Abstract
Purpose
Studies performed in spontaneously breathing patients with mild to moderate respiratory failure suggested that prone position (PP) in COVID-19 could be beneficial.
Materials and methods
Consecutive critically ill patients with COVID-19 were enrolled in four ICUs. PP sessions lasted at least 3 h each and were performed twice daily. A Cox proportional hazard model identified factors associated with the need of intubation. A propensity score overlap weighting analysis was performed to assess the association between spontaneous breathing PP (SBPP) and intubation.
Results
Among 379 patients, 40 underwent SBPP. Oxygenation was achieved by high flow nasal canula in all but three patients. Duration of proning was 2.5 [1.6;3.4] days. SBPP was well tolerated hemodynamically, increased PaO2/FiO2 (78 [68;96] versus 63 [53;77] mm Hg, p = 0.004) and PaCO2 (38 [34;43] versus 35 [32;38] mm Hg, p = 0.005). Neither day-28 survival (HR 0.51, 95% CI 0.16–1.16] nor risk of invasive ventilation [sHR 0.96; 95% CI 0.49;1.88] differed between patients who underwent PP and others.
Conclusions
SBPP in COVID-19 is feasible and well tolerated in severely hypoxemic patients. It did not induce any effect on risk of intubation and day-28 mortality.
Keywords: COVID-19, Prone position, Mechanical ventilation, High-flow nasal cannula
1. Introduction
The COVID-19 pandemic has led to a massive influx of patients in intensive care units (ICUs) [1], as severe forms have been frequently reported, including acute respiratory distress syndrome (ARDS) [2,3]. In a large series of patients hospitalized for severe COVID-19, ARDS was reported to be the cause of death in 98% of cases [4]. In the context of potential ventilator shortage, and based on the characteristics of this specific ARDS [5], limiting mechanical ventilation to only patients with obvious clinical indications in order to avoid complications of mechanical ventilation was proposed [6,7]. COVID-19 patients tolerate profound hypoxemia well with a rate of invasive ventilation worldwide ranging from 29 to 90% [8].
Recent single-centre studies have suggested the feasibility of prone position (PP) in spontaneously breathing COVID-19 patients with acute respiratory failure and its ability to increase oxygenation, resulting in clinical improvement [[9], [10], [11], [12], [13]]. However, these studies were performed in selected patients with mild to moderate decrease in oxygenation, most of them being hospitalized outside the ICU and treated with positive airway pressure. Before large implementation, more studies evaluating the impact of such a strategy on the outcome are required [14]. Ferrando et al. recently reported that PP did not affect the need for intubation [15], while this remains to be confirmed.
We sought to report prevalence PP in spontaneously breathing critically ill COVID-19 patients -with severe acute respiratory failure, as well as its impact on outcomes.
2. Methods
This retrospective observational study was performed in four university-affiliated hospitals in Paris [3]. All consecutive patients with laboratory-confirmed SARS-CoV-2 infection admitted to one of the ICUs between February 20 and April 24, 2020 were enrolled. The appropriate IRB approved this study and, due to the nature of retrospective chart review, waived the need for informed consent from individual patients.
Laboratory confirmation of SARS-Cov-2 was defined as a positive result of real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs. The four participating ICUs applied Parisian guidelines regarding the provision of standard of care, the use of non-invasive ventilation, antibiotic treatment, as well as the use of rescue therapy as ECMO. All mechanically ventilated patients had a protective lung approach. These guidelines were approved by all and shared on the Health Regional Agency website (https://www.iledefrance.ars.fr/coronavirus-covid-19-information-aux-professionnels-de-sante). There were no guidelines regarding the use of anti-viral agents, steroids, or cytokine-blockade that were mostly used in RCTs. Prone position in spontaneously breathing patients was protocolized including at least twice daily physiotherapy. It was left to the discretion of the attending physician when it was expected as potentially useful, as well as the decision to move to intubation, mainly based on clinical status as respiratory exhaustion, encephalopathy, need to start or to increase vasopressors, and rejection by patients to continue proning.
