UP2 activity tracker at the end of the study.
Device-Based Measures
Sedentary behavior and physical activity were measured using an activPAL3 research-grade monitor (PAL Technologies Ltd). activPAL3 is a lightweight device worn on the thigh and includes both an inclinometer (to detect changes in position) and a triaxial accelerometer. activPAL is the gold standard in sedentary behavior research and provides accurate measures of sitting (or lying), standing, and stepping [69-72]. Participants wore the device for 24 hours per day for 7 days, before and after the intervention. The device was only removed for bathing or swimming or if an adverse reaction occurred to the Tegaderm dressing used to attach the device. Participants recorded in their diary, the day/time when the device was attached, each time it was removed and reattached, and the time they went to bed at night and woke up in the morning.
Outcomes and Measurements
Feasibility and Acceptability Outcomes
The feasibility and acceptability of the mHealth intervention were determined by achieving the following goals: (1) to recruit 60 older cancer survivors; (2) to retain 80% of the sample; (3) to achieve 80% adherence to the intervention; (4) to have no serious adverse events attributable or possibly attributable to the intervention, defined as any condition that is life threatening and results in overnight hospitalization or a physical or cardiac event serious enough to require medical attention; and (5) to achieve high satisfaction (acceptability) rates with the intervention; to have 75% or more of participants report agree or strongly agree on a 5-point Likert scale.
Retention was calculated as the percentage of participants who completed the follow-up clinic visits and accelerometer assessment. Adherence to wearing the Jawbone UP2 tracker, checking the app daily, and acting on the idle alert was assessed with 4 questions. Response items included never, rarely, sometimes, often, or very often. For adherence to the intervention, we calculated the percentage of intervention group participants who responded often or very often to the 4 questions regarding their use of the Jawbone tracker and app. In addition, the completion of telephone support calls was tracked. Acceptability and evaluation of the Jawbone UP2 technology (UP2 tracker and app) were assessed using 7 questions. Response items included strongly disagree, disagree, neutral, agree, or strongly agree. For acceptability, we calculated the percentage of respondents who responded agree or strongly agree to the 7 questions regarding ease of use, motivation, intention for continued use, and recommendations of this technology. Adherence and acceptability were stratified based on whether participants received a replacement Jawbone tracker owing to severe malfunctioning.
Primary Preliminary Efficacy Outcomes
The primary behavioral outcomes of interest were changes in total sedentary time (average minutes per day) and number of breaks from sitting (average breaks per day). As the opportunity to interrupt sitting while standing or stepping is dependent on the amount of sedentary time, the break ratio was also calculated. The break ratio was defined as the number of absolute breaks divided by total sedentary time.
Secondary Preliminary Efficacy Outcomes
Device-Based Measures of Sedentary Behavior and Physical Activity
activPAL was also used to assess changes in total minutes spent in prolonged sedentary bouts, minutes per day spent standing, number of steps per day, and minutes of light- and moderate-intensity physical activity (reported separately). A prolonged sedentary bout was defined as 30 or more continuous minutes in a seated or lying position [73]. LPA was defined as stepping at a cadence equivalent to 1.5 to 3.0 METs [73]. A MET is a multiple of resting energy expenditures. With resting (sitting quietly) energy expenditure defined as 1 MET, a 3-MET activity expends the energy of rest by 3 times, whereas a 5-MET activity expends the energy of rest by 5 times. Standing is also considered an LPA and has been reported separately from light stepping. Moderate-intensity physical activity was similarly defined, but with MET values from 3.0 to 5.9. Vigorous-intensity physical activity was defined as MET values of ≥6.0 or higher. As the guidelines at the time this intervention were designed specified that MVPA be accumulated in minimum bouts of 10 minutes, we also evaluated guideline bouts of MVPA [17,62]. The activPAL monitor provides accurate and precise categorization of sedentary time, LPA, and MVPA in a free-living setting (96.2% accuracy compared with direct observation) [73].
Objectively Measured Physical Performance
The emphasis on frequent interruptions of sedentary behavior with standing and stepping has the potential to improve lower extremity physical function. This was measured using the Short Physical Performance Battery (SPPB). The SPPB includes tests of standing balance, walking speed (timed 8-ft walk at usual speed), and lower body strength (time to rise from a chair 5 times) [6,74]. Scores range from 0 (not attempted) to 4 (highest score) for each test, with a total score ranging from 0 to 12. This battery has strong predictive validity and is responsive to changes [6,74].
Subjective Measures
Given the inverse association reported between sedentary behavior and QoL [29,75,76], we evaluated changes in QoL as a secondary outcome. The Medical Health Outcomes Study Short Form 36-item survey (SF-36, version 2) was used to assess health-related QoL. The SF-36 includes 8 individual scale scores and 2 component summary scores for physical and mental health and well-being. This instrument is valid and reliable for use in healthy and chronically ill adults [77,78]. Surveys were scored using QualityMetric [79]. Raw scores range from 0 to 100, with higher scores indicating better functioning and well-being. T-scores represent a linear transformation, normed to the US population, with a mean of 50 (SD 10). Pain and fatigue were assessed using the patient-reported outcomes measurement information system (PROMIS) Pain Interference Short Form 8A and the functional assessment of chronic illness therapy (FACIT)-Fatigue scale (version 4) [80,81]. The pain interference survey included 8 questions on whether and the degree to which pain interfered with various activities during the past 7 days. The fatigue scale included 13 questions on whether fatigue affected a person’s life during the past 7 days and the degree to which fatigue affected a person’s life during the past 7 days.
Other Measures
In addition, sociodemographics, cancer-related data, comorbidities, and simple anthropometrics were ascertained via paper surveys to characterize the study population. Sociodemographic data were assessed via questionnaires at baseline, including age, sex, race/ethnicity, education, income range, and marital status. Smoking status (current, former, or never smoker) was also assessed at baseline. Cancer data were obtained from the New Mexico Tumor Registry (cancer type, stage, and date of diagnosis) and from self-reported surveys (treatment [yes/no]: surgery, chemotherapy, radiation, hormone therapy, and date primary therapy completed). The Self-Administered Comorbidity Questionnaire [82] was used to assess the number of conditions and their impact on usual activities. The number of comorbidities and whether they limited activities were summed and categorized as 0 or 1 comorbidity (activities not limited), 1 comorbidity (activities limited), and 2 or more comorbidities (activities limited). Height (nearest 0.5 cm) was measured at the baseline clinic visit. Weight (nearest 0.1 kg) was measured at both the baseline and follow-up clinic visits. BMI (kg/m2) was calculated and categorized as normal weight (18.5 kg/m2-24.9 kg/m2), overweight (25.0 kg/m2-29.9 kg/m2), and obese (≥30 kg/m2).
Data Processing and Statistical Analysis
Processing of activPAL Data
activPAL3 data were downloaded using activPAL software (version 7; PAL Technologies Limited). The event files (start/stop time for sitting/lying, standing, and stepping) were processed using the activPALProcessing R package (version 1.0.2) [73,83]. After converting the event file into a second-by-second data file (second-by-second R function), other R functions were used to calculate the sedentary behavior and physical activity metrics. Only days with 10 or more hours of wear per awake time were included, and only the first 7 valid days were included (extra days were excluded). To be included in the analyses, a participant needed at least one valid day of activPAL3 data from baseline, which is consistent with the intention-to-treat principle and similar to other recent trials [58,84]. Owing to the large variability in the within- and between-person average number of awake per wear hours, all activPAL metrics were standardized to a 15-hour awake per wear day (average in this study sample). Additional details of the activPAL data collection and processing are included in Multimedia Appendix 2 [69-73,83,85], similar to other studies [59,85].
Efficacy Outcomes
Baseline descriptive characteristics (mean, SD or frequency, %) were used to characterize the study population. Intent-to-treat analyses were conducted to evaluate changes in sedentary behavior metrics and secondary outcomes. Linear mixed methods were used to estimate the within- and between-group differences for each outcome. Each model included a fixed effect for group (tech support, health coaching, and waitlist control), time (before and after the intervention), and group by time interaction. A subject-level random effect was included to account for the correlation between repeated measurements of the same individuals over time. Statistical analyses were performed using SAS (version 9.4) and R (v.3.4.3).
Complete case analyses were conducted that only included individuals with complete data (12 tech support, 17 health coaching, and 18 controls). A sensitivity analysis was conducted that excluded individuals with fewer than 4 valid days of activPAL data (3 participants from the tech support only group). In addition, a sensitivity analysis was conducted by excluding the 12 intervention participants who experienced major problems with their Jawbone tracker (ie, required 1 or more tracker replacements, excluding 6 participants in each intervention group). For this sensitivity analysis, the control group was restricted to control participants who completed their baseline visit during the same period as the intervention participants, to account for potential seasonality effects (ie, before mid-February 2017, excluding 6 controls).
The proposed pilot intervention was a feasibility and acceptability intervention and thus was not powered to detect small effect sizes for change in any outcome. However, for sedentary time, with 20 people per group, assuming a 2-sided alpha level of 0.05 and an SD of 1.4 hours, there was 80% power to detect a difference of 1.3 hours in sedentary time between 2 groups [86,87].
