Abstract
Basement membranes help to establish, maintain, and separate their associated tissues. They also provide growth and signaling substrates for nearby resident cells. The internal limiting membrane (ILM) is the basement membrane at the ocular vitreoretinal interface. While the ILM is essential for normal retinal development, it is dispensable in adulthood. Moreover, the ILM may constitute a significant barrier to emerging ocular therapeutics, such as viral gene therapy or stem cell transplantation. Here we take a neurodevelopmental perspective in examining how retinal neurons, glia, and vasculature interact with individual extracellular matrix constituents at the ILM. In addition, we review evidence that the ILM may impede novel ocular therapies and discuss approaches for achieving retinal parenchymal targeting of gene vectors and cell transplants delivered into the vitreous cavity by manipulating interactions with the ILM.
Keywords: Inner limiting membrane, basement membrane, barrier, cell signaling, neurodevelopment, retinal ganglion cell, transplantation, cell replacement
1. Introduction
Our understanding of the internal limiting membrane (ILM) at the vitreoretinal junction has evolved with the advancement of microscopy. Almost two centuries ago, Gustav Retzius used silver nitrate staining and light microscopy to examine human and animal retinas, leading him to postulate that the terminal footplates of Müller glia form the ILM (Retzius, 1871). This speculation was later challenged using Mallory trichrome stain, which labels the ILM blue and radial cellular fibers red, indicating the ILM and Müller glial processes are separate structures (Wolff, 1937). Advanced imaging techniques in the modern era further catalyzed a revolution in observational capabilities, which helped paint the current prevailing view that the Müller glia plasma membranes do not constitute the ILM, but are likely involved in ILM formation (Cohen, 1961; Heegaard et al., 1986; Pedler, 1961).
Understanding the biochemical composition and mechanical properties of the ILM is scientifically and clinically relevant. ILM components function as key substrates in molecular and cellular interactions between the basement membrane and the developing retina. These interactions play a central role in developmental patterning of the retinal vasculature and neuroretinal lamellae. In addition, the ILM components define its biomechanical properties including thickness, stiffness, and elasticity. These attributes influence cellular interactions at the vitreoretinal interface (Halfter et al., 2014), and can potentially contribute to pathologic tractional forces on the mature retina. Modern proteomic analyses combined with ultrastructural imaging have identified compositional and structural changes in the ILM that occur with advancing age and in pathologic disease states (Halfter et al., 2015, 2013), underscoring the need to understand the roles that the ILM plays in clinical vitreoretinal disorders. Indeed, the ILM is involved in the development of vitreomacular traction, epiretinal membrane, macular hole, diabetic fibrovascular proliferation, and proliferative vitreoretinopathy, as has been reviewed extensively elsewhere, and will not be further discussed here (Chatziralli et al., 2018; Pastor et al., 2016; Pournaras et al., 2011; Schechet et al., 2017; Steel and Lotery, 2013).
In recent years, clinical interest in the ILM has expanded with increasing recognition that its barrier properties may hamper translation of novel ophthalmic therapies. Emerging evidence suggests that the ILM poses a significant impediment to retinal transduction by intravitreally delivered viral gene therapy vectors and to the retinal integration of cell transplants. The ILM also poses an obstacle to intravitreally administered antibodies and nanoparticles, as elegantly reviewed recently (Peynshaert et al., 2019).
Understanding the biochemical composition of the ILM and identifying its molecular binding partners could accelerate the development of innovative treatments for overcoming the ILM barrier. In fact, modifying surface proteins on viral capsids and nanoparticles to enhance ILM diffusion has improved retinal therapeutic targeting (Woodard et al., 2016). Furthermore, surgical removal or bypass of the ILM has been shown to enhance viral transduction in intravitreally administered gene therapy (Comander et al., 2016).
Mitigating the ILM barrier in cell replacement therapy for treating optic neuropathies is a more complex challenge. Lessons from retinal development teach us that neuronal migration, cellular polarization, and axonal growth all depend on specific molecular interactions with the extracellular matrix (ECM) (see below). Thus, modulating the interactions between cellular transplants and the ILM, rather than completely removing the ILM, may be key to advancing cell transplantation therapy.
