Abstract
Randomized clinical trials are the foundation of evidence-based medicine and central to practice guidelines and patient care decisions. Nonetheless, randomized trials in heart failure (HF) populations have become increasingly difficult to conduct and are frequently associated with slow patient enrollment, highly selected populations, extensive data collection, and high costs. The traditional model for HF trials has become particularly difficult to execute in the United States (US), where challenges to site-based research have frequently led to modest US representation in global trials. In this context, the TRANSFORM-HF (Torsemide Comparison with Furosemide for Management of Heart Failure) trial aims to overcome traditional trial challenges and compare the effects of torsemide versus furosemide among patients with HF in the US. Loop diuretics are regularly used by the majority of patients with HF and practice guidelines recommend optimal use of diuretics as key to a successful treatment strategy. Long-time clinical experience has contributed to dominant use of furosemide for loop diuretic therapy, although pre-clinical and small clinical studies suggest potential advantages of torsemide. However, due to the lack of appropriately powered clinical outcome studies, there is insufficient evidence to conclude that torsemide should be routinely recommended over furosemide. Given this gap in knowledge and the fundamental role of loop diuretics in HF care, the TRANSFORM-HF trial was designed as a prospective, randomized, event-driven, pragmatic, comparative effectiveness study to definitively compare the effect of a treatment strategy of torsemide versus furosemide on long-term mortality, hospitalization and patient-reported outcomes among patients with HF.
Keywords: heart failure, diuretic, clinical trial, pragmatic
Randomized clinical trials represent the cornerstone of evidence-based medicine and provide the foundation for modern clinical practice guidelines and patient care. In the field of heart failure (HF), several clinical trials over the past 30 years have directly led to availability of multiple effective therapies for HF with reduced ejection fraction (HFrEF).(1) However, despite these landmark trials in HFrEF populations, the current model for large-scale HF clinical trials faces ongoing and escalating challenges. For example, high costs and slow enrollment associated with site-based research in the United States (U.S.) have led to minimal US representation in most large trials, generating questions regarding the generalizability of overall trial results to routine US practice.(2) To ensure future programs support the continued need for high-quality, timely, and efficient evidence generation and are conducive to robust US participation, examination and reassessment of the contemporary HF clinical trial enterprise is warranted. With a focus on trials for patients hospitalized for HF, this article will review challenges encountered by traditional trial programs and propose a conceptual framework for overcoming barriers through a pragmatic and patient-centered approach to HF trial design. To illustrate these concepts in practice, we conclude with the rationale and design of the TRANSFORM-HF (Torsemide Comparison with Furosemide for Management of Heart Failure) trial, a pragmatic, randomized, comparative effectiveness trial of torsemide versus furosemide among patients hospitalized for HF.
CHALLENGES WITH THE TRADITIONAL MODEL FOR HEART FAILURE TRIALS
Low Enrollment Rates
A recent review of phase II to phase IV HF trials in hospitalized patients from 2001-2016 demonstrated an enrollment rate of only 0.68 patients/site/month.(3) Moreover, such enrollment rates have often been lower among the subset of large global trials. For example, in the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) trial, 77 (18%) of the 436 activated sites did not enroll a single patient.(4) Among sites that enrolled ≥1 patient, the enrollment rate was 0.41 patients/site/month and >60% of sites enrolled 10 or fewer patients.(4) Although enrollment efficiency is a widespread concern, the problem is particularly severe in the U.S. Across 4 global HF trials in hospitalized patients published since 2013, the proportion of patients enrolled from North America was 8-15%.(5-8)
Reasons for slow enrollment rates and low US representation in HF trials are likely multifactorial. Traditional protocols require detailed and longitudinal data collection and a rigorous schedule of on-site patient follow-up assessments. These elements generally include testing beyond what patients would otherwise receive as standard care, introducing a potentially unappealing or infeasible burden for patients and caregivers (especially in the setting of the physical disability and comorbidities common to the HF population). Likewise, traditional protocols routinely carry with them a significant workload for enrolling sites. This sizeable workload is in the setting of diminishing incentives for site investigators to participate in clinical research. This is especially the case in the US, where investigator salaries are commonly tied to production of relative value units derived from clinical work, making the role of site investigator potentially unappealing compared with mandatory or better compensated clinical activities.(2) In a survey of investigator perceptions of research participation, 86% of respondents were less likely to perform activities that did not directly count towards their clinical work target.(2,9) Compounding the general lack of financial incentive, site investigators and coordinators typically have little academic incentive, often receiving minimal recognition and/or promotion within their home institution and inconsistent authorship on trial manuscripts or any subsequent academic output.
