Abstract
Aim:
To examine whether anesthesia exposure is associated with neurocognitive decline in pediatric medulloblastoma.
Methods:
Patients were treated at St. Jude Children’s Research Hospital and completed ≥2 protocol-directed neurocognitive assessments (n=107), as part of a multi-site clinical trial for pediatric medulloblastoma (NCT00085202). Patients received risk-adapted craniospinal photon irradiation, followed by four cycles of high-dose chemotherapy and stem cell rescue. Neurocognitive testing was completed at study baseline (after surgery and <2 weeks of starting radiation therapy) and annually for 5 years. Data on anesthesia exposure during treatment was abstracted from medical records.
Results:
Patients were 10.2 years at diagnosis on average (SD=4.5; 37% female, 73% average-risk). Mean cumulative anesthesia duration was 20.4 hours (SD=15.2; range 0.7–55.6 hours). In the overall group, longer anesthesia duration was associated with greater declines in IQ (Estimate=−0.08, P<.001), attention (Estimate=−0.10, P<.001), and processing speed (Estimate=−0.13, P<.001). Similar results were shown in subgroups of patients who were <7 years at diagnosis (IQ=−0.14, P=.027; Attention=−0.25: P=.011), ≥7 years at diagnosis (Attention=−0.07, P=.039; Processing Speed=−0.08, P=.022), treated for high-risk disease (IQ=−0.09, P=.024; Attention=−0.11, P=.034; Processing Speed=−0.13, P=.001), or treated for average-risk disease (IQ=−0.05, P=.022; Attention=−0.08, P=.011; Processing Speed=−0.10, P<.001).
Conclusion:
Greater anesthesia exposure is a risk factor for clinically significant neurocognitive decline, in addition to factors of age at diagnosis and treatment risk arm. This result is notable as there are evidence-based strategies that can limit the need for anesthesia. Limiting anesthesia exposure, as feasible, may mitigate neurocognitive late effects and thus improve quality of life for survivors.
Keywords: neurocognitive, longitudinal, children, medulloblastoma, brain tumor, anesthesia
Medulloblastoma is the most common malignant pediatric brain tumor, which is currently treated with surgery, risk-adapted radiation therapy, and adjuvant chemotherapy[1]. Survival rates have increased with contemporary treatment; however, survivors show neurocognitive decline following treatment[2, 3]. Established risk factors for neurocognitive problems include younger age and higher intensity treatments[4, 5], and deficits may increase over time[6].
Another risk factor for neurocognitive impairment may be exposure to general anesthesia. Pre-clinical studies have suggested that anesthesia impacts brain development[7], which led to warnings that repeated or lengthy use of anesthesia should be avoided in children younger than 3 years[8]. Clinical studies generally suggest that a single, brief exposure to anesthesia is not associated with cognitive deficits[9]. However, there have been mixed results[10, 11], owing to methodological concerns such as using retrospective or birth cohort designs[12]. Furthermore, most studies have not examined children with complex medical conditions. These children are particularly vulnerable because of their disease and treatment, but also because they have multiple exposures to anesthesia. Importantly, anesthesia exposure is a modifiable risk factor, such that children can complete some procedures without sedation given sufficient training[13, 14]. Knowledge of whether multiple exposures to anesthesia impacts long-term neurocognitive outcomes may help to guide current clinical practice.
This longitudinal study examined associations between anesthesia exposure and neurocognitive outcomes in patients treated for pediatric medulloblastoma on a clinical trial. These patients had multiple exposures to anesthesia, such as for radiation or diagnostic imaging. Neurocognitive functioning was followed prospectively from baseline (after surgery and <2 weeks of starting radiation) and annually up to 5 years post-diagnosis. Results from a cross-sectional analysis at 3 years post-diagnosis showed that cumulative anesthesia exposure was associated with poorer performance on overall IQ, attention, working memory, processing speed, and reading[15]. Anesthesia exposure predicted poorer cognitive performance, separately from age at diagnosis, risk group, and experiencing posterior fossa syndrome (PFS). The current study focused on a subset of neurocognitive measures in order to evaluate changes over time that may be associated with anesthesia exposure. We hypothesized that longer durations or frequency of anesthesia would be associated with larger declines over time.
Methods
This research was approved by Institutional Review Board (IRB) at St. Jude Children’s Research Hospital. All participants or their parent/guardian gave written informed consent. Children gave their assent.
Participants
There were 155 patients (3–21 years) with histologically confirmed medulloblastoma who were treated at St. Jude Children’s Research Hospital as part of a multi-site clinical trial from 2003–2013 (ClinicalTrials.gov: NCT00085202). There were 38 patients who were ineligible for testing (n=3 no consent; n=13 limited English proficiency or sensorimotor condition; n=22 off study/off treatment). Of the 117 eligible patients, 107 were included in the current analyses as they completed at least 2 time points of neurocognitive testing (i.e., at baseline, 1, 2, 3, 4, or 5-year time points). Ten patients were excluded from analyses because they did not complete testing due to refusal or scheduling conflicts (n=8) or they had received prolonged sedation necessary for mechanical ventilation (n=2).
Protocol-Directed Treatment
Patients underwent surgical resection and were subsequently placed into groups of average-risk or high-risk disease[16]. After enrollment, risk-adapted radiation therapy started within 31 days after surgery. Treatment for high-risk disease included craniospinal photon irradiation (CSI; M0–1: 36 Gy; M2–3: 39.6 Gy) and a focal boost to the tumor bed (total dose: 55.8 Gy). For metastatic disease, local sites received supplemental irradiation (total dose: 50.4–54 Gy). Treatment for average-risk disease included CSI (23.4 Gy) and boost to the tumor bed (total dose: 55.8 Gy). All clinical target volumes were 1.0 cm and all radiation protocols were delivered over 30 fractions (~30–45 min/fraction). Patients subsequently received four cycles of high-dose chemotherapy (cyclophosphamide, cisplatin, vincristine) with peripheral blood stem cell rescue.