Cell transplantation for RGC replacement is still very much in an exploratory phase, but experimental evidence suggests that targeting the ILM may facilitate functional integration of donor neurons into the recipient neurocircuitry. Methods that have proven beneficial for circumventing the ILM for AAV delivery might be repurposed for cell delivery. This review examines the composition of ILM, its functions during development, and the rationale for ILM disruption or circumvention to aid in development of innovative ocular therapies.
2. Properties of the ILM
2.1. Morphology and composition
The ILM is the basement membrane (BM) that separates the retina from the vitreous cavity (Heegaard et al., 1986). The retinal side of the ILM faces the plasma membrane of Müller glial endfeet. Müller glia are radial macroglia with cell somas and nuclei residing in the inner nuclear layer and radial processes spanning the entire depth of the neuroretina, from the ILM to the outer limiting membrane (OLM). In contrast to the ILM, the OLM is not a true basement membrane but rather a cellular structure composed of junctional complexes (including heterotypic adherens junctions and desmosomes) between Müller glia and photoreceptor inner segment plasma membranes (Omri et al., 2010). Essential for maintaining ionic balance of the extracellular space, Müller glia also regulate acid-base balance and provide metabolic support to inner retinal neurons (Guidry, 2005). The vitreous side of the ILM anchors a loose network of collagen fibers emanating from the vitreous cortex (Hogan, 1963; Rhodes, 1982). Under high resolution transmission electron microscopy (TEM), the ILM appears as a layer of electron dense lamina densa sandwiched in between two thin layers of lamina lucida, and external to cellular Müller footplates (Halfter et al., 2008; Kröger and Mann, 1996). Fibrillar deposits from the vitreous cortex rest on top of the ILM. Our lab has shown that ex vivo cultures of organotypic retinal explants maintain the integrity of a continuous ILM for at least 7 days, as demonstrated by TEM (Figure 1), making this a good model system in which to study the ILM.
Figure 1. Transmission electron microscopy demonstrating internal limiting membrane (ILM) integrity during ex vivo organotypic retinal explant culture.
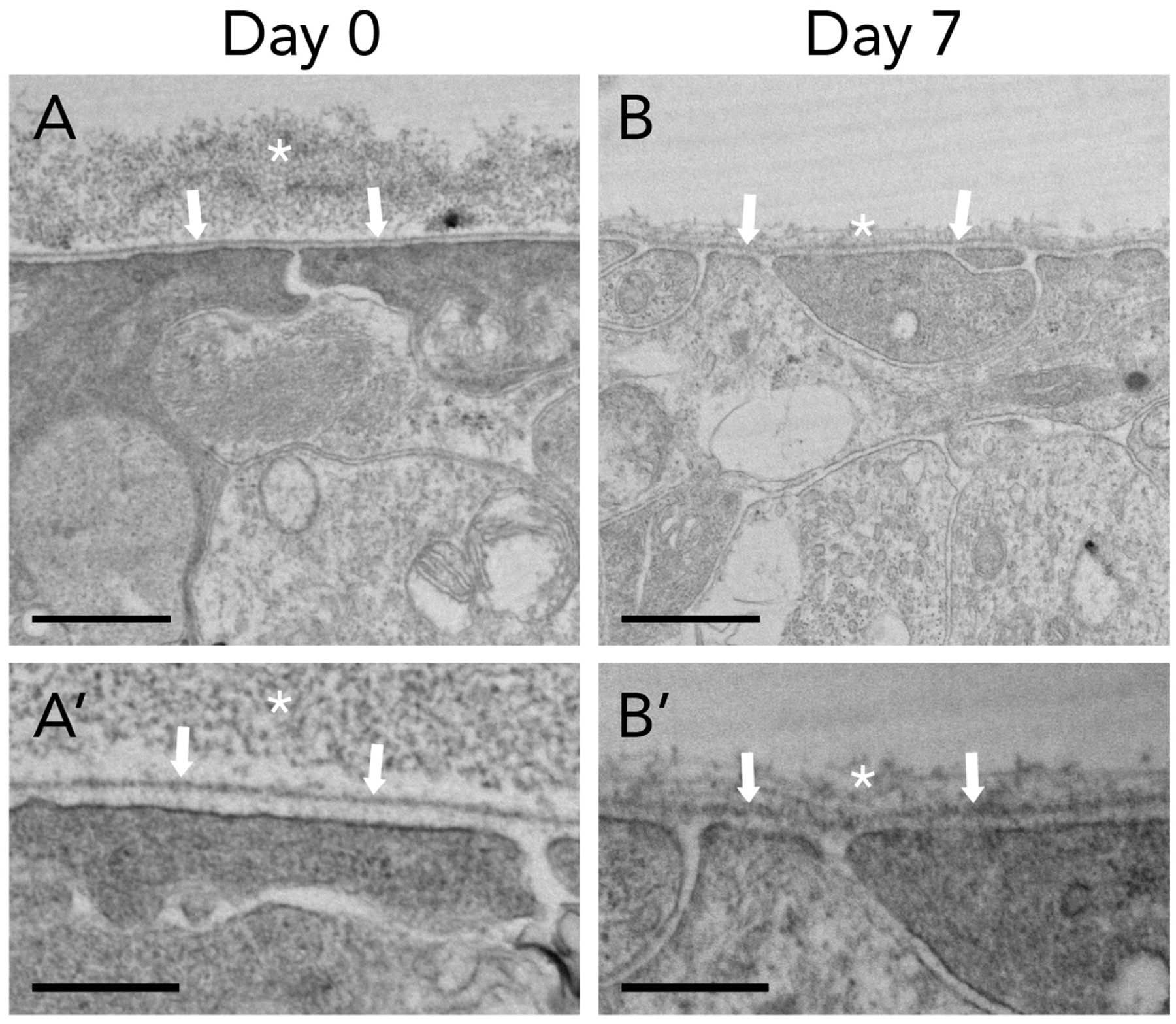
Vitreoretinal interface of adult organotypic mouse retinal explants demonstrates ILM (arrows) separating posterior vitreous cortex (asterisk) and neuroretina. Müller glial footplates underlie the ILM. The ILM remains intact in culture from day 0 (A) to day 7 (B). Scalebars: 1μm (A, B); 400nm (A’, B’).
