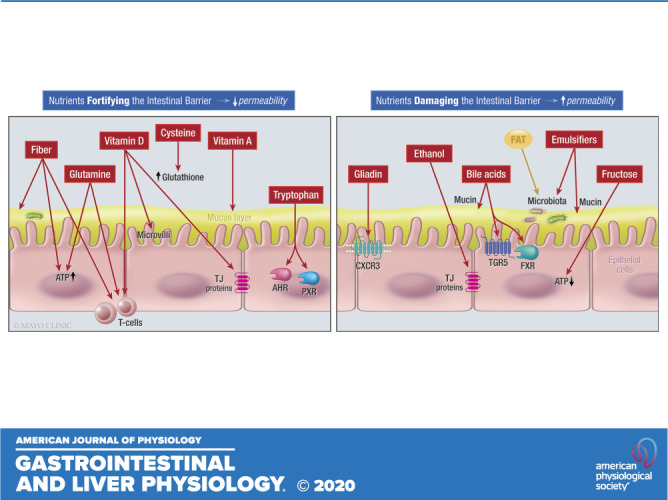
Keywords: diet, food, gut barrier, nutrition, microbiota
Abstract
Altered intestinal permeability plays a role in many pathological conditions. Intestinal permeability is a component of the intestinal barrier. This barrier is a dynamic interface between the body and the food and pathogens that enter the gastrointestinal tract. Therefore, dietary components can directly affect this interface, and many metabolites produced by the host enzymes or the gut microbiota can act as signaling molecules or exert direct effects on this barrier. Our aim was to examine the effects of diet components on the intestinal barrier in health and disease states. Herein, we conducted an in-depth PubMed search based on specific key words (diet, permeability, barrier, health, disease, and disorder), as well as cross references from those articles. The normal intestinal barrier consists of multiple components in the lumen, epithelial cell layer and the lamina propria. Diverse methods are available to measure intestinal permeability. We focus predominantly on human in vivo studies, and the literature is reviewed to identify dietary factors that decrease (e.g., emulsifiers, surfactants, and alcohol) or increase (e.g., fiber, short-chain fatty acids, glutamine, and vitamin D) barrier integrity. Effects of these dietary items in disease states, such as metabolic syndrome, liver disease, or colitis are documented as examples of barrier dysfunction in the multifactorial diseases. Effects of diet on intestinal barrier function are associated with precise mechanisms in some instances; further research of those mechanisms has potential to clarify the role of dietary interventions in treating diverse pathologic states.
INTRODUCTION
Food entering the gastrointestinal tract provides nutrition to the organism. In addition, there are many metabolites produced by enzymatic conversion of nutrients, either by the host enzymes or by the gut microbiota, or by stimulating release of nonenzymatic molecules that influence diverse functions, including alterations in the intestinal barrier. The metabolites produced in the lumen may enter the bloodstream and reach sufficient concentrations to affect the functions of body organs (51). These interactions between diet, digestion, microbiota, and the intestinal barrier may impact gut homeostasis (56); indeed, disruption of the intestinal barrier and increased intestinal permeability may play a role in many pathological or inflammatory states, such as obesity and diabetes (26), rheumatoid arthritis (66), and ulcerative colitis (46). On the other hand, many disease states associated with mucosal injury, such as celiac disease or inflammatory bowel disease, as well as irritable bowel syndrome, with immune activation but modest levels of inflammation and no morphological evidence of loss of mucosal integrity (26, 27), are all reported to be associated with increased intestinal permeability. Given these observations suggesting that loss of barrier function may be an integral part of disease pathogenesis, it is relevant to appraise the effects of nutritional components on gut barrier function.
Diet is a crucial regulatory factor for the gut microbiota, providing a source of energy. Diverse disease states have been associated with alterations in intestinal barrier function and alterations in gut microbiota (26). Having a better understanding of the diet-microbiota-permeability correlations may shed light on many aspects of existing pathological conditions (179). However, as reviewed elsewhere, alterations in both barrier function and changes in microbiota have only rarely been documented in the same patients or disease models in the reports available in the literature to date (26). Therefore, although beyond the scope of the current article, the additional role of the microbiota and their products must also be considered as potential modulators of barrier function (56).
The normal intestinal barrier allows absorption of beneficial dietary elements, while preventing the passage of harmful ingested substances, particularly, pathogens or toxins. Disruption of the barrier function may lead to pathological states (17).
Several nuclear receptors are involved in signaling pathways for dietary metabolites and include farnesoid X receptor (FXR), aryl hydrocarbon receptor (AHR), pregnane X receptor (PXR), and specific G protein-coupled receptors (GPCRs) (27, 175).
There are diverse mechanisms that may lead to the effects of diet on gut permeability: alterations in microbiota composition, expression of tight junction (TJ) proteins and/or epithelial cell transporters and ion channels, and activation of receptors when dietary factors or their metabolites act as ligands or signaling molecules (45).
In this review, we discuss the effects of various nutrients in the diet on the intestinal barrier function, focusing mostly on in vivo animal and human gastrointestinal tract. Many nutrients may affect intestinal permeability only in pathological states, and further studies are needed to assess whether these nutrients can affect permeability in the healthy state as well. An outline and summary of the topic covered appear in Table 1.
Table 1.
Effects of ingested components with reported effects on barrier function in vivo (human or animal studies)
| Dietary Component | Effects on Barrier Function | References |
|---|---|---|
| Fiber: inulin, pectin, fructo-oligosaccharides | NAFLD, environmental enteropathy, obesity/metabolic syndrome, dextran sulfate colitis | (42, 74, 96, 162) |
| Vitamins and minerals: vitamins A and D, zinc | Vitamin D receptor knockout and DSS-induced colitis in mice; vitamin A or zinc deficiency in children | (36, 94, 127, 166, 172) |
| Others: polyphenols, flavonones, and anthocyanins | Exercise-related oxidative stress and inflammation | (75) |
| Probiotics | Obesity; primary sclerosing cholangitis and IBD | (98, 115) |
| Synbiotics: probiotics and prebiotics | Indomethacin-induced increased permeability; obesity | (57, 186) |
| Prebiotics | Nonobese diabetic mice; Western diet-induced increased intestinal permeability in mice; aspirin-induced intestinal permeability in humans; children with Type 1 diabetes mellitus; preterm infants; burns | (21, 37, 72, 98, 126, 184) |
| Arabinoxylans | Overweight and obesity | (143) |
| Macronutrients |
||
| Protein | Protein-restricted diet or dexamethasone-induced permeability in chickens; dietary meat and fish (and fat) in epidemiological studies of IBD | (11, 78) |
| Gluten | Mice with DSS-induced colitis; human nonceliac gluten sensitivity and IBS-D | (117, 145, 174) |
| Sulfur-containing amino acids: cysteine and methionine | Rats infected with Salmonella enteritidis; ischemia-reperfusion injury in rats; high-fat diet with methionine restriction in mice | (159, 169, 193) |
| Other amino acids | ||
| Glutamine | Glutamine: mice with activity-based anorexia; malnourished children; Crohn’s disease; burn patients; acute pancreatitis | (9, 14, 81, 101, 132) |
| l-Tryptophan | Tryptophan: colitis in mice | (77) |
| Arginine | Arginine: intestinal injury induced by heat stress; ischemia-reperfusion; methotrexate; NASH or intestinal obstruction in rodents; mouse colitis | (7, 95, 148, 157, 171, 176, 190) |
| Sugars | ||
| Glucose | Hyperglycemia in mice and obese humans | (165) |
| Fructose | Human inflammation; alcoholic liver disease; NAFLD/NASH | (55, 167, 197) |
| Fat | ||
| Fat-restricted diets | Polyunsaturated fat intake and ulcerative colitis (epidemiology) | (70) |
| Obesity and hepatic steatosis | (16, 48) | |
| Alcohol | Alcohol abuse and alcoholic liver disease | (18, 91) |
| Intraluminal emulsifiers |
||
| Endogenous bile acids | Detergent effects in mammalian colon | (124) |
| Dietary emulsifiers | Mouse colon permeability and inflammatory changes | (34) |
| Receptors associated with intraluminal factors |
||
| Aryl hydrocarbon receptor | Anti-inflammatory in diverse animal models of immune diseases | (64) |
| Pregnane X receptor | DSS-colitis in mice; metabolic syndrome-obesity; T2DM; RYGB | (32, 77, 79, 168) |
DSS, dioctyl sodium sulfosuccinate; IBD, inflammatory bowel disease; IBS-D, diarrhea-predominant irritable bowel syndrome; NAFLD, nonalcoholic fatty liver disease; RYGB, Roux-en Y gastric bypass; T2DM, Type 2 diabetes mellitus.
To facilitate understanding, the methods used to assess the effects of diet on barrier function, we briefly define intestinal barrier and intestinal permeability, and describe methods to assess permeability.
METHOD OF LITERATURE SEARCH
A literature search was conducted by a librarian, with key words: gut barrier function, barrier function, gut permeability, intestinal barrier, intestinal permeability, food, diet, dietary factors, nutrients, fiber, amino acids, proteins, sugar, fat, emulsifiers, alcohol. The search was conducted for all literature published after 2010. In addition, the cross references from those articles were reviewed.
INTESTINAL BARRIER: DEFINITIONS AND STRUCTURE
Intestinal permeability is a vital component of the intestinal barrier. This barrier is a dynamic structure that interacts with external factors (54). The intestine is one of the largest interfaces between what is outside the human body and the inside. In particular, the small intestine is the main locus of barrier vulnerability, due to its massive surface area, resulting from the villous architecture, the higher permeability of the enterocyte layer [average 8 Angstrom (Ǻ), being 4–5 Ǻ at villus tip to over 20 Ǻ at base of the crypt] relative to the stomach and colon (3 Ǻ), and the availability of food, contaminants, and digestive products before absorption in the small bowel. The intestinal barrier has multiple layers to enforce defense mechanisms against external intruders (88). The major component of the intestinal barrier is the epithelial layer, but before the intruders reach the intestinal epithelium, there are three layers of defense. In the lumen, secretions from the stomach, pancreas, and gallbladder help destroy bacteria or antigens entering the body. The “commensal bacteria” that colonize the lumen prevent the growth of other pathogens (135). A “microclimate” consisting of unstirred water, glycocalyx, mucus, products of Paneth cells, such as lysozymes, α-defensins, and secretory IgA (secreted by plasma cells in the lamina propria) provides defense against bacteria and precludes adhesion of bacteria to the epithelial layer underneath (88).
There are two mucus layers in the intestinal barrier: an outer layer that is thick and loose and has numerous bacteria trapped in it and an inner layer that is firm with scant bacteria under physiological conditions (135). Under the microclimate, there is the “intestinal epithelium,” which consists of epithelial cells that are linked by junctional complexes (88). These cells secrete chloride in response to harmful stimuli and also antimicrobial peptides. The last layer of the intestinal barrier is the lamina propria with its immune cells, submucosal neurons that may influence the TJs between epithelial cells (121), myofibroblasts that reside immediately beneath the epithelial basement membrane and play a role in closure of the interepithelial TJs and the paracellular space (19), and enteroendocrine cells that produce bioactive substances (peptides, amines) that alter epithelial functions, such as secretions that can “flush” away the intruders (88).
Epithelial cells are an extremely important component of the intestinal barrier; they are continually undergoing cell death and renewal, being constantly replaced from the pluripotent stem cells located at the base of the crypts. Various lineages of epithelial cells allow for the diverse functions of epithelial cells in the intestinal barrier; this topic is further reviewed by Peterson and Artis (134). Nevertheless, this constant death and renewal of epithelial cells emphasizes the concept that there is more to the barrier than the epithelial layer.
Epithelial cells are linked by intercellular junctions, tight junctions, and adherence junctions, which together constitute the apical junctional complex (26). The literature has documented intercellular pore pathways (that allow passage of small molecules, ions, and water), leak pathways (that permit passage of macromolecules, bacterial products, and food antigens) and, finally, apoptosis of epithelial cells that provides “unrestricted” access for intraluminal content (17, 125).
ASSESSMENT OF INTESTINAL BARRIER FUNCTION IN VIVO
There are various methods to assess permeability. The most commonly used methods involve the use of probe molecules. However, the literature includes other methods, and these will be briefly described to facilitate the subsequent discussion. It should be noted that the methods that assess the permeability in vivo do not represent the same functions in permeability by in vitro measurements, such as Ussing chambers, which lack contribution from neural input or vascular permeability and Caco2 monolayers, which consist exclusively of epithelial cells.
Orally Ingested Probe Molecules
This is the most commonly used method to assess permeability; it measures permeability indirectly by the urinary excretion of orally administered probe molecules. These molecules pass through the intestinal barrier via paracellular pathways without being digested and, after circulating in the blood, are excreted in the urine without reabsorption in the kidneys. Saccharides are the most frequently used probes and include sucrose, mannitol, rhamnose, lactulose, and sucralose (estimated molecular diameters 6–10 Ǻ) (27). Sometimes, all saccharide markers are used together to appraise pan-gastrointestinal permeability. Although it was originally thought that sucrose was a marker for gastric permeability and sucralose for colonic permeability (particularly, because it is not metabolized by colonic bacteria), it was subsequently shown in children and in adults that sucralose is also absorbed in the small intestine and is not exclusively a marker of colonic permeability (6, 114).
There are important precautions to be taken when using these probes because of environmental contamination with mannitol or sucralose. Nonsaccharide probe molecules are polyethylene glycol 400 (PEG) and radioactive chromium complexed with ethylene diamine tetracetic acid (51Cr-labeled EDTA). In general, these tests lack complete standardized and validated normal values from healthy volunteers (26).
Other in vivo tests of permeability involve administration of probe molecules, such as fluorescein isothiocyanate linked to dextran 4000 [FITC-dextran 4 kDa (estimated molecular diameter 30 Ǻ)] (27), followed by measurement of “serum” appearance of the probe molecule. The molecular weight and estimated or observed molecular diameters of all these probe molecules are summarized elsewhere (26). Lucifer yellow (LY) is a fluorescent marker (457.25 Da, estimated diameter 11 Ǻ) used to appraise paracellular permeability (199).