Demographic and Medical Variables
Demographic and medical information was collected as part of the clinical trial. Anesthesia exposure was extracted from medical records, which included frequency/duration, agents/type of anesthetic, and other related procedures for 12 months after diagnosis[15].
Neurocognitive Measures
Neurocognitive testing was obtained at baseline (after surgery and <2 weeks of starting radiation) and annually for 5 years. We focused on index scores from the Woodcock-Johnson III Tests of Cognitive Abilities (WJ-III COG)[17], which included measures of overall intelligence (General Ability), attention and working memory (Broad Attention), and processing efficiency (Processing Speed). Age standardized normative data were available for patients ≥2 years for overall IQ and attention, and ≥3 years old for processing speed. Higher scores indicate better performance (M=100, SD=15).
Analysis
Fisher’s exact, likelihood ratio, and Wilcoxon rank-sum tests were used to examine differences in demographic and clinical information for eligible participants versus non-participants. Descriptive statistics were used to describe anesthesia exposure. Relationships between age, baseline cognitive performance, and cumulative anesthesia frequency/duration were examined with Pearson correlations. Differences between risk groups for cumulative anesthesia frequency/duration were examined with independent samples t-tests. Non-parametric tests were used to evaluate differences in demographic and clinical groups for total number of completed neurocognitive tests over time.
Linear mixed models were used to evaluate associations between age at diagnosis, treatment risk, and anesthesia exposure with neurocognitive functioning. Outcomes of interest included overall IQ, attention, and processing speed. First, separate models were estimated for each predictor along with its’ interaction with time in the overall group. This included: age at diagnosis (continuous in years); risk category (high vs. average); cumulative duration of anesthesia (continuous in hours); and cumulative frequency of anesthesia (continuous N events). Multivariate models that included all predictors together were not examined due to the high correlations between variables. Next, we estimated models for anesthesia effects within subgroups similar to the literature (i.e., <7 years old, ≥7 years old, high-risk group, average-risk group[6, 18]). All models included random intercepts and slopes.
Primary analyses focused on the overall group (n=107). Supplementary analyses included the non-PFS group (n=88), as PFS is a risk factor for neurocognitive deficits[19]. All statistical comparisons were two-tailed and were considered significant at P<.05. Analyses were conducted in R-4.0.0 (R-Core Team, Vienna, Austria).
Results
Demographic and Clinical Information
Eligible participants versus non-participants were older (M=10.2 vs. 7.5 years; P=.05) and the proportion of males to females was higher (64% vs. 30%, P=.05); however, the number of patients excluded from analyses was small (Table 1). There were no differences between participants and non-participants for race (P=.33), risk arm (P=.47), PFS status (P>.99), or anesthesia exposure (frequency P=.31; duration P=.28).
Table 1.
Demographic and clinical information for participants and non-participants
| Variable | Category | Participants (No. = 107) | Non-Participants (No. = 10) | |
|---|---|---|---|---|
| No. (%) | No. (%) | P † | ||
| Sex | Female | 39 (37) | 7 (70) | .05 |
| Race | White | 83 (78) | 5 (50) | .33 |
| Risk Group | Average | 78 (73) | 6 (60) | .47 |
| Posterior Fossa | No | 99 (82) | 9 (90) | >.99 |
| Mean (SD) | Mean (SD) | P ‡ | ||
| Age at Diagnosis | Years | 10.2 (4.5) | 7.5 (3.1) | .05 |
| Anesthesia Exposure | Cumulative Frequency | 19.0 (15.5) | 22.9 (16.3) | .31 |
Abbreviations: SD: standard deviation; No: sample size; %: percent
Notes: Participants were included in analyses if they had at least 2 time points of neurocognitive data. Percentages may not equal 100 due to rounding.
Difference between groups based on Fisher’s exact test or likelihood ratio test.
Difference between groups based on Wilcoxon rank-sum tests.
Anesthesia Exposure
Mean cumulative frequency of anesthesia was 19.0 events (SD=15.5; range 1–52) and mean cumulative duration was 20.4 hours (SD=15.2; range 0.7–55.6; Table 1). Indications for anesthesia included radiation, imaging, or procedures such as lumbar punctures. Radiation therapy was the most common indication (52% of events, 41% of patients), followed by imaging (25% of events, 85% of patients). Administrations included intravenous, inhalation, or mixed methods (Table 2). Most common agents were propofol and fentanyl for intravenous (100% of patients) and sevoflurane for inhalation (91% of patients) methods.
Table 2.
Anesthesia exposure during first year after diagnosis (overall group)
| Anesthetic Agent | Overall Group (No. = 107) | |
|---|---|---|
| Patient Received Agent | Cumulative Exposure per Patient | |
| No. (%) | Mean (SD) | |
| Intravenous | ||
| Propofol (mg/kg) | 107 (100) | 6026.4 (5393.7) |
| Fentanyl (μg/kg) | 107 (100) | 333.1 (303.2) |
| Midazolam (mg/kg) | 66 (62) | 9.0 (12.5) |
| Morphine (mg/kg) | 26 (24) | 11.3 (21.0) |
| Pentobarbitol (mg/kg) | 22 (21) | 289.8 (213.5) |
| Merperidine (mg/kg) | 16 (15) | 109.8 (87.3) |
| Ketamine (mg/kg) | 9 (8) | 270.1 (296.5) |
| Lorazepam (mg/kg) | 5 (5) | 1.4 (1.1) |
| Hydromorphone (mg/kg) | 2 (2) | 1.1 (1.3) |
| Clonidine (mg/kg) | 1 (1) | 0.1 (0.0) |
| Dexmedetomidine (mg/kg) | 0 (0) | 0.0 (0.0) |
| Inhaled | ||
| Sevoflurane | 97 (91) | 2.0 (1.2) |
| Nitrus oxide | 52 (49) | 1.4 (0.8) |
| Isoflurane | 15 (14) | 1.2 (0.6) |
| Desflurane | 6 (6) | 1.0 (0.0) |
| Halothane | 1 (1) | 1.0 (0.0) |
Abbreviations: SD: standard deviation; No: sample size; %: percent
Notes: Anesthesia exposure was extracted from medical records for the first year after diagnosis.