3. Data collection
3.1. Patient characteristics
Demographic characteristics were collected with age, weight, size, gender, body mass index (BMI), and comorbidities (hypertension, immunosuppression, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, chronic heart failure, asthma). Use of non-steroidal anti-inflammatory drugs before ICU admission was reported. COVID-19 disease history (symptom onset, in-hospital admission and in-ICU admission) was also collected, thereby allowing calculation of the interval between symptom onset and admission to the ICU. SOFA score [16] was recorded, as were the need for vasopressors, the serum lactate level, the presence of acute kidney injury at admission [17], the need for renal replacement therapy, and the main laboratory findings. PaO2/FiO2 and respiratory rate (RR) were collected, as were the modalities of oxygenation in spontaneously breathing patients: non-invasive ventilation (NIV), continuous positive airway pressure (CPAP), high-flow nasal cannula (HFNC) and conventional low flow devices (nasal cannula, simple face mask). Need for intubation until day 28, ICU discharge and day-28 mortality were evaluated.
3.2. Collection of data regarding prone position
We recorded the duration in days of the PP strategy. In the spontaneous breathing PP (SBPP) group, PP session lasted between 3 and 6 h and was performed twice a day when possible. PP tolerance was evaluated by reporting respiratory rate (RR), mean arterial pressure (MAP), heart rate (HR) and serum lactate before and at the end of the first proning session, just before resupination. FiO2, PaO2/FiO2, PaCO2, and pH were reported before the first proning session and at the end of the last proning session.
3.3. Statistical analysis
Quantitative variables were described as median (interquartile range [IQR]) and were compared between groups using the non-parametric Wilcoxon rank-sum test. Qualitative variables were described as frequency (percentages) and were compared between groups using Fisher's exact test.
Factors associated with day-28 mortality were assessed using survival analysis. Risk of invasive ventilation was assessed using a time-dependent Cox model and competing risk cumulative incidence analysis taking into account competing risk of mortality and discharge alive from the ICU. Data are reported as hazard ratios (HR; 95% CI) or sub-hazard ratios (sHR; 95% CI) according to the model used. Adjustment for a centre effect was assessed using frailty models.
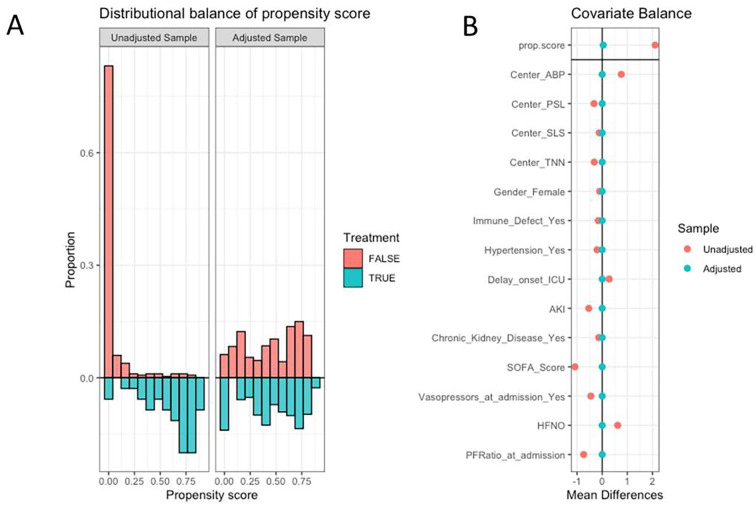
Then propensity score weighting analysis was performed to assess the association between SBPP and the outcome of interest. Briefly, variables associated with SBPP and believed to have influenced its choice were entered in a propensity score overlap weighting. This strategy allows weighting of patients from each treatment group by the probability of being assigned the other treatment group [18]. This allows higher weight to be assigned to patients with intermediate risk and lower weight to outliers in both treatment groups, the analysis emphasizing the proportion of the population where the most treatment equipoise exists in clinical practice [19]. Covariates included in the model were centre, underlying immune defect, history of hypertension, underlying chronic kidney disease, interval between onset of symptoms and ICU admission, SOFA score, acute kidney injury, need for vasopressors and PaO2/FiO2 at ICU admission, and use of HFNC as oxygenation modality at day 1. Quality of matching was assessed using propensity score distribution before and after matching and standardized mean difference of variables of interest before and after matching. The influence of SBPP on mortality and risk of invasive ventilation was assessed using survival analyses as previously described.
Statistical analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing), ‘survival’,’ cmprisk’ and ‘WeightIt’ and ‘Survey’ packages and statistical significance was considered using two-sided tests with a critical alpha risk of 0.05.
