Keywords: bimanual choice, decision-making, motor adaptation, movement vigor, reaction time, virtual reality
Abstract
The decision regarding which arm to use to perform a task reflects a complex process that can be influenced by many factors, including effort requirements of acquiring the goal. In this study, we considered a virtual reality environment in which people reached to a visual target in three-dimensional space. To vary the cost of reaching, we altered the visual feedback associated with motion of one arm but not the other. This altered the extent of motion that was required to reach, thus changing the effort required to acquire the goal. We then measured how that change in effort affected the decision regarding which arm to use, as well as the preparation time for the movement that ensued. As expected, with increased visual amplification of one arm (reduced effort to reach the goal), subjects increased the probability of choosing that arm. Surprisingly, however, the reaction times to start these movements were also reduced: despite constancy of the visual representation of the target, reaction times were shorter for movements with less effort. Thus, as the perceived effort associated with accomplishing a goal was reduced for a given limb, the decision-making process was biased toward use of that limb. Furthermore, movements that were perceived to be less effortful were performed with shorter reaction times. These results suggest that visual amplification can alter the perceived effort associated with using a limb, thus increasing frequency of use. This may provide a useful method to increase use of a limb during rehabilitation.
NEW & NOTEWORTHY We report that visual amplification may serve as an effective means to alter the perceived effort associated with use of a limb. This method may provide an effective tool with which use of the affected limb can be encouraged noninvasively after neurological injury.
INTRODUCTION
In daily activities, people routinely make decisions as to which arm to use to perform an action. Aside from handedness or task context, choice of arm can be influenced by its physical impairments, such as those after neurological/orthopedic injuries. For instance, upper extremity impairment is common and persistent after stroke (1) because functional impairment of affected limbs is often exacerbated by their behavioral maladaptation, i.e., “learned” nonuse. This learned nonuse exacerbates the symptoms, leading to further neurophysiological problems (e.g., muscle atrophy) and functional degradation (2).
Despite its significance, the idea of countering learned nonuse of the more impaired limb has not been a primary target for therapy. Rather, rehabilitation practices have focused on improving functionality of more-impaired limbs of stroke patients. While functional improvement of the more-impaired limbs could promote their use in their daily activities, functional use of the more-impaired arms outside the laboratory/clinic can also be affected by various factors other than their degree of impairment, such as perceived/subjective confidence (3), sensory loss (4), or pain (2). Indeed, functional gains achieved by intensive training (e.g., robot-assisted therapy) tend to disappear at follow-up examination (5). These results suggest that following a stroke, frequent, voluntary use of the more-impaired limbs may be critical in functional recovery.
Preferred choice of arm use (or handedness) in humans reflects complex neuropsychological processes formed by genetic factors (6), prior experiences (7), and parental modeling (8) over time. However, arm choice is also influenced by the context of the task; for instance, the probability of using the dominant arm is correlated with task precision and efficiency (9, 10), while the nondominant arm is predominantly used for stabilization (11). Several studies have found that arm choice is also influenced by sensory feedback (12), body movements (13), or task performance (14), suggesting that arm choice can be altered by changing task conditions or sensory information provided to subjects. Recent studies have found that in chronic stroke patients, “visual” amplification of movements of their paretic limbs toward the targets (in the virtual reality environment) promotes use of those limbs (15, 16).
In theory, visual amplification of the movement of one limb, theoretically, could produce two contrasting patterns of adaptation. “Virtual” amplification of the movements of one limb can certainly reduce the cost associated with its movement (i.e., energy), thereby promoting its use (17). Conversely, alteration of the visual feedback could also increase the task error when performed by the “visually-amplified” arm, which may discourage the use of this limb. For instance, a previous study found that people often prioritize task performance over energy reduction (18). Furthermore, subject-specific (inherent) factors, e.g., degree of handedness, may affect the degree of adaptation of different individuals in response to the same visual amplification.
We posited that the choice of arm use may be viewed as a decision-making problem, in which both reward and effort associated with the choice are considered (8, 17). Recent studies developed a framework that examines characteristics of movements in the context of a “value-based” choice problem (i.e., “utility” of the movements; 19–25), in which the reward and effort of the choice “not only affect the decision but also the motor control” (i.e., movement characteristics) (17). Utility of each movement represents a measure that balances the efforts and reward, which determines the optimal level of “vigor (speed as a function of distance)” of the movement (26, 27). A previous study (23) found that expectation of reward reduced reaction time and increased velocity of reaching movements. However, it remains unknown whether manipulation of effort associated with movements affect reaction time and movement vigor. Behavioral adaptation of subjects under altered visual feedback, i.e., kinematic changes (reaction time, duration, velocity) and their response rate (frequency of amplified arm use), will allow us to better understand the decision-making process of individuals under altered sensory feedback, which could also lead to a better technique to promote the impaired arm use among stroke survivors.
Here, we report on an experiment in which subjects had the option of using their left or right arm to reach a target. Using virtual reality (VR), we manipulated the effort costs associated with using their dominant arm. We anticipated that, similar to previous studies, visual amplification of the movements of one limb would affect its frequency of use. Furthermore, if the visual manipulation affected perceived effort, then there may also be changes in movement vigor, as reflected in reaction time and peak velocity. We also expected that the degree of motor adaptation would vary significantly across subjects, as it may be influenced by subject-specific factors, such as handedness (14, 28).
METHODS
We allowed subjects to choose which arm to use in order to reach with a handheld tool to a target displayed in a VR environment. We manipulated the energetic cost of the reaching movements by virtually amplifying the range of motion of the hand with the altered visual feedback.
Participants
Thirty-one right-handed participants (age: 22±3 yr; 15 females, 16 males), naive to the experiment, participated in this study. All participants were right-handed according to the Edinburgh Handedness Inventory (score greater than 75%). The experimental protocol was approved by the Institutional Review Board at the Catholic University of America, and the written informed consent was obtained from all participants before their participation.
Experimental Setting
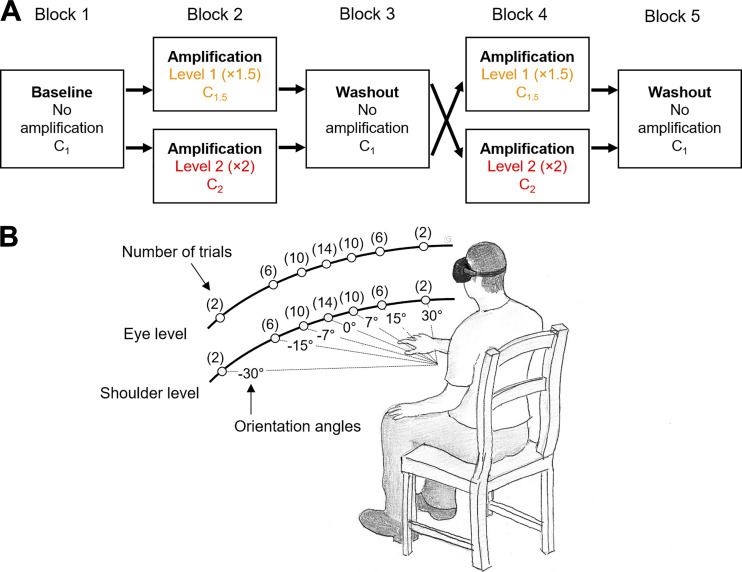
Subjects were instructed to perform reaching movements toward targets that appear in the virtual reality environment using a head-mounted display (Oculus Rift; Facebook Technologies, LLC., C). A cube-shaped target (size: 12 cm × 12 cm × 12 cm) appeared in a random order at one of the fourteen possible locations defined by seven orientation angles (0°, ±7°, ±15°, and ±30°), with respect to the subject’s midsagittal plane, and two height levels (eye level/shoulder level) (Fig. 1A). Subjects held two controllers, one in each hand. They were told that they could use their right or left arm to reach for the target.
