Abstract
Typhrasa is a rare genus that comprises two species and that has previously been reported only from Europe and North America. The present study expands the geographical scope of the genus by describing two new species – T. polycystis and T. rugocephala – from subtropical China. The new species are supported by morphological characteristics and phylogenetic analyses (ITS, LSU and tef-1α). The new species have very similar morphological characteristics and are 98% similar in their ITS region. However, T. rugocephala has two types of long gills at the same time, rarely fusiform pleurocystidia with rostrum. Detailed descriptions, colour photos, illustrations and a key to related species are presented in this paper.
Keywords: Basidiomycota , macromycetes, morphology, phylogenetic analysis, taxonomy
Introduction
The genus Typhrasa Örstadius & E. Larss. was established in 2015. It is characterised by a hygrophanous cap, crowded gills with white edge, small-to-medium-sized spores, large hymenial cystidia with intracellular oily drops or globules and a hymeniderm or paraderm pileipellis (Örstadius et al. 2015). The genus includes two species, T. gossypina (Bull.) Örstadius & E. Larss. and T. nanispora Örstadius, Hauskn. & E. Larss., the former being previously reported occasionally from some countries in Europe, North America and Asia within the genus Psathyrella (Fr.) Quél (Smith 1972; Kits van Waveren 1985; Knudsen and Vesterholt 2012; Örstadius et al. 2015). In addition, Psathyrella delineata (Peck) A.H. Sm., P. canadensis A.H. Sm. and P. subtenacipes A.H. Sm. are also reported to have oily drops in their cystidia (Smith 1972; Kits van Waveren 1985) and seems to be a candidate for Typhrasa. However, P. delineata and P. canadensis were combined into T. gossypina, based on the morphology (Örstadius et al. 2015). During investigations in subtropical China during 2018–2020, Typhrasa was recorded for the first time in China with two unrecorded species, which were frequently collected. Based on morphological characters and phylogenetic analyses, they are described as new species in this paper.
Materials and methods
Morphological studies
Macromorphological characters and habitat details were noted from fresh, young to mature basidiomata (over five basidiomata for each species) in the field. The location of the collection point is marked on the map (Suppl. material 1: Fig. S1). Colour codes are from the Methuen Handbook of Colour (Kornerup and Wanscher 1978). Micromorphological characters were observed with a light microscope (Olympus BX53). Sections from dry specimens were observed in water, 5% aqueous potassium hydroxide (KOH) solution, 10% aqueous ammonia (NH3·H2O) solution and Melzer’s Reagent, separately. More than fifty basidiospores, cystidia and basidia in 5% aqueous KOH solution were measured under the microscope. Basidiospore measurements were recorded in front and profile view. The measurements and Q values are given as (a)b–c(d), in which “a” is the lowest value, “b–c” covers a minimum of 90% of the values and “d” is the highest value. “Q” represents the ratio of length to width of a spore (Bas 1969; Ge et al. 2017; Na and Bau 2019). Specimens are deposited in the Herbarium of Fungi, Jiangxi Agricultural University (HFJAU) and Herbarium of Mycology, Jilin Agricultural University (HMJAU).
DNA extraction and sequencing
DNA was extracted from dried specimens with the NuClean Plant Genomic DNA kit (CWBIO, China). Three regions (ITS, LSU and Tef-1α) were generated for the study, which were amplified with primers ITS1/ITS4 (White et al. 1990), LR0R/LR7 (Hopple and Vilgalys 1999) and EF983F/EF2218R (Örstadius et al. 2015), respectively. PCR was performed using a touchdown programme: 5 min at 95 °C; 1 min at 95 °C; 30 s at 65 °C (add -1 °C per cycle); 1 min at 72 °C; 15 cycles; 1 min at 95 °C; 30 s at 50 °C; 1 min at 72 °C; 20 cycles; and 10 min at 72 °C (Yan and Bau 2018). The DNA sequencing was done by Qing Ke Biotechnology Co. Ltd. (Wuhan City, China).
