Abstract
Stopping action depends on the integrity of the right inferior frontal gyrus (rIFG). Electrocorticography from the rIFG shows an increase in beta power during action stopping. Scalp EEG shows a similar right frontal beta increase, but it is unknown whether this beta modulation relates to the underlying rIFG network. Demonstrating a causal relationship between the rIFG and right frontal beta in EEG during action stopping is important for putting this electrophysiological marker on a firmer footing. In a double-blind study with a true sham coil, we used fMRI-guided 1-Hz repetitive transcranial magnetic stimulation (rTMS) to disrupt the rIFG and to test whether this reduced right frontal beta and impaired action stopping. We found that rTMS selectively slowed stop signal reaction time (SSRT) (no effect on Go) and reduced right frontal beta (no effect on sensorimotor mu/beta related to Go); it also reduced the variance of a single-trial muscle marker of stopping. Surprisingly, sham stimulation also slowed SSRTs and reduced beta. Part of this effect, however, resulted from carryover of real stimulation in participants who received real stimulation first. A post hoc between-group comparison of those participants who received real first compared with those who received sham first showed that real stimulation reduced beta significantly more. Thus, real rTMS uniquely affected metrics of stopping in the muscle and resulted in a stronger erosion of beta. We argue that this causal test validates right frontal beta as a functional marker of action stopping.
NEW & NOTEWORTHY Action stopping recruits the right inferior frontal gyrus (rIFG) and elicits increases in right frontal beta. The present study now provides causal evidence linking these stopping-related beta oscillations to the integrity of the underlying rIFG network. One-hertz transcranial magnetic stimulation (TMS) over the rIFG impaired stopping and reduced right frontal beta during a stop-signal task. Furthermore, the effect on neural oscillations was specific to stopping-related beta, with no change in sensorimotor mu/beta corresponding to the Go response.
Keywords: inhibitory control, oscillations, stop-signal task, transcranial magnetic stimulation
INTRODUCTION
Action stopping is a critical component of evervyday cognitive control that allows us to rapidly cancel inappropriate responses and adapt to changes in our goals and environment. The ability to quickly stop an action is thought to require a right lateralized fronto-basal ganglia network in which the right inferior frontal gyrus (rIFG) is a critical node (reviewed by Aron et al. 2014; Bari and Robbins 2013; Cai et al. 2014; Coxon et al. 2009, 2012; Garavan et al. 1999; Jahanshahi et al. 2015). Identifying a scalp electrophysiological marker of this network would provide a high-temporal resolution, portable, and inexpensive tool. For example, it could be used for testing recent theories that a right frontal executive system is also involved in stopping memory retrieval, emotional responsiveness, and gait (Berkman et al. 2009; Castiglione et al. 2019; Depue et al. 2007; Guo et al. 2018; Wagner et al. 2016).
An apparent electrophysiological marker of the rIFG-mediated action-stopping process is increased beta band power (∼16 Hz). Evidence for this comes from both electrocorticography (ECoG) recorded from the rIFG (Swann et al. 2009; Wessel et al. 2013) and scalp EEG studies that recorded neural activity during a stop-signal task (Hannah et al. 2020; Jana et al. 2020; Wagner et al. 2018). In a stop-signal task, participants press a button to respond to Go cues (i.e., a rightward or leftward pointing arrow). On a minority of trials (i.e., 25%), a stop signal occurs, requiring participants to try to rapidly cancel their prepotent response. Critically, for the abovementioned EEG and ECoG studies, the beta increase occurred in a specific time window important for stopping, i.e., after the stop signal appears and before the participant’s stop signal reaction time (SSRT), a latent measure of the stopping process. Although ECoG provides a more anatomically specific link between stopping-related beta and the rIFG compared with EEG, neither method shows that these beta modulations depend on the integrity of the rIFG during stopping. Another benefit of better understanding the role of this oscillatory signature and how it relates to the underlying network is the potential to entrain neural oscillations and hypothetically affect inhibitory control.
Here we used fMRI-guided 1-Hz repetitive transcranial magnetic stimulation (rTMS) to disrupt the rIFG and to test whether this selectively reduces right frontal beta power and stopping performance in a simple stop-signal task. Although prior studies have shown impaired stopping after 1-Hz rTMS over rIFG (Chambers et al. 2006, 2007; for meta-analysis see Yang et al. 2018), here we present the first study to use a “true sham” double-blind approach (that also elicits facial discomfort) and to test how disruption of the rIFG affects right frontal beta in EEG during action stopping.
In the present study, each participant first underwent fMRI while performing the stop-signal task. We used participant-specific activation maps and structural scans to localize our rIFG target stimulation site. On a subsequent visit, the participants underwent a TMS-EEG protocol. This started with a baseline session of the stop-signal task with EEG recorded. There then followed, in each participant, two TMS sessions, either in the order real TMS then sham TMS or the order sham TMS then real TMS, on the same day for each participant, with a 30-min “washout period.” For each of these TMS sessions, we first delivered 1-Hz rTMS over the rIFG and then recorded scalp EEG during the stop-signal task. Although ideally sham and real stimulation would have been conducted on two separate days, we did them here on the same day in order to have a single right frontal EEG spatial filter per subject that we could compare across task sessions.1 Furthermore, although the duration of 1-Hz rTMS effects varies across studies, stimulation sites, and TMS protocols, multiple studies report effects that last <30 min (Boroojerdi et al. 2000; Chen et al. 1997; Lang et al. 2008; Nyffeler et al. 2006; but also see Siebner et al. 2003). Even so, we did anticipate, in our preregistration document, that we might have to deal with an order effect, i.e., that real TMS would “leak” into the sham session.
Our neural measures were beta activity in a right frontal spatial filter to track changes in stopping-related beta and mu/beta activity in a left sensorimotor spatial filter (contralateral to the responding hand) to test for effects on Go-related neural activity.
Behaviorally, our measures were stop signal reaction time (SSRT) and Go latency. We also included an electromyography (EMG) metric of stopping based on recent work (Jana et al. 2020) showing that this indexes a pseudo-single-trial metric of stopping latency called “CancelTime” (also see Raud et al. 2020; Raud and Huster 2017; Thunberg et al. 2019). EMG CancelTime reflects the time at which EMG activity is shut down on stop trials that show a small burst of muscle activity that is subsequently silenced as the action is successfully withheld.
We preregistered the following key predictions for real versus sham stimulation: 1) a reduction in stopping-related right frontal beta; 2) a prolongation of both behavioral measures of stopping (SSRT and CancelTime); 3) no change in Go behavior or Go-related left sensorimotor mu/beta activity.
MATERIALS AND METHODS
Methods
The study design and predictions were preregistered as an Open Science Framework (OSF) document (https://osf.io/9vynp/?view_only=7ecf843dfcd247eebe4885f4aff39bc5).
Power.
Two prior studies showed that 1-Hz rTMS over rIFG compared with control sites increased SSRT with a combined effect size of 0.76 (Chambers et al. 2006, 2007). Based on this effect size, we estimated a required sample size of 17 (to achieve 90% power) to detect a real vs. sham effect on SSRT with a one-tailed test. However, because we did not have sufficient information for a power analysis of our EEG hypothesis, we planned to collect 30 participants.
Participants.
Per our preregistration document, we aimed to analyze 30 participants who had a right frontal component to test our EEG hypothesis. However, we anticipated running more participants, as a right frontal spatial filter is typically found in ∼85% of people for this task (Wagner et al. 2018). Participants completed three visits: an orientation visit with a rTMS demonstration (visit 1), a fMRI visit to localize the site of stimulation (visit 2), and the EEG-rTMS visit (visit 3). Five participants withdrew from the study after the rTMS demonstration on visit 1 because of a level of discomfort that would be intolerable on visit 3 (see visit 1: safety screening and rtms demonstration below for more details). One participant withdrew after visit 2 because of a head injury that occurred outside of the study. In total, we collected fMRI, EEG, and behavioral data in 33 participants to achieve the 30-participant goal for EEG analysis. All 33 were included in the behavioral analysis (18 women, 15 men; right-handed; mean age = 20 yr, SD = 1.91 yr). All participants provided written informed consent according to a protocol approved by the University of California San Diego (UCSD). They were compensated $20/h.
Visit details.
visit 1: safety screening and rtms demonstration.
Participants came to the laboratory for an fMRI and TMS safety screening and for a demonstration of the 1-Hz rTMS procedure. Safety screening confirmed that all participants met our fMRI and TMS safety requirements. To demonstrate the rTMS procedure, we first delivered single-pulse TMS over left primary motor cortex (M1) to estimate each participant’s resting motor threshold (RMT). We then delivered a brief train of rTMS over rIFG at 110% of this RMT value to ascertain whether it would be tolerable for them in visit 3. If it was too uncomfortable, the participant was withdrawn from the study.
visit 2: fmri scanning.
fMRI was used to identify an rTMS target for each participant for visit 3 (Fig. 1C). We scanned participants with a 3T GE scanner at the Center for Functional Magnetic Resonance Imaging at UCSD. Each scanning session included an anatomical scan and two 6-min blocks of a visual stop-signal task (Fig. 1A) that reliably activates the pars opercularis of the rIFG (Aron and Poldrack 2006). We collected 182 functional T2-weighted echo planar images (EPIs) for each of the task blocks [repetition time (TR) 2 s].
Figure 1.