Results
Feasibility
The New Mexico Tumor Registry identified 421 potentially eligible participants and, after accounting for a 3-week opt-out period, forwarded contact information on 354 individuals to study staff. Of the 364 individuals (including 10 self-referrals) we attempted to contact by telephone, 76 refused to participate, 101 were ineligible, and 118 were considered passive refusals after 3 to 4 attempts to contact via telephone (Figure 2; see Multimedia Appendix 3 for CONSORT [Consolidated Standards of Reporting Trials] checklist). The overall response rate was 20.5%. The top 3 reasons for ineligibility included not owning a smartphone, volunteering or working for more than 20 hours per week, and mobility limitations. The top 2 reasons for refusal included a lack of interest and feeling that they were already active enough. An additional 15 individuals were eligible and interested but were unable to begin the intervention before the end of the enrollment period. Owing to the major malfunctions with the Jawbone UP2 monitors during the second half of the study, enrollment was stopped early with a final enrollment of 54 participants.
Figure 2.
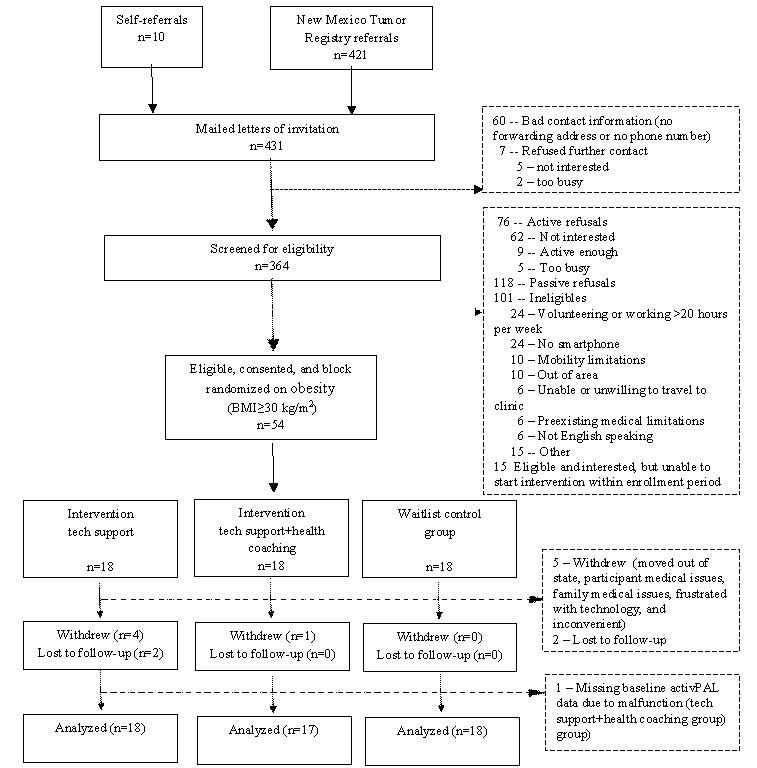
CONSORT (Consolidated Standards of Reporting Trials) diagram.
Retention in this 13-week intervention for older cancer survivors was moderately high (47/54, 87%). All of the dropouts occurred in the intervention groups, with the majority in the tech support group (6 of 7). The reasons included personal or severe family illness (n=2), move out of state (n=1), inconvenience (n=1), frustration with technology (n=1), and loss to follow-up (n=2). Notably, 3 of the 7 dropouts occurred among individuals who experienced malfunctioning with their Jawbone monitor (tech support group). Individuals who dropped out or were lost to follow-up were more likely to be female (5/7, 71% vs 25/47, 53%), have a higher BMI (34.4 kg/m2 vs 29.5 kg/m2), and report poor or fair health at baseline (3/7, 43% vs 5/47, 11%) compared with individuals who completed the study.
The characteristics of the 54 cancer survivors enrolled in this study are presented in Table 2. The mean age at study enrollment was 69.6 years (SD 4.8, range 60-84 years), 44% (24/54) were male, 24% (13/54) were Hispanic, and 57% (31/54) had graduated from college. Most study participants (44/54, 81%) were overweight or obese, 44% (24/54) reported very good or excellent general health, and 50% (27/54) reported 1 or more comorbidities that limited their general activity. There were no significant differences between groups. Among the participants, 39% (21/54) had been diagnosed as having breast cancer, 30% (16/54) had prostate cancer, and 31% (17/54) had a variety of other cancer types. Most patients (40/53, 75%) had been diagnosed as having local-stage disease. The mean age at diagnosis was 65.2 (SD 4.8) years, and the mean number of years between diagnosis and study enrollment was 4.4 (SD 1.6) years.
Table 2.
Baseline characteristics of the mobile health intervention study participants.
| Characteristic | Combined groups (N=54) | Intervention group: tech support (n=18) | Intervention group: tech support+health coaching (n=18) | Waitlist control group (n=18) | ||||||||||
| Sociodemographic characteristics | ||||||||||||||
|
|
Age (years), mean (SD) | 69.6 (4.8) | 69.6 (4.5) | 69.1 (4.0) | 70.2 (5.9) | |||||||||
|
|
BMI, mean (SD) | 30.1 (5.7) | 30.2 (6.0) | 29.8 (4.8) | 30.4 (6.5) | |||||||||
|
|
BMI, n (%) | |||||||||||||
|
|
|
Normal weight | 10 (18) | 4 (22) | 2 (11) | 4 (22) | ||||||||
|
|
|
Overweight | 21 (39) | 6 (33) | 9 (50) | 6 (33) | ||||||||
|
|
|
Obese | 23 (43) | 8 (44) | 7 (39) | 8 (44) | ||||||||
|
|
Male, n (%) | 24 (44) | 10 (56) | 6 (33) | 8 (44) | |||||||||
|
|
Ethnicity, n (%) | |||||||||||||
|
|
|
Hispanic | 13 (24) | 5 (28) | 4 (22) | 4 (22) | ||||||||
|
|
|
Non-Hispanic | 41 (76) | 13 (72) | 14 (78) | 14 (78) | ||||||||
|
|
Race, n (%) | |||||||||||||
|
|
|
Non-White | 4 (7) | 1 (6) | 2 (11) | 1 (6) | ||||||||
|
|
|
White | 50 (93) | 17 (94) | 16 (89) | 17 (94) | ||||||||
|
|
College degree, n (%) | 31 (57) | 11 (61) | 11 (61) | 9 (50) | |||||||||
|
|
Household income, n (%) | |||||||||||||
|
|
|
<US $50,000 | 19 (35) | 7 (39) | 8 (44) | 4 (22) | ||||||||
|
|
|
≥US $50,000 | 32 (59) | 10 (56) | 9 (50) | 13 (72) | ||||||||
|
|
|
Missing or refused | 3 (6) | 1 (6) | 1 (6) | 1 (6) | ||||||||
| Health and physical functioning | ||||||||||||||
|
|
Ever smoker, n (%)a | 24 (44) | 8 (44) | 7 (39) | 9 (50) | |||||||||
|
|
General health status, n (%) | |||||||||||||
|
|
|
Fair or poor | 8 (15) | 4 (22) | 2 (11) | 2 (11) | ||||||||
|
|
|
Good | 22 (41) | 9 (50) | 6 (33) | 7 (39) | ||||||||
|
|
|
Very good or excellent | 24 (44) | 5 (28) | 10 (56) | 9 (50) | ||||||||
|
|
Number of comorbidities, n (%) | |||||||||||||
|
|
|
0-1; does not limit activities | 27 (50) | 10 (56) | 8 (44) | 9 (50) | ||||||||
|
|
|
1-2; limits activities | 16 (30) | 6 (33) | 5 (28) | 5 (28) | ||||||||
|
|
|
≥3; limits activities | 11 (20) | 2 (11) | 5 (28) | 4 (22) | ||||||||
|
|
Self-reported physical function, mean (SD) |
|
|
|
||||||||||
|
|
|
Raw score (0-100) | 73.7 (20.7) | 68.1 (22.4) | 77.5 (15.4) | 75.6 (23.2) | ||||||||
|
|
|
T-scoreb | 47.5 (7.9) | 45.3 (8.6) | 48.9 (5.9) | 48.2 (8.9) | ||||||||
|
|
Short Physical Performance Battery (0-12), mean (SD) | 10.7 (1.6) | 10.4 (2.2) | 11.1 (0.9) | 10.7 (1.5) | |||||||||
| Clinical characteristics | ||||||||||||||
|
|
Cancer type, n (%) | |||||||||||||
|
|
|
Breast | 21 (39) | 7 (39) | 9 (50) | 5 (28) | ||||||||
|
|
|
Prostate | 16 (30) | 7 (39) | 3 (17) | 6 (33) | ||||||||
|
|
|
Otherc | 17 (31) | 4 (22) | 6 (33) | 7 (39) | ||||||||
|
|
Stage at diagnosisd, n (%) | |||||||||||||
|
|
|
Local | 40 (75) | 14 (78) | 14 (78) | 12 (71) | ||||||||
|
|
|
Regional | 13 (25) | 4 (22) | 4 (22) | 5 (29) | ||||||||
|
|
Treatment receivede, n (%) | |||||||||||||
|
|
|
Surgery | 42 (78) | 13 (72) | 13 (72) | 14 (78) | ||||||||
|
|
|
Chemotherapy | 10 (18) | 3 (17) | 3 (17) | 4 (22) | ||||||||
|
|
|
Radiation | 30 (56) | 12 (67) | 10 (56) | 8 (44) | ||||||||
|
|
|
Hormone therapy | 12 (22) | 2 (11) | 6 (33) | 4 (22) | ||||||||
|
|
Time since diagnosis (years), mean (SD) | 4.4 (1.6) | 4.3 (1.4) | 4.2 (1.9) | 4.6 (1.4) | |||||||||
| Other characteristics | ||||||||||||||
|
|
Comfort level with using smartphone, n (%) | |||||||||||||
|
|
|
Very or extremely comfortable | 38 (70) | 11 (61) | 13 (72) | 14 (78) | ||||||||
|
|
|
Slightly or not comfortable | 16 (30) | 7 (39) | 5 (28) | 4 (22) | ||||||||
|
|
activPAL data, mean (SD) | |||||||||||||
|
|
|
Number of valid wear daysf | 6.7 (0.7) | 6.6 (1.0) | 6.8 (0.4) | 6.7 (0.5) | ||||||||
|
|
|
Average awake hours | 14.5 (1.0) | 14.1 (1.1) | 14.6 (0.6) | 14.6 (0.9) | ||||||||
aOnly 1 participant was currently smoking at baseline.
bT-scores represent a linear transformation, normed to the US population, with a mean of 50 (SD 10).
cOther cancers include bladder, cervical, colon, endometrium, kidney, lymphoma, or melanoma cancers.
dStage at diagnosis is missing for 1 participant.
ePercentages do not add up to 100% because participants may have had more than 1 type of treatment.
fUp to the first 7 days of 10 hours or more of awake/wear time were included in the analyses; additional days of wear beyond the first 7 days were excluded.