Similar to BMs elsewhere in the body, the ILM is composed of networks of secretory ECM proteins including collagen IV, laminins, nidogens, and heparan sulfate proteoglycans (Table 1) (Kleinman et al., 1982; Timpl et al., 1979; Vracko, 1974). The general assembly of the BM is built on collagen IV and laminin protein scaffolds, with nidogens facilitating their molecular linkage (Fox et al., 1991; Yurchenco and Patton, 2009). These macromolecules contain multiple peptide domains that self-polymerize or bind to other BM proteins (Timpl and Brown, 1996). Basement membrane assembly also requires binding of ECM components to cellular receptors such as integrins and dystroglycan (Fässler and Meyer, 1995; Henry and Campbell, 1998).
Table 1.
Summary of ILM structural components and their digestive enzymes
| Major ILM components | Digestive Enzyme | References |
|---|---|---|
| collagen IV (α5α5α6) | collagenase | Bu et al., 2015 |
| collagen IV (α3α4α5) | ||
| collagen VI | ||
| collagen XVIII | ||
| laminin (LNα1β2γ1) | pronase | Dalkara et al., 2009 |
| laminin (LNα5β2γ1) | ||
| hyaluronic acid | hyaluronidase | Rhodes, 1982 |
| fibronectin | dispase | Rhodes, 1982 |
| nidogen 1 | PNGase F, GluC, trypsin | Kunze et al., 2010; Lössl et al., 2014; Patel et al., 2014 |
| perlecan | plasmin, stromelysin, collagenase |
Timpl and Brown, 1996; Whitelock et al., 1996 |
| dystroglycan | ureafaciens sialidase, beta(1,4)galactosidase, beta-N-acetylglucosaminidase, Bgus, Xylsa |
Briggs et al., 2016; Clements et al., 2017; Combs and Ervasti, 2005 |
| agrin | neurotrypsin |
Kröger and Mann, 1996; Reif et al., 2007 |
| CSPG | chondroitinase ABC | Clark et al., 2011 |
Although considered the BM of the retinal neuroepithelium, the ILM and its components are not produced by the retina itself. Based on in situ hybridization of oligonucleotide probes that recognize laminin and collagen IV mRNAs, the genes that encode these proteins are primarily transcribed by, and proteins ultimately secreted from, the lens epithelium and ciliary body, respectively (Dong et al., 2002; Halfter et al., 2008). In fact, all of the ILM proteins in chick, except for agrin (Mann and Kröger, 1996), are of extraretinal origin. All ILM proteins can be detected in the vitreous at high concentrations during early embryogenesis and rapidly decline postnatally (Halfter et al., 2008, 2005), suggesting that the vitreous acts as a temporary reservoir of molecular components for ILM assembly during stages of rapid eye growth. The ILM does not reconstitute after surgical removal in adult humans (Piven and Moisseiev, 2010), further supporting the temporary extrinsic reservoir hypothesis.
