Abstract
Introduction:
Subperiosteal orbital abscess (SPOA) is a serious suppurative complication of pediatric sinusitis. The objective of this study is to stratify patient selection into those best treated medically versus surgically based on clinical outcomes.
Methods:
This is a retrospective review of patients diagnosed with SPOA complicating sinusitis treated at a tertiary care pediatric hospital from 2002 through 2016. SPOA was diagnosed by CT scan. Characteristics evaluated include demographics, abscess size, location, and measurements, length of hospital stay, medical and surgical interventions, presenting symptoms, and complications.
Results:
A total of 108 total SPOA secondary to sinusitis patients were included. A majority, 72.2%, were male with an average age at presentation of 6.8 years. The mean ± standard deviation abscess cubic volume was 0.98 ± 1.27 cm3 (median(range) = 0.44(0.01–7.34 cm3)). With an abscess volume of .510cm3, there was a sensitivity of 71.2% and a specificity of 84.4% for needing surgical drainage. Those with large abscesses at our volume threshold were 13 times more likely to require surgery than those with small abscesses, OR: 13.41, 95%CI: 5.02–35.86, p<.001. Patients that required surgery had an abscess closer to the orbital apex with the majority, 25 (61.0%), being the most proximal to the apex, p=.004. The likelihood of surgery decreased with increased distance from the orbital apex in medial abscesses (OR:.92, 95%CI: .86–.98, p=.009).
Conclusion:
In the pediatric population, SPOA is a serious consequence of sinusitis. This study provides evidence supporting that larger abscess size is a significant risk factor for requiring surgery. The appeal of our study is that it provides evidence and support that employ clinical parameters already assessed as standard practice in evaluating these patients. In summarizing the clinical translational relevance of our study, when determining whether to treat a patient with surgery and antimicrobial/medical therapy vs. non-surgical medical therapy alone, the clinician should focus on size of.510 cm3 or larger for abscesses in any location as a relative indication for surgery.
Keywords: Subperiosteal orbital abscess, pediatric, sinusitis, surgical intervention
1. Introduction
“Never allow the sun to rise and set on an abscess!” This time-honored dictum was held sacred by surgeons for decades until the modern antibiotic era ushered in a challenge to this precept in selected cases. Subperiosteal orbital abscesses (SPOA) are one such specific scenario in otolaryngology where controversy exists. Orbital complications from sinusitis are uncommon but require prompt and efficient treatment in order to avoid serious consequences including permanent blindness. Both medical and surgical treatments are options, however, the necessity, timing and type of surgical intervention remains controversial. SPOA is a progression of periosteal cellulitis of the frontal, maxillary and ethmoid bones secondary to bacterial extension from the nasal cavity and sinuses via neurovascular foramina or bony dehiscence.
In the literature, there is much discrepancy in volume thresholds for surgical intervention versus the patient being conservatively managed with antibiotics. Using a cutoff volume of ≥1cm3 as a reference point for a large abscess with an increased likelihood of surgical intervention has been used previously1, however, in our sample there were many patients that had an abscess sizes below this value that required surgery. A preliminary analysis proposed 12/16 (75%) patients that had an abscess within 0.50–0.99cm3 still needed surgery. A more detailed analysis is necessary to pinpoint an increased likelihood of surgical intervention with the largest case series for subperiosteal abscesses secondary to sinusitis treated at a tertiary care pediatric hospital to date.
The objectives of the study were to 1) characterize pediatric patients with SPOA using demographics and proxy socioeconomic status measures 2) predict an abscess cutoff volume from computerized tomography (CT) scan measurements for surgical intervention decision making 3) use abscess characteristics measured on CT scans to predict abscess size, abscess location, and need for surgery 4) associate presenting symptoms with predicted abscess size, abscess location and need for surgery 5) assess conservative versus surgical management with predicted abscess size and location.
2. Methods
An IRB protocol (STUDY20060022) was approved to perform a retrospective chart review on patients diagnosed with SPOA secondary to sinusitis treated at a tertiary care pediatric hospital from 2002 through 2016. SPOA was diagnosed by contrasted CT scan. Patients were excluded from the study if they were over the age of 18, did not have a SPOA, if no contrasted CT scan was available, and if the CT scan was unmeasurable. Scans were deemed unmeasurable if there was not a clear reconstruction in the dimensions for measurements. Abscesses were measured on all three CT scan planes (Anteroposterior (AP), transverse, and craniocaudal) by one physician for consistency. The three dimensions were multiplied to compute cm3. An ellipsoid volume is most relevant to the volume of an abscess, however, due to the complexity of this equation and limited time in surgical decision making, cm3 is the simplest and most clinically relevant data that can be calculated quickly by radiologists’ measurements. The ellipsoid volume (4/3π·abc) was computed in addition to the cubic (cm3) volume for comparison. The length from the orbital apex to the abscess, middle of the abscess, and the orbital rim were measured on the CT scan. To adjust for the differences in the total length from apex to abscess, the apex to abscess measurement was divided by the total length from the apex to the rim.
Proxy socioeconomic status measures were used to describe the sample. Insurance type, distance to the hospital, and zip code characteristics using US Census data was collected. Each patient’s zip code information for income and poverty level were compared to the levels on a national scale. For comparison, the US median income at the time of data collection was $55,322 and the percentage of individuals at poverty level in the US was 15.1%. Patient data was dichotomized to below and above the US levels for data analysis.
Additional data collected included patient demographics, abscess size as measured on CT scan, location within orbit, and microbiology, symptoms, length of hospital stay, surgical intervention(s) including external and transnasal approaches, and prolonged antibiotics use.
Statistics were performed using SPSS version 24 with p<.05 denoting statistical significance2. Likelihood ratio, Fisher’s Exact test, Chi-Square Test, Logistic regression, receiver operating curve (ROC) curve analysis, Kruskal-Wallis test and Mann-Whitney U test were used appropriately for analysis.
3. Results
108 patients with subperiosteal orbital abscess (SPOA) secondary to sinusitis were used for analysis. Inclusion and exclusion criteria are illustrated in Figure 1. 78 (72.2%) patients were male with an average age at presentation of 6.8 years (SD = 4.5) and a median (range) of 6 years (0–17 years). The majority, 72 (66.7%), of patients were between the ages of 0–8 and 36 (33.3%) were between 9–17 years of age. There was no significant difference in the season that the patients presented to the hospital, although winter and spring that the highest frequencies, 32 (29.6%) patients each season, p=.224.
Figure 1.