Low site enrollment rates have implications on overall trial costs, duration, and generalizability, but accumulating research suggests enrollment inefficiency can have deeper consequences that directly impact trial data. Data from 2 global hospitalized HF trials found that patients enrolled from centers with lower enrollment rates had higher rates of mortality and hospitalization endpoints.(4,10) In addition, these same patients from low-enrolling sites tended to have higher rates of protocol discontinuation (e.g., protocol deviations, withdrawal of consent, lost to follow-up).(4,10) Therefore, from the perspective of trial conduct, poorly enrolling sites naturally make minimal contribution towards overall recruitment targets, but may also contribute data of lesser quality or completeness.
Generalizability of Trial Results
Questions over trial generalizability to US clinical practice partly stem from the above-mentioned quantitative underrepresentation of US patients in global trials dominated by enrollment from abroad. However, these concerns are amplified by qualitative features of the limited US patients ultimately included.(11) By virtue of numerous inclusion and exclusion criteria and the requirement for rigorous longitudinal data collection and in-person visits, US patients enrolled in a hospitalized HF trial may differ substantially from US patients seen in routine clinical practice. Moreover, among US HF trials in hospitalized patients, questions of clinical trial generalizability may particularly apply to older patients, women, and racial/ethnic minorities.(11) Among trials for patients hospitalized for HF conducted between 2001 and 2016 with partial or exclusive participation from North America, the mean age of participants was 62 years compared with 73 years among US registry/community-based studies.(12) The proportion of women in such trials was only 31% relative to 50% for US epidemiologic cohorts.(12) Given potentially important biologic differences by comorbidity status, age, sex, and race/ethnicity, risks and benefits of tested therapies could conceivably vary based on these differing patient characteristics. In aggregate, these systematic differences in representation between trial and real-world cohorts may limit the ability of the traditional HF trial model to best inform care of the general HF community in the US.
High Costs
Financial Costs
The financial costs of clinical trials are a concern for study sponsors. An analysis including 7 major pharmaceutical companies and 726 clinical trials conducted across several medical conditions from 2010-2015 found that median costs for conducting a study from protocol approval to final report were US$3.4 million for phase I trials, US$8.6 million for phase II, and US$21.4 million for phase III.(13) However, these costs are generally much higher for HF trials. For example, among 138 pivotal clinical trials leading to regulatory approval from 2015 to 2016, PARADIGM-HF was the most expensive at US$347 million, compared to the median of US$19 million across all such trials.(14)
Opportunity Costs
From time of program inception to primary publication, large multicenter HF trials commonly take several years to complete. For phase II-IV HF trials, the enrollment period alone is frequently >2 years and this duration has generally increased over time.(15) The time and resources needed for site activation in the U.S. are also substantial, with institutional review board (IRB) approval tending to take >3 months and contract completion 3-6 months at many HF sites.(16) The collective duration of trial activities requires substantial sustained resource allocation from sponsors, possibly limiting investment in additional programs and limiting the number of scientific questions that can simultaneously be addressed. Patients and clinicians share this opportunity cost; the slow pace of evidence generation delays use of therapies ultimately shown to be efficacious, while in other cases may prolong exposure to routinely used interventions subsequently proven ineffective or harmful. Furthermore, long trial duration lengthens the necessary commitment from enrolling sites and oftentimes participants as well. This prolonged obligation, coupled with limited site resources and frequent exclusions against patient co-enrollment in multiple trials, has the negative consequence of increased competition between trials for the already limited pool of potential sites and patients.
THE CASE FOR PRAGMATIC RANDOMIZED TRIALS FOR HEART FAILURE
Traditional randomized HF trials may be termed “explanatory” in their design and intent.(17) Specifically, these programs have generally aimed to determine efficacy and safety of an intervention under ideal conditions, a proposition that carries the aforementioned challenges.(17,18) Although the need to assess treatment effects under real-world conditions has long been recognized, the standard approach across cardiovascular medicine has involved coupling a “positive” explanatory randomized trial with a large observational comparative effectiveness study in a more representative population. While potentially valuable for many purposes such as defining real-world uptake, treatment patterns, and possible treatment-related safety signals, these observational analyses are fundamentally unable to determine causality and confirm treatment effect. Despite sophisticated statistical methods, random treatment assignment is mandatory to eliminate inherent selection bias and confounding when patients and clinicians choose the therapy received. Across medicine, there exist several examples of discordance between results from randomized explanatory trials and observational studies of the identical therapy.(19,20) Acknowledging no substitute for randomization, pragmatic randomized trials may be the “best of both worlds,” offering potential to more accurately and efficiently determine effectiveness of interventions under real-world “usual” conditions. Moreover, in the setting of challenges associated with the traditional model for HF trials, scientific and methodologic advantages can be combined with substantial operational benefits.