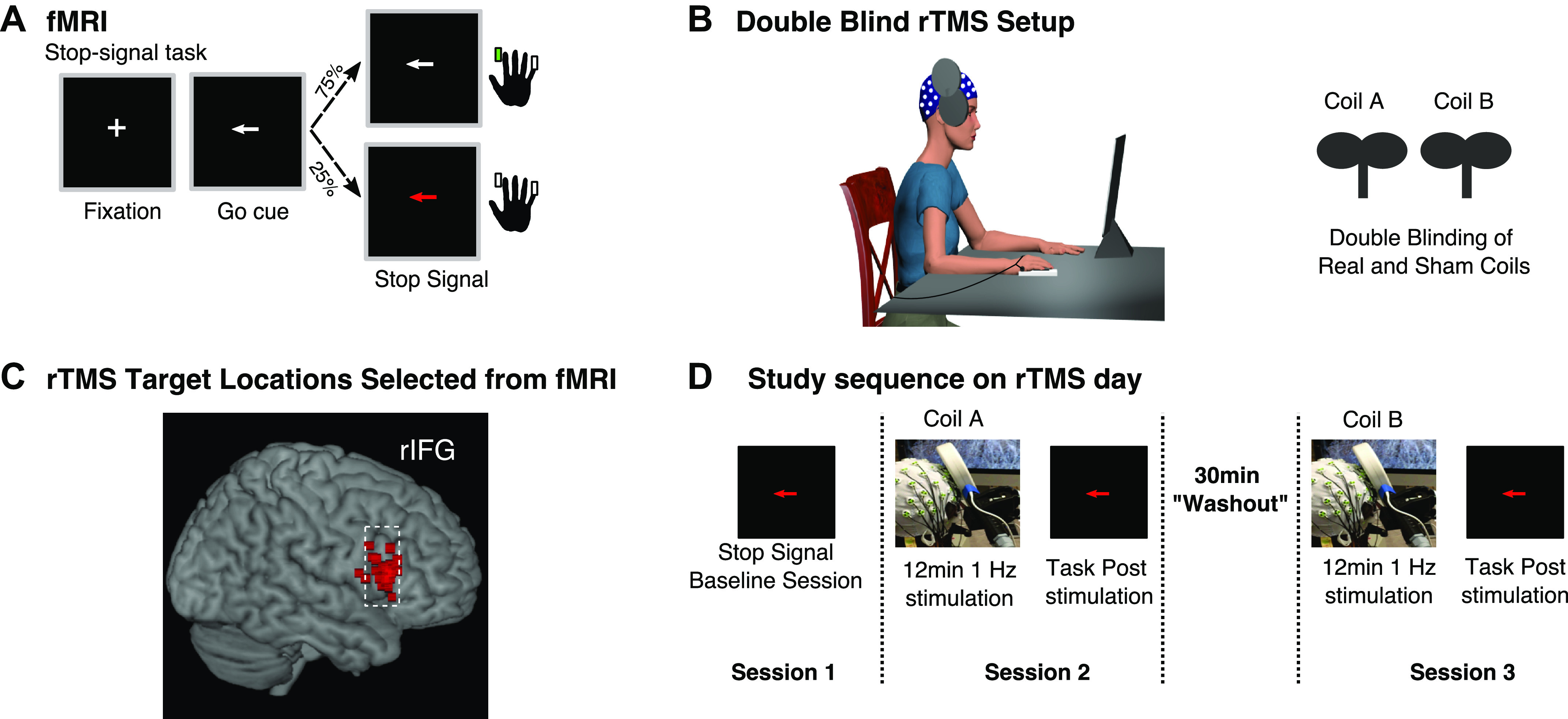
Task, fMRI targeting, and EEG-transcranial magnetic stimulation (TMS) procedure. A: diagram of the stop-signal task. B: example of EEG-repetitive TMS (rTMS) setup and double-blinding procedure. C: rTMS targets selected from voxels in right inferior frontal gyrus (rIFG) with peak activation during the stop-signal task in fMRI scanner. D: sequence of events during the EEG-rTMS study visit.
We used FSL software (https://www.fmrib.ox.ac.uk/fsl) for all preprocessing and analysis steps. To identify the optimal target for rTMS stimulation, we created activation maps by contrasting stop trials versus go trials for each participant. We derived these activation maps with the exact steps described in Aron and Poldrack (2006) except that we included all stop trials for the contrast Stop vs. Go to obtain more observations, as we had only two runs per participant (the typical contrast is Successful Stop vs. Go). We were able to pool across all stop trials for our target localization because the pars opercularis shows activation on both successful and failed stop trials (Aron and Poldrack 2006). Each participant’s activation map was aligned into the participant’s native anatomical space and then masked with the pars opercularis region of interest from the Harvard-Oxford Atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). We selected our specific target by identifying the voxels within the pars opercularis mask that showed peak activation related to stopping. For one participant who had no discernible right pars opercularis activation, apparently because of poor task performance, we used the Montreal Neurological Institute (MNI) coordinates (x: 61, y: 21, z: 13) reported by Chambers et al. (2006), a prior study that used similar rTMS procedures to target the rIFG.
visit 3: rtms and eeg.
The visit 3 procedure used two TMS coils (PowerMag Lab100; MAG & More GmbH, Munich, Germany): a real coil (Double coil PMD70-pCool) and a sham coil (Double coil PMD70-pCool-Sham). The position of the coil was guided with neuronavigation (Brainsight, Rogue Research Inc., Montreal, QC, Canada; Brain Science Tools, The Netherlands) to the pars opercularis activation spot. The sham coil is designed to produce physical sensations (i.e., facial twitches) that are comparable to the real coil, allowing for a true double-blind procedure. At the start of visit 3, we first placed an EEG cap on each participant. Next, we determined the RMT by delivering single-pulse TMS over left M1, over the EEG cap. RMT was defined as the lowest pulse intensity to produce a motor evoked potential (MEP) of at least 0.05 mV in 5 of 10 consecutive pulses. The subsequent rTMS intensity was set to 110% of each participant’s RMT. After EEG capping and the TMS threshold procedure, participants performed several minutes of practice (80 total practice trials) of the stop-signal task. After practice, the main experimental procedure was as follows: 1) A designated laboratory member (not the experimenter delivering TMS) labeled the sham and real TMS coils (coil A and coil B according to the blinding document) while the experimenter waited outside of the room (Fig. 1B). 2) The participant completed a 12-min baseline session of the stop-signal task. This first task session provided baseline measurements for stopping performance as measured by SSRT and EMG CancelTimes and the stopping-related right frontal beta signature. 3) Twelve minutes of 1-Hz rTMS was delivered with coil A. 4) Immediately afterwards, the participant performed another 12-min session of the stop-signal task as we recorded EEG. 5) The participant then rested for a 30-min “washout” period (ideally this would be much longer, to ensure that the effects of the real coil dissipate, but the overall session was already several hours long). 6) Twelve minutes of 1-Hz rTMS stimulation was then done with coil B. 7) Immediately afterwards, the participant performed another 12-min session of the stop-signal task as we recorded EEG (Fig. 1D). Overall, visit 3 typically lasted between 3.5 and 4.5 h depending on a number of factors (especially the time it took to cap with EEG, to identify the M1 hotspot, to find the resting motor threshold, and to implement the Brainsight TMS coil localization method).
In all three task sessions, participants responded to the Go cues on a button box with their right first dorsal interosseus (FDI; index finger) for rightward arrows and their right abductor digiti minimi (ADM; pinky finger) for leftward arrows. We placed surface EMG electrodes on both of these task-relevant muscles to record EMG as participants performed the task (see Fig. 6A). Requiring participants to respond with their pinky and index fingers was important because it allowed us to record EMG data from two task-relevant muscles in the same hand.
Figure 6.
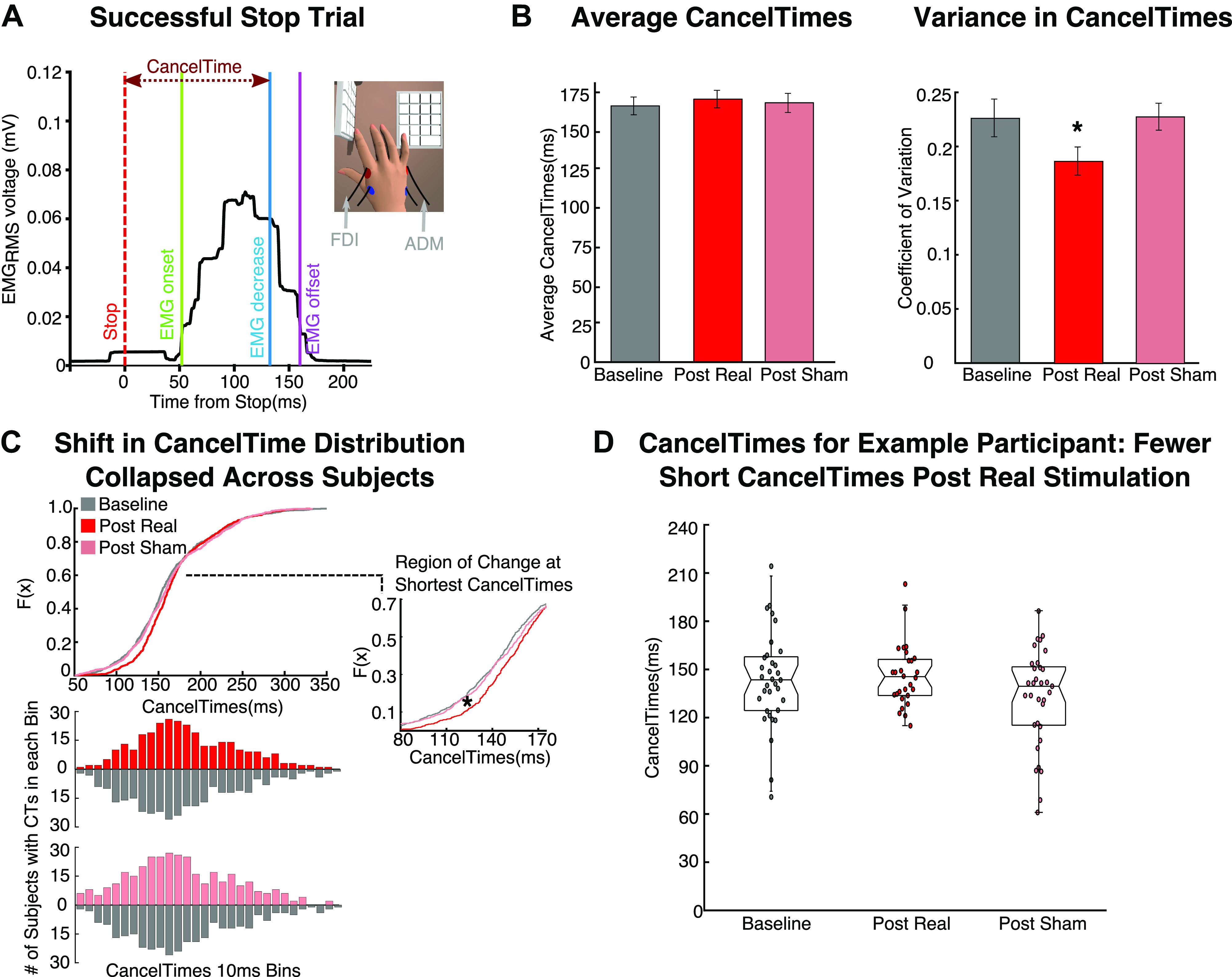
Analysis of the CancelTime metric of stopping from electromyography. A: example EMG recording from the responding hand on a successful stop trial with partial muscle activity. EMG onset marks the earliest detection of muscle activity after the stop signal. The CancelTime is the point at which EMG activity begins to decrease from the peak activity on trials when the action was successfully canceled. CancelTimes served as an alternative metric of stopping speed. RMS, root mean square. B: there was no difference between sessions in average CancelTimes (left), but there was in CancelTime variability measured by the coefficient of variation (right). C, top: the cumulative distribution function (CDF) illustrates the distribution of CancelTimes for each session (collapsed across all participants). The inset shows the portion of the CDF with a significant shift after real stimulation (i.e., the shortest CancelTimes are less frequent after real stimulation). Bottom: the number of participants contributing to each CancelTime (CT) bin included in the CDF. These data show that fewer participants overall have data points in the earliest CancelTime bins after real stimulation and that the effect on the CDF is not driven by only a few participants. D: example participant illustrating how the shortest CancelTimes are largely abolished after real stimulation.