Figure 1.
Experimental design. A: experimental protocol with randomized crossover design. B: target locations and frequency of appearance per block. The order of appearance was randomized within each block.
Each trial began with the subject placing their hands on their lap. Once the system detected their hands at the starting position, a cube target (12 cm × 12 cm × 12 cm) was displayed with an auditory cue. At this point the subject decided which arm they wished to use for reaching to the target, and then performed the movement. The trial ended by the disappearance of the target once the handheld tool reached inside the target. At this point the subject returned their hand to their lap and after 3 s, the next trial started. The positions of the two hand-held controllers and the headset were recorded at 90 Hz.
Three visual feedback conditions were implemented: 1) no amplification (C1); 2) ×1.5 amplification (C1.5); and 3) ×2 amplification (C2) of the right arm. When the visual amplification was implemented, the displacement (or movement) of the right arm from the initial position was amplified by a factor of 1.5 (C1.5) or of 2 (C2). The movement of the right arm was amplified regardless the direction of movement. In contrast to previous studies that selectively applied a higher gain to the goal-directed components of the reaching movements (15, 16), in our experiment the hand movement was amplified in all directions. Thus the amplification did not necessarily adjust the task difficulty as the movements in the task-irrelevant dimension were amplified to the same degree with the movements toward the target. The amplification in our experiment was designed to only reduce the energetic cost associated with the movements of the amplified arm.
Experimental Protocol
Participants completed five blocks of trials, each consisting of 100 reaching movements. Within each block, the target appeared eight times at the locations with ±30° orientation angles (2 trials/target), 24 times at ±15° angles (6 trials/target), 40 times at ±7° angles (10 trials/target), and 28 times at 0° angle (trials/target), half assigned at eye level, and half at shoulder level. At each height (eye/shoulder), the seven targets were placed at the same distance from the body center of each subject (i.e., circular path), and their locations were adjusted so that they are placed at approximately 80% of the maximum reaching distance (arm length) (Fig. 1B). This target distribution was designed to collect more movement data at locations in which participants were likely to use either arms (i.e., ambiguous locations near the center). The eccentric locations (at ±30° angle) were included to prevent subjects from adopting a strategy of using the same arm to reach to all targets. Within each block, the target appearance order was randomized.
Subjects were randomly assigned to two groups, for which the experimental conditions were implemented in a crossover design (Fig. 1B). Out of five blocks, two levels of visual amplification (×1.5 and ×2) were implemented to the right arm movements in the blocks 2 and 4, while the no amplification was implemented in the blocks 1, 3, and 5. For one group, the lower level of visual amplification (×1.5) was implemented in the block 2, and the higher level (×2) in the block 4. Conversely, for the other group, the higher level of amplification (×2) was implemented first (block 2) and then the lower level (×1.5) in the block 4. A minimum of 5-min rest periods were given between the blocks.
We also collected data on the subjects’ awareness about the altered visual feedback in the VR environment. At the end of the experimental session, subjects were asked to fill out a debriefing survey to assess their awareness of the visual amplification in the experimental blocks. Specifically, they were asked 1) if they had noticed any changes in the VR system over the course of the experiment; 2) if the task got easier, harder, or stayed the same for the right arm and left arm; 3) whether they felt they had used one arm more than the other; and 4) if this perceived pattern had changed over the course of the experiment.
Data Analysis
Our primary outcome measure was the right-arm use percentage (RUP) for each block. Two a priori assumptions were first tested: 1) whether a significant difference in the RUP was observed between the no-amplification blocks (after effects); and 2) whether a significant difference in the RUP was observed between the subject groups, for which the two levels of the visual amplification were delivered in a different order (order effects). Then, the RUP values were compared between the three amplification conditions (C1, C1.5, and C2). Lastly, kinematic analyses were performed to examine difference in the movement parameters between these amplification levels.
Significant level was set to 0.05 for all statistical tests. Generalized eta square () was used to report the effect size in case a statistical significance was found in the ANOVA. Cohen’s d values were used to gauge the effect size of the post hoc pairwise comparisons. Descriptive statistics are reported as means ± SD.
Right-arm use percentage.
Within each block, the frequency of the right arm use for each target, target-specific right-arm use percentage (RUP), was first calculated by dividing the number of trials that the right arm was used to reach the target by the total number of the target appearance. Then, the RUP for a given block was computed by averaging the target-specific RUP values across all targets. In addition, the target-specific RUP values were used to evaluate the workspace of the right hand.
Pretest: aftereffects and order effects.
In order to test whether the visual amplification had any residual effects on the arm selection (aftereffects), a one-way ANOVA with three blocks without visual amplification (baseline and two washout blocks) as the within-subject factor was performed on the RUP values from the blocks 1, 3, and 5. The order effects of the different amplification conditions were tested by a one-way ANOVA on the RUP values from the blocks 2 and 4, with the subject group as the between-subjects factor.
Effects of visual amplification on arm selection.
A repeated-measures ANOVA was performed on the RUP values with the amplification condition as the within-subject factor. When found significant, post hoc comparisons between the amplification levels were made with the level of significance adjusted for multiple comparison (Bonferroni correction). Sphericity was tested by Mauchley’s test, and when the sphericity assumption was found violated, the Greenhouse-Geiser correction was applied to adjust the P value.
In addition, learning effects on the arm selection under visual amplification were examined by comparing the RUP values in first half of each block (trials 1–50) with those in the second half (trials 51–100).
Kinematic analysis.
The following kinematic parameters were estimated from the movement data of the hand-held controllers of both sides: 1) peak velocity of each hand, 2) reaction time, 3) task duration time, 4) path length ratio, and 5) overshoot ratio for each reaching movement. All kinematic parameters were computed from the movement data in the VR environment.