Circulating Endotoxins
Endotoxins are lipopolysaccharides (LPSs) that are abundant in the outer membrane of gram-negative bacteria. Their estimated molecular diameters are 45.7–62.8 Ǻ (27). Almost 70% of the gut bacteria are gram negative, and these result in the production of endotoxins in the gut. Alterations in the intestinal barrier may lead to entry of these endotoxins into the bloodstream. Lipid-A is a major component of LPS; it activates proinflammatory mechanisms and induces oxidative stress. Toll-like receptor-4 (TLR-4) is a receptor that binds to lipid-A; this receptor is available on diverse cells, including immune cells, and plays a major role in connecting gut bacteria, endotoxins, and inflammatory pathways (22).
Endotoxins are recognized as a causative factor for inflammation in animal studies of obesity and may contribute to nonalcoholic steatohepatitis (NASH) (32). It has been proposed that a low-grade level of systemic inflammation in diabetes and obesity is attributable to LPS (28). In physiological states, there is a low-grade endotoxemia during different times of the day, and this is increased with a high-fat diet (HFD) (131). Dietary changes may exacerbate this endotoxemia and lead to inflammation and further pathological conditions in the body (28).
Serum Zonulin Levels
Zonulin is a protein that modulates the TJ structure. In a cross-sectional study of nonpregnant women, higher zonulin levels were associated with higher inflammatory markers (serum C-reactive protein and IL-6). Significant associations between serum zonulin levels and body mass index (BMI), waist and hip measurements, and fat mass were proposed (118).
Endoscopy
Two methods can be applied for endoscopic measurements and are reviewed elsewhere: first, confocal endomicroscopy, which evaluates leaking of intravenously administered fluorescein into the gut lumen, while conducting an endoscopy; and second, endoscopic mucosal impedance performed with a catheter passed through the endoscope and placed in contact with duodenal mucosa (26).
ASSESSMENT OF INTESTINAL BARRIER FUNCTION IN VITRO
In vitro measurements include measurements that assess permeability based on tissue biopsies, either by measuring the movement of probe molecules in Ussing chambers or by assessing protein expression of TJ morphologically (by immunohistochemistry) or by quantitative assays such as Q-PCR. Some of the differences between in vitro and in vivo measurements and their potential impact on the interpretation of intestinal barrier function are reviewed elsewhere (26).
EFFECTS OF DIET COMPONENTS ON BARRIER FUNCTION
Here, we discuss the chemical nature of dietary factors, the rationale or mechanism believed to mediate the effect on the barrier, as well as results of studies of dietary factors (beneficial or possibly deleterious) in healthy or nonhealthy human gastrointestinal tract. A summary of the effects of all nutrients on the intestinal barrier is discussed in the Table 2.
Table 2.
Proposed mechanisms of the effects of diet on permeability
| Nutrient | Effect on Permeability | Proposed Mechanisms |
|---|---|---|
| SCFAs | ↓ | ↑ATP, Treg regulation, cytokine production, HIF-1 regulation, relocation of ZO-1, and occludin |
| Vitamin D | ↓ | Regulation of innate and adaptive immunity, ↑Ezrin, altered villous morphology |
| Vitamin A | ↓ | ↑ Mucus and defensin production, ↑TLRs |
| Zinc | ↓ | ↓Phosphorylated occludin and claudin-1, ↑claudin-2 |
| Anthocyanins | ↓ | ↑ GLP-2 and MUC-2 |
| Cysteine | ↓ | ↑ GSH |
| Methionine | ↓ | ↑Occludin, ZO-1 and claudin-3 |
| Glutamine | ↓ | ↑ATP, ↑ERK1/2, and JNK, growth factors EGF, TGF, and IGF-1 pathways |
| Tryptophan | ↓ | AHR and PXR pathways |
| Arginine | ↓ | NOS pathway |
| Gluten | ↑ | Binding to CXCR3 |
| Glucose | ↑ | Altering AJ proteins |
| Fructose | ↑ | ↓ATP |
| Bile acids | ↑ | TGR5 and FXR pathways |
| Fat | ↑ | Change the microbiota composition |
| Ethanol | ↑ | Direct damage to epithelia, altering TJ proteins |
| Emulsifiers | ↑ | Change the microbiota composition |
↓, Decrease; ↑, Increase; AJ, adherens junction; AHR, aryl hydrocarbon receptor; ATP, adenosine triphosphate; CXCR3, chemokine receptor CXCR3; EGF, epithelial growth factor; ERK1/2, extracellular signal-regulated protein kinase; FXR, Farnesoid X receptor; GLP-2, glucagon-like peptide-2; GSH, glutathione; HIF-1, hypoxia-inducible factor-1; IGF-1, insulin-like growth factor; JNK, c-Jun NH2-terminal kinases; MUC-2, gene coding for mucin-2 protein; NOS, nitric oxide synthase; PXR, pregnane X receptor; SCFA, short-chain fatty acid; TGF, transforming growth factor; TGR5, Takeda G protein-coupled receptor 5; TJ, tight junction; TLR, Toll-like receptor; Treg, regulatory T cells; ZO, zonula occludens.
Fiber
Multiple definitions and categories of dietary fiber have been proposed; however, there is no consensus between different references. According to the CODEX Alimentarius Commission (CAC) (85), dietary fiber is defined as “carbohydrate (CHO) polymers with 10 or more monomeric units, which are not hydrolyzed by the endogenous enzymes in the small intestine of humans.” Fiber may be soluble or insoluble, and microbiota can potentially ferment both groups (92). Insoluble fiber increases stool bulk, whereas soluble fiber undergoes fermentation by gut microbiota to metabolites, including short-chain fatty acids (SCFAs) (111). The term, microbiota-accessible carbohydrates (MACs) refers to the dietary carbohydrates that cannot be digested by human digestive enzymes and will later be fermented by microbiota (27, 151). Consumption of fiber may be one of the few dietary interventions with evidence of reinforcement of the intestinal barrier in health (27). Dietary fiber may influence the intestinal barrier by altering the gut microbiota and/or the mucus layer integrity and the fermentation products (Fig. 1).
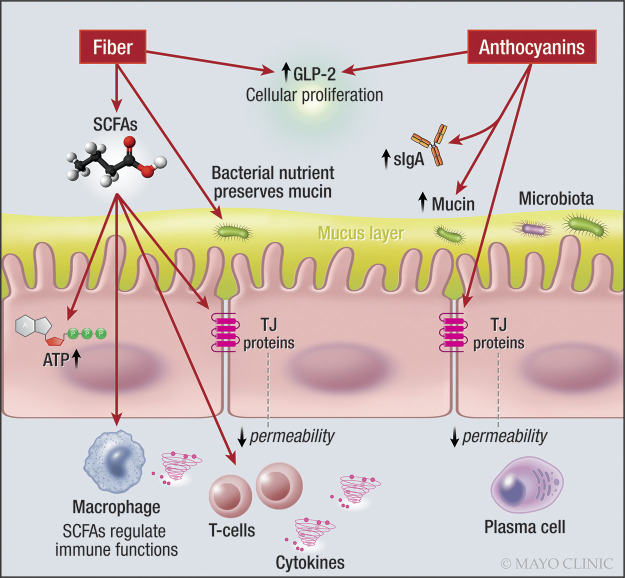
Fig. 1.
Effects of fibers and anthocyanins on the intestinal barrier. Note effects of SCFA on intracellular production of ATP, mucus layer, GLP-2 production, and regulation of immune cells including T cells. ATP, adenosine triphosphate; GLP-2, glucagon-like peptide-2; s-IgA, secretory immunoglobulin-A; SCFA, short-chain fatty acid. One-sided arrow shows activation.
Mucus layer integrity may be altered by dietary fiber. The outer mucus layer interacts with gut microbiota and is degraded to glycans that are foraged by microbiota (83, 160). In vivo studies in mice revealed that glycan-consuming microbiota use the glycans of the mucus layer, as part of their diet in the absence of enough amounts of dietary fiber (152, 160). Thus, availability of dietary fiber can modify the mucus layer by changing the function of the microbiota (160).
Fermentation products (mainly SCFAs) support host cellular metabolism and gut immune system colonocytes, neutrophils, and T cells (129), which are components of the intestinal barrier function. The SCFAs acetate, propionate, and butyrate enter colonocytes via simple diffusion or via facilitated diffusion by solute transporters, SLC16a1, or SLC5a8. Butyrate provides energy for the colonocytes. Acetate and propionate enter the bloodstream and go through metabolic pathways to generate energy in organs, including liver and muscles. SCFAs regulate neuroimmune functions, including direct enhancement of regulatory T-cell (Treg) differentiation and function (150), and production of chemokines and cytokines (93), leading to additional protective effects to the intestinal barrier. Dysfunctional Treg may lead to abnormal immune responses, especially through T-helper 17 cell (Th17) cells (27) and cytokine production, leading to inflammation (63). Th17 cells play a major role in inflammatory bowel disease (IBD) (63).
Butyrate can improve the paracellular permeability of 3-kDa molecule regulating hypoxia-inducible factor-1 (HIF-1), which modulates the efficiency of epithelial TJ CLDN1 (gene coding for claudin-1) (90, 141) and decreases intestinal permeability at concentrations of 2 and 4 mM in a monolayer model (181). Studies using the Caco-2 cell monolayer model in vitro have shown that butyrate enhances transepithelial electrical resistance (TEER) and affects the relocation of zonula occludens-1 (ZO-1) and occludin. Effects of butyrate on other TJ proteins require further study. Paradoxically, there is evidence that 8 mM butyrate is detrimental for barrier function in a monolayer preparation in the presence of inflammatory mediators, TNF-α and interferon (IFN)-γ (170).
Healthy state.
Inulin is an oligosaccharide or polysaccharide (based on its chain length) that is present in plant sources such as wheat, barley, and garlic (116). In a study performed in healthy male volunteers (140), participants who took inulin for 8 wk had significantly lower lactulose/mannitol (L/M) ratio and serum zonulin levels. Inulin also induced higher levels of mucosal GLP-2, a 33-amino acid peptide released from the mucosal enteroendocrine L-cells of the intestine. GLP-2 has a trophic role within the intestine, inducing cellular proliferation, as well as being protective during inflammatory states (195) (Fig. 1).
Pectin is a polysaccharide available in plant cell walls, such as citrus peel or apples. In the upper gastrointestinal tract, pectin is resistant to digestion and hydrolysis. Because of its complex structure, pectin serves as a substrate for fermentation by the microbiota in both the proximal and distal colon, resulting in the production of SCFAs by colonic bacteria (187). In experimental studies using cell lines, pectin protected the intestinal barrier, as shown by TEER measurement (178). Pectin also prevented inflammation in doxorubicin-induced ileitis in mice in vivo (142). However, in a randomized, controlled trial conducted in healthy young and elderly participants who received pectin for 4 wk, a pan-gastrointestinal permeability test with sugars showed no significant effects of pectin (187). Therefore, it is unclear whether pectin actually changes human intestinal barrier function.
Fructo-oligosaccharides (FOS) are carbohydrates derived from sucrose that are used as a new form of sweetener in the food industry (194). In a human placebo-controlled trial (163), urinary excretion of 51Cr-labeled EDTA did not change after taking FOS.
Disease states.
Dietary fiber and its components have been investigated in the following disease states:
Nonalcoholic Fatty Liver Disease: A randomized, controlled trial of the effects of dietary fiber on nonalcoholic fatty liver disease (NAFLD) patients used the serum zonulin level as a marker for intestinal permeability. Zonulin levels decreased significantly after increased intake of dietary fiber. This implies a change in intestinal permeability after fiber intake, and it coincided with improvement in the steatosis demonstrated by reduction in liver enzymes and improved Hamaguchi scores (96).
Environmental enteropathy: In developing countries, starting complementary feeding (when breast milk alone is no longer sufficient to meet the nutritional requirements of infants) is associated with multiple episodes of enteric infections. These infections may lead to intestinal barrier damage and chronic immune activation, thus causing a condition called environmental enteropathy (EE). EE is associated with impaired growth in children. In a cohort study of healthy newborns done in Brazil (42), lower energy intake, and dietary fiber were linked with intestinal barrier dysfunction, measured by urinary L/M ratio, and higher intestinal inflammation, respectively. These findings suggest that dietary intake from complementary feeding is associated with decreased intestinal barrier function and EE in children, but do not necessarily prove the benefit of fiber on intestinal permeability (P = 0.40).
Obesity/metabolic syndrome: In a study comparing two groups of lean and obese women, intestinal permeability was assessed by urinary L/M ratio. Although mannitol and lactulose urinary excretions were both higher in obese individuals, a statistically significant difference in L/M ratio was not found. In a further analysis, higher urinary lactulose excretion was associated with more urinary mannitol, higher weight, BMI, waist, and abdominal measurements and body fat. Dividing the groups based on L/M ratio median did not yield any significant difference between the aforementioned anthropometric measurements, but higher L/M ratio was associated with higher homeostatic model assessment, insulin and low-density lipoprotein/high-density lipoprotein (HDL) levels, and lower HDL levels.
Colitis: In a dextran sulfate sodium (DSS)-induced colitis model in mice (74), fermentable fiber was introduced to the diet in the form of guar gum (GG) that contains galactomannan. In the group with induced colitis, GG had a protective effect on TJ proteins and suppressed the colitis. In a separate study in mice (58), the chemotherapeutic agent, 5-fluoro-uracil (5-FU), increased intestinal permeability. Administration of butyrate reduced intestinal permeability and intestinal damage. Although there are studies of the effects of fiber in different forms of colitis, such as radiation-induced gastrointestinal toxicity following pelvic radiotherapy (182), and in patients with IBD (5), none of the studies evaluated barrier function, except for one that showed no increased mucin gene expression in response to butyrate enemas (68).