Data analyses
The ITS, LSU and Tef-1α datasets were assembled following Örstadius et al. (2015) and BLAST in GenBank. Sequences from a total of 24 taxa were analysed using five data partitions (ITS, LSU, Tef 1st, Tef 2nd and Tef 3rd). The details are presented in Table 1. Sequences were aligned separately in MAFFT v.7 (Katoh and Standley 2013). The best-fit models of nucleotide evolution for ITS, LSU, Tef 1st, Tef 2nd and Tef 3rd datasets (GTR+G, GTR+I, SYM, SYM and GTR+G, respectively) were obtained in MrModeltest v.2.3 (Nylander et al. 2008). Phylogenetic analysis was conducted using Bayesian Inference (BI) in MrBayes v.3.2.6 (Ronquist et al. 2012). Gaps were treated as missing data following Örstadius et al. (2015). Four Monte Carlo Markov Chains (MCMC) were run for five million generations, sampling every 100th generation. The first 25% of trees were discarded as burn-in (Ronquist et al. 2012). The sequence alignment is deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S27860).
Table 1.
Sequences used in this study. Newly generated sequences are given in bold. Type material is indicated in the column Voucher.
| Taxa | Voucher | Locality | ITS | LSU | tef-1α |
|---|---|---|---|---|---|
| Cystoagaricus hirtosquamulosus | Ramsholm800927 | Finland | KC992945 | KC992945 | – |
| C. olivaceogriseus | WK 8/15/63-5 (MICH) Type | USA | KC992948 | KC992948 | – |
| C. sylvestris | LÖ191-92 | Sweden | KC992949 | KC992949 | – |
| C. squarrosiceps | Laessoe44835 | Ecuador | KC992950 | – | – |
| C. strobilomyces | E. Nagasawa 9740 | AY176347 | AY176348 | – | |
| Kauffmania larga | LAS97-054 | Sweden | DQ389695 | DQ389695 | – |
| K. larga | LÖ223-90 | Sweden | DQ389694 | DQ389694 | KJ732824 |
| Psathyrella delineata | CCB171 | USA | KY744151 | ||
| P. delineata | TMW02 | USA | MF686534 | ||
| P. delineata | MGW1406 | USA | KY777378 | ||
| Typhrasa gossypina | 180524-H08 | Korea | MN082538 | – | – |
| T. gossypina | BRNM:705622 | Austria | AM712293 | – | – |
| T. gossypina | BRNM:705609 | Czech | AM712292 | – | – |
| T. gossypina | WU:25069 | Austria | AM712294 | – | – |
| T. gossypina | Schumacher024 | Germany | KC992946 | KC992946 | KJ732825 |
| T. nanispora | Barta980706 Type | Austria | KC992947 | KC992947 | – |
| T. polycystis | HFJAU1454 Type | China:Jiangxi | MW466538 | MW466544 | MW475280 |