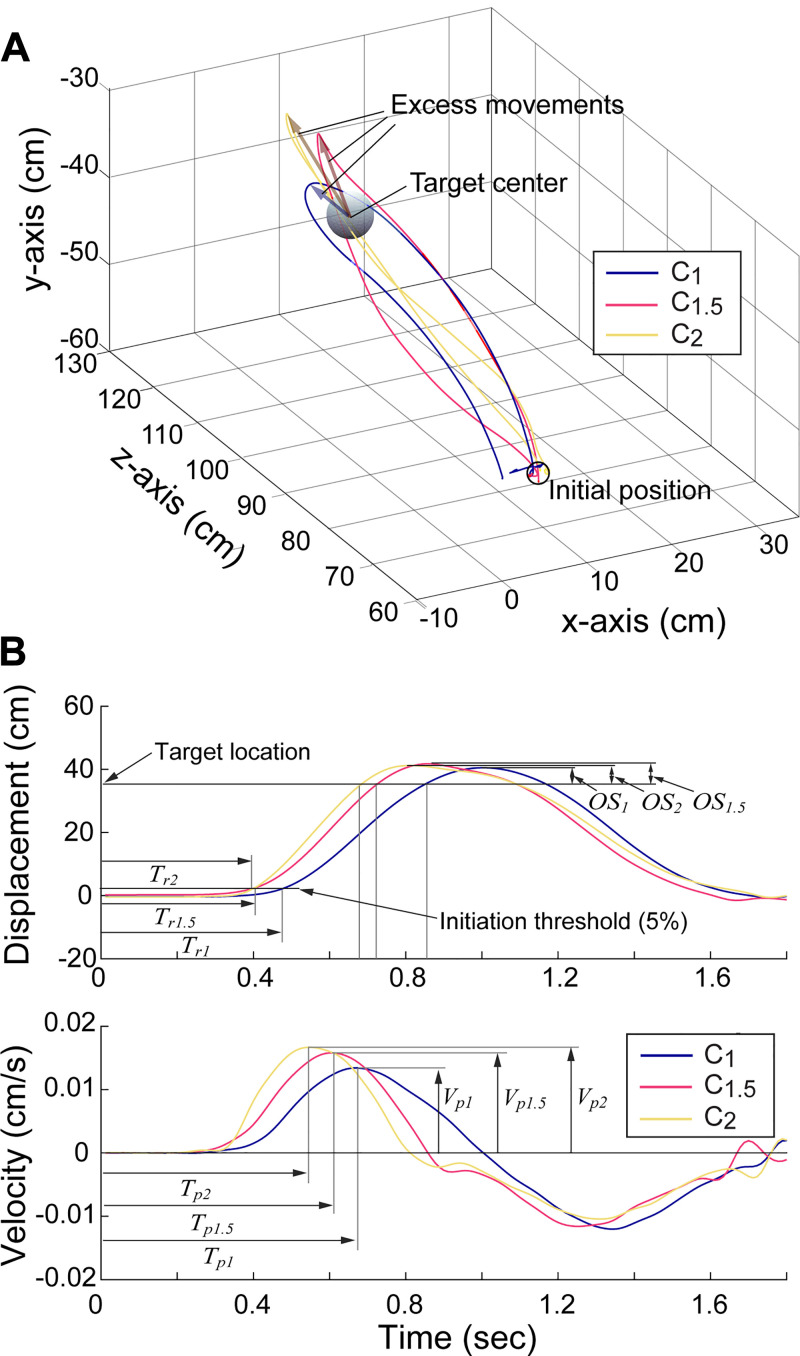
The displacement at a given time was computed as the Cartesian distance between the initial position (position of the controller at the initiation of the trial) and its current position in the VR environment. The speed of each hand/controller was then computed via numerical differentiation (i.e., central difference method) of its displacement. For each trial, first, the time points were determined from the displacement of each controller. Other than the two time points logged by the system, target appearance time (ta) and the task completion time (tf), two additional time points were determined; the movement initiation time (ti) was determined as the time point that the hand moved 5% ti of the total displacement, determined by the initial hand location and the target location. As subjects often continued the movement after reaching the target, the maximum distance time tmax was also determined as the time point at which the maximum distance from the initial position was achieved (see Fig. 2, top). As the movement threshold for the movement initiation time was determined as percentage of the total movement, the ti values estimated from the actual movements and those from the amplified movements were not different.
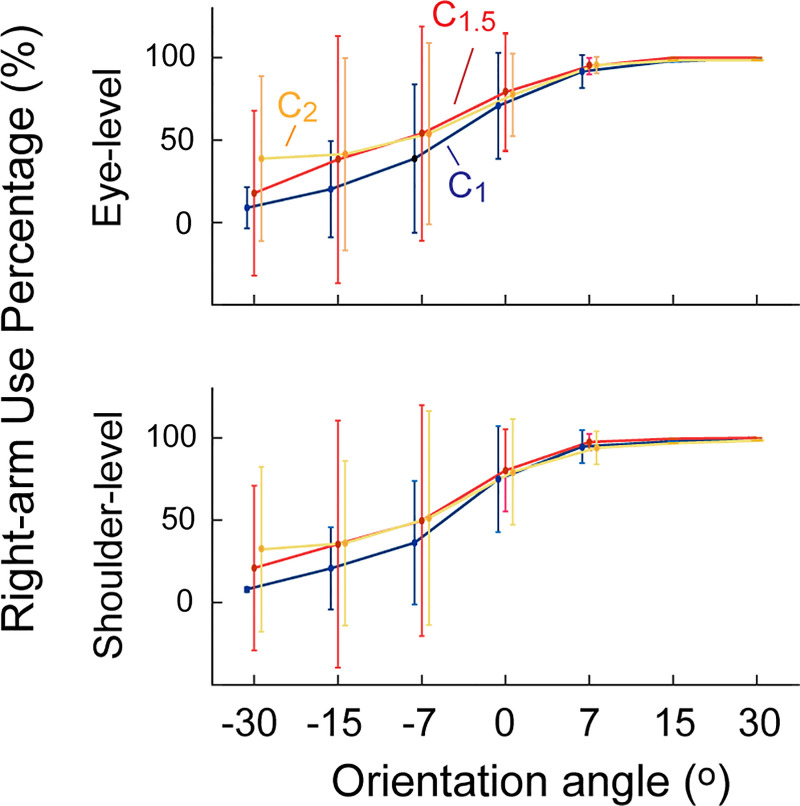
Figure 2.
Change in the right-hand use under visual amplification. Use of the right hand (amplified side) increased across all orientation angles under visual amplification at both levels. A repeated-measures ANOVA showed significant increase in the right-hand use under both conditions (n = 31).
Peak velocity (Vp) was then determined as the maximum velocity observed between the target appearance time (ta) and the task completion time (tf). Reaction time (Tr) was computed as the time elapsed between the target appearance (ta) and the movement initiation (ti). Task duration time (Td) was defined as the total time elapsed from the target appearance (ta) and until the task completion (ti).
The path length ratio (RPL) and the overshoot ratio (ROS) was calculated from the displacement data. RPL was computed by dividing the total distance the hand traveled by the shortest distance between the initial hand location and the target location. ROS was computed by dividing the excess movements of the hand (movement made after reaching the target) by the distance between the initial hand location and the target location (see Fig. 2A).
For each of the variables (VP, Tr, Td, RPL, and ROS), a repeated-measures multivariate analysis-of-variance (rmMANOVA) was performed with the amplification condition (C1, C1.5, and C2) as the within-subject variable, and the parameters computed from the movements of the right and the left hands as the independent variables. Follow-up analyses were conducted on the variables with significant amplification effects, with Bonferroni adjustments for multiple comparisons. Learning effects on the kinematic variables were tested by comparing these values from first half of each block (trials 1–50) with those from its second half (trials 51–100).
Movement vigor.
The movement vigor of individual subjects can be identified based on the “across-subject” relationship between the peak velocity and the movement amplitude (2, 29). The distance to the targets were normalized to be approximately the same distance from the body center (i.e., 80%). To estimate vigor of each reaching movement, we first found the across-subject mean of the peak velocity normalized by the distance across all targets and all conditions.
| (1) |
In the above equation, νi(Ck) denotes the average peak velocity of the subject i under visual amplification condition k(Ck) (k = 1; k = 1.5: or k = 2). Then, the peak velocity of each subject i under visual amplification k can be represented as a scaled value of the across-subject mean of the normalized peak velocity.
| (2) |
Thus the scale factor αi(Ck) was used as a proxy for reach vigor of subject i under condition (Ck).