RATIONALE FOR THE TRANSFORM-HF TRIAL
Use of Loop Diuretics for Heart Failure
Since furosemide gained approval from the U.S. Food and Drug Administration (FDA) in 1966, loop diuretics (e.g., furosemide, torsemide, bumetanide) have remained a cornerstone therapy for the symptomatic management of HF and treatment of congestion. Practice guidelines highlight diuretics as the primary medications to control fluid retention in HF, despite unknown effects on morbidity and mortality.(1) Specifically, the American College of Cardiology and American Heart Association provide a Class I indication for diuretics among patients who have evidence of hypervolemia, with loop diuretics the preferred agents.(1) HF treatment guidelines have changed substantially over time in many key areas to acknowledge new evidence and therapies, but the recommendations for diuretics have remained essentially unchanged for decades.
Potential Advantages of Torsemide
Although furosemide and torsemide are both generic medications of generally comparable cost, approximately 90% of patients with HF who are prescribed a loop diuretic receive furosemide.(21) This may stem from furosemide being first to market combined with long-time clinical experience. Nonetheless, despite dominant use of furosemide in HF care, accumulating data has generated the hypothesis of torsemide as the loop diuretic of choice (Table 1).(22) Potential advantages include a more favorable pharmacologic profile; compared with furosemide, torsemide is 2-4 times more potent, offers consistent 80-100% bioavailability irrespective of food intake, may provoke less hypokalemia, and carries a longer half-life and duration of effect. These collective properties suggest torsemide may be more effective and reliable in the routine management of congestion. Moreover, and perhaps less recognized, torsemide has been associated with a host of favorable effects seemingly independent of its diuretic effect.(21) Notably, these additional non-diuretic effects are consistent with the purported mechanisms by which existing guideline-directed HF therapies improve clinical outcomes. For example, pre-clinical and small clinical studies have supported the ability of torsemide to downregulate activity of the renin-angiotensin-aldosterone system through both inhibition of aldosterone release and aldosterone antagonist-like blockade of the receptor.(21,23) In addition, potentially through its effect as a neurohormonal modulator or through other properties, a series of observational and small randomized experiences have consistently shown torsemide to reduce myocardial fibrosis and foster reverse ventricular remodeling.(24,25) Such mechanistic findings have been extended to possible clinical benefits for patients with HF, where observational and small randomized trials have suggested torsemide may reduce HF hospitalization, improve functional status, and improve survival, as compared with furosemide.(26-28)
Table 1.
Potential Advantages of Torsemide Over Furosemide for Treatment of Heart Failure
| Pharmacologic Properties Better Suited for Managing Congestion |
|
| Favorable Effects on Neurohormones |
|
| Favorable Effects on Cardiac Remodeling |
|
Adapted with permission from Greene SJ and Mentz RJ.(22)
Despite basic science, pre-clinical, observational, and small randomized studies providing a rationale for preferential use of torsemide, HF research contains numerous examples where favorable mechanistic, surrogate, and limited clinical endpoint data are followed by neutral or negative results in large clinical outcome trials.(29) Indeed, although a meta-analysis suggests torsemide may reduce all-cause mortality compared with furosemide, the pooled data include only 101 total death events.(30) Thus, despite the Class I indication for use of diuretics in HF, treatment guidelines appropriately do not provide a specific recommendation for routine use of any specific agent.(1) This lack of clear evidence is paradoxical to the near ubiquitous use of loop diuretics in routine HF care. To fill this knowledge gap, the TRANSFORM-HF trial was designed as a pragmatic, prospective, randomized, comparative-effectiveness study to definitively compare the effects of torsemide and furosemide on clinical outcomes for patients with HF.
DESIGN OF THE TRANSFORM-HF TRIAL
Objectives
The primary objective of the TRANSFORM-HF trial is to determine whether a torsemide treatment strategy is superior to a furosemide treatment strategy in increasing time to all-cause mortality among patients hospitalized for HF and receiving standard care. Secondary objectives include determining whether torsemide is superior to furosemide in reducing the composite of all-cause mortality or all-cause readmission over 30 days and 12 months, reducing the total number of hospitalizations over 12 months, improving health-related quality of life over 12 months (as measured by the Kansas City Cardiomyopathy Questionnaire [KCCQ]), and improving symptoms of depression over 12 months (as measured by the Patient Health Questionnaire-2 [PHQ-2]).
