Abstract
Investigations have shown that infection from the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) is responsible also for initiating severe inflammatory responses that can lead macrovascular and microvascular thrombosis.
Several studies have already described acute limb ischemia and peripheral arterial disease in critically ill patients with Coronavirus disease 2019 (Covid-19), as well as coronary artery disease and ischemic stroke as a manifestation usually associated with respiratory distress. However, what still remains unclear is how long inflammation and thrombotic derangements can last after recovery from the symptoms of Covid-19. Hence, in this article we report 3 cases of arterial thrombotic sequalae after this viral infection. To the best of our knowledge, this is the first cases’ series that had described different delayed vascular arterial complications, which occurred after the index infection, with a negative nasopharyngeal swab and Covid-19 systemic symptoms resumption. A better understanding of the coagulopathy in Covid-19 could have an essential role to guide prevention and treatment of arterial thromboembolic events, both during and after the viral infection.
Further investigations are required to confirm these data and to estabilish the type, dose and duration of anticoagulant/antiplatelet therapy not just during but also after Covid-19 infection.
KEY WORDS: SARS-CoV-2, Arterial thrombotic sequalae, delayed vascular complication
The association of Covid-19 with coagulopathy has gained attention and several studies have already described acute limb ischemia and peripheral arterial disease in critically ill patients with this viral infection,1 , 2 , 3 , 4 as well as coronary artery disease and ischemic stroke as a manifestation usually associated with respiratory distress.5
Actually no information is currently available on how long inflammation and thrombotic derangements can last after recovery from Covid-19 symptoms.
In this article we report 3 cases of arterial thrombotic sequalae after this viral infection.
CASES PRESENTATION
CASE 1
A 70-year-old woman presented to our emergency department with acute abdominal pain. She had a known Covid-19 exposure approximately 45 days before, even if she didn't do any Covid-19 swab at home. On admission, the patient reported an influenza-like illness 20 days earlier, for which she had received at-home treatment with antibiotic and low dose of prednisone. During clinical examination, she hadn't had fever, cough, dyspnea, ageusia and anosmia and no other symptoms related to an upper airway infection. Her main comorbidities were hypertension and hypothyroidism.
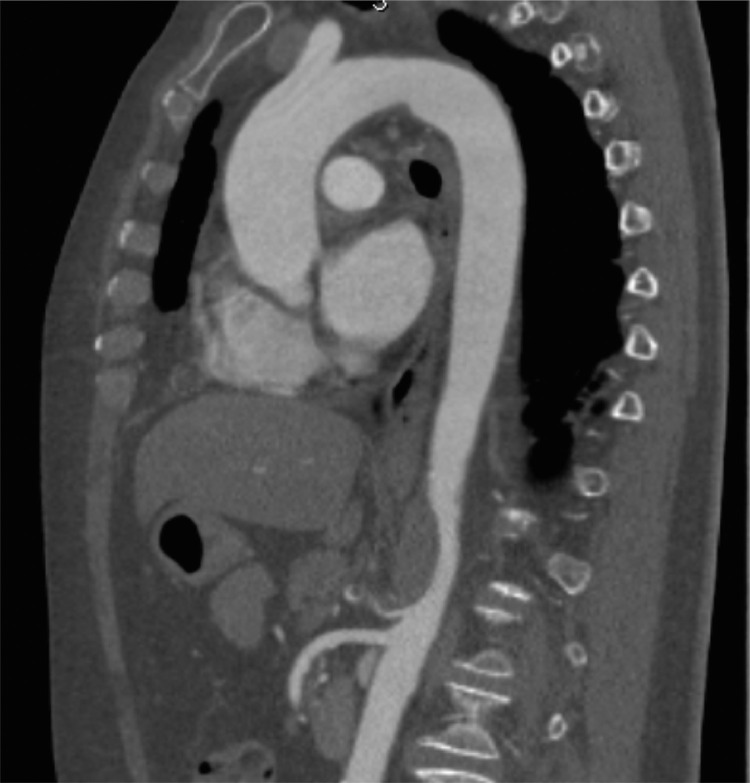
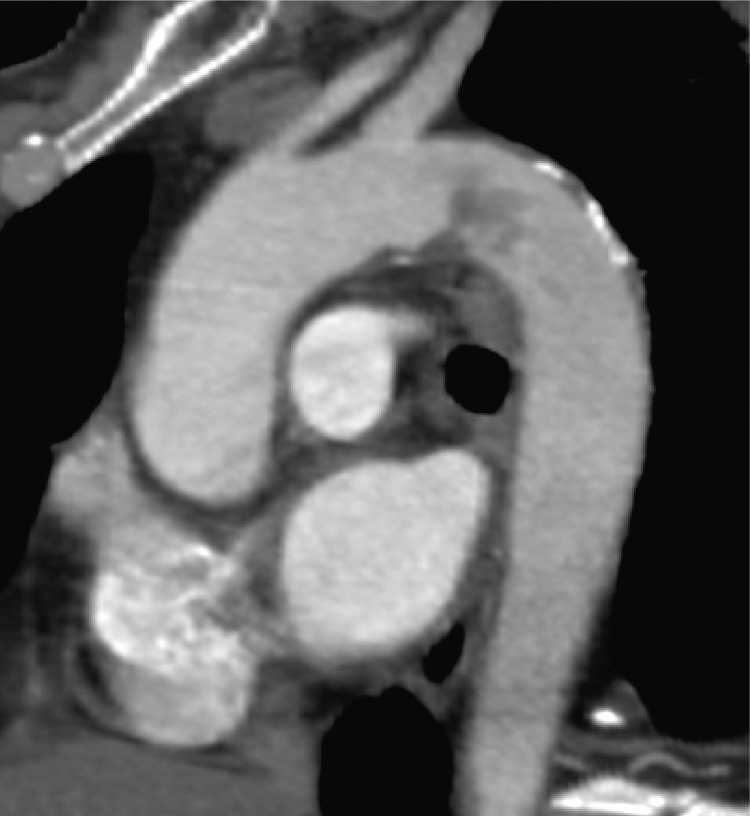
A computed tomography angiogram (Angio-CT) of the thoraco-abdominal aorta demonstrated an occlusive thrombosis of the celiac trunk with a floating aortic thrombus immediately above it in an otherwise healthy aorta (Fig. 1 ). In addition, there were no radiological findings of bowel ischemia and distal branches of the celiac trunk were regularly reperfused.