Adherence
Adherence during the intervention was moderately high for wearing the Jawbone activity monitor most days of the week (100% very often) and checking the app daily for the number of steps taken (23/29, 79% very often and 5/29, 17% often; Figure 3). However, few participants checked the app for the longest idle time (aka longest sedentary bout; 7/29, 24% often or very often), and on a typical day, most participants ignored the vibration on their tracker and remained seated when reminded to stand up and move (18/29, 62% sometimes and 6/29, 21% often or very often). As indicated in Figure 3, adherence related to the sedentary features of the tracker and app was lower in participants who experienced malfunctions with their initial Jawbone UP2 monitor. Among the participants who completed the trial, 93% (27/29) completed all 5 coaching calls.
Figure 3.
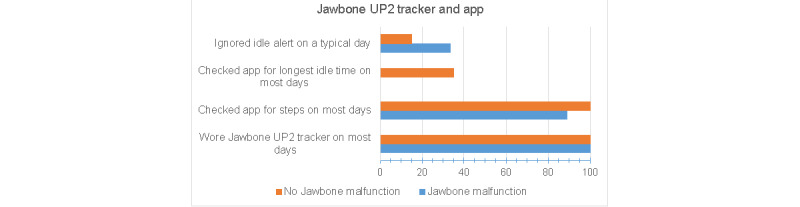
Adherence to wearing the Jawbone UP2 activity tracker and using the smartphone app, stratified by whether the intervention participant experienced malfunctions with the Jawbone UP2 tracker.
Adverse Events
There were no serious adverse events attributable or possibly attributable to the intervention.
Acceptability
Despite initial Jawbone UP2 malfunctions among one-third of the intervention group, the acceptability of the intervention was moderately high (Figure 4). Overall, 79% (23/29) of the participants agreed or strongly agreed that the Jawbone UP2 technology (monitor plus app) was easy to use and the same percentage indicated that they would use the Jawbone UP2 in the future. Despite the lack of tracking of sedentary data, most participants agreed or strongly agreed that this technology made them more aware of how much time they spent sitting and motivated them to decrease their sedentary time (27/29, 93% and 24/29, 83%, respectively). Participants who started with a malfunctioning Jawbone tracker reported lower acceptability scores than those with properly functioning trackers, with the greatest difference related to ease of use and recommending the tracker and app to others.
Figure 4.
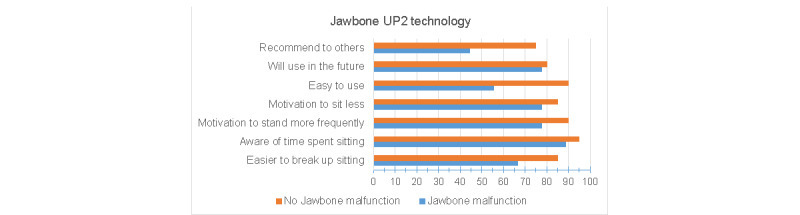
Acceptability and participant evaluation of the mobile health intervention using the Jawbone UP2 activity tracker and smartphone app to sit less, stand more, and move more, throughout the day, and every day. Results are stratified by whether the intervention participant experienced malfunctions with the Jawbone UP2 tracker.
Efficacy Primary Outcomes
Of the 54 cancer survivors enrolled in the study, data for the primary and secondary outcomes for sedentary behavior and physical activity were available for 53 participants (1 monitor malfunction at baseline). On average, participants wore the activPAL monitor for 6.7 days (SD 0.7, range 3-7 days), for an average of 14.5 (SD 1.0) awake/wear hours per day. During a standardized 15 hour awake/wear day, study participants spent 9.6 hours (SD 1.7 h) in sedentary (sitting/lying) activities. Approximately half (5.1, SD 1.7 h) of the number of sedentary minutes were spent in prolonged bouts (30 minutes or longer). The average number of breaks from sitting was 46.6 (SD 14.0) per 15 hour day. Standing accounted for one-quarter of the awake hours (3.8, SD 1.5 h). The remaining time was spent in light- and moderate-intensity stepping (36.8, SD 14.8 minutes and 56.5, SD 25.5 min, respectively; zero minutes in vigorous-intensity stepping). At baseline, only 5 participants met the physical activity guidelines that were recommended at the time the study began (150 minutes per week of moderate-intensity or 75 minutes of vigorous-intensity physical activity, minimum bout duration of 10 min) [17]. On the basis of current guidelines, which no longer require that activity occurs in bouts of at least 10 minutes, 46 participants met the minimum recommendation of at least 150 minutes per week of moderate-intensity activity [88,89].
Between- and within-group comparisons of changes in sedentary behavior are presented in Table 3. The tech support and the tech support plus health coaching groups did not reduce their daily sedentary time compared with the control group (least square means 8.5 min, 95% CI −50.5 to 67.5; P=.77 and least square means 10.4 min, 95% CI −43.5 to 64.3; P=.70, respectively). There were no significant differences between the intervention and control groups in the daily number of breaks from sitting (least square means −0.1, 95% CI −7.6 to 7.4; P=.97 and least square means −2.2, 95% CI −9.0 to 4.7; P=.52, respectively). There were no significant or meaningful changes in these sedentary behavior outcomes within any of the 3 groups.
Table 3.
Between- and within-group comparisons of change in sedentary behavior and physical activity after a 13-week mobile health intervention.a,b
| Sedentary behavior and physical activity metrics | Baseline, least square mean (95% CI) | Follow-up, least square mean (95% CI) | Within-group change, least square mean difference (95% CI) | P value | Between-group changec, least square mean difference (95% CI) | P value | |
| Sedentary, minutes per 15 hours awake | |||||||
|
|
Tech support | 598.5 (550.1 to 646.9) | 604.6 (549.1 to 660.0) | 6.0 (−39.5 to 51.6) | .79 | 8.5 (−50.5 to 67.5) | .77 |
|
|
Health coaching | 567.7 (517.9 to 617.5) | 575.6 (525.0 to 626.1) | 7.9 (−30.8 to 46.6) | .68 | 10.4 (−43.5 to 64.3) | .70 |
|
|
Control | 555.4 (507.0 to 603.8) | 552.9 (503.8 to 602.0) | −2.5 (−40.0 to 35.0) | .89 | N/Ad | N/A |
| Prolonged sedentary bouts (≥30 min), minutes per 15 hours awake | |||||||
|
|
Tech support | 319.8 (258.8 to 380.8) | 331.9 (263.8 to 400.0) | 12.1 (−38.2 to 62.4) | .63 | 4.7 (−60.3 to 69.7) | .88 |
|
|
Health coaching | 287.0 (224.2 to 349.7) | 305.5 (242.0 to 369.0) | 18.5 (−23.9 to 61.0) | .38 | 11.2 (−48.0 to 70.3) | .71 |
|
|
Control | 289.7 (228.7 to 350.7) | 297.1 (235.4 to 358.8) | 7.4 (−33.8 to 48.6) | .72 | N/A | N/A |
| Breaks from sitting, number per 15 hour awake | |||||||
|
|
Tech support | 50.6 (44.2 to 57.1) | 50.5 (43.2 to 57.9) | −0.1 (−5.9 to 5.8) | .97 | −0.1 (−7.6 to 7.4) | .97 |
|
|
Health coaching | 48.8 (42.2 to 55.4) | 46.6 (39.9 to 53.4) | −2.2 (−7.1 to 2.7) | .38 | −2.2 (−9.0 to 4.7) | .52 |
|
|
Control | 46.2 (39.7 to 52.6) | 46.2 (39.6 to 52.7) | 0.0 (−4.7 to 4.8) | 1.00 | N/A | N/A |
| Break ratio, number of breaks per sedentary hour | |||||||
|
|
Tech support | 5.4 (4.5 to 6.2) | 5.4 (4.5 to 6.4) | 0.06 (−0.66 to 0.77) | .87 | −0.08 (−1.00 to 0.85) | .87 |
|
|
Health coaching | 5.3 (4.4 to 6.2) | 4.9 (4.0 to 5.8) | −0.39 (−0.99 to 0.21) | .20 | −0.52 (−1.36 to 0.32) | .22 |
|
|
Control | 5.1 (4.2 to 5.9) | 5.2 (4.3 to 6.0) | 0.13 (−0.45 to 0.72) | .65 | N/A | N/A |
| Standing, minutes per 15 hours awake | |||||||
|
|
Tech support | 213.9 (174.2 to 253.7) | 202.8 (157.6 to 248.0) | −11.2 (−47.4 to 25.0) | .54 | −8.7 (−55.6 to 38.2) | .71 |
|
|
Health coaching | 243.0 (202.1 to 283.9) | 220.4 (178.9 to 261.8) | −22.6 (−53.3 to 8.1) | .14 | −20.1 (−62.9 to 22.6) | .35 |
|
|
Control | 241.9 (202.2 to 281.6) | 239.4 (199.2 to 279.7) | −2.5 (−32.3 to 27.3) | .87 | N/A | N/A |
| Steps per 15 hour awake | |||||||
|
|
Tech support | 6686 (5166 to 8206) | 7339 (5594 to 9085) | 654 (−794 to 2101) | .37 | 420 (−1456 to 2297) | .65 |
|
|
Health coaching | 6663 (5099 to 8227) | 8338 (6749 to 9926) | 1675 (444 to 2906) | .009e | 1441 (−273 to 3156) | .10 |
|
|
Control | 7898 (6378 to 9418) | 8132 (6590 to 9674) | 233 (−961 to 1428) | .70 | N/A | N/A |
| Light-intensity physical activity, minutes per 15 hours awake | |||||||
|
|
Tech support | 34.4 (27.2 to 41.5) | 33.1 (25.4 to 40.9) | −1.2 (−6.0 to 3.6) | .61 | −4.2 (−10.4 to 2.0) | .18 |
|
|
Health coaching | 37.3 (29.9 to 44.7) | 36.9 (29.5 to 44.4) | −0.3 (−4.4 to 3.7) | .86 | −3.3 (−8.9 to 2.3) | .24 |
|
|
Control | 38.8 (31.6 to 45.9) | 41.7 (34.5 to 49.0) | 3.0 (−0.9 to 6.9) | .13 | N/A | N/A |
| Moderate-intensity physical activity (MPA), minutes per 15 hours awake | |||||||
|
|
Tech support | 53.2 (40.2 to 66.1) | 59.5 (44.5 to 74.6) | 6.4 (−6.6 to 19.3) | .33 | 4.6 (−12.2 to 21.4) | .58 |