Younger age at diagnosis was positively associated with frequency (r=−0.58, P<.001) and duration (r=−0.59, P<.001) of anesthesia. Patients with high-risk disease had greater anesthesia duration compared to the average-risk group (M=26.5 vs. 18.1 hours, P=.02); differences did not reach significance for anesthesia frequency (M=23.7 vs. 17.3 events, P=.08). There were no significant correlations between baseline cognitive performance and anesthesia exposure (r range=−0.03 to 0.14, P>.20).
Anesthesia Exposure and Neurocognitive Performance in Overall Group
Most participants (80%) completed ≥4 neurocognitive assessments (Table A1). Patients with PFS completed fewer baseline (P<.001) and Year 1 (P=.03) assessments than the non-PFS group. There were no other demographic or clinical predictors for number of completed assessments (P>.05).
Table 3 shows results from linear mixed models in the overall group. All models showed significant interactions with time. Attention performance is illustrated as an example (Figure 1). These results suggest that older children had increasing neurocognitive performance over time (Overall IQ: P<.001; Attention: P<.001; Processing Speed: P<.001); however, graphs illustrate that younger children also had declining performance. Declines across neurocognitive domains were shown in patients who were treated for high-risk (vs. average-risk) disease (Overall IQ: P<.001; Attention: P=.029; Processing Speed: P<.001) and for those who had greater anesthesia exposure (Overall IQ: P<.001; Attention: P<.001; Processing Speed: P<.001).
Table 3.
Age, treatment risk, and cumulative anesthesia duration/frequency as predictors of neurocognitive decline (overall group)
| Model (No.) | Predictor Variable | Outcome Variable | |||||
|---|---|---|---|---|---|---|---|
| Overall IQ | Attention | Processing Speed | |||||
| Estimate (SE) | P † | Estimate (SE) | P † | Estimate (SE) | P † | ||
| Age (No.=107) | Intercept | 95.29 (3.96) | <.001 | 95.01 (4.15) | <.001 | 78.61 (4.72) | <.001 |
| Age at diagnosis | 0.06 (0.35) | .86 | −0.26 (0.37) | 0.49 | 0.24 (0.42) | .58 | |
| Time | −2.41 (0.74) | .002 | −3.91 (0.97) | <.001 | −3.61 (0.89) | <.001 | |
| Age * time | 0.25 (0.07) | <.001 | 0.30 (0.09) | <.001 | 0.44 (0.08) | <.001 | |
| Risk group (No.=107) | Intercept | 94.72 (1.85) | <.001 | 91.56 (1.91) | <.001 | 78.39 (2.14) | <.001 |
| Risk (high vs. average) | 4.06 (3.55) | .25 | 1.96 (3.68) | .60 | 9.51 (4.12) | .023 | |
| Time | 0.81 (0.34) | .021 | −0.31 (0.45) | .50 | 1.75 (0.43) | <.001 | |
| Risk * time | −2.33 (0.67) | <.001 | −1.98 (0.89) | .029 | −3.52 (0.86) | <.001 | |
| Anesthesia duration (No. = 107) | Intercept | 101.22 (2.57) | <.001 | 96.23 (2.65) | <.001 | 85.67 (3.08) | <.001 |
| Anesthesia duration | −0.26 (0.10) | .013 | −0.19 (0.11) | .08 | −0.22 (0.12) | .07 | |
| Time | 1.76 (0.48) | <.001 | 1.20 (0.60) | .050 | 3.50 (0.58) | <.001 | |
| Anesthesia * time | −0.08 (0.02) | <.001 | −0.10 (0.02) | <.001 | −0.13 (0.02) | <.001 | |
| Anesthesia frequency (No.=107) | Intercept | 101.26 (2.41) | <.001 | 96.36 (2.49) | <.001 | 85.23 (2.91) | <.001 |
| Anesthesia frequency | −0.28 (0.10) | .006 | −0.22 (0.11) | .045 | −0.22 (0.12) | .07 | |
| Time | 1.54 (0.46) | .001 | 0.79 (0.59) | .18 | 3.17 (0.56) | <.001 | |
| Anesthesia * time | −0.07 (0.02) | <.001 | −0.09 (0.03) | .001 | −0.12 (0.02) | <.001 | |
Abbreviations: No: sample size; SE: standard error
Notes:
In the overall group (n=107), linear mixed models were estimated for WJ III indices of overall IQ, attention, and processing speed. Separate models were conducted to evaluate effects of age at diagnosis (continuous in years), protocol-directed risk arm (high-risk vs. average-risk), total cumulative duration of anesthesia (continuous in hours), and total cumulative frequency of anesthesia (continuous). Interactions with time included years since diagnosis.
Figure 1.