Fig. 1.
Floating aortic thrombus.
Blood test showed just a slight platelet count increasing and a leukocytosis.
All four nasopharyngeal swabs for SARS-CoV-2, performed by real-time reverse transcription-PCR (RT-PCR), were negative (nasopharyngeal swab was performed on patient's admission to the emergency department, 3 and 7 days after hospitalization and before her discharge, according to our hospital protocol). Her chest CT angiography showed bilateral peripheral consolidation areas as a result of a previous pneumonia.
During medical examination performed after Angio-CT scan, patient reported abdominal pain relief and full regression of this symptom.
Based on this mild symptomatology, we decided for a conservative approach treating patient by intravenous unfractionated heparin infusion at therapeutic dose (checking aPTT every 6 hours to achieve the target dose) plus antiplatelet therapy with Cardio-aspirin 100 mg/die. We decided to use unfractionated heparin instead of low molecular weight heparin in the hypothesis that we needed to rapidly stop anticoagulation for urgent surgical and/or endovascular recanalization of the celiac trunk.
During hospitalization she was asymptomatic for abdominal pain and laboratory test showed normal lactate levels.
At day 5, unfractionated heparin was discontinued in favor of Fondaparinux 2.5 mg/die which was given for the next 30 days, according to the consulting of thrombotic diseases’ specialist.
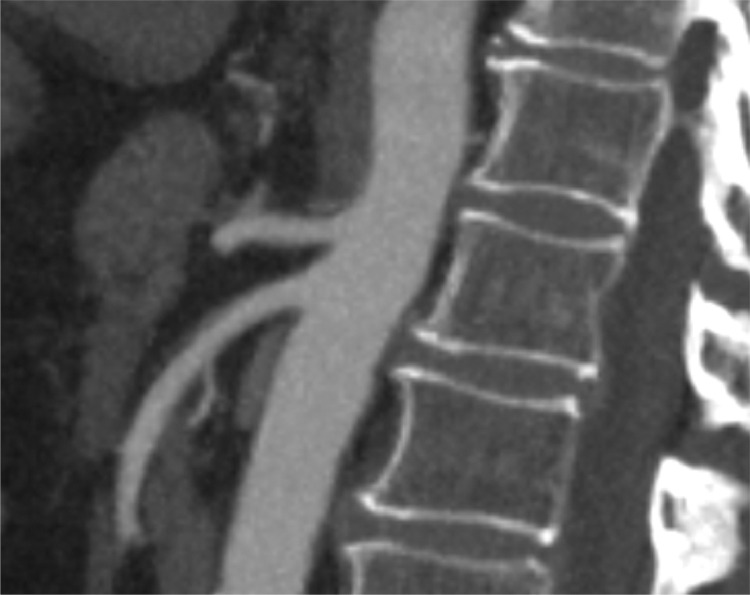
An Angio-CT scan performed after 7 days and at one month, showed a gradual improvement of radiological signs and the complete absence of thrombus at 30 days (Fig. 2 ).
Fig. 2.
Angio-CT scan at 1 month.
Considering that the thrombotic event was unexplained and based on the high clinical suspicion, an antibody testing for SARS-CoV-2 was performed and it showed a significantly high concentration of IgG, even if it was not possible to accurately date how long ago she became infected.
CASE 2
A 54-year-old man was admitted to our emergency department for severe acute left lower limb pain, reporting during clinical examination that pain and foot hypothermia occurred approximately 12 hours before. At admission left foot motility was present, but patient complained of big toe hypoesthesia and paresthesia affecting the remaining toes. Left dorsal pedis artery and posterior artery pulses were abolished, while left popliteal artery pulse was valid.
The patient presented moderate respiratory symptoms of Covid-19 infection, which required hospitalization, approximately 20 days before (confirmed by a former positive nasopharyngeal swab) and was discharged from hospital 3 days before the admission in our vascular department for acute limb ischemia, with a negative nasopharyngeal swab.
He was previously a healthy man and had only dyslipidemia as cardiovascular risk factor.
Laboratory test at admission revealed a slight increase of C-reactive protein level.
All the four nasopharyngeal swabs for SARS-CoV- 2 carried out by RT-PCR (according to our hospital protocol) were negative.