4. Results
Of 379 consecutive patients included, 40 (10.5%) underwent SBPP (Fig. 1 ). Oxygenation in these patients was achieved with HFNC in 37 patients, CPAP in 1 patient and 2 patients received standard oxygen. The main characteristics of these 40 patients at admission, and their comparison with the rest of the cohort, are reported in Table 1 . Briefly, age was 59.5 [56;64] and BMI 28.5 [26;30.9]. SOFA score was 4 [3;4], PaO2/FiO2 90 [71;125] mm Hg, RR 31 [25;36] /min and 7.5% received vasopressor infusion.
Fig. 1.
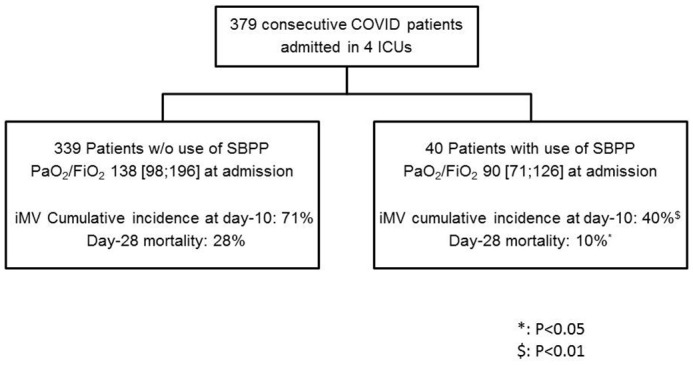
Flow chart of the study.
SBPP: spontaneously breathing prone position, iMV: invasive mechanical ventilation.
Table 1.
Main characteristics of the 40 patients of the SBPP group and their comparison with the rest of the cohort.
| No SBPP |
SBPP |
P Value | |
|---|---|---|---|
| n = 339 | n = 40 | ||
| Age (years) | 62 [53–69] | 59.5 [56–64] | 0.15 |
| Female gender, n (%) | 84 (24.8%) | 4 (10.0%) | 0.06 |
| Comorbidities | |||
| No comorbidities, n (%) | 29 (8.6%) | 6 (15.0%) | 0.30 |
| History of COPD, n (%) | 17 (5.0%) | 3 (7.5%) | 0.77 |
| History of asthma, n (%) | 20 (5.9%) | 3 (7.5%) | 0.96 |
| Hypertension, n (%) | 175 (51.6%) | 13 (32.5%) | 0.03 |
| Diabetes, n (%) | 103 (30.4%) | 11 (27.5%) | 0.85 |
| Underlying immune deficiency, n (%) | 66 (19.6%) | 2 (5.0%) | 0.04 |
| LV dysfunction, n (%) | 30 (8.8%) | 2 (5.0%) | 0.60 |
| CKD, n (%) | 63 (18.6%) | 2 (5.0%) | 0.05 |
| Body mass index (kg/m2), n (%) | 28 [25–32] | 28.5 [26–31] | 0.56 |
| Overweight | 0.89 | ||
| None, n (%) | 105 (33.0%) | 12 (31.6%) | |
| Overweight, n (%) | 113 (35.5%) | 15 (39.5%) | |
| Morbid obesity, n (%) | 100 (31.4%) | 11 (28.9%) | |
| Features since onset of disease | |||
| Use of NSAIDs, n (%) | 9 (2.7%) | 2 (5.0%) | 0.74 |
| Interval since onset of symptoms (days) | 8 [6–11] | 10 [7–12] | 0.07 |
| Interval since hospital admission (days) | 1 [0–3] | 1 [0–4] | 0.84 |
| Temperature at admission (°C) | 37.9 [37.1–38.7] | 38.4 [38–39] | 0.004 |
| Main characteristics at ICU admission | |||