Vitamins and Minerals
Fat-soluble vitamins affect several components of the mucosal barrier: epithelial integrity, innate and adaptive immune systems, and gut microbiota. Epithelial cells and several types of immune cells in the gastrointestinal tract express vitamin D and vitamin A receptors. In vitro studies show that vitamin A and vitamin D modify the expression of TJ molecules. Intestinal epithelial cell lines treated with vitamin A or D have shown increased TEER, with upregulation of ZO-1, occluding, and several claudins (94, 99). Deletion of vitamin D receptors (VDR) in knockout (KO) mice increased the susceptibility to DSS-induced colitis, with increased colonic permeability to ions, as demonstrated by reduced TEER (94), and increased paracellular permeability to 4 kDa dextran (127). The regulation of innate and adaptive immunity mediated by vitamin A and vitamin D and the stimulation of antibacterial peptides occur at different levels of the mucosal barrier of the gastrointestinal tract. While acknowledging the importance of the immune mechanisms to barrier function, a detailed description of the multitude of effects of vitamin A and D on these immune functions is beyond the scope of the current article, and the reader is referred to a recent scholarly narrative review on this topic (56).
As described above, it seems reasonable to postulate that vitamins may help reduce the increased intestinal permeability described in other pathological functional gastrointestinal disorders, celiac disease, and liver disease, among others. However, this does not appear to have been investigated in published controlled clinical studies.
Vitamin D
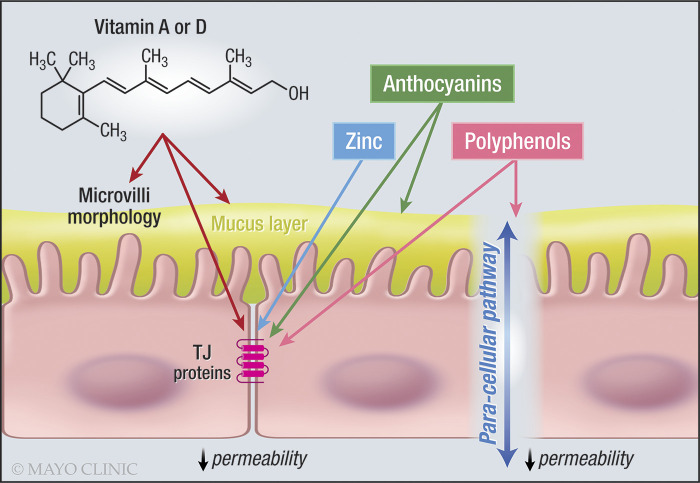
Vitamin D regulates calcium homeostasis in the body. In addition, it can affect other tissues, including the intestine (Fig. 2).
Fig. 2.
Effects of vitamins, zinc, anthocyanins, and polyphenols on the intestinal barrier. Note the effects of vitamins A and D, zinc and anthocyanins on TJ proteins. TJ, tight junction. One-sided arrow shows activation.
Healthy state.
The potential association between vitamin D deficiency and intestinal barrier function has been tested in experimental animals. In a study (100) conducted in VDR KO mice compared with wild-type mice, longer microvilli were observed in the duodenal mucosa, and higher messenger RNA and protein expression of ezrin (involved in the regulation of microvillus morphogenesis) were observed. Although there was reduced expression of claudin-2, serum FITC-dextran levels and morphology of the TJs did not show any significant difference in intestinal permeability. On the other hand, another mouse study of the effect of vitamin D-deficient diet during pregnancy on the offspring (192) demonstrated no change in villous morphology. Serum levels of FITC-dextran 4 kDa during 4 h after oral administration were twofold higher, and claudin-1 protein expression was decreased.
Disease state.
In a mouse model of DSS-induced colitis, the severity of colitis was greater in VDR KO mice, which also had disrupted TJs and desmosomes in comparison to VDR-positive mice (198). There was also severe disruption in epithelial junctions and lowering of TEER; increased intestinal permeability was also observed with small interfering (si)RNA-induced knockdown of the vitamin D receptor. Administration of vitamin D to mice in another DSS-colitis model (172), improved colitis symptoms, sustained the expression of TJ proteins (expression of ZO-1, occluding, and claudin-1), and improved barrier function (e.g., decreased the FITC-dextran permeability and the circulating levels of LPS). Short-term treatment with 2,000 international units (IU)/day of vitamin D or placebo for 3 mo in patients with Crohn’s disease (136) has shown that vitamin D may improve gastroduodenal permeability (sucrose excretion) relative to the deterioration observed on placebo; however, there was no significant improvement in small intestinal or colonic permeability with vitamin D treatment for 3 mo.
Vitamin A
Vitamin A deficiency has been associated with altered mucus production, downregulated defensin-6, and upregulated TLR in rats, thereby, damaging both humoral and cellular immunity in the mucosa and increasing susceptibility to intestinal infection (4) (Fig. 2).
Healthy state.
A randomized, controlled trial of 4 month’s duration (109) conducted in children compared the effects of vitamin A treatment with placebo and documented no differences in L/M ratio, although the vitamin A-treated group had significantly lower urinary lactulose and mannitol excretion at the end of the 4-mo study. This was associated with lower prevalence of parasitic and Giardia infections, although the relationship between reduced infections and changes in barrier function is unclear. In a cross-sectional study (36) of Brazilian children, higher intestinal permeability (L/M ratio) was documented in children with vitamin A deficiency.
In vitamin A-deficient Gambian and Indian children, vitamin A supplementation improved the intestinal barrier function, assessed by the L/M ratio test, although this was still not normal when compared with healthy infants in the United Kingdom (166).
Zinc
There are two types of zinc transporters conserved in mammals: 1) Zrt-/Irt-like protein (ZIP) zinc transporters [SLC30A], which increase the cytoplasmic zinc level by zinc import. ZIP14 is a member of this family of proteins. 2) ZnTs (zinc transporters, SLC39A) decrease the cytoplasmic zinc level by zinc export.
Healthy state.
A study (67) of ZIP14 KO mice showed increased intestinal permeability by serum FITC-dextran 4,000 Da levels and endotoxins, reduced expression of phosphorylated occludin and claudin-1, and increased claudin-2. These findings suggest a role for zinc in maintaining intestinal barrier function. In fact, coadministration of vitamin A and zinc (36) normalized intestinal permeability and restored linear growth in a cross-sectional sample of 75 children in northeastern Brazil (Fig. 2).
Polyphenols
Polyphenols are secondary metabolites available in plants in which they have many functions, such as being involved in defense mechanisms (60). They are abundant in many fruits, vegetables, and wine. Flavonoids are derivatives of polyphenols. Some subgroups of flavonoids include anthocyanins and flavonones (Fig. 2).
Healthy State.
A polyphenolic extract obtained from Portuguese red wine (123), (which includes catechins, oligomeric procyanidins, flavonols, anthocyanins, phenolic acid, and tannins) decreased the paracellular permeability across a HT-29 colon epithelial cell monolayer by promoting a significant increase of the messenger RNA (mRNA) of key barrier-forming TJ proteins (occludin, claudin-5, and ZO-1), and reducing claudin-2 mRNA, which would lead to a channel-forming TJ protein that increases permeability.
Flavonones
Flavonones are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides. Total phenolic extracts of Citrus aurantium L. (TPE-CA) are rich in dietary flavanones. In mice treated with antibiotics, TPE-CA counteracted most of the dysbiosis, reshaped intestinal permeability (upregulation of ZO-1 and occluding-associated proteins), and reduced serum endotoxin. Interestingly, there was also stimulation of FXR, resulting in reduced hepatic synthesis of bile acids (110). More trials in humans are needed to further assess the effects of citrus flavanones. In vitro studies in Caco-2 cell monolayers have shown improved intestinal barrier function (increase in TEER and a decrease in FITC-dextran flux) and increased TJ proteins after administration of citrus flavanones (hesperetin and naringenin) (155).
Anthocyanins
Anthocyanin pigments are a subfamily of flavonoids that are responsible for colors in fruits; they play a major role in the antioxidant function of fruits, such as berries and grapes (52).
Healthy state.
Anthocyanin supplementation reduced the effects of an HFD on the gut barrier in a mouse model, as shown by lower plasma endotoxin levels, increased GLP-2 levels (with upregulation of occludin, ZO-1, and claudin-1 TJ protein expression), microbiota composition, and MUC2 (gene coding for Mucin-2 protein) expression. In addition, in Caco-2 monolayers, anthocyanin reduced intestinal permeability measured with FITC-dextran flux and TEER (43). In another cell model (15) of the intestinal barrier with inflammation consisting of cocultured Caco-2 and HT29-MTX cells, as well as activated macrophages, procyanidin B-2 prevented the decrease in occludin expression and markedly increased claudin-7 expression (Figs. 1 and 2). Daily consumption of New Zealand black currant anthocyanin-rich extract for 5 wk promoted beneficial/protective antioxidant/anti-inflammatory cellular events that facilitated recovery from exercise-mediated oxidative stress, as well as increased plasma IL-10, salivary β-defensin 2 and secretory IgA (75).
Probiotics
Probiotics are live microorganisms available in dietary sources and may exert beneficial effects on the host (144), such as maintaining homeostasis (86) in gut mucosa by enhancing integrity of gut barrier, increasing the production of butyrate, and strengthening the TJ proteins (such as occludin and claudin 3) by strains of Lactobacillus reuteri (138).
Disease state.
Several studies in animals have shown increased TJ proteins and attenuated effects of LPS on intestinal barrier after probiotic administration (86). Some Saccharomyces species are probiotic yeasts that have a beneficial effect on intestinal barrier in animal models (138, 164). Specific examples are S. boulardii reduction in intestinal permeability (mannitol flux) when colitis was induced by Citrobacter rodentium strain DBS100 in mice (189), and partial restoration of claudin-1 expression and barrier integrity in T-84-polarized cell monolayers (colonic cells from humans) apically infected with Shigella flexneri (120).
In a randomized, double-blind, placebo-controlled, parallel-arm clinical trial in obese humans (98), different strains of Bifidobacterium probiotics and the prebiotic galactooligosaccharide (GOS) reduced postaspirin excretion of sucralose: lactulose ratios or sucralose alone with no effects on circulating endotoxins. In another placebo-controlled trial in healthy human volunteers (119), Lactobacillus plantarum TIFN101 induced specific gene transcription of barrier proteins that are involved in repair processes in the intestine damaged by indomethacin. For example, the reduced claudin-5 observed may conceivably enhance the intestinal barrier, given that increased claudin-5 expression is associated with a decrease in alveolar epithelial barrier function (146).
The effects of a combination probiotic, Ecologic 641, have been tested in a trial (177) of 13 male patients with primary sclerosing cholangitis and IBD. Patients who received probiotics showed no significant improvement in liver function indices. Another combination probiotic, VSL#3, reduced barrier disruption by IFN-γ in polarized monolayers of T84 intestinal epithelial cells, as measured by TEER and FITC-dextran permeability (97). This beneficial effect of VSL#3 was confirmed in an in vivo model of DSS-induced acute colitis in mice. Thus, VSL#3 protects the epithelial barrier by preventing decreased TJ protein expression (occludin, ZO-1, and several claudins) and preventing the increased apoptotic ratio (115).
Synbiotics
Synbiotics consist of a combination of specific probiotic strain(s) with the prebiotics that feed them (147).
Healthy state.
In a study in humans (183), healthy males participating in physical activity took either a synbiotic supplement consisting of multiple probiotic organisms, including several Lactobacillus and Bifidobacterium species, two prebiotics, bovine whey-derived lactoferrin and immunoglobulins with acacia gum, or a prebiotic (acacia gum) for 3 wk. No significant changes in intestinal permeability measured by L/M ratio were documented in either group. In a double-blind, controlled, randomized, parallel design study (186) in 20 adults, who were administered indomethacin to increase intestinal permeability, participants received either synbiotic (1.5 × 1010 CFU Ecologic 825 + 10 g FOS per day) or control supplements for two weeks. There was increased stool frequency, but no significant alteration of intestinal permeability.
Disease state.
Another trial (57) showed beneficial effects of the synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on intestinal permeability (by L/M urinary ratio or serum LPS) in a population with NASH.
As summarized above, probiotic Bifidobacterium strains and GOS improved intestinal barrier function in obese adults, but showed no synergism when used together as synbiotics (98). Nevertheless, salmosan, a β-galactomannan-rich product, in combination with Lactobacillus Plantarum, restored intestinal epithelial barrier function measured by TEER in monolayers of intestinal epithelial cells primed with either infectious agents or inflammatory cells (25).
Prebiotics
These are nondigestible dietary components that have beneficial effects for the host through affecting the colonic bacterial activity (113).
Disease states.
Prebiotics have been used in animal models of disease to assess whether the underlying disease is altered in association with changes in intestinal permeability. For example, effects of xylo-oligosaccharides (XOS) on pancreatic islet and salivary gland inflammation were investigated in nonobese diabetic (NOD) mice; XOS reduced intestinal permeability markers (serum FITC-dextran and zonulin) in the small and large intestine and inflammatory markers locally and systemically (69). Another study of XOS in rats showed changes in microbiota, but not in intestinal integrity (37). Bovine milk oligosaccharides reduced Western diet-induced increase in intestinal permeability in mice, as measured by markers of paracellular and transcellular flux (FITC-dextran and horseradish peroxidase) in mucosa studies in vitro (21). In a study on severe acute pancreatitis in rats (200), prebiotic GOS added to enteral nutrition decreased bacterial translocation and epithelial apoptosis and, thus, improved disrupted intestinal permeability. It was proposed that the improved growth of epithelial cells may be due to the GOS metabolite, butyrate.
In human studies, the prebiotic GOS reduced postaspirin excretion of sucralose:lactulose ratios or sucralose (98). Only marginal effects were noted in intestinal permeability of children with Type 1 diabetes mellitus (72), and there were no significant effects of enteral supplementation of a prebiotic mixture to decrease intestinal permeability in preterm infants who were being fed breast milk or mixed breast milk/formula feeding in the first week of life (184). Prebiotic ingestion did not improve gastrointestinal barrier function (measured by L/M ratio) in burn patients (126).
