Abstract
Dysregulated kynurenine (KYN) pathway has been implicated in the pathophysiology of depression. In this systematic review, we examined the relationship between kynurenine pathway metabolites (KYN, kynurenic acid KYNA, tryptophan TRP, quinolinic acid QUIN, KYN to TRP ratio) and depression symptoms in the context of pro-inflammatory activation and immune response. Out of 5,082 articles, fifteen studies were suitable; ten studies (N=315 medically ill patients treated with Interferon-alpha IFN-α) reported baseline and post-intervention plasma KYN, TRP and KYN/TRP ratios which were included in quantitative meta-analysis. Data from five studies were summarized (Interferon-alpha IFN-α, Interferon-beta IFN-β, and lipopolysaccharide LPS). We found that IFN-αtreatment in patients with chronic illnesses was associated with decreased TRP, increased levels of KYN and KYN/TRP ratio and depression scores from baseline to follow-up at both 4 and 24 weeks. Our findings suggest that increased risk of depression observed after immune-activating agents in patients with chronic medical illnesses likely is mediated by the kynurenine pathway. Further prospective studies are required to investigate the exact pathophysiology of the KYN pathway in depression.
Keywords: Depression, Kynurenine, Kynurenic acid, Tryptophan, Interferon, Inflammation, Mood Disorder
Introduction
Major Depressive Disorder (MDD) is the most common mental health problem and the second major contributor to the global disease burden worldwide (Ferrari et al., 2013). Patients with depression have an increased risk of mortality, which persists for up to two decades, even after symptomatic remission (Cuijpers et al., 2014). Only 50-60% of depressed patients achieve full remission with usual pharmacotherapy, and the need for more treatment options is increasingly imperative (Rush et al., 2006).
An increasing number of studies demonstrate an association between inflammatory markers and altered mood (Harrison et al., 2009; Wright et al., 2005). Notably, patients with a history of MDD have been shown to have elevated levels of interleukin-6 (IL-6) despite the presence of comorbidities (Dowlati et al., 2010). Inflammatory markers such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) may also be associated with depressive symptoms (Leonard and Maes, 2012). One of the possible mechanisms by which these cytokines induce depression is the kynurenine pathway (Dantzer, 2017; Dantzer et al., 2011; Tanaka et al., 2020).
The kynurenine (KYN) pathway is the process by which tryptophan (TRP) is broken down into KYN metabolites leading to the production of nicotinamide adenine dinucleotide (NAD+) (Figure 1). TRP is an essential amino acid and precursor of serotonin, a key neurotransmitter involved in the regulation of mood and a target for most antidepressant treatments. The KYN pathway accounts for 95% of TRP metabolism (Gál and Sherman, 1980), produces metabolites collectively known as kynurenines, and altered levels of kynurenines have been implicated in psychiatric and neurodegenerative diseases (Lovelace et al., 2017; Maddison and Giorgini, 2015). Altered KYN metabolites have been found in MDD, cancer, diabetes, cardiovascular disease, autoimmune syndromes, and other neurodegenerative diseases such as Alzheimer’s, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) (Lovelace et al., 2017).
Figure 1. Degradation of Tryptophan (TRP) in the Kynurenine pathway and the main metabolites.
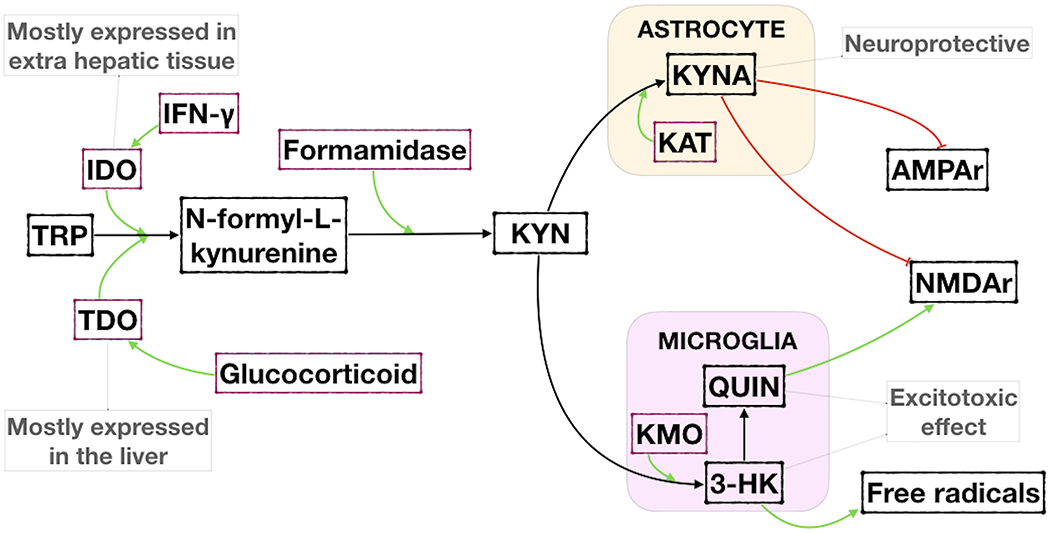
IFN-γ = Interferon-γ; IDO = Indoleamine 2,3-dioxygenase; TDO = Tryptophan 2,3-dioxygenase; KYN = L-kynurenine; KAT = Kynurenine aminotransferase; KMO = Kynurenine 3-monooxygenase; KYNA = Kynurenic acid; QUIN = Quinolinic acid; 3-HK = 3-hydroxykynurenine; AMPAr = Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype glutamate receptor; NMDAr = N-methyl-D-aspartate receptor.
The KYN pathway begins with the conversion of TRP to N-formylkynurenine by tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO) in the liver or extrahepatic tissues, respectively (Fujiwara et al., 1978). IDO is more specifically found in macrophages, monocytes, microglia, astrocytes, and neurons (Guillemin et al., 2007; Jones et al., 2013). IDO has two isoforms, IDO1 and IDO2, and IDO1 is considered the major enzyme involved in tryptophan degradation (Löb et al., 2009). Kynurenic acid (KYNA) is formed from L-kynurenine, a known N-methyl-d-aspartate (NMDA) antagonist that may exhibit antidepressant and neuroprotective effects (Müller and Schwarz, 2008a; Stone, 2000). Quinolinic acid (QUIN) is an NMDA agonist and, together with 3-hydroxykynurenine (3-HK) is considered to be neurotoxic (Guillemin, 2012; Müller et al., 2009). Chronic exposure of neurons to elevated QUIN disrupts the structure of dendrites and decreases microtubule-associated protein 2 immunoreactivity, which perpetuates neurodegenerative disease (Kerr et al., 1998). Picolinic acid is also an NMDA agonist, although it is considered neuroprotective (Guillemin et al., 2007).
Evidence shows that inflammatory mediators increase the activity of IDO, as well as its gene expression (Werner-Felmayer et al., 1989). Inflammatory cytokines, such as IL-6, TNF-α, and IFN-γ have been shown to activate the KYN pathway (Leonard and Maes, 2012). Increases in IL-6 result in an elevation of KYN and KYNA (Schwieler et al., 2015). The release of TNF-α by macrophages activates nuclear factor κB (NFκB), which triggers an increase in IL-6 as well as the production of IFN-γ by T cells (Leonard and Maes, 2012). IFN-γ, produced in response to infection, has been shown to activate IDO specifically (Pfefferkorn, 1984). Activation of IDO promotes the catabolism of TRP and increases the production of KYN, 3-HK, and 3-hydroxylanthranilic acid, which inhibits the proliferation of pathogens (O’Farrell and Harkin, 2017). Furthermore, inflammation can activate kynurenine 3-monooxygenase (KMO) that can potentially favor the neurotoxic metabolites of the KYN pathway (Connor et al., 2008).