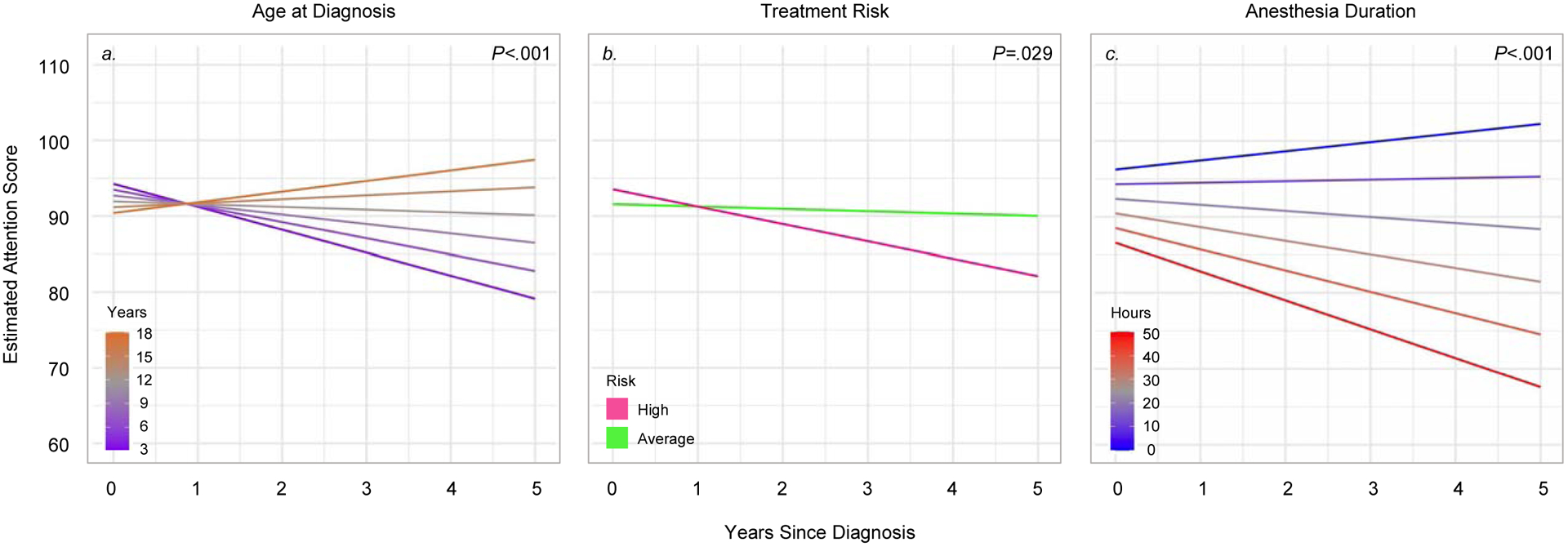
Association between time and age, treatment risk, and anesthesia duration on neurocognitive decline (overall group)
Notes: Estimated performance on the WJ III Attention index is shown as age standardized norms (M=100, SD=15) in the overall group (n=107). Linear mixed models showed significant interactions between time and age at diagnosis (P<.001), time and treatment risk group (P=.029), and time and anesthesia duration (P<.001). Colored lines represent estimated scores for: 3, 6, 9, 12, 15, or 18 years old at diagnosis (panel a); high risk or average risk treatment (panel b); and 0, 10, 20, 30, 40, or 50 hours of cumulative anesthesia exposure (panel c).
Anesthesia Exposure and Neurocognitive Performance in Age and Treatment Risk Groups
In the next analyses, linear mixed models were conducted for separate age and risk groups. Similarly to the overall group, significant interactions were shown between time and anesthesia exposure (Table 4; Table A2). Attention performance is illustrated as an example (Figures 2 and 3).
Table 4.
Cumulative anesthesia duration as predictor of neurocognitive decline (age at diagnosis and treatment risk groups)
| Model (No.) | Predictor Variable | Outcome Variable | |||||
|---|---|---|---|---|---|---|---|
| Overall IQ | Attention | Processing Speed | |||||
| Estimate (SE) | P † | Estimate (SE) | P † | Estimate (SE) | P † | ||
| <7 years old (No.=27) | Intercept | 94.47 (10.66) | <.001 | 89.95 (11.48) | <.001 | 84.37 (12.17) | <.001 |
| Anesthesia duration | −0.01 (0.28) | .99 | 0.06 (0.30) | .84 | 0.02 (0.31) | .96 | |
| Time | 3.20 (2.32) | .18 | 6.70 (3.39) | .06 | 3.20 (2.87) | .28 | |
| Anesthesia * time | −0.14 (0.06) | .027 | −0.25 (0.09) | .011 | −0.15 (0.07) | .058 | |
| ≥7 years old (No.=80) | Intercept | 103.10 (2.87) | <.001 | 98.07 (2.93) | <.001 | 89.90 (3.33) | <.001 |
| Anesthesia duration | −0.44 (0.15) | .005 | −0.38 (0.16) | .019 | −0.68 (0.18) | <.001 | |
| Time | 1.19 (0.50) | .021 | 0.72 (0.60) | .23 | 3.04 (0.63) | <.001 | |
| Anesthesia * time | −0.02 (0.03) | .51 | −0.07 (0.03) | .039 | −0.08 (0.03) | .022 | |
| High risk (No.=29) | Intercept | 108.81 (6.19) | <.001 | 100.21 (6.56) | <.001 | 97.78 (6.10) | <.001 |
| Anesthesia duration | −0.38 (0.20) | .07 | −0.25 (0.21) | .25 | −0.35 (0.20) | .09 | |
| Time | 0.88 (1.18) | .46 | 0.75 (1.57) | .64 | 1.58 (1.03) | .15 | |
| Anesthesia * time | −0.09 (0.04) | .024 | −0.11 (0.05) | .034 | −0.13 (0.03) | .001 | |
| Average risk (No.=78) | Intercept | 99.72 (2.79) | <.001 | 95.62 (2.93) | <.001 | 83.64 (3.48) | <.001 |
| Anesthesia duration | −0.27 (0.12) | .031 | −0.22 (0.14) | .11 | −0.29 (0.16) | .07 | |
| Time | 1.76 (0.49) | <.001 | 1.10 (0.63) | .09 | 3.64 (0.65) | <.001 | |
| Anesthesia * time | −0.05 (0.02) | .022 | −0.08 (0.03) | .011 | −0.10 (0.03) | <.001 | |
Abbreviations: No: sample size; SE: standard error
Notes:
Linear mixed models were estimated for WJ III indices of overall IQ, attention, and processing speed within subgroups of younger age at diagnosis (<7years), older age at diagnosis (≥7 years), high risk treatment, and average risk treatment. Predictor variables included total cumulative duration of anesthesia (continuous in hours) within first year after diagnosis. Interactions with time included years since diagnosis.