Arabinoxylans
Arabinoxylans are nondigestible carbohydrates that are readily available in wheat. They are metabolized in the colon by bacteria (122) and are gradually fermented in the colon (143). A randomized, controlled trial in healthy volunteers with BMI of 28–35 kg/m2 showed that, compared with lean controls, intestinal permeability was increased in overweight or obese individuals. After 6 wk of arabinoxylans therapy, the fermentation profile was improved, but the intestinal permeability did not change (143). There is a need for more studies to investigate the effects of arabinoxylans on intestinal permeability.
EFFECTS OF MACRONUTRIENTS
Protein
Dietary protein is derived from animal or plant-based sources. Protein from sources such as eggs, meat, fish, dairy products (milk and cheese), and nuts is enzymatically metabolized to amino acids and peptides, and products are subsequently fermented to hydrogen sulfide (H2S) by gut microbiota.
Disease state.
In a study (11) in broiler chickens with leaky gut induced by dexamethasone (DEX), intestinal permeability was measured by FITC-dextran, and TJ protein expression was measured by PCR. The study compared standard diet, reduced protein diet, or reduced protein diet supplemented by amino acids, glutamine, glycine, or arginine. There were inconsistent effects on TJ protein expression in jejunum and ileum; the most consistent finding was that arginine supplementation to a reduced protein diet showed benefits on permeability, including increased claudin-3 expression. These results need to be further assessed in human subjects.
Protein intake in patients with IBD has been evaluated in several retrospective and cohort studies. There was no consensus about the association between protein consumption and IBD in the studies, which are compromised by potential recall bias in filling out questionnaires about diet. An epidemiological study (78) in >67,500 middle-aged women followed for >705,000 patient-years in France reported increased risk of IBD associated with consumption of meat and fish; this was not reported for eggs or dairy products. Nevertheless, the relationship between protein intake and alterations in intestinal permeability has not been demonstrated in healthy humans or patients with IBD, in contrast to the significant effects of other dietary components, such as high-fat diets in IBD, discussed below.
Gluten
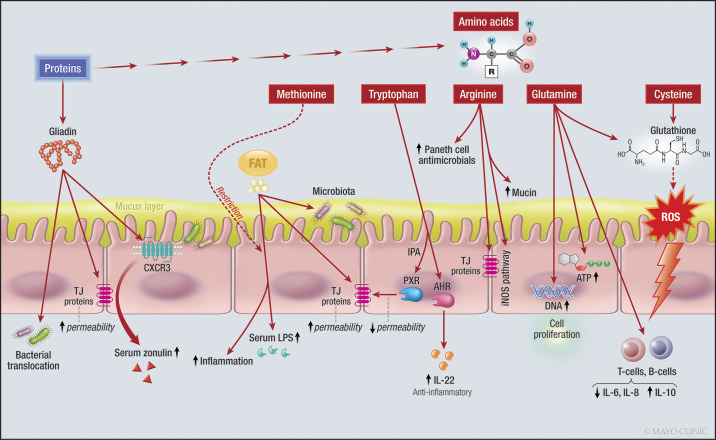
Gluten proteins can be divided into two main fractions, according to their solubility in aqueous alcohols: the soluble gliadins and the insoluble glutenins. Both fractions consist of numerous partially closely related protein components characterized by high glutamine and proline contents. Gliadins are mainly monomeric proteins with molecular weights (MWs) around 28,000–55,000 and can be classified according to their different primary structures (185) (Fig. 3). Gluten is clearly implicated in the damaged barrier in celiac disease; several studies have reported altered TJ proteins in active celiac disease that were partly reversed with a gluten-free diet (31). This discussion will consider effects of gluten on the intestinal barrier in nonceliac disease patients or disease models.
Fig. 3.
Effects of fat, proteins and amino acids on the intestinal barrier. Note the effect of amino acids on different intracellular signaling pathways and enzymes, and the effect of gliadin on CXCR3 and TJ proteins. IL-22, interleukin-22, LPS, lipopolysaccharides, TJ, tight junction, ATP, adenosine triphosphate, DNA, deoxyribonucleic acid, CXCR3, chemokine receptor CXCR3; ROS, reactive oxygen species; iNOS, inducible nitric oxide synthase; PXR, pregnane X receptor; AHR, aryl hydrocarbon receptor. One sided arrow shows activation, while dashed arrow shows inhibitory effects.
Disease state.
In mice with DSS-induced colitis, those fed with gluten had elevated intestinal permeability, translocation of bacteria, and worsening of colitis that resulted from gluten impairing the desmosomes and adherent junctions and damaging the colonic mucosa (117). In another study, increased permeability in intestinal epithelial cell lines from mice and human biopsies was associated with gliadin binding to CXCR3 (chemokine receptors in intestinal epithelia) and increasing zonulin release (103).
Two other studies investigated intestinal permeability in nonceliac gluten sensitivity. Sapone et al. (145) showed no increase in intestinal permeability in the small bowel of nonceliac gluten-sensitive individuals (n = 13) using the L/M ratio test compared with dyspeptic controls (n = 14) at baseline; however, in response to gluten withdrawal, the permeability was significantly decreased, and claudin-4 expression was increased in jejunal biopsies of gluten-sensitive patients compared with the dyspeptic controls. Another study documented permeability of duodenal biopsies ex vivo after exposure to gliadin; the permeability was higher in biopsies from patients with active celiac disease, but not in biopsies from nonceliac gluten-sensitive subjects compared with biopsies from healthy controls (73).
The celiac disease-permissive human leukocyte antigen (HLA) genotype is associated with abnormal barrier function (increased small intestinal permeability, and reduced mRNA expression of TJ proteins) in irritable bowel syndrome with diarrhea (IBS-D) relative to health. This may be, in part, related to immunogenotype, given that reduced ZO-1 protein expression in rectosigmoid mucosa was observed in HLA-DQ2/8-positive, relative to HLA-DQ2/8-negative, patients (174). In a clinical trial of nonceliac disease patients with IBS-D, a gluten-free diet (GFD) demonstrated symptomatic improvement relative to gluten-containing diet (GCD), and this was associated with reduced small intestinal permeability (173). Diet-induced changes were assessed and correlated with urinary mannitol excretion (after oral administration). In the small intestine, epithelial myosin light-chain (MLC) phosphorylation was increased and decreased by GCD and GFD, respectively, and this correlated with increased intestinal permeability. Colonocytes expression of the paracellular Na+ channel claudin-15 was also markedly augmented following GCD challenge. Conversely, colonic claudin-2 expression correlated with reduced intestinal permeability. These data show that alterations in barrier protein expression are associated with gluten-induced symptomatology and intestinal permeability changes in IBS-D (188).
Sulfur-Containing Amino Acids: Cysteine and Methionine
Cysteine is a semiessential amino acid that can be produced from methionine or serine in the body. Cysteine is required to produce glutathione (GSH) in the body. GSH, a tripeptide composed of γ-glutamic acid, cysteine, and glycine, is a potent antioxidant in epithelial cells of the gastrointestinal tract, and it serves as a reservoir of cysteine and as a means for transporting cysteine to extrahepatic tissues (156). Cystine is formed from the oxidation of two cysteine molecules, which results in the formation of a disulfide bond.
Disease state.
In a study performed in rats infected with Salmonella enteritidis (169), a control diet was compared with a diet supplemented with cysteine as a precursor of GSH, or buthionine sulfoximine, which inhibits GSH. Intestinal permeability was assessed by 51Cr-labeled EDTA, and the cysteine-treated group showed restoration of the intestinal permeability that was impaired after the Salmonella infection without altering inflammatory markers. Damage to epithelial barrier function has been investigated in models of ischemia-reperfusion injury in rats; the severity of reperfusion injury in the intestinal, endothelial, and epithelial barrier seems to correlate with the period of ischemia, the pathway of cell damage and death, together with proteinase-antiproteinase imbalance (159). N-acetyl-l-cysteine pretreatment resulted in improved endothelial and epithelial barrier integrity, and a decrease in protease inhibitor consumption (158).
Methionine (Met) is an essential amino acid that is used in several important methylation pathways in the body (61).
Healthy state.
In a study done in mice, the effects of Met restriction plus an HFD on the intestinal permeability were assessed (193). There were three groups: normal diet, HFD, and Met-restricted HFD. HFD-induced gut microbiota dysbiosis, reduced SCFA production, and increased intestinal permeability. Met-restricted HFD improved intestinal barrier function, and it was reported that Met-restricted HFD reduced LPS levels in the plasma and induced the mRNA expression of TJ proteins, including occludin, ZO-1, and claudin-3 both in ileal and colonic samples. Met-restricted HFD altered the gut microbiota in favor of increased production of SCFAs and decreased proinflammatory bacteria.
Glutamine
Glutamine is a conditionally essential amino acid because its consumption increases during adverse conditions. Glutamine is a significant source of energy for intestinal mucosa, for both enterocytes and immune cells. Deprivation of exogenous and endogenous glutamine in a Caco-2 monolayer cell model decreased TEER, increased the paracellular permeability to mannitol, and downregulated and redistributed the TJ proteins, claudin-1, occludin, and ZO-1 (105, 106). Glutamine enhances intestinal epithelial proliferation, as it is a source of adenosine triphosphate (ATP) for the cells and a substrate for the metabolic pathways to produce nucleotides and GSH. It also controls cell proliferation via signaling pathways, such as extracellular signal-regulated protein kinase 1/2 and JNK, and interacts with growth factors, such as epithelial growth factor (EGF), transforming growth factor (TGF), and insulin-like growth factor-1 (180). An extensive review in the literature (180) appraised various roles of glutamine in the gastrointestinal tract, which included enhancing the cellular growth, prevention of epithelial atrophy in catabolic states, enhancing the cellular morphology and immune response, and improving the barrier function by regulating the expression of TJ. Glutamine improved histological scores and inflammation, and increased intestinal permeability of 4-kDa dextran in everted sacs of ileum of a preclinical model of ischemia-reperfusion (IR) injury induced by superior mesenteric artery occlusion (133) (Fig. 3).
Disease state.
The effects of acetaldehyde and interleukin-13 (IL-13), which damage the intestinal barrier in a Caco-2 epithelial model in vitro, were reversed in the presence of glutamine (149) (104). Glutamine also reduced the production of proinflammatory IL-6 and IL-8, enhanced anti-inflammatory IL-10 level in T- and B-lymphocytes and epithelial cells (40), and is required for T cell activation. In an in vitro study (12) on colonic mucosal biopsies from human subjects, EGF and glutamine prevented the negative effects of acetaldehyde on adherens junction and TJ proteins. In a study done in mice (101), activity-based anorexia was induced. The effects of supplementation with glutamine versus branched-chain amino acids (BCAA) were assessed. BCAA included leucine, isoleucine, and valine. The activity-based anorexia was associated with an alteration of intestinal barrier in previous studies (81). Intestinal permeability was assessed by FITC-dextran in Ussing chambers. Glutamine, but not BCAA, restored permeability to the level of the control group.
Another study (108) comparing glutamine supplement with standard formula for 10 days in malnourished children showed that the L/M ratio decreased significantly in the glutamine-treated group compared with the same standard formula plus glycine, demonstrating an improvement in gut permeability. A randomized, controlled trial (14) showed that glutamine improved intestinal permeability, assessed by the L/M ratio test, and morphology in patients with Crohn’s disease; however, the control group receiving whey protein (to match net protein intake) had similar results. In patients with postinfectious IBS-D (201), glutamine supplementation for 8 wk was evaluated and compared with placebo. Elevated intestinal permeability, as shown by high urinary L/M ratio, was restored toward normal in the glutamine-treated group compared with no change in the control group. There were reduced symptoms, frequency of defecation, and improved consistency of stools in the glutamine-treated group. Peng et al. (132) studied effects of oral glutamine administered to patients with burns; urinary L/M ratios were lower compared with burn patients who did not receive glutamine, suggesting that glutamine supplements reversed the intestinal barrier disruption (132). In addition, the glutamine-treated group had a shorter hospital course. In a randomized, controlled trial (9), in patients with acute pancreatitis, enteral glutamine added to the standard nutrition was compared with the standard nutrition alone. Permeability was assessed by plasma PEG levels. Glutamine treatment was associated with significant decline in plasma PEG compared with levels in the beginning of the study. In-hospital mortality or infected necrosis outcomes did not change with glutamine treatment.
l-Tryptophan
95% of l-tryptophan (Trp) in the body enters the kynurenine pathway in immune cells and epithelial cells of the gastrointestinal tract; the remaining Trp is metabolized by the enzyme, tryptophanase, in many gut microbiota, producing tryptamine and other indole derivatives, including 3-indole propionic acid (IPA), and a small percentage enters the serotonin pathway in enterochromaffin cells (161). Trp can affect gut permeability through the action of nuclear receptors, including aryl hydrocarbon receptor (AHR) and PXR (27) discussed below (Fig. 3). Trp improved colitis in wild-type mice (77).
Arginine
Arginine (Arg) is a semiessential amino acid and a substrate for different enzymes, such as arginases and nitric oxide synthases (NOS). It can enter pathways to be metabolized by host enzymes, or it may be consumed by gut bacteria (2). The constitutive form of NOS (c-NOS) produces the small amounts of nitric oxide (NO) necessary for specific physiological cell functions, including maintenance of normal epithelial barrier in the intestine (3). Dietary Arg supplementation can also affect the innate immune system by increasing production of proinflammatory cytokines, IL-1β, IFN-γ, and TNF-α, secretory IgA, mucin-2, mucin-4, and Paneth cell antimicrobial secretions in the small intestine of mice (139) (Fig. 3).
Disease states.
Only a few studies have shown that Arg supplementation protects the intestinal epithelial integrity against different damaging conditions in different epithelial cell and animal models of disease:
Heat-stress: Pretreatment with 4 mM of l-Arg partially reverted the drop on TEER and the increase in Lucifer yellow (457.25 Da, estimated diameter 11A°) paracellular permeability induced by heat stress conditions (171). l-Arg improved the jejunal morphology in a rodent model of heat stress-induced intestinal damage. This amelioration was associated with the upregulation of ZO-1 at mRNA and protein levels (190).