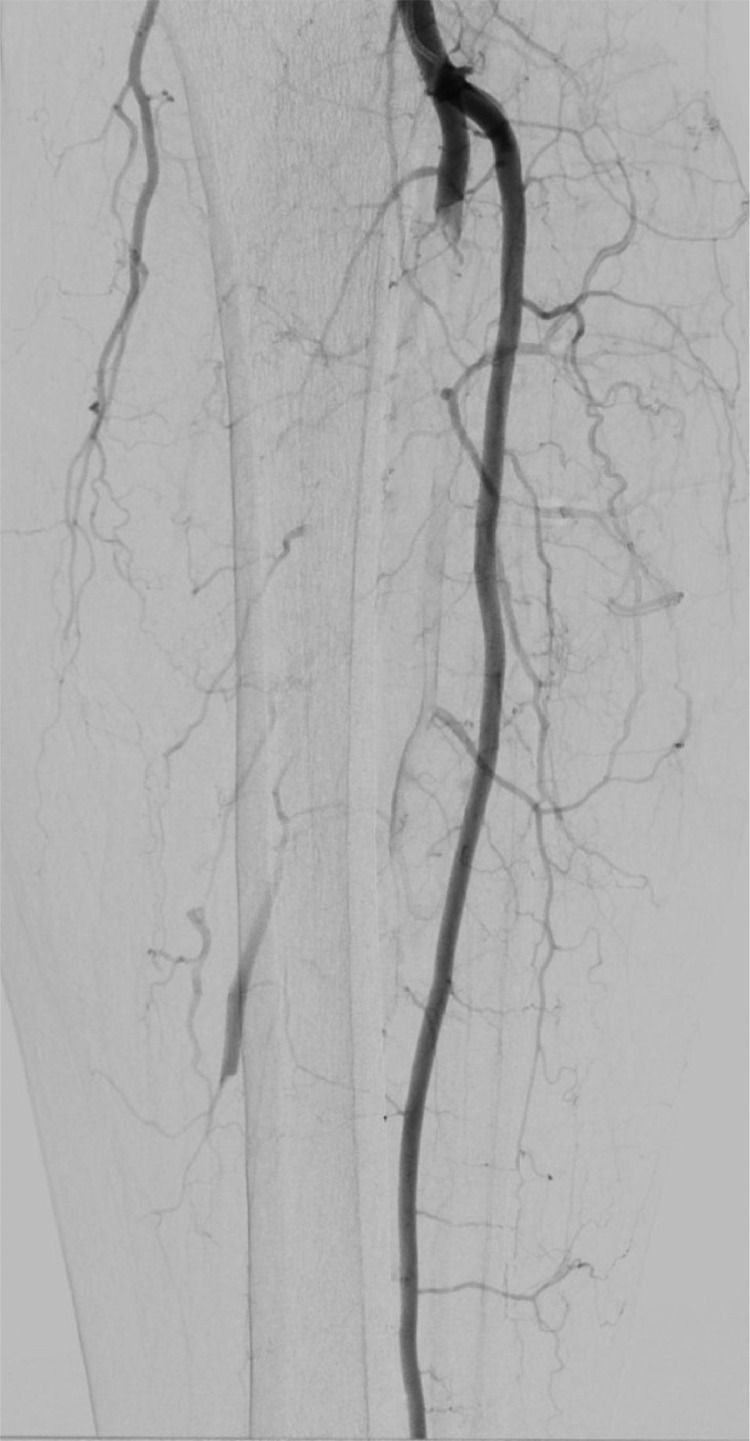
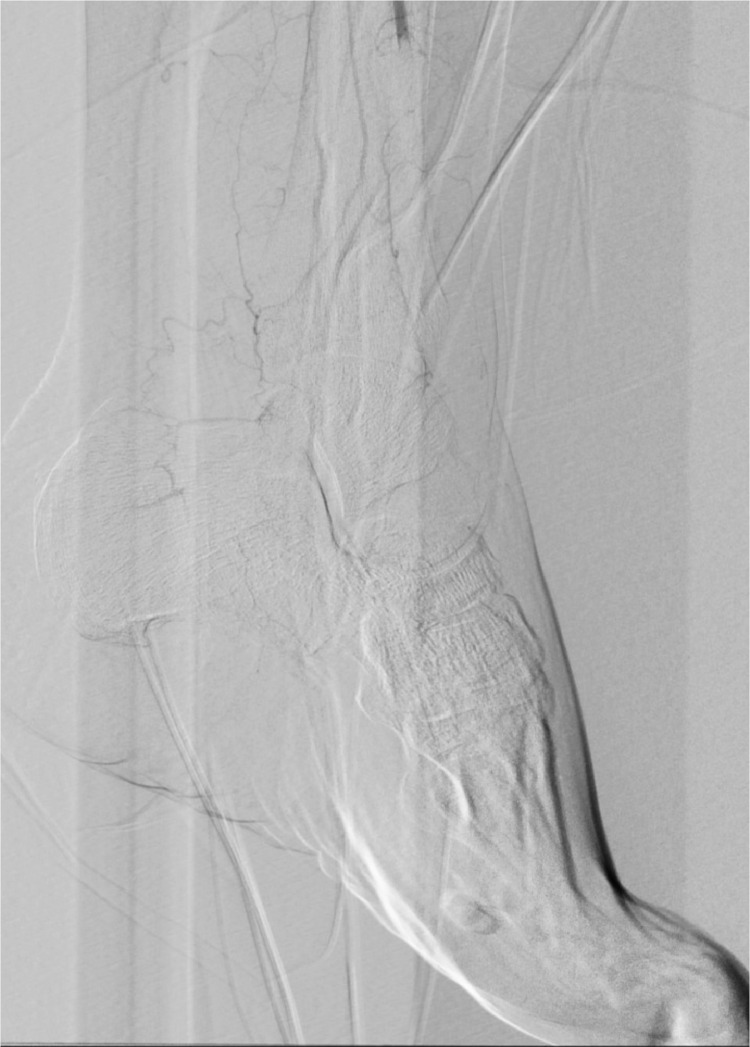
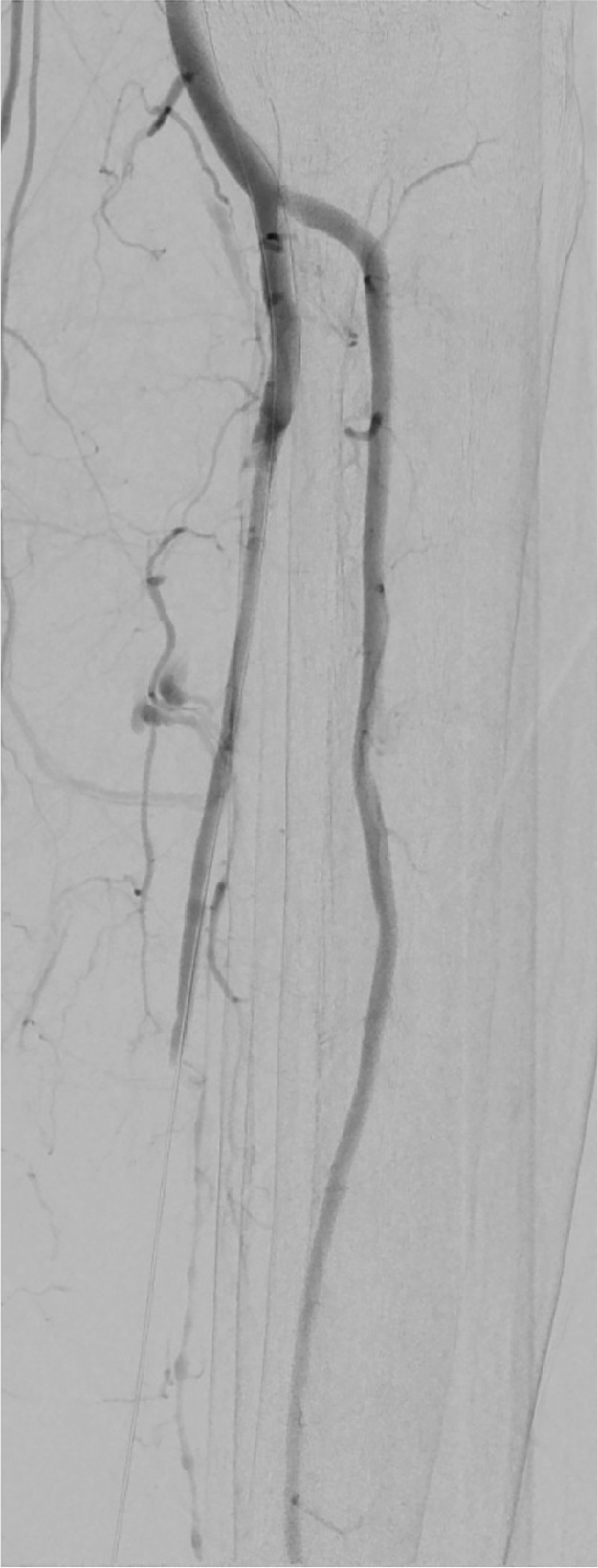
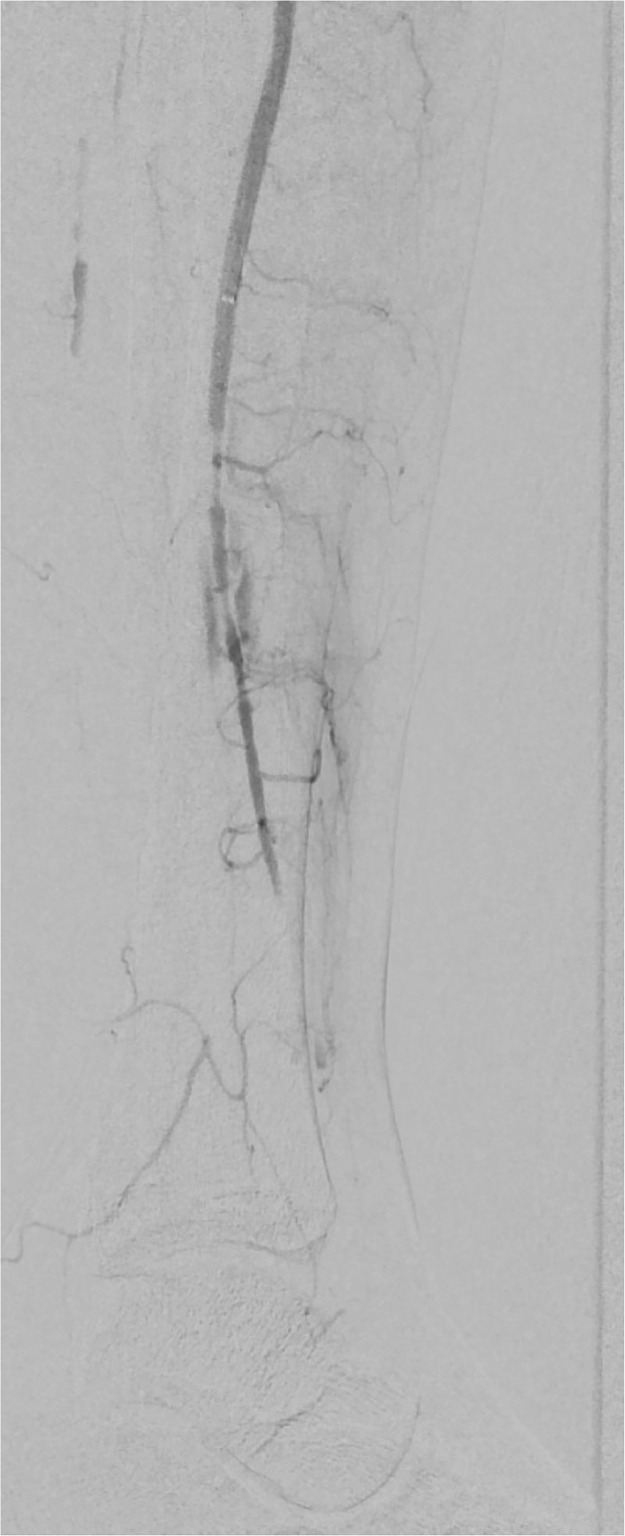
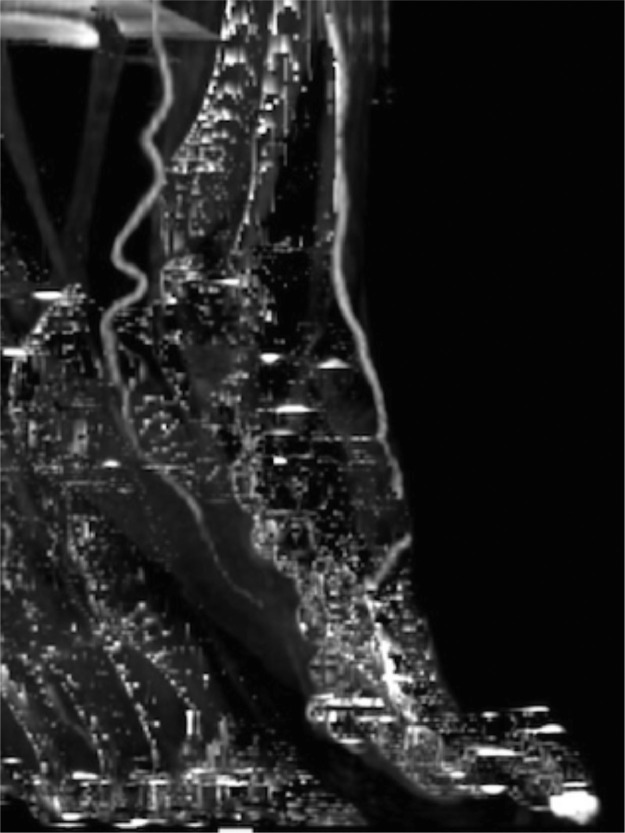
A left leg Duplex ultrasound evaluation, performed at admission, highlighted acute tibio-peroneal and posterior tibial artery occlusions from their origin and an abrupt thrombosis of anterior tibial artery at ankle level without flow into the left foot. These findings were confirmed by an emergent angiography (Fig. 3, Fig. 4 ). Considering the extremely peripheral arteries’ involvement, we decided on an endovascular approach: under local anesthesia, antegrade access was achieved using an ultrasound-guided puncture to the left common femoral artery. A 6-Fr sheath was inserted and patient received 100U/Kg of intravenous heparin; a