Abstract
A systematic literature review was performed to summarize the frequency and nature of renal complications in patients with chronic hypoparathyroidism managed with conventional therapy. Methodology was consistent with the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Peer-reviewed journal articles with specified medical subject heading terms were identified using the PubMed, EMBASE, and Cochrane databases. Data were extracted from eligible articles based on prespecified parameters for clinical outcomes of renal calcifications and disease. Because of the heterogeneity of the data, a meta-analysis could not be conducted. From 1200 potentially relevant articles, data were extracted from 13 manuscripts that reported data for ≥1 of the 19 predefined renal outcomes for ≥10 adult patients (n = 11 manuscripts) or pediatric patients (n = 2 manuscripts). The collective data provide evidence that adult and pediatric patients with chronic hypoparathyroidism and treated with conventional therapy (oral calcium and active vitamin D) had an increased risk of renal complications. The reported rate of nephrolithiasis was up to 36%, with the lowest rates in studies reporting shorter duration of disease. The rate of nephrocalcinosis was up to 38%. Some studies reported a combined nephrolithiasis/nephrocalcinosis outcome of 19% to 31%. Data for renal disease that encompassed a range of renal insufficiency to chronic kidney disease were reported in 10 articles; the reported rates ranged from 2.5% to 41%. In patients who receive long-term treatment with oral calcium and active vitamin D, chronic hypoparathyroidism may be associated with an increased risk of renal complications compared with the general population.
Keywords: Hypoparathyroidism, Chronic kidney disease, Nephrocalcinosis, Nephrolithiasis
Introduction
Hypoparathyroidism is a rare endocrine disorder caused by absent or inappropriately low levels of parathyroid hormone (PTH) [1]. Mineral homeostasis cannot be maintained because of the loss of the PTH-controlled pathways involving bone, kidney and the gastrointestinal (GI) tract. The absorption of calcium in the GI tract is greatly decreased because of the loss of activation of 25-hydroxyvitamin D (25[OH]D) to 1,25-dihydroxyvitamin D (1,25 [OH]2D), which stimulates absorption of both calcium and phosphate [2]. The skeleton ceases to be a ready source of calcium because of exceedingly low bone turnover [3]. There is reduced calcium reabsorption and urinary phosphate excretion in the kidney because of the loss of the effect of PTH [2, 4]. The results of these pathophysiological factors in hypoparathyroidism are hypocalcemia and hyperphosphatemia [1, 2, 5].
Conventional treatment in patients with chronic hypoparathyroidism is oral calcium and active vitamin D (eg, calcitriol), as well as parenteral forms of vitamin D and thiazide diuretics as needed [1, 4, 5]. Over the lifetime of an individual, chronic hypoparathyroidism and long-term therapy with oral calcium and active vitamin D appear to be associated with an increase in the risk of renal complications based on a number of retrospective studies in adult or pediatric patients with chronic hypoparathyroidism [6–10]. A case-controlled retrospective study found that increased calcium-phosphate product (ie, [serum calcium concentration] × [serum phosphate concentration]) was associated with increased risk of renal disease in patients with hypoparathyroidism [8]. Mitchell et al. suggested that conventional treatment may increase the risk of hypercalciuria, itself a risk factor for nephrolithiasis, nephrocalcinosis, and impaired renal function [9].
The objective of this systematic literature review is to summarize the reported frequency and nature of renal complications in patients with chronic hypoparathyroidism managed conventionally with calcium and active vitamin D. The specific renal outcomes investigated were nephrolithiasis/kidney stones, nephrocalcinosis, and chronic kidney disease (CKD). In addition, estimated glomerular filtration rate (eGFR) levels were also investigated. Any reported associations between each of the renal outcomes and relevant biochemical or disease parameters in the published selected articles are included in this review.
Systematic literature search
Data sources
Methodology was consistent with the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11]. A methodology protocol specified the process. Primary eligibility criteria for the inclusion of peer-reviewed journal articles are listed in Table 1. Searches were conducted in PubMed®/MEDLINE® for English-language abstract-containing articles published in peer-reviewed journals from database inception to 15 November 2018. Additional peer-reviewed journal articles not in PubMed®/MEDLINE® were identified by similar searches conducted in EMBASE® and Cochrane® databases for the same timeframe.
Table 1.
Primary Eligibility Criteria for Relevant Peer-Reviewed Journal Articles
| Inclusion criteria | |
|---|---|
| Patient population | Adults, children, infants with hypoparathyroidism |
| Other population | Preclinical (hypoparathyroidism relevant) |
| Language | English language |
| Exclusion criteria | |
| Treatment interventions | PTH, PTH analogs, rhPTH(1–84), rhPTH(1–34) |
| Type of article | Review |
PTH, parathyroid hormone; rhPTH, recombinant human parathyroid hormone
Search strategy
The strategy employed a database search string composed of free text and controlled vocabulary terms (ie, medical subject headings [MeSH] for PubMed®/MEDLINE®). Selected MeSH terms included nephrocalcinosis, nephrolithiasis, kidney calculi, and all related terms for kidney stones, renal insufficiency, and chronic kidney/renal disease. This approach was broad based but also included precise terminology to capture publications that would potentially have data values for the predefined relevant clinical outcomes (Table 2). The following is the search string that was used, formatted for PubMed®/MEDLINE®: “Hypoparathyroidism”[Title] OR ((Hypoparathyroidism[MESH] AND kidney diseases[MESH] AND kidney[MESH]) OR (Hypoparathyroidism[MESH] AND hypercalciuria[MESH]) OR (Hypoparathyroidism[MESH] AND morbidity[MESH]) OR (hypoparathyroidism[MESH] AND hypercalcemia[MESH]) OR (hypoparathyroidism[MESH] AND hyperphosphatemia[MESH])) NOT (Hyperparathyroidism [MESH] OR adynamic bone disease[MESH]) AND ((Clinical Study[ptyp] OR Clinical Trial[ptyp] OR Clinical Trial, Phase I[ptyp] OR Clinical Trial, Phase II[ptyp] OR Clinical Trial, Phase III[ptyp] OR Clinical Trial, Phase IV[ptyp] OR Comparative Study[ptyp] OR Controlled Clinical Trial[ptyp] OR Dataset[ptyp] OR Meta-Analysis[ptyp] OR Observational Study[ptyp] OR Pragmatic Clinical Trial[ptyp] OR Randomized Controlled Trial[ptyp] OR Research Support, N I H, Extramural[ptyp] OR Research Support, N I H, Intramural[ptyp] OR Research Support, Non U S Gov’t[ptyp] OR Research Support, U S Gov’t, Non P H S[ptyp] OR Research Support, U S Gov’t, P H S[ptyp] OR Research Support, U.S. Government[ptyp]) AND has abstract[text] AND (“humans”[MeSH Terms] OR “animals”[MeSH Terms:noexp])).
Table 2.
Predefined Clinical Outcomes for Data Extraction
| Renal-Related Outcomes | Biochemical-Related Outcomes |
|---|---|
| Chronic kidney disease | Calcium |
| eGFR levels | Serum levels |
| Nephrocalcinosis | Urine levels |
| Nephrolithiasis/kidney stones | Hypocalcemia |
| Other terms | Hypercalcemia |
| Acute kidney injury | Hypercalciuria |
| Acute renal failure | Phosphate |
| Acute renal injury | Serum levels |
| Dehydration | Urine levels |
| Polyuria | Hyperphosphatemia |
| Transient renal impairment | Calcium-phosphate product |
eGFR, estimated glomerular filtration rate
Duplicate publication abstracts in the search output were removed to create a combined pool of identified articles that were used for inclusion screening.
Screening and data extraction process
Articles with abstracts were reviewed according to the Table 1 eligibility criteria by two independent reviewers; a third reviewer resolved any nonconsensus selections. Articles excluded were assigned a reason for rejection. Eligible articles that remained after abstract screening underwent a full article review by each of the two independent reviewers to extract all data reported for the 19 predefined relevant clinical outcomes (Table 2). Extracted data were reviewed, and a subset of articles containing data for the most relevant renal-related outcomes (ie, nephrolithiasis/kidney stones, nephrocalcinosis, and CKD) and eGFR levels was selected. One reviewer conducted a second round of extraction, not specified in the protocol, to capture data reported for associations between renal outcomes and predefined biochemical-related outcomes. One reviewer conducted a third round of extraction, not specified in the protocol, to capture available data for thiazide use, blood pressure in the context of reported hypertension, and diabetes mellitus. The individual eligible articles reported data that were collected using differing heterogeneous methodologies that precluded any aggregation of the extracted data and a meta-analysis.