Between-subject difference in response to visual amplification.
Subjects were categorized based on their responsiveness to the visual amplification. Namely, for each subject, we computed the normalized RUP change (ΔRUP) by comparing the RUP values of both amplification levels to the prior block. Based on the mean ΔRUP values, subjects were categorized into three groups: Adaptors (mean ΔRUP > 5%); Nonresponders (−5% < mean ΔRUP < 5%); and Maladaptors (mean ΔRUP < −5%).
To examine the between-group difference in the pattern of their motor adaptation, normalized changes in the kinematic parameters (peak velocity, reaction time, task duration time, path length ratio, and overshoot ratio) under amplification were compared by RMANOVA with the subject group as a between-subject factor. Follow-up analyses were conducted on the variables with significant amplification effects, with Bonferroni adjustments for multiple comparisons.
Additionally, to test whether subject-specific factors affect the degree of motor adaptation, both Spearman’s and Pearson correlation tests were performed between ΔRUP and the four subject-specific parameters: height, weight, body mass index (BMI), and handedness score. For each correlation test, normality of the correlation was tested by the Shapiro-Wilk test on the independent variable. In case a nonlinearity was found, a nonparametric Spearman’s test was used instead of the Pearson correlation test. Additionally, the normal probability plot of the residuals was inspected. Linearity and homoscedasticity were examined from the scatter plot between the residual and the predicted value for each regression. A Chi-square test was conducted to examine correlations between ΔRUP and their awareness of the visual amplification.
RESULTS
All subjects successfully completed the reaching tasks in all five blocks. Reaching trials with the reaction time larger than 1,000 ms (“distraction”; 121 out of 15,500) or smaller than 100 ms (“anticipatory action”; 147 out of 15,500) were excluded from analysis.
Pretest: Aftereffects and Order Effects
No significant differences in the use frequency of the right hand (i.e., RUP) were found between the three baseline blocks (i.e., no aftereffects; block 1: 65.4% ± 14.5%; block 3: 64.6 ± 18.7%; block 5: 63.3% ± 17.7%; P = 0.752). The RUP values under the visual amplification were not significantly different between the two groups (P = 0.199), indicating that the order did not affect the adaptation patterns.
Increase in Right Arm Usage: Frequency of Use and Workspace
Under both amplification levels, the right-hand use percentage (RUP) significantly increased (F2,60 = 7.909, P < 0.001, = 0.054); when compared with the baseline (61.7% ± 3.3%), the RUP increased to 73.9% ± 4.6% under ×1.5 amplification (t30 = 3.44, P = 0.003, Cohen’s d = 3.05) and to 69.0% ± 5.8% under ×2 amplification (t30 = 3.15, p = 0.015, Cohen’s d = 1.547). The increase in the right-arm usage was observed across all orientation angles and at both height levels (Fig. 2).
We found that the workspace of the right hand expanded toward the left side under visual amplification. For the eye-level targets, the left boundary of the right-arm workspace significantly expanded to 15.4°± 10.7° from 11.4°± 9.9° in the prior block (P = 0.028) under ×1.5 amplification, and to −20.1°± 11.8° from −12.7°± 9.4° in the prior block (P < 0.001) under ×2 amplification. For the shoulder-level targets, the left boundary of the workspace significantly expanded to −18.6°± 11.6° only with the ×2 amplification.
We found some evidence for a learning-related effects in the frequency of use for the amplified arm: RUP in the second half of the ×1.5 amplification block (means ± SD = 68.5 ± 17.8%) was significantly higher than that in its first half (means ± SD = 74.6 ± 17.9%) (P = 0.005). However, no difference in the RUP values was found between the first and second half of the ×2 amplification block.
In summary, visual amplification of motion of the right arm was followed by increased frequency of use and increased territory of use.
Behavioral Changes: Movement Kinematics
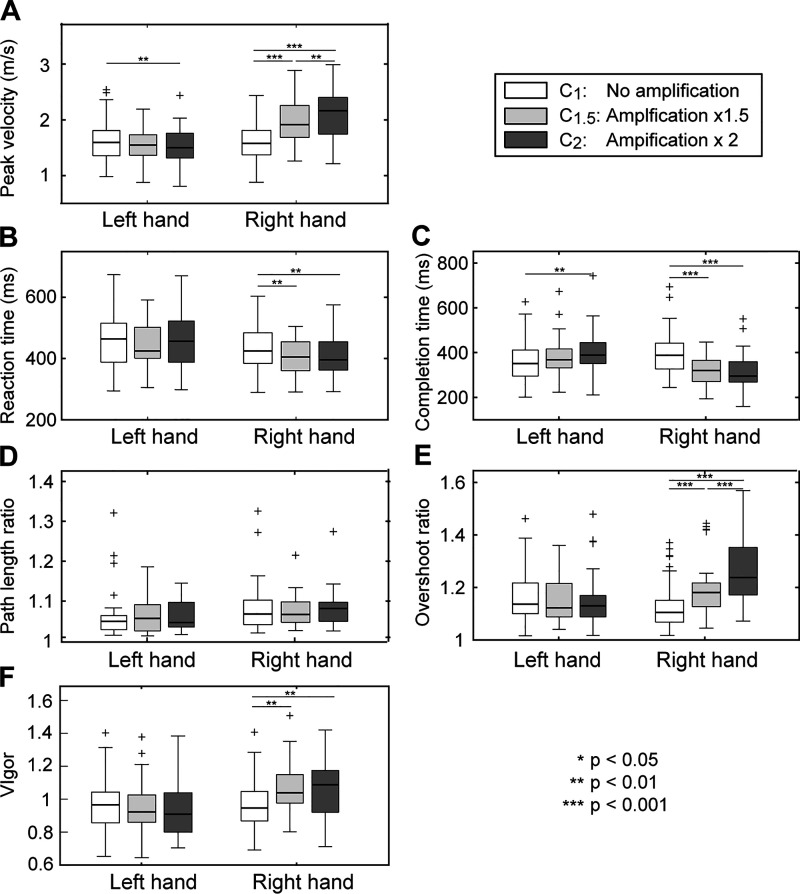
Kinematics of the right-hand movements in VR significantly changed under visual amplification (Fig. 3, A and B). Significant changes were observed in all three temporal parameters (reaction time, peak velocity, and completion time) of the amplified arm, indicating that subjects responded more quickly to the visual cue (shorter reaction time) and moved more swiftly (higher peak velocity/shorter completion time) (reaction time: F2,60 = 8.51, P = 0.001, = 0.05; peak velocity: F2,60 = 75.43, P < 0.001, = 0.21; completion time: F2,60 = 30.22, P < 0.001, = 0.18; Fig. 4, A–C). Similar movement patterns were employed across conditions, as no significant differences between conditions were found in the spatial characteristics of the movement trajectories (path length ratio; all Ps > 0.130; Fig. 4D). However, the overshoot ratio for the amplified arm significantly increased under visual amplification (F2,60 = 36.00, P < 0.001, = 0.22; Fig. 4E). The movement vigor (scale factor ) also significantly increased under the visual amplification (F2,60 = 27.00, P < 0.001, = 0.08; Fig. 4F).
Figure 3.