| T. polycystis | HFJAU1520 | China:Fujian | MW466539 | MW466545 | MW475281 |
| T. polycystis | HFJAU1349 | China:Jiangxi | MW466540 | – | – |
| T. rugocephala | HFJAU1467 Type | China:Zhejiang | MW466541 | MW466546 | MW475282 |
| T. rugocephala | HFJAU1455 | China:Zhejiang | MW466542 | MW466547 | MW475283 |
| T. rugocephala | HFJAU1476 | China:Zhejiang | MW466543 | MW466548 | – |
| Outgroup | |||||
| Psathyrella oboensis | DED 8234 Type | SãoTomé | NR148107 | – | – |
| P. pertinax | LO259-91 Neotype | Sweden | DQ389701 | DQ389701 | KJ732809 |
Results
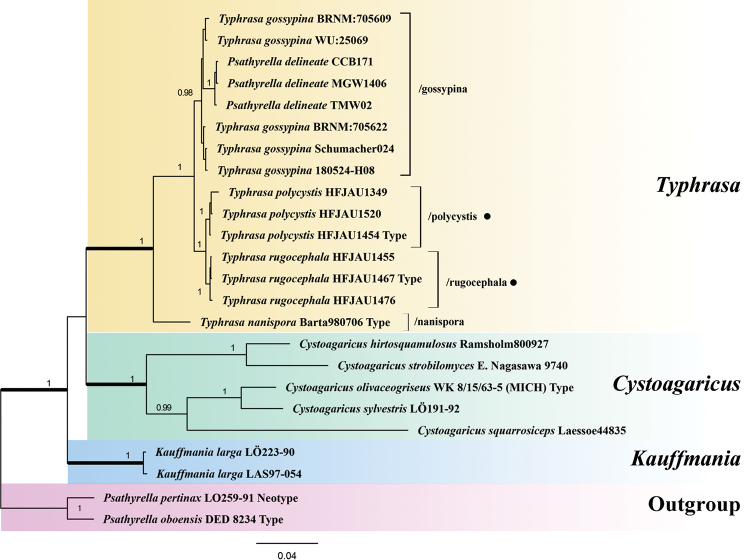
Based on the BLAST results of the full length of the ITS region, two new species were found sharing less than 98.0% similarity with the known species of Typhrasa, respectively: 97% with T. gossypina and 92% with T. nanispora. The Bayesian analysis (Figure 1) comprised material from three major genera, all of which are identified as monophyletic with very strong support (BPP = 1), viz. Typhrasa, Cystoagaricus Singer and Kauffmania Örstadius & E. Larss., in agreement with the study published by Örstadius et al. (2015). Our collections of the two new Typhrasa species formed a joint, strongly supported clade (BPP = 1) and both new species received strong support as monophyletic (BPP ≥ 0.97). The collections of T. gossypina (Bull.) Örstadius & E. Larss. clustered together and appeared as a sister to the group consisting of the two new species.
Figure 1.
Phylogram generated by Bayesian Inference (BI) analysis, based on sequences of a concatenated dataset from three nuclear markers (ITS, LSU and tef-1α) rooted with Psathyrella spp. Bayesian posterior probabilities ≥ 0.95 are shown. ● indicates the newly-described species.
Taxonomy
Typhrasa polycystis
J.Q Yan & S.N. Wang sp. nov.
11360C03-9F5B-50D7-A968-94A3D152A7EA
838482
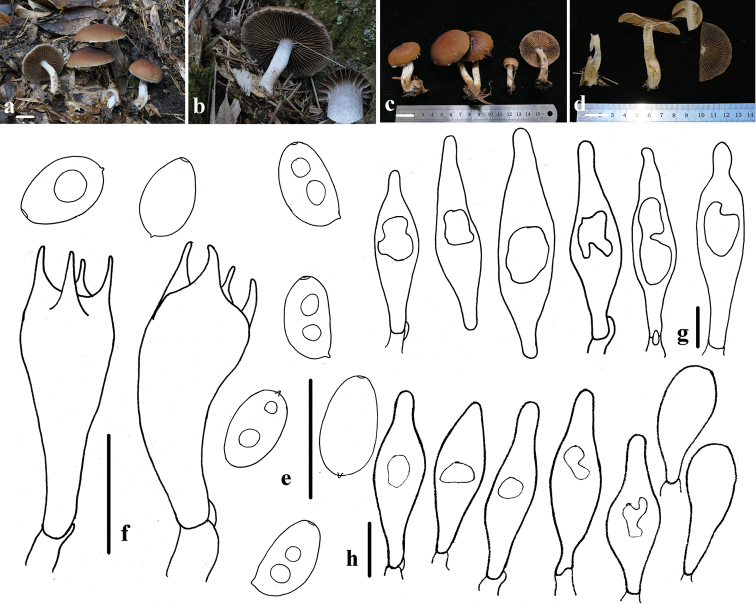
Figure 2.
Basidiomata and microscopic features of Typhrasa polycystisa–c basidiomata d basidiospores e basidia f pleurocystidia g cheilocystidia. Scale bars: 20 mm (a–c); 10 μm (d–g).
Diagnosis.
Differs from Typhrasa gossypina by its smaller spores (7.1–8.2 × 4.3–5.1 μm).
Holotype.
China. Jiulianshan National Nature Reserve, Jiangxi Province, 25 June 2020, Jun-Qing Yan, HFJAU1454.
Etymology.
Referring to the characteristics of the pleurocystidia.
Description.