Articles selected
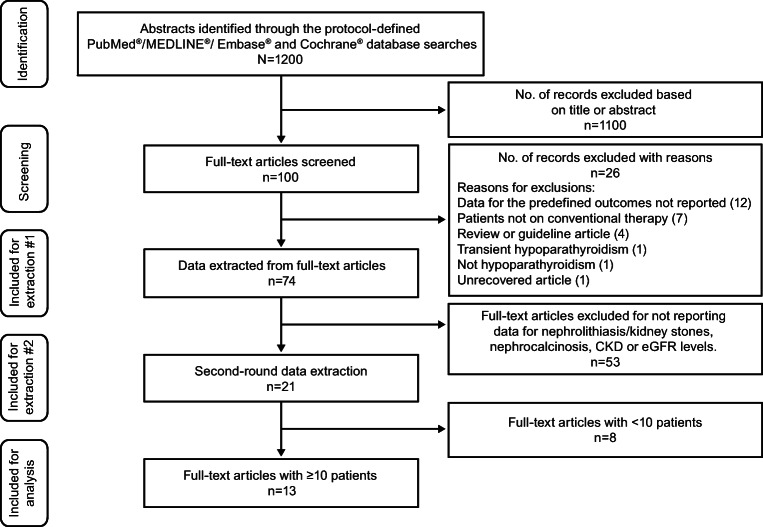
The process of the peer-review database search for data of interest yielded 1200 articles (Fig. 1). Following screening and assessment for eligibility, 74 of the 1200 articles had data that reported one or more of the 19 predefined clinical outcomes listed in Table 2. Of the 74 articles with data for one or more of the 19 predefined clinical outcomes, 21 reported data for nephrolithiasis/kidney stones, nephrocalcinosis, CKD, or eGFR levels. Of these 21 papers, 13 articles were the ultimate focus of this review because they reported data for nephrolithiasis/kidney stones, nephrocalcinosis, CKD, or eGFR levels from studies of ≥10 adult (n = 11) or pediatric patients (n = 2) with chronic hypoparathyroidism.
Fig. 1.
Flow Diagram of Article Selection for Data Extraction
Renal calcifications
Treatment of hypoparathyroidism with conventional therapies may result in hypercalciuria, which is a risk factor for nephrolithiasis and nephrocalcinosis [9]. Nephrolithiasis is defined by the appearance of solid calcium-containing stones in the collecting system of the kidney [12–14]. Nephrocalcinosis refers to the parenchymal deposition of calcium salt crystals within the interstitium of the kidney and usually involves the renal medulla [12, 15–17]. Nephrolithiasis and nephrocalcinosis are conditions that may coexist [12, 16]. Nephrolithiasis is commonly diagnosed by abdominal computed tomography (CT) or ultrasonography [18]. Nephrocalcinosis is typically detected using ultrasound imaging as increased bilateral, symmetrical echogenicity within renal pyramids, or by abdominal CT [12, 16].
Nephrolithiasis/kidney stones
Among the six articles with data on the percentage of patients with nephrolithiasis/kidney stones, rates varied from 0% [10] to 35.5% [19] (Table 3, Fig. 2) [6, 7, 10, 19–21]. Differences in study designs, patient populations, and overall size of the studies may help explain some of the variation in the reported rates of this renal complication. Furthermore, the considerably different assessment methods used in the six studies, ranging from diagnostic codes [6, 7], ultrasound [10, 20, 21], and patient self-reporting [19], likely contributed substantially to the heterogeneity in the reported rates. In the pediatric study of Levy et al., the lowest outcome rate of nephrolithiasis was reported (0%) based on ultrasound [10]. In adult studies, rates were reported using diagnostic codes (1%–2%) [6, 7], ultrasound (8%–30%) [20, 21], and patient self-reporting (35.5%) [19]. These findings suggest that current understanding regarding the frequency of this complication in patients with hypoparathyroidism is likely affected by the choice of diagnostic coding or ultrasound. Meola et al. evaluated a cross-section of patients with chronic hypoparathyroidism on conventional treatment to determine the proportion who met biochemical parameter targets defined by the European Society of Endocrinology (ESE) treatment guidelines [22] in order to meet the ESE treatment goal to relieve symptoms of hypocalcemia and maintain calcium levels in the low or slightly below normal reference range. As part of that study, Meola et al. reported that 30% of the study population had nephrolithiasis detected by renal ultrasound and that most were asymptomatic [21]. Meola et al. used a study design with age- and gender-matched healthy normative controls and determined that the rate and risk of nephrolithiasis/kidney stones was significantly higher in patients with postsurgical chronic hypoparathyroidism versus controls (30% vs 5%, P < 0.0001; odds ratio, 8.2). In the two studies that used age- and gender-matched controls, there was an increased risk of nephrolithiasis/kidney stones in patients with postsurgical chronic hypoparathyroidism, but not in patients with nonsurgical chronic hypoparathyroidism (hazard ratios, 4.82 and 0.80, respectively; Table 3) [6, 7]. Underbjerg et al. suggest that for renal outcomes there may be an interaction between the age of the individual and duration of disease, with increased risk in older patients [7].
Table 3.
Nephrolithiasis/Kidney Stones (6 studies)
| Article Study Design |
Population | Disease Duration/Follow-Up (years) | Supplementation (%) | Methods | Kidney Stones (% of patients) | Reported Association Data Between Those Renal Outcomes and the Predefined Biochemical-Related Outcomes | Serum Calcium | Urinary Calcium | Serum Phosphate | Urine Phosphate | Calcium-Phosphate Product |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Underbjerg et al. 2015 [7] Retrospective follow-up study using national health registry data |
180 Danish pts with nonsurgical HypoPT, mean age, 49.7 years 540 age- and gender-matched controls |
Not reported |
Calcium, 71% Active vitamin D analogs, 70% |
ICD-8 and ICD-10 codes | 1% |
Not reported Relevant finding stated in the article: Risk of nephrolithiasis was not increased in pts compared with controls (HR: 0.80 [95% CI, 0.17–3.85]) |
Not reported | Not reported | Not reported | Not reported | Not reported |
|
Outcome Hypocalcemia: 27% pts (9 pts) | |||||||||||
|
Underbjerg et al. 2013 [6] Retrospective follow-up study using national health registry data |
688 Danish pts with postsurgical HypoPT, median (range) age, 49 (17–87) years 2064 age- and gender-matched controls |
Median (IQR) duration of disease: 8 (4;12) |
Calcium, 93% Alfacalcidol, 93% |
Determined by ICD-8 or ICD-10 codes | 2% |
Not reported Relevant finding stated in the article: Compared with controls, pts had increased risk of renal stones HR (unadjusted): 4.82 (95% CI, 2.0–11.64) HR (adjusted for prior renal diseases): 4.22 (1.73–10.30) HR (adjusted for prior diabetes mellitus and renal disease): 4.02 (1.64–9.90) |
Not reported | Not reported | Not reported | Not reported | Not reported |
|
Arlt et al. 2002 [20] Cross-sectional study |
25 women with postsurgical HypoPT, mean (SD) age, 48.4 (13.6) years 25 sex-, age-, and surgery-matched controls,a mean (SD) age, 49.5 (13.2) years |
Median (range) duration of disease: 3 (0.5–38) | Calcium and oral vitamin D, vitamin D metabolites or analogs, 100% | Renal ultrasound | 8% | Not reported | 2.15 ± 0.21 mmol/L | 5.51 ± 4.17 mmol/24 h | 1.32 ± 0.22 mmol/L | 26.1 ± 8.8 mmol/24 h | Not reported |
|
Outcome Hypocalcemiab: 12% pts (3 pts) |
Outcome Hypercalciuriac: 23% pts (5/22 pts) |
||||||||||
|
Meola et al. 2018 [21] Prospective study |
90 pts with postsurgical HypoPT Mean (SD) age, females: 50 (14) years; males: 57 (14) years 142 sex- and age-matched healthy normative controls Mean (SD) age, females: 53 (8) years; males: 50 (6) years |
Mean ± SD disease duration: 9 ± 7 |
Calcium, 38.9% Calcitriol, 100% |
Renal ultrasound | 30% | No significant correlation (P = 0.98) between presence of kidney stones and duration of HypoPT, 24-h urinary calcium excretion, total Alb-sCa or vitamin D status |
Alb-sCa 8.9 ± 0.5 mg/dL (range 7.5–10.1) |
Male: 359 ± 178 mg/24 h Female: 290 ± 155 mg/24 h |
3.6 ± 0.7 mg/dL (range 2.2–5.9) | Not reported | Normal, <55 mg2/dL2 in all pts |
|
Outcome Hypocalcemiad: 14% pts (13 pts) |
Outcome Hypercalciuriae: Female: 52% pts (33/63 pts) Male: 63% pts (12/19 pts) |
Outcome Hyperphosphatemia: 8% pts (7 pts) |
|||||||||
|
Hypercalcemiad: 20% pts (18 pts) | |||||||||||
|
Hadker et al. 2014 [19] Patient self-reporting in a cross-sectional survey |
374 pts with chronic HypoPT, mean (SD) age, 49.4 (11.6) years | Mean ± SD duration of disease: 12.6 ± 12.4 |
Calcium, 25% Calcitriol, 44% Ergocalciferol vitamin D2 or cholecalciferol vitamin D3, 20% Combination of calcium/calcitriol, 67% |
Self-report |
35.5% (since diagnosis) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
|
Levy et al. 2015 [10] Long-term retrospective follow-up study |
29 pediatric pts with chronic HypoPT, mean (SD) age, 11.1 (5.9) years |
Mean ± SD duration of disease: 9.1 ± 5.5 Mean ± SD duration of follow-up: 7.4 ± 5.0 |
Calcitriol/calcium, 100% Cholecalciferol, 79% |
Renal ultrasound | 0 | Not reported |
Total calcium: 8.9 ± 0.8 mg/dL Ionized calcium: 4.6 ± 0.5 mg/dL |
Average urine calcium/creatinine ratio: 0.27 ± 0.25 mg/mg | 5.9 ± 1.2 mg/dL | Not reported | Not reported |
Alb-sCa, albumin-corrected serum calcium; ESE, European Society of Endocrinology; HR, hazard ratio; HypoPT, hypoparathyroidism; ICD, international classification of diseases and related health problems; IQR, interquartile range; pt, patient; ULN, upper limit of normal
Note: The following superscripted-letter footnotes are based on information contained in the indicated manuscript
aSubtotal thyroidectomy for goiter with intact parathyroid function (n = 23) or parathyroid surgery for hyperparathyroidism (n = 2)
bBelow 2.00 mmol/L
c>ULN 3–8 mmol/day
dESE target ranges used with hypocalcemia being below the recommended ranges and hypercalcemia above
eValues above the ULN (≥300 mg/24 h in males and ≥ 250 mg/24 h in females)
Fig. 2.