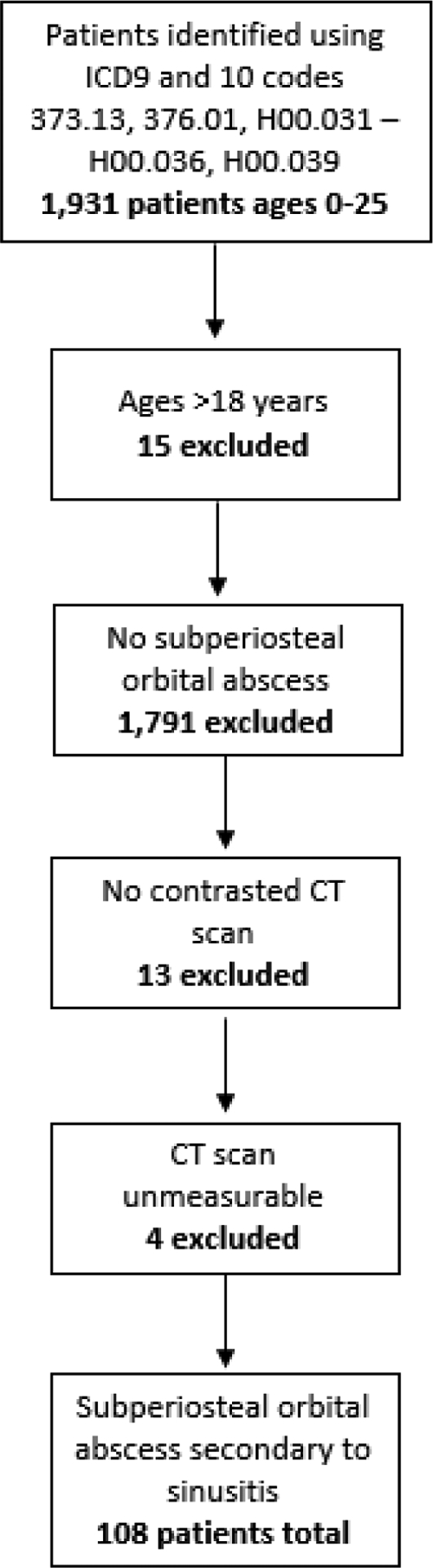
Inclusion an exclusion flowchart for subperiosteal orbital abscess in the pediatric population
54 (59.3%) of the patients had private insurance. The median (range) distance from patient zip code to UPMC Children’s Hospital of Pittsburgh was 18.5 miles (2.6–124.0 miles). Using US Census data, a majority of the patients, 67 (62.0%), lived in zip codes that were below the US median income and a third, 33 (30.6%), of the patients lived in zip codes that were above the US median poverty level.
3.1. Subperiosteal Orbital Abscess
Most of the patients, 90 (83.3%), were diagnosed with sinusitis the same day as the SPOA. A mean of 2.17 days (SD = 1.34; range = 1–6) lapsed between the date of sinusitis diagnosis and date of abscess presentation for those previously diagnosed with sinusitis (n=18). Patient history sino-nasal comorbidities were few in frequency with 14 (13.0%) having a history of environmental or seasonal allergies, 5 (4.6%) adenoidectomy, 4 (3.7%) facial trauma, 1 (0.9%) previous periorbital abscess, and 0 (0%) with sino-nasal surgery.
The mean ± SD abscess cubic volume was 0.98 ± 1.27 cm3 (median(range) = 0.44(0.01–7.34 cm3)). When calculating the ellipsoid volume from these statistics for reference, the mean ± SD was 4.10 ± 5.32 (median(range) = 1.83(0.03–30.76). Using ROC curve analysis, we assessed an abscess volume cutoff value in order to anticipate surgical intervention, AUC = .813, 95%CI: .730–.896, p<.001. With an abscess volume of .510cm3, there was a sensitivity of 71.2% and a specificity of 84.4%. We did not have any volumes between .495 and .525 cm3, therefore a reference of .500cm3 was denoted for small (<.500cm3) and large (≥.500cm3) abscesses. 42/49 (85.7%) patients with an abscess volume at and over .500cm3 required surgical intervention, whereas 17/55 (30.9%) patients needed surgery with a volume of less than .500cm3. Those with large abscesses at our volume threshold were 13 times more likely to require surgery than those with small abscesses, OR: 13.41, 95%CI: 5.02–35.86, p<.001.
A majority of abscesses were medial within the orbit, 84/108 (77.8%), half needed surgical intervention, 59/108 (54.6%) and were considered small in our study, 55/104 (52.9%). One scan dimension was missing for 4 patients and size could not be computed for these patients. Abscess characteristics measured on CT scans are shown in Table 1. Statistical significance was computed for small versus large abscess, no OR versus OR, and medial versus non-medial abscesses. Of the non-medial abscesses, 2 (8.3%) were inferior, 2 (8.3%) were lateral, and 20 (83.3%) were superior. Those with large abscesses were more likely to have involvement with the medial rectus muscle, p=.027. There were no differences between small and large abscesses in terms of ethmoid air cell involvement, whether the abscess reached the orbital apex, or if there was preseptal swelling, p>.05. Large abscesses were significantly closer to the orbital apex (mm) than smaller abscesses, p=.001. 24 (66.7%) large abscesses were in the first third, most proximal to the apex, whereas a majority, 30 (68.2%), of small abscesses were in the second third, p=.001. Similar results were found between those who went to the OR versus conservative management. Patients with surgery had a significantly higher percentage of medial rectus muscle involvement, p=.017. Surgical patients had an abscess closer to the orbital apex with the majority, 25 (61.0%), being the most proximal to the apex, p=.004. Figure 2 shows how the likelihood of surgery decreases with increased distance from the orbital apex in medial abscesses, OR:.92, 95%CI: .86–.98, p=.009.
Table 1.