Pileus 20–35 mm, extending hemispherically to expanded, plane with or without umbo, surface with slightly ridge-like folds or smooth, hygrophanous, brown (7D6–7C6), pale brown (6B6–6C6) at the margin. Veil distinct, fibrous or fluffy, white (7C1), markedly appendiculate at margin, falling off easily. Context thin and fragile, hygrophanous at pileus, about 3.0 mm at the centre. Gills 4.0–5.0 mm broad, moderately close, pale cinnamon (6C6–6D5) with a white (6C1) edge, adnexed. Stipe 30–45 mm long, 5.0–8.0 mm thick, white (6C1), hollow, pulverulent at apex, with fibrils and fluffy from pellicular veil remnants below, falling off easily.
Spores (6.9)7.1–8.2 × (4.2)4.3–5.1(5.2) μm, Q = (1.4)1.5–1.8(1.9), ellipsoid to oblong-ellipsoid, profile flattened on one side, 4.1–5.0(5.2) μm broad, smooth, reddish-brown (8C5–8C6) in water, yellow-brown (7D6–7D7) in 5% KOH or 10% NH3·H2O, becoming darker (7E4–7F4) in 5% KOH, germ pore small and indistinct, 1–2 guttulate, inamyloid. Basidia 21–24 × 7.5–8.5 μm, 4-spored, clavate, hyaline. Pleurocystidia 55–81(87) × 11–17(18) μm, variously shaped, often fusiform and lageniform, with rostrum; rarely fusiform with subacute to acute apex or cystiform with rostrum, with one or two large internal oily drops, oily drops colourless and distinct or indistinct in 5% KOH, glassy-yellow (5B6–5B7) and very distinct in Melzer’s Reagent. Cheilocystidia (24)30–54(58) × (9)10–15(16) μm, similar to pleurocystidia, abundant, rarely mixed with pyriform or clavate cells. Trama of gills consisting of parallel hyphae. Pileipellis a 2–3 cells deep layer of subglobose or pyriform cells which are 24–36 μm wide. Veil composed of hyphae 6.5–14.5 μm-broad, thin-walled and fawn (5A2-A3) hyphae in 5% KOH. Clamps present in trama of gills, hyphae of stipe and at the base of the basidia and cystidia.
Ecology and distribution.
Saprotrophic, solitary to slightly caespitose on rotten hard wood or humus in mixed forests.
Other specimens examined.
China. Jiulianshan National Nature Reserve, Jiangxi Province, 28 May 2018, Guang-Hua Huo, Lin-Ping Zhang, HFJAU1349. Wuyishan National Nature Reserve, Fujian Province, 27.748888°N, 117.7625°E, 761 alt., 12 June 2020, Liangliang Qi, Yupeng Ge, HFJAU1520, HMJAU58461.
Typhrasa rugocephala
J.Q Yan & S.N. Wang sp. nov.
0BF3F3E7-9EE6-5283-9CCB-10FC36A5A2AE
838483
Figure 3.
Basidiomata and microscopic features of Typhrasa rugocephalaa–d basidiomata e basidiospores f basidia g pleurocystidia h cheilocystidia. Scale bars: 20 mm (a–d); 10 μm (e–h).
Diagnosis.
Differs from Typhrasa polycystis by having two types of long gills and rarely rostrum can be found in fusiform pleurocystidia.
Holotype.
China. Baishanzu National Nature Reserve, Zhejiang Province, 27.734233°N, 119.186943°E, 1184 m alt. 28 June 2020, Sheng-Nan Wang, HFJAU1467.
Etymology.
Referring to the surface of the pileus.
Description.
Pileus 35–55 mm, spreading hemispherically to oblate with a slight umbo, surface with distinct ridge-like folds, hygrophanous, reddish-brown (8E5–8F6), pale brown (6D7–6C7) at the margin, drying tawny (7D6–7E6), striate, sometimes faintly, at margin. Veil distinct, fibrous or fluffy, white (7C1), markedly appendiculate at margin, falling off easily. Context thin and fragile, hygrophanous, about 2.5 mm at the centre. Gills 5.0–7.0 mm broad, moderately close; when young, dirty white (7B1), becoming cinnamon (7C6–7D5) with a white edge (7C1); two types of long gills arranged at intervals: A: adnate to slightly decurrent, B: emarginate- adnexed. Stipe 40–60 mm long, 5.0–10 mm thick, white (7C1), hollow, pulverulent at apex, with fibrils and fluffy from pellicular veil remnants below, falling off easily.