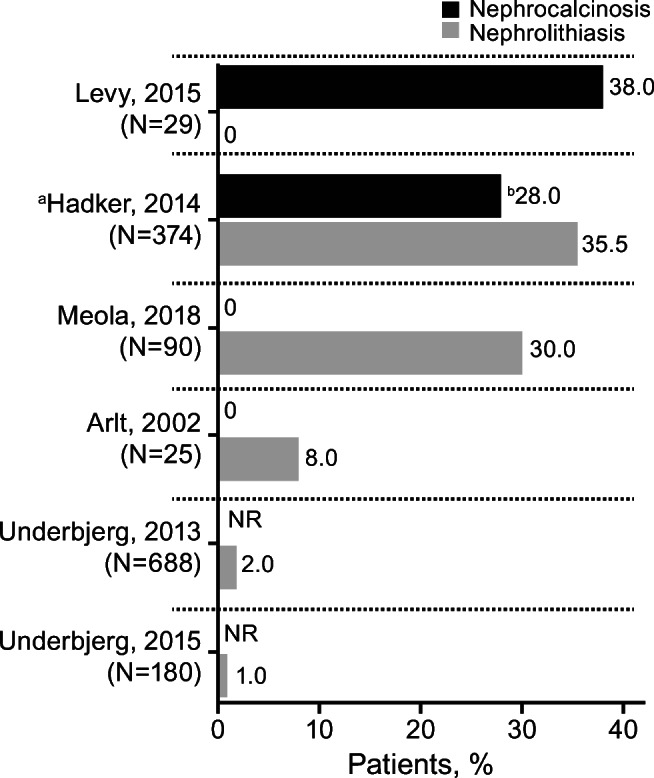
Percentages of Patients With Renal Calcifications. Bars and values represent the percentage of patients with nephrolithiasis or nephrocalcinosis. Patient numbers (N) refer to the number of patients with hypoparathyroidism in the study. aPatients self-reporting in a cross-sectional survey. bIncluded patients with severe hypoparathyroidism (22%) and patients with milder hypoparathyroidism (6%). NR, not reported
A separate study by Bohrer et al. used a new Likert-scale questionnaire to capture patient self-reported impairment reported as distress level scores [23]. Patients with higher total symptom or distress level scores had more illness manifestations, including kidney stones (plus bone changes, basal ganglia calcification, and cataracts), and had lower serum calcium levels than patients with lower total symptom or distress level scores (both P < 0.05).
Nephrocalcinosis
Among the four articles reporting specifically the percentage of patients with nephrocalcinosis, rates varied from 0% to 38% (Table 4, Fig. 2) [10, 19–21]. Similar to the nephrolithiasis/kidney stones outcome, between-study differences in design, methodology and population size may explain the large discrepancies in the reported rates of nephrocalcinosis. In order of lowest to highest, the reported occurrences in the three adult studies used ultrasound (0%) and patient self-reporting (28%); none of the studies reported diagnostic codes [19–21]. Hadker et al. used a patient self-reporting cross-sectional survey of adult patients and found that those who indicated severe disease on the questionnaire (no definition or description of severity grades was provided) reported a significantly higher occurrence of nephrocalcinosis versus patients who reported having mild disease (22% vs 6%; P ≤ 0.05) [19].
Table 4.
Nephrocalcinosis (4 studies)
| Article Study Design |
Population | Disease Duration/Follow-Up (years) | Supplementation (%) | Methods | Nephrocalcinosis (% of patients) |
Reported Association Data Between Those Renal Outcomes and the Predefined Biochemical-Related Outcomes | Serum Calcium | Urinary Calcium | Serum Phosphate | Urine Phosphate | Calcium-Phosphate Product |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Arlt et al. 2002 [20] Cross-sectional study |
25 women with postsurgical HypoPT, mean (SD) age, 48.4 (13.6) years 25 sex-, age-, and surgery-matched controls,a mean (SD) age, 49.5 (13.2) years |
Median (range) duration of disease: 3 (0.5–38) | Calcium and oral vitamin D, vitamin D metabolites or analogs, 100% | Renal ultrasound | 0 | Not reported | 2.15 ± 0.21 mmol/L | 5.51 ± 4.17 mmol/24 h | 1.32 ± 0.22 mmol/L | 26.1 ± 8.8 mmol/24 h | Not reported |
|
Outcome Hypocalcemiab: 12% pts (3 pts) |
Outcome Hypercalciuriac: 23% pts (5/22 pts) |
||||||||||
|
Meola et al. 2018 [21] Prospective study |
90 pts with postsurgical HypoPT Mean (SD) age, females: 50 (14) years; males: 57 (14) years 142 sex- and age-matched healthy normative controls, mean (SD) age, females: 53 (8) years; males: 50 (6) years |
Mean ± SD disease duration: 9 ± 7 |
Calcium, 38.9% Calcitriol, 100% |
Renal ultrasound | 0 | Not reported |
Alb-sCa 8.9 ± 0.5 mg/dL (range 7.5–10.1) |
Male: 359 ± 178 mg/24 h Female: 290 ± 155 mg/24 h |
3.6 ± 0.7 mg/dL (range 2.2–5.9) | Not reported | Normal, <55 mg2/dL2 in all pts |
|
Outcome Hypocalcemiad: 14% pts (13 pts) |
Outcome Hypercalciuriae: Female: 52% pts (33/63 pts) Male: 63% pts (12/19 pts) |
Outcome Hyperphosphatemia: 8% pts (7 pts) |
|||||||||
|
Hypercalcemiad: 20% pts (18 pts) | |||||||||||
|
Hadker et al. 2014 [19] Patient self reporting in a cross-sectional survey |
374 pts with chronic HypoPT, mean (SD) age, 49.4 (11.6) years |
Mean ± SD duration of disease: 12.6 ± 12.4 |
Calcium, 25% Calcitriol, 44% Ergocalciferol vitamin D2 or cholecalciferol vitamin D3, 20% Combination of calcium/calcitriol, 67% |
Self-report | Pts with severe HypoPT: 22% vs pts with milder HypoPT: 6% (P ≤ 0.05) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
|
Levy et al. 2015 [10] Long-term retrospective follow-up study |
29 pediatric pts with chronic HypoPT, mean (SD) age, 11.1 (5.9) years |
Mean ± SD duration of disease: 9.1 ± 5.5 Mean ± SD duration of follow-up: 7.4 ± 5.0 |
Calcitriol/calcium, 100% Cholecalciferol, 79% |
Renal ultrasound | 38% |
Multivariate analysis: degrees of relative hypercalcemiaf and hyperphosphatemiag most significant predictors for nephrocalcinosis (R2 = 0.47, P < 0.01) Relevant finding stated in the article: Nephrocalcinosis resolved after initial ultrasound (n = 2); remained in early stage I (n = 3), progressed to stage III (n = 6) Pts with non-resolved (n = 9) vs w/o (n = 18) nephrocalcinosis had a greater: degree of hypercalcemiaf (P = 0.005); degree of hypocalcemiah (P = 0.004); duration of hypocalcemia (P = 0.003); degree of hyperphosphatemiag (P = 0.01) |
Total calcium: 8.9 ± 0.8 mg/dL Ionized calcium: 4.6 ± 0.5 mg/dL Total calcium: Pts with nephrocalcinosis: 8.5 ± 1.1 mg/dL Pts w/o nephrocalcinosis: 9.2 ± 0.6 mg/dL |
Average urine calcium/creatinine ratio: 0.27 ± 0.25 mg/mg |
5.9 ± 1.2 mg/dL Pts with nephrocalcinosis: 6.0 ± 1.9 mg/dL Pts w/o nephrocalcinosis: 5.8 ± 0.9 mg/dL |
Not reported | Not reported |
|
Outcome Hypocalcemiai: Percentage of time with total calcium <8.0 mg/dL: Pts with nephrocalcinosis: 29.4 ± 20.4% Pts w/o nephrocalcinosis: 10.5 ± 11.3% |
Outcome Hyperphosphatemiak: Percentage of time with phosphate concentrations above age-adjusted levels: Pts with nephrocalcinosis: 50 ± 36.2% Pts w/o nephrocalcinosis: 29 ± 29.4% |
||||||||||
|
Outcome Hypercalcemiaj: Percentage of time with total calcium >9.6 mg/dL: Pts with nephrocalcinosis: 22.8 ± 23.8% Pts w/o nephrocalcinosis: 35.3 ± 31.7% |
Alb-sCa, albumin-corrected serum calcium; AUC, area under the curve; HypoPT, hypoparathyroidism; pt, patient; ULN, upper limit of normal
Note: the following superscripted-letter footnotes are based on information contained in the indicated manuscript
aSubtotal thyroidectomy for goiter with intact parathyroid function (n = 23) or parathyroid surgery for hyperparathyroidism (n = 2)
bBelow 2.00 mmol/L
c>ULN 3–8 mmol/day
dESE target ranges used with hypocalcemia being below the recommended ranges and hypercalcemia above
eValues above the ULN (≥300 mg/24 h in males and ≥ 250 mg/24 h in females)
fAUC of total calcium concentrations >9.6 mg/dL
gAUC above age-adjusted phosphate levels
hAUC of total calcium concentrations <8.0 mg/dL
iPercentage of time with total calcium <8.0 mg/dL
jPercentage of time with total calcium >9.6 mg/dL
kPercentage of time with phosphate concentrations above age-adjusted levels
Levy et al. pediatric study that used renal ultrasound reported 38% of patients had nephrocalcinosis, in contrast to the finding that no patients had nephrolithiasis/kidney stones [10]. This study also enabled evaluation of changes in nephrocalcinosis using a staging system developed by Boyce et al., in which stage 0 was no echogenicity; stage I, mild echogenicity around medullary pyramid borders; stage II, moderate echogenicity around and inside pyramids; and stage III, severe echogenicity of entire pyramids [12]. Of the 11 patients with nephrocalcinosis after the initial ultrasound, the nephrocalcinosis resolved in two patients (18%), remained in early stage I in three patients (27%), and progressed from stage I to III in six patients (55%). In the two patients with resolved nephrocalcinosis, both had DiGeorge syndrome, and calcium concentrations were more frequently within the target range versus patients in whom nephrocalcinosis persisted (81% ± 7.6% vs 56% ± 8.5%; P = 0.01).