|
|
Health coaching | 52.1 (38.8 to 65.4) | 67.2 (53.7 to 80.8) | 15.2 (4.1 to 26.2) | .008e | 13.4 (−2.0 to 28.8) | .09 |
|
|
Control | 64.0 (51.0 to 76.9) | 65.7 (52.6 to 78.9) | 1.8 (−9.0 to 12.5) | .74 | N/A | N/A |
| MPA (guideline bouts), minutes per 15 hours awake | |||||||
|
|
Tech support | 5.8 (−3.2 to 14.8) | 13.0 (2.2 to 23.8) | 7.3 (−3.1 to 17.6) | .17 | 7.1 (−6.4 to 20.6) | .30 |
|
|
Health coaching | 3.0 (−6.3 to 12.2) | 19.7 (10.2 to 29.1) | 16.7 (7.8 to 25.7) | <.001e | 16.6 (4.1 to 29.0) | .01e |
|
|
Control | 12.1 (3.1 to 21.1) | 12.3 (3.1 to 21.4) | 0.2 (−8.5 to 8.8) | .97 | N/A | N/A |
aIntent-to-treat analyses.
bAll variables were standardized to a 15-hour awake per wear day before calculating the pre- to postintervention changes.
cComparisons are between each intervention group and the control group.
dN/A: not applicable.
eStatistically significant (P<.05) results.
Secondary Outcomes
Between- and within-group comparisons of changes in daily steps and time spent stepping are presented in Table 3. Although time spent standing is considered an LPA, it was evaluated separately from the time spent stepping at a light intensity. There were no significant between-group changes in the time spent standing for either intervention group compared with controls (tech support vs control: least square means −8.7 min, 95% CI −55.6 to 38.2; P=.71 and health coaching vs control: least square means −20.1 min, 95% CI −62.9 to 22.6; P=.35). There were no significant changes in daily steps between the intervention groups and the control group (tech support vs control: least square means 420 steps, 95% CI −1456 to 2297; P=.65 and health coaching vs control: least square means 1441 steps, 95% CI −273 to 3156; P=.10). There was a borderline significant difference between moderate-intensity stepping in the health coaching group compared with the control group (least square means 13.4 min, 95% CI −2.0 to 28.8; P=.09), but there was no difference between the tech support and control groups (least square means 4.6 min, 95% CI −12.2 to 21.4; P=.58). The between-group differences for moderate-intensity stepping accumulated in guideline bouts of 10 minutes or longer were least square means of 16.6 minutes (95% CI 4.1-29.0; P=.01) and 7.1 minutes (95% CI −6.4 to +20.6; P=.30), respectively, for health coaching group vs controls and tech support group vs controls.
The only significant within-group change occurred in the health coaching group. There was a significant increase of 1675 daily steps (95% CI 444-2906; P=.009). Although there was no appreciable change in light-intensity stepping, there was a significant increase in moderate-intensity stepping overall and guideline bouts among the health coaching group (least square means 15.2 extra minutes per day, 95% CI 4.1-26.2; P=.008 and least square means 16.7 extra minutes per day, 95% CI 7.8-25.7; P<.001). There was neither a significant decrease in sedentary time (least square means 7.9 min, 95% CI −30.8 to 46.6; P=.68) nor increase in standing (least square means −22.6 min/day, 95% CI −53.3 to 8.1; P=.14). There were no significant within-group changes for either the tech support group or the control group.
QoL Analysis
There were no significant between-group changes in subjectively measured health-related QoL (Multimedia Appendix 4). However, between-group differences of 4 or more points, representing the minimally clinically significant difference for the SF-36 QoL survey [90], occurred in several subscales. For health coaching compared with controls, these scales included general health, role physical, social functioning, and vitality. For tech support compared with controls, these scales included physical function and social functioning (favoring tech support) and mental health and role emotional (favoring controls). No significant or meaningful between- or within-group differences were observed for the FACIT-Fatigue or the PROMIS pain scales.
Physical Performance
The average baseline scores on the SPPB were relatively high at baseline for each of the 3 groups (tech support: 10.4, health coaching: 11.2, and control: 10.7). There were no significant between-group changes (P>.4); the difference between the health coaching and control groups was at the lower limit of the minimally meaningful change for this scale (0.3-0.8 points) [91].
Additional Analyses
The results of the complete case analyses, including participants with both baseline and follow-up data, did not differ substantially from the intent-to-treat analyses regarding sedentary behavior and physical activity (data not shown). The results of a sensitivity analysis excluding people with fewer than 4 days of valid activPAL data were not appreciably different from the intention-to-treat analyses (data not shown). No significant between-group differences were found in a sensitivity analysis, excluding participants who experienced issues/failures with the Jawbone tracker. The results for tech support versus controls were as follows (least square mean, 95% CI): sedentary time (−28 min, −99 to 43), standing (17 min, −42 to 76), total daily steps (1290 steps, −403 to 2982), and moderate-intensity stepping (13 min, −2 to 28). The results for health coaching versus controls were as follows: sedentary time (10 min, −56 to 76), standing (−18 min, −72 to 36), total daily steps (1102 steps, −460 to 2663), and moderate-intensity stepping (11 min, −3 to 25).
Discussion
Principal Findings
This study explored the feasibility, acceptability, and preliminary efficacy of a home-based mHealth intervention to disrupt and replace sedentary time with LPA (standing and stepping) among older cancer survivors. Despite technical issues with one-third of the Jawbone UP2 activity trackers, an mHealth intervention in older cancer survivors was feasible (high retention and adherence) and acceptable. However, although participants reported that the mHealth intervention increased their awareness of sedentary behavior, this did not translate into a reduction in total sedentary time, prolonged sedentary time, or an increase in breaks from sitting in either intervention group.
The lack of a reduction in total sedentary time was an unexpected finding, given ample room for improvement (nearly 10 hours of sedentary time per day at baseline). In contrast, this group of older, primarily retired, cancer survivors was already taking frequent breaks from sitting, averaging 3 breaks per hour. However, despite the average number of hourly breaks, the amount of time spent in prolonged sedentary bouts (≥30 min) was not reduced, suggesting that there is room for improvement in this metric. Only a few studies have reported a significant increase in the number of breaks from sitting [66]. A large proportion of our study participants reported ignoring the idle alert on a typical day. Whether this represented a valid opportunity to stand up and move (eg, alerted while watching television) or an inopportune time (eg, eating, driving, or in a social setting) is unknown. Other studies using the Jawbone tracker reported overall acceptability, including the usefulness or interest in continued use of the idle alert [92,93]; however, other studies noted that some participants found the idle alert very irritating and inaccurate [94].
In our study, both the postintervention evaluation and comments received from many participants during coaching calls support their focus on the step goal. Similar to other activity tracker apps, the predominant tracking features of the Jawbone apps are related to daily steps rather than sedentary behavior, which may have reinforced the step goal. More support for replacing rather than merely disrupting sedentary time with a suggested minimal bout duration may have been more helpful for individuals already taking frequent breaks from sitting. In addition, research suggests that given the automaticity of sedentary behavior, different and more effortful strategies are required to break existing habits compared with forming new habits [95-97].