Characteristics of subperiosteal orbital abscesses on CT scans
| Small (<.500cm3) n=55 | Large (≥.500cm3) n=49 | p value | No OR n=49 | OR n=59 | p value | Medial n=84 | Non-Medial n=24 | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Length (AP dimension), cm, M (SD) | 1.08±0.33 | 2.08±0.67 | <.001 | 1.14±0.37 | 1.87±0.75 | <.001 | 1.58±0.74 | 1.40±0.60 | .358 |
| Width (Transverse dimension), cm, M (SD) | 0.31±0.12 | 0.68±0.47 | <.001 | 0.31±0.12 | 0.61±0.46 | <.001 | 0.39±0.19 | 0.77±0.64 | .002 |
| Thickness (Craniocaudal dimension), cm, M (SD) | 0.72±0.27 | 1.32±0.48 | <.001 | 0.83±0.33 | 1.13±0.55 | .003 | 1.09±0.50 | 0.70±0.29 | <.001 |
| Involved Ethmoid Air Cells, n (%) | 43/52 (82.7%) | 38 (84.4%) | .519 | 36/44 (81.8%) | 45/53 (84.9%) | .445 | 66/80 (82.5%) | 15/17 (88.2%) | .435 |
| Involved Intramedial Rectus, n (%) | 2/52 (3.8%) | 8/45 (17.8%) | .027 | 1/44 (2.3%) | 9/53 (17.0%) | .017 | 9/80 (11.3%) | 1/17 (5.9%) | .444 |
| Reached Apex, n (%) | 2/55 (3.6%) | 5/46 (10.9%) | .151 | 2/49 (4.1%) | 5/56 (8.9%) | .277 | 7/84 (8.3%) | 0/21 (0.0%) | .199 |
| Preseptal Swelling, n (%) | 54/55 (98.2%) | 46/46 (100%) | .545 | 48/49 (98.0%) | 56/56 (100%) | .467 | 83/84 (98.8%) | 21/21 (100%) | .800 |
| Measurements for medial abscesses with coronal CT dimension | |||||||||
| n=44 | n=36 | n=43 | n=41 | ||||||
| Total length from apex to rim (mm), M±SD | 36.31±4.48 | 38.40±4.91 | .070 | 36.77±4.20 | 37.84±5.10 | .301 | – | – | – |
| Length from apex to middle of abscess (mm), M±SD | 21.05±7.40) | 21.80±5.18 | .608 | 20.98±7.29 | 21.82±5.47 | .720 | – | – | – |
| Length from apex to abscess (mm), M±SD | 15.87±7.81 | 10.47±6.54 | .001 | 15.78±7.58 | 11.28±7.15 | .005 | – | – | – |
| Apex to abscess divided by total length from apex to rim, M±SD | 0.43±0.20 | 0.27±0.17 | <.001 | 0.43±0.19 | 0.30±0.18 | .003 | – | – | – |
| Apex to abscess divided by total length from apex to rim dichotomized into thirds, n (%) | – | – | – | ||||||
Abbreviations: AP, anteroposterior; M, mean; SD, standard deviation;
Figure 2.
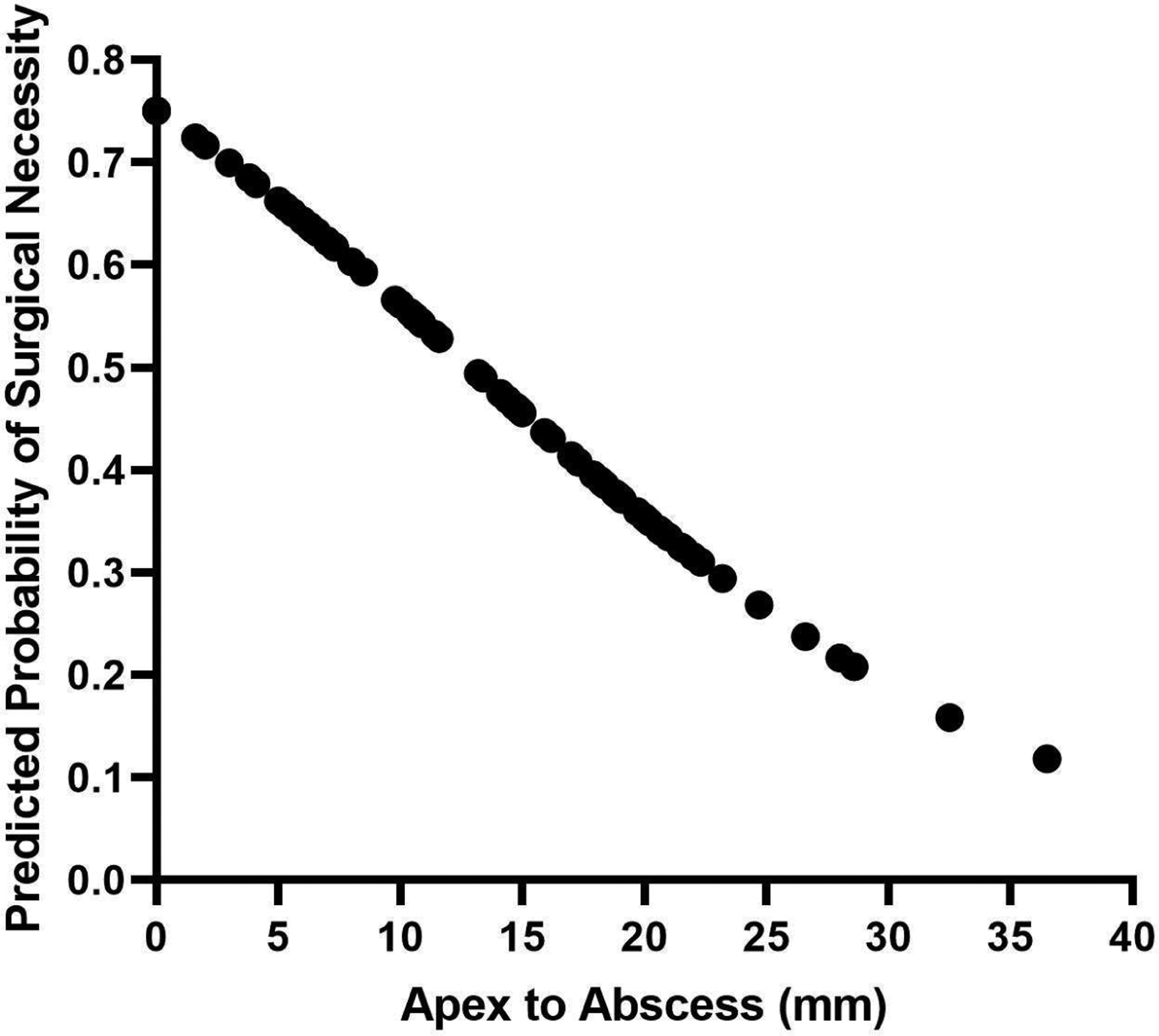
The likelihood of surgery decreases with distance from orbital apex for medial abscesses (n=84)
Those with non-medial abscesses were 3 times were more likely to need surgery than medial, (75.0% versus 48.8%; OR: 3.15, 95%CI:1.14–8.71, p=.027). There was no difference in likelihood between abscess location and size, p=.431. In a multivariable model, non-medial abscess location and larger abscess size significantly predicted the need for surgery, p<.001. When assessing only small non-medial abscesses, 54.5% of patients still required surgical intervention, compared to only 25% of small medial abscesses.
3.2. Symptoms
Abscesses were present in left and right eyes equally, 54 (50%). There were no differences in intra-ocular pressure in the abscess eye for small versus large abscesses (M ± SD = 23.29 ± 8.46 versus 22.94 ± 8.24) mm Hg), p=.955, conservative versus surgical management (M ± SD = 20.57 ± 5.26 versus 23.80 ± 8.81 mm Hg), p=.562, or medial versus non-medial (M ± SD = 23.36 ± 7.48 versus 22.14 ± 11.08 mm Hg), p=.302.