In our recent systematic review, we found that people with unipolar major depression had lower KYN levels than healthy controls, although TRP levels and KYN/TRP ratio were not significantly different (Arnone et al., 2018). However, most of the studies included in the review were cross-sectional case-control studies that likely are affected by illness heterogeneity and other confounders. A prospective clinical study will help clarify the relationship between KYN and the emergence of mood symptoms. Prospective follow-up studies investigating human subjects treated with drugs that activate the immune system (such as IFN-α) or administered an inflammatory challenge (such as lipopolysaccharides LPS) have been widely used as experimental paradigms to study the relationship between elevated cytokines, and KYN metabolites in depression (Dantzer, 2017). Prospective follow-up studies usingIFN-α in healthy humans to study the emergence of depression symptoms is not feasible due to ethical reasons. IFN-α has been used as a treatment for a wide range of chronic illnesses such as hepatitis C, malignancies, and multiple sclerosis (George et al., 2012). IFNtreatment-induced emergence of depression is a common iatrogenic side effect of IFN therapy, occurring in up to 60% of patients (Capuron and Miller, 2004). Increases in plasma KYN metabolite levels in hepatitis C and cancer patients undergoing IFN-αtherapy have been associated with depressed mood symptoms (Capuron and Miller, 2004; Smith et al., 2011). Although a previous history of depression and other physical comorbidities are predictors of the early development of depression duringIFN treatment (Smith et al., 2011), the precise role of KYN metabolites in the causation of treatment-induced or secondary, depression is less clear.
In this systematic review, we evaluated the effect of immune activation treatments (IFN-α or Interferon-beta IFN-β) administered to patients treated for chronic medical conditions and experimental inflammatory challenge (LPS or vaccines) on KYN metabolites in humans. We predict that the administration of inflammatory challenge results in decreased TRP, KYNA, elevated KYN, KYN/TRY ratio (as proxy measure of IDO activity), and QUIN. In addition, we assessed the relationship between KYN metabolites and depression symptoms, with the prediction of a positive correlation between elevated KYN and depression symptoms.
Methods
Search Strategy
The research was conducted following the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Moher et al., 2009). The following eligibility criteria were designated to study the relationship between agents that activate the immune system and inflammation and KYN pathway metabolite levels: 1) English language articles, 2) human in vivo studies, 3) clinical or experimental studies in which an immune activation agent or inflammatory challenge was administered, and serum KYN metabolites were periodically measured before and after the intervention. We excluded any studies that were 1) animal studies, 2) in vitro, 3) administered antidepressants or anti-inflammatory drugs to patients without clear discrimination between groups exposed to these drugs, 4) only measured non-serum levels of KYN. For the secondary analysis, we also collected data on mood, as it was available from the selected studies.
A systematic search was conducted on Ovid Medline, Elsevier EMBASE, Cochrane library, and Ovid PsycINFO on June 21, 2019. The search terms included: “Vaccines” [MESH] OR “vaccines” [ti,ab,kw] OR “endotoxins” [MESH] OR “endotoxins” [ti,ab,kw] OR “lipopolysaccharides” [MESH] OR “lipopolysaccharides” [ti,ab,kw] OR “Interferon-alpha” [MESH] OR “Interferon-alpha” [ti,ab,kw] OR “Interferon-beta” [MESH] OR “Interferon-beta” [ti,ab,kw] OR “inflammatory challenge” [ti,ab,kw] AND “Kynurenine” [MESH] OR “kynurenine” [ti,ab,kw] OR “Quinolinic Acid” [MESH] OR “quinolinic acid” [ti,ab,kw] OR “Picolinic Acids” [MESH] OR “picolinic acid” [ti,ab,kw] OR “3-Hydroxyanthranilic Acid” [MESH] OR “3-Hydroxyanthranilic Acid” [ti,ab,kw] OR “kynurenine metabolite” [ti,ab,kw] OR “Tryptophan” [MESH] OR “tryptophan” [ti,ab,kw] OR “Indoleamine-Pyrrole 2,3,-Dioxygenase” [MESH] OR “Indoleamine-Pyrrole 2,3,-Dioxygenase” [ti,ab,kw]. A detailed search strategy can be made available upon request. Articles were deduplicated then assessed by two independent reviewers (CH and VACL) for eligibility. Demographic information and KYN metabolite data were then systematically extracted for analysis. Specifically, we extracted sample size, demographic variables of the study participants such as age and sex, type of inflammatory challenge and agents, patient illness, medications in the protocol, KYN biomarkers and mood rating scores. Study authors were contacted for missing or additional data.
Summary Measures and Data Extraction
Meta-analysis was performed to determine standardized mean change (SMC) over time from baseline for four relevant measures (KYN, KYN/TRP ratio, TRP, and Hamilton Depression Rating scores (HAM-D)) to two follow-up points (4 weeks later; 24 weeks later). The follow-up points, which were selected due to the cumulative incidence of significant depressive symptoms in 1,391 euthymic patients with chronic hepatitis C who were started IFN plus ribavirin, were 6% and 25% after 4 and 24 weeks respectively (Udina et al., 2012). We investigated both the early and late changes in KYN metabolites and their association with the onset of depression. Positive SMC values reflected increases from baseline. Data were extracted from each article and checked for accuracy. Where possible, raw values were extracted from tables, descriptive text or plots for baseline and follow-up measurements. Given the assumption of some meaningful degree of study-level variability, random (as opposed to fixed) effects were used to determine the SMC in each model. As the SMC requires each model to stipulate a correlation between time points for each measure, and this correlation is not typically known a priori (Cuijpers et al., 2017), a generic value was set for each model. This generic value was set to r = 0.54, following from a reported median value for within-group correlation across studies of continuous outcomes for individuals receiving active treatments in the literature (Balk et al., 2012). Follow-up sensitivity analyses evaluated the degree to which each model was robust to this assumption by also testing lower and higher values for the correlation (r = 0.34 and r = 0.74, respectively).
Forest plots were generated for each model to visualize the relative contribution of each study to the SMC. Funnel plots with trim and fill were used to explore the possibility of publication bias. Asymmetry was assessed visually, as Egger’s test of asymmetry was considered inappropriate for the present analysis because fewer than ten studies were included in each model. Individual study influence was investigated via leave-one-out jackknife sensitivity analysis, whereby SMC values were calculated by omitting each study in turn. All analyses were performed using the metafor package (Viechtbauer, 2010) in the R statistical computing environment [34].