Stop-signal task (visits 2 and 3).
We used a visual stop-signal task (Logan and Cowan 1984) programmed in MATLAB 2015b (The MathWorks, United States) and Psychtoolbox (Brainard 1997). On each trial participants saw a left or right white arrow on the screen and pressed a button according to the direction of the arrow (Go trials). However, on 25% of the trials (stop trials), the arrow suddenly turned red after a stop signal delay (SSD), indicating that the participant must try to cancel their imminent response. We used an algorithm to adjust the SSD to achieve ∼50% successful stops; this reduced or increased the SSD by 50 ms depending on the outcome of the previous stop trial. Participants were instructed to respond as quickly and as accurately as possible.
On visit 2 (fMRI) there were two blocks of the task, of 128 trials each, of which 32 trials (25%) were stop signal trials and 96 were Go (for more details see Aron and Poldrack 2006). Each of the task sessions during visit 3 (baseline, post coil A, and post coil B) was 12 min long. Each of these sessions had 320 trials, of which 25% were stop signal trials (i.e., 80 stop trials and 240 Go trials per session). Participants received a 10-s break after every 80 trials.
EEG recording (visit 3).
We used a 64-electrode EasyCap slim electrode system (EasyCap and BrainVision actiCHamp amplifier; Brain Products GmbH, Gilching, Germany) with electrode placement in the International 10-20 System and PyCorder (Brain Products) to record the EEG data. Electrode Fpz served as the ground. We recorded the data reference free with EEG electrodes placed bilaterally over mastoids for later off-line re-referencing. We reduced electrode impedances to <10 kΩ before recording. We placed additional electrodes at each canthus and one electrode below the right eye to monitor for eye movements. The EEG and EOG data were sampled at 1,000 Hz.
EMG recording (visit 3).
We used a Grass QP511 AC amplifier (Glass Technologies, West Warwick, RI) with a frequency cutoff between 30 and 1,000 Hz to collect the EMG data. Data were sampled at 2 kHz with a CED Micro 1401 mk II acquisition system and recorded in CED Signal v4 software (Cambridge Electronic Design Limited, Cambridge, UK).
Preregistered Predictions
Behavior.
rtms effects on stop.
We predicted that real rTMS stimulation would impair stopping, reflected by a lengthening of both SSRT and EMG CancelTimes.
rtms effects on go.
We expected no effect on the Go process as indicated by both no change in Go response times and EMG onset times. Alternatively, we speculated that real rTMS could quicken Go responding, on the view that rIFG also acts as a precautionary brake in contexts where stopping might be required (Swann et al. 2012; Wessel et al. 2013). Thus, eroding this brake could result in quicker overall responding.
EEG.
baseline session.
Consistent with our recent report (Wagner et al. 2018), we expected right frontal beta during the baseline session of the stop-signal task (before any stimulation) to increase in power after the stop signal and before SSRT. Furthermore, we expected the stopping-related increase in right frontal beta power to be greater for successful compared with failed stop trials.
rtms effects on stop.
Our core hypothesis was that disrupting rIFG would reduce a putative electrophysiological marker of the executive system, namely, right frontal beta power. Specifically, we expected reduced right frontal beta power in a time window critical for stopping (i.e., after the stop signal and before SSRT) in the task session following real stimulation.
rtms effects on go.
We predicted no change in left sensorimotor mu/beta (7–25 Hz) band desynchronization associated with the Go process for the three task sessions.
Analysis
Behavior.
baseline session exclusion criteria.
We preregistered our plan to exclude any participants who violated the following sanity checks during the baseline session of the stop-signal task: 1) Stop % > 30% and < 70% (percentage of successful stops should be close to 50%), 2) mean response time (RT) < 700 ms (>700-ms RTs indicate a strategy of waiting for the stop signal to appear), 3) Go error rate < 10%, 4) Go RT > Failed Stop RT (this latter is a required assumption of the race model). On the basis of these criteria, all participants were included.
calculating ssrt.
Stop signal reaction time (SSRT) was calculated with the integration method (Verbruggen et al. 2019). This estimates when the stop process finishes by integrating the response time (RT) distribution and identifying the point at which the integral equals P(respond|stop signal). When using a tracking method to adjust stop signal delays during the task, this point in the distribution corresponds to the nth RT, where n = the number of RTs in the distribution multiplied by the overall probability of stopping. SSRT is calculated by subtracting the participants’ mean SSD from the nth RT. For the most reliable and unbiased SSRTs, we included all Go trials with a response in the RT distribution (including incorrect responses). Furthermore, per the recommended methods (Verbruggen et al. 2019), we replaced any Go omission trials with the participant’s maximum RT.
calculating emg canceltime and emg onset.
EMG data were collected to determine EMG onsets, a secondary measure of the Go process, and EMG CancelTimes, a pseudo-single-trial metric of the Stop process. We analyzed the EMG data according to methods described in Jana et al. (2020). EMG data were filtered with a fourth-order Butterworth filter (roll-off 24 dB/octave) to eliminate 60-Hz line noise. The data were then full-wave rectified, and the root mean square (RMS) was computed with a 50 ms centered window. For each trial we selected the EMG peak by identifying periods of EMG activity that exceeded a trial specific threshold. This threshold was set to 8 standard deviations above the trial’s mean baseline EMG activity between the fixation cross and the Go cue. If more than one section of EMG activity exceeded the threshold, we selected the maximum as the peak.
We next examined the EMG activity before the peak and marked the EMG onset as the moment at which EMG activity dipped below 20% of peak amplitude and remained below this threshold for at least 5 ms. Using these trial-specific thresholds provides superior detection of onsets compared with a fixed threshold (e.g., X number of standard deviations above an average) that is applied to all trials and does not account for trial-by-trial noise in the EMG data. Our approach is particularly useful for detecting EMG onsets on the successful stop trials with smaller partial EMG bursts. Next, EMG offset was marked as the moment after the peak EMG when EMG activity decreased for five consecutive milliseconds. We removed trials as outliers if the EMG onset or the EMG offset were 1.5 times the interquartile range (IQR) of the first and third quartiles of their respective distribution. Finally, we marked EMG CancelTimes on all successful stop trials with a partial EMG response. CancelTime reflects the period of time between the stop signal and the point at which the EMG burst starts declining consistently. More specifically, the moment of EMG decline is defined as the first point after the EMG peak where EMG declines consistently for at least five consecutive milliseconds. For CancelTimes, outliers were rejected if they fell below a 50-ms cutoff or above Q3 + 1.5 × IQR.
Because EMG amplitude for the FDI and ADM muscles varied considerably, we normalized the muscle activity by the peak activity for each muscle before averaging the EMG data across the muscles.
Our laboratory developed these EMG analysis methods after we had begun data collection for the present study. Consequently, EMG data were only collected for 29 of the 33 participants who completed the study.
EEG.
preprocessing.
We analyzed the EEG data with EEGLAB 14.1.1b (Delorme and Makeig 2004) in MATLAB 2015b (The MathWorks). Data were downsampled to 512 Hz and low-pass filtered with a MATLAB polyphaser antialiasing filter. We then re-referenced the data to an average of the two mastoid electrodes. We applied a high-pass filter at 2 Hz [finite impulse response (FIR) order 3,300] and a notch filter at 60 and 180 Hz (FIR order 846) to remove electrical noise. We then visually inspected the continuous data and manually rejected any stretches contaminated with excessive artifact. After preprocessing, we concatenated the three sessions of EEG data and submitted it to independent component analysis (ICA) decomposition (Makeig et al. 1996). We identified and removed artifact components (e.g., blinks, muscle tension) before further analysis. After preprocessing, we identified a left sensorimotor and a right frontal spatial filter to test our EEG hypotheses related to the Go and stop processes, respectively. We used linear spatial filters rather than select electrodes to boost the signal-to-noise ratio in these analyses (Cohen 2017).
left sensorimotor filter and go frequency of interest.
Based on the EEG data for Go trials in the stop-signal task (Wagner et al. 2018) and also a substantial literature on EEG correlates of action execution (Kilavik et al. 2013), we expected the Go response to elicit a mu/beta desynchronization in a left sensorimotor component contralateral to the responding hand. Furthermore, we predicted that the effects of rTMS would be specific to the stopping system and not impact this signature of the Go process. To test this prediction, we first identified a left sensorimotor component from the ICA decomposition for each participant. This component was selected according to spatial topography (left central). We then epoched the data relative to the Go cue on all Go trials and generated event-related perturbations (ERSPs) (see Fig. 2A for group ERSP). Relative changes in spectral power were computed by calculating the mean difference between each single-trial log spectrogram and the mean baseline spectrum (the log spectrum 1,000 ms before the Go cue averaged across all trials). We used the ERSP from each participant’s baseline EEG session to identify the frequency of interest, the participant’s mu/beta (7–25 Hz) frequency band that showed the greatest reduction in the time window surrounding the Go response (Go cue to the participant’s average Go RT) (across participants, mean frequency of interest = 13.83, SD = 5.19). We narrowed our time window of interest by identifying all time points between the Go cue and the participant’s average Go response time (using the baseline EEG session) in which the participant’s selected frequency (±2) dropped below zero, indicative of a Go-related desynchronization.