Figure 3 depicts dichotomized abscess size, surgical necessity, and abscess location with presenting symptoms including fever, conjunctival injection, chemosis, proptosis, impaired vision, restricted mobility, pain with extraocular movements (EOM), and photophobia. In addition, 99.1% of patients had erythema, 100% had a swollen eye, and 0% had hypoglobus upon presentation. Significant differences are displayed with an asterisk and a p value. Large abscesses were more likely to be presented with chemosis, proptosis, and impaired compared to small abscesses, p<.05. Patients who were surgically managed also were more likely to present with chemosis, proptosis, and impaired vision with the addition of restricted mobility and pain with extraocular movements compared to conservative management, p<.05. Non-medial abscesses were more likely to present with chemosis and restricted mobility, p<.05.
Figure 3.
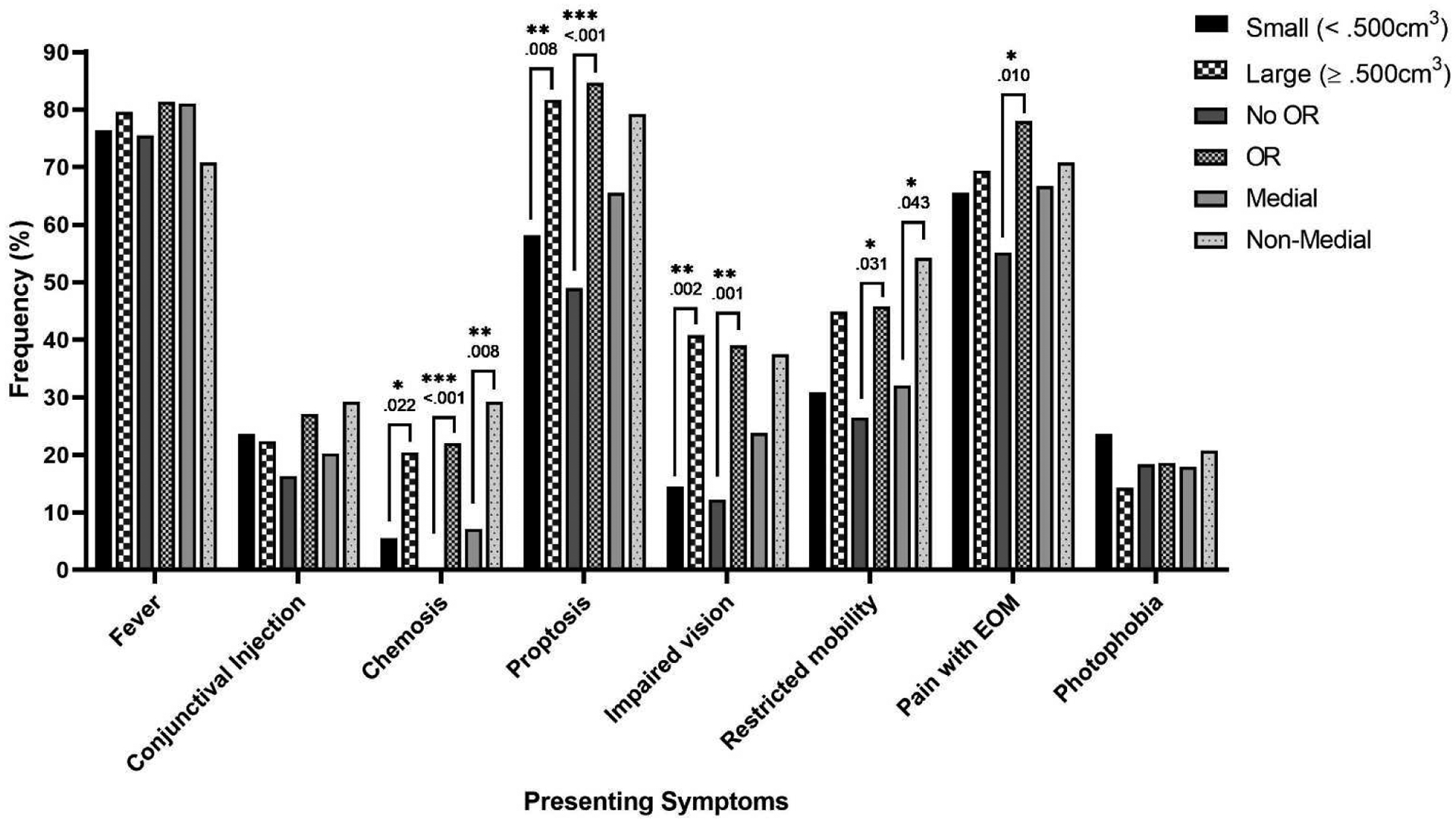
Subperiosteal orbital abscess presenting symptoms and abscess size, surgical necessity, and location
*p<.05, **p<.01, ***p<.001
Abbreviations: EOM, Extraocular movements
3.3. Microbiology
Of those patients with surgical intervention (n=59), 46 (78.0%) patients had positive microbiology results, 12 (20.3%) were negative, and 1 (1.7%) patient was not cultured or missing results from the chart. 34/46 (73.9%) positive cultures were single strain (Streptococcus (n=25/34, 73.5%), Staphylococcus (n=6/34, 17.6%), Haemophilus influenzae (n=2/34, 5.9%)), Eikenella corrodens (n=1/34, 2.9%) and 12/46 (26.1%) cultures were multiple strains with combinations of Streptococcus, Staphylococcus, Haemophilus influenzae, Eikenella corrodens, Aggregatibacter aphrophilus, Moraxella catarrhalis, and Prevotella. All 46 patients with positive microbiology were aerobic cultures and only 3 (6.5%) cultures were also anaerobic positive (Streptococcus (n=2) and Prevotella (n=1)).
Including the whole sample, 4/7 (57.1%) patients that also had a respiratory culture performed were positive (75% with surgical intervention). Out of the 51 total blood cultures performed, there were five positives (60% with surgical intervention). Haemophilus influenzae, Eikenella corrodens, Streptococcus pneumoniae, Streptococcus pyogenes, Aggregatibacter aphrophilus, and Staphylococcus aureus were found.
3.4. Conservative versus surgical management
The average ± SD length of stay (LOS) was 4.01 ± 2.43 days (median(range) = 3(1 – 22) days). Patients who went to the OR had a longer LOS compared to conservative management (M ± SD = 4.85 ± 2.94 versus 3.00 ± 0.89 days), p<.001. Patients with non-medial abscesses had a longer LOS than those with medial, p<.001, however there were no differences when LOS and abscess size were analyzed, p=.067. Surgical patients stayed an average of 3.81 (SD=2.97) days after the surgery (median(range) = 3(1 – 21)).
Surgical interventions included transnasal, 32 (54.2%), external, 4 (6.8%), and a combined transnasal + external approach, 23 (39.0%). 24/59 (40.7%) patients had a drain placement: 2/32 (6.3%) transnasal, 3/4 (75.0%) external, and 19/23 (82.6%) combined.