Additional unexpected findings were the 6-fold higher time spent standing compared with light-intensity stepping (both before and after intervention) and the suggested decrease in standing, especially in the health coaching group (22 fewer minutes per day). Interventions that report LPA separately indicate that cancer survivors spend 2 to 5 hours per day in these activities [59,60,98,99]. In comparison, our study measured, on average, only 30 to 40 minutes per day. This likely involves measurement differences. Importantly, many interventions have not been able to determine the amount of time spent standing, and standing still is often combined with sedentary time. The activPAL monitor, which is worn on the upper thigh and includes both an inclinometer and accelerometer, provides a more accurate measure of sedentary time (sitting or lying) and standing compared with the ActiGraph accelerometer [70,72], which is the gold standard in MVPA research.
Another research challenge is measuring daily steps in a free-living population (vs in a controlled lab setting), especially if all steps are of interest rather than just higher intensity steps (ie, MVPA). In a free-living population measured during awake hours, stepping ranges from slow, intermittent stepping to fast, continuous stepping. The accuracy of step accumulation by research-grade monitors varies according to walking speed (less accurate at slower speeds) and intermittent (less accurate) versus continuous (more accurate) stepping [100,101]. Therefore, slow or intermittent stepping may be classified as standing rather than light-intensity stepping [100,101]. In our study, overall, there was no reduction in sedentary time, which was measured with high accuracy. Instead, the increased step accumulation among each group, especially the health coaching group, likely represents a shift from standing and slow or intermittent stepping to moderate-intensity and continuous stepping.
There was much flexibility allowed to achieve the goals of the study, that is, no minimum bout duration (standing or stepping) or intensity level (stepping) was provided to participants. The results suggest that most of the intervention group participants focused on the step goal rather than standing more frequently. Furthermore, participants self-selected to accumulate steps in longer bouts and at a moderate versus light intensity. However, only the intervention group with additional health coaching (vs only tech support) achieved significant and meaningful increases in the total daily steps and number of moderate-intensity steps. Although the average number of additional daily steps was below the 3000 goal, it is similar to that reported from meta-analyses using consumer wearable activity trackers, which report 400 to 475 additional daily steps [52,102].
Comparison With Previous Work
On the basis of recent reviews, interventions with a sedentary behavior focus were more effective (greater reduction in sedentary time) than interventions with a focus on increasing MVPA or both increasing MVPA and reducing sedentary time [103,104]. Reviews of interventions with device-based measurement of sedentary behavior (eg, activPAL and ActiGraph) report, on average, a decrease of 35 minutes per day of sedentary time; however, there was significant heterogeneity detected [51,52,102]. Although device-based measures of sedentary behavior are more accurate than self-report measures, there are also differences in accuracy between device-based measures. For example, hip-worn accelerometers estimate sedentary behavior based on lack of movement (eg, <100 counts per minutes on an ActiGraph), whereas thigh-worn monitors base their estimation on posture (eg, activPAL) [105]. As a result, a hip-worn accelerometer cannot distinguish between standing and sedentary time and can overestimate the change in sedentary time if both sitting and standing are reduced.
To date, few interventions have been designed specifically to decrease sedentary behavior in cancer survivors [106]. In contrast to our findings, several studies have reported a reduction in sedentary time among breast, prostate, and colorectal cancer survivors [58-60]. However, our study compares favorably with the increase in daily steps, especially moderate-intensity stepping. Lynch et al [58] designed an RCT to both reduce sedentary behavior and increase MVPA using the Garmin Vivofit activity tracker among 80 breast cancer survivors (mean age 62 years, SD 6.4). They reported a 37 minutes per day decrease in sitting (95% CI −72.0 to −2.0), which was primarily replaced with standing (27 minutes; 95% CI −2 to 56), and an increase of 933 steps per day (95% CI −215 to 2082). Gomersall et al [59] designed a text-message enhanced clinical exercise intervention (RCT) to reduce sitting time and increase activity among 36 participants, representing several cancer types, primarily colorectal and prostate cancer. The significant decrease in total daily sitting (mean difference −48 minutes/16 h awake day; 95% CI −90 to −6) was primarily replaced with standing (mean difference 42 minutes; 95% CI −4 to 88) and light-intensity stepping (mean difference 7.0 minutes; 95% CI 0.4-14). The RiseTx web-based program designed by Trinh et al [60] included 46 prostate cancer survivors (mean age 73.2 years, SD 7.3) who were given a Jawbone UP 24 activity monitor (model preceding the UP2). The goal was to increase daily steps by 3000 and to reduce sedentary time over a 12-week period in a single-arm trial. There was a significant decrease in sitting time (−455.4 minutes per week; 95% CI −766.6 to −144.2), a nonsignificant decrease in LPA (−91.0 minutes per week; 95% CI −236.4 to 54.4), and a significant increase in MVPA (44.1 minutes per week; 95% CI 11.1-77.0; all measured with the hip-worn ActiGraph). There was also an increase in daily steps (1535; P<.001), which was measured using the Jawbone wearable activity tracker rather than a research-grade accelerometer.
Limitations and Strengths
The limitations of our feasibility study include the potential for selection bias because smartphone ownership was an eligibility criterion. Individuals not familiar with a smartphone (if provided with a loaner phone) may have had more difficulty with adherence or uptake of an mHealth intervention. In addition, individuals who were enrolled were likely more motivated to change their inactivity. The results of this study may not be generalizable to cancer survivors who are less healthy, less physically active, or less comfortable with smartphones than those enrolled in the study. Recruitment was more challenging than anticipated, resulting in a low response rate. Another limitation is the lack of fidelity measures to ensure that the intervention components were delivered as intended. The use of a consumer activity monitor, in this case the Jawbone UP2, is both a limitation and a strength. We experienced substantial technical issues/failures with the device, affecting one-third of the intervention group, as the manufacturing company quit the production, stopped providing support, and eventually closed. While adversely affecting intervention delivery (starting over with tech support/health coaching calls) and possibly retention (3 of 7 dropouts had issues with their Jawbone UP2 monitor; all tech support group), the intervention acceptability scores were moderately high. Most importantly, as reported during follow-up interviews, many intervention participants switched to a different consumer activity monitor to track their steps (Fitbit or Garmin), suggesting a transfer of knowledge and skills gained during the intervention. The strengths of this study include the RCT design and a diverse study sample in terms of sociodemographics, cancer type, and health characteristics. Another strength is the measurement of sedentary behavior with the activPAL research-grade monitor, which is the gold standard for distinguishing between sitting, standing, and stepping [69-72].
Several lessons were learned from this pilot study. First, despite the tremendous growth in the consumer wearable activity tracker market, the disadvantages of using these devices for research studies include technical issues/failures, changes in availability, changes in the user interface or algorithms behind the app, and the potential lack of support from the manufacturer. However, this mHealth approach has been popular among researchers because of its low cost, the ability to reach a large number of participants, and the potential for maintenance of behavior change. The advantages for participants include receiving feedback in real time to prompt change and reducing the burden of tracking weekly/monthly steps (eg, participant recording steps in diary vs automated recording and tracking with app).
Second, sedentary behavior is a strongly ingrained habit that is mostly initiated subconsciously [94]. Research suggests that, given the automaticity of sedentary behavior, different and more effortful strategies are required to break existing habits compared with forming new habits [95-97]. This may require different or multiple behavioral theories to inform the intervention. Although many consumer activity trackers have several behavioral change techniques built into the tracker and/or the app, including Jawbone [54,55,107], accumulating evidence suggests that additional behavior change techniques are needed to achieve meaning change [92,102]. Until activity tracker apps advance to provide features for tracking daily sedentary behavior, researchers will need to provide participants with other strategies. Finally, the daily step goal (+3000 steps above baseline) may have been too high, although participants were able to self-select the minimum bout duration and intensity level for stepping. Nevertheless, the step goal may have competed with messaging to reduce sedentary time.
Conclusions
This low-touch, home- and technology-based intervention designed to disrupt and replace sedentary time with LPA (standing and stepping) was feasible and acceptable for a diverse group of older cancer survivors. Future studies are warranted to evaluate strategies for replacing sedentary time with standing and/or physical activity.
Acknowledgments
This research was supported by the American Cancer Society Institutional Review Grant (#IRG-14-187-19) and the University of New Mexico Comprehensive Cancer Center Support Grant (NCI P30CA118100) and the Behavioral Measurement and Population Sciences Shared Resource and the Biostatistics Shared Resource. This project was also supported by Contract HHSN261201800014I, Task Order HHSN26100001, from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or the American Cancer Society. CB is currently supported by the NIH K07 grant CA215937.
Abbreviations
- CONSORT
Consolidated Standards of Reporting Trials
- LPA
light-intensity physical activity
- MET
metabolic equivalent
- mHealth
mobile health
- MVPA
moderate-to-vigorous physical activity
- NIH
National Institutes of Health
- QoL
quality of life
- RCT
randomized controlled trial
- SF-36
Short Form 36-item survey
- SPPB
Short Physical Performance Battery
Appendix
An example of the activPAL monitor summary data from baseline discussed with intervention group participants in an mHealth study.
activPAL3 data collection and processing details.
CONSORT (Consolidated Standards of Reporting Trials)-EHEALTH checklist (v 1.6).
Effects of mHealth intervention on health-related Quality of Life.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Cancer Treatment & Survivorship: Facts & Figures 2019-2021. American Cancer Society. 2019. [2021-03-22]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf.