Enzymatic disruption of the ILM followed by microscopic observations of ECM structural changes provided early evidence of its biochemical composition. For instance, upon fixation with glutaraldehyde and TEM visualization, the mouse ILM exhibits focal globular electron dense material on the inner lamina lucida, as well as electron dense fibrils in the outer vitreous cortex (Rhodes, 1982). The globular material in the lamina lucida disappears after hyaluronidase treatment, but the vitreal filamentous network is not affected, indicating that the globular material along the BM is composed of hyaluronic acid. Furthermore, the fibrillary meshwork was postulated to contain oligosaccharide chains associated with vitreous proteins, based on its structural stability after glutaraldehyde fixation, the staining patterns with Alcian blue, and its association with collagen fibrils. In nonhuman primate eyes, hyaluronidase digestion causes the globular material on the lamina lucida to become scattered, and the fibrillary meshwork to be irregular (Heegaard et al., 1986). However, chondroitinase treatment does not alter the ILM morphology, suggesting that chondroitin sulfate plays a minor role in ILM structure. Neither hyaluronidase nor chondroitinase treatment results in significant structural changes to the remaining retina. However, collagenase treatment considerably distorts the fibril meshwork and alters retinal layers. Pronase-E, a mixture of proteolytic enzymes derived from Streptomyces griseus, produces even more marked disruption. Most of the ILM is digested without leaving traces of the fibril meshwork, but at a concentration of 1%, it also resulted in significant structural compromise to the neuroretinal layers, particularly the photoreceptor layer (Heegaard et al., 1986). Of note, the proteolytic effects from Pronase-E are concentration dependent, as our laboratory has demonstrated that Pronase-E concentration can be titrated to produce areas of focal breaks in the ILM without damaging underlying retinal neurons or glia in organotypic retinal explant cultures (Zhang et al., 2021) (Figure 2).