Combined data for nephrolithiasis and/or nephrocalcinosis
Four articles reported data on the percentage of patients with nephrolithiasis and/or nephrocalcinosis as a combined outcome; all studies used ultrasound or CT scans [9, 24–26]. Among the four studies, the rates were similar to those reported in the studies using separated nephrolithiasis and nephrocalcinosis outcomes (19% to 31%; Table 5). In a study by Leidig-Bruckner et al. of 33 patients with postsurgical hypoparathyroidism (and medullary thyroid carcinoma), there were two cases noted for hospitalization for symptomatic nephrolithiasis [26]. The article also provided details about the nine patients (27%) with documented renal calcifications; five patients had initially received high cholecalciferol dosages, and two patients had received dihydrotachysterol. Also, three of the nine patients receiving cholecalciferol/dihydrotachysterol experienced transient renal failure.
Table 5.
Nephrolithiasis and/or Nephrocalcinosis (4 studies)
| Article Study Design |
Population | Disease Duration/Follow-Up (years) | Supplementation | Methods | Nephrolithiasis and/or Nephrocalcinosis (% of patients) | Reported Association Data Between Those Renal Outcomes and the Predefined Biochemical-Related Outcomes | Serum Calcium | Urinary Calcium | Serum Phosphate | Urine Phosphate | Calcium-Phosphate Product |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lopes et al. 2016 [25] Retrospective observational study |
55 pts with chronic HypoPT, mean (SD) age, 44.5 (19.3) years 41 (74.5%) with post-surgical HypoPT 5 (9.1%) with pseudoHypoPT 9 (16.4%) with autoimmune HypoPT |
Mean ± SD duration of disease: 11.2 ± 7.5 (range 1–32) |
Calcium, 92% Calcitriol, 80% Cholecalciferol, 75% |
Renal ultrasound |
25% (10/40 with imaging) |
No correlation between serum and urinary levels of calcium and the presence of calcification Relevant finding stated in the article: Weight-adjusted urinary calcium in 24 h was higher in pts with renal calcification vs those without (3.3 mg/kg/d vs 1.8, respectively; P < 0.05) |
6.87–8.62 mg/dL (mean, first to last visit) |
Outcome Hypercalciuriaa: 27% pts (15 pts) |
6.14–4.89 mg/dL(mean, first to last visit) | Not reported | Not reported |
|
Leidig-Bruckner et al. 2016 [26] Retrospective, longitudinal chart review |
33 pts with medullary thyroid carcinoma and postsurgical HypoPT, mean (SD) age 52.8 (13.7) years: Classified as having partial HypoPTb (n = 20) or complete HypoPTb (n = 13) |
Mean ± SD duration of disease: 15.9 ± 9.4 Mean ± SD follow-up: 11.9 ± 6.6 |
Calcium, 72.7% Cholecalciferol, 18.1% Calcitriol, 33.3% Alfacalcidol, 6.1% Dihydrotachysterol, 18.2% |
Radiological imaging (ultrasound, CT, and/or MRI) Calcification group: documented calcifications, renal stones, medullary sponge kidney |
27% Partial HypoPTb: 25% Complete HypoPTb: 31% 2 pts hospitalized for symptomatic nephrolithiasis |
Not reported Relevant finding stated in the article: Incidence was higher in pts who initially received high cholecalciferol dosages Of the 9 pts with renal calcifications, 2 were treated with calcitriol from the beginning of treatment, 5 initially received high cholecalciferol doses, and 2 received dihydrotachysterol [see Table 6 for the reported eGFR data and renal calcifications] |
Partial HypoPTb: 2.13 ± 0.10 mmol/L Complete HypoPTb: 2.12 ± 0.12 mmol/L |
Partial HypoPTb: 3.13 ± 1.9 mmol/L (range 1–10, n = 17, end of study) Complete HypoPTb: 5.20 ± 3.22 mmol/L (range 1–10, n = 10, end of study) |
Partial HypoPTb: 1.4 ± 0.18 mmol/L Complete HypoPTb: 1.51 ± 0.22 mmol/L |
Not reported |
Partial HypoPTb: 2.98 ± 0.32 mmol2/L2 Complete HypoPTb: 3.16 ± 0.42 mmol2/L2 |
|
Outcome Hypocalcemia: 27% pts (9 pts) | |||||||||||
|
Mitchell et al. 2012 [9] Retrospective, longitudinal chart review |
120 pts with chronic HypoPT mean (SD) [range] age, 52 (19) [2–87] years |
Mean ± SD duration of disease: 17 ± 16 (range 1–59) Mean ± SD follow-up: 7.4 ± 5.1 |
Calcium, 94% Calcitriol, 88% High-dose vitamin D, 6% Thiazide, 20% Relevant finding stated in the article: Pts on a thiazide diuretic had higher urinary calcium levels (mean 318 vs 197 mg, P = 0.02) |
Renal/abdominal ultrasound and abdominal CT |
31% (17/54 with imaging) |
2 pts required renal transplant because of nephrocalcinosis | Mean±SD 8.6 ± 1.1 mg/dL (range 5.3–11.5) |
Mean±SD 216 ± 140 mg/24 h (range 8–557) |
Mean±SD 4.2 ± 0.9 (range 1.3–7.8) mg/dL | Not reported |
Mean±SD 35.4 ± 9.0 mg2/dL2 22% pts: >55 mg2/dL2 (at least once during study period) |
|
Outcome Hypocalcemia: 16% pts (most recent measurement) |
Outcome Hypercalciuria: 38% pts overall 26% pts (most recent measurement) |
||||||||||
|
Outcome Hypercalcemia: 13% pts (most recent measurement) Frank hypercalcemiac: 2% pts 3 episodes of mild hypercalcemia associated with elevated 25-OH vitamin D levels |
|||||||||||
|
Kim et al. 2015 [24] Retrospective |
37 pediatric pts with primary HypoPT median (range) age, 1.7 months (1 day–17 years) |
Mean ± SD duration of follow-up: 7.0 ± 5.3 (range 0.5–22) |
Calcium and calcitriol or calcitriol alone, 57% | Renal ultrasound in 26 pts (conducted every ~2.5 years) | 19% |
Not reported Relevant finding stated in the article: Developed after 3.5 years (range 1.6–12.5) after calcium and calcitriol supplementation |
Total Ca: 2.1 ± 0.2 mmol/L (range 1.8–2.5) Ionized Ca: 1.1 ± 0.1 mmol/L(range 0.9–1.4) |
Not reported |
1.7 ± 0.3 mmol/L (range 1.3–2.2) |
Not reported | Not reported |
CT, computed tomography; eGFR, estimated glomerular filtration rate; HypoPT, hypoparathyroidism; MRI, magnetic resonance imaging; pt, patient; PTH, parathyroid hormone
Note: the following superscripted-letter footnotes are based on information contained in the indicated manuscript
a>250 mg/24 h for females and > 300 mg/24 h for males
bPartial hypoparathyroidism defined ≥1 PTH measurement >10 ng/L; complete hypoparathyroidism defined as all PTH measurements ≤10 ng/L
c>10.5 mg/dL
Chronic kidney disease
Data for CKD, renal insufficiency, and eGFR levels were reported in 10 articles [6–10, 19, 21, 25–27]. Eight adult studies reported the percentage of patients with CKD based on standard methods of eGFR <60 mL/min/1.73 m2 or ≥ stage 3 classification [28], or renal insufficiency international classification of diseases (ICD) ICD-8 and ICD-10 codes. One survey reported CKD based on adult patients self-reporting for chronic kidney failure [19]. The pediatric study of Levy et al. used the revised Schwartz estimating equation for nonchronic kidney disease populations. The methods used by each study are detailed in Table 6. The rates of CKD varied among studies from 2.5% to 41% (Fig. 3 and Table 6).