| AKI, n (%) | 190 (56.0%) | 5 (12.5%) | <0.001 |
| O2 flow (L) | 15 [9–15] | 15 [12–15] | 0.08 |
| HFNC during first 24 h, n (%) | 109 (32.2%) | 37 (92.5%) | <0.001 |
| CPAP during first 24 h, n (%) | 5 (1.5%) | 1 (2.5%) | 1.00 |
| NIV during first 24 h, n (%) | 27 (8.0%) | 0 (0.0%) | 0.13 |
| Invasive MV during first 24 h, n (%) | 200 (59.0) | 4 (10.0) | <0.001 |
| SOFA score at day 1, n (%) | 5 [3–8] | 4 [3–4] | 0.004 |
| PaO2/FiO2 ratio (worst at day 1), n (%) | 138 [98–196] | 91 [71–126] | <0.001 |
| Vasopressors at day 1, n (%) | 162 (47.8%) | 3 (7.5%) | <0.001 |
| Renal replacement therapy at day 1, n (%) | 73 (21.5%) | 1 (2.5%) | 0.008 |
| Laboratory results at ICU admission | |||
| Leucocytes (G/L) | 7.9 [5.5–10.7] | 9.8 [6.3–11.9] | 0.14 |
| Lymphocytes (G/L) | 0.77 [0.54–1.10] | 0.78 [0.63–0.98] | 0.88 |
| Platelets (G/L) | 209 [155–272] | 209 [156–283] | 0.97 |
| Fibrinogen (g/L) | 6.85 [5.80–7.75] | 6.70 [5.90–7.20] | 0.27 |
| Ferritin | 1255 [688–2271] | 1195 [839–2268] | 0.65 |
| Prothrombin time (%) | 84 [74–94] | 70 [61–80] | <0.001 |
| D-dimers | 1747 [918–3684] | 1465 [760–2390] | 0.47 |
| Troponin level below threshold | 170 (56.7%) | 36 (90.0%) | <0.001 |
| Lactate level (mmol/L) | 1.2 [1.1–1.7] | 1.4 [1.3–1.7] | 0.02 |
| Outcome | |||
| ICU mortality, n (%) | 94 (32.4%) | 4 (13.3%) | 0.05 |
| Hospital mortality, n (%) | 98 (41.4%) | 5 (16.7%) | 0.02 |
| Day-28 mortality, n (%) | 96 (28.3%) | 4 (10.0%) | 0.02 |
COPD: chronic obstructive pulmonary disease, CKD: chronic kidney disease, AKI: acute kidney injury, NSAIDs: non-steroidal anti-inflammatory drugs, NIV: non-invasive ventilation, CPAP: continuous positive airway pressure.
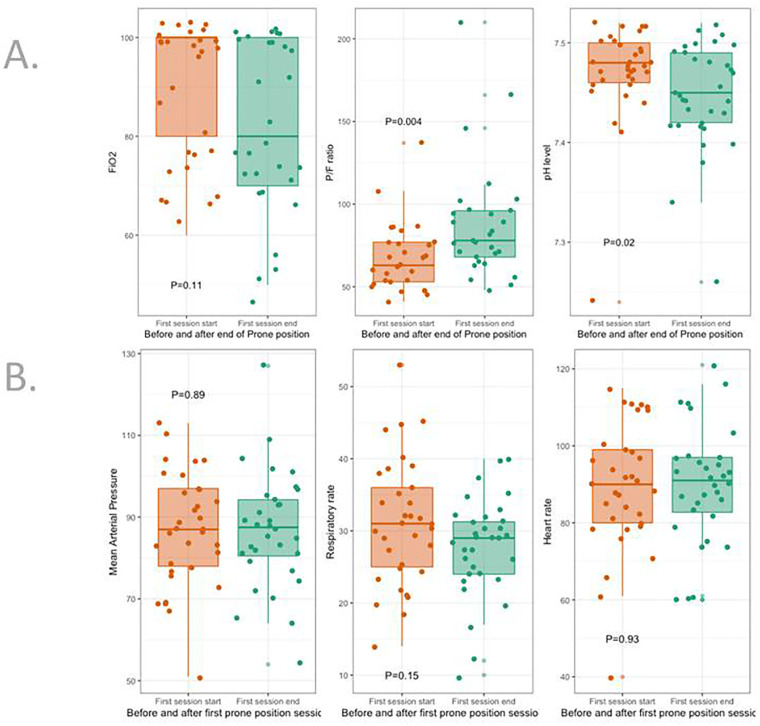
In the SBPP group, PP was started 1 [1;2] day after ICU admission. Its duration was 2.5 [1.6;3.4] days for a total of 3 [2;5] sessions. PaO2/FiO2 just before SBPP was 63 [53–77]. SBPP was well tolerated, without change in HR, RR, MAP and serum lactate (Table 2 , Fig. 2 ), increased PaO2/FiO2 (p = 0.004) and PaCO2 (p = 0.005) (Table 3 , Fig. 2).
