Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by motor and gastrointestinal (GI) deficits. Despite its prevalence, the pathophysiology of PD is not well understood. Recent studies highlight the role of gut microbiota in neurological disorders. In this review, we summarize the potential role of gut microbiota in the pathophysiology of PD. We first describe how gut microbiota can be influenced by factors predisposing individuals to PD, such as environmental toxins, aging, and host genetics. We then highlight the effect of gut microbiota on mechanisms implicated in the pathophysiology of PD, including disrupted microbiota gut brain axis (GBA), barrier dysfunction, and immune dysfunction. It is too early to connect the dots between gut microbiota and PD to establish causation, and experiments focused on investigating interrelationship between gut microbiota and associated metabolites on GBA, barrier dysfunction, and immune activation will be crucial to fill in the gaps.
Keywords: gut brain axis, gut microbiota, Parkinson’s disease
INTRODUCTION
Parkinson’s disease (PD) is a commonly occurring progressive neurodegenerative disorder characterized by both motor (16) and nonmotor deficits, including olfactory deficits, cognitive decline, and gastrointestinal (GI) deficits (134). GI dysfunction is a prevalent nonmotor deficit in PD, which affects up to 60–80% of patient population and can be accompanied by alterations in the gut microbiota (1, 82, 102). The known role of gut microbiota alterations in GI dysfunction along with alterations described in PD patients has brought to attention the potential role of gut microbiota in pathophysiology of PD (1, 23, 41, 50). There is now accumulating evidence that links gut microbiota to central nervous system (CNS) diseases such as PD (17, 95, 123). In this review, we have examined in detail, the available evidence of GI dysfunctions and gut microbiota alterations in PD patients. Upon examining these studies, we believe that microbiota plays a central role in etiology of PD, primarily through mechanism that involve modulation of GI epithelial barrier integrity, immune function, and the microbiota gut-brain axis, which will be described in detail in the later part of the review (84, 108).
GI DYSFUNCTION IS PREVALENT IN PD PATIENTS
GI dysfunctions including abnormal salivation, nausea, dysphagia, altered gastric emptying, constipation, and defecatory dysfunction, are ubiquitous in PD patients (31). These GI abnormalities are a common nonmotor symptom of PD and may precede the onset of motor symptoms by several years, adding significantly to the healthcare burden and disrupted quality of life in PD patients (1, 82, 102, 119). The high prevalence (∼80%) and early onset of these GI dysfunctions in PD certainly raises a question regarding the role of GI tract in the pathogenesis of PD. In a large population-based study of more than 6,000 patients without PD that enrolled in the “Honolulu Heart Program,” Abott et al. (1) found that patients who suffered from constipation (i.e., <1 bowel movement/day) had fourfold greater risk of developing PD in the future. Subsequent autopsy on 245 of these constipated subjects, with no clinical signs of parkinsonism and dementia showed higher incidental Lewy body (α-synuclein aggregates) formation in the substantia nigra, suggesting a possible link between delayed GI transit and PD (2).
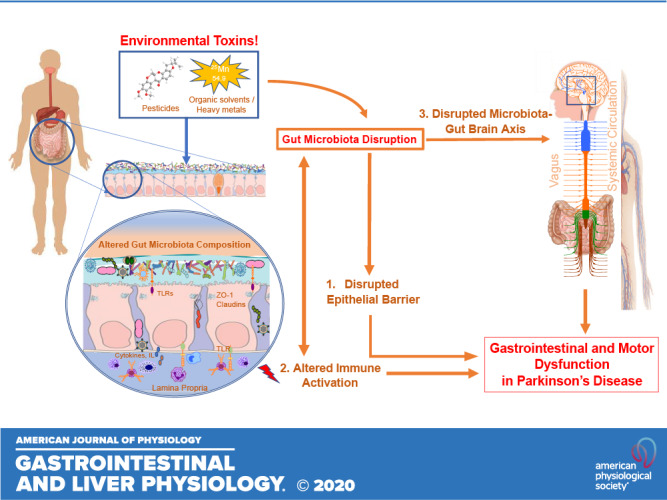
In addition to GI symptoms, physiological defects, such as disrupted intestinal permeability and altered immune activation, have also been observed in PD (see Fig. 1). Clairembault et al. (29) reported nearly 50% lower occludin expression in lysates of colon biopsies of PD patients, and Perez Pardo et al. (98) observed a reduction in the average intensity of ZO-1 immunolabeling in sigmoid colon biopsies from PD patients. Together, these studies indicate potential disruption of mechanisms that regulate epithelial paracellular permeability in PD patients. Schwiertz et al. (113) observed a simultaneous increase in markers of intestinal inflammation (fecal calprotectin) and disruption of the intestinal barrier (fecal zonulin and α-1-antitrypsin) in PD patients compared with age-matched controls. Since disruption in intestinal barrier function and intestinal inflammation are characteristics feature of inflammatory bowel disease (IBD), it is not surprising that three separate studies by Lin et al. (75), Weimers et al. (132), and Villumsen et al. (128) in distinct population cohorts all show an association between IBD and increased risk of PD later in life. Although it is tempting to speculate on the basis of these studies that risk of PD would be lower in patients that undergo colectomy, paucity of clinical data in IBD patients and controversial results in patients with other GI conditions make it difficult to evaluate whether this is, indeed, the case. Recently published studies that evaluated risk of PD in patients who underwent surgical procedures, such as colectomy and appendectomy for various GI conditions, including malignant neoplasms, benign tumors, noninfective inflammation, or disease of the appendix, found that the risk of developing PD increased in some cases, while decreased in others (66, 87, 120). These contradictory findings suggest that specific modulation of colonic factors, rather than full thickness colonic resection, is necessary to decrease risk of PD. Further studies are required to assess the role of colectomy in IBD patients and determine how colonic factors, such as microbiota, barrier function, and intestinal inflammation precisely play a role in PD pathophysiology. This will help us understand whether increased occurrence of GI-related physiological defects is a potential risk factor for PD or simply PD-related pathologies that precede motor symptoms.
Fig. 1.
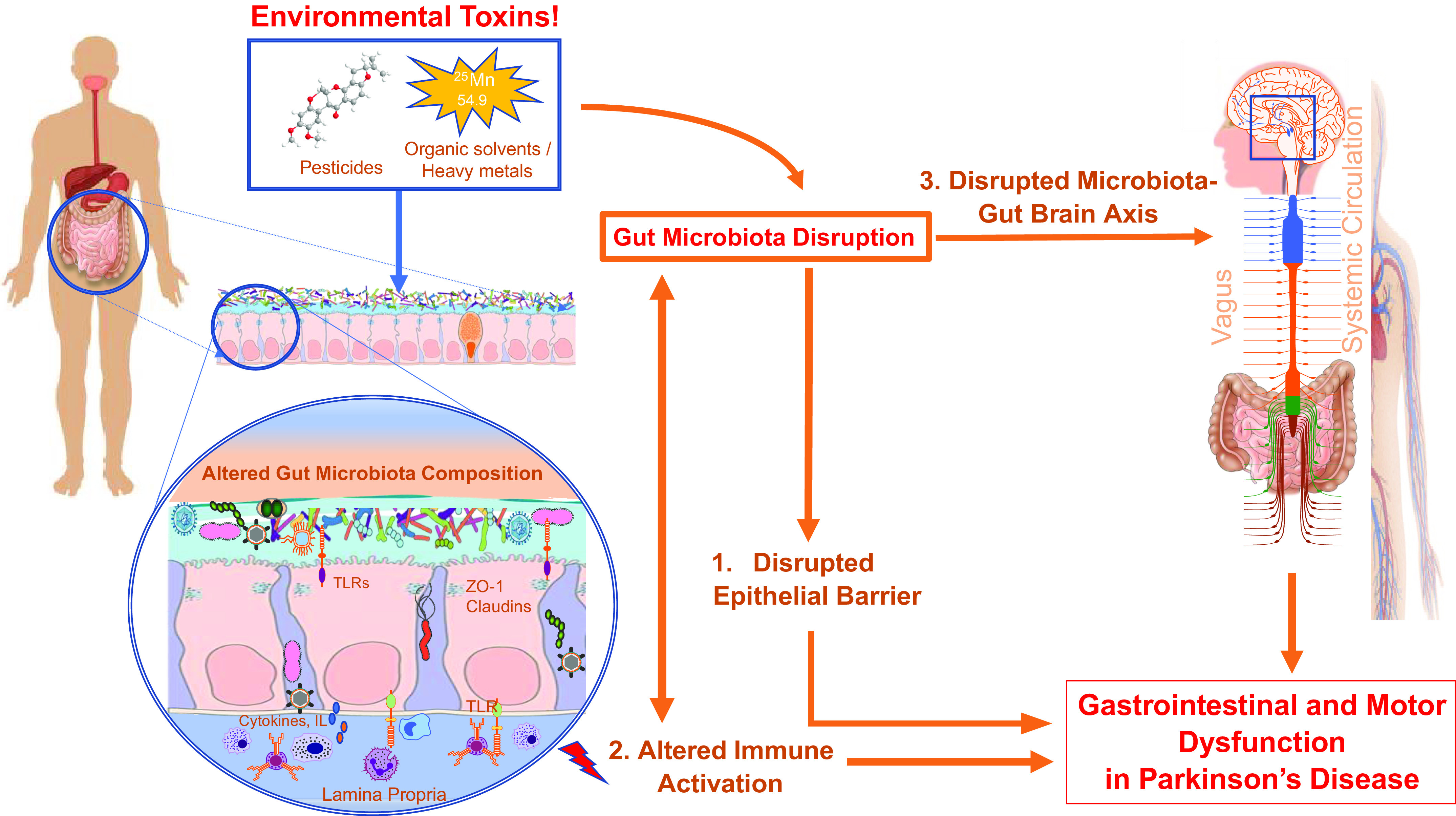
Figure highlights gut microbiota-associated mechanistic pathways that regulate gastrointestinal and motor dysfunction in Parkinson’s disease. Environmental toxins consumption alters gut microbiota composition and leads to disruption in epithelial barrier (1), alteration in immune activation (2), and disrupted microbiota-gut-brain axis communication through vagal and nonvagal pathways (3) to cause gastrointestinal and motor dysfunction in Parkinson’s disease. TLRs, Toll-like receptors; ZO-1, zona occludins 1.