Results
Study Characteristics
The database search identified 5,082 eligible studies, of which 1,281 duplicates were removed. Following assessment for eligibility and inclusion of one additional study from hand search, 15 studies were included in the final review. Of these 15 studies, ten IFN-αstudies were included in the quantitative meta-analysis (Bannink et al., 2007; Baranyi et al., 2015; Bonaccorso et al., 2002; Capuron et al., 2003; Comai et al., 2011; Frick et al., 2004; Pawlowski et al., 2018; Van Gool et al., 2008; Wichers et al., 2005; Zignego et al., 2007). A total of 315 patients (200 men and 152 women; mean age: 47 standard deviation (SD): 4.3) that received IFN-α and took part in the prospective follow up. Data from the other five studies are summarized in Table 1(one IFN-α (Raison et al., 2010), two IFN-β (Amirkhani et al., 2005; Durastanti et al., 2011), two LPS (Kruse et al., 2019; Padberg et al., 2012) (Figure 2). Also, due to limitations on QUIN and KYNA data, full quantitative analysis was not possible. Table 1 summarizes the details of all the studies included in this review.
Table 1.
Effect of Immune Activation on the Kynurenine Pathway and Depression symptoms - Clinical characteristics and main findings of studies
| Author | Type of Study | Patients/Controls | Clinical context | Drug administered/ Inflammatory challenge |
Biomarkers | Mood Scale | Main findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Patients Age | ||||||||||
| Total | Mean | SD | M | F | |||||||
| Bonaccorso et al., 2002 | Prospective Nonrandomized Study | 18 | 40.5 | 11.5 | 10 | 8 | HCV | IFN-α | KYN, Amino acids and 5-HT | MADRS, HAM-A | ↓ plasma TRP and ↑ serum KYN and the K/T ratio ↓ serum 5-HT ↑ MADRS and HAM-A |
| Capuron et al., 2003∞ | Randomized Controlled Double-blind Trial (prospective longitudinal arm of the study) | 26 | 53 | 10 | 14 | 12 | Melanoma | IFN-α,+ Paroxetine or placebo | Free TRP, KYN and neopterin | DSM-IV, HAM-D | ↑ plasma KYN and neopterin concentrations; ↑ KYN/TRP ratio. |
| Frick et al., 2004 | Prospective Nonrandomized Study | 15 | 53 | 13 | 9 | 6 | Melanoma | IFN-α | TRP, KYN, KYN/TRP ratio, homocysteine, cysteine, folate, vitamin B12 and neopterin. | NR | ↑ neopterin and ↓ TRP, ↔homocysteine |
| Wichers et al., 2005 | Prospective Nonrandomized Study | 16 | 42 | 7.7 | 12 | 4 | HCV | IFN-α, Ribavirin | TRP, KYN, KA and competing amino acids | MADRS | ↑ KYN/TRP ratio, ↓TRP ↑ KYN/KA ratio; + correlation with higher MADRS scores. |
| Bannink et al., 2007 | Prospective Nonrandomized Study | 43* | 58 (median) | NR | 25 | 18 | Renal Cell Carcinoma, Melanoma | IFN-α | TRP, KYN, 5-HIAA, biopterin, neopterin, TRP/LNAA ratio, KYN/LNAA ratio and, KYN/TRP ratio | MADRS, BAS, SCL-90 | ↓ TRP at all-time points and ↓TRP/LNAA ratios at 2 out of 3 time points |
| Van Gool et al., 2008 | Prospective Nonrandomized Study | 24** | 60,5 (median) | NR | 16 | 8 | Renal Cell Carcinoma | IFN-α | KYN, KA, 3-HAA, KA/3-HAA ratio, KA/KYN ratio, KYN/LNAA ratio | SCL-90 | ↑ KYN and KYN/LNAA ratio. ↓ KA/3-HAA ratio and the KA/KYN ratio No correlations with depression |
| Zignego et al., 2007 | Prospective Nonrandomized Study | 39 | 46 | 11 | 22 | 17 | HCV | IFN-α, Ribavirin | Serum TRP and KYN; and IDO activity in macrophages | HDRS | ↓ serum TRP and more frequent anxiety and depression |
| Comai et al., 2011 | Prospective Nonrandomized Controlled Study | 45 | 46.9 | 11.8 | 26 | 19 | HCV | IFN-α, Ribavirin | TRP, KYN, 5-HTP, KYN/TRP ratio | BDI | ↓serum total non-protein TRP; ↑ KYN levels and KYN/TRP; ↓ 5-HTP. |
| Baranyi et al., 2015 | Prospective Nonrandomized Study | 35 | 46.8 | 12.7 | 21 | 14 | HCV | IFN-α, Ribavirin | TRP, KYN, QUIN, KYN/TRP ratio | HAMD-17 | ↑ HAMD-17; ↑ KYN/TRP ratio. ↑ QUIN. |
| Pawlowski, Tomasz et al., 2018*** | Prospective Nonrandomized Study | 101 | 46.6 | 11.1 | 51 | 50 | HCV | IFN-α, Ribavirin | TRP, KYN, AA, KYNA, KYN/TRP ratio | MADRS | ↑ KYN/TRP ratio persisting 6 months after the end of treatment. ↑ KYNA and AA concentration ↓Serum total TRP and 5-HT. |
| Amirkhani et al., 2005 $ | Prospective Nonrandomized Controlled Study | 27 | 39 | 1 | 5 | 22 | Multiple sclerosis | IFN-β | TRP, KYN, KYNA | NONE | ↑ KYN/TRP ratio |
| Durastanti et al., 2011 $$ | Prospective Randomized Study | 101**** | 34.8 | 8.3 | 30 | 71 | Multiple sclerosis | IFN-β | TRP, KYN and neopterin | NONE | ↑ Neopterin and ↑ KYN/TRP ratio over time vs baseline in both treatment groups |
| Padberg et al., 2012 | Prospective Nonrandomized Study | 6 | 32 | 4 | 6 | 0 | Healthy | E coli LPS | TRP, KYN, KYN/TRP ratio | NONE | ↑ KYN/TRP ratio |
| Raison et al., 2010 | Prospective Nonrandomized Controlled Study | 16 | 47.7 | 6.8 | 10 | 6 | HCV | IFN-α, Ribavirin | TRP, KYN, KYNA, QUIN, IFN-a, sTNFR2, IL-6, sIL-6R | SCID and MADRS | ↑ KYN in peripheral blood and ↑ increased KYN, QUIN and KA in the CSF. ↓ TRP and ↑ KYN/TRP ratio in peripheral blood ↔TRP levels in the CSF. ↑ MADRS scores. |
| Kruse et al., 2019 | Randomized Controlled Double-blind Trial (prospective longitudinal arm of the study) | 61 | 24.9 | 7.1 | 38 | 23 | Healthy | Escherichia coli group O:113 | TRP, KYN, KYNA, QUIN, KYN/TRP ratio, IL-6 and TNF-α | Profile of Mood States | ↑KYN/TRP ratio |
| Quantitative and Qualitative analysis | |||||||||||
| Qualitative analysis | |||||||||||
M=male; F=female; SD= standard deviation; NR=not reported; TRP - Tryptophan; KYN - Kynurenine; KRY/TRP ratio – Kynurenine/Tryptophan ratio; KYNA - Kynurenine Acid; QUIN - Quinolinic acid; IDO – indoleamine 2,3-dioxygenase; IFN-α – Interferon alpha; IFN-β – Interferon beta
HAM-D – Hamilton depression scale; MADRS – Montgomery-Asberg depression rating scale; HAM-A – Hamilton anxiety scale; HCV – Hepatitis C virus; TNF-α – Tumor Necrosis Factor alpha; 5-HT – 5-hydroxytryptopamine; 5-HIAA – 5-hydroxyindoleacteicacid; CRP – c-reactive protein; IL-6 – Interleukin-6; LPS – lipopolysaccharides; E-coli – Escherichia coli; AA – Amino Acid;; sTNFR – soluble tumor necrosis factor receptor; LNAA – large neutral amino acids;
= decreased as study progressed; 3 depressed patients excluded at baseline n=40; 4 weeks n=34; 8 weeks n=31; 6 months n=18
= decreased as study progressed; baseline n=24; 4 weeks n=22; 8 weeks n=20; 6 months n=10 (continuation of Bannink 2007)
= decreased as study progressed; total patient drop out: n= 14
= decreased as study progressed; total patient drop out: n= 23
=quantitative analysis based in data from the 15 patients who received placebo (n=11 on paroxetine)
=The study evaluated two subgroups of IFN intervention. One with first time exposed to IFN: n=16, 4 males, 12 females, mean age=39/SD=1; and one with long term treatment with IFN: n=11, 1 male, 10 females, mean age=40/SD=1.