Table 6.
Chronic Kidney Disease and eGFR Levels (10 studies)
| Article Study Design |
Population | Disease Duration/Follow-Up (years) | Supplementation | Methods | CKD (% of Patients) |
Reported Association Data Between Those Renal Outcomes and the Predefined Biochemical-Related Outcomes | Serum Calcium | Urinary Calcium | Serum Phosphate | Urine Phosphate | Calcium-Phosphate Product |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hadker et al. 2014 [19] Patient self-reporting in a cross-sectional survey |
374 pts with chronic HypoPT, mean (SD) age, 49.4 (11.6) years |
Mean ± SD duration of disease: 12.6 ± 12.4 |
Calcium, 25% Calcitriol, 44% Ergocalciferol vitamin D2 or cholecalciferol vitamin D3, 20% Combination of calcium/calcitriol, 67% |
Self-report; CKD reported as chronic kidney failure |
CKD 2.5% with mild HypoPTa vs 19% with severe HypoPTa (P ≤ 0.05) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
|
Underbjerg et al. 2013 [6] Retrospective follow-up study using national health registry data |
688 Danish pts with postsurgical HypoPT, median (range) age, 49 (17–87) years 2064 age- and gender-matched controls |
Median (IQR) duration of disease: 8 (4;12) |
Calcium, 93% Alfacalcidol, 93% |
Reported as renal insufficiency defined using ICD codes |
5% (35 pts) |
Not reported Relevant finding stated in the article: Compared with controls, pts had increased risk of renal insufficiency HR (unadjusted): 4.95 (95% CI, 2.88–8.50) HR (adjusted for prior renal diseases): 4.54 (2.63–7.84) HR (adjusted for prior diabetes mellitus and renal disease): 3.10 (1.73–5.55) |
Not reported | Not reported | Not reported | Not reported | Not reported |
|
Underbjerg et al. 2015 [7] Retrospective follow-up study using national health registry data |
180 Danish pts with nonsurgical HypoPT, mean age, 49.7 years 540 age- and gender-matched controls |
Not reported |
Calcium, 71% Active vitamin D analogs, 70% |
Reported as renal insufficiency, defined using ICD codes |
8% (15 pts) |
Not reported Relevant finding stated in the article: Compared with controls, pts had increased risk of renal insufficiency HR (unadjusted): 6.01 (95% CI, 2.45–14.75) |
Not reported | Not reported | Not reported | Not reported | Not reported |
|
Outcome Hypocalcemia: 27% pts (9 pts) | |||||||||||
|
Meola et al. 2018 [21] Prospective study |
90 pts with postsurgical HypoPT Mean (SD) age, females: 50 (14) years; males: 57 (14) years 142 sex- and age-matched healthy normative controls, mean (SD) age, females: 53 (8) years; males: 50 (6) years |
Mean ± SD disease duration: 9 ± 7 |
Calcium, 38.9% Calcitriol, 100% |
CKD-EPI equation |
<60 mL/min/1.73 m2 12% pts (11 pts) Mean ± SD 82 ± 20 mL/min/1.73 m2 (range 33–148) |
Not reported |
Alb-sCa 8.9 ± 0.5 mg/dL (range 7.5–10.1) |
Male: 359 ± 178 mg/24 h Female: 290 ± 155 mg/24 h |
3.6 ± 0.7 mg/dL (range 2.2–5.9) | Not reported | Normal, <55 mg2/dL2 in all pts |
|
Outcome Hypocalcemiab: 14% pts (13 pts) |
Outcome Hypercalciuriac: Females: 52% pts (33/63 pts) Males: 63% pts (12/19 pts) |
Outcome Hyperphosphatemia: 8% pts (7 pts) |
|||||||||
|
Outcome Hypercalcemiab: 20% pts (18 pts) | |||||||||||
|
Astor et al. 2016 [27] Pt survey using hospital registry |
283 pts with chronic HypoPT in Norway, median (range) age, 53 (9–89) years 25% pts (70/283 pts) Nonsurgical HypoPT 70% pts (197/283 pts) Postsurgical HypoPT 6% pts (16/283 pts) PseudoHypoPT |
Not reported |
Calcium, 70% Calcitriol, 40% Alphacalcidiol, 44% Ergocalciferol, 19% Cholecalciferol, 29% |
MDRD formula: calculated eGFR (MDRD formula) × (0.20247 × height (m)0.725 × weight (kg)0.425 )/1.73, where the MDRD formula is 175 × (s-Creatinine/88.4) −1.154 × (age)−0.203 × 0.742 (if female) |
<60 mL/min/1.73 m2 18% pts (51 pts) Median eGFR 80.8 mL/min/1.73 m2 (range 14.6–215.7) |
Not reported Relevant findings stated in the article: Despite conventional therapy, 18% had kidney failure (eGFR <60 mL/min/1.73 m2), of whom 98% had an eGFR level > 30 mL/min/1.73 m2 |
Alb-sCa 2.08 mmol/L (range 1.47–2.84) |
0.51 mmol/mmol creatinine (range 0.02–2.29) |
1.29 mmol/L (range 0.76–2.55) |
Not reported | Not reported |
|
Underbjerg et al. 2018 [8] Case-controlled retrospective study using national health registry data |
431 Danish pts with postsurgical or nonsurgical HypoPT, mean (range) age, 41 (0–87) years |
Median (range) duration of disease: 12.7 (0.5–87.1) |
Calcium, 95.3% Alfacalcidol, 94.4% |
MDRD equation [sex-specific eGFR using MDRD equation, converted to stages of CKD according to criteria defined by the NKF] eGFR <60 mL/min/1.73 m2 as threshold limit for renal insufficiency |
<60 mL/min/1.73 m2 21% pts (91 pts) 60–90 mL/min/1.73 m2 45% pts (194 pts) >90 mL/min/1.73 m2 34% pts (147 pts) |
Not reported |
Time-weighted avgd: Ionized Ca 1.17 mmol/L (range 1.14–1.21 (431 pts) |
Not reported |
Time-weighted avgd 1.21 mmol/L(range 1.11–1.32) (353 pts) |
Not reported |
Time-weighted avgd 2.80 mmol2/L2 (range 2.51–3.03) (304 pts) |
|
Outcome Hypercalcemia: ≥1 episodes 41% pts (177/431 pts); ≥ 4 episodes 13% pts (58/431 pts) |
Outcome Hyperphosphatemia: 7% pts (26 pts) |
||||||||||
|
Leidig-Bruckner et al. 2016 [26] Retrospective, longitudinal chart analysis |
33 with medullary thyroid carcinoma and postsurgical HypoPT, mean (SD) age, 52.8 (13.7) years: classified as having partial HypoPTe (n = 20) or complete HypoPTe (n = 13) |
Mean ± SD duration of disease: 15.9 ± 9.4 Mean ± SD follow-up: 11.9 ± 6.6 |
Calcium, 72.7% Cholecalciferol, 18.1% Calcitriol, 33.3% Alfacalcidol, 6.1% Dihydrotachysterol, 18.2% |
Cockcroft-Gault formula |
<60mL/min/ 1.73 m2 Partial HypoPTe: 5% pts (1 pt) Complete HypoPTe: 23% pts (3 pts) >90 mL/min/1.73 m2 Partial HypoPTe: 45% pts (9 pts) Complete HypoPTe: 61.5% (8 pts) |
Not reported Relevant findings stated in the article: The eGFR was negatively correlated with the duration of hypoparathyroidism (r = −0.62; P = 0.0001). This correlation remained significant after adjusting for chronological age (partial correlation, adjusted for age r = −0.35, P = 0.04). The correlation between eGFR and duration of hypoparathyroidism was independent from the degree of hypoparathyroidism (partial or complete) and also independent from the radiological presence of calcification More pts with calcifications had eGFR <60 mL/min/1.73 m2 (ie, CKD) 22% (2/9 pts) than those without calcifications 8% (2/24 pts); differences were not significant At last visit, eGFR was lower in pts with calcifications (9/33 pts) than in those without calcifications (24/33 pts) (77 ± 17 vs 95 ± 29 mL/min/1.73 m2; P = 0.07) |
Partial HypoPTe: 2.13 ± 0.10 mmol/L Complete HypoPTe: 2.12 ± 0.12 mmol/L |
Partial HypoPTe: 3.13 ± 1.9 mmol/L (n = 17) Complete HypoPTe: 5.20 ± 3.22 mmol/L (n = 10) |
Partial HypoPTe: 1.4 ± 0.18 mmol/L Complete HypoPTe: 1.51 ± 0.22 mmol/L |
Not reported |
Partial HypoPTe: 2.98 ± 0.32 mmol2/L2 Complete HypoPTe: 3.16 ± 0.42 mmol2/L2 |
|
Outcome Hypocalcemia: 27% pts (9 pts) | |||||||||||
|
Lopes et al. 2016 [25] Retrospective observational study |