Figure 2. Effects of Pronase-E digestion on ILM laminin.
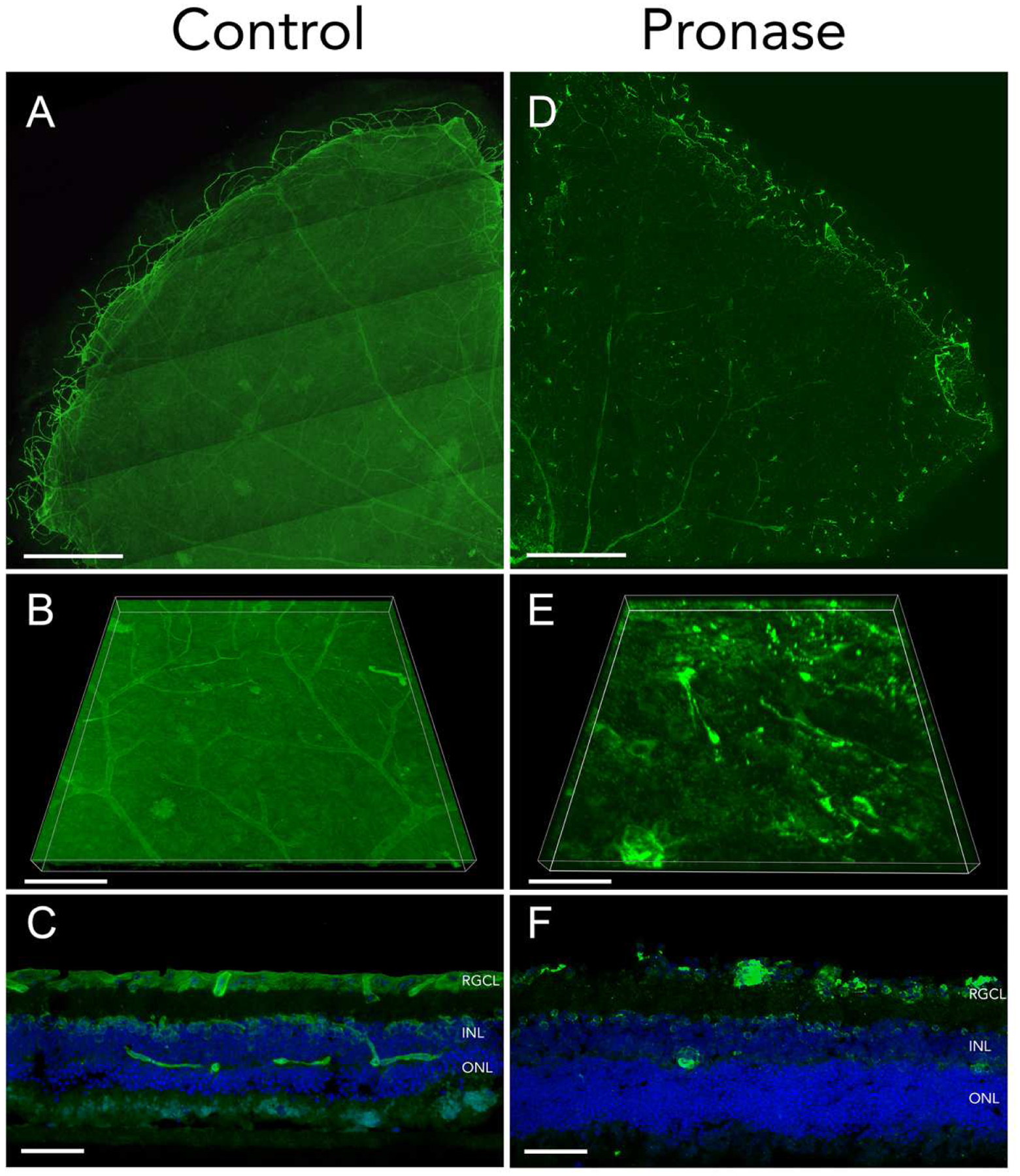
Adult organotypic mouse retinal explants treated with BSS (A-C) or Pronase-E at a concentration of 0.6 U/mL (D-F) were cultured for 7 days. Fixed tissue stained with laminin (green) shows ILM organization. Tiled confocal microscopy of flat mount retina quadrants are shown (A, D). Three-dimensional reconstruction of confocal microscopy z-stacks detail ILM surfaces in control (B) and Pronase (E) treated retinas. Retinal explant cryosections exhibit intact (C) and linear interruptions (F) in ILM, with preserved retinal layers indicated by DAPI counterstain of retinal cell nuclei in blue. Scale bars: 500μm (A, D); 100μm (B, E); 50μm (C, F).
2.2. ILM mechanical properties and polarity
Traditional imaging methods requiring tissue fixation and dehydration contributed to an underestimation of ILM thickness (Candiello et al., 2007), which had been cited as measuring less than 100nm in humans (Candiello et al., 2010). More recent examinations of ILM thickness with confocal microscopy and atomic force microscopy (AFM) detail this structure in its native, undehydrated form. According to AFM measurements, the ILM thickness is up to four times thicker than previously recorded, with water-bound glycosaminoglycan (GAG) chains making up a significant proportion of BM thickness (Candiello et al., 2010)(Balasubramani et al., 2010). ILM thickness can also vary substantially across different regions of the retina and with age. The ILM is thickest near the fovea at approximately 400nm, and the thinnest in regions near the optic disc and the periphery of the retina, measuring only 70nm (Bu et al., 2015; Matsuo et al., 1963). ILM thickness and rigidity increase with age, owing to a relative increase in collagen and decrease in laminin concentrations (Candiello et al., 2010; Halfter et al., 2008). Interestingly, enzymatic digestion reveals no regional differences in the biochemical composition of the ILM (Heegaard et al., 1986).