Figure 2.
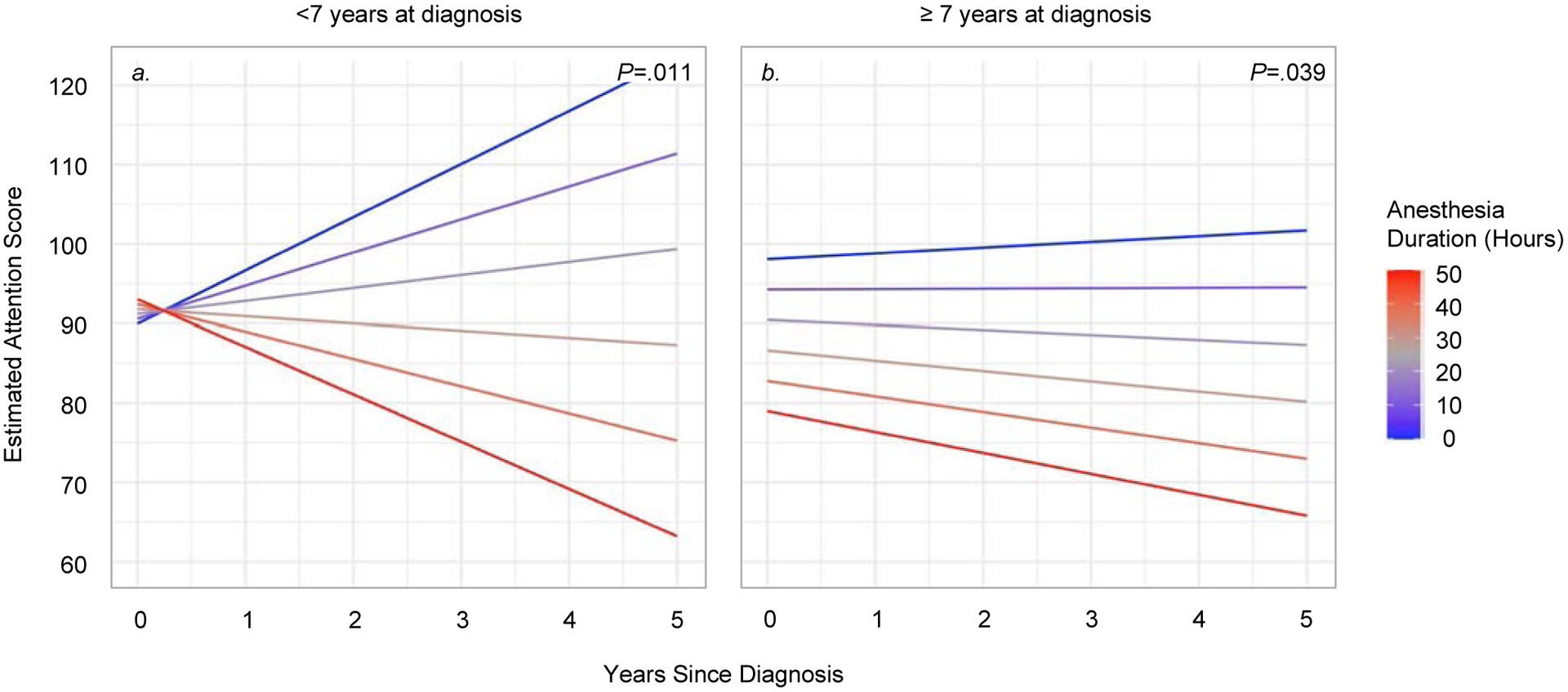
Association between time and anesthesia duration on neurocognitive decline (age groups)
Notes: Estimated performance on the WJ III Attention index is shown as age standardized norms (M=100, SD=15) within the <7 years old at diagnosis group (n=27, panel a) and ≥ 7 years old at diagnosis group (n=80, panel b). Linear mixed models showed significant interactions between time and anesthesia exposure (young age: P=.011; older age: P=.039). Colored lines represent estimated scores for: 0, 10, 20, 30, 40, and 50 hours of cumulative anesthesia exposure.
Figure 3.