GUT MICROBIOTA AND ITS METABOLITES ARE ALTERED IN PD PATIENTS
Several studies have examined alteration in gut microbiota composition in PD patients, but as observed in other disease states, the results are heterogenous in terms of differences in specific taxa (Table 1). There are several factors that contribute to such variability, including lack of standardization in sample collection and sequencing techniques (V3-V4, V4, or V4 and V5), differences in study design, sample size, geographical diversity of patient population, and heterogeneous nature of PD (73, 103).
Table 1.
Gut microbiota alterations in Parkinson’s disease
| Study Number | Study (Ref.) | Participants | Sample Used | Detection Method | Sequence Region | Overall Changes (α/β Diversity) | Increased in PD | Decreased in PD | GI Deficits? |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Aho et al. (2019) (5) | Control: 64 PD: 64 |
Stool | 16S rRNA | V3-V4 | α diversity no change β diversity no change |
Bifidobacterium | Prevotella (G), Roseburia (G), | |
| 2. | Bedarf et al. (2017) (12) | Control: 28 PD: 31 |
Stool | Metagenomic shotgun analysis Illumina Hiseq4000 |
α diversity no change β diversity different |
Verrucomicrobiaceae (F), Firmicutes (F), Akkermansia (G) | Prevotellaceae (F), Erysipelotrichaceae (F), Prevotella (G), Eubacterium (G) | No constipation | |
| 3. | Hasegawa et al. (2015) (49) | Control: 36 PD: 52 |
Stool | 16S or 23S rRNA (qRT-PCR) |
Lactobacillus (G) | Species: Clostridium coccoides group, Clostridium leptum subgroup, Bacteroides fragilis group | |||
| 4. | Heintz-Buschart et al. (2018) (53) | Control: 78 PD: 76 |
Stool and nasal wash samples | 16S and 18S rRNA | V4 | α diversity no change β diversity different |
Verrucomicrobia (P), Verrucomicrobia (O), Verrucomicrobiaceae (F), Akkermansia (G) | ||
| 5. | Hill-Burns et al. (2017) (55) | Control: 130 PD: 197 |
Stool | 16S rRNA | α diversity no change β diversity different |
Bifidobacteriaceae (F), Lactobacillaceae (F), Tissierellaceae (F), Christensenellaceae (F), Verrucomicrobiaceae (F), Bifidobacterium (G), Lactobacillus (G), Akkermansia (G) | Lachnospiraceae (F), Pasteurellaceae (F), Blautia (G), Roseburia (G), Faecalibacterium (G) | ||
| 6. | Hopfner et al. (2017) (56) | Control: 29 PD: 29 |
Stool | 16S rRNA | V1, V2 | α diversity no change β diversity different |
Barnesiellaceae (F), Enterococcaceae (F), Lactobacillaceae (F) | ||
| 7. | Keshavarzian et al. (2015) (63) | Control: 34 PD: 38 |
Sigmoidmucosal biopsies and stool samples | high‐throughput rRNA sequencing | V4 | α diversity increase in PD β diversity different | Bacteroidetes (P), Proteobacteria (P), Verrucomicrobia (P), Clostridiaceae (F), Oscillospira (G), Akkermansia (G) | Firmicutes (P), Lachnospiraceae (F), Coprobacillaceae (F), Blautia (G), Coprococcus (G), Dorea (G), Roseburia (G) | |
| 8. | Li et al. (2017) (72) | Control: 14 PD: 24 |
Stool | 16S rRNA | V3, V4, V5 | Actinobacteria (P), Proteobacteria (P), Enterobacteriaceae (F), Streptococcaceae (F), Veillonellaceae (F), Acidaminococcus (G), Acinetobacter (G), Enterococcus (G), Escherichia-shigella (G), Megamonas (G), Megasphaera (G), proteus (G), Streptococcus (G) | Bacteroidetes (P), Pasteurellaceae (F), Blautia (G), Faecalibacterium (G), Ruminococcus (G) | ||
| 9. | Li C et al., 2019 (71) | Control: 48 PD: 51 |
Stool | 16S rRNA | V4 | α diversity no change reduced β-diversity in PD patients |
Akkermansia | Lactobacillus | |
| 10. | Lin et al. (2018) (73) | Control: 75 PD: 45 |
Stool | 16S rRNA | V4 | α diversity no change β diversity different |
Eubacteriaceae (F), Bifidobacteriaceae (F), Aerococcaceae (F), Desulfovibrionaceae (F) | Tenericutes (P), Euryarchaeota (P), Firmicutes (P), Streptococcaceae (F), Methylobacteriaceae (F), Comamonadaceae (F), Halomonadaceae (F), Hyphomonadaceae (F), Brucellaceae (F), Xanthomonadaceae (F), Lachnospiraceae (F), Actinomycetaceae (F), Sphingomonadaceae (F), Pasteurellaceae (F), Micrococcaceae (F), Intrasporangiaceae (F), Methanobacteriaceae (F), Idiomarinaceae (F), Brevibacteriaceae (F), Gemellaceae (F) | Constipation |
| 11. | Lin et al. 2019 (74) | Control: 77 PD: 80 |
Stool | 16S rRNA | V3-V4 | Parabacteroides, Verrucomicrobia (P), Akkermansia, Butyricimonas, Veillonella, Odoribacter, Mucispirillum, Bilophila, Enterococcus, and Lactobacillus | Prevotella (G) | Constipation | |
| 12. | Petrov et al. (2017) (99) | Control: 66 PD: 89 |
Stool | 16S rRNA | V3-V4 | α diversity decrease β diversity different |
Christensenella (G), Catabacter (G), Lactobacillus (G), Oscillospira (G), Bifidobacterium (G) | Dorea (G), Bacteroides (G), Prevotella (G), Faecalibacterium (G) | |
| 13. | Qian et al. (2018) (104) | Control: 45 PD: 45 | Stool | 16S rRNA | V3-V4 | α diversity increase β diversity different |
Clostridium IV (G), Aquabacterium (G), Holdemania (G), Sphingomonas (G), Clostridium XVIII (G), Butyricicoccus (G), Anaerotruncus (G) | Constipation | |
| 14. | Scheperjans et al. (2015) (112) | Control: 72, PD: 72 |
Stool | 16S rRNA | V1-V3 | α diversity no change β diversity different |
Lactobacillaceae (F), Verrucomicrobiaceae (F), Bradyrhizobiaceae (F), Clostridiales (F), Incertae Sedis IV (F) | Prevotellaceae (F) | Constipation |
| 15. | Unger et al. (2016) (126) | Control: 34 PD: 34 |
Stool | qPCR using bacterial primers | Enterobacteriaceae (F), Bifidobacterium (G) | Bacteroidetes (P), Prevotellaceae (F, descriptively reduced), Lactobacillaceae (F), Enterococcaceae, Species: Faecalibacterium prausnitzii | Constipation |
PD, Parkinson’s disease; qPCR, quantitative PCR.