Like most BMs, the ILM has a polarity, or “sidedness” that is demonstrable by molecular and physiological assays. Gross examination of ILM from human donors reveals a smooth vitreal surface opposite a highly irregular retinal surface (Halfter et al., 2013). When isolated, the ILM naturally curls according to the native curvature of the retina by rolling in toward the vitreal side. This observation supports biomechanical studies showing that the retinal surface has a 50% higher stiffness than the vitreal side (Halfter et al., 2013).
Cell adhesion assays also differentiate the functional sidedness in the ILM. Plating corneal epithelial, retinal, and vascular endothelial cell suspensions on flat-mounted human ILM for 15 minutes results in cellular attachment only to the retinal surface of the ILM. Pretreatment of the ILM with either chondroitinase or hyaluronidase does not affect cell adhesion, suggesting that the interaction is mostly dependent on ILM protein components (Halfter et al., 2013).
Side-specific distribution of ECM proteins in the human ILM can be demonstrated by immunohistochemistry (Halfter et al., 2013). Laminin staining in retinal whole mounts and cross sections shows higher abundance of laminin on the retinal surface of the ILM, while polyclonal antibody directed against collagen IV uniformly labels both sides of the ILM. However, monoclonal antibody against the C-terminus of the collagen polypeptide preferentially labeled the vitreal side, whereas monoclonal antibody against the N-terminal domain almost exclusively labeled the retinal side. These results, together with asymmetric mechanical and functional properties, reveal inherent structural polarity in the ILM.
The ILM permits passive diffusion of various ions, glucose, lactate, and ascorbate (Levin, 2011). These small molecules freely cross the ILM through a meshwork of porous channels varying from 10–25nm in diameter (Nishihara, 1991). The upper size limit for exogenous materials delivered into the vitreous cavity to traverse the ILM is unclear, but large particles have longer vitreal retention times thus hindering diffusion rate before even reaching the ILM (Xu et al., 2013). In a bovine explant model studying nanoparticle delivery system, negatively charged 40nm polystyrene particles could pass through the ILM, while similar beads of 100–200nm size were blocked by the ILM (Peynshaert et al., 2018). Another study comparing the particle exclusion limit of the human ILM to those of porcine, bovine, and rabbit ILM found a common range of 60–90kDa (Jackson et al., 2003).
3. Role of the ILM in neuroretinal development
The embryonic eye develops as an outpouching of the neural tube that then invaginates, forming the optic cup. At this stage the neuroectoderm is composed of two monolayers of epithelial cells, the outer retinal pigment epithelium (RPE) and the inner retinal neuroepithelium. The neuroblasts of the inner monolayer become tightly packed and attached to each other by adherens junctions on their apical side nearest RPE (Harris and Tepass, 2010). The basal surface, facing the vitreous cavity, becomes anchored to the basal lamina of the ILM. It is on the ILM that the RGCs, the first differentiated retinal cell type (Austin et al., 1995; McCabe et al., 1999), extend their growing efferent axons toward the optic nerve head. Accessing the BM provides signals that define RGC polarity. In zebrafish with defective junctional complex protein Pals-1, the retinal pigment epithelium is disrupted, allowing RGCs to extend axons towards Bruch’s membrane, the basal lamina of the RPE (Zolessi et al., 2006). Aberrant contact with Bruch’s membrane reverses RGC polarity. Studies elucidating the roles that retinal basement membrane plays in retinal development have utilized a number of genetic knockout paradigms, and relevant animal models are listed in Table 2.
Table 2.