Study Design
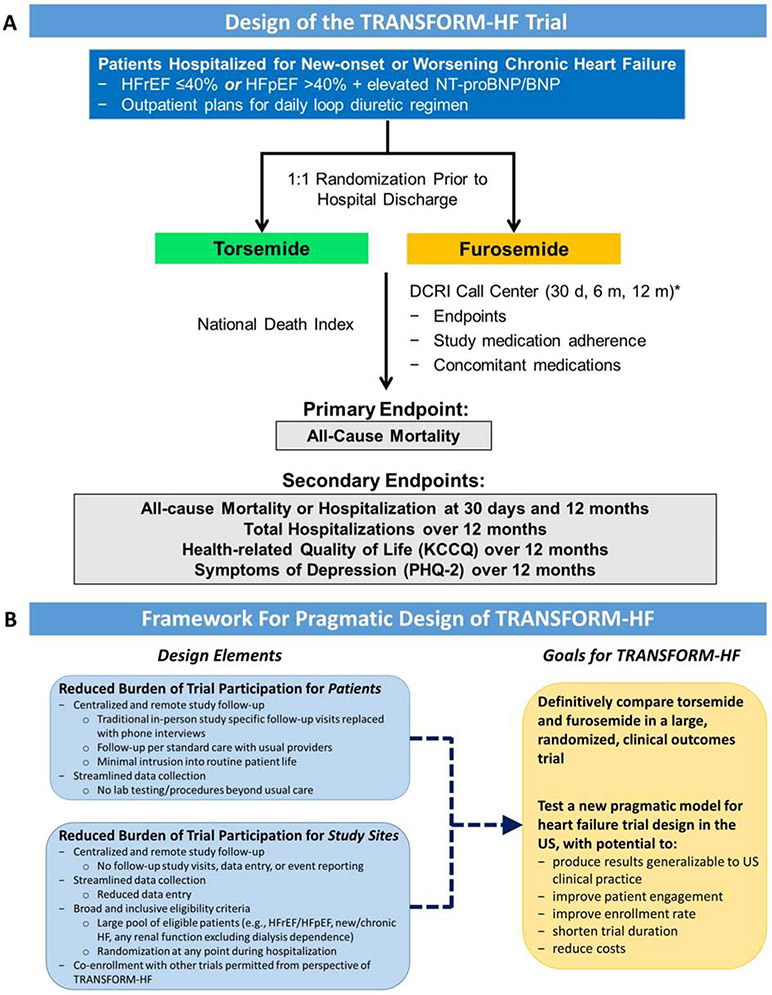
TRANSFORM-HF is an event-driven comparative-effectiveness trial. Given the unbiased nature of the all-cause mortality primary endpoint and the frequency with which loop diuretics are titrated in routine HF care, the trial is unblinded at both participant and investigator levels. Enrollment will occur entirely within the U.S. and the event-driven trial is projected to randomize up to 6,000 patients across approximately 50 sites. Patients hospitalized for HF will be randomized in a 1:1 ratio to torsemide or furosemide prior to hospital discharge, with continuation of therapy post-discharge (Central Illustration). Dosing and frequency of the randomized therapy during hospitalization and at hospital discharge will be at the discretion of local investigators. Dosing and frequency changes to the randomized therapy after hospital discharge are at the discretion of the patient’s usual outpatient clinicians.
Central Illustration. Study Design and Conceptual Framework of the TRANSFORM-HF Trial.
(A) TRANSFORM-HF is a prospective, randomized, comparative effectiveness trial designed to definitively compare the effect of a treatment strategy of torsemide versus furosemide on long-term mortality. (B) TRANSFORM-HF was designed to lower the traditional barriers for patient and site participation in HF trials and support a robust enrollment rate several fold higher than seen in prior studies. These collective strategies are intended to produce trial results widely applicable to routine US clinical practice at substantially lower cost and over a shorter timeline than traditional large HF programs. BNP, B-type natriuretic peptide; DCRI, Duke Clinical Research Institute; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT-proBNP, N-terminal pro-B-type natriuretic peptide; Patient Health Questionnaire-2, PHQ-2. *Patients enrolled early in the trial will have additional phone interviews at 6-month intervals.
TRANSFORM-HF will use a centralized follow-up procedure with no study-specific, in-person follow-up visits. All study participants will follow up on a usual care basis with routine clinical providers. Study follow-up is anchored by the Duke Clinical Research Institute (DCRI) Call Center and all participants will have phone interviews with the Call Center at 30 days, 6 months, and 12 months following randomization. Those enrolled in the early phase of the trial will have additional phone interviews at 6-month intervals. During interviews, the Call Center will collect information from participants or approved proxies regarding vital status, hospitalization events, KCCQ and PHQ-2 data, and adherence to the randomized therapy. Information gained regarding hospitalization events will be verified by the Call Center using hospitalization records. To confirm and supplement vital status data obtained by the Call Center and ensure complete capture of primary endpoint events, the National Death Index (NDI) will be searched at regular intervals.