Figure 2.
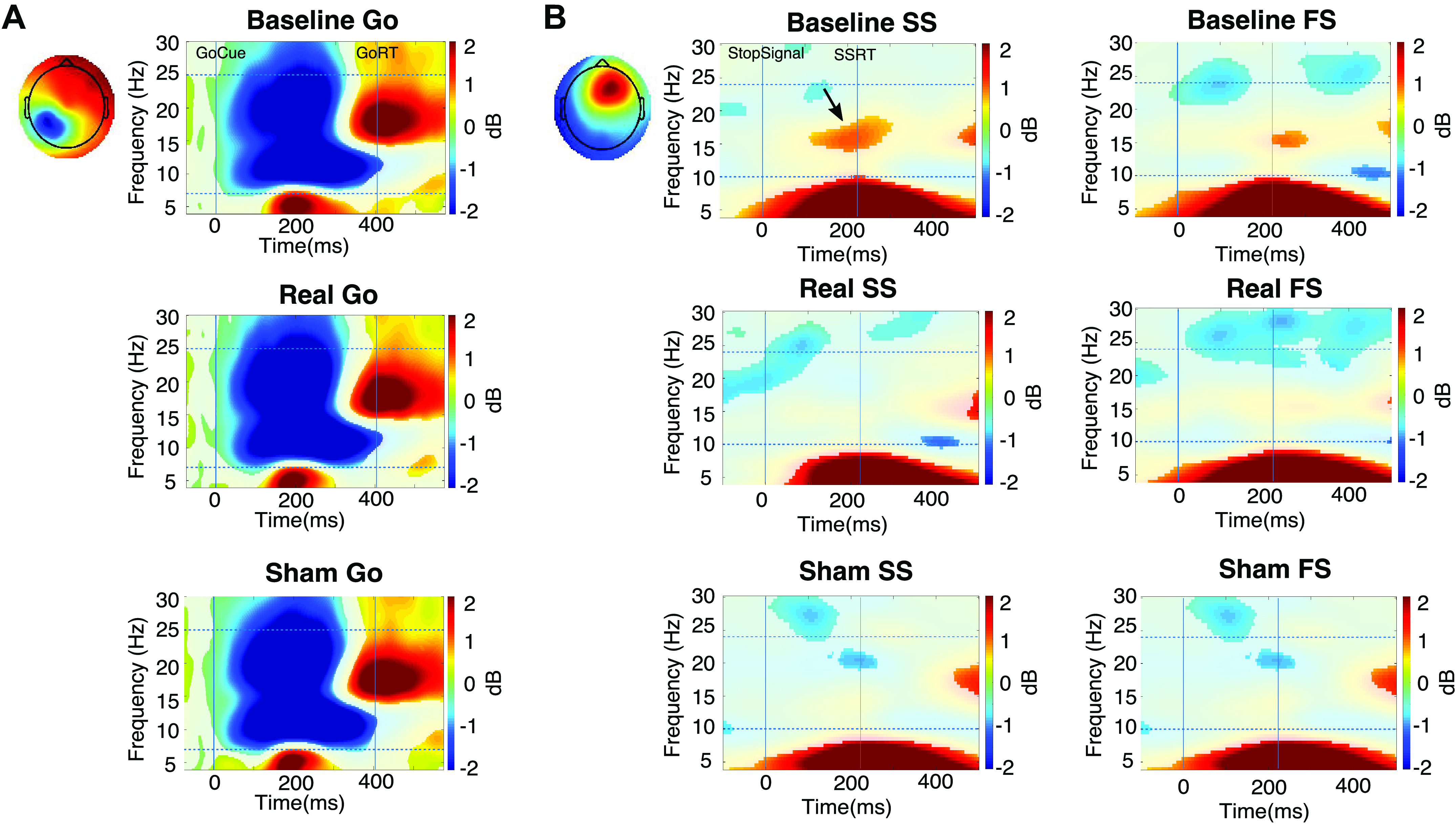
Event-related spectral perturbation (ERSP) results for Going and Stopping. A: group ERSPs for the left sensorimotor filter for Go trials in all 3 task sessions. Time 0 on the x-axis is the time of the Go cue. The ERSP is generated relative to the baseline, which is −1,000 ms before the Go cue. For each of baseline and real and sham transcranial magnetic stimulation (TMS), there is a typical event-related desynchronization in mu/beta followed by a postmovement beta rebound. RT, response time. B: group ERSPs for the right frontal filter for stop trials (SS, successful stop; FS, failed stop) in all 3 task sessions. Time 0 on the x-axis is the time of the stop signal. SSRT, stop signal reaction time. The ERSP is generated relative to the baseline, which is from −1,000 to −500 ms before the stop signal. For the baseline session, successful stop trials show an increase in beta band power (indicated by arrow) between the stop signal and SSRT, consistent with several prior studies, whereas this is absent after real and sham TMS.
Extracting average power. We then extracted the power for the frequency of interest in the time window of interest for each EEG session (baseline, post real stimulation, post sham stimulation). We conducted a one-way repeated-measures ANOVA to test for changes in left sensorimotor mu/beta power across task sessions.
Right Frontal Filter and Stop Frequency of Interest
We expected stopping-related modulations of beta to occur in right frontal electrodes, consistent with prior work (Castiglione et al. 2019; Wagner et al. 2018) and the putative right-lateralized anatomy of the underlying network. Based on these studies, we planned to identify a right frontal spatial filter (a weighting applied to all electrodes) from each participant’s EEG data (being mindful, as explained above, that not every participant would provide such a filter). Before unblinding the data, we used two different source separation techniques to try to identify a right frontal spatial filter in the first 30 participants who completed the study. As specified in the OSF document, we planned to adopt the source separation technique that provided the largest number of participants with a right frontal filter to test our hypothesis.
1. ICA clustering approach. We first submitted the data to the ICA clustering approach used in Wagner et al. (2018). This approach yielded 18 participants with a right frontal IC of the initial 30 collected.
2. GED approach. Next we applied a guided multivariate source separation method using generalized eigenvalue decomposition (GED) (Cohen 2017). This approach is “guided” because it uses priors about topography (i.e., right frontal), frequency of interest (i.e., beta band 12–24 Hz), and time window of interest (i.e., time between stop signal and SSRT). This approach allowed us to compute the weights or spatial filters over the right prefrontal cortex that optimally separated a time window critical for stopping from a baseline time window. We used successful stop trials from the baseline EEG session to identify this filter in a manner that was independent of our core hypothesis (i.e., right frontal beta would be modulated after real stimulation).
We ultimately used the GED approach, as it was able to identify filters for 27 of the initial 30 participants, only requiring data collection for 3 more. Next, we computed ERSPs for failed stop trials and successful stop trials for each session (baseline, real, sham) (see Fig. 2B for group ERSPs). Relative changes in spectral power were computed by calculating the mean difference between each single-trial log spectrogram and the mean baseline spectrum (the log spectrum 1,000 ms to 500 ms before the stop signal averaged across all trials). Using the baseline session alone, we then selected a participant-specific frequency of interest and time window of interest between the stop signal and SSRT on successful stop trials. Specifically, we selected the frequency of interest as the participant’s beta frequency band (12–24 Hz) that showed the greatest increase in the time between the stop signal and SSRT (across participants, mean frequency of interest = 16.53, SD = 3.70). We narrowed our time window of interest by identifying all time points between the stop signal and the participant’s SSRT (using the successful stop trials in the baseline EEG session) in which the participant’s selected frequency (±2) increased above zero.
1. Extracting average power. We then extracted the mean power of the frequency of interest from baseline, post-sham and post-real EEG sessions on both successful and failed stop trials. We conducted a 3 × 2 ANOVA with session (baseline, post real, post sham) and stop trial outcome (successful, failed) as factors and mean power of the frequency of interest as the dependent measure.
2. Beta bursts. Recent studies on beta oscillations show that changes in average power are the result of brief bursting events in the beta band rather than a prolonged modulation. Analyses of beta bursts provide a rich set of features including burst timing relative to an event of interest (see Hannah et al. 2020; Jana et al. 2020; Little et al. 2019; Wessel 2019). A study that examined beta bursts during stopping showed that earlier bursts were associated with quicker SSRTs and CancelTimes (Jana et al. 2020). Although our experiment was designed to focus on beta power, we extracted beta bursts in the baseline task session to test whether we could replicate this relationship between beta burst timing and stopping behavior in our data. We only conducted this analysis on the baseline data because we only had 12 min of data per session and we expected beta bursts to occur less frequently after real stimulation. [Measures like burst timing require many observations to achieve a reliable estimate.] We extracted beta bursts from the baseline task session in the epoched data after filtering at the participant’s frequency of interest (±2.5) (frequency-domain Gaussian, fwhm = 5 Hz). We then defined the burst threshold according to the beta amplitude in a baseline period (−1,000 to −500 ms before the Stop Signal; mean SSD −1,000 ms to mean SSD −500 ms before Go cue). A Hilbert transform was used to derive the complex analytic time series. We took the absolute of the analytic signal to compute beta amplitude during the baseline period. We calculated the median and standard deviation of the baseline period beta after pooling across all trial types (successful stop, failed stop, and Go trials). Bursts were then defined as any period in a trial when beta amplitude exceeded the baseline median value + 1.5 SD (for diagram see supplemental material of Jana et al. 2020). After identifying the bursts, we marked burst time as the time (relative to the stop signal) of each burst’s peak amplitude.
Statistical analyses.