Movement kinematics under visual amplification. Spatial trajectories of the hand (A) and displacement and velocity of the hand (B) in the 3 conditions (representative case; subject 2). Under amplification, subjects responded more quickly, and moved faster to the target, which resulted in a higher degree of overshoot.
Figure 4.
Kinematic parameters across 3 conditions. A: peak velocity. B: reaction time. C: completion time. D: path length ratio. E: overshoot ratio. F: vigor. Repeated-measures ANOVAs showed significant changes in the peak velocity, reaction time, overshoot ratio, and vigor of the amplified arm (n = 31). The plus symbols are outliers, placed outside the whiskers (interquartile range).
Significant learning-related effects were also found in a subset of kinematic parameters (peak velocity and movement vigor), as subjects moved faster with a greater vigor in the second half of the experimental blocks with both visual amplification levels; the peak velocity increased from 1.82±0.38 m/s to 1.87±0.38 m/s under ×1.5 amplification (P = 0.032) and from 1.93±0.50 m/s to 2.01±0.48 m/s under ×2 amplification (P = 0.026).
Visual amplification of the right-arm movements also significantly affected a subset of the kinematic parameters of the contralateral side (left-side), particularly in the ×2 amplification condition. In contrast to the changes in the kinematics of the amplified arm, movements of the nonamplified arm became slower, as indicated by the significantly lower peak velocity (F2,60 = 6.18, P = 0.004, = 0.01; Fig. 4A) and the longer completion time (F2,60 = 6.66, P = 0.009, = 0.05; Fig. 4C).
Indicator for the Degree of Adaptation: Kinematics of Nonamplified Side
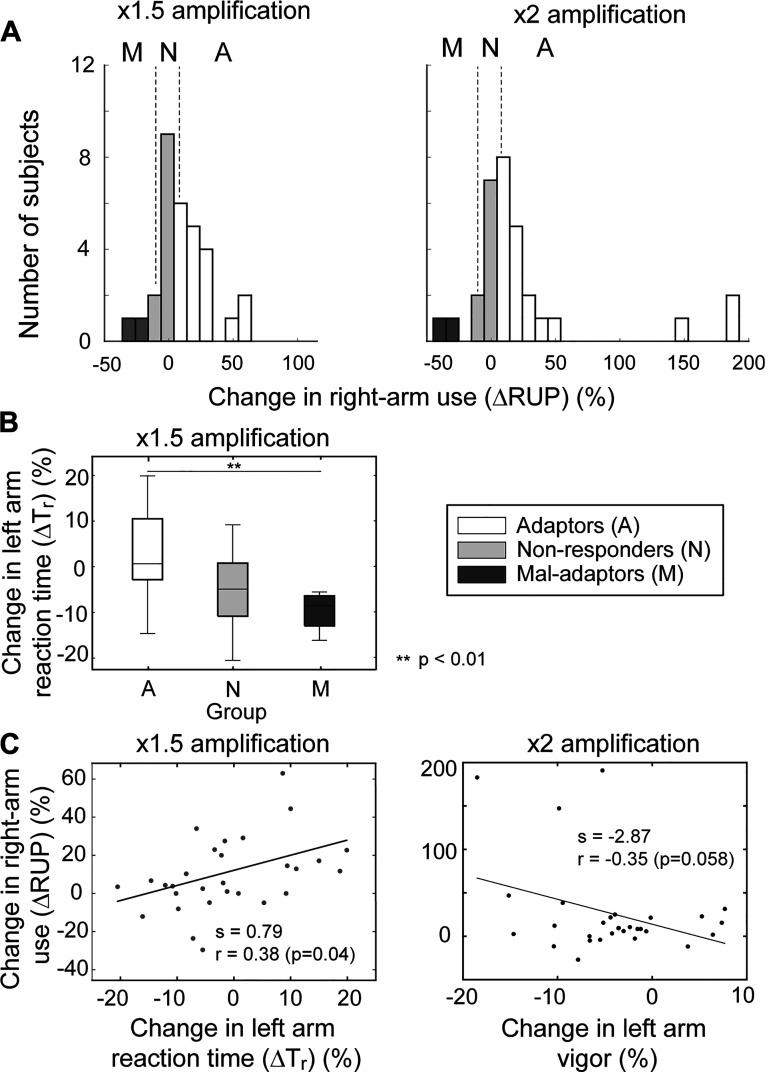
A considerable degree of between-subject variability was observed in their adaptation patterns. The normalized right-arm use change under amplification (ΔRUP) was considerably high for about two-thirds of the subjects (ΔRUP > 5%; Adaptors: 18/31 under ×1.5 amplification, and 20/31 under ×2 amplification), while the changes were relatively small in some subjects (−5% < ΔRUP < 5%; Nonresponders: 9/31 under ×1.5 amplification, and 7/31 under ×2 amplification) (see Fig. 5A). For a small number of subjects, the right-arm usage decreased under amplification (ΔRUP < −5%; Mal-adaptors: 4/31 under ×1.5 amplification and 4/31 under ×2 amplification).
Figure 5.
Between-subject variability in the motor adaptation and kinematics. A: between-subject variability in the level of adaptation (Adaptor: change in right hand use > 5%; nonadaptor: −5% < change < 5%; mal-adaptor: change < −5%). B: change in reaction time (%) between 3 subject groups. C: correlation between the change in left arm kinematics and the change in the right-arm usage. A significant positive correlation was observed between the changes in the reaction time of the left hand and the right arm under ×1.5 amplification condition; a negative correlation (trending toward significance; P = 0.058) was also observed between the vigor of the left arm and the right arm in ×2 amplification condition.
When the patterns of kinematic changes were compared between these subject groups, significant between-group differences were mainly found in the kinematics of the nonamplified side (left). Under ×1.5 amplification, the reaction time of the nonamplified side (left arm) increased among the adaptors but decreased for the mal-adaptors (t11=3.820, P = 0.02, Cohen’s d = 18.15; Fig. 5B). A significant positive correlation was also found between the reaction time change of the left arm (ΔTr) and the change in the right-arm usage (ΔRUP) (r = 0.44, P = 0.04; Fig. 5C); the reaction time of the left arm was greater for those who increased the use of their right arm. Under ×2 amplification, a nonsignificant, but trending toward significance, negative correlation was found between ΔRUP and the change in vigor (r = −0.35, P = 0.058; Fig. 5C), which indicates that the movement vigor of the left arm was significantly lower for those who increased the use of their right arm. However, no such significant differences or correlations were found in the kinematic parameters of the amplified side (right arm) (all Ps > 0.10).