=The study evaluated two subgroups of IFN intervention. One group with IFN-beta-1a 44 mcg three times weekly: n = 48, 18 males, 30 females, mean age=34.2/SD=8,4; and one with IFN-beta-1a 22 mcg three times weekly: n=53, 12 males, 41 females, mean age=35.3/SD=8.2.
Bonaccorso et al – (N=15) for final analysis, 3 dropped out; Wichers et al – (N=21) total subjects recruited for the study; Raison – (N=16) had interferon and the others are waiting list controls; Kruse et al – 115 randomized to endotoxin (N=61) whose data is included
Figure 2. Systematic Search Flow Chart.
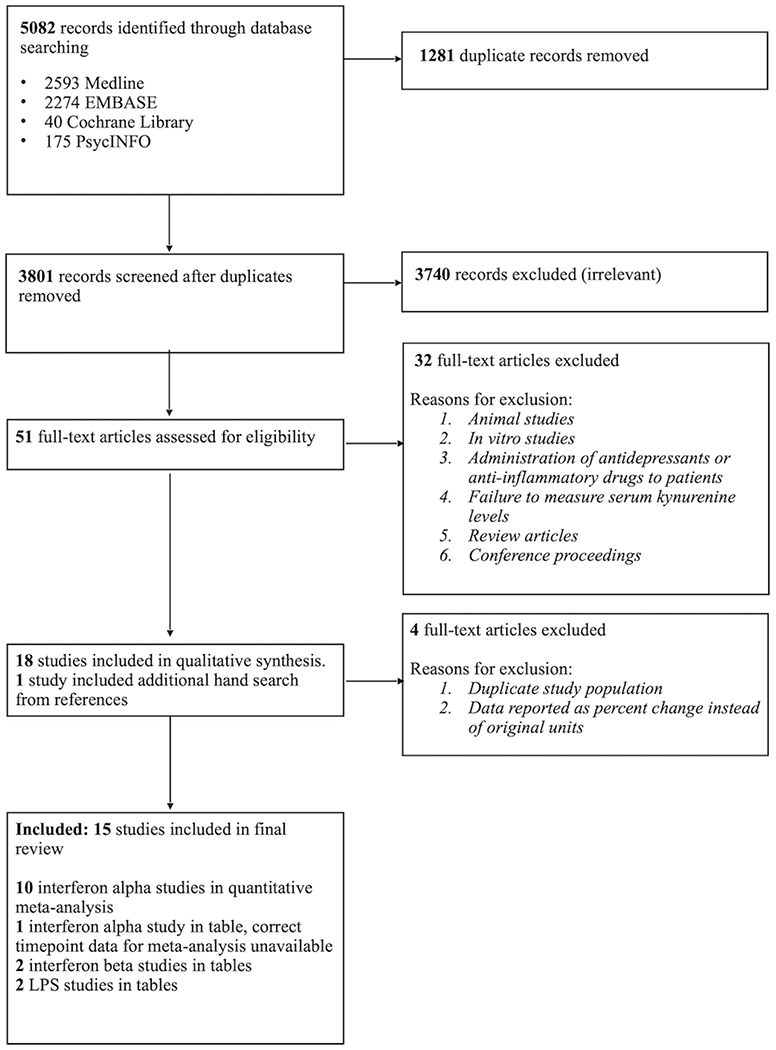
Study flow for the systematic review.
Methodological Quality
Most of the studies included in this review were nonrandomized prospective studies. Only three were randomized studies. The quality of all studies was assessed using the Newcastle-Ottawa Scale (GA Wells, B Shea, D O’Connell, J Peterson, V Welch, M Losos, P Tugwell, n.d.) and the results are described in Supplementary Table 1.
Primary Analyses
Eight models derived the standardized mean change (SMC) from baseline to follow-up at either 4 weeks or 24 weeks for each of 4 measures: KYN, TRP, KYN/TRP ratio, and HAM-D scores. Random (rather than fixed) effects were used to determine SMC in each model. Moderate-to-large effect sizes were determined for each model. Figures 3 (KYN),4 (TRP) and 5 (KYN/TRP ratio) provide forest plots for each measure; these plots describe change over time (with 95% confidence intervals) for each study as well as the overall SMC, including indices of between-study variability (i.e., heterogeneity: I2; Q). Overall effect sizes are summarized here. KYN values increased over time such that SMC = 0.68 (95% CI = [0.40, 0.96]) and 0.55 (95% CI = [0.39, 0.71]) were found for the change over time from baseline to follow-up at week 4 and week 24, respectively (Figure 3). TRP values decreased such that SMC = −0.77 (95% CI = [−1.03, −0.51]) and −0.83 (95% CI = [−1.08, −0.58]), respectively (Figure 4). For KYN/TRP ratio values increased over time such that SMC = 0.84 (95% CI = 0.56, 1.13]) and 0.99 (95% CI = [0.84, 1.15]) were found for the same follow-up points (Figure 5). For HAM-D scores, values increased such that SMC = 0.69 (95% CI = [0.54, 0.84]) and 0.57 (95% CI = [0.44, 0.71]).
Figure 3. Forest plot for Kynurenine levels analysis.