Roughly three-quarters of those with and without surgery sought out prior medical attention from an outside hospital (OSH) or pediatrician before arriving to our hospital (43 (72.9%) with surgery versus 39 (79.6%) without). There were no patients on saline or Afin sprays prior to diagnosis at our hospital. Only one patient was on a steroid spray and this was prescribed prior to presenting symptoms. Of those who were on previous oral or intramuscular injection (IM) antibiotics before presenting to our hospital (n=43), 20/43 (46.5%) still required surgery compared to 39/65 (60.0%) who were not on oral or IM and required surgery, p=.119. There was also no difference in number of days patients were on oral or IM antibiotics for first line of treatment between those who required surgery versus conservative management at our hospital (surgical versus non-surgical median (range) = 1.5 (1–3) versus 1.0 (1–4) days). Of those that had oral or IM antibiotics previously, 8/43 (18.6%) of these patients also had prior intravenous (IV) antibiotics for an average of 0.63 (SD=0.52) days before being transferred to our hospital. 28 (25.9%) total patients had IV antibiotics and were transferred from an OSH. Of these patients, 19/28 67.9% of them needed surgery and 9/28 (32.1%) patients were conservatively managed at our hospital. Including all patients on prior IV antibiotics, there was an average of 0.20 (SD=0.58) days before being transferred to our hospital with 12/28 (42.9%) being transferred to our hospital the same day. Only 4/108 (3.7%) patients who had both prior oral and IV antibiotics required surgery. These four abscesses were large with 75% being medial.
Half of the patients who had surgery were on previous antibiotics (any form) before arriving to the hospital (n=29, 49.2%). Of these patients, over half, 17 (58.6%) had IV antibiotics at our hospital for a median (range) of 1 (1–4) days before surgery. The remaining twelve patients (41.4%) had surgery the same day as admission.
Table 2 shows the breakdown of antibiotic use for abscess size, medical management, and abscess location groups. Patients who had large abscesses were on IV antibiotics including inpatient (IP) and at an OSH longer and were on antibiotics including both oral and IV at IP and OSH longer than those that had small abscesses, p=.007 and p=.011, respectively. Those that went to the OR compared to conservatively managed were on IV antibiotics during inpatient longer (p<.001), on IV antibiotics including both IP and OSH longer (p<.001), and on both oral and IV antibiotics, p<.001. Those with non-medial abscesses had similar LOS results to those that went to the OR. Of those who went to the OR, patients with large abscesses were on IV antibiotics a significantly shorter period until surgery compared to patients with small abscesses, p=.013. Table 3 outlines descriptive statistics antibiotic use of conservative versus surgical management for both abscess size and location.
Table 2.
Prior antibiotic use before presentation to our hospital; median (range)
| Small (<.500cm3) n=55 | Large (≥.500cm3) n=49 | p value | No OR n=49 | OR n=59 | p value | Medial n=84 | Non-Medial n=24 | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Days on IV abx in our hospital | 4 (2 – 9) | 5 (3 – 22) | .055 | 4 (2 – 6) | 5 (3 – 22) | <.001 | 4 (2–10) | 6 (3–22) | .001 |
| Days on both prior IV and oral abx from OSH | 1 (0 – 4) | 1 (0 – 4) | .860 | 1 (0 – 4) | 0 (0 – 4) | .872 | 1 (0–4) | 0 (0–3) | .255 |
| Total days on abx IV only (Both OSH and IP) | 4 (2 – 9) | 5 (3 – 22) | .007 | 4 (2 – 7) | 6 (3 – 22) | <.001 | 4 (2–10) | 6 (3–22) | <.001 |
| Total days on all abx OSH and IP | 5 (2 – 9) | 6 (3 – 22) | .011 | 5 (2 – 8) | 6 (3 – 22) | <.001 | 5 (2–10) | 6 (3–22) | .004 |
| OR Patients Only | |||||||||
| n=17 | n=42 | n=59 | n=41 | n=18 | |||||
| Days IV abx IP from admission to OR | 1 (0 – 6) | 0.5 (0 – 3) | .013 | – | 1 (0 – 6) | – | 1 (0 – 4) | 1 (0 – 6) | .153 |
| Days all IV abx to OR | 1 (0 – 6) | 1 (0 – 4) | .144 | – | 1 (0 – 6) | – | 1 (0 – 4) | 1 (0 – 6) | .284 |
| Total days on all abx IP and OSH to OR | 2 (0 – 6) | 2 (0 – 4) | .073 | – | 2 (0 – 6) | – | 2 (0 – 5) | 2 (0 – 6) | .710 |
| Days OR to discontinued IV† | 3 (2 – 6) | 3 (1 – 21) | .795 | – | 3 (1 – 21) | – | 3 (2 – 9) | 4 (1 – 21) | .034 |
Abbreviations: IV, intravenous; IP, inpatient; abx, antibiotics; OSH, outside hospital
Inpatient, not including PICC line
Table 3.
Conservative versus surgically managed patient antibiotic use for both abscess size and location, n (%)
| Abscess Size | Abscess Location | |||
|---|---|---|---|---|
| Small n=55 | Large n=49 | Medial n=84 | Non-Medial n=24 | |
| Conservatively managed with IV abx therapy in hospital - No OR (n=49) | ||||
| n=38 | n=7 | n=43 | n=6 | |
| No prior abx (n=21) | 16/19 (84.2%) | 3/19 (15.8%) | 17/21 (81.0%) | 4/21 (19.0%) |
| No oral but prior IV (n=5) | 3/4 (75.0%) | 1/4 (25.0%) | 5/5 (100%) | 0/5 (0%) |
| Prior oral but no IV (n=19) | 16/18 (88.9%) | 2/18 (11.1%) | 18/19 (94.7%) | 1/19 (5.3%) |
| Prior oral and IV (n=4) | 3/4 (75.0%) | 1/4 (25.0%) | 3/4 (75.0%) | 1/4 (25.0%) |
| Surgical intervention needed - OR (n=59) | ||||
| n=17 | n=42 | n=41 | n=18 | |
| No prior abx to OR (n=9) | 0/9 (0%) | 9/9 (100%) | 7/9 (77.8%) | 2/9 (22.2%) |
| No oral but prior IV before OR (n=30) | 10/30 (33.3%) | 20/30 (66.7%) | 19/30 (63.3%) | 11/30 (36.7%) |
| Prior oral but no IV before OR (n=6) | 2/6 (33.3%) | 4/6 (66.7%) | 6/6 (100%) | 0/6 (0%) |
| Prior oral and IV before OR (n=14) | 5/14 (35.7%) | 9/14 (64.3%) | 9/14 (64.3%) | 5/14 (35.7%) |
Abbreviations: IV, intravenous; Abx, antibiotics
3.5. Discharge Medications and Surgical Complications
Table 4 shows patient frequencies and significance value for medications prescribed at discharge and complications. Patients with large abscesses and those who had surgery were more likely to be discharged home with a peripherally inserted central catheter (PICC) line for antibiotics compared to their counterparts, p=.016 and p=.001, respectively. There were no increased likelihoods for surgical complications between abscess size, surgical necessity, and abscess location groups, p>.05. The two patients who were treated with antibiotics initially and needed their first surgery at a later admission had small abscesses that were medial. There was an overall success rate of 89.8% determined by the need for initial or additional surgery. Success rates for abscess size and surgical necessity are seen in Figure 4, p=.014. Kruskal-Wallis test with Bonferroni correction for multiple tests was used for analysis.