Other Subject-Specific Factors
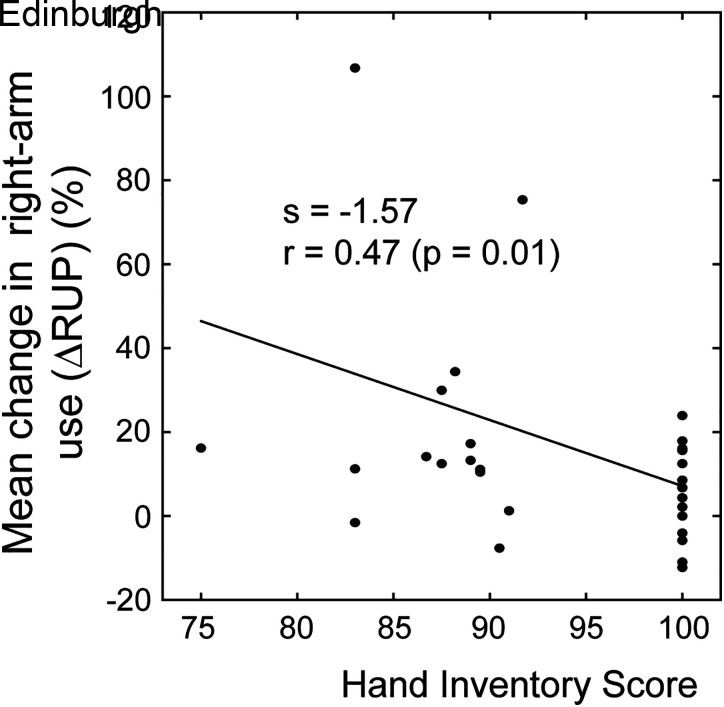
A significant correlation was found between the handedness scores of the subjects and their change in the right-arm usage (ΔRUP) under ×1.5 amplification (Spearman’s ρ = −0.42, P = 0.019; Fig. 6), which showed that strong right-handers did not change their right-arm usage under the visual amplification. Here, nonparametric Spearman test was used as normality was found violated (Shapiro-Wilk test). However, no significant correlations were found between the ΔRUP values and the other subject-specific factors (weight, height, and BMI). In addition, at the baseline, a significant (negative) correlation between the reaction time and peak velocity of the movements (both arms) were found across subjects (r = −0.52; P < 0.01), representing between-subject variability in their response speeds and the initial level of vigor. No significant correlations were found between subjects’ awareness and their change in the right-arm use (ΔRUP).
Figure 6.
Correlation analysis. Negative correlation was observed between the degree of adaptation of subjects and their handedness scores.
DISCUSSION
We found that visual amplification of the movements of one arm leads to an increase in the vigor of its movements in VR environment (velocity, reaction time), resulting in the subsequent increase in the frequency of use. A significant degree of between-subject variability was also observed in the degree of the adaptation (i.e., the change in the right-arm usage), and the dynamics of the nonamplified arm were found to be a strong indicator of the adaptation efficiency of individual subjects.
Mechanisms Underlying Adaptation: Change in Vigor and Reaction Time
The kinematic changes observed under visual amplification (higher velocity, shorter reaction time) confirmed that subjects produced movements with higher vigor when their movements were amplified in the VR environment. Here, significant changes in the higher peak velocity, shorter completion time, and shorter reaction time were observed only in the amplified side (right-arm), indicating that the asymmetric modulation of the visual feedback induced changes selectively in the motor control of the amplified side. Note that, while the shorter completion time and higher peak velocity may be explained in part by the visual amplification of the movements (as the actual range of motion was smaller), the shorter reaction time cannot be explained by such difference in physical demands.
Previous studies showed that the utility of a movement could be increased by manipulating the reward associated with its outcome, for instance, by providing “pleasing” visual/auditory stimuli (23, 25). Movement effort, on the other hand, has been typically considered as a discounting factor that reduce the utility of movements (17); for instance, a significant increase in reaction time was observed when higher effort was required when producing isometric force (30, 31) or movements (32, 33).
The findings from our study demonstrate that reduction in the effort associated with a movement, by means of altered visual feedback, leads to a reduction in reaction time, as well as an increase in velocity of the ensuing movement. Such kinematic changes reflect an increase in the perceived utility of the movement of the amplified arm, which in turn affects their choice of arm selection (i.e., increased use of the amplified arm). Previous studies also showed that different visual feedback conditions can affect arm choice, albeit through a different mechanism; for instance, removing visual feedback can affect task performance perceived by subjects, not their effort (12). In this case, altering visual feedback conditions promoted the use of the nondominant arm, as the perceived task performance (“relative performance”) of the nondominant arm improved under the no-vision condition. In our case, altered visual feedback could have also degraded the task performance due to the mismatch between the visual and proprioceptive information of arm movements. However, subjects still increased the use of amplified arm under visual amplification, indicating that the benefit of reduced effort outweighed potential task degradation.
Altered visual feedback in our study did not directly affect task difficulty (or expected success), unlike the previous studies (15, 16) that further amplified the goal-directed movement components, which could have lowered task difficulty by guiding the movements toward the target. However, it is possible that, as the movements were amplified regardless of the target location in our study, task difficulty could have slightly increased under the visual amplification if subjects had difficulty controlling the altered hand movements.
On the other hand, the kinematics of the nonamplified side (left arm) exhibited an opposite pattern of changes under visual amplification of the right arm, albeit to a lesser degree, as their movements became slower (i.e., lower peak velocity, longer completion time). This can be interpreted in two different ways; first, the reduction in movement velocity may represent lower vigor of perceived movements of the nonamplified arm, as a relative effort level required to produce the reaching movements was higher (when compared with the amplified arm). However, note that the reaction time of the nonamplified arm did not significantly change under amplification. As the reaction time tend to covary with the movement speed (42), which reflects the change in the overall utility of the movement (26, 27), the decrease in the velocity of the nonamplified arm may not be solely due to lower vigor associated with the nonamplified movements. Second, such changes could be interpreted as an attempt [by the central nervous system (CNS)] toward bimanual symmetry. Movements of two limbs in bimanual movements may be planned to achieve “perceptual” symmetry (34), and cross talk occurs at the “programming” (or planning) level (35). Even when the movements of two arms were executed separately, generalization from the reaching movements of the dominant arm to those of the nondominant arm was observed (36). As the actual movements of the amplified arm became much slower under visual amplification, movements of the nonamplified arm may have become slower to achieve “symmetry” in the motor execution and its proprioceptive feedback. Such “transfer” would affect the movement velocity, but not the reaction time, as observed in our data.
Movement Characteristics: Visual Versus Somatosensory Feedback
Our results also suggest that the visual feedback of movements dictated the pattern of behavioral adaptation of the movements. Under visual amplification, the actual displacements of the hand decreased significantly as the hand traveled only 50% (×2 amplification) or 67% (×1.5 amplification) of the distance of the no-amplification condition. However, the degree of reduction in the completion time was not as great (about 90% or the time under ×1.5 amplification; about 80% under ×2 amplification), when compared with the reduction in the reaching distance. This indicates that the movement speed was adjusted to maintain similar movement patterns presented via visual feedback.
Both visual and proprioceptive feedback can guide motor learning process during reaching (e.g., under external perturbation). However, depending on how these two modalities are presented, they could be either well integrated to improve different aspects of motor task (37), or interfere with each other, resulting in one modality (visual feedback) degrading the influence of the other (proprioception) (38). Different brain regions are involved in reaching movements as they receive and process visual and proprioceptive information (39), and the decisions of arm choice can be altered by disrupting how information from different sensory modalities are integrated (via transcranial magnetic stimulation delivered to the posterior parietal cortex; 40). During our experiments (visual amplification of arm movements), in which conflicting information were presented by the two modalities (visual vs. proprioceptive), the CNS appears to have relied more on the visual feedback to determine its movement patterns, as indicated by previous studies (38, 41).