Hypoxia: Arg also prevents reduction of TEER and increases inulin paracellular permeability that is induced by hypoxia in jejunal intestinal epithelial J2 cells monolayers. These functional changes are associated with a downregulation of the TJ protein, ZO-1 (33).
Ischemia-reperfusion: In a study in rats with mucosal injury due to ischemia-reperfusion (157), treatment with oral Arg improved cell proliferation and recovery from mucosal injury.
Colitis: Arg-treated mice with colitis showed a reduction in myeloperoxidase-positive neutrophils and of colonic paracellular permeability. In this model, these effects were exerted through inducible NOS (iNOS)-NO system, as the KO mice for iNOS did not show the benefits of Arg therapy (39). In a DSS-induced colitis model (7) in mice, Arg was administered before or during the induction of colitis. Both groups showed decreased intestinal permeability and bacterial translocation compared with the control group. In another study (95), Arg was given to rats that had intestinal mucosal injuries due to methotrexate, resembling the effects of chemotherapy. Arg decreased epithelial cell apoptosis and mucosal injury.
Steatohepatitis: Arg supplementation mitigated the decreased TJ protein expression of occludin and ZO-1 in the small intestine and promoted levels of bacterial endotoxin in plasma in a rodent model of non-alcoholic steatohepatitis (148).
Intestinal obstruction: Arg supplementation reduced intestinal paracellular permeability of a molecule of 400 Da and bacterial translocation, assessed by gavaging with E. coli, in a model of intestinal obstruction (176).
Although there is potential benefit for the use of Arg in the treatment of patients with an altered mucosal barrier, based on the studies in epithelial cell models and preclinical models described above, to the best of our knowledge, no clinical trials have been reported.
Glucose and Fructose
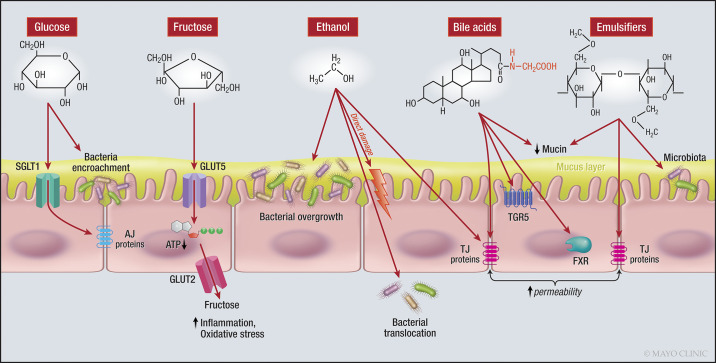
A high-glucose diet and a high-fructose diet were both associated with increased intestinal permeability and altered TJ proteins in mice; these effects were also associated with a change in gut microbiota and fat deposition in the liver (50) (Fig. 4).
Fig. 4.
Effects of sugars, ethanol, bile acids, and emulsifiers on the intestinal barrier. Note the effects of glucose on AJ proteins and bacteria, fructose reduces the intracellular ATP, and ethanol alters the bacteria and directly damages the epithelial cells. Note how bile acids affect signaling pathways and emulsifiers exert their effect on TJ proteins. AJ, adherens junction; ATP, adenosine triphosphate; BA, bile acid; FXR, farnesoid X receptor; GLUT5, glucose transporter 5; SGLT1, sodium-glucose linked transporter 1; TGR5, Takeda G protein-coupled receptor 5; TJ, tight junction. One-sided arrow shows activation.
Intestinal glucose absorption occurs mainly via the Na+/glucose cotransporter (SGLT-1) on intestinal epithelia. Another pathway for absorption of high concentrations of luminal glucose occurs via a paracellular pathway between epithelial cells (10), or via the carrier protein, GLUT-2, in the apical membranes (89). As monosaccharide absorption is completed 100% in the 100 cm of proximal jejunum, it is not clear whether any glucose reaches the distal parts of the gastrointestinal system to affect intestinal barrier function (20).
In a mouse model (165), elevated serum glucose levels, in the absence of obesity, were associated with dysfunction of adherens junction protein between intestinal epithelial cells, rendering the mice more susceptible to intestinal pathogens and the appearance of bacterial products in the circulation. Hyperglycemia increases intestinal cell transcription of apical GLUT-2, thereby, increasing glucose absorption, whereas treatment of hyperglycemia restores barrier function and further metabolism of glucose. In 27 healthy individuals (165), hemoglobin A1C levels had the strongest association with microbial pattern recognition receptor ligands in the serum. This association was not seen in association with BMI, showing a direct independent effect of hyperglycemia on intestinal barrier dysfunction.
In patients with diabetes, there was encroachment of bacteria in the dense inner layer of mucus close to the epithelium, whereas in nonobese, nondiabetic controls, bacteria were only present in the outer layers of mucus. This encroachment of bacteria was independent of BMI and obesity, suggesting the effect was related to a cellular effect of glucose on barrier function (35).
Fructose is found in fruits, honey, and table sugar (sucrose) (80); it is also added to many food products such as soft drinks in the form of high-fructose corn syrup (HFCS). With the increase in the use of HFCS (23), it is crucial to investigate the possible effects of fructose on barrier function. Fructose is absorbed via GLUT-5 in the intestinal brush border and transported to the portal bloodstream via basolateral GLUT-2 (197). In the liver, fructose is metabolized by the enzyme, fructokinase-c, for which there is no negative feedback control. Thus, the delivery of fructose leads to further metabolism, depletion of ATP, generation of uric acid, mediators of inflammation and oxidative stress, and ultimately the disruption of protein production. Fructokinase-C is also present in the small intestine where unchecked fructose metabolism ultimately leads to ATP depletion and decreased expression of TJ proteins, leading to increased permeability and endotoxemia. These principles were confirmed in fructokinase-C gene KO mice (84).
Disease states.
Fructose consumption is associated with inflammation and liver disease. An increased level of inflammatory cytokines in patients after fructose consumption may cause barrier dysfunction and allow endotoxins to enter the bloodstream, leading to mitochondrial dysfunction, inducing insulin resistance and oxidative stress that, in turn, leads to an inflammatory response (197).
In liver disease, it is proposed that fructose may cause similar effects as ethanol intake (153); fructose causes increased permeability which leads to translocation of endotoxins in the portal circulation that eventually activates Kupffer cells in the liver and leads to NAFLD. Endotoxins can further trigger oxidative stress in the liver and lead to a progression from NAFLD to NASH and cirrhosis (55). A study by Thuy et al. (167) in patients with NAFLD and a control group reported that higher dietary intake of fructose was associated with NAFLD, higher markers of intestinal permeability, and plasma endotoxins, and higher expression of endotoxin receptor, TLR4, on hepatocytes (167). In a randomized, controlled trial (82) in adolescents with NAFLD, consumption of fructose compared with glucose for 4 wk led to increased endotoxins in the blood. In adults (41) with NASH, there was significant liver ATP depletion after intravenous fructose in comparison to healthy controls. Similarly, in healthy adult men, oral fructose also caused ATP depletion in the liver (13).
Fat
Fat is an essential macronutrient source of energy and is important for the structure of cellular lipid membranes (87). Fat can induce changes in intestinal permeability via altering barrier structure and/or the gut microbiota. HFD altered TJ proteins and microbiota and induced inflammation in the gut in a study in rats (44), all of these leading to increased intestinal permeability as documented by the appearance of FITC-dextran in plasma and the translocation of occludin into the cytoplasm of ileal epithelial cells after 10 wk of the diet. Another manifestation of HFD or chronic metabolic syndrome is the well-documented endotoxemia induced in mice (28). A high-fat diet is associated with changes in the microbiota, such as increases in sulfur-reducing bacteria (SRB), with increased production of H2S leading to decreased butyrate oxidation (30) or reduced Bifidobacteria with increased inflammation and barrier dysfunction (196) (Fig. 4).
The type of fat in the diet may exert different effects on microbiota and permeability, as shown by Devkota et al. (47); thus, a diet high in saturated fat (milk) exerted different effects on microbiota compared with a diet high in polyunsaturated fat (safflower oil). A high-milk fat diet promoted an increase in Bilophila wadsworthia, a sulfite-reducing bacterium, producing H2S. These changes were mediated in part by an increase in taurocholic acid in bile, which is a nutritional source for B. wadsworthia to reduce sulfur and produce H2S, thereby disrupting barrier function. Abulizi et al. (1) reported effects of different sources of fats in mice; from the perspective of barrier function, the most relevant effects were observed with corn oil [a source of omega-6-polyunsaturated fatty acids (PUFA)], which decreased integrin-linked kinase, which is indispensable for barrier function and was associated with decreased expression of several TJ proteins of the intestinal barrier.
Healthy state.
A prospective interventional study (128) in healthy normal-weight men, who received 1,000 kcal/day (added as whipping cream) above their calculated energy need for 7 days, showed no effects on intestinal permeability, measured using sugar and PEG absorption tests, and plasma zonulin.
Disease state.
A prospective epidemiological human study (70) conducted in >260,000 European men and women sought associations between ulcerative colitis and diet, and none were detected, apart from a possible increased risk with a higher total PUFA intake.
Changes in permeability were compared in two groups of patients with NAFLD taking a Mediterranean diet or a low-fat diet (16). Permeability was assessed by 51Cr-labeled EDTA at baseline and at the end of the diet. Although the Mediterranean diet improved body weight and liver transaminases, there was no significant change in permeability indices after the intervention in any of the groups.
Di Palo et al. (48) studied pan-gastrointestinal permeability in three groups based on BMI: normal, overweight, and obese. Obesity and liver steatosis were associated with increased colonic permeability, and there was a significant difference in colonic permeability among the three BMI groups. Participants who reported less compliance with the Mediterranean diet had significantly increased small intestinal permeability compared with those who were more adherently following the diet. It is, therefore, still unclear whether the Mediterranean diet significantly impacts intestinal barrier function.
Alcohol (Ethanol)
Animal and human studies suggest alteration of small intestinal barrier in both acute and chronic alcohol use (154) e.g., at dosages ranging from 6 g·kg−1·day−1 to 8 g·kg−1·day−1 in animal studies. Ethanol concentrations in the gastrointestinal tract reach the same level as blood levels after coming to equilibrium, suggesting that ethanol must increase intestinal permeability (53). Ethanol disrupts intestinal permeability by directly damaging the cells and altering TJ structures, as proposed below (Fig. 4).
Healthy state.
Single oral intake of alcohol (1 g/kg) induced altered histology in duodenum (65), and consumption of an excessive amount of alcohol led to a change in rectal mucosal cells and inflammation (24).
Disease state.
A study done (130) in men with chronic alcohol abuse and alcoholic liver disease compared with nonalcoholic controls showed increased permeability to larger molecules up to a molecular mass of 10,000 (estimated diameter 46 Å) to mimic the structure of endotoxins. Endotoxins need a molecular mass of ≥1,900 to maintain biological activity. PEG 10,000 was not found in the urine of controls, whereas it was found in the urine of many alcoholics, and plasma endotoxin was increased fivefold in the alcoholics in comparison to control.
There was a significant correlation between PEG 4000 permeability measures and endotoxins in the plasma in the alcoholic group. Keshavarzian et al. (91) assessed the role of permeability in inducing alcoholic liver disease in chronic alcohol abusers. Interestingly, only alcoholics with chronic liver disease showed increased markers of permeability (L/M ratio in urine) compared with alcoholics without liver disease or subjects who had liver disease unrelated to alcohol abuse. These data indicate increased intestinal permeability, which is related to the alcohol rather than a nonspecific effect of liver disease. In another study (18), chronic alcohol abuse was associated with increased intestinal permeability, measured using 51Cr-labeled EDTA. The increased permeability persisted even after 2 wk of cessation. In mice (191), chronic alcohol administration led to altered microbiota composition, intestinal bacterial overgrowth, and bacterial translocation, compared with the control group.
INTRALUMINAL EMULSIFIERS
Endogenous Bile Acids
Bile acids are produced in the liver from cholesterol. These steroid molecules act as mediators for fat digestion and absorption by having detergent properties, and they also act as signaling molecules (59) (Fig. 4).
Direct action of bile acids.
Some bile acids may exert direct toxic effects on the intestinal epithelium through detergent properties of the primary bile acid, chenodeoxycholic acid and the secondary bile acid, deoxycholic acid, which are both di-α-hydroxy bile acids (124), or may induce cytotoxicity through apoptosis, independent of their detergent effects (8). An in vitro study showed that bile acids modify TJ proteins and, thus, increased intestinal permeability (137).
Indirect action as signaling molecules.
As signaling molecules, bile acids exert their effects mainly through two receptors: Takeda G protein-coupled receptor (a GPCR) and FXR (59). The lack of Takeda G protein-coupled receptor 5 in knockout mice led to altered mucous cells and disruption of TJs, leading to increased intestinal permeability (38). FXR affects barrier function through two mechanisms: first, through anti-inflammatory effects and immunoregulation, leading to production of antibacterial products in the gut, and, thus, indirectly affecting barrier function (49, 62); second, an FXR agonist prevents bacterial overgrowth and mucosal injury to the ileum in a bile duct ligation model (76) that disrupts barrier function, as shown by higher numbers of bacteria in mesenteric lymph nodes and breaks in the mucosal surface with bacteria moving into deeper parts of the epithelium.
Dietary Emulsifiers and Surfactants
Emulsifiers, also called surfactants (71), are food additives commonly used in the processed food industry. Emulsifiers make noncompatible components of food, such as oil and water, and blend smoothly rather than remaining separate. Two common emulsifiers are carboxymethylcellulose and polysorbate-80 (34). Administration of these two emulsifiers in mice changed the microbiota composition to a more proinflammatory combination and increased intestinal permeability (34). Although a change in permeability by surfactants is proposed, it is still unclear whether, under physiological conditions and when combined with other food and liquids, surfactants, in fact, affect permeability (27) (Fig. 4).