55 pts with chronic HypoPT, mean (SD) age, 44.5 (19.3) years 41 (74.5%) with postsurgical HypoPT, 5 (9.1%) with pseudoHypoPT, and 9 (16.4%) with autoimmune HypoPT |
Mean ± SD duration of disease: 11.2 ± 7.5 (range 1–32) |
Calcium, 92% Calcitriol, 80% Cholecalciferol, 75% |
Cockcroft-Gault formula (for patients with weight and creatinine available for the last visit) CKD stages per KDIGO |
CKD Stage 2 33% pts (15 pts) Stage 3 9% pts (4 pts) Stage 4 2% pts (1 pt) Stage 5 2% pts (1 pt) Mean ± SD 92.9 ± 36.2 mL/min/1.73 m2 (range 14–223) |
Not reported | 6.87–8.62 mg/dL (mean, first to last visit) |
Outcome Hypercalciuriaf: 27% pts (15 pts) |
6.14–4.89 mg/dL (mean, first to last visit) | Not reported | Not reported |
|
Mitchell et al. 2012 [9] Retrospective, longitudinal chart review |
120 pts with chronic HypoPT, mean (SD) [range] age, 52 (19) [2–87] years |
Mean ± SD duration of disease: 17 ± 16 (range 1–59) Mean ± SD follow-up: 7.4 ± 5.1 |
Calcium, 94% Calcitriol, 88% High-dose vitamin D, 6% Thiazide, 20% Relevant finding stated in the article: Pts on a thiazide diuretic had higher urinary calcium levels (mean 318 vs 197 mg, P = 0.02) |
MDRD equation |
<60 mL/min/1.73 m2 41% pts (44/107 pts) This parameter analysis had age-matched normative controls |
eGFR Univariate analyses: age (P < 0.001), duration of disease (P < 0.001), avgtw calcium (P < 0.001), and estimated proportion of time with serum calcium higher than 9.5 mg/dL (P < 0.001) negatively correlated with eGFR Multivariate regression analyses: age (P < 0.001), duration of disease (P = 0.032), and proportion of time with relative hypercalcemia (P = 0.005) remained significantly associated with eGFR |
Mean±SD 8.6 ± 1.1 mg/dL (range 5.3–11.5) |
Mean± SD 216 ± 140 mg/24 h (range 8–557) |
Mean±SD 4.2 ± 0.9 (range 1.3–7.8) mg/dL | Not reported |
Mean±SD 35.4 ± 9.0 mg2/dL2 22% pts (25 pts): >55 mg2/dL2 (at least once during study period) |
|
Outcome Hypocalcemia: 16% pts (most recent measurement) |
Outcome Hypercalciuria: 38% pts overall 26% pts (most recent measurement) |
||||||||||
|
Outcome Hypercalcemia: 13% pts (most recent measurement) Frank hypercalcemiag: 2% pts 3 episodes of mild hypercalcemia associated with elevated 25-OH vitamin D levels | |||||||||||
|
Levy et al. 2015 [10] Long-term retrospective follow-up study |
29 pediatric pts with chronic HypoPT, mean (SD) age, 11.1 (5.9) years |
Mean ± SD duration of disease: 9.1 ± 5.5 Mean ± SD duration of follow-up: 7.4 ± 5.0 |
Calcitriol/calcium, 100% Cholecalciferol, 79% |
eGFRRevised Schwartz estimating equation for nonchronic kidney disease populations |
<60 mL/min/1.73 m2 0% pts (0 pts) >60 mL/min/1.73 m2 100% pts (29 pts) 60–90 mL/min/1.73 m2 45% pts (13 pts) Mean ± SD 92 ± 18 mL/min/1.73 m2 Males: Mean ± SD 85.1 ± 11.9 mL/min/1.73 m2 Females: Mean ± SD 99.3 ± 20.4 mL/min/1.73 m2 |
Univariate analysis: Higher calcium concentrations (r = −0.42, P = 0.02) and a greater percentage of time with total calcium >9.6 mg/dL (r = −0.41, P = 0.03) were associated with lower eGFR |
Total calcium: 8.9 ± 0.8 mg/dL Ionized calcium: 4.6 ± 0.5 mg/dL |
Average urine calcium/creatinine ratio: 0.27 ± 0.25 mg/mg | 5.9 ± 1.2 mg/dL | Not reported | Not reported |
Alb-sCa, albumin-corrected serum calcium; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; ESE, European Society of Endocrinology; HR, hazard ratio; HypoPT, hypoparathyroidism; ICD codes, international statistical classification of diseases and related health problems; IQR, interquartile range; KDIGO, Kidney Disease Outcomes Quality Initiative; MDRD, Modification of Diet in Renal Disease; NKF, National Kidney Foundation; NR, not reported; PTH, parathyroid hormone; ULN, upper limit of normal
Note: the following superscripted-letter footnotes are based on information contained in the indicated manuscript
aHypoPT severity was self-reported
bESE target ranges used with hypocalcemia being below the recommended ranges and hypercalcemia above
cValues above the ULN (≥300 mg/24 h in males and ≥ 250 mg/24 h in females)
dFrom first available biochemical measurement after index date to end of follow-up
ePartial hypoparathyroidism defined ≥1 PTH measurement >10 ng/L; complete hypoparathyroidism defined as all PTH measurements ≤10 ng/L
f>250 mg/24 h for females and > 300 mg/24 h for males
g>10.5 mg/dL
Fig. 3.
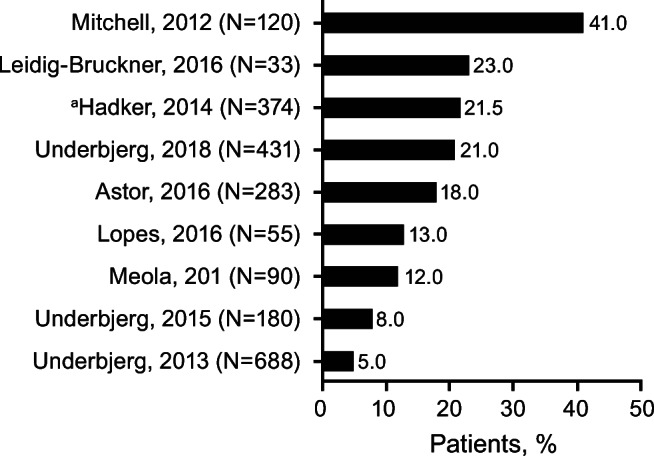
Percentages of Patients With Chronic Kidney Disease. Bars and values represent the percentage of patients with chronic kidney disease determined by eGFR <60 mL/min/1.73 m2, ≥ stage 3, or renal insufficiency ICD-8 and ICD-10 codes. The methods used by each study are detailed in Table 6. Patient numbers (N) refer to the number of patients with hypoparathyroidism in the study. aPatients self-reporting in a cross-sectional survey. eGFR, estimated glomerular filtration rate; ICD, international classification of diseases and related health problems
In a survey by Hadker et al., 2.5% of patients with milder hypoparathyroidism symptoms and 19% of patients with severe hypoparathyroidism symptoms self-reported having CKD [19]. In the two studies that used age- and gender-matched controls, there was an increased risk of renal insufficiency in both patients with postsurgical or nonsurgical chronic hypoparathyroidism (hazard ratios, 4.95 and 6.01, respectively; Table 6) [6, 7].
There were five adult studies that reported eGFR data using either the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, Cockcroft-Gault (eCrCl) or Modification of Diet in Renal Disease (MDRD) formula [8, 9, 21, 26, 27]. Four articles reported similar percentages of patients with eGFR <60 mL/min/1.73 m2, ranging from 12% to 23% of patients; the overall populations had a mean duration of disease of 9 to 15.9 years [8, 21, 26, 27]. In a study by Mitchell et al., 41% of 120 patients had eGFR <60 mL/min/1.73 m2; the overall population had a mean duration of disease of 17 years [9].
Thiazide use, hypertension, and diabetes mellitus
Renal outcomes may be affected by thiazide use, blood pressure in the context of reported hypertension, and diabetes mellitus. However, an examination of the 13 articles in this review found that very few reported any data on the following indices: thiazide use (n = 2) [9, 21], blood pressure in the context of reported hypertension (n = 2) [8, 21], and diabetes mellitus (n = 3) [6, 8, 21]. In the two studies that reported thiazide data, the percentages of patients prescribed this medication were 2% and 20%; the authors of the paper with the higher percentage noted they were unable to determine whether the patients were prescribed thiazides for hypertension, hypercalciuria, or both [9, 21]. In the two studies that reported the percentage of patients with hypertension or diabetes mellitus type 1 or 2, ranges were 3.5% to 18% and 1% to 8.4%, respectively [8, 21]. The authors of these four articles did not infer any relationship with renal outcomes based on the limited data. These conditions may have been underreported in the articles because they were not the primary focus of the publications and these diagnoses were based on hospital records.