Kinematics of Nonaffected Side: Indicator of Between-Subject Variability in Adaptation
Results from our study also indicate a “structured” between-subject variability in the motor adaptation under visual amplification. Across subjects, a substantial difference was found in their degree of adaptation (i.e., increase in the right arm usage; ΔRUP), and some subjects even decreased the use of their amplified arms under amplification. Interestingly, we found that the degree of adaptation (i.e., increase in the right-arm use) covaried with the pattern of motor adaptation of the nonamplified (left) arm; namely, the movement vigor of the nonamplified arms was significantly higher among the mal-adaptors (when compared with the adaptors). Previous studies have also found a significant degree of between-subject variability in the motor behavior in decision-making (e.g., variability in movement vigor; 42), which were typically explained by the difference in their subjective reward system, such as temporal discount of reward (29). In case of the bimanual decision-making, which requires a subjective comparison/evaluation of the two actuators/systems (arms) to choose subsequent actions, our results suggest that the CNS may rely on the perceived utility (or difficulty) of the “less-efficient” system (left arm), rather than on the efficiency of the “improved/enhanced” system (right arm), to make decisions. “Expected” effort levels are shown to affect arm choice (14), and the relatively higher expected effort associated with the left arm among adaptors seemed to have increased their reaction time and decreased the movement vigor.
The between-subject variability in the degree of adaptation (ΔRUP) is, in part, also related to the handedness of the subjects, as the degree of handedness affected the arm choice in reaching (43). In our experiment, the less the subject was right-handed (i.e., lower Edinburgh score), the higher degree of changes (i.e., increase in the right-hand use) were observed under amplification. Although this seems counterintuitive (as the strong right-handers use their right hands less frequently), such correlation may be explained by the laterality. Right-handedness was found to be associated with a stronger degree of “lateralization” in their information processing (44); in part, this could be explained by the paradigm of brain lateralization (45), which hypothesizes that a left-hemisphere specialize for well-established behavioral patterns while a right-hemisphere is better equipped to deal with rather unfamiliar environments (46). Thus, the less subjects were right-handed, the better they adapted to the new environment, taking advantage of the altered sensory feedback. Left-handers are also shown to have less attentional bias for the hand use (47) and show a lack of lateralization to the dominant hand-hemisphere system (48), indicating that their choice of hand use may be easier to be modified by the task context or sensory feedback.
Implications in Stroke Rehabilitation
After neurological injures such as stroke, functional degradation of the more impaired arm of patients is often accelerated by the behavioral maladaptation, i.e., learned nonuse (49). The results of our study suggest that the visual amplification of one arm could increase its frequency of use during reaching tasks by changing its perceived “utility.” Note that the modulation of visual feedback in our experiment did not directly affect task performance/difficulty as some previous studies (15, 16); thus, our results suggest that the reduction in the energetic cost (associated with the use of one arm) alone can increase its perceived utility, leading to a significant increase in its usage. Indeed, our pilot study with a small number of stroke survivors (n = 2), in which the same visual amplification was implemented, showed that patients significantly increased the use of their more-impaired arm when the range of motion was virtually amplified, suggesting that the visual amplification of the impaired hand can potentially be used to modify/reverse their behavioral maladaptation. However, questions remain whether increased use of the impaired hand of patients in the laboratory will translate to the behavioral adaptation (i.e., use of their more-impaired hands) in their daily activities. Similar to physical rehabilitation (50), the duration and frequency of the training, as well as the training condition (e.g., degree of amplification), are likely to affect the efficacy of the training.
Limitations of the Study
The size of the targets used (12 cm × 12 cm × 12 cm) was relatively large; thus the effects of endpoint accuracy on bimanual choice were relatively small. Previous studies on bimanual choice (17) showed that intrinsic biomechanical properties and endpoint accuracy both could influence limb choice and use and that the handedness bias was correlated with the expected effort and overall difference in success between the dominant and nondominant hands. However, as the effects of endpoint accuracy were small due to the experimental design of the current study, it is possible that the behavioral adaptation under altered visual feedback could be different when targets with a smaller size were used.
We only recruited right-handed subjects in this study and the visual amplification was only applied their dominant hand. It is possible that a larger degree of between-subject variability is observed when the visual amplification is applied to the nondominant hand, in which case the endpoint accuracy could also play a larger role in the adaptation rate.
Due to the relatively short duration of the testing, we could not verify whether the increase in the amplified hand use would be retained after the visual amplification was removed. To examine the long-term retention of the observed behavioral modification, the altered visual feedback should be administered in multiple sessions, with a varying degree of time intervals between the sessions.
GRANTS
J.W., P.S.L., and S.W.L. were supported by National Institute on Disability, Independent Living, and Rehabilitation Research Grant 90REGE0004. R.S. was supported by National Institutes of Health Grants R01-NS078311, R01-EB028156, and R01-NS096083 and the National Science Foundation Grant CNS-1714623.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.S.L., R.S., and S.L. conceived and designed research; J.W. performed experiments; J.W., P.S.L., and S.L. analyzed data; P.S.L., R.S., and S.L. interpreted results of experiments; S.L. prepared figures; J.W. and S.L. drafted manuscript; J.W., P.S.L., R.S., and S.L. edited and revised manuscript; J.W., P.S.L., R.S., and S.L. approved final version of manuscript.
REFERENCES
- 1.Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke 33: 1840–1844, 2002. doi: 10.1161/01.STR.0000019289.15440.F2. [DOI] [PubMed] [Google Scholar]
- 2.Raghavan P. Upper limb motor impairment post stroke. Phys Med Rehabil Clin N Am 26: 599–610, 2015. doi: 10.1016/j.pmr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxbaum LJ, Varghese R, Stoll H, Winstein CJ. Predictors of arm non-use in chronic stroke: a preliminary investigation. bioRxiv 702159: 2019. doi: 10.1101/702159v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub E, Heitmann RD, Barro G. Alertness, level of activity, and purposive movement. Ann NY Acad Sci 290: 348–365, 1977. doi: 10.1111/j.1749-6632.1977.tb39737.x. [DOI] [PubMed] [Google Scholar]
- 5.Norouzi-Gheidari N, Archambault PS, Fung J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: systematic review and meta-analysis of the literature. J Rehab Res Dev 49: 479–496, 2012. doi: 10.1682/JRRD.2010.10.0210. [DOI] [PubMed] [Google Scholar]
- 6.Levy J, Nagylaki T. A model for the genetics of handedness. Genetics 72: 117–128, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren JM. Handedness and laterality in humans and other animals. Psychobiology 8: 351–359, 1980. doi: 10.3758/BF03337470. [DOI] [Google Scholar]
- 8.Sainburg RL. Lateralization of goal directed movements. In: Vision and Goal-Directed Movement, edited by Elliott D, Khan M.. Champaign, IL: Human Kinetics Publishers, 2010. [Google Scholar]