RECEPTORS ASSOCIATED WITH INTRALUMINAL FACTORS
Aryl Hydrocarbon Receptor
Aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that integrates environmental, dietary, microbial, and metabolic cues to control complex transcriptional programs in a ligand-specific, cell-type-specific, and context-specific manner. Kynurenines, tryptamine, and some indole derivative are AHR ligands that enhance the production of IL-22, an anti-inflammatory molecule, in the gastrointestinal tract. Microbiota metabolites affect AHR and, in return, AHR affects survival of gut microbiota populations via alterations of the immune system (64).
Pregnane X Receptor
Pregnane X receptor (PXR) is a ligand-activated nuclear receptor whose primary function is to sense the presence of foreign toxic substances and, in response, to upregulate the proteins involved in detoxification and clearance of these substances from the body. Interaction of indole propionic acid (IPA) produced by specific gut microbiota with PXR upregulates the production of junctional proteins, thus reducing intestinal permeability (175). In addition, there is a role for IPA in immune responses (51).
Disease state.
In mice models, modified gut microbiota causing impaired metabolism of Trp to ligands that bind AHR reduce the levels of IL-22 in the gut and predispose to IBD (102). In DSS-induced colitis in mice, Trp improved colitis in wild-type mice, but not in AHR KO mice (77). In a cross-sectional study (112), patients with metabolic syndrome and obesity had elevated KYN/Trp ratio, possibly from increased conversion of Trp to KYN. Obese subjects with Type 2 diabetes had lower serum IPA in comparison to lean subjects (79). IPA levels were reversed after Roux-en-Y gastric bypass surgery (RYGB) (79). Obesity is associated with inflammation and endotoxemia, which is attributed to increased permeability, all of which are decreased after RYGB (168); there is also evidence of TJ protein upregulation and decreased paracellular permeability in the small intestine (32). In postweaning piglets, Trp supplementation was compared with a control diet; Trp changed microbiota diversity in the small intestine and increased the expression of TJ proteins, ZO-1 and ZO-3, and claudin-1 in the jejunum and duodenum, as well as occludin in the duodenum (107).
CONCLUSION
Individual items in alteration of the diet may impact one or more elements of the intestinal barrier. It should be emphasized that various dietary factors may also alter the functions of other structures of the gastrointestinal tract, including the smooth muscle or the ENS and the immune apparatus and, therefore, the overall effect of the dietary factor. Hence, it should be noted that nutrients or other dietary factors may have only limited or no effect on health or reversal of disease, even mucosal diseases. Further research is needed to elucidate which dietary factors are most beneficial and cost-effective for dietary interventions of gastrointestinal or systemic diseases that alter intestinal barrier function. Similarly, it is important to continue to examine the mechanisms of barrier dysfunction in health and disease states. Further research of those mechanisms has the potential to clarify the role of dietary intervention in treating diverse pathological states.
GRANTS
M. Camilleri was supported by Grant R01-DK115960 from National Institutes of Health, and he has received a research grant to study intestinal permeability in humans from the International Life Sciences Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K. prepared figures; K.K. and M.C. drafted manuscript; K.K. and M.C. edited and revised manuscript; K.K. and M.C. approved final version of manuscript.
REFERENCES
- 1.Abulizi N, Quin C, Brown K, Chan YK, Gill SK, Gibson DL. Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients 11: 418, 2019. doi: 10.3390/nu11020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander M, Ang QY, Turnbaugh PJ. A diet-dependent enzyme from the human gut microbiome promotes Th17 accumulation and colitis (Preprint). bioRxiv 766899, 2019. doi: 10.1101/766899. [DOI]
- 3.Alican I, Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol Gastrointest Liver Physiol 270: G225–G237, 1996. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- 4.Amit-Romach E, Uni Z, Cheled S, Berkovich Z, Reifen R. Bacterial population and innate immunity-related genes in rat gastrointestinal tract are altered by vitamin A-deficient diet. J Nutr Biochem 20: 70–77, 2009. doi: 10.1016/j.jnutbio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 145: 970–977, 2013. doi: 10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand 182: 171–177, 2004. doi: 10.1111/j.1365-201X.2004.01347.x. [DOI] [PubMed] [Google Scholar]
- 7.Andrade ME, Santos RD, Soares AD, Costa KA, Fernandes SO, de Souza CM, Cassali GD, de Souza AL, Faria AM, Cardoso VN. Pretreatment and treatment with l-arginine attenuate weight loss and bacterial translocation in dextran sulfate sodium colitis. JPEN J Parenter Enteral Nutr 40: 1131–1139, 2016. doi: 10.1177/0148607115581374. [DOI] [PubMed] [Google Scholar]
- 8.Araki Y, Fujiyama Y, Andoh A, Nakamura F, Shimada M, Takaya H, Bamba T. Hydrophilic and hydrophobic bile acids exhibit different cytotoxicities through cytolysis, interleukin-8 synthesis and apoptosis in the intestinal epithelial cell lines. IEC-6 and Caco-2 cells. Scand J Gastroenterol 36: 533–539, 2001. doi: 10.1080/003655201750153430. [DOI] [PubMed] [Google Scholar]
- 9.Arutla M, Raghunath M, Deepika G, Jakkampudi A, Murthy HVV, Rao GV, Reddy DN, Talukdar R. Efficacy of enteral glutamine supplementation in patients with severe and predicted severe acute pancreatitis- A randomized controlled trial. Indian J Gastroenterol 38: 338–347, 2019. doi: 10.1007/s12664-019-00962-7. [DOI] [PubMed] [Google Scholar]
- 10.Ballard ST, Hunter JH, Taylor AE. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr 15: 35–55, 1995. doi: 10.1146/annurev.nu.15.070195.000343. [DOI] [PubMed] [Google Scholar]
- 11.Barekatain R, Chrystal PV, Howarth GS, McLaughlan CJ, Gilani S, Nattrass GS. Performance, intestinal permeability, and gene expression of selected tight junction proteins in broiler chickens fed reduced protein diets supplemented with arginine, glutamine, and glycine subjected to a leaky gut model. Poult Sci 98: 6761–6771, 2019. doi: 10.3382/ps/pez393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and L-glutamine. Am J Physiol Gastrointest Liver Physiol 289: G367–G375, 2005. doi: 10.1152/ajpgi.00464.2004. [DOI] [PubMed] [Google Scholar]
- 13.Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, Aithal GP, Morris PG, Gowland PA. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: A 31P MRS study. Clin Nutr 35: 645–649, 2016. doi: 10.1016/j.clnu.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin J, Makharia G, Ahuja V, Anand Rajan KD, Kalaivani M, Gupta SD, Joshi YK. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig Dis Sci 57: 1000–1012, 2012. doi: 10.1007/s10620-011-1947-9. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi MG, Chiu M, Taurino G, Brighenti F, Del Rio D, Mena P, Bussolati O. Catechin and procyanidin B2 modulate the expression of tight junction proteins but do not protect from inflammation-induced changes in permeability in human intestinal cell monolayers. Nutrients 2271: 11, 2019. doi: 10.3390/nu11102271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biolato M, Manca F, Marrone G, Cefalo C, Racco S, Miggiano GA, Valenza V, Gasbarrini A, Miele L, Grieco A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J Gastroenterol 25: 509–520, 2019. doi: 10.3748/wjg.v25.i4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 14: 189, 2014. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1: 179–182, 1984. doi: 10.1016/S0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 19.Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87: 545–564, 2007. doi: 10.1152/physrev.00012.2006. [DOI] [PubMed] [Google Scholar]
- 20.Borgström B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest 36: 1521–1536, 1957. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudry G, Hamilton MK, Chichlowski M, Wickramasinghe S, Barile D, Kalanetra KM, Mills DA, Raybould HE. Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice. J Dairy Sci 100: 2471–2481, 2017. doi: 10.3168/jds.2016-11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 124: 11–20, 2016. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79: 537–543, 2004. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 24.Brozinsky S, Fani K, Grosberg SJ, Wapnick S. Alcohol ingestion-induced changes in the human rectal mucosa: light and electron microscopic studies. Dis Colon Rectum 21: 329–335, 1978. doi: 10.1007/BF02586661. [DOI] [PubMed] [Google Scholar]
- 25.Brufau MT, Campo-Sabariz J, Carné S, Ferrer R, Martín-Venegas R. Salmosan, a β-galactomannan-rich product, in combination with Lactobacillus plantarum contributes to restore intestinal epithelial barrier function by modulation of cytokine production. J Nutr Biochem 41: 20–24, 2017. doi: 10.1016/j.jnutbio.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 68: 1516–1526, 2019. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol 317: G17–G39, 2019. doi: 10.1152/ajpgi.00063.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti J-F, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 30.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol 3: 448, 2012. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso-Silva D, Delbue D, Itzlinger A, Moerkens R, Withoff S, Branchi F, Schumann M. Intestinal barrier function in gluten-related disorders. Nutrients 11: 2325, 2019. doi: 10.3390/nu11102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casselbrant A, Elias E, Fändriks L, Wallenius V. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 11: 45–53, 2015. doi: 10.1016/j.soard.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Chapman JC, Liu Y, Zhu L, Rhoads JM. Arginine and citrulline protect intestinal cell monolayer tight junctions from hypoxia-induced injury in piglets. Pediatr Res 72: 576–582, 2012. doi: 10.1038/pr.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–96, 2015. [Erratum in Nature 536: 238, 2016.]. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 4: 205–221, 2017. doi: 10.1016/j.jcmgh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Soares AM, Lima AA, Gamble MV, Schorling JB, Conway M, Barrett LJ, Blaner WS, Guerrant RL. Association of vitamin A and zinc status with altered intestinal permeability: analyses of cohort data from northeastern Brazil. J Health Popul Nutr 21: 309–315, 2003. [PubMed] [Google Scholar]
- 37.Christensen EG, Licht TR, Leser TD, Bahl MI. Dietary xylo-oligosaccharide stimulates intestinal bifidobacteria and lactobacilli but has limited effect on intestinal integrity in rats. BMC Res Notes 7: 660, 2014. doi: 10.1186/1756-0500-7-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One 6: e25637, 2011. [Erratum in PLoS One 8: 2013.] doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP, Piazuelo MB, Casero RA Jr, Chaturvedi R, Zhao Z, Wilson KT. l-Arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One 7: e33546, 2012. doi: 10.1371/journal.pone.0033546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coëffier M, Marion R, Ducrotté P, Déchelotte P. Modulating effect of glutamine on IL-1β-induced cytokine production by human gut. Clin Nutr 22: 407–413, 2003. doi: 10.1016/S0261-5614(03)00040-2. [DOI] [PubMed] [Google Scholar]
- 41.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 282: 1659–1664, 1999. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 42.Costa PN, Soares AM, Filho JQ, Junior FS, Ambikapathi R, Rogawski McQuade ET, Guerrant RL, Caulfield LE, Lima AAM, Maciel BLL. Dietary intake from complementary feeding is associated with intestinal barrier function and environmental enteropathy in Brazilian children from the MAL-ED cohort study. Br J Nutr 123: 1003–1012, 2020. doi: 10.1017/S0007114520000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cremonini E, Daveri E, Mastaloudis A, Adamo AM, Mills D, Kalanetra K, Hester SN, Wood SM, Fraga CG, Oteiza PI. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox Biol 26: 101269, 2019. doi: 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Santis S, Cavalcanti E, Mastronardi M, Jirillo E, Chieppa M. Nutritional keys for intestinal barrier modulation. Front Immunol 6: 612, 2015. doi: 10.3389/fimmu.2015.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Den Hond E, Hiele M, Evenepoel P, Peeters M, Ghoos Y, Rutgeerts P. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology 115: 584–590, 1998. doi: 10.1016/S0016-5085(98)70137-4. [DOI] [PubMed] [Google Scholar]
- 47.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Palo DM, Garruti G, Di Ciaula A, Molina-Molina E, Shanmugam H, De Angelis M, Portincasa P. Increased colonic permeability and lifestyles as contributing factors to obesity and liver steatosis. Nutrients 12: 564, 2020. doi: 10.3390/nu12020564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B 5: 135–144, 2015. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Do MHL, Lee E, Oh M-J, Kim Y, Park H-YE, Oh , M-J, Kim Y, Park , H-Y. High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10: 761, 2018. doi: 10.3390/nu10060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551: 648–652, 2017. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Einbond LS, Reynertson KA, Luo X-D, Basile MJ, Kennelly EJ. Anthocyanin antioxidants from edible fruits. Food Chem 84: 23–28, 2004. doi: 10.1016/S0308-8146(03)00162-6. [DOI] [Google Scholar]
- 53.Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev 71: 483–499, 2013. doi: 10.1111/nure.12027. [DOI] [PubMed] [Google Scholar]
- 54.Etienne-Mesmin L, Chassaing B, Desvaux M, De Paepe K, Gresse R, Sauvaitre T, Forano E, de Wiele TV, Schüller S, Juge N, Blanquet-Diot S. Experimental models to study intestinal microbes-mucus interactions in health and disease. FEMS Microbiol Rev 43: 457–489, 2019. doi: 10.1093/femsre/fuz013. [DOI] [PubMed] [Google Scholar]
- 55.Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, Keshavarzian A. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int 28: 1026–1033, 2008. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farré R, Fiorani M, Abdu Rahiman S, Matteoli G. Intestinal permeability, inflammation and the role of nutrients. Nutrients 1185: 12, 2020. doi: 10.3390/nu12041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferolla SM, Couto CA, Costa-Silva L, Armiliato GNA, Pereira CAS, Martins FS, Ferrari M, Vilela E, Torres H, Cunha A, Ferrari T. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients 8: 397, 2016. doi: 10.3390/nu8070397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferreira TM, Leonel AJ, Melo MA, Santos RR, Cara DC, Cardoso VN, Correia MI, Alvarez-Leite JI. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-fluorouracil administration. Lipids 47: 669–678, 2012. doi: 10.1007/s11745-012-3680-3. [DOI] [PubMed] [Google Scholar]