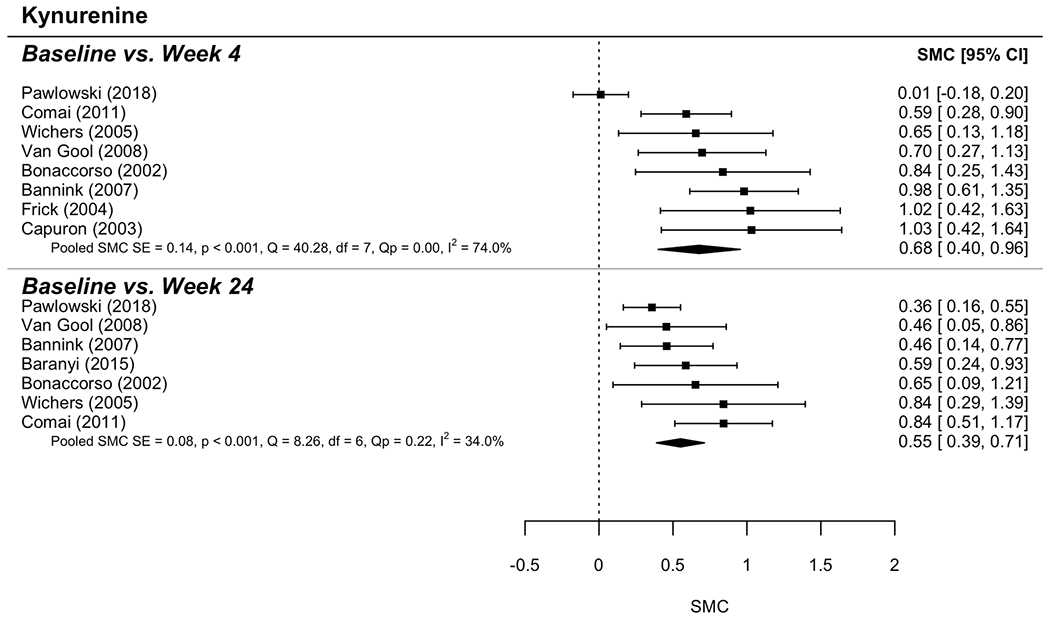
The graphic shows the forest plot for Kynurenine levels analysis comparing baseline and two-time points: 4 weeks and 24 weeks.
Figure 4. Forest plot for Tryptophan activity analysis.
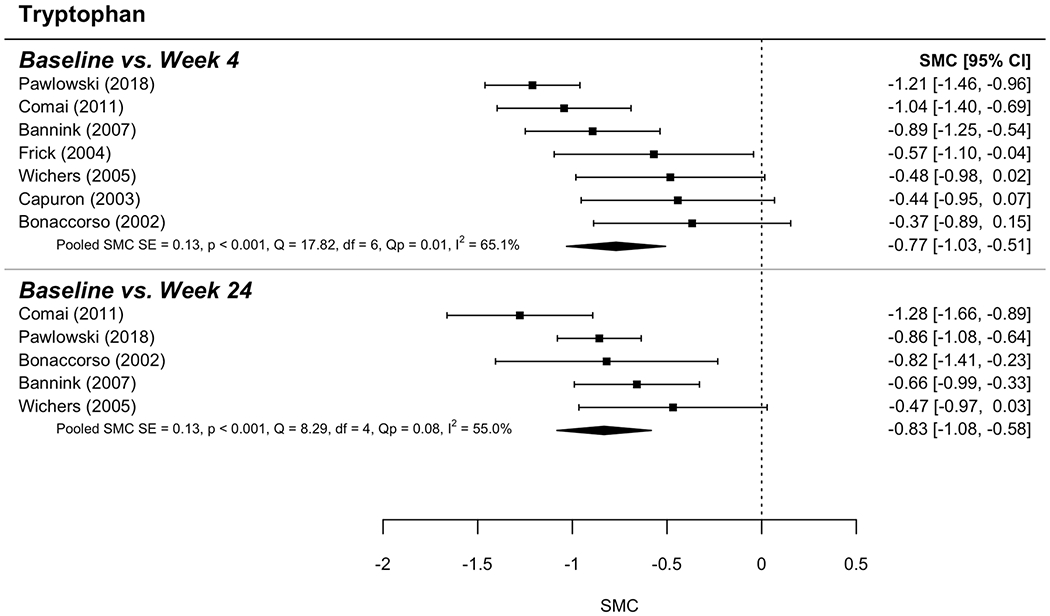
The graphic shows the forest plot for TDO activity analysis comparing baseline and two-item points: 4 weeks and 24 weeks.
Figure 5. Forest plot for Kynurenine/Tryptophan ratio activity analysis.
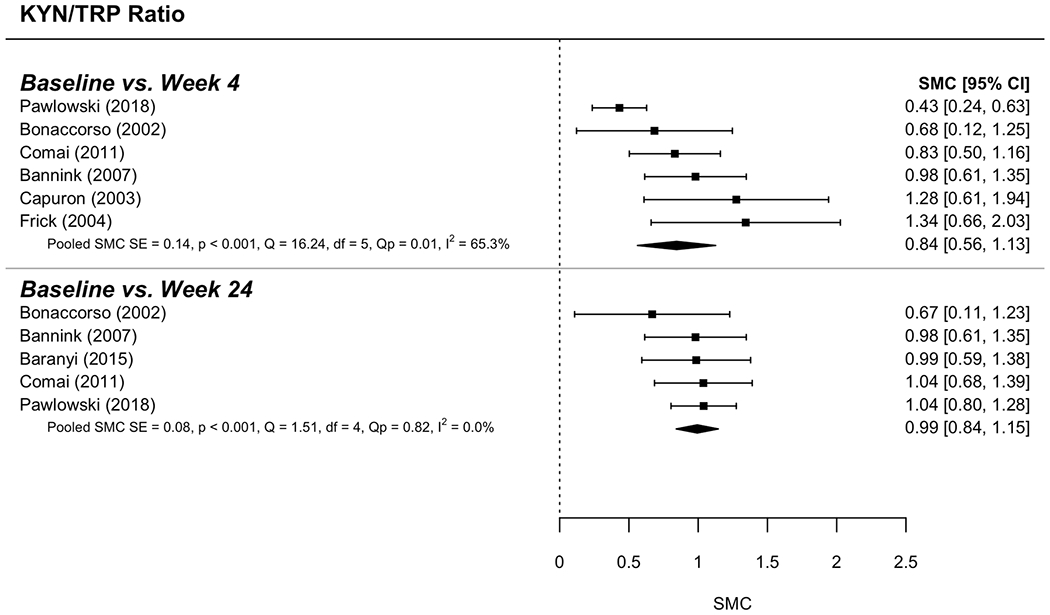
The graphic shows the forest plot for TDO activity analysis comparing baseline and two-item points: 4 weeks and 24 weeks.
Risk of Bias and Sensitivity Analyses
Visual inspection of funnel plots found that for four of the models, one study was outside the margin; however, in each case, the study demonstrated low standard error, potentially indicating reliable studies with divergent results (i.e., (Pawlowski et al., 2018)). Trim and fill indicated that for six of the eight models, a small number of studies (2 to 3) were implied to be missing due to publication bias. Results adjusting for these biases did not result in any changes to inferences. Jackknife leave-one-out sensitivity analyses similarly indicated no changes in inference from leaving out any given study in any of the models. Supplementary Tables 2 and 3 summarize the results from the trim and fill and the jackknife analyses, respectively. Sensitivity analysis for the correlation between baseline and follow-up indicated no differences in inferences resulting from setting the value lower or higher.
Discussion
The primary aim of this research was to evaluate the potential effect of immune activation or experimental inflammatory challenge in the levels of KYN pathway metabolites in humans. Our results indicate that: (1) there was an association between KYN metabolites levels and immune activation interventions, and (2) IFN-α treatment was associated with a reduction in TRP levels and an increase in KYN levels and KYN/TRP ratio activity in quantitative analysis. TRP level analysis effect size was medium and decreased between 4 and 24 weeks’ time points. KYN levels and KYN/TRP ratio activity analysis had an elevated effect size that increased between 4 and 24 weeks’ time points.