Table 2.
Hemodynamic and respiratory tolerance of SBPP in the 40 patients after the first proning session (SBPP). BP: blood pressure.
| Before SBPP initiation | End of first SBPP session | P value | |
|---|---|---|---|
| Lactate (mmol/L) | 1.30 [1.00–1.48] | 1.10 [0.90–1.48] | 0.39 |
| Heart rate (bpm) | 90 [80–99] | 91 [83–97] | 0.93 |
| Systolic BP (mm Hg) | 114 [103−130] | 116 [107–129] | 0.79 |
| Diastolic BP (mm Hg) | 74 [63–82] | 72 [63–81] | 0.52 |
| Mean BP (mm Hg) | 87 [78–97] | 88 [81–94] | 0.89 |
| Respiratory rate (breaths/min) | 31 [25–36] | 29 [24–31] | 0.15 |
Fig. 2.
Response to SBPP in the 40 patients.
Panel A regards changes in blood gas and FiO2 after the last proning session.
Panel B regards clinical tolerance after the first session, just before resupination.
Table 3.
Impact of SBPP on blood gas exchange after the last session in the 40 patients.
| Before SBPP initiation | End of last SBPP session | P value | |
|---|---|---|---|
| FiO2 (%) | 100 [80–100] | 80 [70–100] | 0.11 |
| PaCO2 (mm Hg) | 35 [32–38] | 38 [34–43] | 0.005 |
| PaO2 (mm Hg) | 59 [53–62] | 62 [56–71] | 0.08 |
| PaO2/FiO2 (mm Hg) | 63 [53–77] | 78 [68–96] | 0.004 |
| SaO2 (%) | 92 [88–93] | 93 [90–95] | 0.34 |
| Bicarbonates (mmol/L) | 26 [23–28] | 26 [25–29] | 0.27 |
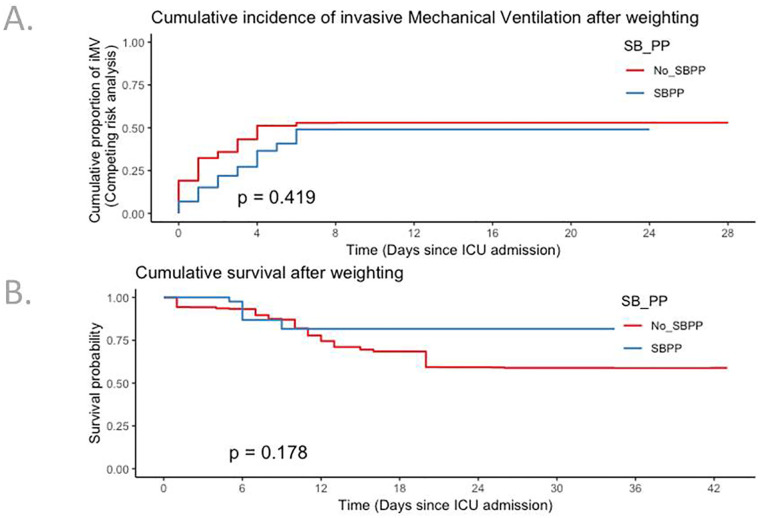
“Do not intubate” decision was negligible in the whole cohort, as 12 patients died without intubation, 1 (2.5%) in the SBPP group and 11 (3.2%) in the non-SBPP group. Twenty-three patients (58%) in the SBPP group were discharged alive without any intubation, while 16 (40%) required invasive ventilation, all within 10 days. After adjustment for centre, SBPP was associated with increased day-28 survival (Fig. S1, panel A, HR 0.25; 95% CI 0.09–0.69) and competing risk adjusted cumulative incidence of invasive mechanical ventilation (Fig. S1, panel B, sHR 0.45; 95% CI 0.26–0.81). After weighting for factors that may have influenced use of PP (Fig. S2), neither day-28 survival (HR 0.51, 95% CI 0.16–1.16) nor risk of invasive ventilation (sHR 0.96; 95% CI 0.49–1.88) differed (Fig. 3). Same result was found after the exclusion of the 204 patients who were intubated during the first 24 h (Table S1).