- 2.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008 Dec 15;113(12 Suppl):3519–29. doi: 10.1002/cncr.23941. doi: 10.1002/cncr.23941. [DOI] [PubMed] [Google Scholar]
- 3.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ, ASCO Cancer Survivorship Expert Panel American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007 Sep 01;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 4.Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57 Suppl 2:289–92. doi: 10.1111/j.1532-5415.2009.02515.x. [DOI] [PubMed] [Google Scholar]
- 5.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003 Jan;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 02;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009 Sep 15;115(18 Suppl):4362–73. doi: 10.1002/cncr.24588. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 8.Guy GP Jr, Ekwueme DU, Yabroff KR, Dowling EC, Li C, Rodriguez JL, de Moor JS, Virgo KS. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013 Oct 20;31(30):3749–57. doi: 10.1200/JCO.2013.49.1241. http://europepmc.org/abstract/MED/24043731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vissers PAJ, Thong MSY, Pouwer F, Zanders MMJ, Coebergh JWW, van de Poll-Franse LV. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: analyses of data from the PROFILES registry. J Cancer Surviv. 2013 Dec;7(4):602–13. doi: 10.1007/s11764-013-0299-1. [DOI] [PubMed] [Google Scholar]
- 10.Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, Bierman AS, Hays RD. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29(4):41–56. http://europepmc.org/abstract/MED/18773613. [PMC free article] [PubMed] [Google Scholar]
- 11.George SM, Alfano CM, Groves J, Karabulut Z, Haman KL, Murphy BA, Matthews CE. Objectively measured sedentary time is related to quality of life among cancer survivors. PLoS One. 2014;9(2):-. doi: 10.1371/journal.pone.0087937. https://dx.plos.org/10.1371/journal.pone.0087937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010 Jul;38(3):105–13. doi: 10.1097/JES.0b013e3181e373a2. http://europepmc.org/abstract/MED/20577058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlop DD, Song J, Arnston EK, Semanik PA, Lee J, Chang RW, Hootman JM. Sedentary time in US older adults associated with disability in activities of daily living independent of physical activity. J Phys Act Health. 2015 Jan;12(1):93–101. doi: 10.1123/jpah.2013-0311. http://europepmc.org/abstract/MED/24510000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011 Mar;32(5):590–7. doi: 10.1093/eurheartj/ehq451. http://europepmc.org/abstract/MED/21224291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyden K, Keadle SK, Staudenmayer J, Braun B, Freedson PS. Discrete features of sedentary behavior impact cardiometabolic risk factors. Med Sci Sports Exerc. 2015 May;47(5):1079–86. doi: 10.1249/MSS.0000000000000499. http://europepmc.org/abstract/MED/25202848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008 Feb;31(2):369–71. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 17.Physical Activity Guidelines Advisory Committee Report 2008. U.S. Department of Health and Human Services. 2008. [2021-03-22]. https://www.europarc.org/wp-content/uploads/2018/03/Physical-Activity-Guidelines-Advisory-Committee-Report-2008.pdf.
- 18.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, Lancet Physical Activity Series Working Group Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012 Jul 21;380(9838):219–29. doi: 10.1016/S0140-6736(12)61031-9. http://europepmc.org/abstract/MED/22818936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blair CK, Robien K, Inoue-Choi M, Rahn W, Lazovich D. Physical inactivity and risk of poor quality of life among elderly cancer survivors compared to women without cancer: the Iowa Women's Health Study. J Cancer Surviv. 2016 Feb;10(1):103–12. doi: 10.1007/s11764-015-0456-9. http://europepmc.org/abstract/MED/26008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007 Nov;56(11):2655–67. doi: 10.2337/db07-0882. https://diabetes.diabetesjournals.org/lookup/pmidlookup?view=long&pmid=17827399. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton MT, Healy GN, Dunstan DW, Zderic TW, Owen N. Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Curr Cardiovasc Risk Rep. 2008 Jul;2(4):292–8. doi: 10.1007/s12170-008-0054-8. http://europepmc.org/abstract/MED/22905272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM, SBRN Terminology Consensus Project Participants Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017 Jun 10;14(1):75. doi: 10.1186/s12966-017-0525-8. https://ijbnpa.biomedcentral.com/articles/10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol. 2011 Jan 18;57(3):292–9. doi: 10.1016/j.jacc.2010.05.065. https://linkinghub.elsevier.com/retrieve/pii/S0735-1097(10)04465-7. [DOI] [PubMed] [Google Scholar]
- 24.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011 Jan;47(2):267–76. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Arem H, Pfeiffer RM, Moore SC, Brinton LA, Matthews CE. Body mass index, physical activity, and television time in relation to mortality risk among endometrial cancer survivors in the NIH-AARP Diet and Health Study cohort. Cancer Causes Control. 2016 Nov;27(11):1403–9. doi: 10.1007/s10552-016-0813-7. http://europepmc.org/abstract/MED/27730319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell MK, Demark-Wahnefried W, Symons M, Kalsbeek WD, Dodds J, Cowan A, Jackson B, Motsinger B, Hoben K, Lashley J, Demissie S, McClelland JW. Fruit and vegetable consumption and prevention of cancer: the Black Churches United for Better Health project. Am J Public Health. 1999 Sep;89(9):1390–6. doi: 10.2105/ajph.89.9.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratjen I, Schafmayer C, di Giuseppe R, Waniek S, Plachta-Danielzik S, Koch M, Burmeister G, Nöthlings Ute, Hampe J, Schlesinger S, Lieb W. Postdiagnostic physical activity, sleep duration, and TV watching and all-cause mortality among long-term colorectal cancer survivors: a prospective cohort study. BMC Cancer. 2017 Oct 25;17(1):701. doi: 10.1186/s12885-017-3697-3. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips SM, Awick EA, Conroy DE, Pellegrini CA, Mailey EL, McAuley E. Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer. Cancer. 2015 Nov 15;121(22):4044–52. doi: 10.1002/cncr.29620. doi: 10.1002/cncr.29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Roekel Elin H, Winkler EA, Bours MJ, Lynch BM, Willems PJ, Meijer K, Kant I, Beets GL, Sanduleanu S, Healy GN, Weijenberg MP. Associations of sedentary time and patterns of sedentary time accumulation with health-related quality of life in colorectal cancer survivors. Prev Med Rep. 2016 Dec;4:262–9. doi: 10.1016/j.pmedr.2016.06.022. https://linkinghub.elsevier.com/retrieve/pii/S2211-3355(16)30072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Television viewing time of colorectal cancer survivors is associated prospectively with quality of life. Cancer Causes Control. 2011 Aug;22(8):1111–20. doi: 10.1007/s10552-011-9786-8. [DOI] [PubMed] [Google Scholar]
- 31.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012 May;35(5):976–83. doi: 10.2337/dc11-1931. http://europepmc.org/abstract/MED/22374636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard BJ, Fraser SF, Sethi P, Cerin E, Hamilton MT, Owen N, Dunstan DW, Kingwell BA. Impact on hemostatic parameters of interrupting sitting with intermittent activity. Med Sci Sports Exerc. 2013 Jul;45(7):1285–91. doi: 10.1249/MSS.0b013e318285f57e. [DOI] [PubMed] [Google Scholar]
- 33.Sardinha LB, Santos DA, Silva AM, Baptista F, Owen N. Breaking-up sedentary time is associated with physical function in older adults. J Gerontol A Biol Sci Med Sci. 2015 Jan;70(1):119–24. doi: 10.1093/gerona/glu193. [DOI] [PubMed] [Google Scholar]
- 34.Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW. Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults. Nutr Metab Cardiovasc Dis. 2014 Sep;24(9):976–82. doi: 10.1016/j.numecd.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Lynch BM, Dunstan DW, Vallance JK, Owen N. Don't take cancer sitting down: a new survivorship research agenda. Cancer. 2013 Jun 01;119(11):1928–35. doi: 10.1002/cncr.28028. doi: 10.1002/cncr.28028. [DOI] [PubMed] [Google Scholar]
- 36.Buman MP, Hekler EB, Haskell WL, Pruitt L, Conway TL, Cain KL, Sallis JF, Saelens BE, Frank LD, King AC. Objective light-intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010 Nov 15;172(10):1155–65. doi: 10.1093/aje/kwq249. http://europepmc.org/abstract/MED/20843864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair CK, Morey MC, Desmond RA, Cohen HJ, Sloane R, Snyder DC, Demark-Wahnefried W. Light-intensity activity attenuates functional decline in older cancer survivors. Med Sci Sports Exerc. 2014 Jul;46(7):1375–83. doi: 10.1249/MSS.0000000000000241. http://europepmc.org/abstract/MED/24389524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Waart Hanna, Stuiver MM, van Harten Wim H, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove Aart, Lustig V, van den Heiligenberg Simone M, Smorenburg CH, Hellendoorn-van Vreeswijk Jeannette AJH, Sonke GS, Aaronson NK. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015 Jun 10;33(17):1918–27. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 39.Van Roekel Elin H, Bours MJL, Breedveld-Peters JJL, Meijer K, Kant I, Van Den Brandt Piet A, Sanduleanu S, Beets GL, Weijenberg MP. Light physical activity is associated with quality of life after colorectal cancer. Med Sci Sports Exerc. 2015 Dec;47(12):2493–503. doi: 10.1249/MSS.0000000000000698. [DOI] [PubMed] [Google Scholar]