- 9.Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88: 2408–2421, 2002. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healey JM, Liederman J, Geschwind N. Handedness is not a unidimensional trait. Cortex 22: 33–53, 1986. doi: 10.1016/S0010-9452(86)80031-4. [DOI] [PubMed] [Google Scholar]
- 12.Pryzbyla A, Coelho CJ, Akpinar S, Kirazci S, Sainburg RL. Sensorimotor performance asymmetries predict hand selection. Neuroscience 228: 349–360, 2013. doi: 10.1016/j.neuroscience.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakker RS, Weijer RHA, van Beers RJ, Selen LPJ, Medendorp WP. Decisions in motion: passive body acceleration modulates hand choice. J Neurophysiol 117: 2250–2261, 2017. doi: 10.1152/jn.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweighofer N, Xiao Y, Kim S, Yoshioka T, Gordon J, Osu R. Effort, success, and nonuse determine arm choice. J Neurophysiol 114: 551–555, 2015. doi: 10.1152/jn.00593.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballester BR, Nirme J, Duarte E, Cuxart A, Rodriguez S, Verschure P, Duff A. The visual amplification of goal-oriented movements counteracts acquired non-use in hemiparetic stroke patients. J Neuroeng Rehabil 12: 50, 2015. doi: 10.1186/s12984-015-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballester BR, Maier M, San Segundo Mozo RM, Castañeda V, Duff A, Verschure PF. Counteracting learned non-use in chronic stroke patients with reinforcement-induced movement therapy. J Neuroeng Rehabil 13: 74, 2016. doi: 10.1186/s12984-016-0178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shadmehr R, Huang HJ, Ahmed AA. A representation of effort in decision-making and motor control. Curr Biol 26: 1929–1934, 2016. doi: 10.1016/j.cub.2016.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian R, Howe RD, Matsuoka Y. Task performance is prioritized over energy reduction. IEEE Trans Biomed Eng 56: 1310–1317, 2009. doi: 10.1109/TBME.2008.2006683. [DOI] [PubMed] [Google Scholar]
- 19.Haith AM, Reppert TR, Shadmehr R. Evidence for hyperbolic temporal discounting of reward in control of movements. J Neurosci 32: 11727–11736, 2012. doi: 10.1523/JNEUROSCI.0424-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLoS Comput Biol 4: e1000133, 2008. doi: 10.1371/journal.pcbi.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niv Y, Daw ND, Dayan P. Choice values. Nat Neurosci 9: 987–988, 2006. doi: 10.1038/nn0806-987. [DOI] [PubMed] [Google Scholar]
- 22.Rigoux L, Guigon E. A model of reward- and effort-based optimal decision making and motor control. PLoS Comput Biol 8: e1002716, 2012. doi: 10.1371/journal.pcbi.1002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shadmehr R. Control of movements and temporal discounting of reward. Curr Opin Neurobiol 20: 726–730, 2010. doi: 10.1016/j.conb.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Summerside EM, Shadmehr R, Ahmed AA. Vigor of reaching movements: reward discounts the cost of effort. J Neurophysiol 119: 2347–2357, 2018. doi: 10.1152/jn.00872.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon T, Geary RB, Ahmed AA, Shadmehr R. Control of movement vigor and decision making during foraging, Proc Natl Acad Sci USA 115: E10476–E10485, 2018. [Erratum in Proc Natl Acad Sci USA 115: E11884, 2018]. doi: 10.1073/pnas.1812979115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shadmehr R, Reppert TR, Summerside EM, Yoon T, Ahmed AA. Movement vigor as a reflection of subjective economic utility, Trends Neurosci 42: 323–336, 2019. doi: 10.1016/j.tins.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shadmehr R, Ahmed AA. Vigor: Neuroeconomics of Movement Control. Cambridge, MA: The MIT Press, 2020. [DOI] [PubMed] [Google Scholar]
- 28.Peters M. Handedness and its relation to other indices of cerebral lateralization. In: Brain Symmetry, edited by Davidson RJ, Hugdahl K.. Cambridge, MA: The MIT Press, 1995, p. 183–214. [Google Scholar]
- 29.Choi JE, Vaswani PA, Shadmehr R. Vigor of movements and the cost of time in decision making. J Neurosci 34: 1212–1223, 2014. doi: 10.1523/JNEUROSCI.2798-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irvy RB. Force and timing components of the motor program. J Mot Behav 18: 449–474, 1986. doi: 10.1080/00222895.1986.10735390. [DOI] [PubMed] [Google Scholar]
- 31.Stelmach GE, Worringham CJ. The preparation and production of isometric force in Parkinson’s disease. Neuropsychologia 26: 93–103, 1988. doi: 10.1016/0028-3932(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 32.Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res 99: 97–111, 1994. doi: 10.1007/BF00241415. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum DA. Human movement initiation: specification of arm direction, and extent. J Exp Psychol Gen 109: 444–474, 1980. doi: 10.1037/0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- 34.Mechsner F, Kerzel D, Knoblich GE, Prinz W. Perceptual basis of bimanual coordination. Nature 414: 69–73, 2001. doi: 10.1038/35102060. [DOI] [PubMed] [Google Scholar]
- 35.Spijkers W, Heuer H. Structural constraints on the performance of symmetrical bimanual movements with different amplitudes. Q J Exp Psychol 48A: 716–740, 1995. doi: 10.1080/14640749508401412. [DOI] [Google Scholar]
- 36.Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. doi: 10.1152/jn.00622.2002. [DOI] [PubMed] [Google Scholar]
- 37.Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. J Neurophysiol 93: 3200–3213, 2005. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- 38.Adams JA, Gopher D, Lintern G. The effects of visual and proprioceptive feedback on motor learning. Proc Hum Factors Soc Annu Meet 19: 162–165, 1975. doi: 10.1177/154193127501900204. [DOI] [Google Scholar]
- 39.Filimon F, Nelson JD, Huang RS, Sereno MI. Multiple parietal reach regions in humans: cortical representations for visual and proprioceptive feedback during on-line reaching. J Neurosci 29: 2961–2971, 2009. doi: 10.1523/JNEUROSCI.3211-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira FT, Diedrichsen J, Verstynen T, Duque J, Irvy RB. Transcranial magnetic stimulation of posterior parietal cortex affects decisions of hand choice. Proc Natl Acad Sci USA 107: 17751–17756, 2010. doi: 10.1073/pnas.1006223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutha PK, Boulinguez P, Sainburg RL. Visual modulation of proprioceptive reflexes during movement. Brain Res 1246: 54–69, 2008. doi: 10.1016/j.brainres.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reppert TR, Rigas I, Herzfield DJ, Sedaghat-Nejad E, Komogortsev O, Shadmehr R. Movement vigor as a traitlike attribute of individuality. J Neurophysiol 120: 741–757, 2018. doi: 10.1152/jn.00033.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stins JF, Kadar EE, Costall A. A kinematic analysis of hand selection in a reaching task. Laterality 6: 347–367, 2001. doi: 10.1080/713754421. [DOI] [PubMed] [Google Scholar]
- 44.Laeng B, Peters M. Cerebral lateralization for the processing of spatial coordinates and categories in left- and right-handers. Neuropsychologia 33: 421–439, 1995. doi: 10.1016/0028-3932(94)00126-A. [DOI] [PubMed] [Google Scholar]
- 45.MacNeilage PF, Rogers LJ, Vallortigara G. Origins of the left & right brain. Sci Am 301: 60–67, 2009. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- 46.Sainburg RL. Convergent models of handedness and brain lateralization. Front Psychol 5: 1092, 2014. doi: 10.3389/fpsyg.2014.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckingham G, Main JC, Carey DP. Asymmetries in motor attention during a cued bimanual reaching task: Left and right handers compared. Cortex 47: 432–440, 2011. doi: 10.1016/j.cortex.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Pool EM, Rehme AK, Fink GR, Eickhoff SB, Grefkes C. Handedness and effective connectivity of the motor system. Neuroimage 99: 451–460, 2014. doi: 10.1016/j.neuroimage.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol 104: 125–132, 1989. doi: 10.1016/S0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 50.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D; EXCITE Investigators . Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 296: 2095–2104, 2006. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]