Fig. 3.
Kaplan-Meier curves for competing risk of invasive ventilation (panel A) and day-28 survival (panel B) after propensity score weighting analysis.
5. Discussion
In a series of 379 patients admitted to the ICU for SARS-Cov-2 pneumonia, SBPP was performed in 40 patients (10.5%). Despite severe respiratory failure with a median PaO2/FiO2 of 63 mm Hg at the time of the first proning session, prone position was feasible, well tolerated and effective as oxygenation significantly improved. After adjustment for confounders, prone position was not significantly associated with intubation or day-28 survival rates. These findings delineate actual benefits that can be expected from SBPP in the real life and could suggest a safe and effective strategy capable to optimize ventilator shortage at a time of surge.
The fact that median PaO2/FiO2 was 63 mm Hg at the time of proning highlights that our patients not only were critically ill, but also presented pictures of severe ARDS. It is somewhat questionable that patients with such low PaO2/FiO2 were not considered for a quick escalation of respiratory support. While it is considered to be specific to COVID-19 patients to well-tolerated profound hypoxemia [6,20], this respiratory strategy has been actually proposed much before the pandemic by Scaravelli et al. in 15 non-COVID patients with “usual” severe respiratory failure, in whom mean PaO2/FiO2 before proning was around 120 mm Hg [21]. Previous studies in COVID-19 suggested the potential value of prone position in awake patients, but they were performed in selected patients with mild to moderate respiratory failure, outside the ICU, and, in 2 of the studies, in patients treated with positive pressure ventilation [[9], [10], [11], [12]]. Elharrar et al. enrolled 24 patients; PaO2/FiO2 was not given, but mean PaO2 before proning was 73 mm Hg with an oxygen delivery below 4 L/min in most patients [9]. Sartini et al. enrolled 15 patients, all non-invasively ventilated [10]. Thomson et al. reported in 25 patients admitted that PP increased oxygenation, but that almost half of the patients finally required intubation, especially among those who did not have oxygen saturation > 95% after 1 h of proning [11]. The largest series was published by Coppo et al. [12]. They enrolled 56 patients with mean PaO2/FiO2 before proning of 180 mm Hg. Most patients were ventilated with a helmet device to enable continuous positive airway pressure. While proning improved oxygenation, around 30% of patients required intubation, with no difference between responders and non-responders to PP [12]. Conversely, our multicenter study, while observational and retrospective, did not select any patients, as the only inclusion criterion was admission to the ICU. Included patients were consecutive and had very severe respiratory failure. We were able to compare survival and the risk of invasive ventilation between patients with and without the SBPP strategy, and not between responders and non-responders to proning, which adds new information on the potential impact of PP in a population of spontaneously breathing COVID-19 patients without any assistance. Interestingly, 58% of our patients in the SBPP group were discharged alive without intubation. Ferrando et al. recently reported in patients with a median PaO2/FiO2 of 125 mm Hg similar results [15]. One difference with our study is that prone position was only considered if the duration was >16 h per day [15].
Besides the absence of association with intubation and survival, patient's safety and oxygenation benefits, namely, increase in PaCO2, an improvement in oxygenation and no increase in RR could also suggest that SBPP did alleviate self-inflicted lung injury. We were however unable to report changes in respiratory compliance in the 16 patients who secondarily required intubation. We also recently reported that oxygen delivery in spontaneously breathing patients using HFNC was able to prevent intubation [22], which also does not support such an injury. However, early intubation and “preemptive mechanical ventilation” to avoid self-inflicted lung injury remains controversial until trials will appropriately assess this hypothesis [6]. Ferrando et al. also reported that prone position in awake patient could induce delay in intubation, while they did not report any impact on day-28 mortality [15].