A consistent finding among studies has been an increase in members of Enterobacteriaceae family and Helicobacter spp. (1.5–3-fold increase in H. pylori in gastric biopsies) (20, 85), as well as an increase in Lactobacillus and Akkermansia (see 18, 20, 27, 43, 57, 99, 112). This is particularly interesting as an increased abundance in members of Enterobacteriaceae family and H. pylori has been associated to severity of postural instability, gait difficulty, and overall worsening of motor functions in PD (57, 107, 112). Furthermore, eradication of H. pylori has been shown to improve absorption of levodopa resulting in better response of motor function to treatment (85). Increase in members of Lactobacillaceae has been associated with reduced concentrations of ghrelin in PD; a gut brain peptide implicated in upregulation of intestinal tight junction proteins (28), stimulation of intestinal motility (115), and maintenance and protection of normal nigrostriatal dopamine function (7, 125). Akkermansia on the other hand has been shown to use mucus as a carbon source and degrade the colonic mucus barrier (42), which might disrupt intestinal permeability and increase pathogen susceptibility causing inflammatory condition on the intestinal wall (114). In addition to the above gut microbial changes, PD patients also display reduced abundance of prominent short-chain fatty acid producers. Strains of Faecalibacterium spp., Blautia spp., Prevotella spp, and Roseburia spp, that are common butyrate producers are more consistently reduced in PD patients (126). Butyrate has been shown to exert barrier protective, anti-inflammatory role in the GI tract (8, 97) and prevent dopaminergic neuron degeneration and subsequent bradykinesia in mice (78). Together, the observed changes in gut microbiota in PD and their association with motor and nonmotor deficits, suggests that altered gut microbiota may play a role in PD (27, 57, 58, 85, 112).
In an attempt to identify potential link between gut microbiota and PD, we will next examine factors that contribute to alterations in gut microbiota, and then discuss how gut microbiota changes could contribute to PD.
FACTORS THAT CONTRIBUTE TO GUT MICROBIOTA ALTERATIONS IN PD
Host Genetics
Host genetics differences have often been linked to differences in microbiome composition (46, 143). Using next generation sequencing approach to analyze fecal bacterial composition (V4 region of 16S rDNA), Goodrich et al. (46) showed that microbiota are more similar within twin pairs compared with unrelated individuals. Among various microbial taxa, studies show that taxa particularly from Firmicutes and Proteobacteria, including members of Christensenellaceae family are the most heritable taxon in humans (25, 45, 131). Several genome-wide association studies or quantitative trait locus (QTL) mapping studies have also successfully identified specific host genes and single nucleotide polymorphisms in the host genome that contribute to the variation of these microbial taxa (21, 32, 124). These studies show that host genetics plays a significant role on determining dominant gut microbiota composition.
In addition to assessing the influence of host genetics on gut microbiota, studies have also been separately conducted to assess the role of host genetics in etiology of PD. Studies suggest that PD is modestly heritable with heritability estimate of around 30% (133). Higher percentage concordance for subclinical striatal dopaminergic dysfunction in sporadic and late-onset PD suggest that host genetics particularly contribute to striatal dopaminergic dysfunction, a primary cause for PD (101). Although these studies individually show that host genetics plays an important role in regulating gut microbiota composition and the pathology of the PD, it remains unclear what precise genetic changes in PD help drive specific changes in microbiota and whether these genetic changes induced microbiota alteration drive PD phenotype.
Earlier studies have successfully associated specific genetic changes in GI diseases to change in specific microbial species (45, 67, 116). However, whether such association between individual genes and gut microbiota exists in PD is not known. In a minority of patients (10–15% of clinically diagnosed cases) where PD is inheritable (Familial PD), mutation in one of the eight genes responsible for aberrant protein formation and disrupted mitochondrial homeostasis are often responsible. They include SNCA (also known as PARK1, a gene that encodes for α-synuclein, a major component of Lewy bodies), PARK2 [also known as PRKN; parkin, a ubiquitin protein ligase involved in the degradation of abnormal proteins by the proteasome (86)], PARK5 (UCHL1; ubiquitin carboxy-terminal hydrolase L1, a gene responsible for generating the ubiquitin monomer), PARK6 (PINK1; encodes for putative serine-threonine kinase), PARK7 (also known as DJ-1; encodes for protein/nucleic acid deglycase DJ-1), PARK8 (LRRK2; encodes for Leucine-rich repeat kinase 2 enzyme), PARK11 (also known as GIGYF2; encodes for GRB10-interacting GYF protein-2), and NR4A2 (encodes for nuclear receptor subfamily 4, group A, member 2 protein) (79a). Recent studies have shown that specific genetic changes in the absence of gut microbiota cannot fully recapitulate the symptom severity seen in PD (3, 110). Using a α-synuclein (SNCA), a presynaptic nerve terminal protein, overexpressing germ-free and conventionally raised specific pathogen-free mice, Sampson et al. (110) showed that germ-free α-synuclein overexpressing mice did not exhibit PD phenotype compared with conventionally raised mice. Given that exposure to bacterial endotoxin, LPS is required to trigger persistent neuroinflammation and generate structurally distinct α-synuclein fibril structure involved in synucleinopathies and neurodegeneration (37, 51, 64), it seems likely that specific genetic mutation though necessary, might not be sufficient and potentially requires gut microbiota interaction to regulate symptom severity and disease outcome in genetic model of PD.
Environmental Toxins
Environmental toxins from different chemical classes can alter both the microbial composition and the metabolic activity of the gut bacteria (reviewed in Refs. 30 and 80). A majority of PD cases are idiopathic and likely caused by exposure to environmental toxins, including herbicides and pesticides, such as rotenone, paraquat, organic solvents, and heavy metals, including vanadium and manganese (4, 70, 122). A recent meta-analysis of data that investigated prospective cohort and case-controlled epidemiological studies found that PD was associated with farming, and the risk of developing PD was increased by exposure to environmental toxins (100). Recent studies have hypothesized that an initial target of these environmental toxin consumption/exposure in PD could be the gut microbiota (55, 137). Hill-Burns et al. (55) found that the gut bacteria responsible for degrading some of these environmental toxins, such as atrazine (herbicide), and naphthalene (insect repellent), are altered in individuals with PD compared with patients that were prescribed PDmedication. Byperforming longitudinal analysis of 16S rRNA gene sequencing of fecal microbiome, Yang et al. (137) showed that three weeks of chronic rotenone administration, at a dose found commonly in pesticides, caused fecal microbiota alterations, mitochondrial disruption, and dopaminergic neuronal loss, along with behavioral and neuropathological features of PD. A direct association between environmental toxin and PD was also shown in a case-controlled prospective study, which assessed the health effects of lifetime exposure to rotenone and paraquat in farmers and found that exposure to these pesticides increases the risk of PD by 2.5-fold (122). Together, these data suggest that common environmental toxins and pesticides lead to behavioral and neuropathological symptoms of PD, via mechanisms that potentially involve gut microbiota alterations and disruption in mitochondrial activity, a cellular organelle that is also evolutionarily a descendant of endosymbiotic bacteria (130). Characteristic microbiota changes after rotenone administration include decreased bacterial diversity and increase in Firmicutes/Bacteroidetes ratio (137), a dysbiotic condition also observed in several other diseases, including colorectal cancer (9), hypertension (136), Type 2 diabetes (T2D), obesity (68), and inflammatory bowel disease (IBD) (9). The similarity in observed microbial changes postrotenone administration with various other diseases suggests that these gut microbial changes that occur in PD are common microbial marker of various chronic diseases. This observation raises two important questions: 1) how do microbiota change after toxin exposure specifically translate to PD, as opposed to other chronic diseases, and 2) is reversing these microbial changes sufficient to reverse the course of PD pathogenesis after toxin administration?
Although these studies certainly suggest that chronic microbiota changes after toxin exposure might be involved in idiopathic PD, future studies using gnotobiotic mice model is necessary to enhance our current mechanistic understanding regarding how gut microbes are able to transform/metabolize different classes of environmental toxins and xenobiotics and alter their pharmacokinetic and pharmacodynamic properties to cause PD. It is also important to carefully study the effects of environmental toxins associated with PD in both conventional and gnotobiotic germ-free animal models to understand the relationship between environmental toxin and microbiota and use this knowledge to open new avenues of treatment options for PD patients.