mechanical thrombectomy of the anterior and tibio peroneal arteries was performed with an Indigo CAT6 catheter (Penumbra), but no satisfactory improvement of distal foot flow was achieved (Figs. 5 ,6 and 7 ). A Fountain-infusion catheter was then placed down into the distal left popliteal artery for a catheter-directed thrombolysis and continuous infusion of 1200,000 UI/24h of Urokinase was given for 48 hours. Meanwhile, therapeutic intravenous unfractionated heparin was continued and antiplatelet therapy with Clopidogrel 75 mg/die was set up.
Fig. 3.
Arteriograms before treatment.
Fig. 4.
Arteriograms before treatment.
Fig. 5.
Not satisfactory improvement of blood flow after mechanical thrombectomy.
Fig. 6.
Not satisfactory improvement of blood flow after mechanical thrombectomy.
Fig 7.
Not satisfactory improvement of blood flow after mechanical thrombectomy.
The patient showed clinical improvement overnight, without evidence of compartment syndrome so a new angiography was performed after 38 hours of therapy: we've found a suboptimal radiological features, so we decided to continue thromboliytic protocol for other 12 hours and, after this time, we agreed to stop continuous infusion as the foot was warm, with a direct Doppler signal in posterior tibial artery.
Intravenous continuous heparin infusion was administrated during hospitalization for extra 48 hours after thrombolysis, then patient was transitioned to therapeutic dose of fractionated heparin and discharged by the hospital on day 9 with anticoagulation plus a single antiplatelet therapy (Clopidogrel 75 mg/die). Before discharging, the patient reported a complete resolution of his symptoms.