For pairwise comparisons, the data were first checked for normality with a Shapiro–Wilk test, and if the data were normally distributed a t test was performed. In the few cases where the data were not normally distributed, when comparing two independent samples we used a Mann–Whitney U test. For analyses with multiple levels, we performed repeated-measures ANOVAs and Bonferroni-corrected t tests for the subsequent pairwise comparisons. All analyses were conducted as two-tailed tests.
RESULTS
Preregistered Analyses
Behavior.
baseline session
All participants met the baseline behavior sanity checks (see baseline session exclusion criteria above) (Table 1).
Table 1.
Behavior
| Session | Go RT | Failed Stop RT | Stop % | Correct Go % | SSRT |
|---|---|---|---|---|---|
| Baseline | 404 (7) | 375 (6) | 48 (1) | 99 (0) | 222 (5) |
| Real | 402 (8) | 378 (7) | 49 (1) | 99 (0) | 232 (4) |
| Sham | 401 (7) | 373 (6) | 47 (1) | 99 (0) | 231 (4) |
Values are means (SE). Stop %, percent of successful stops; RT, response time; SSRT, stop signal reaction time.
efficacy of double blind
To test for consistent coil placement for both sham and real coils on visit 3, we measured the distance between the coil and the target (locus on pars opercularis) throughout each stimulation session. Unfortunately, this software update only became available midway through data collection, allowing us to collect measurements for 14 of the participants. In this sample, the coil placement was highly similar (mean ± SE: real = 2.32 ± 0.13 mm, sham = 2.35 ± 0.11), with no significant difference [t(13) = 0.213, P = 0.834].
Furthermore, after visit 3, participants were also asked to describe their experience during each TMS session “including any sensations in facial muscles, how intense the stimulation felt, or anything else that comes to mind.” Participants were not explicitly informed that there were two different coils but simply that they would be receiving two sessions of rTMS. The free response answers were then recoded into three categories: discomfort, intensity, or twitching. Multiple participants did distinguish between discomfort and intensity (the level of pounding or strength of the pulse). Only seven participants reported a difference in discomfort between the two coils, with four participants reporting greater discomfort during real stimulation and three reporting greater discomfort during sham stimulation. Thirteen participants experienced a difference in “intensity,” in which 10 described the real stimulation as more intense. Finally, 12 participants mentioned facial twitching, with 9 reporting more facial twitching during the real stimulation. Importantly, in the free response answers, no participant mentioned any suspicion of a sham coil or even two different coils. Together these data demonstrate a rather successful double-blind procedure.
metrics of the stop process
1. SSRT. As predicted, a repeated-measures ANOVA showed a main effect of task session (baseline, post sham, post real) on SSRT (see Table 1 for session values) [F(2,64) = 6.818, P = 0.002]. We next conducted two paired-sample t tests with a Bonferroni adjusted alpha level of 0.025 (0.05/2). SSRT was prolonged after real stimulation compared with the baseline session [t(32) = 2.885, P = 0.007, 2-tailed, d = 0.502]. However, surprisingly, SSRT was also prolonged after sham stimulation compared with baseline SSRT [t(32) = 3.111, P = 0.004, 2-tailed, d = 0.542], and there was no difference between SSRT after real stimulation and after sham stimulation [t(32) = 0.416, P = 0.680] (Fig. 3A).
Figure 3.
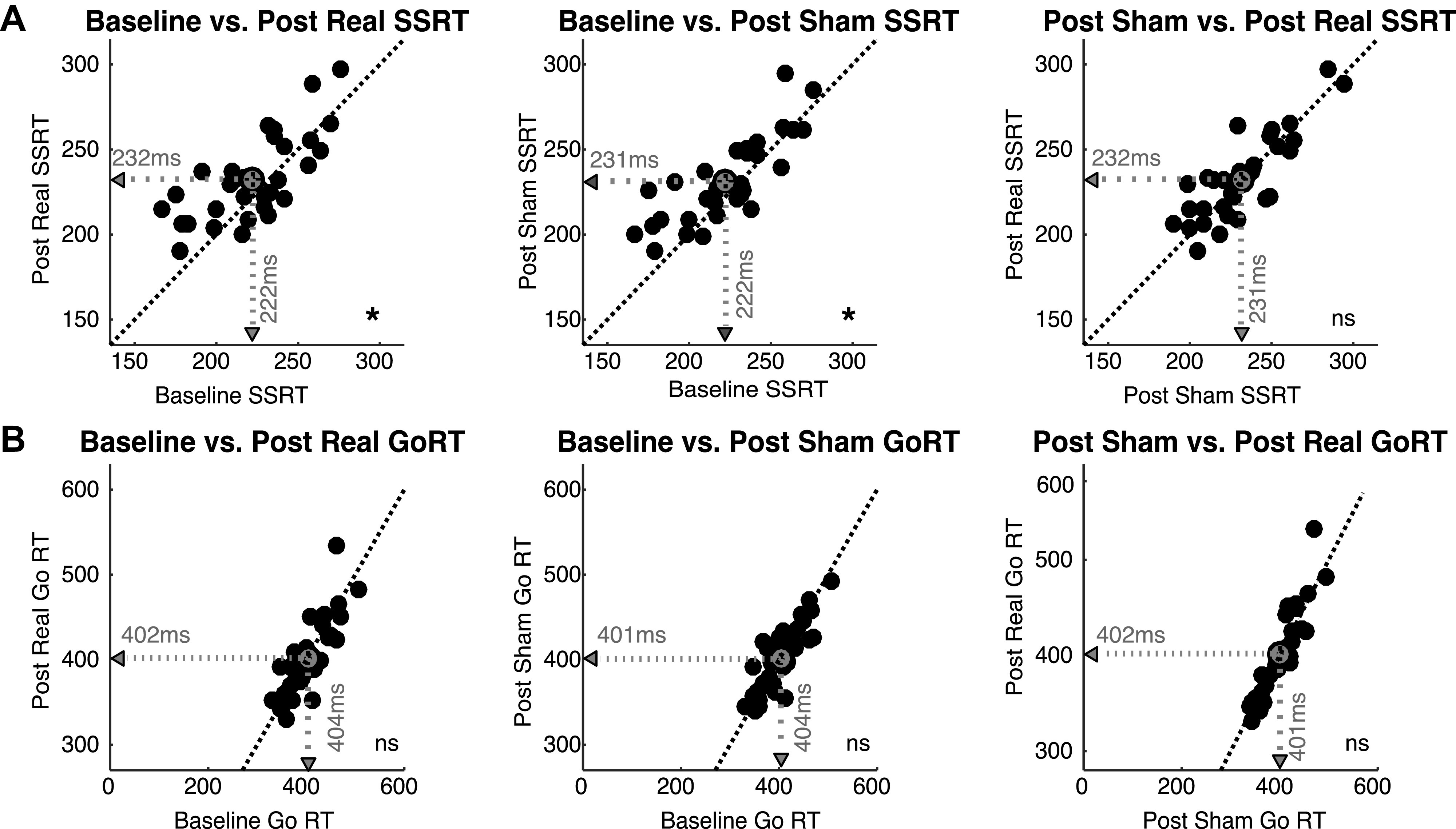
Effect of real vs. sham transcranial magnetic stimulation (TMS) on Stopping and Going. A: the change in stop signal reaction time (SSRT) across task sessions. After real TMS, SSRT is lengthened relative to baseline, but SSRT is also lengthened after sham TMS relative to baseline. There is no difference in SSRT following real vs. sham TMS. B: Go response times (RTs) plotted for each task session. Neither real nor sham TMS affected Go RT relative to baseline. *P < 0.05; ns, Not significant.
2. EMG. Contrary to our prediction, we found no main effect of task session on mean CancelTimes [F(2,56) = 0.783, P = 0.462; Fig. 6B, left; mean ± SE: baseline = 165.36 ± 5.74 ms; sham = 167.17 ± 6.28 ms, real = 169.73 ± 5.65 ms].
metrics of the go process
We performed a repeated-measures ANOVA on Go response times and found no change in response times across the three task sessions (see Table 1 for session values) [F(2,64) = 0.206, P = 0.814; Fig. 3B]. We also did not observe any change in EMG onset, a secondary behavioral measure of the Go process [F(2,56) = 1.618, P = 0.207].
EEG.
baseline predictions.
Consistent with prior work, we expected to observe a greater increase in right frontal beta for successful stop trials compared with failed stop trials in the baseline task session data (i.e., task data before any stimulation) (Castiglione et al. 2019; Wagner et al. 2018). This result did replicate in our data [t(29) = 4.062, P < 0.001, 2-tailed, d = 0.742; Fig. 4A] (bearing in mind that this result is somewhat biased by the fact that we had selected the right frontal (RF) filter, time window of interest, and frequency of interest based on the successful stop trials alone).
Figure 4.