Aging
Besides, environmental toxins and genetics, aging is the most important factor that contributes to alterations in gut microbiota and PD. Recent studies have indicated clear differences in gut microbiota composition among infants, toddlers, adults, and the elderly (18, 91). Using high-throughput sequencing of the 16S rRNA gene (amplicons derived from the V3-V4 region), Odamaki et al. (91) investigated the sequential changes in fecal microbiota composition samples from 367 healthy Japanese subjects between the ages of 0 and 104 yr. They found that the transition from infant to centenarian was accompanied by distinctive coabundance group (CAG; identifies species that are phylogenetically and/or functionally related on the basis of gene abundance) dominance at different stages of life. Significant abundance of Bifidobacterium coabundance groups (CAGs) was observed in infants and children; Lachnospiraceae CAGs were observed in adults; Eubacterium and Clostridiaceae CAGs were observed in the elderly, and Enterobacteriaceae CAGs were observed in both infants and the elderly (91). In addition to Odamaki et al. (91), Biagi et al. (18) from Northern Italy also consistently observed an increase in the relative abundance of Proteobacteria, a major phylum of facultative anaerobic gram-negative bacteria, which include several members, including Escherichia, Pseudomonas, Salmonella, Vibrio, Helicobacter, Yersinia, and Legionellales in subjects that were over 70 yr of age. Although it is still not clear whether these aging-associated changes in gut microbiota increase susceptibility to PD, these studies do, however, suggest that changes in bacterial composition with age consistently favor an increase in pathogenic species with age irrespective of geographical location. Pathogenic species from Enterobacteriaceae CAGs and from Proteobacteria phylum, such as Helicobacter, have been associated with neurodegenerative diseases, including PD (summarized in review in Ref. 43). Future studies need to investigate the contribution of aging-associated factors such as dietary modification, life style changes, and weakened immune function on gut microbiota that could lead to changes in gut microbiota composition. This will help determine whether it is “normal” age-related microbial changes seen in a “healthy” individual or whether it is external factors that promote microbial changes at an older age, which increases PD susceptibility in aging individuals.
MECHANISMS THAT LINK ALTERED GUT MICROBIOTA TO PD
Disrupted Barrier Function
The GI epithelial barrier comprises a thick mucus layer aloft a monolayer of epithelial cells that are interconnected through a system of junction proteins (Fig. 1). The proper maintenance of epithelial barrier integrity is crucial to prevent translocation of pathogenic luminal bacteria and bacterial metabolites that acts as a mediator of diseases, all the while providing regulatory signals to induce immune tolerance toward commensal microbes. Studies show that gut microbes play an important role in maintaining the epithelial barrier, particularly through butyrate production, which modulates expression of mucin-associated genes in goblet cells and regulates expression and distribution of epithelial tight junction proteins (54). Thus, it is not surprising that altered gut microbial composition, as observed in various GI and CNS diseases, including IBD (44), stress-related psychiatric disorders (61), autoimmune disorders (33), and PD (109) is more often accompanied by disrupted barrier function and increased mucosal colonization of adherent and invasive, pathogenic species in many of these diseases (36, 138).
Although a disrupted intestinal barrier is a common denominator implicated in various GI and CNS diseases besides PD, the specific changes in gut microbiota and the microbial pathways that give rise to disrupted intestinal permeability needs to be investigated in the specific context of a PD. Gut microbiota changes as seen in PD can disrupt epithelial permeability through one of three ways, which include, increase in microbial enterotoxin production, increased luminal endotoxin expression, and decreased butyrate production (19). Helicobacter pylori, a gram-negative pathogen, more commonly found in PD patients and aged individuals, for example, produces enterotoxin, such as the vacuolating cytotoxin (VacA), and cytotoxin-associated gene A (CagA) and directly releases them on to the host epithelium to modulate expression of tight junction proteins, including claudin-1 and ZO-1 and disrupt intestinal permeability (35, 39, 85). Additionally, increased mucosal exposure to bacterial endotoxin (lipopolysaccharide, LPS) as observed in early “Hoehn & Yahr Stage II” PD patients (36) could also cause epithelial barrier disruption by downregulating tight junction occludin and ZO-1 mRNA expression (13), and by directly inducing phosphorylation of the 20-kDa myosin light chain (MLC20) (140). Finally, loss of butyrate producing probiotic strain, as seen in PD microbiota, could lead to disruption in epithelial barrier permeability through decreased mucus production and secretion, and by impairing proper localization of tight junction proteins assembly (76, 97, 106).
Although it is becoming increasingly clear that altered gut microbiota can contribute to disrupted intestinal barrier permeability in PD through the mechanisms discussed above, how this leads to PD is not well understood. Recent studies show that disrupted intestinal permeability strongly correlates to increased luminal endotoxin translocation and expression of α-synuclein aggregates in the intestine of both humans and a mouse model of PD (36, 62). This is particularly interesting given the biophysical characteristics of α-synuclein as an antimicrobial peptide within the GI tract (10, 96). Overexpression of α-synuclein as a result of microbial infection or endotoxin induced epithelial damage has also been hypothesized as potential mechanism which leads to formation of α-synuclein aggregates in the ENS and subsequent α-synuclein trafficking to the CNS (10, 62). Future studies are necessary to understand the underlying molecular mechanisms of α-synuclein aggregation after barrier dysfunction, as well as its role on gut microbiota, and test whether prevention of barrier disruption through manipulation of gut microbes is sufficient to prevent gut α-synuclein pathology and thwart PD progression.
Altered Immune Activation
The GI tract holds the largest number of immune cells in the body, which includes the intestinal epithelial cells, macrophages, dendritic cells, B cells, and regulatory T cells. Intestinal epithelial cells, in particular, not only form a physical barrier for the gut microbes but are also dynamically active immune cells (83) that express pattern recognition receptors on the epithelial surface. Subsets of intestinal epithelial cells, especially the Paneth cells and microfold (M) cells, sample the microbe-laden luminal environment by recognizing conserved bacterial motifs and participate in downstream immune regulation by producing relevant antimicrobial peptides and proinflammatory cytokines, and by delivering bacterial antigens to dendritic cells (79, 92). Gut microbial interaction with the intestinal epithelial cells is, therefore, a crucial contributor to immune development and a potent regulator of homeostatic immune response (83).
A mechanistic example of gut microbiota influencing host immunity to induce PD phenotype is through elevated gram negative bacteria expressing LPS in PD patients (47), which acts on the Toll-like receptors (TLRs) present in the epithelial, immune, and nerve cells of the GI tract to modulate innate immune response (79). Dysregulation of LPS-TLR signaling has, thus, been simultaneously implicated in both intestinal inflammation and PD pathogenesis. Perez-Pardo et al. (98), using TLR4-knockout (KO) mice, observed that while rotenone causes dysbiosis in both TLR4-KO and wild-type (WT) mice, TLR4-KO mice had decreased intestinal and motor dysfunction, lower intestinal inflammation, and motor neuron degeneration relative to WT TLR4-expressing mice. These data show that peripheral immune function regulated, at least in part, by LPS-TLR signaling drives altered immune activation and motor neuron degeneration in toxin-induced PD mice. In humans, a direct link between serum LPS and PD was observed in a 22-yr-old laboratory worker who developed Parkinson’s syndrome (exhibiting cardinal signs: bradykinesia, rigidity, and tremor) 3 wk after accidental exposure to Salmonella minnesota LPS through an open wound (90). The proof that LPS exposure could directly lead to parkinsonism in an otherwise healthy individual reasserted the extraordinary ability of microbial endotoxin to directly induce PD. Since the symptom of neuroinflammation that is associated with upregulation of LPS-induced disrupted TLR signaling is often seen in an animal model of PD, it is plausible that altered immune activation as a result of disrupted TLR signaling likely contributes to development of PD phenotype in an LPS-exposed patient (14).
Bacterial LPS-induced immune activation could lead to nigral dopaminergic neuron loss and Parkinsonism by causing disruption of blood-brain barrier function and increasing peripheral α-synuclein translocation into the brain (105, 117). Increased α-synuclein translocation may further exacerbate immune activation by inciting the production of proinflammatory molecules and increasing the expression of TLRs, eventually causing neurodegeneration and motor dysfunction (14, 117). How exactly disrupted LPS-TLR signaling alters blood-brain barrier function remains to be fully understood. According to one hypothesis, the disruption in blood-brain barrier permeability in PD might occur through MyD88-immune pathway activation, which results in increased proinflammatory cytokine production (89) and potentially cause destruction of tight junctions of microvascular endothelial cells that form the blood-brain barrier (139). This pathway of blood-brain barrier disruption through microbiota-dependent immune modulation is supported by evidence from both living PD individuals and postmortem studies, which not only demonstrate elevated levels of proinflammatory cytokines, including TNF-α, IFNγ, IL-1β, and IL-8 in parkinsonian brain tissue, cerebrospinal fluid (reviewed in 26) and plasma, but also directly correlate these increases in proinflammatory cytokines to changes in gut microbiota (74).