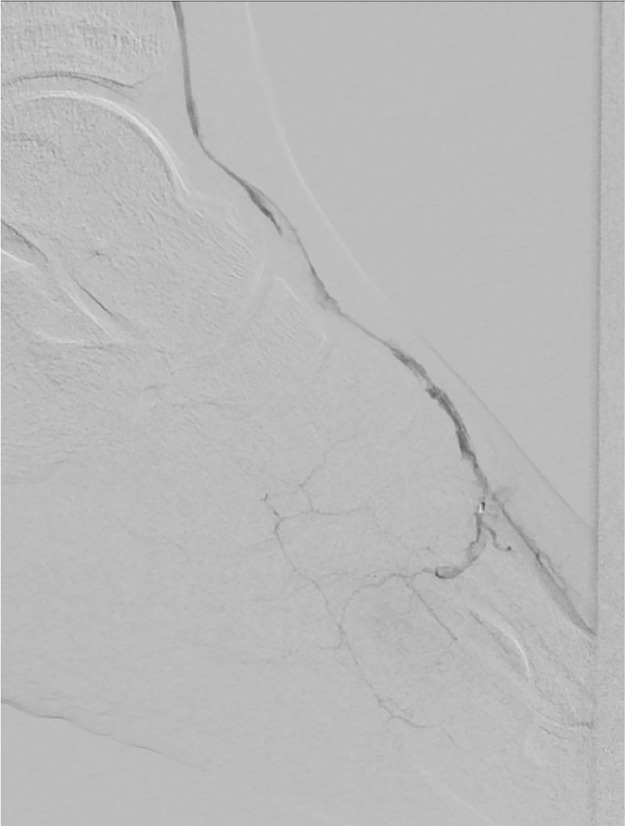
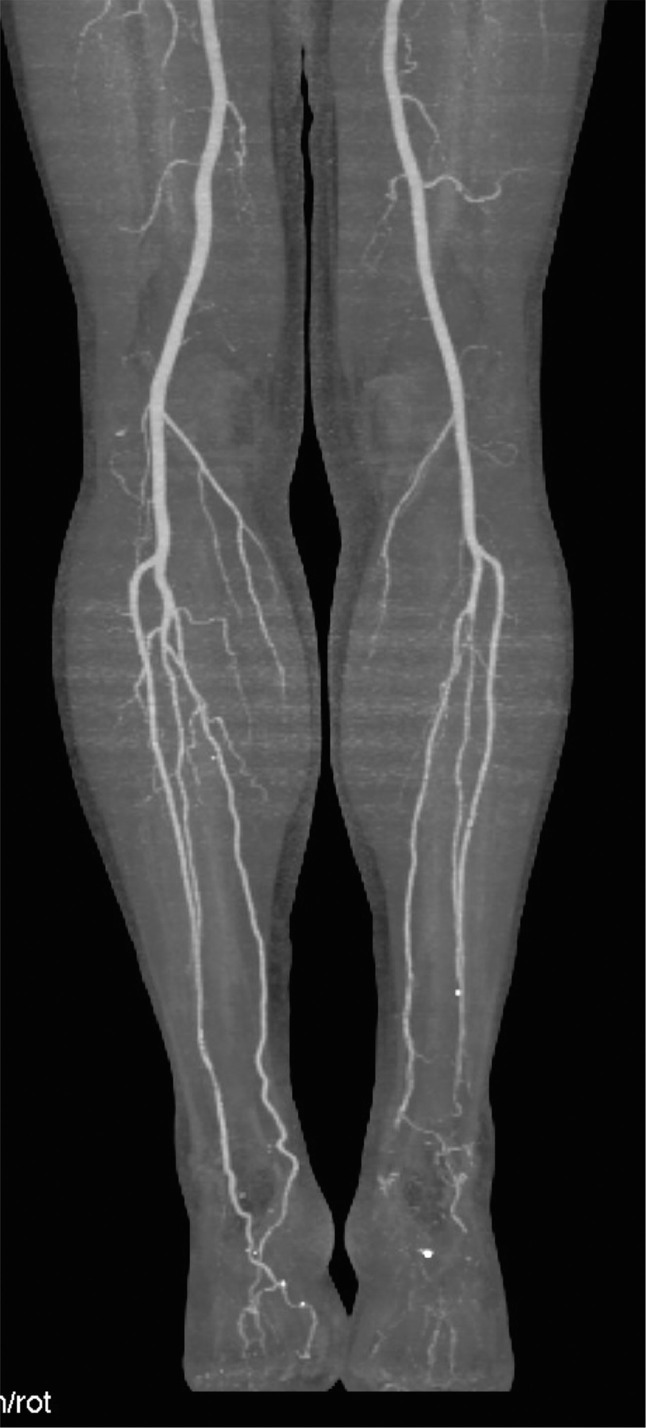
After one month an Angio-CT scan demonstrated patency of treated vessels with a lateral plantar artery as the main source for left foot vascularization (Figs. 8 , and 9 ).
Fig. 8.
Angio-CT control after 1 month.
Fig. 9.
Angio-CT control after 1 month.
Patient's therapy was shifted, discontinuing heparin products, in favor of antiplatelet therapy (Clopidogrel 75 mg/die) and vasodilating products (Cilostazolo 200mg/die). Patient did not complaint rest pain or intermittent claudication during standard vascular follow-up performed at 45 days and Duplex ultrasound exam revealed a triphasic wave in both left anterior and posterior artery.
CASE 3
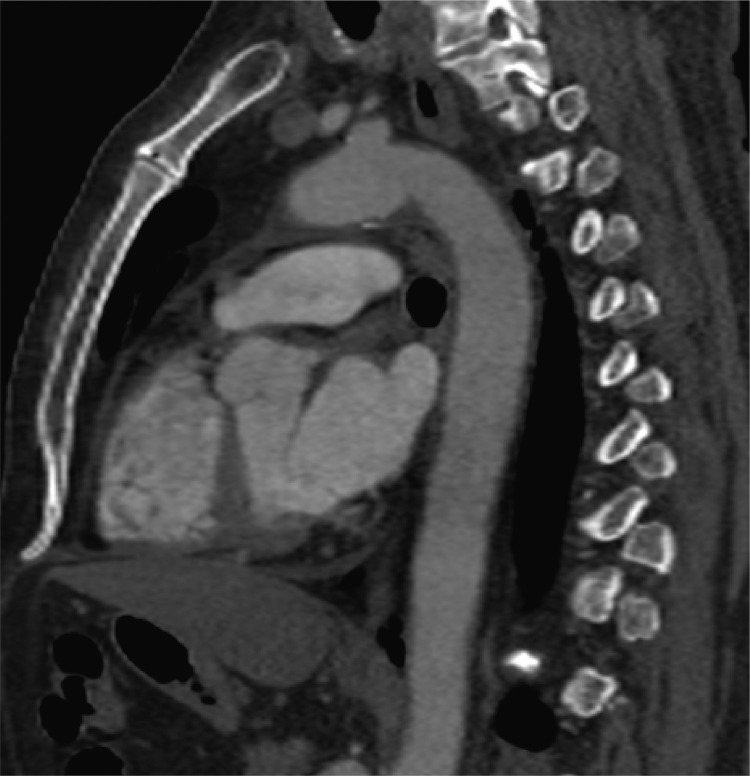
A 58-year-old man was centralized in our hub vascular Center for acute left forearm paresthesia and motility impairment associated with fingers pain and coldness, appeared about 10 hours before his admission in our department. His main comorbidities were hypertension and diabetes. In addition, patient contracted mild respiratory symptoms of Covid-19 infection approximately 30 days before (confirmed by a former positive nasopharyngeal swab; its negativization occurred 6 days before the admission in our department). A brain CT scan and cardiologic consult were performed during patient's permanence in the peripheral spoke Center and any heart or neurological problems were excluded. An Angio-CT thoracic scan was then performed demonstrating a floating thrombus in the aortic arch (Fig. 10 ): so an empirical treatment with prophylactic dose of low molecular weight heparin was somministrated.