Table 4.
Medications prescribed upon discharge and complications, n (%)
| Small (<.500cm3) n=55 | Large (≥.500cm3) n=49 | p value | No OR n=49 | OR n=59 | p value | Medial n=84 | Non-Medial n=24 | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Abx at home | 53 (96.4%) | 41 (83.7%) | .053 | 49 (100%) | 49 (83.1%) | .003 | 79 (95.2%) | 19 (79.2%) | .026 |
| Abx PICC at home | 2 (3.6%) | 9 (18.4%) | .016 | 0 (0%) | 11 (18.6%) | .001 | 6 (7.1%) | 5 (20.8%) | .064 |
| Steroids | 1 (1.8%) | 3 (6.1%) | .252 | 1 (2.0%) | 3 (5.1%) | .368 | 2 (2.4%) | 2 (8.3%) | .205 |
| Oxymetazoline | 46 (83.6%) | 38 (77.6%) | .549 | 40 (81.6%) | 48 (81.4%) | .381 | 71 (84.5%) | 17 (70.8%) | .262 |
| Nasal steroids | 29 (52.7%) | 22 (44.9%) | .346 | 24 (49.0%) | 29 (49.2%) | .500 | 41 (48.8%) | 12 (50.0%) | .500 |
| Surgical complications | 5 (9.1%) | 6 (12.2%) | .418 | 2 (4.1%) | 9 (15.3%) | .053 | 8 (9.5%) | 3 (12.5%) | .460 |
| Additional surgery needed | 3 (5.5%) | 6 (12.2%) | .190 | – | 9/9 (100%) | – | 6/8 (75.0%) | 3/3 (100%) | .319 |
| First surgery needed in later admission | 2 (3.6%) | 0 (0%) | .277 | 2/2 (100%) | – | – | 2/8 (25.0%) | 0 (0%) | .603 |
| Neurologic sequalae | 0 (0%) | 0 (0%) | – | 0 (0%) | 0 (0%) | – | 0 (0%) | 0 (0%) | – |
| Additional admission | 2 (3.6%) | 4 (8.2%) | .286 | 2 (4.1%) | 4 (6.8%) | .433 | 6 (7.1%) | 0 (0%) | .212 |
Figure 4.
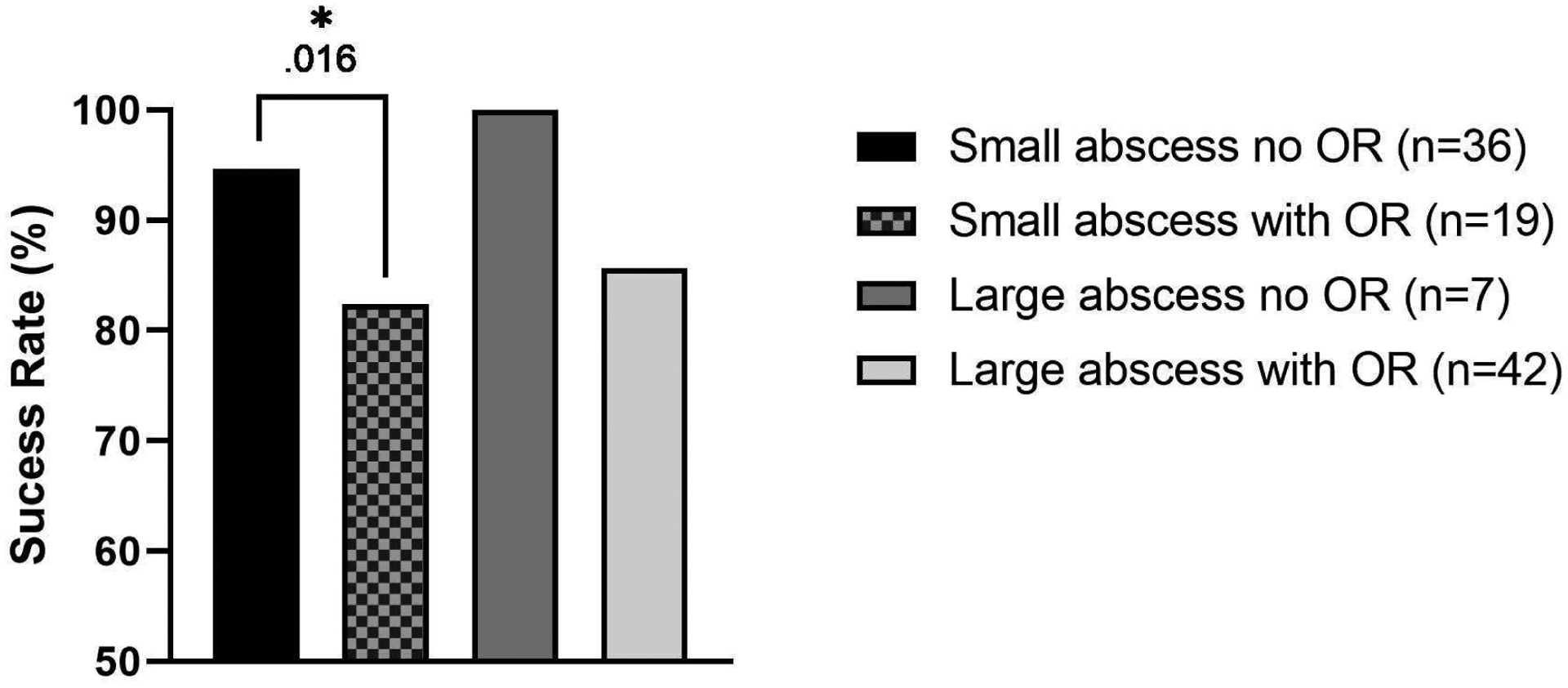
Success rates of small and large subperiosteal orbital abscesses with and without surgery
4. Discussion
Only 6% of pediatric patients presenting to our hospital with an orbital infection, such as cellulitis, had a subperiosteal orbital abscess (SPOA) in a span of 14 years. Subperiosteal and orbital abscesses are a serious consequence that can lead to dangerous complications, including neurologic sequalae leading to death. A recent meta-analysis by Adil et al. outlined SPOA secondary to sinusitis studies from the last thirty years3. While they have established that abscess volume is the most predictive variable for surgical intervention, the authors concluded that more detailed data regarding radiology scans and ophthalmology are necessary to better understand failed conservative treatment3. Our study is detailed in that it predicts surgical intervention, abscess location, and abscess volume from CT scan measurements, and presents data for presenting symptoms, microbiology, and prior exposure to antibiotics before surgery. An abscess reference volume was predicted from ROC curve analysis for surgical intervention decision making in a large sample size for the given population over 14 years.