Although most studies have focused on how environmental toxin-dependent changes in “healthy” microbial community drives alterations in microbe-epithelial cell interaction and disrupted homeostatic immune balance in PD, it is also plausible that chronic consumption of environmental toxins, and not the gut microbiota, directly drives disruption of homeostatic immune balance, which could then alter gut microbiota (69, 141). Chronic rotenone administration in rats, for example, has been shown to increase the nitric oxide (NO) levels within the CNS and increase production of 3-nitrotyrosine, a biomarker for endogenous peroxynitrite-induced oxidative stress activity, ultimately causing damage to nigrostriatal motor pathway (6, 11, 52, 135). Because NO is an important mediator of immune homeostasis and host defense against various GI pathogens, altered NO production and its downstream metabolites as a result of chronic toxin consumption can directly contribute to alterations in gut microbiota and pathological states. A better understanding of how disrupted immune activation translates to altered gut microbiota, neurodegeneration, and motor dysfunction in PD is also an active area of research. Future studies using gnotobiotic mice is crucial to conclusively resolve this uncertainty.
Microbiota-Gut-Brain Axis
Disruption in gut-brain axis has been previously associated to pathogenesis of several CNS and the peripheral nervous system disorders ranging from anxiety, autism, and depression to IBS and IBD. While the gut-brain axis is often viewed as an information highway that mediates bidirectional communication between the brain and the gut, it is now well known that this bidirectional communication is markedly influenced by the gut microbiota and their metabolites through neurological and neuroendocrine pathways.
Evidence shows that a major neurological pathway via which PD pathogenesis once initiated in the gut ascends to the brain is through the vagal route along the gut-brain axis. The evidence in support of this route was observed in a mice study, which showed that intragastrically administered rotenone causes progressive accumulation and propagation of α-synuclein from the ENS to the CNS in a “prion-like” fashion (93, 94). Another recent study also observed that a direct injection of pathologic α-synuclein oligomers into the GI tract of mice causes spread of α-synucleinopathy into the CNS, which was preventable by truncal vagotomy (65). This pattern of pathological staging in mice mimics similar pattern of α-synuclein progression seen in post mortem PD patients (22, 93) and potentially explains why progression of motor symptoms, but not GI symptoms, can be delayed by partial sympathectomy and hemi-vagotomy, and also why full truncal vagotomy in humans could be protective against PD (77).
Gut microbiota could regulate the bidirectional vagus nerve communication by directly producing neurotransmitter such as serotonin, norepinephrine, GABA and dopamine (40, 88, 127, 129, 142) and affecting neurotransmitter receptor expression in the brain and in the intestinal lumen (24, 59). A study shows that subdiaphragmatic vagotomy in turn could prevent some of these microbiota dependent effects on vagal excitability and disrupt PD progression (24). In addition to directly modulating vagus nerve activity through disrupted neurotransmitter production and altered receptor expression, gut microbes could also affect the vagus nerve indirectly by inducing release of gut peptides, and hormones from enteroendocrine epithelial cells. Enteroendocrine epithelial cells in response to microbial activation releases effector molecules such as cholecystokinin (CCK), glucagon like peptide-1 (GLP-1), PYY and ghrelin on to the vagal afferent fibers to modulate excitability and central motor behavior (111). These data suggest that vagus nerve represents a crucial route for gut brain cross talk in PD.
While gut microbiota is capable of driving CNS dysfunction through the vagal pathway, it is also important to note that gut microbes can modulate CNS function independent of vagus by releasing SCFAs and stimulating the release of luminal peptides and gut hormones, such as VIP, secretin, ghrelin and GLP-1 from the epithelial cells into the systemic circulation (15). These luminal signals can directly strengthen the blood brain barrier to prevent toxin translocation in PD (78). Some of these peptides can also passively cross the blood brain barrier to control various peripheral and central physiologic functions such as mucosal defense, inflammatory response and maintenance and protection of nigrostriatal dopamine function (7).
MICROBIOTA AS A THERAPEUTIC TARGET IN PD
Given the role of gut microbiota in pathogenesis of PD, recent studies are beginning to identify gut microbiota as a potential therapeutic target to treat PD. Limited clinical and preclinical studies suggest that three potential microbiota-dependent therapeutic approaches could potentially be employed to treat PD. First, drugs could be developed to prevent microbial uptake of l-DOPA and degradation within GI tract. l-DOPA, a commonly prescribed dopamine precursor drug is extensively metabolized in the gut by aromatic amino acid decarboxylase (AADC) and by gut bacteria that express PLP-dependent tyrosine decarboxylase. Significant degradation of l-DOPA within the gut reduces l-DOPA bioavailability for uptake to the brain, necessitating a higher dose of drug administration for effective PD treatment, which often leads to side effects such as l-DOPA-induced motor fluctuations and dyskinesia (38, 81, 127). Targeted depletion of these gut bacteria or prevention of l-DOPA uptake by the gut bacteria through administration of drugs such as tyrosine mimic (S)-α-fluoromethyltyrosine (AFMT), along with AADC inhibitor are, therefore, likely to significantly improve drug efficacy and prevent motor side effects (81). Second, probiotics could be used as a potential therapeutic in PD. In a randomized, double-blind, placebo-controlled clinical trial, Tamtaji et al. (121) found that PD patient consuming probiotic product containing Lactobacillus and Bifidobacterium species for 12 wk had significantly decreased movement disorder, as measured by Movement Disorders Society-Unified Parkinson’s Disease Rating Scale compared with placebo group. While the mechanism via which these probiotics causes improved motor function in PD patients is not completely understood, a recent study suggests that other probiotics, for example, Bacillus subtilis, exert the therapeutic effect by inhibiting and reversing α-synuclein aggregation (48). Third, fecal microbiota transplantation (FMT) could be employed as a potential therapeutic in treating PD. This approach is based on the evidence from animal studies that show that FMT from PD patients to α-synuclein-overexpressing mice exacerbates physical impairments (110), and microbiota transplants from healthy mice is protective against PD in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxic mice model (118). Clinical trials (see NCT03808389) are now being conducted in early (Hoehn & Yahr score 2–3) PD patients to test whether restoration of gut microbiota by FMT helps prevent the development and progression of PD in humans. While FMT-derived therapies has garnered high success and attention in C. difficile infection, IBD, and autism, FMT therapies for PD are at a very early stages, and benefits of FMT must be weighed against ethical issues, short-term and long-term safety, and psychological consequences before it can be used in PD. While it is still very early to determine which of these therapeutic options present a greater potential against PD moving forward, it is critically important to properly assess individual needs, patient’s health, including stage of PD pathology, and the results of clinical trials to determine what microbial therapeutic strategy might represent a better option for a PD patient.
CONCLUSIONS
The observation that GI dysfunction precedes sensory motor impairment and continues to affect PD patients during the course of disease progression highlights the potential importance of GI tract in initiation and progression of PD pathogenesis. Although it is well established that gut microbes are altered during the course of PD pathogenesis, the considerable debate in the field has been whether altered gut microbiota is a driving force in neurodegeneration or simply represents a response to environmental toxin. The fact that microbial changes seen in PD are often heterogenous makes it very complicated to understand whether microbial dysbiosis precede or succeed GI dysfunction in PD. Future studies using gnotobiotic mice will be crucial to decipher the complicated relationship between gut microbiota and PD that will help us identify microbiota-dependent mechanisms that contribute to PD pathogenesis and devise potential therapeutic strategies.
DISCLOSURES
P. Kashyap is a member of the Advisory Board of Novome and a consultant for Otsuka Pharmaceuticals, Pendulum Therapeutics, and IP Group, Inc. Y. Bhattarai is a scientist at Takeda Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Y.B. prepared figures; Y.B. drafted manuscript; Y.B. and P.C.K. edited and revised manuscript; Y.B. and P.C.K. approved final version of manuscript.