Associations between renal outcomes and biochemical or disease parameters
The collation of the predefined renal outcomes and disease-relevant biochemical parameters affords the opportunity to reveal relationships not previously described. However, it was recognized that there are limitations to this approach in that an individual study with single biochemical measures (24-h urine collection) may not characterize the longitudinal status of that parameter.
The association of nephrolithiasis/kidney stones with a number of biochemical or clinically relevant parameters was only reported by one study of 90 patients [21]. No significant correlation (P = 0.98) was seen between the presence of kidney stones and the duration of hypoparathyroidism, 24-h urinary calcium excretion, total albumin-corrected serum calcium, or vitamin D status (Table 3).
Association data with nephrocalcinosis and a number of biochemical or clinically relevant parameters were reported by the Levy et al. pediatric study (n = 29) [10]. In a multivariate analysis, the most significant predictors for nephrocalcinosis were the degree of relative hypercalcemia (area under the curve [AUC] of total calcium concentrations >9.6 mg/dL) and the degree of hyperphosphatemia (AUC above age-adjusted phosphate concentrations; R2 = 0.47, P < 0.01; Table 4). Odds ratios for the association between nephrocalcinosis and degree of hypercalcemia and degree of hyperphosphatemia were 1.027 (95% CI, 1.003–1.052) and 1.004 (95% CI, 1.001–1.008), respectively. Compared with 18 patients in the study without nephrocalcinosis, the nine patients with unresolved nephrocalcinosis had a greater degree of hypercalcemia (AUC of total serum calcium concentrations >9.6 mg/dL; P = 0.005), hyperphosphatemia (AUC above age-adjusted phosphate; P = 0.01), and hypocalcemia (AUC of total serum calcium concentrations <8.0 mg/dL; P = 0.004) and a greater duration of hypocalcemia (percentage of time with total calcium <8.0 mg/dL; P = 0.003).
Association data with the combined nephrolithiasis and/or nephrocalcinosis outcome and biochemical parameters were only reported in a study by Lopes et al. of 55 patients [25]. Weight-adjusted 24-h urinary calcium was higher in patients with renal calcification versus those without (3.3 vs 1.8 mg/kg/day, respectively; P < 0.05; Table 5). However, there was no correlation between serum and urinary levels of calcium and the presence of calcification.
Mitchell et al. in their study of 120 patients examined correlations between CKD and a number of biochemical or clinically relevant parameters; eGFR <60 mL/min/1.73 m2 was compared with age-matched normative controls (Table 6) [9]. Univariate analyses found that eGFR levels were negatively correlated with age (P < 0.001), duration of disease (P < 0.001), average time-weighted serum calcium (P < 0.001), and estimated proportion of time with serum calcium higher than 9.5 mg/dL (P < 0.001). Average time-weighted serum phosphate and average calcium-phosphate product were not correlated with eGFR levels. Multivariate regression analyses of the predictors from the univariate analyses found that eGFR levels remained significantly associated with age (P < 0.001), duration of disease (P = 0.032), and proportion of time with relative hypercalcemia (P = 0.005). No association was seen between eGFR levels and either 24-h urine calcium values or presence of renal calcification. In a univariate analysis of the pediatric study, lower eGFR was associated with higher calcium concentrations (r = −0.42, P = 0.02) and a greater proportion of time with relative hypercalcemia (r = −0.41, P = 0.03) [10].
In a case-controlled retrospective study of 431 patients with national health registry data of long-term complications, Underbjerg et al. applied a composite endpoint of renal stones (defined by ICD codes) and renal insufficiency (defined by eGFR <60 mL/min/1.73 m2) to describe renal disease in patients with hypoparathyroidism [8]. This study showed that a decreased risk of any renal disease was associated with a higher dose of alfacalcidol supplementation (>1 vs ≤1 μg/day; P = 0.03). An increased risk of any renal disease was associated with an increased serum calcium-phosphate product (>2.80 mmol2/L2), increased number of hypercalcemic episodes, and long duration of disease. Predictors of any incidence of renal disease were disease duration (≥12.7 vs <12.7 years; P < 0.01) and increased calcium-phosphate product (≤2.80 vs >2.80 mmol2/L2; P < 0.01). Although the articles used divergent outcome measures and methodologies to assess biochemical parameters, they nevertheless revealed valuable insights into factors associated with renal outcomes in patients with chronic hypoparathyroidism.
Limitations
There was a significant heterogeneity in the data and in the methods of reporting data for each of the renal outcomes within published articles of clinical data studies of adult and pediatric patients with chronic hypoparathyroidism. Given the relatively low prevalence of hypoparathyroidism, it is not surprising that there are large gaps in the reporting of the key disease-related biochemical parameters studies of patients with chronic hypoparathyroidism; prospective studies are needed to address these knowledge gaps. The methodology for the collection of CKD information based on eGFR data was heterogeneous and often unclear. Only one study explicitly reported a collection method according to the CKD definition (ie, low eGFR levels on ≥2 occasions with an interval of ≥3 months) [26]. However, the low eGFR rate reported in the majority of the articles suggests a common comorbidity in patients with chronic hypoparathyroidism. There are limitations intrinsic to the detection method used. For example, it is possible that imaging for the assessment of nephrolithiasis and nephrocalcinosis may have selected patients who were at higher risk of developing these conditions. These limitations precluded a meta-analysis with data extracted from the selected studies.
Discussion
This systematic review of the literature found evidence that patients with chronic hypoparathyroidism managed with conventional therapy of oral calcium and active vitamin D supplementation have adverse renal outcomes of nephrolithiasis/kidney stones, nephrocalcinosis, and CKD. While there was a wide range for the frequency rate of each outcome, generally one-third of the patients had these renal complications.
Compared with publications on the general population, rates of nephrolithiasis (up to 36%) and CKD (up to 41%) are higher in patients with chronic hypoparathyroidism. Romero et al. reported overall population nephrolithiasis prevalence data from five countries ranging from 2% to 15% [29]. Of note, all studies reporting separate outcomes for nephrolithiasis and nephrocalcinosis used diagnostic codes or kidney ultrasound. The latter method has limited ability to detect kidney stones of a smaller size that may in part depend on the operator; therefore, the true prevalence of kidney stones may be underestimated using the ultrasound technique [30]. The range of rates reported in the two pediatric studies for nephrolithiasis, nephrocalcinosis, or the combined outcome may reflect the difficulty in distinguishing between small stones and parenchymal calcifications. We are unable to provide an obvious explanation for the dramatic difference in the rates of nephrolithiasis and nephrocalcinosis between the Meola et al. adult study and the Levy et al. pediatric study, but we speculate that it was a classification choice by each study group. It is our opinion that this differing classification does not detract from the collective data indicating an increased risk of these renal complications. Epidemiologic data from the Global Burden of Disease study reported a 4% age-standardized prevalence rate for CKD [31]. Only four studies had age- and gender-matched control groups, which is an important limitation to consider when making any cross-study comparisons, or with rates of renal outcomes in the general population [6, 7, 20, 21]. Three of the four studies found an increased risk of nephrolithiasis in patients with postsurgical chronic hypoparathyroidism compared with patients with nonsurgical chronic hypoparathyroidism or general population controls [6, 7, 21]. In two of the four studies, there was an increased risk of renal insufficiency in patients with either postsurgical or nonsurgical chronic hypoparathyroidism [6, 7].
Only a few studies formally analyzed associations between any of the key renal outcomes and clinical or biochemical features of chronic hypoparathyroidism. The most significant predictors for nephrocalcinosis in pediatric patients were degree of relative hypercalcemia and degree of hyperphosphatemia (P < 0.01) [10]. In pediatric patients, lower eGFR was associated with higher serum calcium concentrations and a greater proportion of time with relative hypercalcemia [10]. Similarly in adult patients, a significant inverse correlation was observed for eGFR levels with average time-weighted serum calcium and estimated proportion of time with hypercalcemia, as well as with age and disease duration (P < 0.001) [9]. In our clinical opinion, hypercalcemia in patients with chronic hypoparathyroidism is almost always attributable to overtreatment, making these factors exceedingly difficult to distinguish experimentally. Relatedly, although there is much debate in the medical community about the difference between ‘not adequately controlled’ and ‘not adequately treated’ with conventional therapy, this also cannot be answered on the basis of the published literature. No correlation was seen between the presence of kidney stones and serum calcium or 24-h urinary calcium excretion or with disease duration [21]. Similarly, there was no correlation between serum and urinary levels of calcium and the presence of the combined nephrolithiasis and/or nephrocalcinosis outcome [25].