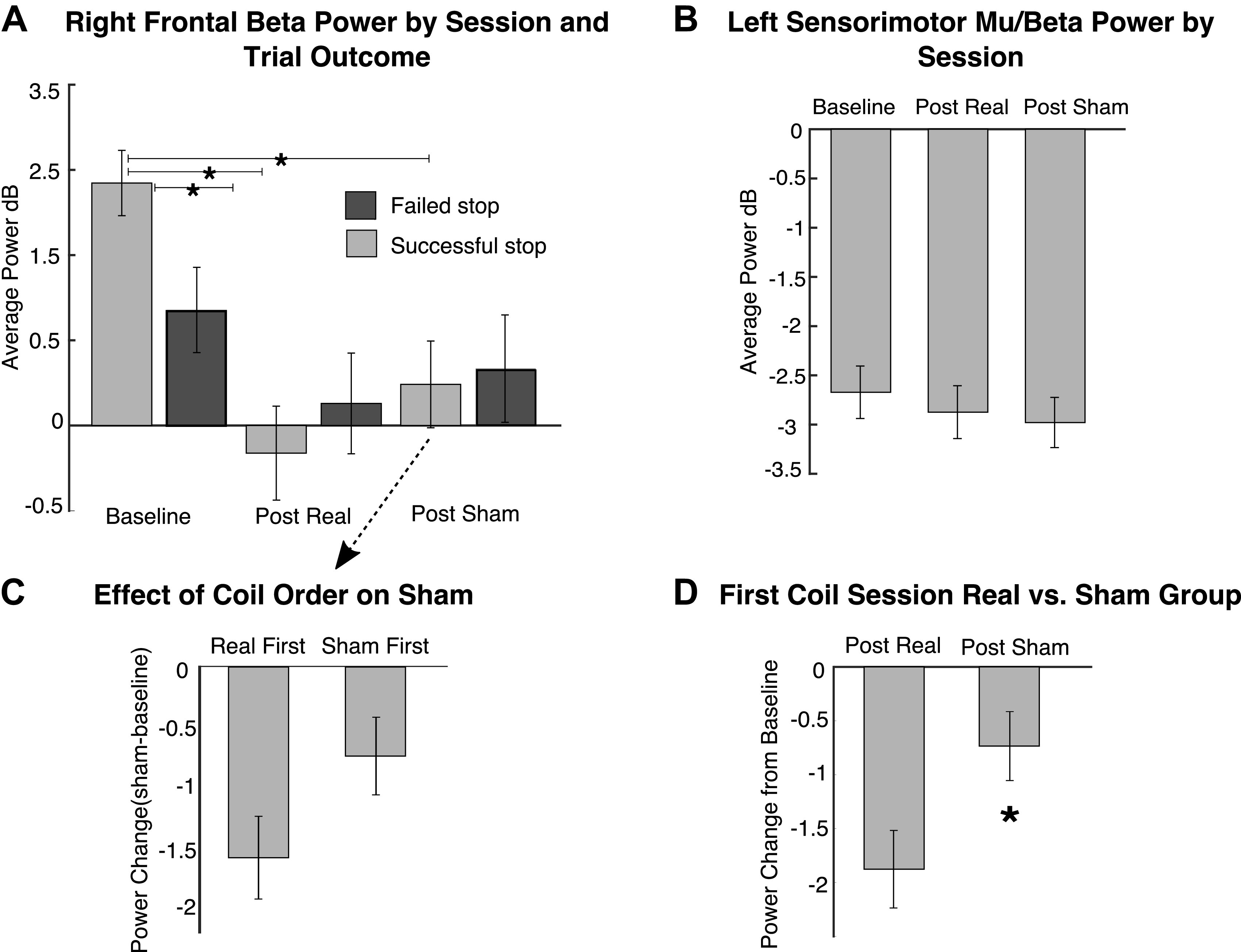
Effect of real vs. sham repetitive transcranial magnetic stimulation (rTMS) on right frontal beta power for Stopping and on left sensorimotor mu/beta power for Going. A: change in right frontal beta power across task sessions and for the comparison of successful and failed stop trials. For each participant, the time and frequency band to extract beta power were determined from the baseline successful stop trials alone and then applied to all other conditions. B: average left sensorimotor mu/beta power related to the Go response for each task session. For each participant, the time and frequency band to extract mu/beta power were determined from the baseline Go trials alone and then applied to the other conditions. C: a comparison of the change in right frontal beta power after sham stimulation for participants who had the real coil first vs those with the sham coil first. As a between-participant analysis, these are expressed relative to each participant’s baseline session: thus in participants with real stimulation first, beta power was much more reduced (from baseline) in the post-sham session compared with participants who had sham first. D: a comparison of the change in right frontal beta after the first stimulation session only, by which we could compare the effects of real vs. sham stimulation without any risk of real stimulation leaking into sham. As a between-participant analysis, these data again represent the change from the baseline session and show that beta power was reduced (from baseline) more for participants in the real coil group (i.e., real first) than participants in the sham group (i.e., sham first). *P < 0.05.
metric of the stop process.
Average power. As described above, we selected a participant-specific beta band by identifying the frequency with peak power between the stop signal and SSRT on successful stop trials during the baseline EEG session. To test whether disrupting the rIFG with 1-Hz stimulation degrades right frontal beta power, we then conducted a 2 × 3 repeated-measures ANOVA with stop trial outcome (successful, failed) and session (baseline, post sham, post real) as the factors. There was a main effect of session [F(2,58) = 9.907, P < 0.001 and a outcome × session interaction [F(2,58) = 8.085, P = 0.001; Fig. 4A]. We focused on successful stop trials and conducted two paired-samples t tests to examine specific effects of real stimulation compared with no stimulation (baseline task session) and compared with sham stimulation. Using a Bonferroni-corrected alpha of 0.025, we found a significant reduction in beta power on successful stop trials after real rTMS stimulation compared with the baseline task session [t(29) = −6.129, P < 0.001, 2-tailed, d = 1.119]. There was not, however, a significant difference between beta power on successful stop trials after real rTMS stimulation and after sham stimulation [t(29) = −1.452, P = 0.157, 2-tailed]. As predicted, these data show that disrupting rIFG with real rTMS results in a reduction of right frontal beta power. Contrary to our hypothesis, however, sham stimulation also had an effect [t(29) = −4.841, P < 0.001, d = 0.884].
metrics of the go process.
Average power. We expected 1-Hz stimulation over rIFG to have effects specific to the stopping process, and not the Go process. To examine Going, we conducted a repeated-measures ANOVA with session (baseline, post sham, post real) as the factor and left sensorimotor mu/beta power on Go trials as the dependent measure. There was no significant change across sessions [F(2,56) = 2.797, P = 0.070; Fig. 4B]; if anything, the event-related desynchronization relative to baseline on each Go trial was even greater in the real and sham task sessions compared with the baseline session. This is the opposite of what one would predict if rTMS reduced an event-related response. Taken together with the stopping analysis above, these results show that the observed changes in neural oscillations were specific to stopping-related right frontal beta (right frontal beta was reduced for stop trials, and left sensorimotor mu/beta was not reduced on Go trials).
Post Hoc Results
Testing coil order effects.
One reason why the difference between real and sham did not come out in the above might have been related to an order effect, wherein real TMS lingered over into the sham session (indeed, our preregistration report anticipated that the 30-min washout period might not be sufficient). This makes the prediction that beta power after sham TMS would be more reduced if the sham session occurred second (i.e., after real) compared with first (i.e., before real): indeed, this prediction held out, albeit at trend level with a two-tailed test: t(28) = 1.78, P = 0.0836, d = 0.651 (Fig. 4C). One concern when interpreting this result is that later task sessions (e.g., the sham sessions that occur after real) may be more susceptible to boredom, fatigue, or lapses in attention that might increase as the visit progresses. However, the same analysis using the real session data (i.e., comparing the effect of real stimulation in those participants who had real first vs. those with real last) did not show a greater reduction in beta for those participants who received real as the final session (U = 87, P = 0.313). We also did not observe any temporal effect on behavior when we compared Go RTs [t(32) = −1.675, P = 0.104] and SSRTs [t(32) = 1.02, P = 0.313] for post-TMS session 1 vs. post-TMS session 2 regardless of coil type (i.e., the final session does not have the longest SSRTs or Go RTs simply because it was last).
This result shows that running both real and sham TMS on the same day introduced a confound—a lingering effect of real TMS on sham TMS. [As noted above, conducting both in 1 day allowed us to use the same EEG spatial filter to compare across sessions and was in line with several estimates in the literature that show effects dissipating within 30 min.] However, one way to test our core idea, that there is a real vs. sham difference, is to do a between-subject test that examines only the first poststimulation session, i.e., to compare the effects of real stimulation in those participants who had real first (n = 16) versus the effects of sham in those who had sham first (n = 14) (for each participant we “normalized” the data by taking the power difference for real/sham minus baseline). We used a nonparametric test because we observed a significant departure from normality after splitting the participants. Indeed, there was a significant between-group difference and beta was reduced on successful stop trials significantly more for the real stimulation group compared with the sham stimulation group [U = 64.0, P = 0.047, 2-tailed test; Fig. 4D]. This between-group result is an important piece of evidence for the idea that the rIFG and underlying circuitry is critical for stopping-related beta oscillations. For completeness, we also conducted this analysis on SSRT (P = 0.704) and CancelTimes (P = 0.425) for the full behavioral sample but found no significant difference between the real and sham groups. Finally, we tested whether there was a correlation across participants between changes in beta power with changes in SSRT and CancelTime. None of the correlations was significant (all P > 0.05).
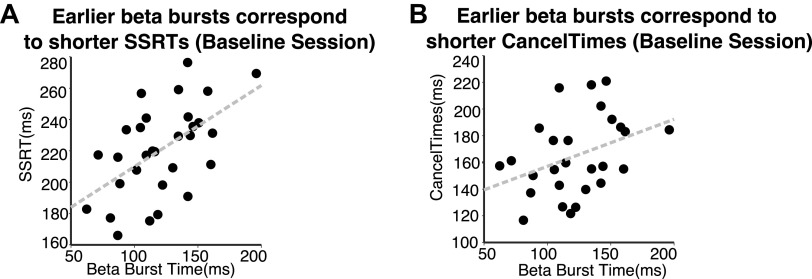
Beta bursts and stopping behavior in baseline task session.
Based on a recent report linking beta burst timing to stopping behavior, we conducted a post hoc analysis of beta bursts in our baseline task data. We expected to replicate the prior finding showing a correlation between stopping behavior (i.e., SSRTs and EMG CancelTimes) and the timing of beta bursts on stop trials (Jana et al. 2020), although this was not a prediction at the time of writing our preregistered document. Consistent with Jana et al. (2020), SSRT was correlated with burst timing (mean ± SE beta burst time = 121 ± 5.56 ms) on successful stop trials (R = 0.549, P = 0.002; Fig. 5A), showing a robust relationship between the standard metric of the stop process (i.e., SSRT) and the timing of stopping-related beta modulations. We also observed a similar relationship between burst timing and EMG CancelTime in the baseline task session (R = 0.370, P = 0.058; Fig. 5B). We did not perform these correlations for the other two task sessions because beta oscillations were reduced and resulted in too few trials with bursts to reliably estimate burst timing (see METRIC OF THE STOP PROCESS. Average power. under EEG above). Finally, we correlated EMG CancelTimes against SSRT, separately for each session. These were significantly correlated in all cases (R = 0.774, P < 0.001 in the baseline session; R = 0.793, P < 0.001 after real stimulation; R = 0.744, P < 0.001 after sham stimulation).