REFERENCES
- 1.Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD, Grandinetti A, Blanchette PL, Popper JS, Ross GW. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57: 456–462, 2001. doi: 10.1212/WNL.57.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Abbott RD, Ross GW, Petrovitch H, Tanner CM, Davis DG, Masaki KH, Launer LJ, Curb JD, White LR. Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 22: 1581–1586, 2007. doi: 10.1002/mds.21560. [DOI] [PubMed] [Google Scholar]
- 3.Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252, 2000. doi: 10.1016/S0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 4.Afeseh Ngwa H, Kanthasamy A, Anantharam V, Song C, Witte T, Houk R, Kanthasamy AG. Vanadium induces dopaminergic neurotoxicity via protein kinase Cδ-dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson’s disease. Toxicol Appl Pharmacol 240: 273–285, 2009. doi: 10.1016/j.taap.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aho VTE, Pereira PAB, Voutilainen S, Paulin L, Pekkonen E, Auvinen P, Scheperjans F. Gut microbiota in Parkinson’s disease: temporal stability and relations to disease progression. EBioMedicine 44: 691–707, 2019. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahsan H. 3-Nitrotyrosine: a biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol 74: 1392–1399, 2013. doi: 10.1016/j.humimm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, Elsworth JD, Savitt JM, DiMarchi R, Tschöp M, Roth RH, Gao XB, Horvath TL. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci 29: 14057–14065, 2009. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, Theil PK, Purup S, Hald S, Schioldan AG, Marco ML, Gregersen S, Hermansen K. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 10: 1499, 2018. doi: 10.3390/nu10101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamola VD, Ghosh A, Kapardar RK, Lal B, Cheema S, Sarma P, Chaudhry R. Gut microbial diversity in health and disease: experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb Ecol Health Dis 28: 1322447, 2017. doi: 10.1080/16512235.2017.1322447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbut D, Stolzenberg E, Zasloff M. Gastrointestinal immunity and alpha-synuclein. J Parkinsons Dis 9: S313–S322, 2019. doi: 10.3233/JPD-191702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashkatova V, Alam M, Vanin A, Schmidt WJ. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp Neurol 186: 235–241, 2004. doi: 10.1016/j.expneurol.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Bedarf JR, Hildebrand F, Coelho LP, Sunagawa S, Bahram M, Goeser F, Bork P, Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med 9: 39, 2017. [Erratum in Genome Med 9: 61, 2017.] doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bein A, Zilbershtein A, Golosovsky M, Davidov D, Schwartz B. LPS induces hyper-permeability of intestinal epithelial cells. J Cell Physiol 232: 381–390, 2017. doi: 10.1002/jcp.25435. [DOI] [PubMed] [Google Scholar]
- 14.Béraud D, Maguire-Zeiss KA. Misfolded α-synuclein and Toll-like receptors: therapeutic targets for Parkinson’s disease. Parkinsonism Relat Disord 18, Suppl 1: S17–S20, 2012. doi: 10.1016/S1353-8020(11)70008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141: 599–609, 2011. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Bereczki D. The description of all four cardinal signs of Parkinson’s disease in a Hungarian medical text published in 1690. Parkinsonism Relat Disord 16: 290–293, 2010. doi: 10.1016/j.parkreldis.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Bhattarai Y. Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil 30: e13366, 2018. doi: 10.1111/nmo.13366. [DOI] [PubMed] [Google Scholar]
- 18.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5: e10667, 2010. [Erratum in PLoS One 5: 2010.] doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 14: 189, 2014. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaecher C, Smet A, Flahou B, Pasmans F, Ducatelle R, Taylor D, Weller C, Bjarnason I, Charlett A, Lawson AJ, Dobbs RJ, Dobbs SM, Haesebrouck F. Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment Pharmacol Ther 38: 1347–1353, 2013. doi: 10.1111/apt.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, Spector TD, Keinan A, Ley RE, Gevers D, Clark AG. Host genetic variation impacts microbiome composition across human body sites. Genome Biol 16: 191, 2015. doi: 10.1186/s13059-015-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396: 67–72, 2006. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 110: 517–536, 2003. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 24.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 108: 16050–16055, 2011. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks AW, Priya S, Blekhman R, Bordenstein SR. Gut microbiota diversity across ethnicities in the United States. PLoS Biol 16: e2006842, 2018. doi: 10.1371/journal.pbio.2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson’s disease. BioMed Res Int 2014: 308654, 2014. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlett A, Dobbs RJ, Dobbs SM, Weller C, Brady P, Peterson DW. Parkinsonism: siblings share Helicobacter pylori seropositivity and facets of syndrome. Acta Neurol Scand 99: 26–35, 1999. doi: 10.1111/j.1600-0404.1999.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Wei Y, Yang W, Cai Y, Chen B, Yang G, Shang H, Zhao W. Ghrelin attenuates intestinal barrier dysfunction following intracerebral hemorrhage in mice. Int J Mol Sci 17: 2032, 2016. doi: 10.3390/ijms17122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F, Heymann MF, Neunlist M, Derkinderen P. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol Commun 3: 12, 2015. doi: 10.1186/s40478-015-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2: 16003, 2016. [Erratum in NPJ Biofilms Microbiomes 3: 17001, 2017.]doi: doi: 10.1038/npjbiofilms.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cloud LJ, Greene JG. Gastrointestinal features of Parkinson’s disease. Curr Neurol Neurosci Rep 11: 379–384, 2011. doi: 10.1007/s11910-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 32.Davenport ER, Cusanovich DA, Michelini K, Barreiro LB, Ober C, Gilad Y. Genome-wide association studies of the human gut microbiota. PLoS One 10: e0140301, 2015. doi: 10.1371/journal.pone.0140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 51: 418–424, 2010. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- 35.Fiorentino M, Ding H, Blanchard TG, Czinn SJ, Sztein MB, Fasano A. Helicobacter pylori-induced disruption of monolayer permeability and proinflammatory cytokine secretion in polarized human gastric epithelial cells. Infect Immun 81: 876–883, 2013. doi: 10.1128/IAI.01406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa α-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6: e28032, 2011. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Treviño I, O’Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci 28: 10825–10834, 2008. doi: 10.1523/JNEUROSCI.3001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas ME, Hess CW, Fox SH. Motor complications of dopaminergic medications in Parkinson’s disease. Semin Neurol 37: 147–157, 2017. doi: 10.1055/s-0037-1602423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuda Y, Bamba H, Okui M, Tamura K, Tanida N, Satomi M, Shimoyama T, Nishigami T. Helicobacter pylori infection increases mucosal permeability of the stomach and intestine. Digestion 63, Suppl 1: 93–96, 2001. doi: 10.1159/000051918. [DOI] [PubMed] [Google Scholar]
- 40.Galland L. The gut microbiome and the brain. J Med Food 17: 1261–1272, 2014. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao X, Chen H, Schwarzschild MA, Ascherio A. A prospective study of bowel movement frequency and risk of Parkinson’s disease. Am J Epidemiol 174: 546–551, 2011. doi: 10.1093/aje/kwr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geerlings SY, Kostopoulos I, de Vos WM, Belzer C. Akkermansia muciniphila in the human gastrointestinal tract: when, where, and how? Microorganisms 6: 75, 2018. doi: 10.3390/microorganisms6030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerhardt S, Mohajeri MH. Changes of colonic bacterial composition in Parkinson’s disease and other neurodegenerative diseases. Nutrients 10: 708, 2018. doi: 10.3390/nu10060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gevers D, Kugathasan S, Denson LA, Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, González A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 15: 382–392, 2014. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, Spector TD, Bell JT, Clark AG, Ley RE. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 19: 731–743, 2016. doi: 10.1016/j.chom.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, Spector TD, Clark AG, Ley RE. Human genetics shape the gut microbiome. Cell 159: 789–799, 2014. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorecki AM, Preskey L, Bakeberg MC, Kenna JE, Gildenhuys C, MacDougall G, Dunlop SA, Mastaglia FL, Akkari PA, Koengten F, Anderton RS. Altered gut microbiome in Parkinson’s disease and the influence of lipopolysaccharide in a human α-synuclein over-expressing mouse model. Front Neurosci 13: 839, 2019. doi: 10.3389/fnins.2019.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goya ME, Xue F, Sampedro-Torres-Quevedo C, Arnaouteli S, Riquelme-Dominguez L, Romanowski A, Brydon J, Ball KL, Stanley-Wall NR, Doitsidou M. Probiotic Bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell Reports 30: 367–380.e7, 2020. doi: 10.1016/j.celrep.2019.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa S, Goto S, Tsuji H, Okuno T, Asahara T, Nomoto K, Shibata A, Fujisawa Y, Minato T, Okamoto A, Ohno K, Hirayama M. Intestinal dysbiosis and lowered serum lipopolysaccharide-binding protein in Parkinson’s disease. PLoS One 10: e0142164, 2015. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33: 599–614, 2007. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Q, Yu W, Wu J, Chen C, Lou Z, Zhang Q, Zhao J, Wang J, Xiao B. Intranasal LPS-mediated Parkinson’s model challenges the pathogenesis of nasal cavity and environmental toxins. PLoS One 8: e78418, 2013. doi: 10.1371/journal.pone.0078418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y, Imam SZ, Dong Z, Jankovic J, Ali SF, Appel SH, Le W. Role of nitric oxide in rotenone-induced nigro-striatal injury. J Neurochem 86: 1338–1345, 2003. doi: 10.1046/j.1471-4159.2003.01938.x. [DOI] [PubMed] [Google Scholar]