We also found increases in depressive symptoms after IFN-α treatment, similar to the increases in KYN pathway metabolites. Although quantitative analyses indicated the elevation of depression scores over time, after IFN-α treatment, it was not possible to establish any associations between depression scores and the levels of KYN metabolites due to the limited number of studies. Our study corroborates pre-clinical evidence of alteration of TRP levels and increased KYN pathway activation after peripheral inflammatory stimuli in humans. The lower levels of TRP were associated with increased levels of KYN, likely reflecting increased IDO activity. It is to be noted, the studies included do not differentiate IDO1 or IDO2, but the majority of tryptophan degradation is regulated by IDO1 (Löb et al., 2009, p. 1). Our findings also support the association between the activation in inflammation/immune response and the development of depressive symptoms.
Systemic inflammation has been shown to trigger depression-like symptoms. Animal and human studies indicate an association between depressive mood and immune pathways, e.g., sickness-related depressive mood and increased prevalence of psychiatric disorders in populations with autoimmune conditions (Dantzer, 2017; Myint et al., 2009). Regarding the KYN pathway, although not fully understood, the models of the possible mechanisms between this metabolic stream and depressive symptoms are either based on the TRP depletion hypothesis or based on the toxic effect of KYN metabolites produced by KMO on the CNS (Dantzer, 2017). According to the TRP depletion hypothesis, the competition between the KYN pathway and the serotonin pathway for TRP uptake could lower the bioavailability of the amino acid to the production of serotonin (which is aligned with the serotonin hypothesis of depression). The risk of depressive symptoms have been associated with low serotonin levels in plasma and elevated KYN/serotonin ratio. Also, a lower serotonin level has been associated with the history and current suicidal behavior in patients with depressive symptoms. (Achtyes, 2020).
On the other hand, toxic metabolites of the KYN pathway, mainly produced in the microglia by the action of KMO, typically exert their effect through excitotoxicity, altering glutamatergic activity, lipid peroxidation and activation of reactive oxygen species in the CNS (Guillemin, 2012; Parrott et al., 2016). Thus, dysregulated KYN mechanism has been linked to the development of inflammation-driven depression (Wichers et al., 2005). KYN is converted to neurotoxic QUIN. It has been reported that microglia in the anterior cingulate cortex of acutely depressed patients contained greater QUIN expression. (Steiner, 2011) QUIN, an NMDA receptor agonist, has been proposed as a target and biomarker of the antidepressant effect of ketamine (Vedonk, 2019).
Due to the limited number of studies reporting downstream metabolites of the KYN pathway (KYNA and QUIN) and heterogeneity between them in the method used to analyze these parameters, we were unable to perform quantitative analysis. Seven of the articles included in this review investigated the levels of downstream metabolites of the KYN pathway. Six studies (Amirkhani et al., 2005; Baranyi et al., 2015; Kruse et al., 2019; Pawlowski et al., 2018; Van Gool et al., 2008; Wichers et al., 2005) analyzed the KYN metabolites in peripheral blood, and only one of the studies (Raison et al., 2010) investigated both peripheral blood and cerebrospinal fluid (CSF). In the blood analysis, KYNA levels or KYNA ratios with other KYN metabolites were measured by six of these studies. KYNA levels were found to be elevated in two studies (Kruse et al., 2019; Pawlowski et al., 2018) and the KYN/KYNA ratio was elevated in two studies (Van Gool et al., 2008; Wichers et al., 2005). The KYNA/3-HAA ratio was reduced in one study (Van Gool et al., 2008), and only one study (Amirkhani et al., 2005) did not show alterations in KYNA. Taken together, while two studies found elevated KYNA levels, that would suggest an increased activation of the neuroprotective KYN-KYNA branch. The ratios between KYNA and KYN or 3-HAA found in the other studies suggest increased levels of metabolites linked to the neurotoxic branch of the KYN pathway in relation to KYNA levels. Of note, a recent meta-analysis investigated the variability of KYN and KYNA metabolites in the setting of depression and found that KYNA and KYN levels were lower in depressed patients when compared to healthy controls (Ogyu et al., 2018). The other primary metabolite investigated was QUIN, which represents the main character in the toxic arm of the KYN pathway regulated by KMO. Three studies reported QUIN blood levels (Baranyi et al., 2015; Kruse et al., 2019; Raison et al., 2010). Only one of them (Baranyi et al., 2015) found it to be increased compared to baseline, but all three studies found associations between QUIN levels and depressive symptoms. Two studies (Baranyi et al., 2015; Raison et al., 2010) found a positive correlation between QUIN levels and depression scores.
Raison et al. (Raison et al., 2010) was the only study that evaluated CSF and blood KYN pathway metabolites. Although this study did not show a statistically significant increase in QUIN blood levels, they found elevated QUIN levels in the CSF, along with the elevated KYN and KYNA levels and no alterations in TRP or QUIN/KYNA ratio. Plasma TRP was reduced and KYN and KYN/TRP ratio was increased, which is in line with the results of our meta-analysis. In this sense, the evidence from the study counters the TRP depletion hypothesis, as the concomitant low blood levels of TRP but normal CSF levels of the amino acid suggest that in the onset of inflammatory driven depression the reduced bioavailability of blood TRP has little or no influence on the brain’s TRP uptake. Raison et al. (Raison et al., 2010) findings are in line with animal models that investigated the effects of QUIN on the CNS and provided evidence for the role of the neurotoxic branch of the KYN pathway on the pathogenesis of inflammation-induced depression in humans.
One of the main limitations of this meta-analysis was that the findings were extended from medically ill patients treated with IFN-αand other immune-activating agents developing depression to MDD etiology. However, it is possible, the mechanisms of inflammation contributing to endogenous depression symptoms may differ between patients with MDD and patients with chronic medical illness treated with immune activating agents. Therefore, the generalizability of these etiological associations should be further investigated. Also, many of the included studies did not control for medications such as psychotropics and anti-inflammatory drugs that could influence the results. Anti-inflammatory agents such as cyclooxygenase-2 (COX-2) inhibitors could contribute to alleviating depressive symptoms (Müller and Schwarz, 2008b), thereby interfering with the mood analysis. From the ten studies included in the quantitative analysis, only one explicitly stated that patients had no influence from other drugs besides the ones included in the study protocol, i.e., IFN-a, ribavirin (Bonaccorso et al., 2002). In the other nine studies, six had exclusion criteria for patients under psychotropic medications that included anti-depressants, antipsychotics, or psychotropics in general (Bannink et al., 2007; Comai et al., 2011; Pawlowski et al., 2018; Van Gool et al., 2008; Wichers et al., 2005; Zignego et al., 2007). However, two studies did not have any clear exclusion criteria for drugs outside their study protocol (Capuron et al., 2003; Frick et al., 2004), and three studies stated that medications for pain, fever and nausea were allowed (Capuron et al., 2003; Frick et al., 2004; Wichers et al., 2005). Likewise, the influence of anti-inflammatory drugs would also contribute to attenuate the influence of inflammatory mediators over IDO activity. Thereby, it is reasonable to infer that the correlations found in our analysis were strong enough to endure the possible influence of these drugs and still reveal statistical significance with an elevated summary effect size.