- 59.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci 30: 570–580, 2009. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 60.Frond AD, Iuhas CI, Stirbu I, Leopold L, Socaci S, Andreea S, Ayvaz H, Andreea S, Mihai S, Diaconeasa Z, Carmen S. Phytochemical characterization of five edible purple-reddish vegetables: anthocyanins, flavonoids, and phenolic acid derivatives. Molecules 24: 1536, 2019. doi: 10.3390/molecules24081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frontiera MS, Stabler SP, Kolhouse JF, Allen RH. Regulation of methionine metabolism: effects of nitrous oxide and excess dietary methionine. J Nutr Biochem 5: 28–38, 1994. doi: 10.1016/0955-2863(94)90006-X. [DOI] [Google Scholar]
- 62.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801: 683–692, 2010. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Gálvez J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm 2014: 928461, 2014. doi: 10.1155/2014/928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 8: 13, 2018. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottfried EB, Korsten MA, Lieber CS. Alcohol-induced gastric and duodenal lesions in man. Am J Gastroenterol 70: 587–592, 1978. [PubMed] [Google Scholar]
- 66.Guerreiro CS, Calado Â, Sousa J, Fonseca JE. Diet, microbiota, and gut permeability—the unknown triad in rheumatoid arthritis. Front Med (Lausanne) 5: 349, 2018. doi: 10.3389/fmed.2018.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guthrie GJ, Aydemir TB, Troche C, Martin AB, Chang S-M, Cousins RJ. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am J Physiol Gastrointest Liver Physiol 308: G171–G178, 2015. doi: 10.1152/ajpgi.00021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamer HM, Jonkers DM, Renes IB, Vanhoutvin SA, Kodde A, Troost FJ, Venema K, Brummer RJ. Butyrate enemas do not affect human colonic MUC2 and TFF3 expression. Eur J Gastroenterol Hepatol 22: 1134–1140, 2010. doi: 10.1097/MEG.0b013e32833a6ca0. [DOI] [PubMed] [Google Scholar]
- 69.Hansen CHF, Larsen CS, Petersson HO, Zachariassen LF, Vegge A, Lauridsen C, Kot W, Krych Ł, Nielsen DS, Hansen AK. Targeting gut microbiota and barrier function with prebiotics to alleviate autoimmune manifestations in NOD mice. Diabetologia 62: 1689–1700, 2019. doi: 10.1007/s00125-019-4910-5. [DOI] [PubMed] [Google Scholar]
- 70.Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T, Appleby P, Bergmann MM, Boeing H, Hallmans G, Danielsson A, Palmqvist R, Sjodin H, Hagglund G, Overvad K, Palli D, Masala G, Riboli E, Kennedy H, Welch A, Khaw KT, Day N, Bingham S. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion 77: 57–64, 2008. doi: 10.1159/000121412. [DOI] [PubMed] [Google Scholar]
- 71.Hasenhuettl GL. Hartel RW. Overview of food emulsifiers. In: Food Emulsifiers and Their Applications. New York: Springer-Verlag, 2019. [Google Scholar]
- 72.Ho J, Nicolucci AC, Virtanen H, Schick A, Meddings J, Reimer RA, Huang C. Effect of prebiotic on microbiota, intestinal permeability, and glycemic control in children with Type 1 diabetes. J Clin Endocrinol Metab 104: 4427–4440, 2019. doi: 10.1210/jc.2019-00481. [DOI] [PubMed] [Google Scholar]
- 73.Hollon J, Puppa EL, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 7: 1565–1576, 2015. doi: 10.3390/nu7031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hung TV, Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J Nutr 146: 1970–1979, 2016. doi: 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- 75.Hurst RD, Lyall KA, Wells RW, Sawyer GM, Lomiwes D, Ngametua N, Hurst SM. Daily consumption of an anthocyanin-rich extract made from New Zealand blackcurrants for 5 weeks supports exercise recovery through the management of oxidative stress and inflammation: a randomized Placebo Controlled Pilot Study. Front Nutr 7: 16, 2020. doi: 10.3389/fnut.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 103: 3920–3925, 2006. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Islam J, Sato S, Watanabe K, Watanabe T, Ardiansyah, Hirahara K, Aoyama Y, Tomita S, Aso H, Komai M, Shirakawa H. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J Nutr Biochem 42: 43–50, 2017. doi: 10.1016/j.jnutbio.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 78.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol 105: 2195–2201, 2010. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 79.Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil e13178: 30, 2018. doi: 10.1111/nmo.13178. [DOI] [PubMed] [Google Scholar]
- 80.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol 68: 1063–1075, 2018. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jésus P, Ouelaa W, François M, Riachy L, Guérin C, Aziz M, Do Rego JC, Déchelotte P, Fetissov SO, Coëffier M. Alteration of intestinal barrier function during activity-based anorexia in mice. Clin Nutr 33: 1046–1053, 2014. doi: 10.1016/j.clnu.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, Kosters A, McClain CJ, Vos MB. Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. Int J Hepatol 2014: 560620, 2014. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108, Suppl 1: 4659–4665, 2011. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson RJ, Rivard C, Lanaspa MA, Otabachian-Smith S, Ishimoto T, Cicerchi C, Cheeke PR, Mcintosh B, Hess T. Fructokinase, fructans, intestinal permeability, and metabolic syndrome: an equine connection? J Equine Vet Sci 33: 120–126, 2013. doi: 10.1016/j.jevs.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joint FAO/WHO Food Standards Programme Secretariat of the CODEX Alimentarius Commission . CODEX Alimentarius (CODEX): Guidelines on Nutrition Labelling, Geneva, Switzerland: WHO, 2020. [Google Scholar]
- 86.Judkins TC, Archer DL, Kramer DC, Solch RJ. Probiotics, nutrition, and the small intestine. Curr Gastroenterol Rep 22: 2, 2020. doi: 10.1007/s11894-019-0740-3. [DOI] [PubMed] [Google Scholar]
- 87.Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr 19: 63–90, 1999. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]
- 88.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 22: 718–733, 2010. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 89.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr 28: 35–54, 2008. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 90.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 92.Kim CH. Immune regulation by microbiome metabolites. Immunology 154: 220–229, 2018. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145: 396–406.e1, 2013. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 94.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294: G208–G216, 2008. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 95.Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I. Dietary l-arginine supplementation reduces methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol 12: 41, 2012. doi: 10.1186/1471-230X-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krawczyk M, Maciejewska D, Ryterska K, Czerwińka-Rogowska M, Jamioł-Milc D, Skonieczna-Żydecka K, Milkiewicz P, Raszeja-Wyszomirska J, Stachowska E. Gut permeability might be improved by dietary fiber in individuals with nonalcoholic fatty liver disease (NAFLD) undergoing weight reduction. Nutrients 10: 1793, 2018. doi: 10.3390/nu10111793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnan M, Penrose HM, Shah NN, Marchelletta RR, McCole DF. VSL#3 probiotic stimulates T-cell protein tyrosine phosphatase-mediated recovery of IFN-γ-induced intestinal epithelial barrier defects. Inflamm Bowel Dis 22: 2811–2823, 2016. doi: 10.1097/MIB.0000000000000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, Walter J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6: 121, 2018. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kubota H, Chiba H, Takakuwa Y, Osanai M, Tobioka H, Kohama G, Mori M, Sawada N. Retinoid X receptor α and retinoic acid receptor γ mediate expression of genes encoding tight-junction proteins and barrier function in F9 cells during visceral endodermal differentiation. Exp Cell Res 263: 163–172, 2001. doi: 10.1006/excr.2000.5113. [DOI] [PubMed] [Google Scholar]
- 100.Kühne H, Hause G, Grundmann SM, Schutkowski A, Brandsch C, Stangl GI. Vitamin D receptor knockout mice exhibit elongated intestinal microvilli and increased ezrin expression. Nutr Res 36: 184–192, 2016. doi: 10.1016/j.nutres.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 101.L’Huillier C, Jarbeau M, Achamrah N, Belmonte L, Amamou A, Nobis S, Goichon A, Salameh E, Bahlouli W, do Rego JL, Déchelotte P, Coëffier M. Glutamine, but not branched-chain amino acids, restores intestinal barrier function during activity-based anorexia. Nutrients 11: 1348, 2019. doi: 10.3390/nu11061348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22: 598–605, 2016. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 135: 194–204.e3, 2008. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li M, Oshima T, Ito C, Yamada M, Tomita T, Fukui H, Miwa H. Glutamine blocks interleukin-13-induced intestinal epithelial barrier dysfunction. Digestion 1–10, 2019. doi: 10.1159/000502953. [DOI] [PubMed] [Google Scholar]
- 105.Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol 287: G726–G733, 2004. doi: 10.1152/ajpgi.00012.2004. [DOI] [PubMed] [Google Scholar]
- 106.Li N, Neu J. Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr 139: 710–714, 2009. doi: 10.3945/jn.108.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang H, Dai Z, Kou J, Sun K, Chen J, Yang Y, Wu G, Wu Z. Dietary l-tryptophan supplementation enhances the intestinal mucosal barrier function in weaned piglets: implication of tryptophan-metabolizing microbiota. Int J Mol Sci 20: 20, 2018. doi: 10.3390/ijms20010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lima AA, Brito LF, Ribeiro HB, Martins MC, Lustosa AP, Rocha EM, Lima NL, Monte CM, Guerrant RL. Intestinal barrier function and weight gain in malnourished children taking glutamine supplemented enteral formula. J Pediatr Gastroenterol Nutr 40: 28–35, 2005. doi: 10.1097/00005176-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 109.Lima AA, Soares AM, Lima NL, Mota RM, Maciel BL, Kvalsund MP, Barrett LJ, Fitzgerald RP, Blaner WS, Guerrant RL. Effects of vitamin A supplementation on intestinal barrier function, growth, total parasitic, and specific Giardia spp infections in Brazilian children: a prospective randomized, double-blind, placebo-controlled trial. J Pediatr Gastroenterol Nutr 50: 309–315, 2010. doi: 10.1097/MPG.0b013e3181a96489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu L, Liu Z, Li H, Cao Z, Li W, Song Z, Li X, Lu A, Lu C, Liu Y. Naturally occurring TPE-CA maintains gut microbiota and bile acids homeostasis via FXR signaling modulation of the liver-gut axis. Front Pharmacol 11: 12, 2020. doi: 10.3389/fphar.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23: 705–715, 2018. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 112.Mallmann NH, Lima ES, Lalwani P. Dysregulation of tryptophan catabolism in metabolic syndrome. Metab Syndr Relat Disord 16: 135–142, 2018. doi: 10.1089/met.2017.0097. [DOI] [PubMed] [Google Scholar]
- 113.Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics. Best Pract Res Clin Gastroenterol 18: 287–298, 2004. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 114.McOmber ME, Ou CN, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr 50: 269–275, 2010. doi: 10.1097/MPG.0b013e3181aa3aa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296: G1140–G1149, 2009. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 116.Mensink MA, Frijlink HW, van der Voort Maarschalk K, Hinrichs WLJ. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr Polym 130: 405–419, 2015. doi: 10.1016/j.carbpol.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 117.Menta PLR, Andrade MER, Leocádio PCL, Fraga JR, Dias MTS, Cara DC, Cardoso VN, Borges LF, Capettini LSA, Aguilar EC, Alvarez-Leite JI. Wheat gluten intake increases the severity of experimental colitis and bacterial translocation by weakening of the proteins of the junctional complex. Br J Nutr 121: 361–373, 2019. doi: 10.1017/S0007114518003422. [DOI] [PubMed] [Google Scholar]
- 118.Mörkl S, Lackner S, Meinitzer A, Mangge H, Lehofer M, Halwachs B, Gorkiewicz G, Kashofer K, Painold A, Holl AK, Bengesser SA, Müller W, Holzer P, Holasek SJ. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr 57: 2985–2997, 2018. doi: 10.1007/s00394-018-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mujagic Z, de Vos P, Boekschoten MV, Govers C, Pieters HH, de Wit NJ, Bron PA, Masclee AA, Troost FJ. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep 7: 40128, 2017. doi: 10.1038/srep40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mumy KL, Chen X, Kelly CP, McCormick BA. Saccharomyces boulardii interferes with Shigella pathogenesis by postinvasion signaling events. Am J Physiol Gastrointest Liver Physiol 294: G599–G609, 2008. doi: 10.1152/ajpgi.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285: G1028–G1036, 2003. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 122.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 6: e20944, 2011. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nunes C, Freitas V, Almeida L, Laranjinha J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: implications for intestinal inflammation. Food Funct 10: 1364–1374, 2019. doi: 10.1039/C8FO02469C. [DOI] [PubMed] [Google Scholar]
- 124.O’Connor CJ, Wallace RG, Iwamoto K, Taguchi T, Sunamoto J. Bile salt damage of egg phosphatidylcholine liposomes. Biochim Biophys Acta 817: 95–102, 1985. doi: 10.1016/0005-2736(85)90072-0. [DOI] [PubMed] [Google Scholar]
- 125.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 14: 9–21, 2017. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olguin F, Araya M, Hirsch S, Brunser O, Ayala V, Rivera R, Gotteland M. Prebiotic ingestion does not improve gastrointestinal barrier function in burn patients. Burns 31: 482–488, 2005. doi: 10.1016/j.burns.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 127.Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr 143: 1679–1686, 2013. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ott B, Skurk T, Lagkouvardos L, Fischer S, Büttner J, Lichtenegger M, Clavel T, Lechner A, Rychlik M, Haller D, Hauner H. Short-term overfeeding with dairy cream does not modify gut permeability, the fecal microbiota, or glucose metabolism in young healthy men. J Nutr 148: 77–85, 2018. doi: 10.1093/jn/nxx020. [DOI] [PubMed] [Google Scholar]