Animals models of ECM component mutations demonstrate roles of the ILM in retinal development
| Gene | Protein name | Mutation description | Species | Phenotype | Reference |
|---|---|---|---|---|---|
| Col4A1 | Type IV collagen, alpha 1 subunit | Autosomal dominant mutation; disrupted splice acceptor site, causing deletion of 17 amino acids in the collagen triple helical domain resulting in improper protein folding | Mouse | ocular dysgenesis, neuronal localization defects | Labelle-Dumais et al., 2011 |
| Col18A1 | Type XVIII collagen, alpha 1 subunit | Homozygous knock-out | Mouse | abnormal outgrowth of retinal vessels; reduced expression of VEGF in retinas; increased retinal astrocytes; lack of vitreous fibril insertion into the ILM |
Fukai, 2002; Hurskainen et al., 2005 |
| ItgaA6 | Integrin, alpha 6 subunit | Homozygous knock-out | Mouse | disorganized basement membrane; ectopic neuroblasts in vitreous | Georges-Labouesse et al., 1998 |
| ISPDL79* | Isoprenoid synthase domain-containing protein | Autosomal recessive; lack mature glycan chains for dystroglycan to bind laminin | Mouse | ILM disruption and, defective axon guidance for RGCs; pups die at P0 due to respiratory failure |
Clements et al., 2017; Wright et al., 2012 |
| Lama1 (bala69) | Laminin, alpha 1 subunit | Dominant-negative mutation; missense mutation affecting disulfide bridge formation | Zebrafish | RGC axonal projection defect; ectopic photoreceptors in inner retina; hyaloid vessels lack capillaries | Semina et al., 2006 |
| Lama1nmf223 | Autosomal recessive point mutation; amino acid substitution at receptor binding and polymerization sites | Mouse | Normal appearing ILM; astrocytes and vasculature migrate ectopically into vitreous; astrocytes persist and proliferate in vitreous, forming dense mesh resembling epiretinal membranes | Edwards et al., 2011 | |
| Lama1tm1.Olf | Conditional knock-out driven by CMV-Cre, bypasses embryonic lethality | Mouse | Very thin ILM; Müller cell processes extend into vitreous through frequent breaks in ILM Similar vascular patterns as those found in Lama1nmf223 |
Alpy et al., 2005; Edwards et al., 2011 |
|
| Lama4 | Laminin, alpha 4 subunit | Homozygous knock-out | Mouse | retinal hemorrhage due to endothelial BM defect; increased vessel branching, reduced vascular maturation, reduced lumen size |
Stenzel et al., 2011; Thyboll et al., 2002 |
| Lamb2 | Laminin, beta 2 subunit | Homozygous nonsense mutation created by inserting stop codons into exon 3; no full length protein produced | Mouse Human |
Mice: abnormal rod photoreceptor synapse formation; abnormal electroretinograms Humans: Pierson syndrome; ocular defects include microcorcia, glaucoma, cataracts, retinal detachment |
Libby et al., 1999
Zenker et al., 2004 |
| Lamc3 | Laminin, gamma 3 subunit | Homozygous knock-out created by vector targeted exon 1 deletion | Mouse | altered vasculature, increased branching in OPL | Li et al., 2012 |
| Lamb2 / Lamc3 | Laminin, beta 2 & Laminin, gamma 3 | Homozygous compound knock-out | Mouse | Müller cells and RGC processes extend into vitreous; thickened Bruch’s membrane; disorganized PR outer segment; synaptic defects | Pinzón-Duarte et al., 2010 |
| Largemyd | Xylosyl- And Glucuronyltransferase 1 | Autosomal recessive allele; intragenic deletion of the glycosyltransferase gene Large | Mouse | disrupted ILM, ectopic cells in vitreous; hypoglycosylation of dystroglycan; retinal vessel tortuosity and leakage | Lee et al., 2005 |
| α-Cre;Ugdhflox | UDP-Glucose 6-dehydrogenase | Connditional knock-out of the GAG synthesis gene Ugdh under the control of the retinaspecific regulatory element alpha of murine Pax6 | Mouse | large ILM defect in peripheral retina; failed astrocyte migration to defective areas |
Marquardt et al., 2001; Tao and Zhang, 2016 |
One significant extracellular signal required for proper RGC migration, lamination, and axon orientation is laminin, a family of heterotrimeric glycoproteins which serves as an ECM ligand for integrins on the RGC plasma membrane (Lei et al., 2012). Laminin activation of RGC integrin signaling through the β1-integrin subunit is sufficient to promote directional microtubule assembly and axon initiation in cultured neurons. Disruption of this signaling cascade by genetically knocking out Itgb1 (the gene encoding β1-integrin) or its Crk-associated substrate (Cas) cytosolic adaptor gene in mice causes loss of RGC layer single-cellularity, RGC aggregation and protrusion ectopically through the ILM, and secondary disorganization of the ILM itself (Riccomagno et al., 2014). Interestingly, ectopic RGCs still project axons to retinorecipient thalamic targets. In zebrafish, contact-mediated cues from laminin both in vitro and in vivo are sufficient to guide axon extensions in newly differentiated RGCs (Randlett et al., 2011). In the absence of laminin α1, RGCs retain a multipolar morphology and exhibit dynamic neurite extension and retraction without axon elongation. Though transgenic deletion of lamb2 or lamc3 alone causes minimal change in the ILM, double knockout of the β2 and γ3 laminin genes in mice disrupts ILM integrity, resulting in a poorly organized ganglion cell layer and ectopic RGCs extruding through the ILM and into the vitreous (Pinzón-Duarte et al., 2010).