The lowest frequency of patients presented to our hospital in the summer (17.6%) compared to 23.1% in the fall and 29.6% in both the spring and winter. Due to sinusitis coinciding with respiratory illnesses in the winter and early spring, this distribution is expected. Nageswaran et al. found 56% of pediatric patients with orbital cellulitis secondary to sinusitis were presented from October to March4.
Socioeconomic status and health disparities are well researched in the literature5. Regarding proxy socioeconomic status (SES) measures, 60% of our patients had private insurance, while over half (62%) lived in zip codes that had a median income below the US median income ($55,322), as well as a third of patients living in zip codes greater than the US median poverty level (15.1%). Although we did not go into detail regarding socioeconomic discrepancies, it has been described in the literature that children with lower socioeconomic status have an increased risk of infection for neck abscesses6,7 and orbital and intracranial infection8,9. Due to our finding that a majority of pediatric patients presenting with SPOA come from low SES communities, increased awareness in infection symptoms for prevention and early intervention is critical for these populations.
A subperiosteal orbital abscess is most closely shaped to an ellipsoid10. Optimally the ellipsoid volume formula (4/3π·abc) would be used, however, this is a complicated equation that is arduous and not useful for real-time decision making. Using given radiological findings is more relevant for clinical decision making, regardless of its imperfections. The method we used is consistent with the literature11.
Ryan et al. characterized a large abscess as >10 mm minimum width in pediatric patients. Their institution’s radiology department uses this measurement as a volumetric reference point to define a large abscess. This study had 47 patients treated conservatively and 21 by surgery and determined that those with larger abscesses had an increased likelihood of surgical intervention compared to smaller abscesses (92% versus 19%). The authors also determined extraocular muscular involvement did not increase surgical likelihood1. However, if we used a similar measurement of >1cm3 as a reference for surgical intervention in our larger sample of 108 patients, a comparative 91% of those with large abscesses needed surgery but a much higher percentage with small abscesses necessitated surgery, 41%, with 75% of patients with abscess sizes 0.5–1cm3 needing surgery. Based on these findings, a predicted abscess cutoff was calculated using ROC curve analysis to facilitate a reference point for anticipated surgical intervention.
Other studies in the literature have investigated surgical volume and the need for surgical intervention in addition to the surgical approach, transnasal, external, or a combined approach12. However, this study is the first to assess the likelihood of surgery and volumetric references for small and large abscesses with specific measurements on CT scans i.e. the distance from the orbital apex to the abscess. We found that medial abscesses closer to the orbital apex were more likely to be large and require surgery, with the likelihood of surgery increasing as the abscess approaches the orbital apex. The finding of large abscesses requiring surgery is consistent with the literature10–14, however our apex-to-abscess measurement is novel. These two factors, however, are not clinically independent if one considers the anatomy and physics of abscess spread in the orbit. Once ethmoiditis by direct extension violates the lamina papyracea and enters the orbit, the path of least resistance for posterior extension is towards the orbital apex. Hence, the larger a medial orbital abscess, the closer to the orbital apex it becomes.
In contrast, non-medial location is likely to serve as an independent risk factor predicting the need for surgical intervention as even 54.5% of smaller non-medial abscesses required surgical intervention. Multivariable analysis of non-medial location and larger abscess size were the most likely to require surgery (p<.001). Our study is the first to report this multivariable surgical risk and is very practical in supporting clinical decision making. In medial abscesses where volume averaging and other practical limitations for precisely determining size are present, proximity to the orbital apex is a reasonable surrogate to also consider albeit not an independent risk factor. Finally, non-medial abscess location of any size should shift the risk-to-benefit shared decision making towards surgery understanding that support for surgery is most compelling for non-medial abscesses that are also large.
Presenting signs and symptoms often determine the clinical course at the hospital and outcomes related to each symptom are important to digest. Interestingly, patients that needed surgery but not those with large abscesses presented to the emergency department with restricted orbital mobility and pain with extraocular movements (EOM) significantly more frequently than their counterparts, those who did not need surgery or had small abscesses. Non-medial abscesses also presented to our hospital with restricted orbital mobility. Along with the result that abscesses found further away from the apex are less likely to require surgery, perhaps larger, medial abscesses that do not present with restricted mobility and pain with EOM can be conservatively managed.
Positive microbiology results were found in 78% of abscess cultures from surgery with a majority being single strain cultures. Interestingly, only 3 (6.5%) cultures were anaerobic positive from patients ages 10, 14, and 15 years old. Liao and Harris also found that anaerobes were only isolated from patients 9 years and older and this age population has an increased variety of pathogens found15. Brook performed a review in 2016 on the microbiology of SPOA secondary to sinusitis and discussed the evolution of positive bacteria cultured due to the current era of increased resistance to antibiotics16.
Most of the patients presenting to UPMC Children’s Hospital of Pittsburgh had sought out medical advice prior to admission from an outside hospital or pediatrician’s office for their SPOA with 43 (39.8%) patients on oral or IM antibiotics and 28 (25.9%) patients on IV antibiotics previously. Regardless of prior antibiotic use, half of the patients still required surgical intervention. Those with large abscesses, who were surgically managed, and with non-medial abscesses all were on antibiotics longer than their counterparts. In a smaller cohort of 39 patients, Sinclair and Berkowitz found only 26% of pediatric patients received antibiotic therapy before admission17. They concluded that antibiotic therapy was not warranted when sinusitis was presented for a short duration and SPOA may be unavoidable17. Interestingly, none of the patients were conservatively managed with saline spray or oxymetazoline prior to diagnosis. Only one patient was prescribed a steroid spray, and this was prior to presenting symptoms. In reality, most SPOA are proceeded by similar symptoms as a viral upper respiratory infection (URI) and short-term use of nasal sprays lessen the use of antibiotics. Education of the use of saline, oxymetazoline, and steroid sprays with sinusitis and orbital symptoms is warranted within pediatrician offices, the emergency department, and outside hospitals.
The likelihood of needing an additional or first surgery after the initial hospital stay for SPOA did not increase between abscess size, surgical necessity, and abscess location groups. With an 89.8% success rate determined by need for initial or additional surgery, SPOA remains an urgent but complicated infectious process. None of our patients had neurologic sequalae or death.