- 53.Heintz-Buschart A, Pandey U, Wicke T, Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel WH, Mollenhauer B, Wilmes P. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33: 88–98, 2018. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, Satokari R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 10: 988, 2018. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32: 739–749, 2017. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopfner F, Künstner A, Müller SH, Künzel S, Zeuner KE, Margraf NG, Deuschl G, Baines JF, Kuhlenbäumer G. Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 1667: 41–45: 2017. doi: 10.1016/j.brainres.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 57.Huang HK, Wang JH, Lei WY, Chen CL, Chang CY, Liou LS. Helicobacter pylori infection is associated with an increased risk of Parkinson’s disease: A population-based retrospective cohort study. Parkinsonism Relat Disord 47: 26–31, 2018. doi: 10.1016/j.parkreldis.2017.11.331. [DOI] [PubMed] [Google Scholar]
- 58.Huang S, Ma J, Zhu M, Ran Z. Status of serum vitamin B12 and folate in patients with inflammatory bowel disease in China. Intest Res 15: 103–108, 2017. doi: 10.5217/ir.2017.15.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jadhav KS, Peterson VL, Halfon O, Ahern G, Fouhy F, Stanton C, Dinan TG, Cryan JF, Boutrel B. Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology 141: 249–259, 2018. doi: 10.1016/j.neuropharm.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 61.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9: 392, 2015. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly LP, Carvey PM, Keshavarzian A, Shannon KM, Shaikh M, Bakay RA, Kordower JH. Progression of intestinal permeability changes and α-synuclein expression in a mouse model of Parkinson’s disease. Mov Disord 29: 999–1009, 2014. doi: 10.1002/mds.25736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord 30: 1351–1360, 2015. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 64.Kim C, Lv G, Lee JS, Jung BC, Masuda-Suzukake M, Hong CS, Valera E, Lee HJ, Paik SR, Hasegawa M, Masliah E, Eliezer D, Lee SJ. Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril. Sci Rep 6: 30891, 2016. doi: 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, Lee JH, Kim WR, Kook M, Foss CA, Shen C, Lee H, Kulkarni S, Pasricha PJ, Lee G, Pomper MG, Dawson VL, Dawson TM, Ko HS. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103: 627–641.e7, 2019. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim YJ, Lee CM, Kim S, Jang JW, Lee SY, Lee SH. Risk of Parkinson’s disease after colectomy: longitudinal follow-up study using a national sample cohort. J Neurol 267: 513–521, 2020. doi: 10.1007/s00415-019-09617-1. [DOI] [PubMed] [Google Scholar]
- 67.Knights D, Silverberg MS, Weersma RK, Gevers D, Dijkstra G, Huang H, Tyler AD, van Sommeren S, Imhann F, Stempak JM, Huang H, Vangay P, Al-Ghalith GA, Russell C, Sauk J, Knight J, Daly MJ, Huttenhower C, Xavier RJ. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med 6: 107, 2014. doi: 10.1186/s13073-014-0107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, Gavalko Y, Dorofeyev A, Romanenko M, Tkach S, Sineok L, Lushchak O, Vaiserman A. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol 17: 120, 2017. doi: 10.1186/s12866-017-1027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kreitinger JM, Beamer CA, Shepherd DM. Environmental immunology: lessons learned from exposure to a select panel of immunotoxicants. J Immunol 196: 3217–3225, 2016. doi: 10.4049/jimmunol.1502149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M. Manganese-induced Parkinsonism and Parkinson’s disease: shared and distinguishable features. Int J Environ Res Public Health 12: 7519–7540, 2015. doi: 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li C, Cui L, Yang Y, Miao J, Zhao X, Zhang J, Cui G, Zhang Y. Gut microbiota differs between Parkinson’s disease patients and healthy controls in northeast China. Front Mol Neurosci 12: 171, 2019. doi: 10.3389/fnmol.2019.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Wu X, Hu X, Wang T, Liang S, Duan Y, Jin F, Qin B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 60: 1223–1233, 2017. doi: 10.1007/s11427-016-9001-4. [DOI] [PubMed] [Google Scholar]
- 73.Lin A, Zheng W, He Y, Tang W, Wei X, He R, Huang W, Su Y, Huang Y, Zhou H, Xie H. Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 53: 82–88, 2018. doi: 10.1016/j.parkreldis.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Lin CH, Chen CC, Chiang HL, Liou JM, Chang CM, Lu TP, Chuang EY, Tai YC, Cheng C, Lin HY, Wu MS. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation 16: 129, 2019. doi: 10.1186/s12974-019-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin JC, Lin CS, Hsu CW, Lin CL, Kao CH. Association between Parkinson’s disease and inflammatory bowel disease: a nationwide Taiwanese retrospective cohort study. Inflamm Bowel Dis 22: 1049–1055, 2016. doi: 10.1097/MIB.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 76.Ling X, Linglong P, Weixia D, Hong W. Protective effects of bifidobacterium on intestinal barrier function in LPS-induced enterocyte barrier injury of Caco-2 monolayers and in a rat NEC model. PLoS One 11: e0161635, 2016. doi: 10.1371/journal.pone.0161635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 88: 1996–2002, 2017. doi: 10.1212/WNL.0000000000003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Wang F, Liu S, Du J, Hu X, Xiong J, Fang R, Chen W, Sun J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci 381: 176–181, 2017. doi: 10.1016/j.jns.2017.08.3235. [DOI] [PubMed] [Google Scholar]
- 79.Liu M, Bing G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinsons Dis 2011: 327089, 2011. doi: 10.4061/2011/327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79a.Ma MJ.Biopsy pathology of neurodegenerative disorders in adults. In: Practical Surgical Neuropathology A Diagnostic Approach 2010, p. 659–680. doi: 10.1016/B978-0-323-44941-0.00027-8. [DOI] [Google Scholar]
- 80.Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555: 623–628, 2018. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 364: eaau6323, 2019. doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Makaroff L, Gunn A, Gervasoni C, Richy F. Gastrointestinal disorders in Parkinson’s disease: prevalence and health outcomes in a US claims database. J Parkinsons Dis 1: 65–74, 2011. doi: 10.3233/JPD-2011-001. [DOI] [PubMed] [Google Scholar]
- 83.Maranduba CM, De Castro SB, de Souza GT, Rossato C, da Guia FC, Valente MA, Rettore JV, Maranduba CP, de Souza CM, do Carmo AM, Macedo GC, de Sa Silva F. Intestinal microbiota as modulators of the immune system and neuroimmune system: impact on the host health and homeostasis. J Immunol Res 2015: 931574, 2015. doi: 10.1155/2015/931574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122: 107–118, 2005. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 85.McGee DJ, Lu XH, Disbrow EA. Stomaching the possibility of a pathogenic role for Helicobacter pylori in Parkinson’s disease. J Parkinsons Dis 8: 367–374, 2018. doi: 10.3233/JPD-181327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat Rev Neurosci 2: 589–594, 2001. doi: 10.1038/35086067. [DOI] [PubMed] [Google Scholar]
- 87.Mendes A, Gonçalves A, Vila-Chã N, Moreira I, Fernandes J, Damásio J, Teixeira-Pinto A, Taipa R, Lima AB, Cavaco S. Appendectomy may delay Parkinson’s disease onset. Mov Disord 30: 1404–1407, 2015. doi: 10.1002/mds.26311. [DOI] [PubMed] [Google Scholar]
- 88.Miraglia F, Colla E. Microbiome, Parkinson’s disease and molecular mimicry. Cells 8: 222, 2019. doi: 10.3390/cells8030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 21: 10609–10620, 2015. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niehaus I, Lange JH. Endotoxin: is it an environmental factor in the cause of Parkinson’s disease? Occup Environ Med 60: 378, 2003. doi: 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16: 90, 2016. doi: 10.1186/s12866-016-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med 49: e338, 2017. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5: e8762, 2010. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pan-Montojo F, Schwarz M, Winkler C, Arnhold M, O’Sullivan GA, Pal A, Said J, Marsico G, Verbavatz JM, Rodrigo-Angulo M, Gille G, Funk RH, Reichmann H. Environmental toxins trigger PD-like progression via increased α-synuclein release from enteric neurons in mice. Sci Rep 2: 898, 2012. doi: 10.1038/srep00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parashar A, Udayabanu M. Gut microbiota: implications in Parkinson’s disease. Parkinsonism Relat Disord 38: 1–7, 2017. doi: 10.1016/j.parkreldis.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park SC, Moon JC, Shin SY, Son H, Jung YJ, Kim NH, Kim YM, Jang MK, Lee JR. Functional characterization of alpha-synuclein protein with antimicrobial activity. Biochem Biophys Res Commun 478: 924–928, 2016. doi: 10.1016/j.bbrc.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 97.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 139: 1619–1625, 2009. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez-Pardo P, Dodiya HB, Engen PA, Forsyth CB, Huschens AM, Shaikh M, Voigt RM, Naqib A, Green SJ, Kordower JH, Shannon KM, Garssen J, Kraneveld AD, Keshavarzian A. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68: 829–843, 2019. doi: 10.1136/gutjnl-2018-316844. [DOI] [PubMed] [Google Scholar]