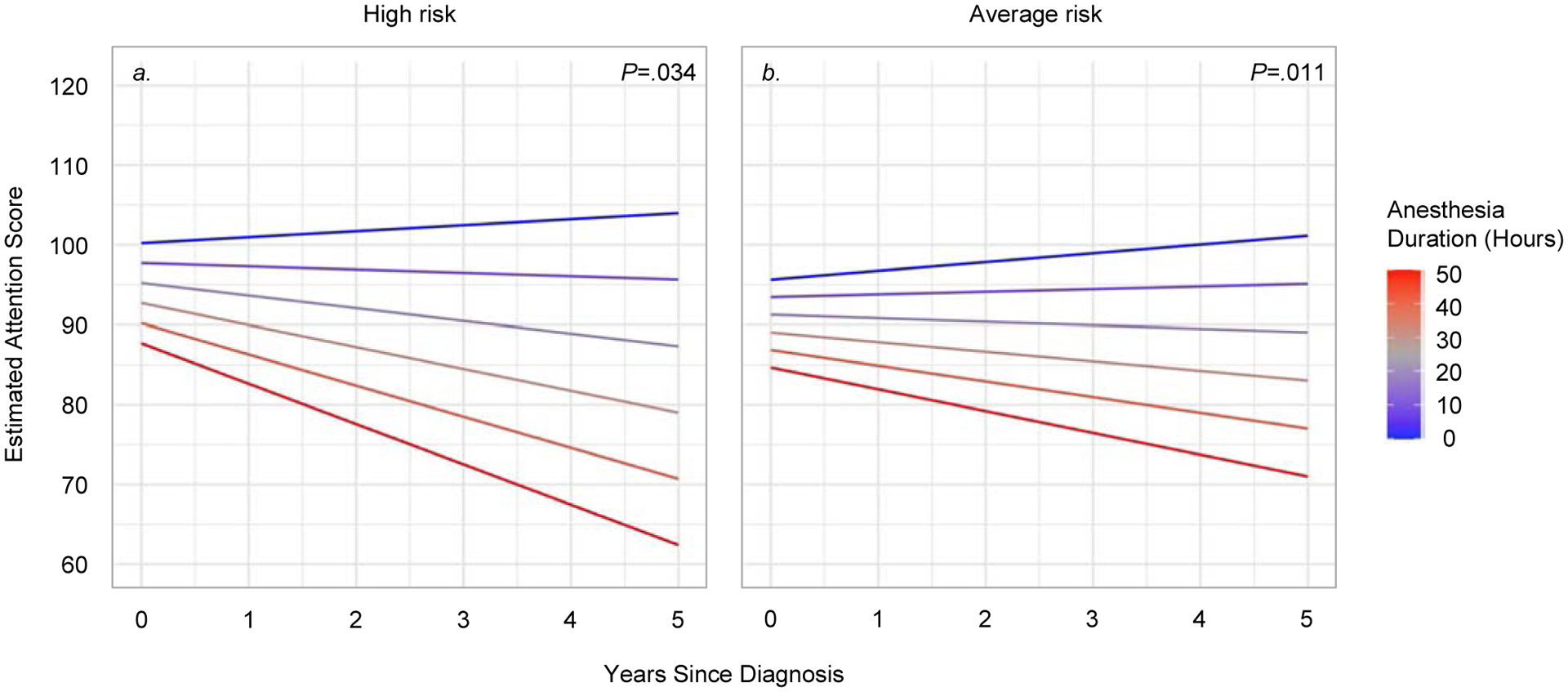
Association between time and anesthesia duration on neurocognitive decline (treatment risk groups)
Notes: Estimated performance on the WJ III Attention index is shown as age standardized norms (M=100, SD=15) within the high risk treatment group (n=29, panel a) and average risk treatment group (n=78, panel b). Linear mixed models showed significant interactions between time and anesthesia exposure (high risk: P=.034; average risk: P=.011). Colored lines represent estimated scores for: 0, 10, 20, 30, 40, and 50 hours of cumulative anesthesia exposure.
Children <7 years at diagnosis who had greater anesthesia durations showed greater declines on measures of overall IQ (P=.027) and attention (P=.011). These results did not meet significance for anesthesia frequency (P>.10). In children ≥7 years at diagnosis, greater anesthesia duration was associated with declines in attention (P=.039) and processing speed (P=.022). A main effect was shown for overall IQ, such that longer anesthesia duration was associated with poorer performance across time (P=.005). Similar results were shown for anesthesia frequency in this age group.
In patients treated for high-risk or average-risk disease, greater anesthesia durations were associated with declines in neurocognitive functioning over time. This result was illustrated across domains for high-risk (Overall IQ: P=024; Attention: P=.034; Processing Speed: P=.001) and average-risk groups (Overall IQ: P=.022; Attention: P=.011; Processing Speed: P<.001). Similar results were shown for anesthesia frequency.
Anesthesia Exposure and Neurocognitive Performance in Non-PFS Group
The non-PFS and PFS groups did not differ in age (P=.13), sex (P=.43), race (P=.87), or treatment risk (P>.99; Table A3). The non-PFS group had lower anesthesia frequency (M=16.3 vs. 31.6 events, P=.001) and duration (M=17.9 vs. 32.1 hours, P=.001) than the PFS group.
In the non-PFS group, primary indications for anesthesia included radiation therapy (47% of events, 35% of patients) and imaging (27% of all events, 82% of patients). Propofol and fentanyl were most common agents for intravenous (100% of patients) and sevoflurane for inhalation (91% of patients) methods (Table A4). Similarly to the overall group, significant correlations were shown between age and anesthesia frequency (r=−0.62, P<.001) and duration (r=−0.62, P<.001). The high-risk group had greater duration of anesthesia than the average-risk group (M=24.4 vs. 15.5 hours, P=.03); differences were marginally significant for anesthesia frequency (M=21.8 vs. 14.3 events, P=.054).
Table A5 shows results from linear mixed models in the non-PFS group. All models showed significant interactions with time. Patients who were older at diagnosis showed increased performance over time (Overall IQ: P<.001; Attention: P=.002; Processing Speed: P<.001), although graphs illustrate declines for younger children. Furthermore, declines across neurocognitive domains were shown for the high-risk group (vs. average-risk; Overall IQ: P<.001; Attention: P=.005; Processing Speed: P<.001) and for those who had greater anesthesia exposures (Overall IQ: P<.001; Attention: P<.001; Processing Speed: P<.001).