Our study has some limitations. First, its retrospective design did not allow a definitive conclusion to be drawn. However, as discussed, patients were not a priori selected for PP, while most of them had single organ failure with lower Sofa score, less acute kidney injury and a lower number of patients with vasopressors. By using adequate statistical analysis with a propensity score, we tried to soften this limitation and especially the absence of a randomized control group and our adjustment between the 2 groups was very good. Moreover, we did not differentiate patients according to predictors of response to proning, as CT-scan or lung ultrasonography. Second, although we report one of the largest series of patients, the sample size was relatively small and a lack of power is not excluded. Another explanation for the absence of impact on outcomes could be related to limited time of patients' exposure to proning. However, in their small observational study, Scaravilli et al. reported that most patients were discharged alive from hospital without intubation and the exposition was also limited with a median duration of proning of 3 h and a mean number of sessions per patient of 2 [21]. Despite low exposition, SBPP could avoid or delay intubation and well-known deleterious effects of positive pressure ventilation [6], especially during the pro-inflammatory process following the admission in the ICU. A recent study which performed a clustering in critically-ill COVID-19 patients based on their inflammatory status reported that patients with high interleukine-6 at ICU admission had the highest requirement to intubation and the highest mortality [23]. How SBPP could be useful in this subgroup of patients could be evaluated. Third, we did not differentiate responders and non-responders to SBPP and then to evaluate whether their outcome could be different. However, this is also one of the strengths of our study as we evaluated a global strategy which is to delay intubation when possible thanks to use of SBPP. Moreover, previous studies have reported that oxygenation improvement (responders) was usually transient and not maintained [[9], [10], [11], [12]]. We also acknowledge that increase in PaO2/FiO2 after proning was probably clinically non-relevant, while statistically significant. However, it was well-demonstrated in ARDS that improvement in gas exchange by prone position did not predict and explain improved survival [24].
6. Conclusion
We report that SBPP was used in around 10% of patients admitted in the ICU for severe respiratory failure and was well-tolerated. After adjusting for confounders, we did not demonstrate any association with intubation and day-28 mortality rates. Randomized controlled trials to assess clinical benefits associated with SBPP are warranted.
Ethics approval
The appropriate IRB approved this study and, due to the nature of retrospective chart review, waived the need for informed consent from individual patients.
Funding
No funding.
Authors' contribution
AVB and RJ designed the study and wrote the manuscript, EA read and corrected the manuscript, MD did the statistical analysis, RJ, FI, GG, AB, MF, JJT, SM and AD included patients, read and accepted the manuscript.
Declaration of Competing Interest
MF declares grant from Biomerieux and personal fees from Pfizer.
EA declares grant from Fisher and Payckle, and Gilead, and personal fees from Gilead, Pfizer, Baxter, Ablynx, Alexion.
GG declares non-financial support from Bard.
JJT declares non-financial support from Pfizer and personal fees from MSD.
MD declares grant from MSD and personal fees from MSD, Astellas and Gilead.
AVB declares grant from GSK.
The other authors did not declare any conflict of interest.
Acknowledgements
None.
Footnotes
Fig. S1.
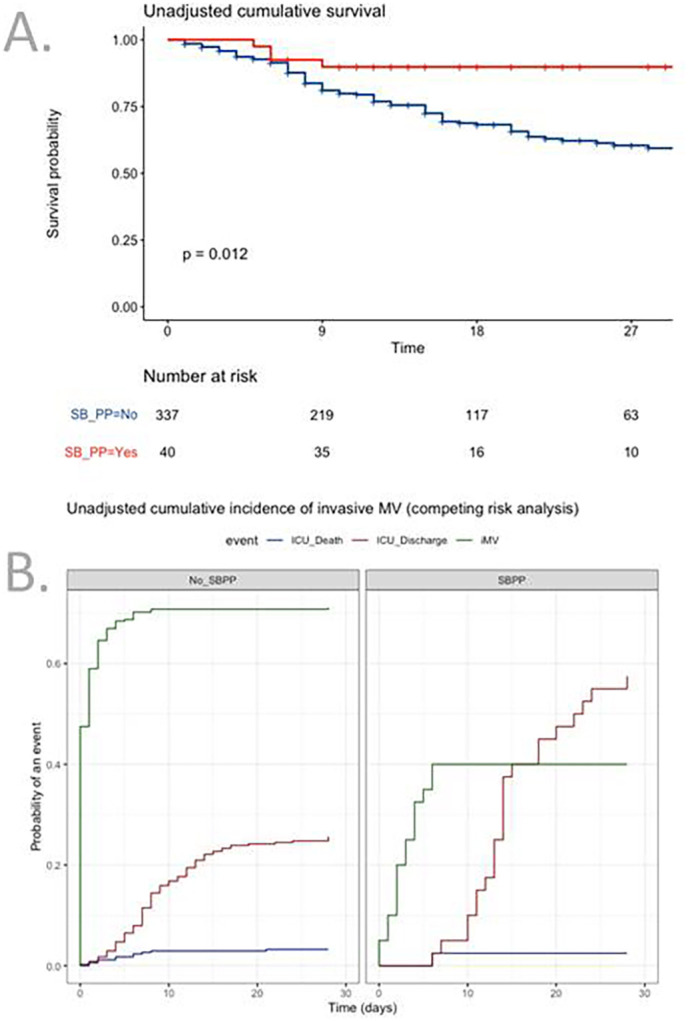
Fig. S2.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcrc.2021.04.014.