Study Population
In summary, adult patients hospitalized for worsening or new-onset HF with anticipated long-term need for ≥1 dose of loop diuretic per day are eligible, provided they have 1) a recently documented ejection fraction (EF) ≤40% and/or 2) an elevated natriuretic peptide level during index hospitalization as measured by the local laboratory (Table 2). Thus, patients are eligible irrespective of EF, and HFpEF patients with EF >40% are eligible if they have an elevated natriuretic peptide concentration. There are no criteria regarding comorbidities, with the exception that patients with malignancy or non-cardiac conditions limiting life expectancy to <12 months and patients with end-stage renal disease requiring dialysis are excluded (given that loop diuretics are not routinely utilized in this patient population).
Table 2.
Eligibility Criteria for the TRANSFORM-HF Trial
| Inclusion Criteria |
|
| Exclusion Criteria |
|
BNP, B-type natriuretic peptide; EF, ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide
Statistical Considerations
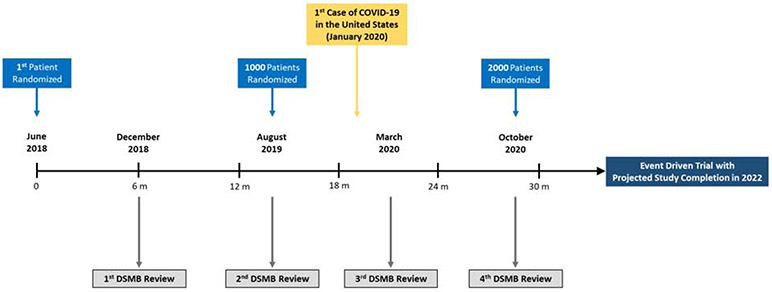
The primary analysis will be based on intention-to-treat with data obtained from the DCRI Call Center supplemented with the NDI search results. For the primary endpoint of all-cause mortality, the statistical comparison of the 2 randomized arms will be a time-to-event analysis, and therefore be based on the time from randomization to death. The Cox proportional hazards regression model will be used to assess outcome differences between the 2 treatment arms and compute a hazard ratio and 95% confidence interval. Pre-specified covariates in the primary model will include randomized treatment, age, sex, EF category (≤40% vs. >40%), and loop diuretic treatment prior to index hospital admission. TRANSFORM-HF is an event-driven trial designed to continue until at least 721 deaths (i.e., primary endpoint events) have been observed (Figure 1). Assuming 1:1 randomization, a two-side Type 1 error of 0.05, and a test statistic based on the log-rank test, 721 events would provide 85% power to detect a 20% relative reduction in all-cause mortality torsemide compared with furosemide.
Figure 1. Timeline for the TRANSFORM-HF Trial.
TRANSFORM-HF trial time lines are shown beginning from the first patient randomized. COVID-19, Coronavirus Disease 2019; DSMB, data safety and monitoring board.
With regard to secondary endpoints, analyses of the composite of all-cause mortality or all-cause hospitalization at 30 days and 12 months will be by time-to-event in a method similar to the primary endpoint analysis. Analyses of longitudinal KCCQ and PHQ-2 data will be conducted using linear mixed models. Secondary analyses will apply the worst-rank approach of Lachin et al to account for missing data related to deaths, and the test statistic will be based on a two-sample nonparametric test and the Win Ratio.(31) The total hospitalizations over 12 months secondary endpoint will be analyzed using Poisson regression and the method of Bang and Tsiatis.(32) Key supportive analyses will include analyses based on the subset of participants discharged alive on the assigned medication. Additional analyses will be presented using Bayesian statistical inference to complement the frequentist approach.(33)
Trial Organization
The TRANSFORM-HF trial organization includes a (1) Steering Committee, (2) Executive Committee, (3) study sponsor, in this case the National Heart Lung and Blood Institute (NHLBI), (4) Clinical Coordinating Center, (5) Data Coordinating Center, and (6) a DSMB (Supplemental Figure 1). An independent Data and Safety Monitoring Board (DSMB) meets approximately every 6 months to monitor enrollment, patient characteristics, trial processes and adherence to randomized therapy, and accruing endpoint data. Because both torsemide and furosemide are existing therapies within current standard of care, TRANSFORM-HF does not include any formal safety endpoints. The DSMB will utilize the Haybittle-Peto-type boundary using two-sided α=0.001 as a guideline for stopping the trial due to differences in all-cause mortality and will apply the guideline in a 2-sided symmetric fashion. Efficacy monitoring by the DSMB will include assessments when approximately 50% and 75% of primary endpoint events have accrued, and a final assessment at the end of the trial. There will be no formal futility analysis.