- 129.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8: 80–93, 2015. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742–747, 2000. doi: 10.1016/S0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 131.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142: 1100–1101.e2, 2012. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 30: 135–139, 2004. doi: 10.1016/j.burns.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 133.Peng Z, Ban K, Sen A, Grill R, Park P, Costantini TW, Kozar R. Syndecan 1 plays a novel role in enteral glutamine’s gut-protective effects of the postischemic gut. Shock 38: 57–62, 2012. doi: 10.1097/SHK.0b013e31825a188a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153, 2014. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 135.Quigley EM. Leaky gut—concept or clinical entity? Curr Opin Gastroenterol 32: 74–79, 2016. doi: 10.1097/MOG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 136.Raftery T, Martineau AR, Greiller CL, Ghosh S, McNamara D, Bennett K, Meddings J, O’Sullivan M. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J 3: 294–302, 2015. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol 294: G906–G913, 2008. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 138.Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci 9: 99–107, 2013. doi: 10.2174/1573401311309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ren W, Chen S, Yin J, Duan J, Li T, Liu G, Feng Z, Tan B, Yin Y, Wu G. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr 144: 988–995, 2014. doi: 10.3945/jn.114.192120. [DOI] [PubMed] [Google Scholar]
- 140.Russo F, Linsalata M, Clemente C, Chiloiro M, Orlando A, Marconi E, Chimienti G, Riezzo G. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr Res 32: 940–946, 2012. doi: 10.1016/j.nutres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 141.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, Campbell EL, Kuhn KA, Furuta GT, Colgan SP, Glover LE. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell 26: 2252–2262, 2015. doi: 10.1091/mbc.E14-07-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sahasrabudhe NM, Beukema M, Tian L, Troost B, Scholte J, Bruininx E, Bruggeman G, van den Berg M, Scheurink A, Schols HA, Faas MM, de Vos P. Dietary fiber pectin directly blocks Toll-like receptor 2-1 and prevents doxorubicin-induced ileitis. Front Immunol 9: 383, 2018. doi: 10.3389/fimmu.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salden BN, Troost FJ, Wilms E, Truchado P, Vilchez-Vargas R, Pieper DH, Jáuregui R, Marzorati M, van de Wiele T, Possemiers S, Masclee AA. Reinforcement of intestinal epithelial barrier by arabinoxylans in overweight and obese subjects: a randomized controlled trial: Arabinoxylans in gut barrier. Clin Nutr 37: 471–480, 2018. doi: 10.1016/j.clnu.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 144.Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis 46, Suppl 2: S58–S61, 2008. doi: 10.1086/523341. [DOI] [PubMed] [Google Scholar]
- 145.Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI, Esposito P, Ferraraccio F, Cartenì M, Riegler G, de Magistris L, Fasano A. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med 9: 23, 2011. doi: 10.1186/1741-7015-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schlingmann B, Molina SA, Koval M. Claudins: gatekeepers of lung epithelial function. Semin Cell Dev Biol 42: 47–57, 2015. doi: 10.1016/j.semcdb.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics–approaching a definition. Am J Clin Nutr 73, Suppl: 361S–364S, 2001. doi: 10.1093/ajcn/73.2.361s. [DOI] [PubMed] [Google Scholar]
- 148.Sellmann C, Degen C, Jin CJ, Nier A, Engstler AJ, Hasan Alkhatib D, De Bandt JP, Bergheim I. Oral arginine supplementation protects female mice from the onset of non-alcoholic steatohepatitis. Amino Acids 49: 1215–1225, 2017. doi: 10.1007/s00726-017-2423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Seth A, Basuroy S, Sheth P, Rao RK. l-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 287: G510–G517, 2004. doi: 10.1152/ajpgi.00058.2004. [DOI] [PubMed] [Google Scholar]
- 150.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20: 779–786, 2014. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307: 1955–1959, 2005. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 153.Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest 92: 1020–1032, 2012. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- 154.Stärkel P, Leclercq S, de Timary P, Schnabl B. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci (Lond) 132: 199–212, 2018. doi: 10.1042/CS20171055. [DOI] [PubMed] [Google Scholar]
- 155.Stevens Y, Rymenant EV, Grootaert C, Camp JV, Possemiers S, Masclee A, Jonkers D. The intestinal fate of citrus flavanones and their efects on gastrointestinal health. Nutrients 11: 1464, 2019. doi: 10.3390/nu11071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24: 539–577, 2004. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 157.Sukhotnik I, Helou H, Mogilner J, Lurie M, Bernsteyn A, Coran AG, Shiloni E. Oral arginine improves intestinal recovery following ischemia-reperfusion injury in rat. Pediatr Surg Int 21: 191–196, 2005. doi: 10.1007/s00383-004-1318-0. [DOI] [PubMed] [Google Scholar]
- 158.Sun Z, Lasson A, Olanders K, Deng X, Andersson R. Gut barrier permeability, reticuloendothelial system function and protease inhibitor levels following intestinal ischaemia and reperfusion–effects of pretreatment with N-acetyl-l-cysteine and indomethacin. Dig Liver Dis 34: 560–569, 2002. doi: 10.1016/S1590-8658(02)80089-5. [DOI] [PubMed] [Google Scholar]
- 159.Sun Z, Wang X, Deng X, Lasson A, Wallén R, Hallberg E, Andersson R. The influence of intestinal ischemia and reperfusion on bidirectional intestinal barrier permeability, cellular membrane integrity, proteinase inhibitors, and cell death in rats. Shock 10: 203–212, 1998. doi: 10.1097/00024382-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 160.Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet 6: 81, 2015. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol 10: 2113, 2019. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teixeira TF, Collado MC, Ferreira CL, Bressan J, Peluzio MC. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res 32: 637–647, 2012. doi: 10.1016/j.nutres.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 163.Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, Katan MB, van der Meer R. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J Nutr 136: 70–74, 2006. doi: 10.1093/jn/136.1.70. [DOI] [PubMed] [Google Scholar]
- 164.Terciolo C, Dapoigny M, Andre F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption. Clin Exp Gastroenterol 12: 67–82, 2019. doi: 10.2147/CEG.S181590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383, 2018. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 166.Thurnham DI, Northrop-Clewes CA, McCullough FS, Das BS, Lunn PG. Innate immunity, gut integrity, and vitamin A in Gambian and Indian infants. J Infect Dis 182, Suppl 1: S23–S28, 2000. doi: 10.1086/315912. [DOI] [PubMed] [Google Scholar]
- 167.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 138: 1452–1455, 2008. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 168.Tuomi K, Logomarsino JV. Bacterial lipopolysaccharide, lipopolysaccharide-binding protein, and other inflammatory markers in obesity and after bariatric surgery. Metab Syndr Relat Disord 14: 279–288, 2016. doi: 10.1089/met.2015.0170. [DOI] [PubMed] [Google Scholar]
- 169.van Ampting MT, Schonewille AJ, Vink C, Brummer RJ, van der Meer R, Bovee-Oudenhoven IM. Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol 9: 6, 2009. doi: 10.1186/1472-6793-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vancamelbeke M, Laeremans T, Vanhove W, Arnauts K, Ramalho AS, Farré R, Cleynen I, Ferrante M, Vermeire S. Butyrate does not protect against inflammation-induced loss of epithelial barrier function and cytokine production in primary cell monolayers from patients with ulcerative colitis. J Crohn’s Colitis 13: 1351–1361, 2019. doi: 10.1093/ecco-jcc/jjz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Varasteh S, Braber S, Kraneveld AD, Garssen J, Fink-Gremmels J. l-Arginine supplementation prevents intestinal epithelial barrier breakdown under heat stress conditions by promoting nitric oxide synthesis. Nutr Res 57: 45–55, 2018. doi: 10.1016/j.nutres.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 172.Vargas-Robles H, Castro-Ochoa KF, Citalán-Madrid AF, Schnoor M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J Gastroenterol 25: 4181–4198, 2019. doi: 10.3748/wjg.v25.i30.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, Burton D, Zinsmeister AR. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144: 903–911.e3, 2013. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, O’Neill J, Carlson P, Lamsam J, Eckert D, Janzow D, Burton D, Ryks M, Rhoten D, Zinsmeister AR. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol 303: G1262–G1269, 2012. doi: 10.1152/ajpgi.00294.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: 296–310, 2014. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Viana ML, Santos RG, Generoso SV, Arantes RM, Correia MI, Cardoso VN. Pretreatment with arginine preserves intestinal barrier integrity and reduces bacterial translocation in mice. Nutrition 26: 218–223, 2010. doi: 10.1016/j.nut.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 177.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol 20: 688–692, 2008. doi: 10.1097/MEG.0b013e3282f5197e. [DOI] [PubMed] [Google Scholar]
- 178.Vogt LM, Sahasrabudhe NM, Ramasamy U, Meyer D, Pullens G, Faas MM, Venema K, Schols HA, de Vos P. The impact of lemon pectin characteristics on TLR activation and T84 intestinal epithelial cell barrier function. J Funct Foods 22: 398–407, 2016. doi: 10.1016/j.jff.2016.02.002. [DOI] [Google Scholar]
- 179.Volynets V, Louis S, Pretz D, Lang L, Ostaff MJ, Wehkamp J, Bischoff SC. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a Western-style diet or drinking water supplemented with fructose. J Nutr 147: 770–780, 2017. doi: 10.3945/jn.116.242859. [DOI] [PubMed] [Google Scholar]
- 180.Wang B, Wu G, Zhou Z, Dai Z, Sun Y, Ji Y, Li W, Wang W, Liu C, Han F, Wu Z. Glutamine and intestinal barrier function. Amino Acids 47: 2143–2154, 2015. doi: 10.1007/s00726-014-1773-4. [DOI] [PubMed] [Google Scholar]
- 181.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci 57: 3126–3135, 2012. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 182.Wedlake L, Shaw C, McNair H, Lalji A, Mohammed K, Klopper T, Allan L, Tait D, Hawkins M, Somaiah N, Lalondrelle S, Taylor A, VanAs N, Stewart A, Essapen S, Gage H, Whelan K, Andreyev HJN. Randomized controlled trial of dietary fiber for the prevention of radiation-induced gastrointestinal toxicity during pelvic radiotherapy. Am J Clin Nutr 106: 849–857, 2017. doi: 10.3945/ajcn.116.150565. [DOI] [PubMed] [Google Scholar]
- 183.West NP, Pyne DB, Cripps AW, Christophersen CT, Conlon MA, Fricker PA. Gut balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 3: 221–227, 2012. doi: 10.4161/gmic.19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Westerbeek EA, van den Berg A, Lafeber HN, Fetter WP, van Elburg RM. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br J Nutr 105: 268–274, 2011. doi: 10.1017/S0007114510003405. [DOI] [PubMed] [Google Scholar]
- 185.Wieser H. Chemistry of gluten proteins. Food Microbiol 24: 115–119, 2007. doi: 10.1016/j.fm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 186.Wilms E, Gerritsen J, Smidt H, Besseling-van der Vaart I, Rijkers GT, Garcia Fuentes AR, Masclee AA, Troost FJ. Effects of supplementation of the synbiotic ecologic® 825/FOS P6 on intestinal barrier function in healthy humans: a randomized controlled trial. PLoS One 11: e0167775, 2016. doi: 10.1371/journal.pone.0167775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wilms E, Jonkers DMAE, Savelkoul HFJ, Elizalde M, Tischmann L, de Vos P, Masclee AAM, Troost FJ. The impact of pectin supplementation on intestinal barrier function in healthy young adults and healthy elderly. Nutrients 11: 1554, 2019. doi: 10.3390/nu11071554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu RL, Vazquez-Roque MI, Carlson P, Burton D, Grover M, Camilleri M, Turner JR. Gluten-induced symptoms in diarrhea-predominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab Invest 97: 14–23, 2017. doi: 10.1038/labinvest.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu X, Vallance BA, Boyer L, Bergstrom KS, Walker J, Madsen K, O’Kusky JR, Buchan AM, Jacobson K. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol 294: G295–G306, 2008. doi: 10.1152/ajpgi.00173.2007. [DOI] [PubMed] [Google Scholar]
- 190.Xia Z, Huang L, Yin P, Liu F, Liu Y, Zhang Z, Lin J, Zou W, Li C. l-Arginine alleviates heat stress-induced intestinal epithelial barrier damage by promoting expression of tight junction proteins via the AMPK pathway. Mol Biol Rep 46: 6435–6451, 2019. doi: 10.1007/s11033-019-05090-1. [DOI] [PubMed] [Google Scholar]
- 191.Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 53: 96–105, 2011. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang K, Zhu J, Wu J, Zhong Y, Shen X, Petrov B, Cai W. Maternal vitamin D deficiency increases intestinal permeability and programs Wnt/β-catenin pathway in BALB/C mice. JPEN J Parenter Enteral Nutr. In press. doi: 10.1002/jpen.1820. [DOI] [PubMed] [Google Scholar]
- 193.Yang Y, Zhang Y, Xu Y, Luo T, Ge Y, Jiang Y, Shi Y, Sun J, Le G. Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high-fat-fed mice. Food Funct 10: 5952–5968, 2019. doi: 10.1039/C9FO00766K. [DOI] [PubMed] [Google Scholar]
- 194.Yun JW. Fructooligosaccharides–Occurrence, preparation, and application. Enzyme Microb Technol 19: 107–117, 1996. doi: 10.1016/0141-0229(95)00188-3. [DOI] [Google Scholar]
- 195.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 119: 744–755, 2000. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 196.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4: 232–241, 2010. [Erratum in ISME J 4: 312–313, 2010.] doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 197.Zhang DM, Jiao RQ, Kong LD. High dietary fructose: direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients 9: 335, 2017. doi: 10.3390/nu9040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol 12: 57, 2012. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao W, Han L, Bae Y, Manickam DS. Lucifer yellow–a robust paracellular permeability marker in a cell model of the human blood-brain barrier. J Vis Exp Aug. 19, 2019. doi: 10.3791/58900. [DOI] [PubMed] [Google Scholar]
- 200.Zhong Y, Cai D, Cai W, Geng S, Chen L, Han T. Protective effect of galactooligosaccharide-supplemented enteral nutrition on intestinal barrier function in rats with severe acute pancreatitis. Clin Nutr 28: 575–580, 2009. doi: 10.1016/j.clnu.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 201.Zhou Q, Verne ML, Fields JZ, Lefante JJ, Basra S, Salameh H, Verne GN. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 68: 996–1002, 2019. doi: 10.1136/gutjnl-2017-315136. [DOI] [PMC free article] [PubMed] [Google Scholar]