Spores (6.5)6.8–7.9(8.3) × 4.5–5.2(5.4) μm, Q = (1.3)1.4–1.7, ellipsoid to oblong-ellipsoid, profile flattened on one side, 4.1–5.0(5.2) μm broad, smooth, reddish-brown (8C6–8C7) in water, yellow-brown (7D6–7D7) in 5% KOH or 10% NH3·H2O, becoming darker (7E4–7E5) in 5% KOH, germ pore small and indistinct, 1–2 guttulate, inamyloid. Basidia 18–23 × 7.0–8.0 μm, 4-spored, clavate, hyaline. Pleurocystidia 42–68 × 13–17 μm, thin-walled, fusiform, apex obtuse to subacute, rarely cystiform with a short rostrum, with one or two large internal oily drops, oily drops colourless and distinct or indistinct in 5% KOH, glassy-yellow (5B6–5B7) and very distinct in Melzer’s Reagent. Cheilocystidia scanty, 33–48 × 10–15 μm, similar to pleurocystidia, few and scattered, mix with pyriform or clavate, 21–39 × 11–13 μm-sized cells. Trama of gills consisting of parallel hyphae. Pileipellis a 2–3 cells deep layer of subglobose or pyriform cells which are 18–32 μm wide. Veil composed of 5.4–8.4 μm-broad hyphae, thin-walled and fawn (5A2-A3) hyphae in 5% KOH. Clamps rare, but observed in trama of gills, hyphae of stipe and at the base of the basidia and cystidia.
Ecology and distribution.
Saprotrophic, solitary or gregarious on soil or humus in broad-leaved forests.
Other specimens examined.
China. Baishanzu National Nature Reserve, Zhejiang Province, 24 June 2020, Ya-Ping Hu, HFJAU1476; 28 June 2020, Sheng-Nan Wang, HFJAU1455, HMJAU58462.
Discussion
Typhrasa was established by Örstadius et al. (2015), based on the main characters of having rostrate, hymenial cystidia with oily drops. Only T. gossypina and T. nanispora Örstadius, Hauskn. & E. Larss. were reported in that study. T. gossypina, as the type species of the genus, was, therefore, separated from Psathyrella (Fr.) Quél. This species can be separated from the two new species through its longer spores up to 9.0 μm long, 5.0–6.0 μm broad in front view and pleurocystidia often with a long rostrum (Kits van Waveren 1985; Örstadius et al. 2015). T. nanispora has smaller spores, 5.0–6.0 × 3.0–4.0 μm and can be thereby easily be distinguished (Örstadius et al. 2015). T. rugocephala is very easily confused with T. polycystis, but the former has two types of long gills arranged at intervals, scanty cheilocystidia and rarely rostrum can be found in fusiform pleurocystidia. In addition, P. subtenacipes are also reported to have oily drops in their cystidia (Smith 1972) and seems to be a candidate for Typhrasa, but study of type material is needed to settle this question. Morphologically, the spores of P. subtenacipes are up to 7.8–9.5 × 5.0–5.6 μm, significantly larger than the two new species (Smith et al. 1950; Smith 1972). A key to these related species is presented below:
Key to related species
| 1 | Spores up to 9.5 μm long, 5.0–5.6 μm broad | P. subtenacipes |
| – | Not as above | 2 |
| 2 | Spores less than 6.0 μm long | T. nanispora |
| – | Spores over 6.0 μm long | 3 |
| 3 | Spores 7.0–9.0 μm long, 5.0–6.0 μm broad in front view | T. gossypina |
| – | Spores smaller, less than 8.0 μm long and 5.0 μm broad in front view | 4 |
| 4 | Long gills have two types concurrently: adnate to slightly decurrent and emarginate-adnexed, fusiform, apex obtuse to subacute, rarely cystiform with a short rostrum, cheilocystidia scanty | T. rugocephala |
| – | Gills adnexed, pleurocystidia variously-shaped, often fusiform and lageniform with rostrum | T. polycystis |
Supplementary Material
Acknowledgements
This work is supported by the National Natural Science Foundation of China (31960008), Jiangxi Provincial Natural Science Foundation (20202BABL213041). The project was supported by the biodiversity investigation, observation and assessment programme (2019–2023) of the Ministry of Ecology and Environment of China (2110404); Central Public-Interest Scientific Institution Basal Research Fund (GYZX200203). Sincere thanks to the anonymous reviewers of the manuscript.