- 40.van Roekel Elin H, Duchâteau J, Bours MJL, van Delden L, Breedveld-Peters JJL, Koole JL, Kenkhuis M, van den Brandt PA, Jansen RL, Kant I, Lima Passos V, Meijer K, Breukink SO, Janssen-Heijnen MLG, Keulen E, Weijenberg MP. Longitudinal associations of light-intensity physical activity with quality of life, functioning and fatigue after colorectal cancer. Qual Life Res. 2020 Nov 2;29(11):2987–98. doi: 10.1007/s11136-020-02566-7. http://europepmc.org/abstract/MED/32617891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunlop DD, Song J, Semanik PA, Sharma L, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Mysiw WJ, Nevitt MC, Chang RW. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. Br Med J. 2014 Apr 29;348:-. doi: 10.1136/bmj.g2472. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=24782514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thraen-Borowski KM, Trentham-Dietz A, Edwards DF, Koltyn KF, Colbert LH. Dose-response relationships between physical activity, social participation, and health-related quality of life in colorectal cancer survivors. J Cancer Surviv. 2013 Sep;7(3):369–78. doi: 10.1007/s11764-013-0277-7. http://europepmc.org/abstract/MED/23546822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conroy DE, Wolin KY, Blair CK, Demark-Wahnefried W. Gender-varying associations between physical activity intensity and mental quality of life in older cancer survivors. Support Care Cancer. 2017 Nov;25(11):3465–73. doi: 10.1007/s00520-017-3769-6. http://europepmc.org/abstract/MED/28620700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews CE, Keadle SK, Troiano RP, Kahle L, Koster A, Brychta R, Van Domelen D, Caserotti P, Chen KY, Harris TB, Berrigan D. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in US adults. Am J Clin Nutr. 2016 Nov;104(5):1424–32. doi: 10.3945/ajcn.116.135129. http://europepmc.org/abstract/MED/27707702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews CE, Moore SC, Sampson J, Blair A, Xiao Q, Keadle SK, Hollenbeck A, Park Y. Mortality benefits for replacing sitting time with different physical activities. Med Sci Sports Exerc. 2015 Sep;47(9):1833–40. doi: 10.1249/MSS.0000000000000621. http://europepmc.org/abstract/MED/25628179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reid N, Daly RM, Winkler EAH, Gardiner PA, Eakin EG, Owen N, Dunstan DW, Healy GN. Associations of monitor-assessed activity with performance-based physical function. PLoS One. 2016;11(4):-. doi: 10.1371/journal.pone.0153398. https://dx.plos.org/10.1371/journal.pone.0153398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elosua R, Redondo A, Segura A, Fiol M, Aldasoro E, Vega G, Forteza J, Martí H, Arteagoitia JM, Marrugat J. Dose-response association of physical activity with acute myocardial infarction: do amount and intensity matter? Prev Med. 2013 Nov;57(5):567–72. doi: 10.1016/j.ypmed.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Gourlan M, Bernard P, Bortolon C, Romain AJ, Lareyre O, Carayol M, Ninot G, Boiché J. Efficacy of theory-based interventions to promote physical activity. A meta-analysis of randomised controlled trials. Health Psychol Rev. 2016 Apr 10;10(1):50–66. doi: 10.1080/17437199.2014.981777. [DOI] [PubMed] [Google Scholar]
- 49.Glanz K, Bishop DB. The role of behavioral science theory in development and implementation of public health interventions. Annu Rev Public Health. 2010;31:399–418. doi: 10.1146/annurev.publhealth.012809.103604. [DOI] [PubMed] [Google Scholar]
- 50.McGarrigle L, Todd C. Promotion of physical activity in older people using mhealth and ehealth technologies: rapid review of reviews. J Med Internet Res. 2020 Dec 29;22(12):-. doi: 10.2196/22201. https://www.jmir.org/2020/12/e22201/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Compernolle S, DeSmet A, Poppe L, Crombez G, De Bourdeaudhuij I, Cardon G, van der Ploeg Hidde P, Van Dyck Delfien. Effectiveness of interventions using self-monitoring to reduce sedentary behavior in adults: a systematic review and meta-analysis. Int J Behav Nutr Phys Act. 2019 Aug 13;16(1):63. doi: 10.1186/s12966-019-0824-3. https://ijbnpa.biomedcentral.com/articles/10.1186/s12966-019-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stockwell S, Schofield P, Fisher A, Firth J, Jackson SE, Stubbs B, Smith L. Digital behavior change interventions to promote physical activity and/or reduce sedentary behavior in older adults: A systematic review and meta-analysis. Exp Gerontol. 2019 Jun;120:68–87. doi: 10.1016/j.exger.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 53.Gardner B, Smith L, Lorencatto F, Hamer M, Biddle SJ. How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol Rev. 2016;10(1):89–112. doi: 10.1080/17437199.2015.1082146. http://europepmc.org/abstract/MED/26315814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014 Aug 15;16(8):e192. doi: 10.2196/jmir.3469. https://www.jmir.org/2014/8/e192/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duncan M, Murawski B, Short CE, Rebar AL, Schoeppe S, Alley S, Vandelanotte C, Kirwan M. Activity trackers implement different behavior change techniques for activity, sleep, and sedentary behaviors. Interact J Med Res. 2017 Aug 14;6(2):e13. doi: 10.2196/ijmr.6685. https://www.i-jmr.org/2017/2/e13/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol. 2006 Nov 10;24(32):5125–31. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 57.Courneya KS, Rogers LQ, Campbell KL, Vallance JK, Friedenreich CM. Top 10 research questions related to physical activity and cancer survivorship. Res Q Exerc Sport. 2015 Jun;86(2):107–16. doi: 10.1080/02701367.2015.991265. [DOI] [PubMed] [Google Scholar]
- 58.Lynch BM, Nguyen NH, Moore MM, Reeves MM, Rosenberg DE, Boyle T, Vallance JK, Milton S, Friedenreich CM, English DR. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer. 2019 Aug 15;125(16):2846–55. doi: 10.1002/cncr.32143. doi: 10.1002/cncr.32143. [DOI] [PubMed] [Google Scholar]
- 59.Gomersall SR, Skinner TL, Winkler E, Healy GN, Eakin E, Fjeldsoe B. Feasibility, acceptability and efficacy of a text message-enhanced clinical exercise rehabilitation intervention for increasing 'whole-of-day' activity in people living with and beyond cancer. BMC Public Health. 2019 Jun 03;19(Suppl 2):542. doi: 10.1186/s12889-019-6767-4. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-6767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trinh L, Arbour-Nicitopoulos KP, Sabiston CM, Berry SR, Loblaw A, Alibhai SMH, Jones JM, Faulkner GE. RiseTx: testing the feasibility of a web application for reducing sedentary behavior among prostate cancer survivors receiving androgen deprivation therapy. Int J Behav Nutr Phys Act. 2018 Jun 07;15(1):49. doi: 10.1186/s12966-018-0686-0. https://ijbnpa.biomedcentral.com/articles/10.1186/s12966-018-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Visser M, Koster A. Development of a questionnaire to assess sedentary time in older persons - a comparative study using accelerometry. BMC Geriatr. 2013 Jul 30;13:80. doi: 10.1186/1471-2318-13-80. https://bmcgeriatr.biomedcentral.com/articles/10.1186/1471-2318-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen Vivian E, Schwartz AL, American College of Sports Medicine American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010 Jul;42(7):1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 63.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute. [2020-02-23]. https://seer.cancer.gov/
- 64.Bandura A. Social learning theory. Prentice Hall, N. J: Englewood Cliffs; 1977. [Google Scholar]
- 65.Bandura A. Social foundations of thought and action : a social cognitive theory. Prentice-Hall, N.J: Englewood Cliffs; 1986. p. A. [Google Scholar]
- 66.Gardiner PA, Eakin EG, Healy GN, Owen N. Feasibility of reducing older adults' sedentary time. Am J Prev Med. 2011 Aug;41(2):174–7. doi: 10.1016/j.amepre.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 67.Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij Ilse, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM, Ramirez-Marrero FA, Rogers LQ, Rowe DA, Schmidt MD, Tully MA, Blair SN. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011 Jul 28;8:80. doi: 10.1186/1479-5868-8-80. https://ijbnpa.biomedcentral.com/articles/10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buman MP, Winkler EAH, Kurka JM, Hekler EB, Baldwin CM, Owen N, Ainsworth BE, Healy GN, Gardiner PA. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005-2006. Am J Epidemiol. 2014 Feb 01;179(3):323–34. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- 69.Chastin SFM, Granat MH. Methods for objective measure, quantification and analysis of sedentary behaviour and inactivity. Gait Posture. 2010 Jan;31(1):82–6. doi: 10.1016/j.gaitpost.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011 Aug;43(8):1561–7. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 71.Grant PM, Ryan CG, Tigbe WW, Granat MH. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br J Sports Med. 2006 Dec;40(12):992–7. doi: 10.1136/bjsm.2006.030262. http://europepmc.org/abstract/MED/16980531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sellers C, Dall P, Grant M, Stansfield B. Validity and reliability of the activPAL3 for measuring posture and stepping in adults and young people. Gait Posture. 2016 Jan;43:42–7. doi: 10.1016/j.gaitpost.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 73.Lyden K, Keadle SK, Staudenmayer J, Freedson PS. The activPALTM accurately classifies activity intensity categories in healthy adults. Med Sci Sports Exerc. 2017 May;49(5):1022–8. doi: 10.1249/MSS.0000000000001177. http://europepmc.org/abstract/MED/28410327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 75.George SM, Alfano CM, Wilder Smith A, Irwin ML, McTiernan A, Bernstein L, Baumgartner KB, Ballard-Barbash R. Sedentary behavior, health-related quality of life, and fatigue among breast cancer survivors. J Phys Act Health. 2013 Mar;10(3):350–8. doi: 10.1123/jpah.10.3.350. http://europepmc.org/abstract/MED/22820125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balboa-Castillo T, León-Muñoz LM, Graciani A, Rodríguez-Artalejo F, Guallar-Castillón P. Longitudinal association of physical activity and sedentary behavior during leisure time with health-related quality of life in community-dwelling older adults. Health Qual Life Outcomes. 2011 Jun 27;9:47. doi: 10.1186/1477-7525-9-47. https://hqlo.biomedcentral.com/articles/10.1186/1477-7525-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baker F, Haffer SC, Denniston M. Health-related quality of life of cancer and noncancer patients in Medicare managed care. Cancer. 2003 Feb 01;97(3):674–81. doi: 10.1002/cncr.11085. doi: 10.1002/cncr.11085. [DOI] [PubMed] [Google Scholar]
- 78.Walters SJ, Munro JF, Brazier JE. Using the SF-36 with older adults: a cross-sectional community-based survey. Age Ageing. 2001 Jul;30(4):337–43. doi: 10.1093/ageing/30.4.337. [DOI] [PubMed] [Google Scholar]
- 79.Johnston RI. Quality Metric. [2021-04-07]. https://www.qualitymetric.com/
- 80.PROMIS: Patient-Reported Outcomes Measurement Information System. [2021-03-22]. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis.