Figure 5.
Relationship between the timing of beta modulations and stopping behavior in the baseline task session. A: consistent with prior work, we found a positive correlation between the timing of beta bursts (relative to the stop signal) and stop signal reaction time (SSRT). B: we observed a similar relationship between burst time and EMG CancelTime.
Shift in EMG CancelTime distribution.
Although mean EMG CancelTime was not different between real and sham stimulation, a different feature of CancelTime was affected, i.e., CancelTime variability. Specifically, we calculated each participant’s coefficient of variation (COV) for each session (COV = mean of session CancelTime/standard deviation of session CancelTime). An ANOVA showed that CancelTime COV was significantly different across sessions [F(2,56) = 5.058, P = 0.010; Fig. 6B, right). Follow-up tests showed that CancelTime COV was reduced after real stimulation compared with both baseline and sham sessions [t(28) = −2.646, P = 0.013, d = 0.491 and t(28) = −3.153, P = 0.004, d = 0.584, respectively]. This suggests that specific portions of the CancelTime distribution were disproportionately affected by real rIFG stimulation. We next aimed to identify the portion of the CancelTime distribution that was affected by real stimulation. Because of the low number of partial EMG trials (mean count = 26.07, SD = 6.11 per participant per session), we did not have a sufficient number of observations to produce reliable session distributions for single participants. Instead, we collapsed across all participants, resulting in a single CancelTime distribution for each of the three sessions: baseline session, post real stimulation, and post sham stimulation. We then produced a cumulative distribution function (CDF) to statistically compare the shape of each distribution. We conducted Kolmogorov–Smirnov tests to compare CDFs for the real session versus baseline, the real session versus sham, and the sham session versus baseline. There was no difference between baseline and sham (P = 0.262). However, there was a significant difference for the real session versus baseline (P < 0.001) and for the real session versus sham (P = 0.004). Specifically, the leftward tail of the distribution (i.e., the quickest CancelTimes) was cut after real stimulation (see Fig. 6, C and D). This suggests that assuming a constant speed for the stopping process may be erroneous and that disrupting rIFG does not simply shift the entire distribution. Instead, our data suggest that rTMS over rIFG may specifically interfere with the quickest/most efficient instances of stopping.
DISCUSSION
In a preregistered double-blind study, we used 1-Hz rTMS to disrupt rIFG and/or the underlying right fronto-basal ganglia stopping network to test the effects on stopping-related beta and stopping performance. In the baseline session before rTMS, we observed the typical increase in right frontal beta power on stop trials that was greater for successful versus failed stop trials. Consistent with our predictions, after real rTMS SSRT was lengthened, and there was a reduction of the stopping-related beta power in a time window critical for stopping. Furthermore, this was a selective effect, as real rTMS did not reliably change Go RT or EEG mu/beta desynchronization in a left sensorimotor region. Surprisingly, however, sham stimulation also led to an increase in SSRT and a reduction in right frontal beta. However, a post hoc between-group analysis that considered the coil order showed that part of this reduction for sham might be due to a lingering effect of real stimulation, when real came first. Another between-group analysis, focused on the first rTMS session only, showed that the real stimulation group had a greater reduction of beta compared with the sham stimulation group. This latter result is one of the more important results of this report, as it shows that real stimulation reduces right frontal beta power significantly more than sham stimulation. We interpret this as evidence that real rTMS altered the neural architecture of the rIFG and/or underlying connected circuitry in a way that selectively effected the generation or propagation of beta oscillations and the event-related recruitment of the stopping system.
To date, there are more than half a dozen electrophysiological studies in humans pointing to an increase of right frontal beta power related to action stopping or other forms of putative inhibitory control (Castiglione et al. 2019; Hannah et al. 2020; Jha et al. 2015; Picazio et al. 2014; Schaum et al. 2020; Swann et al. 2009; Wagner et al. 2016, 2018; Wessel et al. 2013). Changes in beta are also observed in other key regions of the stopping network during inhibitory control, further supporting beta as a functionally relevant signature of the stopping process (e.g., Brittain et al. 2012; but also see Errington et al. 2020). The present study, however, is the first to use a causal test to demonstrate that stopping-related modulations of beta are related to the integrity of the rIFG and/or the underlying circuitry. This has several important implications. First, it motivates the use of right frontal beta recorded in scalp EEG as a tool for testing theories of a putative right IFG network in other domains of inhibitory control such as emotional responsiveness and memory retrieval (Berkman et al. 2009; Castiglione et al. 2019; Depue et al. 2007; Guo et al. 2018). Scalp EEG has the advantage, over fMRI, of temporal resolution and portability. Furthermore, right frontal beta can be decomposed into beta bursts that have richer features such as trial-specific timing that other studies have used in research on action stopping (Hannah et al. 2020; Jana et al. 2020; Little et al. 2019; Wessel 2019). Although the present experiment was designed to focus on beta power, we did replicate prior work showing a relationship between the timing of beta bursts and stopping behavior in our baseline task session. Second, this work joins a growing body of literature that uses causal methods to test how changing neural circuits affects oscillations. By showing that disrupting a node in the stopping network reduced beta oscillations, our results support the idea that these oscillations are functionally relevant rather than a mere by-product of cognitive or behavioral processing (e.g., Fetz 2013; Hanslmayr et al. 2014, 2019; Joundi et al. 2012).
Although real rTMS did have selective effects on electrophysiology, the effects on behavior were only subtle. However, in both task sessions (sham and real) in which we observed a reduction in beta power relative to the baseline task session, we also found impaired stopping performance. As predicted, we observed an increase in SSRT after real stimulation. However, we also observed an effect after sham stimulation, and, unlike the EEG results, there was no clear distinction in the behavioral effect when we conducted a between-group analysis that removed coil order as a potential confound or when we analyzed the first session only (those who got real TMS first vs. those who got sham TMS first). We suspect that sham rTMS affected subsequent stopping behavior, at least in part, as a side effect of physical discomfort. Unlike earlier rIFG TMS studies that used a sham where the coil is turned away from the head (Chambers et al. 2006, 2007), our sham coil also activated the facial muscles, which is quite uncomfortable for 12 min. This discomfort may have put participants into a subsequently altered cognitive set that affected stopping performance. Indeed, other studies have shown that discomfort from stimulation can affect motor and cognitive task performance (Abler et al. 2005; Meteyard and Holmes 2018). Another reason that SSRT may not have been sensitive to coil type is that it is a single number calculated per participant. Although our alternative behavioral measure of stopping speed, mean CancelTime measured with EMG, was not different between coil types, a post hoc analysis showed differences in the CancelTime distribution. Specifically, there was a lengthening of the shortest CancelTimes after real versus sham stimulation, whereas the remainder of the distribution was unchanged. We consider two accounts of why only the shortest CancelTimes were affected. First, although 1-Hz rTMS is thought to have inhibitory effects on the underlying cortex, the affected region may still function, albeit less efficiently. As a result of a sluggish rIFG, the stopping process may show a slight lag, pushing the quickest instances of stopping into the next “bin” but leaving the remainder of the distribution largely intact. Specifically, because the longer CancelTimes are already relatively slow, the effect may get washed out for longer CancelTimes and affect only the quickest instances of stopping (i.e., the shortest CancelTimes). Second, the rIFG is only one node in a putative neural architecture for action stopping. Although other brain regions may compensate for an impaired rIFG, this may not happen quickly enough to affect the shortest CancelTimes.
One interpretational limitation is that we only stimulated one site, the rIFG, with the sham and real coils. Therefore, we cannot rule out that stimulating other prefrontal areas or nodes of the stopping network might also have yielded similar effects. Indeed, several studies have shown effects of TMS on stopping behavior when stimulating other nodes of the stopping network (e.g., Obeso et al. 2017). Our study, however, was hypothesis driven based on prior scalp EEG and ECoG studies (Swann et al. 2009; Wagner et al. 2018; Wessel et al. 2013), and going beyond one prefrontal site would have made our study intractable. It already required three visits per participant (and the TMS visit, involving EEG capping, was already very long). Future work should probe other stimulation sites and test for changes in right frontal beta to examine region specificity.
Finally, it is pertinent to note that several studies also implicate the rIFG in attention orienting and stimulus detection (Corbetta et al. 2002; Sharp et al. 2010) and it is possible that impaired stopping occurred because rTMS interfered with the role of the rIFG in detecting the stop signal as opposed to implementing the stopping process. Although we cannot definitively rule out this possibility, evidence from selective stopping tasks that control for attentional capture clearly show that rIFG recruitment and beta modulations are linked specifically to the stopping process, in contrast to other right prefrontal subregions that contribute more to attentional capture (Schaum et al. 2020; Sebastian et al. 2016). There is also now further anatomical evidence for a hyperdirect pathway in humans that provides a route for the rIFG to rapidly inhibit thalamocortical drive to the motor cortex via the subthalamic nucleus and cancel actions (Chen et al. 2020). Based on the current literature, our effects of rTMS over rIFG are likely the result of disrupting a stopping-specific process. Future work, however, should continue to tease apart the relationship between right frontal beta and action stopping versus attention and stimulus detection.
In conclusion, our study showed a real vs. sham effect on stopping-related beta, and it also showed across participants that the timing of beta bursts correlated with SSRT (just like Hannah et al. 2020; Jana et al. 2020). But we did not see a specific influence of real versus sham TMS on SSRT, nor did we find that those participants with more TMS-reduced beta had longer SSRTs, so our study does still leave open the important question of whether beta is causal to stopping ability. To our knowledge, however, this is the first causal evidence linking stopping-related changes in right frontal beta to the integrity of the underlying rIFG-related network. This advances our understanding of the role of beta in inhibitory control over actions and supports the use of this EEG signature as a tool for testing the role of a rIFG prefrontal network in other domains of executive control.