- 99.Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, Perevozchikova TV, Fait EA, Babenko VV, Vakhitova MT, Govorun VM, Sazonov AE. Analysis of gut microbiota in patients with Parkinson’s disease. Bull Exp Biol Med 162: 734–737, 2017. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 100.Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 80: 2035–2041, 2013. doi: 10.1212/WNL.0b013e318294b3c8. [DOI] [PubMed] [Google Scholar]
- 101.Piccini P, Burn DJ, Ceravolo R, Maraganore D, Brooks DJ. The role of inheritance in sporadic Parkinson’s disease: evidence from a longitudinal study of dopaminergic function in twins. Ann Neurol 45: 577–582, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 102.Poirier AA, Aubé B, Côté M, Morin N, Di Paolo T, Soulet D. Gastrointestinal dysfunctions in Parkinson’s disease: symptoms and treatments. Parkinsons Dis 2016: 6762528, 2016. doi: 10.1155/2016/6762528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qian Y, Yang X, Xu S, Wu C, Qin N, Chen SD, Xiao Q. Detection of microbial 16S rRNA gene in the blood of patients with Parkinson’s disease. Front Aging Neurosci 10: 156, 2018. [Erratum in Front Aging Neurosci 11: 4, 2019]. doi: 10.3389/fnagi.2018.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qian Y, Yang X, Xu S, Wu C, Song Y, Qin N, Chen SD, Xiao Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 70: 194–202, 2018. doi: 10.1016/j.bbi.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 105.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55: 453–462, 2007. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci 9: 99–107, 2013. doi: 10.2174/1573401311309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rees K, Stowe R, Patel S, Ives N, Breen K, Clarke CE, Ben-Shlomo Y. Helicobacter pylori eradication for Parkinson’s disease. Cochrane Database Syst Rev (11): CD008453, 2011. doi: 10.1002/14651858.CD008453.pub2. [DOI] [PubMed] [Google Scholar]
- 108.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9: 313–323, 2009. [Erratum in Nat Rev Immunol 9: 600, 2009.] doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci 39: 185–188, 2012. doi: 10.1017/S0317167100013202. [DOI] [PubMed] [Google Scholar]
- 110.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167: 1469–1480.e12, 2016. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Santos SF, de Oliveira HL, Yamada ES, Neves BC, Pereira A Jr. The gut and Parkinson’s disease: a bidirectional pathway. Front Neurol 10: 574, 2019. doi: 10.3389/fneur.2019.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord 30: 350–358, 2015. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 113.Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, Schäfer KH, Unger MM. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50: 104–107, 2018. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 114.Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, Baxter NT, Zhao L, Schloss PD, Martens EC, Eaton KA, Chen GY. NLRP6 protects Il10−/− mice from colitis by limiting colonization of Akkermansia muciniphila. Cell Reports 19: 733–745, 2017. [Erratum in Cell Rep 19: 2174, 2017.] doi: 10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sessenwein JL, Lomax AE. Ghrelin receptors as targets for novel motility drugs. Neurogastroenterol Motil 27: 589–593, 2015. doi: 10.1111/nmo.12562. [DOI] [PubMed] [Google Scholar]
- 116.Sidiq T, Yoshihama S, Downs I, Kobayashi KS. Nod2: a critical regulator of ileal microbiota and Crohn’s disease. Front Immunol 7: 367, 2016. doi: 10.3389/fimmu.2016.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sui YT, Bullock KM, Erickson MA, Zhang J, Banks WA. Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 62: 197–202, 2014. doi: 10.1016/j.peptides.2014.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav Immun 70: 48–60, 2018. doi: 10.1016/j.bbi.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 119.Sung HY, Park JW, Kim JS. The frequency and severity of gastrointestinal symptoms in patients with early Parkinson’s disease. J Mov Disord 7: 7–12, 2014. doi: 10.14802/jmd.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Svensson E, Horváth-Puhó E, Stokholm MG, Sørensen HT, Henderson VW, Borghammer P. Appendectomy and risk of Parkinson’s disease: A nationwide cohort study with more than 10 years of follow-up. Mov Disord 31: 1918–1922, 2016. doi: 10.1002/mds.26761. [DOI] [PubMed] [Google Scholar]
- 121.Tamtaji OR, Taghizadeh M, Daneshvar Kakhaki R, Kouchaki E, Bahmani F, Borzabadi S, Oryan S, Mafi A, Asemi Z. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr 38: 1031–1035, 2019. doi: 10.1016/j.clnu.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 122.Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 119: 866–872, 2011. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tillisch K. The effects of gut microbiota on CNS function in humans. Gut Microbes 5: 404–410, 2014. doi: 10.4161/gmic.29232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turpin W, Espin-Garcia O, Xu W, Silverberg MS, Kevans D, Smith MI, Guttman DS, Griffiths A, Panaccione R, Otley A, Xu L, Shestopaloff K, Moreno-Hagelsieb G, Paterson AD, Croitoru K; GEM Project Research Consortium . Association of host genome with intestinal microbial composition in a large healthy cohort. Nat Genet 48: 1413–1417, 2016. doi: 10.1038/ng.3693. [DOI] [PubMed] [Google Scholar]
- 125.Unger MM, Möller JC, Mankel K, Eggert KM, Bohne K, Bodden M, Stiasny-Kolster K, Kann PH, Mayer G, Tebbe JJ, Oertel WH. Postprandial ghrelin response is reduced in patients with Parkinson’s disease and idiopathic REM sleep behaviour disorder: a peripheral biomarker for early Parkinson’s disease? J Neurol 258: 982–990, 2011. doi: 10.1007/s00415-010-5864-1. [DOI] [PubMed] [Google Scholar]
- 126.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32: 66–72, 2016. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 127.van Kessel SP, Frye AK, El-Gendy AO, Castejon M, Keshavarzian A, van Dijk G, El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun 10: 310, 2019. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Villumsen M, Aznar S, Pakkenberg B, Jess T, Brudek T. Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut 68: 18–24, 2019. doi: 10.1136/gutjnl-2017-315666. [DOI] [PubMed] [Google Scholar]
- 129.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 817: 221–239, 2014. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 130.Wallin IE. Bacteria and the origin of species. Science 64: 173–175, 1926. doi: 10.1126/science.64.1651.173. [DOI] [PubMed] [Google Scholar]
- 131.Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol 17: 83, 2019. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weimers P, Halfvarson J, Sachs MC, Saunders-Pullman R, Ludvigsson JF, Peter I, Burisch J, Olén O. Inflammatory bowel disease and Parkinson’s disease: a nationwide Swedish cohort study. Inflamm Bowel Dis, 25: 111–123, 2019. doi: 10.1093/ibd/izy190. [DOI] [PubMed] [Google Scholar]
- 133.Wirdefeldt K, Gatz M, Reynolds CA, Prescott CA, Pedersen NL. Heritability of Parkinson disease in Swedish twins: a longitudinal study. Neurobiol Aging 32: 1923.e1–1923.e8, 2011. doi: 10.1016/j.neurobiolaging.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu SL, Liscic RM, Kim S, Sorbi S, Yang YH. Nonmotor symptoms of Parkinson’s disease. Parkinsons Dis: 2017: 4382518, 2017. doi: 10.1155/2017/4382518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiong ZK, Lang J, Xu G, Li HY, Zhang Y, Wang L, Su Y, Sun AJ. Excessive levels of nitric oxide in rat model of Parkinson’s disease induced by rotenone. Exp Ther Med 9: 553–558, 2015. doi: 10.3892/etm.2014.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang X, Qian Y, Xu S, Song Y, Xiao Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front Aging Neurosci 9: 441, 2018. doi: 10.3389/fnagi.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y, Jobin C. Microbial imbalance and intestinal pathologies: connections and contributions. Dis Model Mech 7: 1131–1142, 2014. doi: 10.1242/dmm.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 6: 18–22, 2009. [PMC free article] [PubMed] [Google Scholar]
- 140.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113: 1873–1882, 1997. doi: 10.1016/S0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 141.Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol 10: 18–26, 2017. doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao L, Xiong Q, Stary CM, Mahgoub OK, Ye Y, Gu L, Xiong X, Zhu S. Bidirectional gut-brain-microbiota axis as a potential link between inflammatory bowel disease and ischemic stroke. J Neuroinflammation 15: 339, 2018. doi: 10.1186/s12974-018-1382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zoetendal EG, Akkermans ADL, Akkermans-van Vliet WM, de Visser JAGM, de Vos WM. The host genotype affects the bacterial community in the human gastronintestinal tract. Microb Ecol Health Dis 13: 129–134, 2001. doi: 10.1080/089106001750462669. [DOI] [Google Scholar]