Communication between the immune system and the brain takes place through multiple pathways, including neural afferents and circulating immune mediators that activate brain endothelial and innate immune cells (perivascular and meningeal macrophages, microglia) (Dantzer et al., 2008; Perry et al., 2010). In the context of this complex interaction, it seems that the immune system modulation of the KYN pathway is intrinsically related to alterations on the CNS, as it is evident from animal and humans studies. Haroon et al., in unmedicated depressed patients (N=72), reported a significant association between plasma TNF and plasma KYN and KYN/TRP (Haroon et al., 2020). Using pathway analysis, they also found a correlation between the plasma and CSF KYN, KYNA, and QUIN, supporting the hypothesis that peripheral inflammation may mediate the depression symptoms through the KYN pathway. In our previous meta-analysis of KYN studies in depression, we found decreased levels of plasma KYN but no differences in KYN/TRP ratios in MDD patients vs. healthy controls and thus suggesting heterogeneity of KYN findings in MDD patients (Arnone et al., 2018). It seems plausible that only the subgroup of MDD patients with higher peripheral inflammation activate the KYN pathway resulting in depression symptoms. Our results provide further evidence from human studies corroborating that the KYN pathway is indeed upregulated in the context of immunotherapy or inflammatory challenge and related to the emergence of depression symptoms. Some questions remain on the contribution of TRP depletion and KP downstream metabolites on the emergence of depressive symptoms.
Future studies should evaluate further the individual effects of KYN metabolites, especially discriminating the balance between KYNA and QUIN/AA and their relationship with KMO and KAT activity, weighing the contribution of the neuroprotective and the neurotoxic branches of the KYN pathway. These findings should also encourage new efforts on research over drugs involved in the modulation of the KYN pathway as possible strategies to approach inflammatory driven depression. The use of IDO inhibitors has been shown to reduce QUIN levels and subsequent neurotoxicity and oligodendrocyte death (Sundaram et al., 2014). KMO inhibitors demonstrate further benefits in that they lower neurotoxic QUIN levels while increasing the levels of neuroprotective KYNA (Chiarugi et al., 2001). Overall, the lowering of neurotoxic KYN metabolites and elevation of the neuroprotective can be an important new method of treatment for neurologic disease.
Supplementary Material
Acknowledgments
Special thanks to Keya Lee, Amy Sisson, and Travis Holder at the Texas Medical Center Library for their assistance with the database search process.
Disclosures of Interest
J.C.S has received grants/research support from BMS, Forrest, J&J, Merck, Compass pathways, Stanley Medical Research Institute, NIH and has been a speaker for Pfizer and Abbott. S.S. has received speaking honoraria from Global Medical Education and honoraria from British Medical Journal Publishing Group; own shares at Flow Med Tech. Research support from Compass pathways. All the other authors have no conflicting interests to disclose.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- Achtyes E, Keaton S, Smart L, Burmeister A, Heilman P, Krzyzanowski S, Nagalla M, Guillemin G, Escobar M, Lim C, Muzik M, Postolache T, Leach R, Brundin L, 2020. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain, Behavior, and Immunity. 83, 239–247. 10.1016/j.bbi.2019.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirkhani A, Rajda C, Arvidsson B, Bencsik K, Boda K, Seres E, Markides KE, Vécsei L, Bergquist J, 2005. Interferon-beta affects the tryptophan metabolism in multiple sclerosis patients. Eur. J. Neurol. 12, 625–631. 10.1111/j.1468-1331.2005.01041.x [DOI] [PubMed] [Google Scholar]
- Arnone D, Saraykar S, Salem H, Teixeira AL, Dantzer R, Selvaraj S, 2018. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci Biobehav Rev 92, 477–485. 10.1016/j.neubiorev.2018.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ, 2012. Empirical Assessment of Within-Arm Correlation Imputation in Trials of Continuous Outcomes, AHRQ Methods for Effective Health Care. Agency for Healthcare Research and Quality (US), Rockville (MD). [PubMed] [Google Scholar]
- Bannink M, Fekkes D, Van Gool AR, Kruit WHJ, Sleijfer S, van der Holt B, Eggermont AMM, Stoter G, Hengeveld MW, 2007. Interferon-alpha influences tryptophan metabolism without inducing psychiatric side effects. Neuropsychobiology 55, 225–231. 10.1159/000108382 [DOI] [PubMed] [Google Scholar]
- Baranyi A, Meinitzer A, Breitenecker RJ, Amouzadeh-Ghadikolai O, Stauber R, Rothenhäusler H-B, 2015. Quinolinic Acid Responses during Interferon-α-Induced Depressive Symptomatology in Patients with Chronic Hepatitis C Infection - A Novel Aspect for Depression and Inflammatory Hypothesis. PLoS ONE 10, e0137022. 10.1371/journal.pone.0137022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M, 2002. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol 22, 86–90. 10.1097/00004714-200202000-00014 [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH, 2004. Cytokines and psychopathology: lessons from interferon-alpha. Biol. Psychiatry 56, 819–824. 10.1016/j.biopsych.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH, 2003. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 54, 906–914. 10.1016/s0006-3223(03)00173-2 [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Cozzi A, Ballerini C, Massacesi L, Moroni F, 2001. Kynurenine 3-monooxygenase activity and neurotoxic kynurenine metabolites increase in the spinal cord of rats with experimental allergic encephalomyelitis. Neuroscience 102, 687–695. 10.1016/s0306-4522(00)00504-2 [DOI] [PubMed] [Google Scholar]
- Comai S, Cavalletto L, Chemello L, Bernardinello E, Ragazzi E, Costa CVL, Bertazzo A, 2011. Effects of PEG-interferon alpha plus ribavirin on tryptophan metabolism in patients with chronic hepatitis C. Pharmacol. Res 63, 85–92. 10.1016/j.phrs.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O’Sullivan JB, Harkin A, 2008. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci. Lett 441, 29–34. 10.1016/j.neulet.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW, 2014. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry 171, 453–462. 10.1176/appi.ajp.2013.13030325 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Weitz E, Cristea IA, Twisk J, 2017. Pre-post effect sizes should be avoided in meta-analyses. Epidemiol Psychiatr Sci 26, 364–368. 10.1017/S2045796016000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, 2017. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. Curr Top Behav Neurosci 31, 117–138. 10.1007/7854_2016_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW, 2011. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36, 426–436. 10.1016/j.psyneuen.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL, 2010. A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Durastanti V, Lugaresi A, Bramanti P, Amato M, Bellantonio P, De Luca G, Picconi O, Fantozzi R, Locatelli L, Solda A, Sessa E, Totaro R, Marino S, Zipoli V, Zorzon M, Millefiorini E, 2011. Neopterin production and tryptophan degradation during 24-months therapy with interferon beta-1a in multiple sclerosis patients. J Transl Med 9, 42. 10.1186/1479-5876-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford HA, 2013. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 10, e1001547. 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick B, Capuron L, Schröcksnadel K, Musselman DL, Lawson DH, Nemeroff CB, Miller AH, Fuchs D, 2004. Plasma homocysteine and immune activation in patients with malignant melanoma undergoing treatment with IFN-alpha. J. Interferon Cytokine Res. 24, 311–317. 10.1089/107999004323065101 [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Shibata M, Watanabe Y, Nukiwa T, Hirata F, Mizuno N, Hayaishi O, 1978. Indoleamine 2,3-dioxygenase. Formation of L-kynurenine from L-tryptophan in cultured rabbit fineal gland. J. Biol. Chem. 253, 6081–6085. [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P, n.d. Ottawa Hospital Research Institute [WWW Document]. URL http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 2.22.20).