Pragmatic Features of TRANSFORM-HF
Aside from addressing a key scientific question for routine HF care, TRANSFORM-HF was designed to overcome obstacles that have increasingly challenged HF site-based research and trial execution (Central Illustration). The burden of study follow-up and data capture in TRANSFORM-HF is shifted centrally to the DCRI Call Center, with a) no patient requirement for in-person study-specific follow-up visits, and b) no site requirement for longitudinal data entry and event reporting after index hospital discharge. Innovative strategies for patient engagement will be a priority throughout the trial, recognizing the responsibility placed with patients for receipt of the study drug. Data collection is streamlined to capture only what is essential, representing both less data entry for enrolling sites and no additional medical testing or procedures for patients beyond usual care. In contrast to most prior hospitalized HF trials, there is no narrow in-hospital enrollment window linked to the time of initial hospital presentation. Rather, patients can be enrolled at any point throughout the hospital stay, thus increasing the pool of eligible patients and allowing site staff maximal flexibility for identifying appropriate patients and completing randomization.
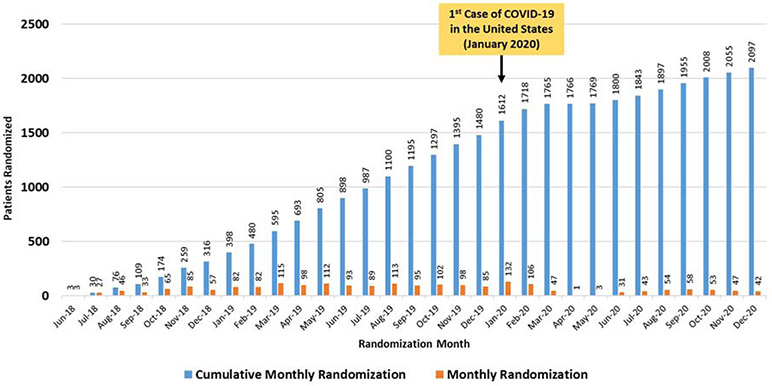
These design elements are also intended to increase enrollment among groups traditionally underrepresented in HF trials. Specifically, the relative lack of conventional comorbidity-based exclusion criteria and the reduced follow-up activities is expected to yield a high proportion of older participants. Likewise, the lack of mandatory in-person follow-up visits may better allow inclusion of patients with lower socioeconomic status, limited caregiver support, or with employment/family responsibilities. In addition, inclusion of HFpEF is expected to improve participation among women. In summary, TRANSFORM-HF was designed to lower the traditional barriers for patient and site participation in HF trials and support a robust enrollment rate several fold higher than seen in prior studies (Figure 2). These collective strategies are intended to produce trial results widely applicable to routine US clinical practice at substantially lower cost and over a shorter timeline than traditional large HF programs.
Figure 2. Cumulative and Monthly Randomization in the TRANSFORM-HF Trial.
Cumulative and monthly randomization in the TRANSFORM-HF trial are shown in the context of timing of the 1st case of COVID-19 in the United States. COVID-19, Coronavirus Disease 2019
LIMITATIONS OF PRAGMATIC HEART FAILURE TRIALS
At the current time, pragmatic trial design may be most readily applied to comparisons of existing therapies in routine use and within contemporary standard of care. For those seeking regulatory approval and labeling, the US FDA and other agencies have historically required efficacy data from an explanatory trial. Although regulatory authorities may adapt over time, the less granular data collection within a pragmatic design may not meet current data standards for confirming efficacy and safety. In addition, although a pragmatic design may favor rapid enrollment of a heterogeneous cohort, neutral results in such populations leave open the possibility that interventions could show benefit in select patient subsets. Likewise, the less stringent approach to patient monitoring and added reliance on patient-reported data could accentuate factors driving pragmatic trials results toward the null. For example, study drug non-adherence (irrespective of patient-reported adherence), failure to comply with study procedures (e.g., telephone follow-up visits), and less complete endpoint capture could reduce study power, neutralizing the magnitude of “true” treatment effect.
CONSIDERATIONS FOR COVID-19
Although the COVID-19 pandemic poses significant challenges across the spectrum of ongoing clinical trials, the pragmatic design of TRANSFORM-HF may be ideally suited for continued operations in the current environment. Specifically, the centralized follow-up procedure through the DCRI Call Center without study specific in-person follow-up mitigates concerns for heightened risk of COVID-19 transmission with patient participation. Likewise, from the study coordinator and investigator perspective, the streamlined case report form and trial protocol may require minimal on-site time for study personnel, or facilitate off-site or virtual completion of study responsibilities. Some TRANSFORM-HF sites have already successfully transitioned to virtual patient consent and data entry, highlighting the feasibility of these innovative approaches for future HF trials.