- 81.FACIT Measurement System. [2021-03-22]. https://www.facit.org/
- 82.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003 Apr 15;49(2):156–63. doi: 10.1002/art.10993. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 83.Lyden K. Package 'activpalProcessing'. [2021-03-22]. https://cran.r-project.org/web/packages/activpalProcessing/index.html.
- 84.Edwardson CL, Yates T, Biddle SJH, Davies MJ, Dunstan DW, Esliger DW, Gray LJ, Jackson B, O'Connell SE, Waheed G, Munir F. Effectiveness of the Stand More AT (SMArT) Work intervention: cluster randomised controlled trial. Br Med J. 2018 Oct 10;363:-. doi: 10.1136/bmj.k3870. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=30305278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Edwardson CL, Winkler EA, Bodicoat DH, Yates T, Davies MJ, Dunstan DW, Healy GN. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci. 2017 Jun;6(2):162–78. doi: 10.1016/j.jshs.2016.02.002. https://linkinghub.elsevier.com/retrieve/pii/S2095-2546(16)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fitzsimons CF, Kirk A, Baker G, Michie F, Kane C, Mutrie N. Using an individualised consultation and activPAL™ feedback to reduce sedentary time in older Scottish adults: results of a feasibility and pilot study. Prev Med. 2013 Nov;57(5):718–20. doi: 10.1016/j.ypmed.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 87.Rosenberg DE, Gell NM, Jones SMW, Renz A, Kerr J, Gardiner PA, Arterburn D. The feasibility of reducing sitting time in overweight and obese older adults. Health Educ Behav. 2015 Oct;42(5):669–76. doi: 10.1177/1090198115577378. http://europepmc.org/abstract/MED/25794518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.2018 Physical Activity Guidelines Advisory Committee Scientific Report. U.S. Department of Health and Human Services. 2018. [2021-03-22]. http://fitnessmedicoitaliano.it/wp-content/uploads/2016/07/ATTIVIT%C3%A0-FISICA-2018-LINEE-GUIDA.pdf.
- 89.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput J, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020 Dec 25;54(24):1451–62. doi: 10.1136/bjsports-2020-102955. http://bjsm.bmj.com/lookup/pmidlookup?view=long&pmid=33239350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001 Jul;33(5):350–7. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 91.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009 Jun;13(6):538–44. doi: 10.1007/s12603-009-0104-z. http://europepmc.org/abstract/MED/19536422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lyons EJ, Swartz MC, Lewis ZH, Martinez E, Jennings K. Feasibility and acceptability of a wearable technology physical activity intervention with telephone counseling for mid-aged and older adults: a randomized controlled pilot trial. JMIR Mhealth Uhealth. 2017 Mar 06;5(3):e28. doi: 10.2196/mhealth.6967. https://mhealth.jmir.org/2017/3/e28/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu HS, Gal R, van Sleeuwen Niek C, Brombacher AC, IJsselsteijn WA, May AM, Monninkhof EM. Breast cancer survivors' experiences with an activity tracker integrated into a supervised exercise program: qualitative study. JMIR Mhealth Uhealth. 2019 Feb 21;7(2):-. doi: 10.2196/10820. https://mhealth.jmir.org/2019/2/e10820/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matson TE, Renz AD, Takemoto ML, McClure JB, Rosenberg DE. Acceptability of a sitting reduction intervention for older adults with obesity. BMC Public Health. 2018 Jun 07;18(1):706. doi: 10.1186/s12889-018-5616-1. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-018-5616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gardner B, Lally P, Wardle J. Making health habitual: the psychology of 'habit-formation' and general practice. Br J Gen Pract. 2012 Dec;62(605):664–6. doi: 10.3399/bjgp12X659466. https://bjgp.org/cgi/pmidlookup?view=long&pmid=23211256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lally P, Wardle J, Gardner B. Experiences of habit formation: a qualitative study. Psychol Health Med. 2011 Aug;16(4):484–9. doi: 10.1080/13548506.2011.555774. [DOI] [PubMed] [Google Scholar]
- 97.Matson TE, Anderson ML, Renz AD, Greenwood-Hickman MA, McClure JB, Rosenberg DE. Changes in self-reported health and psychosocial outcomes in older adults enrolled in sedentary behavior intervention study. Am J Health Promot. 2019 Sep;33(7):1053–57. doi: 10.1177/0890117119841405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003-2006) Cancer Causes Control. 2010 Feb;21(2):283–8. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 99.Lynch BM, Dunstan DW, Winkler E, Healy GN, Eakin E, Owen N. Objectively assessed physical activity, sedentary time and waist circumference among prostate cancer survivors: findings from the National Health and Nutrition Examination Survey (2003-2006) Eur J Cancer Care (Engl) 2011 Jul;20(4):514–9. doi: 10.1111/j.1365-2354.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 100.Dall PM, McCrorie PRW, Granat MH, Stansfield BW. Step accumulation per minute epoch is not the same as cadence for free-living adults. Med Sci Sports Exerc. 2013 Oct;45(10):1995–2001. doi: 10.1249/MSS.0b013e3182955780. [DOI] [PubMed] [Google Scholar]
- 101.Stansfield B, Hajarnis M, Sudarshan R. Characteristics of very slow stepping in healthy adults and validity of the activPAL3™ activity monitor in detecting these steps. Med Eng Phys. 2015 Jan;37(1):42–7. doi: 10.1016/j.medengphy.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Brickwood KJ, Watson G, O'Brien J, Williams AD. Consumer-based wearable activity trackers increase physical activity participation: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2019 Apr 12;7(4):-. doi: 10.2196/11819. https://mhealth.jmir.org/2019/4/e11819/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin A, Fitzsimons C, Jepson R, Saunders DH, van der Ploeg Hidde P, Teixeira PJ, Gray CM, Mutrie N, EuroFIT consortium Interventions with potential to reduce sedentary time in adults: systematic review and meta-analysis. Br J Sports Med. 2015 Aug;49(16):1056–63. doi: 10.1136/bjsports-2014-094524. [DOI] [PubMed] [Google Scholar]
- 104.Prince SA, Saunders TJ, Gresty K, Reid RD. A comparison of the effectiveness of physical activity and sedentary behaviour interventions in reducing sedentary time in adults: a systematic review and meta-analysis of controlled trials. Obes Rev. 2014 Nov;15(11):905–19. doi: 10.1111/obr.12215. doi: 10.1111/obr.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edwardson CL, Henson J, Biddle SJH, Davies MJ, Khunti K, Maylor B, Yates T. activPAL and Actigraph assessed sedentary behavior and cardiometabolic health markers. Med Sci Sports Exerc. 2020 Feb;52(2):391–7. doi: 10.1249/MSS.0000000000002138. [DOI] [PubMed] [Google Scholar]
- 106.Swain CTV, Nguyen NH, Eagles T, Vallance JK, Boyle T, Lahart IM, Lynch BM. Postdiagnosis sedentary behavior and health outcomes in cancer survivors: a systematic review and meta-analysis. Cancer. 2020 Feb 15;126(4):861–9. doi: 10.1002/cncr.32578. doi: 10.1002/cncr.32578. [DOI] [PubMed] [Google Scholar]
- 107.Chia GLC, Anderson A, McLean LA. Behavior change techniques incorporated in fitness trackers: content analysis. JMIR Mhealth Uhealth. 2019 Jul 23;7(7):-. doi: 10.2196/12768. https://mhealth.jmir.org/2019/7/e12768/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An example of the activPAL monitor summary data from baseline discussed with intervention group participants in an mHealth study.
activPAL3 data collection and processing details.
CONSORT (Consolidated Standards of Reporting Trials)-EHEALTH checklist (v 1.6).
Effects of mHealth intervention on health-related Quality of Life.