References
- 1.COVID19-APHP Group Assistance Publique-Hôpitaux de Paris’response to the COVID-19 pandemic. Lancet. 2020;395:1760–1761. doi: 10.1016/S0140-6736(20)31210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Du R., Fan G., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay E., Fartoukh M., Darmon M., et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intens Care Med. 2020;11:1–9. doi: 10.1007/s00134-020-06202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Liu T., et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;0:1–10. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobin M. Basing respiratory management of COVID-19 on physiological principles. Am J Respir Crit Care Med. 2020;201:1319–1336. doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intens Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson W.R., Amato M.B.P., Brochard L.J. Fifty years of research in ARDS. Respiratory mechanics in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:822–833. doi: 10.1164/rccm.201612-2495CI. [DOI] [PubMed] [Google Scholar]
- 8.Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202:1–21. doi: 10.1164/rccm.202004-1385ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elharrar X., Trigui Y., Dols A.M., et al. Use of prone position in nointubated patients with COVID-19 and hypoxemic respiratory failure. JAMA. 2020;323:2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartini C., Tresoldi M., Scarpellini P., et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323:2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson A., Ranard B., Wei Y., et al. Prone positioning in awake, nonintubated patients with COVID-19 hypoxemic respiratory failure. JAMA Intern Med. 2020;180:1537–1539. doi: 10.1001/jamainternmed.2020.3030. e203030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppo A., Bellani G., Winterton D., et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8:765–774. doi: 10.1016/S2213-2600(20)30268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng Z., Tay W.C., Ho C.H.B. Awake prone positioning for non-intubated oxygen dependent COVID-19 pneumonia patients. Eur Respir J. 2020;56:2001198. doi: 10.1183/13993003.01198-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNicholas B., Cosgrave D., Giacomini C., et al. Prone position in COVID-19 acute respiratory failure: just do it? Br J Anaesth. 2020;125:440–443. doi: 10.1016/j.bja.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrando C., Mellado-Artigas R., Gea A., et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicentre, adjusted cohort study. Crit Care. 2020;24:597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intens Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 17.Thomas M.E., Blaine C., Dawnay A., et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 18.Thomas L., Li F. Pencina M using propensity score methods to create target populations in observational clinical research. JAMA. 2020 doi: 10.1001/jama.2019.21558. [DOI] [PubMed] [Google Scholar]
- 19.Li F., Thomas L.E., Li F. Addressing extreme propensity scores via the overlap weights. Am J Epidemiol. 2019;188:250–257. doi: 10.1093/aje/kwy201. [DOI] [PubMed] [Google Scholar]
- 20.Guan W.J., Ni Z.Y., Liang W.H., et al. Clinical characteristics of coronavirus disease in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scavarilli V., Grasselli G., Castagna L., et al. Prone position improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30:1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Demoule A., Vieillard-Baron A., Darmon M., et al. High flow nasal canula in critically ill severe COVID-19 patients. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azoulay E., Zafrani L., Mirouse A., et al. Clinical phenotypes of critically ill Covid-19 patients. Intens Care Med. 2020;46:1651–1652. doi: 10.1007/s00134-020-06120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albert R.K., Keniston A., Baboi L., et al. Prone position-induced improvement in gas exchange does not predict improved survival in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2014;189:494–496. doi: 10.1164/rccm.201311-2056LE. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis assessing influence of prone positioning on day-28 mortality and cumulative incidence of invasive mechanical ventilation after exclusion of 204 patients requiring invasive ventilation within first 24 h.