Nonetheless, TRANSFORM-HF study leadership will remain attentive to potential consequences of the COVID-19 pandemic on trial enrollment, event rates, and operations. Recent analyses have suggested that the number of cardiovascular hospitalizations in US hospitals has fallen in some health systems during the pandemic. This observation, combined with site-level reductions in clinical research activities, has reduced the TRANSFORM-HF enrollment rate compared with earlier in the trial and may affect the rate of all-cause hospitalization during follow-up. In addition, the COVID-19 pandemic may have uncertain effects on the rate of the all-cause mortality primary endpoint. Patients with HF and cardiovascular disease have a high risk of mortality when infected with COVID-19, which could increase the observed mortality rate seen in this trial. Alternatively, the trial mortality rate may be expected to decrease if enrollment rate slows and the proportion of participants in the early post-discharge phase of follow-up falls. This early post-discharge “vulnerable” phase represents the highest risk period for mortality and rehospitalization, and a higher relative contribution of patient-years of follow-up from participants surviving beyond this highest risk period could decrease the overall observed mortality rate. These considerations notwithstanding, the event-driven nature of TRANSFORM-HF offers some protection against the potential impact of the pandemic on the event rate for the primary endpoint. The trial will continue until the specified 721 deaths are reached. The independent DSMB will continue to convene as usual during the pandemic and continue to monitor enrollment, event rates, and all relevant trial processes.
CONCLUSIONS
The efficient execution of traditional HF trials remains hindered by slow patient enrollment, time-intensive protocols, extensive study-specific data collection and procedures, and high costs. Associated with these operational barriers are further concerns that results from these highly-selected trial cohorts may not be fully generalizable to real-world HF care in the U.S. Although such real-world evidence can be readily obtained from observational studies, randomization is fundamentally required to determine treatment effects. Thus, while randomized trials and real-world evidence have frequently been regarded as distinct entities, there is an increasingly apparent need to merge the rigor of randomized trials with the capacity for efficient generation of generalizable evidence. The TRANSFORM-HF trial was designed to introduce pragmatic and innovative randomized trial design to HF clinical research while addressing a fundamental, yet unanswered, clinical question: what is the best loop diuretic for routine use in HF? In this context, results of TRANSFORM-HF are expected to serve both as a model for future pragmatic HF trials, and directly inform routine clinical care and practice guidelines.
Supplementary Material
HIGHLIGHTS:
Conducting heart failure (HF) clinical trials in the United States (US) has become increasingly difficult, and frequently challenged by slow patient enrollment, highly selected patient populations, and high costs.
Furosemide is the predominant loop diuretic used in HF care, but there is insufficient evidence for guidelines to recommend routine use of a specific loop diuretic agent.
TRANSFORM-HF is a pragmatic, randomized, comparative effectiveness trial of torsemide versus furosemide among patients hospitalized for HF in the US.
TRANSFORM-HF was designed to lower the traditional barriers for patient and site participation in HF trials, support a robust enrollment rate several fold higher than prior studies, and produce results generalizable to US clinical practice.
ACKNOWLEDGEMENTS
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; The National Institutes of Health; or the U.S. Department of Health and Human Services.
FUNDING SOURCES
TRANSFORM-HF is supported through cooperative agreements from NHLBI: U01-HL125478 and U01-HL125511.
ABBREVIATIONS:
- DSMB
Data and Safety Monitoring Board
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NDI
National Death Index
- PHQ-2
Patient Health Questionnaire-2
- TRANSFORM-HF
Torsemide Comparison with Furosemide for Management of Heart Failure
Footnotes
DISCLOSURES
Dr. Greene receives research support from the American Heart Association, Amgen, AstraZeneca, Bristol-Myers Squibb, Merck, and Novartis; serves on advisory boards for Amgen and Cytokinetics; and serves as a consultant for Amgen and Merck. Dr. Mentz receives research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Eli Lilly, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Medtronic, Merck, Novartis, Relypsa, Respicardia, Roche, Sanofi, Vifor, and Windtree Therapeutics. All other authors report no disclosures.