Torretta et al. touched on the difficulty of comparing abscess volume results across studies due to the differences in SPOA cutoff volumes used for analysis18. While this is true, our study with a large sample and 14-year study period allows us to have a representative sample to base our abscess cutoff point on. While many studies have looked at abscess size and location, surgical intervention, presenting symptoms, microbiology, antibiotic treatment and complications separately as individual studies, this study brings all these variables together in the largest sample size in the literature for the pediatric population with SPOA. Another strength of this study is that we follow SPOA in each patient at the start of first presentation to a provider, regardless of being an outside hospital or not. By looking at outside provider notes, we have a clearer picture of the length of time on antibiotics and until surgery if required. This study is limited in that it is retrospective. A prospective study assessing the rate of surgical necessity based on our cutoff point would be helpful to standardize a decision-making tree for treating SPOA. It would also be optimal to have a total abscess volume computed by radiology for decision making purposes, possibly by region of interest (ROI) analysis.
5. Conclusion:
Subperiosteal orbital abscess continues to be a prevalent complication of sinusitis in the pediatric population and can have life-changing consequences if not treated promptly. A clear protocol for when surgical intervention is necessary has not been determined previously. This study has shown measurements found on CT scans can help aid in the decision-making process. Abscess size, location, and proximity to orbital apex as measured on CT scans are significant predictors of requiring surgery. Complex mathematical models to predict risk and guide medical decision making are statistically appealing but clinically impractical as they are simply too tedious to recall and apply to best practice clinical pathways. The appeal of our study is that it provides evidence and support that employ clinical parameters already assessed as standard practice in evaluating these patients. In summarizing the clinical translational relevance of our study, when determining whether to treat a patient with surgery and antimicrobial/medical therapy vs. non-surgical medical therapy alone, the clinician should focus on size of.510 cm3 or larger for abscesses in any location as a relative indication for surgery.
Acknowledgments
The project described was supported by the National Institutes of Health through Grant Number UL1 TR001857.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report for any author.
References:
- 1.Ryan JT, Preciado DA, Bauman N, et al. Management of pediatric orbital cellulitis in patients with radiographic findings of subperiosteal abscess. Otolaryngol Head Neck Surg. 2009;140(6):907–911. doi: 10.1016/j.otohns.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 2.Corp IBM. IBM SPSS Statistics for Windows. 2016.
- 3.Adil EA, Muir ME, Kawai K, Dombrowski ND, Cunningham MJ. Pediatric Subperiosteal Abscess Secondary to Acute Sinusitis: A Systematic Review and Meta-analysis. Laryngoscope. February 2020. doi: 10.1002/lary.28570 [DOI] [PubMed] [Google Scholar]
- 4.Nageswaran S, Woods CR, Benjamin DKJ, Givner LB, Shetty AK. Orbital Cellulitis in Children. Pediatr Infect Dis J. 2006;25(8). https://journals.lww.com/pidj/Fulltext/2006/08000/Orbital_Cellulitis_in_Children.7.aspx. [DOI] [PubMed] [Google Scholar]
- 5.Fiscella K, Williams DR. Health Disparities Based on Socioeconomic Inequities: Implications for Urban Health Care. Acad Med. 2004;79(12). https://journals.lww.com/academicmedicine/Fulltext/2004/12000/Health_Disparities_Based_on_Socioeconomic.4.aspx. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RF, Jiang ZY. Socioeconomic Factors in Pediatric Neck Abscesses. Otolaryngol Head Neck Surg. 2011;145(2_suppl):P113–P114. doi: 10.1177/0194599811416318a231 [DOI] [Google Scholar]
- 7.Angajala V, Hur K, Jacobson L, Hochstim C. Geographic health disparities in the Los Angeles pediatric neck abscess population. Int J Pediatr Otorhinolaryngol. 2018;113:134–139. doi: 10.1016/j.ijporl.2018.07.043 [DOI] [PubMed] [Google Scholar]
- 8.Sedaghat AR, Wilke CO, Cunningham MJ, Ishman SL. Socioeconomic disparities in the presentation of acute bacterial sinusitis complications in children. Laryngoscope. 2014;124(7):1700–1706. doi: 10.1002/lary.24492 [DOI] [PubMed] [Google Scholar]
- 9.Eggart MD, Greene C, Fannin ES, Roberts OA. A 14-Year Review of Socioeconomics and Sociodemographics Relating to Intracerebral Abscess, Subdural Empyema, and Epidural Abscess in Southeastern Louisiana. Neurosurgery. 2016;79(2):265–269. doi: 10.1227/NEU.0000000000001225 [DOI] [PubMed] [Google Scholar]
- 10.Gavriel H, Yeheskeli E, Aviram E, Yehoshua L, Eviatar E. Dimension of subperiosteal orbital abscess as an indication for surgical management in children. Otolaryngol Head Neck Surg. 2011;145(5):823–827. doi: 10.1177/0194599811416559 [DOI] [PubMed] [Google Scholar]
- 11.Rahbar R, Robson CD, Petersen RA, et al. Management of Orbital Subperiosteal Abscess in Children. Arch Otolaryngol Neck Surg. 2001;127(3):281–286. doi: 10.1001/archotol.127.3.281 [DOI] [PubMed] [Google Scholar]
- 12.Tabarino F, Elmaleh-Bergès M, Quesnel S, Lorrot M, Van Den Abbeele T, Teissier N. Subperiosteal orbital abscess: volumetric criteria for surgical drainage. Int J Pediatr Otorhinolaryngol. 2015;79(2):131–135. doi: 10.1016/j.ijporl.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 13.Todman MS, Enzer YR. Medical Management Versus Surgical Intervention of Pediatric Orbital Cellulitis: The Importance of Subperiosteal Abscess Volume as a New Criterion. Ophthalmic Plast Reconstr Surg. 2011;27(4). [DOI] [PubMed] [Google Scholar]
- 14.Oxford LE, McClay J. Medical and surgical management of subperiosteal orbital abscess secondary to acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2006;70(11):1853–1861. doi: 10.1016/j.ijporl.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 15.Liao JC, Harris GJ. Subperiosteal Abscess of the Orbit: Evolving Pathogens and the Therapeutic Protocol. Ophthalmology. 2015;122(3):639–647. doi: 10.1016/j.ophtha.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 16.MicrobiologyBrook I. and choice of antimicrobial therapy for acute sinusitis complicated by subperiosteal abscess in children. Int J Pediatr Otorhinolaryngol. 2016;84:21–26. doi: 10.1016/j.ijporl.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 17.Sinclair CF, Berkowitz RG. Prior antibiotic therapy for acute sinusitis in children and the development of subperiosteal orbital abscess. Int J Pediatr Otorhinolaryngol. 2007;71(7):1003–1006. doi: 10.1016/j.ijporl.2007.02.013 [DOI] [PubMed] [Google Scholar]
- 18.Torretta S, Guastella C, Marchisio P, et al. Sinonasal-Related Orbital Infections in Children: A Clinical and Therapeutic Overview. J Clin Med. 2019;8(1):101. doi: 10.3390/jcm8010101 [DOI] [PMC free article] [PubMed] [Google Scholar]