Fig. 10.
Aortic floating thrombus.
Laboratory test at admission in our hub Center revealed a slight increase of C-reactive protein and D-dimer levels.
Again, all the four nasopharyngeal swabs for SARS-CoV- 2 by RT-PCR, were negative.
During our vascular visit, patient did not reported any pain or functional deficit, left arm motility and sensivity were present as left radial and ulnar pulse.
Based on this mild symptomatology, we decided for a conservative approach treating patient by intravenous unfractionated heparin infusion at therapeutic dose (checking aPTT every 6 hours to achieve the target dose) plus antiplatelet therapy with Cardioaspirin 100 mg/die.
An Angio-CT scan performed after 3 days showed aortic thrombus reduction in sizing (Fig. 11 ).
Fig. 11.
Angio-CT scan after 3 days of therapy.
During hospitalization the patient remained asymptomatic and we decided at day 4 to shift continuous endovenous heparin infusion to therapeutic dose of fractionated heparin plus single antiplatelet therapy (Cardioaspirin 100 mg/die). At day 10 patients was discharged with dual antiplatelet therapy (Cardiaspirin 100 mg/die + Clopidogrel 75 mg/die) plus a prophylactic dose of fractionated heparin.
DISCUSSION
The association of Covid-19 with coagulopathy has gained attention as some patients affected by this viral disease have disseminated intravascular coagulation (CID) and the subsequent consumption coagulopathy.
The pathophysiology of Covid-19-induced thrombosis is complex and likely has multiple contributing factors. The virus acts on vascular endothelium via angiotensin-converting enzyme-2 (ACE2) receptor that is highly expressed in vascular endothelium and other cells.6 SARS-CoV-2 infiltrates these cells by binding to ACE2 receptor and therefore potentiates an intense immune response known as the cytokine storm, which precipitates the onset of systemic inflammatory response syndrome (SIRS). The subsequent SIRS can induce an endotheliopathy and hypercoagulability state, leading to both systemic macrothrombosis and microthrombosis.7
Another suggested mechanism involves elevation of D- dimer levels and presence of anti phospholipid antibodies.8
On the other hand, no information is currently available on how long inflammation and thrombotic derangements can last after recovery from Covid-19 symptoms.
All three vascular arterial complications reported herein occurred after completely Covid-19 systemic symptoms resumption and with a negative nasopharyngeal swab at hospital admission. Moreover, our patients were admitted without any suspected findings of currently active disease at chest CT, which suggested that at the moment of the acute vascular event there was no Covid-19 infection.
Another worrying aspect that deserves to be pointed out is that these reported vascular arterial delayed complications occurred in previously healthy patients with no history of vascular disease, relatively young, with previously healthy artery without evidence of atherosclerosis. Moreover, all patients had no other comorbidity which could explain increased risk for vascular arterial thrombi: platelets count was in range and all patients were in sinus rhythm when accepted in our department and they hadn't pre-existing blood clotting disorders.
We must underline that our patients were symptomatic for peripheral embolism/thrombosis and for this reason a prompt diagnosticiter and therapeutic approach has been carried out.
Silingardi et al9 had already raised out what should be the correct preoperative workup in Covid-19 patients presenting with embolic complications and concluded that thoraco-abdominal Angio-CT scan should be considered a routine evaluation in Covid-19 patients presenting with embolic complications. In fact, in their experience, they reported 2 cases of Covid-19 patients with asymptomatic aortic floating thrombus detected incidentally. From this perspective, vascular screening and follow-up should be discussed both during and after Covid-19 systemic symptoms resumption, since in the future we could detect vascular manifestations which are completely silent during viral infection.
Analyzing current literature, only a few case reports are describing a delayed manifestation of Covid-19 presenting as a lower extremity arterial thrombosis.
Schweblin et al10 reported a case of acute lower limb ischemia in a young man with an influenza-like illness occurred 15 days before, who presented a negative nasofaringeal swab and a positivity of antibody testing for SARS-CoV-2. In this case, limb ischemia was due to a multilevel arterial thrombosis (common iliac, profunda femoral and popliteal artery) and managed with a hybrid approach (surgical embolectomy followed by below the knee endovascular intervention due to distal embolization post-surgery).
Renaud-Picard et al11 described a case of cardio-embolic limb ischemia in a lung transplant recipient who had a recent history of upper respiratory tract infection and was admitted with negative nasopharyngeal swab but with strongly positive IgM and mild positive IgG at serology check. Moreover, in this patient a chest Angio-CT scan showed radiological findings suggestive of Covid-19 infection.