ClinicalTrials.gov Identifier: NCT03296813
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Harinstein ME, Butler J, Greene SJ et al. Site selection for heart failure clinical trials in the USA. Heart Fail Rev 2015;20:375–83. [DOI] [PubMed] [Google Scholar]
- 3.Samman Tahhan A, Vaduganathan M, Kumar S, Okafor M, Greene SJ, Butler J. Design Elements and Enrollment Patterns of Contemporary Trials in Heart Failure With Preserved Ejection Fraction: A Systematic Review. JACC Heart Fail 2018;6:714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler J, Subacius H, Vaduganathan M et al. Relationship between clinical trial site enrollment with participant characteristics, protocol completion, and outcomes: insights from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) trial. J Am Coll Cardiol 2013;61:571–9. [DOI] [PubMed] [Google Scholar]
- 5.Teerlink JR, Cotter G, Davison BA et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Bohm M, Greene SJ et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 2013;309:1125–35. [DOI] [PubMed] [Google Scholar]
- 7.Packer M, O'Connor C, McMurray JJV et al. Effect of Ularitide on Cardiovascular Mortality in Acute Heart Failure. N Engl J Med 2017;376:1956–1964. [DOI] [PubMed] [Google Scholar]
- 8.Metra M, Teerlink JR, Cotter G et al. Effects of Serelaxin in Patients with Acute Heart Failure. N Engl J Med 2019;381:716–726. [DOI] [PubMed] [Google Scholar]
- 9.Summer R, Wiener RS, Carroll D, Sager A. Physician perception of the impact of productivity measures on academic practice. Arch Intern Med 2012;172:967–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene SJ, Hernandez AF, Sun JL et al. Influence of Clinical Trial Site Enrollment on Patient Characteristics, Protocol Completion, and End Points: Insights From the ASCEND-HF Trial (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure). Circ Heart Fail 2016;9. [DOI] [PubMed] [Google Scholar]
- 11.Greene SJ, DeVore AD, Sheng S et al. Representativeness of a Heart Failure Trial by Race and Sex: Results From ASCEND-HF and GWTG-HF. JACC Heart Fail 2019;7:980–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahhan AS, Vaduganathan M, Greene SJ et al. Enrollment of Older Patients, Women, and Racial and Ethnic Minorities in Contemporary Heart Failure Clinical Trials: A Systematic Review. JAMA Cardiol 2018;3:1011–1019. [DOI] [PubMed] [Google Scholar]
- 13.Martin L, Hutchens M, Hawkins C, Radnov A. How much do clinical trials cost? Nat Rev Drug Discov 2017;16:381–382. [DOI] [PubMed] [Google Scholar]
- 14.Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated Costs of Pivotal Trials for Novel Therapeutic Agents Approved by the US Food and Drug Administration, 2015-2016. JAMA Intern Med 2018;178:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samman Tahhan A, Vaduganathan M, Greene SJ, Okafor M, Kumar S, Butler J. Evolving Landscape of Clinical Trials in Heart Failure: Patient Populations, Endpoint Selection, and Regions of Enrollment. Curr Heart Fail Rep 2018;15:10–16. [DOI] [PubMed] [Google Scholar]
- 16.Psotka MA, Ammon SE, Fiuzat M et al. Heart Failure Site-Based Research in the United States: Results of the Heart Failure Society of America Research Network Survey. JACC Heart Fail 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 18.Mentz RJ, Hernandez AF, Berdan LG et al. Good Clinical Practice Guidance and Pragmatic Clinical Trials: Balancing the Best of Both Worlds. Circulation 2016;133:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Federspiel JJ, Anstrom KJ, Xian Y et al. Comparing Inverse Probability of Treatment Weighting and Instrumental Variable Methods for the Evaluation of Adenosine Diphosphate Receptor Inhibitors After Percutaneous Coronary Intervention. JAMA Cardiol 2016;1:655–65. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez AF, Mi X, Hammill BG et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA 2012;308:2097–107. [DOI] [PubMed] [Google Scholar]
- 21.Buggey J, Mentz RJ, Pitt B et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J 2015;169:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene SJ, Mentz RJ. Potential advantages of torsemide in patients with heart failure: more than just a 'water pill'? Eur J Heart Fail 2018;20:471–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasama S, Toyama T, Hatori T et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart 2006;92:1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez B, Querejeta R, Gonzalez A, Sanchez E, Larman M, Diez J. Effects of loop diuretics on myocardial fibrosis and collagen type I turnover in chronic heart failure. J Am Coll Cardiol 2004;43:2028–35. [DOI] [PubMed] [Google Scholar]
- 25.Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol 2007;50:859–67. [DOI] [PubMed] [Google Scholar]
- 26.Murray MD, Deer MM, Ferguson JA et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med 2001;111:513–20. [DOI] [PubMed] [Google Scholar]
- 27.Cosin J, Diez J, investigators T. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail 2002;4:507–13. [DOI] [PubMed] [Google Scholar]
- 28.Muller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV--efficacy and quality of life. Eur J Heart Fail 2003;5:793–801. [DOI] [PubMed] [Google Scholar]
- 29.Greene SJ, Mentz RJ, Fiuzat M et al. Reassessing the Role of Surrogate End Points in Drug Development for Heart Failure. Circulation 2018;138:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bikdeli B, Strait KM, Dharmarajan K et al. Dominance of furosemide for loop diuretic therapy in heart failure: time to revisit the alternatives? J Am Coll Cardiol 2013;61:1549–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lachin JM. Worst-rank score analysis with informatively missing observations in clinical trials. Control Clin Trials 1999;20:408–22. [DOI] [PubMed] [Google Scholar]
- 32.Bang H AAT Estimating medical costs with censored data. Biometrika 2000;87:329–343. [Google Scholar]
- 33.Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol 2009;62:13–21 e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.