Discussion
This study examined longitudinal outcomes after exposure to anesthesia in a medically complex pediatric population. Patients were homogeneous in terms of tumor type and risk-adapted treatment, and their exposure to anesthesia was well-characterized. Results showed that survivors had declines in neurocognitive functioning, particularly if a patient was younger at diagnosis, treated for high-risk disease, or exposed to longer cumulative duration or frequency of anesthesia. Importantly, similar results for the effect of anesthesia were shown in the overall group as well as in age, treatment risk, and non-PFS subgroups. Therefore, the results suggest that anesthesia may affect neural or cognitive development with increasing time from diagnosis and treatment.
Our results are consistent with previous research, which has shown that attention and processing speed are particularly impacted following medulloblastoma treatment[2]. In clinical studies focusing on anesthesia exposure, larger doses, length, or frequency of anesthesia was associated with cognitive or learning impairments in children who needed surgery for various reasons[20–22]. Most children were exposed to anesthesia for surgical procedures in early childhood, and the frequency or duration of anesthesia was relatively lower than the current study (i.e., 1–4 events previous vs. 1–52 events current). We extend this literature by including patients who were medically complex, had a broad age range, and had varied anesthesia exposures. The current results and our previous cross-sectional study[15] suggest that multiple anesthesia exposures are associated with poorer neurocognitive functioning, in addition to known factors of young age and treatment intensity. Furthermore, lower baseline IQ was not associated with greater frequency of anesthesia exposure, suggesting that the need for anesthesia was not related to the cognitive functioning of the patient. However, large, prospective trials will be needed to determine safe limits for anesthesia dose, duration, or frequency while considering the potential confounding factors with anesthesia exposure (e.g., shunt revisions, infections).
Few investigations have used a longitudinal design to measure changes in cognitive functioning following anesthesia exposure (e.g., [23, 24]). Studies of young children receiving anesthesia for inguinal surgery showed no pre- to post-surgery decline in cognition, although the follow-up times were relatively short (4 weeks-18 months). In contrast, one group used a prospective design to evaluate infant development following excision of benign facial growths[25]; results showed decreased cognitive and motor functioning over time, but this was only shown after 3 exposures to ketamine and not 1–2 exposures.
Our results suggest that neurocognitive decline is more prominent in patients who had greater frequency or duration of anesthesia. In pediatric brain tumor, some evidence suggests that anesthesia exposure is associated with poorer IQ; this association was shown in a secondary analysis an average of 3.6 years after diagnosis[26]. We extend this literature by evaluating a longitudinal cohort of patients who completed testing up to 5 years into survivorship. Further studies will be needed to examine the potential interactions between anesthetic agent, administration method, dose, frequency/duration of exposure, and related complications in patients who have complex medical histories.
The mechanism by which anesthesia exposure could impact cognitive functioning has been explored in pre-clinical studies. Anesthesia may induce neuroapoptosis and cell death, which subsequently affects neural and cognitive development[27]. It may also selectively target the hippocampus and learning performance, although other studies have shown more diffuse changes in the brain associated with cognitive or behavioral impairment[28]. The exact mechanisms or pathways of anesthesia-induced neurotoxicity remain an area of research.
This study retrospectively extracted anesthesia records, and there was no control group available due to the nature of the study. However, it would not be feasible to recruit a control group with similar diagnoses and treatment who did not receive anesthesia. Also, anesthesia data were not available for neurosurgical procedures, as these were conducted at an outside institution. Future studies should examine anesthesia exposure through a prospective, longitudinal method to determine the onset and timing of cognitive deficits that may be associated with anesthesia and related complications. The current study completed subgroup analyses to examine outcomes in different age, treatment risk, and non-PFS groups. Due to relatively small samples, it was not possible to examine both age and treatment risk groupings together (i.e., younger high-risk vs. older high-risk). Furthermore, patients with PFS had greater anesthesia exposures and they also completed fewer baseline and year 1 assessments than those without PFS; these results align with the clinical presentation and severity of this syndrome[29]. Multi-site studies will be needed to obtain sufficient sample sizes to examine the relationship between anesthesia exposure and cognitive outcomes within separate age, treatment risk, and PFS groups. This study did not examine the impact of ototoxicity on neurocognitive performance, as there were multiple confounding variables and a relatively small sample size to examine complex interactions between variables. Previous studies have shown that hearing loss can impact cognitive functioning in pediatric brain tumor [18, 30, 31], and thus future research should include this factor (along with other complicating factors) in larger, multi-site trials. Additionally, it will be important to examine these questions in other complex medical conditions and across multiple institutions, which will determine if cognitive declines can be generalized to other conditions.
This research highlights the importance of limiting anesthesia exposure in childhood medulloblastoma, when possible and not medically contraindicated, as repetitive anesthesia exposure may negatively impact neurodevelopment. Furthermore, higher costs and risk for complications[32, 33] are other reasons to limit anesthesia in these groups. Children who receive appropriate training and practice may be able to complete procedures without exposure to anesthesia. For example, involving child life specialists[13] and incorporating play-based behavioral training[14] can be useful ways for children to practice prior to radiation treatments or imaging studies.
Supplementary Material
Highlights.
Children with medulloblastoma often receive anesthesia during treatment
Anesthesia exposure is associated with neurocognitive declines over time
Limiting anesthesia exposure, as feasible, may mitigate neurocognitive decline
Role of the Funding Source
Research support was provided by the NIH National Cancer Institute grants(s) (P30-CA21765 and GM92666 to St. Jude Children’s Research Hospital) and the American Lebanese Syrian Associated Charities (ALSAC). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official view of the sponsors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing
Deidentified participant data and study protocol will be available beginning 9 months and ending 36 months following article publication. These data will be available to investigators whose proposed use of the data has been approved by an independent review committee identified for this purpose. Requests should be directed to corresponding author; to gain access, data requestors will need to sign a data access agreement.