- Gál EM, Sherman AD, 1980. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem. Res. 5, 223–239. 10.1007/bf00964611 [DOI] [PubMed] [Google Scholar]
- George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA, 2012. Pharmacology and therapeutic potential of interferons. Pharmacology & Therapeutics 135, 44–53. 10.1016/j.pharmthera.2012.03.006 [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, 2012. Quinolinic acid, the inescapable neurotoxin. FEBS J. 279, 1356–1365. 10.1111/j.1742-4658.2012.08485.x [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ, 2007. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 27, 12884–12892. 10.1523/JNEUROSCI.4101-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, Felger JC, Miller AH, 2020. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology. 10.1038/s41386-020-0607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD, 2009. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 66, 407–414. 10.1016/j.biopsych.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SP, Guillemin GJ, Brew BJ, 2013. The kynurenine pathway in stem cell biology. Int J Tryptophan Res 6, 57–66. 10.4137/IJTR.S12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SJ, Armati PJ, Guillemin GJ, Brew BJ, 1998. Chronic exposure of human neurons to quinolinic acid results in neuronal changes consistent with AIDS dementia complex. AIDS 12, 355–363. 10.1097/00002030-199804000-00003 [DOI] [PubMed] [Google Scholar]
- Kruse JL, Cho JH-J, Olmstead R, Hwang L, Faull K, Eisenberger NI, Irwin MR, 2019. Kynurenine metabolism and inflammation-induced depressed mood: A human experimental study. Psychoneuroendocrinology 109, 104371. 10.1016/j.psyneuen.2019.104371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M, 2012. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36, 764–785. 10.1016/j.neubiorev.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Löb S, Königsrainer A, Zieker D, Brücher BLDM, Rammensee H-G, Opelz G, Terness P, 2009. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother 58, 153–157. 10.1007/s00262-008-0513-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace MD, Varney B, Sundaram G, Lennon MJ, Lim CK, Jacobs K, Guillemin GJ, Brew BJ, 2017. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 112, 373–388. 10.1016/j.neuropharm.2016.03.024 [DOI] [PubMed] [Google Scholar]
- Maddison DC, Giorgini F, 2015. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 40, 134–141. 10.1016/j.semcdb.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62, 1006–1012. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Müller N, Myint A-M, Schwarz MJ, 2009. The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders--relation to drug treatment. Dialogues Clin Neurosci 11, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ, 2008a. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci 258 Suppl 2, 97–106. 10.1007/s00406-008-2012-3 [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ, 2008b. COX-2 inhibition in schizophrenia and major depression. Curr. Pharm. Des. 14, 1452–1465. 10.2174/138161208784480243 [DOI] [PubMed] [Google Scholar]
- Myint AM, Schwarz MJ, Steinbusch HWM, Leonard BE, 2009. Neuropsychiatric disorders related to interferon and interleukins treatment. Metab Brain Dis 24, 55–68. 10.1007/s11011-008-9114-5 [DOI] [PubMed] [Google Scholar]
- O’Farrell K, Harkin A, 2017. Stress-related regulation of the kynurenine pathway: Relevance to neuropsychiatric and degenerative disorders. Neuropharmacology 112, 307–323. 10.1016/j.neuropharm.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y, Wada M, Tarumi R, Plitman E, Moriguchi S, Miyazaki T, Uchida H, Graff-Guerrero A, Mimura M, Nakajima S, 2018. Kynurenine pathway in depression: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 90, 16–25. 10.1016/j.neubiorev.2018.03.023 [DOI] [PubMed] [Google Scholar]
- Padberg J-S, Van Meurs M, Kielstein JT, Martens-Lobenhoffer J, Bode-Böger SM, Zijlstra JG, Kovesdy CP, Kümpers P, 2012. Indoleamine-2,3-dioxygenase activity in experimental human endotoxemia. Exp Transl Stroke Med 4, 24. 10.1186/2040-7378-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC, 2016. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 6, e918. 10.1038/tp.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski T, Malyszczak K, Inglot M, Zalewska M, Radkowski M, Laskus T, Pawlak D, 2018. Alterations in the metabolism of tryptophan in patients with chronic hepatitis C six months after pegylated interferon-α 2a treatment. Psychoneuroendocrinology 97, 1–7. 10.1016/j.psyneuen.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JAR, Holmes C, 2010. Microglia in neurodegenerative disease. Nat Rev Neurol 6, 193–201. 10.1038/nrneurol.2010.17 [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. U.S.A. 81, 908–912. 10.1073/pnas.81.3.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403. 10.1038/mp.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163, 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, Finn A, Bhat M, Samuelsson M, Lundberg K, Dahl M-L, Sellgren C, Schuppe-Koistinen I, Svensson C, Erhardt S, Engberg G, 2015. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J Psychiatry Neurosci 40, 126–133. 10.1503/jpn.140126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Norris S, O’Farrelly C, O’Mara SM, 2011. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat 7, 275–292. 10.2147/NDT.S13917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, 2000. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol. Sci. 21, 149–154. 10.1016/s0165-6147(00)01451-6 [DOI] [PubMed] [Google Scholar]
- Sundaram G, Brew BJ, Jones SP, Adams S, Lim CK, Guillemin GJ, 2014. Quinolinic acid toxicity on oligodendroglial cells: relevance for multiple sclerosis and therapeutic strategies. J Neuroinflammation 11, 204. 10.1186/s12974-014-0204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Bohár Z, Vécsei L, 2020. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 25. 10.3390/molecules25030564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Langohr K, Solà R, Vieta E, Martín-Santos R, 2012. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 73, 1128–1138. 10.4088/JCP.12r07694 [DOI] [PubMed] [Google Scholar]
- Van Gool AR, Verkerk R, Fekkes D, Bannink M, Sleijfer S, Kruit WHJ, van der Holt B, Scharpé S, Eggermont AMM, Stoter G, Hengeveld MW, 2008. Neurotoxic and neuroprotective metabolites of kynurenine in patients with renal cell carcinoma treated with interferon-alpha: course and relationship with psychiatric status. Psychiatry Clin. Neurosci. 62, 597–602. 10.1111/j.1440-1819.2008.01854.x [DOI] [PubMed] [Google Scholar]
- Viechtbauer W, 2010. Conducting Meta-Analyses in R with the metafor Package. Journal of Statistical Software 36, 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H, 1989. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta 1012, 140–147. 10.1016/0167-4889(89)90087-6 [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpé S, Maes M, 2005. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry 10, 538–544. 10.1038/sj.mp.4001600 [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A, 2005. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav. Immun. 19, 345–350. 10.1016/j.bbi.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Zignego AL, Cozzi A, Carpenedo R, Giannini C, Rosselli M, Biagioli T, Aldinucci A, Laffi G, Moroni F, 2007. HCV patients, psychopathology and tryptophan metabolism: analysis of the effects of pegylated interferon plus ribavirin treatment. Dig Liver Dis 39 Suppl 1, S107–111. 10.1016/s1590-8658(07)80021-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