Citation
Wang S-N, Hu Y-P, Chen J-L, Qi L-L, Zeng H, Ding H, Huo G-H, Zhang L-P, Chen F-S, Yan J-Q (2021) First record of the rare genus Typhrasa (Psathyrellaceae, Agaricales) from China with description of two new species. MycoKeys 79: 119–128. https://doi.org/10.3897/mycokeys.79.63700
Funding Statement
This work is supported by the National Natural Science Foundation of China (31960008), Jiangxi Provincial Natural Science Foundation (20202BABL213041). The project was supported by the biodiversity investigation, observation and assessment programme (2019-2023) of the Ministry of Ecology and Environment of China (2110404); Central Public-Interest Scientific Institution Basal Research Fund (GYZX200203)
Contributor Information
Fu-Sheng Chen, Email: chenfush@hotmail.com.
Jun-Qing Yan, Email: yanjunqing1990@126.com.
Supplementary materials
Figure S1. Collection site of Typhrasa rugocephala and T. polycystis
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Sheng-Nan Wang, Ya-Ping Hu, Jun-Liang Chen, Liang-Liang Qi, Hui Zeng, Hui Ding, Guang-Hua Huo, Lin-Ping Zhang, Fu-Sheng Chen, Jun-Qing Yan
Data type
image
References
- Bas C. (1969) Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5(1): 96–97. [Google Scholar]
- Ge Y, Yang S, Bau T. (2017) Crepidotus lutescens sp. nov. (Inocybaceae, Agaricales), an ochraceous salmon colored species from northeast of China. Phytotaxa 297(2): 189. 10.11646/phytotaxa.297.2.6 [DOI] [Google Scholar]
- Hopple JJ, Vilgalys R. (1999) Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ribosomal subunit RNA: divergent domains, outgroups, and monophyly. Molecular Phylogenetics & Evolution 13(1): 1–19. 10.1006/mpev.1999.0634 [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology & Evolution 30(4): 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kits van Waveren E. (1985) The Dutch, French and British species of Psathyrella. Persoonia 2: 1–284. [Google Scholar]
- Knudsen H, Vesterholt J. (2012) Funga Nordica. Agaricoid, boletoid, cyphelloid and gasteroid genera. Nordsvamp, Copenhagen.
- Kornerup A, Wanscher JHK. (1978) The Methuen Handbook of Colour 3rd edn. Eyre Methuen Ltd. Reprint., London.
- Na Q, Bau T. (2019) Mycena section Sacchariferae: three new species with basal discs from China. Mycological Progress 18(3): 483–493. 10.1007/s11557-018-1456-8 [DOI] [Google Scholar]
- Nylander J. (2004) MrModeltest 2.3. Computer program and documentation distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala.
- Örstadius L, Ryberg M, Larsson E. (2015) Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: introduction of three new genera and 18 new species. Mycological Progress 14(5): 1–42. 10.1007/s11557-015-1047-x [DOI] [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Stuntz D. (1950) New or Noteworthy Fungi from Mt. Rainier National Park. Mycologia 42(1): 80–134. 10.2307/3755245 [DOI] [Google Scholar]
- Smith AH. (1972) The North American species of Psathyrella. The New York Botanical Garden 24: 1–633. [Google Scholar]
- White TJ, Bruns TD, Lee SB, Taylor JW, Innis MA, Gelfand DH, Sninsky JJ. (1990) Amplification and direct sequencing of Fungal Ribosomal RNA Genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR Protocols: a guide to methods and applications.Academic Press, San Diego, 315–322. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Yan JQ, Bau T. (2018) Psathyrella alpina sp. nov. (Psathyrellaceae, Agaricales), a new species from China. Phytotaxa 349(1): 85–91. 10.11646/phytotaxa.349.1.11 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Collection site of Typhrasa rugocephala and T. polycystis
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Sheng-Nan Wang, Ya-Ping Hu, Jun-Liang Chen, Liang-Liang Qi, Hui Zeng, Hui Ding, Guang-Hua Huo, Lin-Ping Zhang, Fu-Sheng Chen, Jun-Qing Yan
Data type
image