Declaration of interests
Authors have no conflicts of interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. The Lancet Oncology. 2006;7:813–20. [DOI] [PubMed] [Google Scholar]
- [2].Robinson KE, Fraley CE, Pearson MM, Kuttesch JF, Jr., Compas BE. Neurocognitive late effects of pediatric brain tumors of the posterior fossa: a quantitative review. J Int Neuropsychol Soc. 2013;19:44–53. [DOI] [PubMed] [Google Scholar]
- [3].Brinkman TM, Ness KK, Li Z, Huang IC, Krull KR, Gajjar A, et al. Attainment of functional and social independence in adult survivors of pediatric CNS tumors: a report from the St. Jude Lifetime Cohort Study. J Clin Oncol. 2018;36:2762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chapman CA, Waber DP, Bernstein JH, Pomeroy SL, LaVally B, Sallan SE, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J Child Neurol. 1995;10:209–12. [DOI] [PubMed] [Google Scholar]
- [5].Mulhern RK, Kepner JL, Thomas PR, Armstrong D, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1723–8. [DOI] [PubMed] [Google Scholar]
- [6].Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–9. [DOI] [PubMed] [Google Scholar]
- [7].Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol. 2017;60:2–23. [DOI] [PubMed] [Google Scholar]
- [8].FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. U.S. Food and Drug Administration; 2016. [Google Scholar]
- [9].Lin EP, Lee JR, Lee CS, Deng M, Loepke AW. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol Teratol. 2017;60:117–28. [DOI] [PubMed] [Google Scholar]
- [10].Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Research and Human Genetics. 2009;12:246–53. [DOI] [PubMed] [Google Scholar]
- [11].Zaccariello MJ, Frank RD, Lee M, Kirsch AC, Schroeder DR, Hanson AC, et al. Patterns of neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. British Journal of Anaesthesia. 2019;122:671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Clausen NG, Kahler S, Hansen TG. Systematic review of the neurocognitive outcomes used in studies of paediatric anaesthesia neurotoxicity. Br J Anaesth. 2018;120:1255–73. [DOI] [PubMed] [Google Scholar]
- [13].Scott MT, Todd KE, Oakley H, Bradley JA, Rotondo RL, Morris CG, et al. Reducing anesthesia and health care cost through utilization of child life specialists in pediatric radiation oncology. Int J Radiat Oncol Biol Phys. 2016;96:401–5. [DOI] [PubMed] [Google Scholar]
- [14].Bharti B, Malhi P, Khandelwal N. MRI customized play therapy in children reduces the need for sedation: a randomized controlled trial. Indian J Pediatr. 2016;83:209–13. [DOI] [PubMed] [Google Scholar]
- [15].Jacola LM, Anghelescu DL, Hall L, Russell K, Zhang H, Wang F, et al. Anesthesia exposure and neurocognitive outcomes in survivors of childhood medulloblastoma treated with risk-adapted protocol therapy. J Pediatr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–9. [DOI] [PubMed] [Google Scholar]
- [17].Woodcock RW, McGrew KS, Mather M. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- [18].Schreiber JE, Gurney JG, Palmer SL, Bass JK, Wang M, Chen S, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schreiber JE, Palmer SL, Conklin HM, Mabbott DJ, Swain MA, Bonner MJ, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19:1673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Andropoulos DB, Ahmad HB, Haq T, Brady K, Stayer SA, Meador MR, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Paediatr Anaesth. 2014;24:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology. 2018;129:89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fan Q, Cai Y, Chen K, Li W. Prognostic study of sevoflurane-based general anesthesia on cognitive function in children. Journal of Anesthesia. 2013;27:493–9. [DOI] [PubMed] [Google Scholar]
- [24].Yin J, Wang SL, Liu XB. The effects of general anaesthesia on memory in children: a comparison between propofol and sevoflurane. Anaesthesia. 2014;69:118–23. [DOI] [PubMed] [Google Scholar]
- [25].Yan J, Li YR, Zhang Y, Lu Y, Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. J Child Neurol. 2014;29:1333–8. [DOI] [PubMed] [Google Scholar]
- [26].Pulsifer MB, Duncanson H, Grieco J, Evans C, Tseretopoulos ID, MacDonald S, et al. Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys. 2018;102:391–8. [DOI] [PubMed] [Google Scholar]
- [27].Bilotta F, Evered LA, Gruenbaum SE. Neurotoxicity of anesthetic drugs: an update. Curr Opin Anaesthesiol. 2017;30:452–7. [DOI] [PubMed] [Google Scholar]
- [28].Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. The Journal of Neuroscience. 2003;23:876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Catsman-Berrevoets CE. Cerebellar mutism syndrome: cause and rehabilitation. Curr Opin Neurol. 2017;30:133–9. [DOI] [PubMed] [Google Scholar]
- [30].Olivier TW, Bass JK, Ashford JM, Beaulieu R, Scott SM, Schreiber JE, et al. Cognitive implications of otoxicity in pediatric patients with embryonal brain tumors. J Clin Oncol. 2019;37:1566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Orgel E, O’Neil SH, Kayser K, Smith B, Softley TL, Sherman-Bien S, et al. Effect of sensorineural hearing loss on neurocognitive functioning in pediatric brain tumor survivors. Pediatr Blood Cancer. 2016;63:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mir Ghassemi A, Neira V, Ufholz LA, Barrowman N, Mulla J, Bradbury CL, et al. A systematic review and meta-analysis of acute severe complications of pediatric anesthesia. Paediatr Anaesth. 2015;25:1093–102. [DOI] [PubMed] [Google Scholar]
- [33].Verma V, Beethe AB, LeRiger M, Kulkarni RR, Zhang M, Lin C. Anesthesia complications of pediatric radiation therapy. Pract Radiat Oncol. 2016;6:143–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