Additional factors important to renal outcomes were identified in articles that did not undertake an association analysis but might be considered as surrogate markers or candidates for further exploration. In adult patients with chronic hypoparathyroidism, higher serum calcium-phosphate product values increased the risk of renal disease (ie, composite renal stones/eGFR <60 mL/min/1.73m2) [8]. The serum calcium-phosphate product level was within generally recommended reference ranges but was relatively high, leading Underbjerg et al. to suggest that treating physicians should target the lower part of the reference range. A similar point was made by the authors for target serum phosphate levels based on their association findings with increased risk for complications and mortality. Other studies in the general population reported that high serum phosphate was associated with harmful effects on renal function [32] and impairment of microvascular function in individuals with normal renal function [33]. In adult patients with chronic hypoparathyroidism, an increased number of hypercalcemic episodes and a longer duration of illness were associated with an increased risk of any incidence of renal disease [8]. In pediatric patients, a greater degree of hypocalcemia or repeated episodes of hypocalcemia were unexpectedly associated with the development of nephrocalcinosis [10], which could be explained by the need for higher doses of oral calcium to maintain normal serum calcium and/or concomitant hyperphosphatemia. However, these assertions require further investigation. The pediatric study provided definitive information about the presence of early mild renal impairment in children with chronic hypoparathyroidism. The authors noted that early renal impairment in childhood aligns with longitudinal studies in adults, and may progress to CKD in adulthood [12].
Concluding remarks
Renal complications and an increased risk of adverse renal events in patients with chronic hypoparathyroidism who receive conventional therapy were observed consistently in a systematic literature review. There is an unmet need for additional large-scale studies, including more studies with standardized CKD definitions methodology, to better establish the factors that increase the risk of renal complications in patients with hypoparathyroidism.
Acknowledgments
This study was funded by Shire International GmbH, a Takeda company, Zurich, Switzerland. Under the direction of the authors, editorial support in the preparation of this manuscript was provided by Alan Storey, PhD (ICON, Abingdon, Oxon, UK), and Sheila Curristin, PhD (ICON, North Wales, PA, USA), and funded by Shire International GmbH, a Takeda company, Zurich, Switzerland.
Author contributions
All authors contributed to the protocol and concept of the review. All authors reviewed the selected articles and analyzed the extracted data. All authors contributed to the preparation of the article and reviewed and approved the final version for publication.
Funding
Shire International GmbH, a Takeda company, Zurich, Switzerland.
Data availability
Not applicable.
Compliance with ethical standards
Conflicts of interest/competing interests
E. Gosmanova has served as a consultant for Shire, a Takeda company; P. Houillier has served as an advisory board member, speaker, and research investigator for Shire, a Takeda company; C. Marelli is an employee of Takeda Pharmaceuticals International AG, Zurich, Switzerland; L. Rejnmark has served as a consultant and speaker for Shire, a Takeda company; J. Bilezikian has served as an advisory committee/board member, received consulting fees and grant/research support, and acted as speaker for Shire, a Takeda company.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babey M, Brandi ML, Shoback D. Conventional treatment of hypoparathyroidism. Endocrinol Metab Clin N Am. 2018;47(4):889–900. doi: 10.1016/j.ecl.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Shoback D. Hypoparathyroidism. N Engl J Med. 2008;359(4):391–403. doi: 10.1056/NEJMcp0803050. [DOI] [PubMed] [Google Scholar]
- 3.Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J, Jr, et al. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res. 2008;23(12):2018–2024. doi: 10.1359/jbmr.080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoback DM, Bilezikian JP, Costa AG, Dempster D, Dralle H, Khan AA, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab. 2016;101(6):2300–12. [DOI] [PubMed]
- 5.Bilezikian JP, Brandi ML, Cusano NE, Mannstadt M, Rejnmark L, Rizzoli R, et al. Management of hypoparathyroidism: present and future. J Clin Endocrinol Metab. 2016;101(6):2313–24. [DOI] [PMC free article] [PubMed]
- 6.Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res. 2013;28(11):2277–2285. doi: 10.1002/jbmr.1979. [DOI] [PubMed] [Google Scholar]
- 7.Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. The epidemiology of nonsurgical hypoparathyroidism in Denmark: a nationwide case finding study. J Bone Miner Res. 2015;30(9):1738–1744. doi: 10.1002/jbmr.2501. [DOI] [PubMed] [Google Scholar]
- 8.Underbjerg L, Sikjaer T, Rejnmark L. Long-term complications in patients with hypoparathyroidism evaluated by biochemical findings: a case-control study. J Bone Miner Res. 2018;33(5):822–831. doi: 10.1002/jbmr.3368. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DM, Regan S, Cooley MR, Lauter KB, Vrla MC, Becker CB, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97(12):4507–14. [DOI] [PMC free article] [PubMed]
- 10.Levy I, Licht C, Daneman A, Sochett E, Harrington J. The impact of hypoparathyroidism treatment on the kidney in children: long-term retrospective follow-up study. J Clin Endocrinol Metab. 2015;100(11):4106–4113. doi: 10.1210/jc.2015-2257. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 12.Boyce AM, Shawker TH, Hill SC, Choyke PL, Hill MC, James R, et al. Ultrasound is superior to computed tomography for assessment of medullary nephrocalcinosis in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(3):989–94. [DOI] [PMC free article] [PubMed]
- 13.Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2010;25(5):831–841. doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weigert A, Hoppe B. Nephrolithiasis and nephrocalcinosis in childhood-risk factor-related current and future treatment options. Front Pediatr. 2018;6:98. doi: 10.3389/fped.2018.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shavit L, Jaeger P, Unwin RJ. What is nephrocalcinosis? Kidney Int. 2015;88(1):35–43. doi: 10.1038/ki.2015.76. [DOI] [PubMed] [Google Scholar]
- 16.Dickson FJ, Sayer JA. Nephrocalcinosis: a review of monogenic causes and insights they provide into this heterogeneous condition. Int J Mol Sci. 2020;21(1):369. [DOI] [PMC free article] [PubMed]
- 17.Priante G, Ceol M, Terrin L, Gianesello L, Quaggio F, Del Prete D, et al. Understanding the pathophysiology of nephrocalcinosis. Updates and advances in nephrolithiasis - pathophysiology, genetics, and treatment modalities. IntechOpen; 2017. 10.5772/intechopen.69895
- 18.Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371(12):1100–10. [DOI] [PubMed]
- 19.Hadker N, Egan J, Sanders J, Lagast H, Clarke BL. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the PARADOX study. Endocr Pract. 2014;20(7):671–679. doi: 10.4158/EP13328.OR. [DOI] [PubMed] [Google Scholar]
- 20.Arlt W, Fremerey C, Callies F, Reincke M, Schneider P, Timmermann W, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. 2002;146(2):215–22. [DOI] [PubMed]
- 21.Meola A, Vignali E, Matrone A, Cetani F, Marcocci C. Efficacy and safety of long-term management of patients with chronic post-surgical hypoparathyroidism. J Endocrinol Investig. 2018;41(10):1221–1226. doi: 10.1007/s40618-018-0857-5. [DOI] [PubMed] [Google Scholar]
- 22.Bollerslev J, Rejnmark L, Marcocci C, Shoback DM, Sitges-Serra A, van Biesen W, et al. European Society of Endocrinology clinical guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173(2):G1–G120. [DOI] [PubMed]
- 23.Bohrer T, Paul J, Krannich H, Hasse C, Elert O. The Wuerzburg scale: a new classification system for permanent postoperative hypoparathyroidism. Eur Surg. 2007(39/1):39–44. 10.1007/s10353-006-0306-0
- 24.Kim JH, Shin YL, Yang S, Cheon CK, Cho JH, Lee BH, et al. Diverse genetic aetiologies and clinical outcomes of paediatric hypoparathyroidism. Clin Endocrinol (Oxf). 2015;83(6):790–6. [DOI] [PubMed]
- 25.Lopes MP, Kliemann BS, Bini IB, Kulchetscki R, Borsani V, Savi L, et al. Hypoparathyroidism and pseudohypoparathyroidism: etiology, laboratory features and complications. Arch Endocrinol Metab. 2016;60(6):532–6. [DOI] [PMC free article] [PubMed]
- 26.Leidig-Bruckner G, Bruckner T, Raue F, Frank-Raue K. Long-term follow-up and treatment of postoperative permanent hypoparathyroidism in patients with medullary thyroid carcinoma: differences in complete and partial disease. Horm Metab Res. 2016;48(12):806–813. doi: 10.1055/s-0042-118181. [DOI] [PubMed] [Google Scholar]
- 27.Astor MC, Lovas K, Debowska A, Eriksen EF, Evang JA, Fossum C, et al. Epidemiology and health-related quality of life in hypoparathyroidism in Norway. J Clin Endocrinol Metab. 2016;101(8):3045–3053. doi: 10.1210/jc.2016-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kidney Disease. Improving Global Outcomes. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2011;2017;7(1):1–59. [DOI] [PMC free article] [PubMed]
- 29.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12(2–3):e86–e96. [PMC free article] [PubMed] [Google Scholar]
- 30.Fowler KA, Locken JA, Duchesne JH, Williamson MR. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology. 2002;222(1):109–113. doi: 10.1148/radiol.2221010453. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567–81. [DOI] [PubMed]
- 32.Moon H, Chin HJ, Na KY, Joo KW, Kim YS, Kim S, et al. Hyperphosphatemia and risks of acute kidney injury, end-stage renal disease, and mortality in hospitalized patients. BMC Nephrol. 2019;20(1):362. [DOI] [PMC free article] [PubMed]
- 33.Ginsberg C, Houben A, Malhotra R, Berendschot T, Dagnelie PC, Kooman JP, et al. Serum phosphate and microvascular function in a population-based cohort. Clin J Am Soc Nephrol. 2019;14(11):1626–1633. doi: 10.2215/CJN.02610319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.