Similarly, Kamel Muhammad et al12 reported a case of acute limb ischemia in a patient with 2-week respiratory symptoms of Covid-19 infection admitted with a negative swab but highly suggestive findings of Covid-19 infection at chest X-ray and CT scan.
As we know, radiological chest signs at CT scan in association with respiratory symptoms are sufficient for diagnosis of Covid-19, even in the absence of positive nasopharyngeal swab13 and for this reason a negative swab does not rule out the disease. In our patients both nasopharyngeal swab and chest CT were negative.
Bozzani et al14 analyzed the clinical outcome of 9 patients who developed limb thromboses related with Covid-19 infection: notably 6 of these thrombotic events occurred during active infectious symptoms, and the other 3 occurred 41 to 149 days after the index infection.
The cases reported in our article aim at raising awareness to the medical community to be vigilant for the thrombotic complications after Covid-19. Moreover, a remarkable aspect to underline is which could be the appropriate choice of therapy to adequately prevent thrombosis, since in literature there are several reports of arterial vascular thrombosis that occurred despite therapeutic dose of anticoagulation or in patient with heparin resistance.15,16,17,18,19
In our experience all cases herein reported were managed according to thrombotic diseases’ specialist advise. Each patient received a different empirical anticoagulation and/or antiplatelet therapy, considering the absence of a standardized medical protocol for this particular group of patients, even if better results with low molecular weight heparin have demonstrated in the literature.20
Other key points which should be further discussed are how long medical therapy deserves to be continued and which could be the best follow-up scheme and its duration.
CONCLUSIONS
This cases’ series underlines that prothrombotic sequela of Covid‐19 are not confined to the initial phase when we can find swab positivity or typical chest CT findings; delayed arterial thrombotic events can also occur in susceptible patients after the index infection, even if it has passed as mild and/or moderate disease with absence of overt features of disseminated intravascular coagulation or severe respiratory manifestations.
A better understanding of the coagulopathy in Covid-19 could have an essential role to guide prevention and treatment of arterial thromboembolic events, both during and after the viral infection.
Further investigations are required to establish the type, dose and duration of anticoagulant/antiplatelet therapy not just during but also after Covid-19 infection.
Footnotes
Authors’ contribution: All Authors read and approved the final version of the manuscript.
REFERENCES
- 1.Putko RM, Bedrin MD, Clark DM, et al. SARS-CoV-2 and limb ischemia: a systematic review. J Clin Orthop Trauma. 2021;12:194–199. doi: 10.1016/j.jcot.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh B, Aly R, Kaur P, et al. COVID-19 infection and arterial thrombosis: report of three cases. Ann Vasc Surg. 2021;70:314–317. doi: 10.1016/j.avsg.2020.08.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VO Costa, de Almeida GBC, Nicolini EM, et al. Acute arterial occlusion of the lower limb as the main clinical manifestation in a patient with Covid-19 - Case Report. Int J Surg Case Rep. 2020;77:454–458. doi: 10.1016/j.ijscr.2020.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Roquetaillade C, Chousterman BG, Tomasoni D, et al. Unusual arterial thrombotic events in Covid-19 patients. Int J Cardiol. 2021;323:281–284. doi: 10.1016/j.ijcard.2020.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silingardi R, Gennai S, Migliari M, et al. Acute limb ischemia in COVID-19 patients: could aortic floating thrombus be the source of embolic complications? J Vasc Surg. 2020;72:1152–1153. doi: 10.1016/j.jvs.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweblin C, Hachulla AL, Roffi M, et al. Delayed manifestation of COVID-19 presenting as lower extremity multilevel arterial thrombosis: a case report. Eur Heart J Case Rep. 2020;4:1–4. doi: 10.1093/ehjcr/ytaa371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renaud-Picard B, Gallais F, Ohana M, et al. Bilateral acute cardioembolic limb ischemia after coronavirus disease 2019 pneumonia in a Lung Transplant Recipient: a case report. Transplant Proc. 2020;52:2715–2718. doi: 10.1016/j.transproceed.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muhammad K, Tantawy TG, Makar RR, et al. Successful catheter-directed thrombolysis for acute lower limb ischemia secondary to COVID-19 infection. Ann Vasc Surg. 2021;71:103–111. doi: 10.1016/j.avsg.2020.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bozzani A, Arici V, Tavazzi G, et al. Acute thrombosis of lower limbs arteries in the acute phase and after recovery from COVID19. Ann Surg. 2021;273:e159–e160. doi: 10.1097/SLA.0000000000004700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mietto C, Salice V, Ferraris M, et al. Acute lower limb ischemia as clinical presentation of COVID-19 infection. Ann Vasc Surg. 2020;69:80–84. doi: 10.1016/j.avsg.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baccellieri D, Bilman V, Apruzzi L, et al. A case of Covid-19 patient with acute limb ischemia and heparin resistance. Ann Vasc Surg. 2020;68:88–92. doi: 10.1016/j.avsg.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gubitosa JC, Xu P, Ahmed A, et al. COVID-19-associated acute limb ischemia in a patient on therapeutic anticoagulation. Cureus. 2020;12:e10655. doi: 10.7759/cureus.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson K, Quail N, Kewin P, et al. COVID-19 associated with extensive pulmonary arterial, intracardiac and peripheral arterial thrombosis. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anwar S, Acharya S, Shabih S, et al. Acute limb ischemia in COVID-19 disease: a mysterious coagulopathy. Cureus. 2020;12:e9167. doi: 10.7759/cureus.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozzani A, Arici V, Tavazzi G, et al. Cute arterial and deep venous thromboembolism in COVID-19 patients: risk factors and personalized therapy. Surgery. 2020;168:987-992 [DOI] [PMC free article] [PubMed]