GRANTS
This work was supported by NIH Grant DA-026452, NIMH Grant MH-020002, and the James S. McDonnell Foundation (220020375).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K.S. and A.R.A. conceived and designed research; K.K.S. and S.J. performed experiments; K.K.S. analyzed data; K.K.S. and S.J. interpreted results of experiments; K.K.S. and S.J. prepared figures; K.K.S. drafted manuscript; K.K.S., S.J., and A.R.A. edited and revised manuscript; K.K.S. and A.R.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank John Rothwell for technical advice on procedures and Seraphina Solders for helping to develop this project as part of a rotation in the laboratory. We also thank Vignesh Muralidharan and Ricci Hannah for advice on study analyses and interpretation and Cynthia Yu for assistance with data collection.
Footnotes
Testing our hypothesis about right frontal beta critically requires first deriving a right frontal spatial filter from each participant’s EEG data, yet our various publications have shown that it is not possible to derive a right frontal spatial filter in every participant (here we used generalized eigenvalue decomposition, but the same applies for other methods such as independent component analysis). We worried that if we had conducted the TMS-EEG protocol on two separate visits per participant this would have resulted in too much subject attrition per expended effort (i.e., we would have to exclude participants for whom we could not identify a reliable filter in either of the sessions). We reasoned that having a single right frontal spatial filter per participant to better compare the effects of real versus sham stimulation trumped the worry about a possible order effect, with real TMS “leaking” into the sham session.
REFERENCES
- Abler B, Walter H, Wunderlich A, Grothe J, Schönfeldt-Lecuona C, Spitzer M, Herwig U. Side effects of transcranial magnetic stimulation biased task performance in a cognitive neuroscience study. Brain Topogr 17: 193–196, 2005. doi: 10.1007/s10548-005-6028-y. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26: 2424–2433, 2006. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18: 177–185, 2014. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108: 44–79, 2013. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. Neuroimage 47: 705–712, 2009. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology 54: 1529–1531, 2000. doi: 10.1212/WNL.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. doi: 10.1163/156856897X00357. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Watkins KE, Joundi RA, Ray NJ, Holland P, Green AL, Aziz TZ, Jenkinson N. A role for the subthalamic nucleus in response inhibition during conflict. J Neurosci 32: 13396–13401, 2012. doi: 10.1523/JNEUROSCI.2259-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci 34: 14652–14667, 2014. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione A, Wagner J, Anderson M, Aron AR. Preventing a thought from coming to mind elicits increased right frontal beta just as stopping action does. Cereb Cortex 29: 2160–2172, 2019. doi: 10.1093/cercor/bhz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol 98: 3638–3647, 2007. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci 18: 444–455, 2006. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403, 1997. doi: 10.1212/WNL.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen W, de Hemptinne C, Miller AM, Leibbrand M, Little SJ, Lim DA, Larson PS, Starr PA. Prefrontal-subthalamic hyperdirect pathway modulates movement inhibition in humans. Neuron 106: 579–588.e3, 2020. doi: 10.1016/j.neuron.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Comparison of linear spatial filters for identifying oscillatory activity in multichannel data. J Neurosci Methods 278: 1–12, 2017. doi: 10.1016/j.jneumeth.2016.12.016. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci 14: 508–523, 2002. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Stop and go: the neural basis of selective movement prevention. J Cogn Neurosci 21: 1193–1203, 2009. doi: 10.1162/jocn.2009.21081. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Van Impe A, Wenderoth N, Swinnen SP. Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. J Neurosci 32: 8401–8412, 2012. doi: 10.1523/JNEUROSCI.6360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21, 2004. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 317: 215–219, 2007. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Errington SP, Woodman GF, Schall JD. Dissociation of medial frontal β-bursts and executive control. J Neurosci JN-RM-2072-20, 2020. doi: 10.1523/JNEUROSCI.2072-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE. Volitional control of cortical oscillations and synchrony. Neuron 77: 216–218, 2013. doi: 10.1016/j.neuron.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306, 1999. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Schmitz TW, Mur M, Ferreira CS, Anderson MC. A supramodal role of the basal ganglia in memory and motor inhibition: meta-analytic evidence. Neuropsychologia 108: 117–134, 2018. doi: 10.1016/j.neuropsychologia.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah R, Muralidharan V, Sundby KK, Aron AR. Temporally-precise disruption of prefrontal cortex informed by the timing of beta bursts impairs human action-stopping. Neuroimage 222: 117222, 2020. doi: 10.1016/j.neuroimage.2020.117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Axmacher N, Inman CS. Modulating human memory via entrainment of brain oscillations. Trends Neurosci 42: 485–499, 2019. doi: 10.1016/j.tins.2019.04.004. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Matuschek J, Fellner M-C. Entrainment of prefrontal beta oscillations induces an endogenous echo and impairs memory formation. Curr Biol 24: 904–909, 2014. doi: 10.1016/j.cub.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci 16: 719–732, 2015. doi: 10.1038/nrn4038. [DOI] [PubMed] [Google Scholar]
- Jana S, Hannah R, Muralidharan V, Aron AR. Temporal cascade of frontal, motor and muscle processes underlying human action-stopping. eLife 9: e50371, 2020. doi: 10.7554/eLife.50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Nachev P, Barnes G, Husain M, Brown P, Litvak V. The frontal control of stopping. Cereb Cortex 25: 4392–4406, 2015. doi: 10.1093/cercor/bhv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joundi RA, Jenkinson N, Brittain J-S, Aziz TZ, Brown P. Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol 22: 403–407, 2012. doi: 10.1016/j.cub.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A. The ups and downs of β oscillations in sensorimotor cortex. Exp Neurol 245: 15–26, 2013. doi: 10.1016/j.expneurol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Lang N, Speck S, Harms J, Rothkegel H, Paulus W, Sommer M. Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiatry 63: 231–233, 2008. doi: 10.1016/j.biopsych.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Little S, Bonaiuto J, Barnes G, Bestmann S. Human motor cortical beta bursts relate to movement planning and response errors. PLoS Biol 17: e3000479, 2019. doi: 10.1371/journal.pbio.3000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 91: 295–327, 1984. doi: 10.1037/0033-295X.91.3.295. [DOI] [PubMed] [Google Scholar]
- Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. In: Advances in Neural Information Processing Systems. Cambridge, MA: MIT Press, 1996, p. 145–151. [Google Scholar]
- Meteyard L, Holmes NP. TMS SMART—scalp mapping of annoyance ratings and twitches caused by transcranial magnetic stimulation. J Neurosci Methods 299: 34–44, 2018. doi: 10.1016/j.jneumeth.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Wurtz P, Lüscher H-R, Hess CW, Senn W, Pflugshaupt T, von Wartburg R, Lüthi M, Müri RM. Repetitive TMS over the human oculomotor cortex: comparison of 1-Hz and theta burst stimulation. Neurosci Lett 409: 57–60, 2006. doi: 10.1016/j.neulet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Obeso I, Wilkinson L, Teo JT, Talelli P, Rothwell JC, Jahanshahi M. Theta burst magnetic stimulation over the pre-supplementary motor area improves motor inhibition. Brain Stimul 10: 944–951, 2017. doi: 10.1016/j.brs.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, Koch G. Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol 24: 2940–2945, 2014. doi: 10.1016/j.cub.2014.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raud L, Huster RJ. The temporal dynamics of response inhibition and their modulation by cognitive control. Brain Topogr 30: 486–501, 2017. doi: 10.1007/s10548-017-0566-y. [DOI] [PubMed] [Google Scholar]
- Raud L, Westerhausen R, Dooley N, Huster RJ. Differences in unity: the go/no-go and stop signal tasks rely on different mechanisms. Neuroimage 210: 116582, 2020. doi: 10.1016/j.neuroimage.2020.116582. [DOI] [PubMed] [Google Scholar]
- Schaum M, Pinzuti E, Sebastian A, Lieb K, Fries P, Mobascher A, Jung P, Wibral M, Tuescher O. Cortical network mechanisms of response inhibition (Preprint). bioRxiv 940841, 2020. doi: 10.1101/2020.02.09.940841. [DOI]
- Sebastian A, Jung P, Neuhoff J, Wibral M, Fox PT, Lieb K, Fries P, Eickhoff SB, Tüscher O, Mobascher A. Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Struct Funct 221: 1635–1651, 2016. doi: 10.1007/s00429-015-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 107: 6106–6111, 2010. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Filipovic SR, Rowe JB, Cordivari C, Gerschlager W, Rothwell JC, Frackowiak RS, Bhatia KP. Patients with focal arm dystonia have increased sensitivity to slow-frequency repetitive TMS of the dorsal premotor cortex. Brain 126: 2710–2725, 2003. doi: 10.1093/brain/awg282. [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 29: 12675–12685, 2009. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, Aron AR, Tandon N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage 59: 2860–2870, 2012. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunberg C, Messel MS, Raud L, Huster R. Stimulating stopping? Investigating the effects of tDCS over the inferior frontal gyri and visual cortices (Preprint). bioRxiv 723296, 2019. doi: 10.1101/723296. [DOI] [PMC free article] [PubMed]
- Verbruggen F, Aron AR, Band GP, Beste C, Bissett PG, Brockett AT, et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 8: e46323, 2019. doi: 10.7554/eLife.46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Makeig S, Gola M, Neuper C, Müller-Putz G. Distinct β band oscillatory networks subserving motor and cognitive control during gait adaptation. J Neurosci 36: 2212–2226, 2016. doi: 10.1523/JNEUROSCI.3543-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Wessel JR, Ghahremani A, Aron AR. Establishing a right frontal beta signature for stopping action in scalp EEG: implications for testing inhibitory control in other task contexts. J Cogn Neurosci 30: 107–118, 2018. doi: 10.1162/jocn_a_01183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR. β-Bursts reveal the trial-to-trial dynamics of movement initiation and cancellation (Preprint). bioRxiv 644682, 2019. doi: 10.1101/644682. [DOI] [PMC free article] [PubMed]
- Wessel JR, Conner CR, Aron AR, Tandon N. Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. J Neurosci 33: 19611–19619, 2013. doi: 10.1523/JNEUROSCI.3468-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Völlm B, Khalifa N. The effects of rTMS on impulsivity in normal adults: a systematic review and meta-analysis. Neuropsychol Rev 28: 377–392, 2018. doi: 10.1007/s11065-018-9376-6. [DOI] [PubMed] [Google Scholar]