RGC-ILM interactions are important for RGC survival. Enzymatic ILM disruption with collagenase in chick embryos on E5 leads to a dramatic decline in RGC survival, but RGC death can be rescued by injection of exogenous laminin protein soon after collagenase treatment (Halfter et al., 2005). Interestingly, the role of ILM on developing RGC survival seems to be temporary, since collagenase treatment on E7 did not cause decline in RGC number. Indeed, in our work transplanting terminally differentiated RGCs onto organotypic retinal explants, pretreatment of recipient retina with Pronase E did not lead to changes in neuronal graft survival (Zhang et al., 2021).
The ILM also contains a diverse group of heparan sulfate proteoglycans (HSPGs) including perlecan, agrin, and collagen XVIII, with important roles in retinal development (Clark et al., 2011). Heparinase treatment of the developing chick retina greatly reduces RGC neurite growth rate and density, suggesting axonal development is partly dependent on HSPGs of the ILM (Chai and Morris, 1999). Based on previous discoveries that proteoglycans can act as a reservoir for growth factors (Hardingham and Fosang, 1992), the same authors demonstrated that HSPGs in the ILM bind basic fibroblast growth factor (bFGF), and heparinase disruption of the ILM eliminates the ability for bFGF to promote neurite outgrowth. Neurite extension in heparinase-treated ILM can be rescued with exogenous administration of heparin and bFGF, an effect not observed when heparin is restored in isolation. These results suggest that the neurotrophic activity provided by HSPGs in the ILM depend on the binding and sequestering of growth factors. Chondroitin sulfate proteoglycans (CSPGs), another ECM component of the ILM, are produced by retinal glia and are implicated in axonal pathfinding in early development. These proteoglycans are heavily glycosylated with GAG side chains, and can facilitate ligand binding interactions and diffusible ligand sequestration (Bishop et al., 2007).
4. Role of the ILM in retinal glial and vasculature development
Normal integrity of the ILM during retinal vascular development ensures intact substrate for astrocyte migration and the formation of astrocytic networks that, in turn, provide the template for endothelial cell migration (Dorrell et al., 2002). Hyaloid vessels first arise from the optic nerve head, extending through the vitreous and wrap around the developing lens (Fruttiger, 2007). These fetal vessels then regress secondarily and are replaced by the retinal vasculature. Failure of hyaloid vascular regression results in persistent fetal vasculature (PFV), a congenital eye disorder that accounts for 5% of childhood blindness (Mets, 1999).
Animal models with transgenic disruption of ILM molecular components exhibit abnormal retinal vasculature (Edwards et al., 2010; Fukai, 2002; Hurskainen et al., 2005; Lee et al., 2005). An example includes the Lamanmf223 mouse strain, in which a point mutation in laminin-α1 leads replacement of tyrosine-265 with cysteine within the N-terminal domain, which is critical for interactions with other laminin subunits and numerous receptors including integrins, perlecan, alpha-dystroglycan, and netrins (Edwards et al., 2010). The ILM in these mice appears normal throughout most of the retina where it is present, but has frequent small breaks (Edwards et al., 2011). Early on in retinal vasculature development, the mutant retina exhibits a vascular apron around the optic nerve head, indicating that normal endothelial cell differentiation and migration from the optic nerve are preserved. However, in the ensuing days, retinal vessels cease to develop, and astrocytes migrate across the ILM breaks to envelope the fetal hyaloid vessels in the vitreous. Hyaloid vasculature that normally regresses instead persists and invades into retinal layers on the scaffold formed by ectopic astrocytes. The dense astrocytic ensheathment around the fetal vasculature persist into adulthood and resembles PFV in humans. Indirect ophthalmoscopy of adult Lamanmf223 mice reveal white retinal spots, vitreal fibroplasia, and vessel tortuosity (Edwards et al., 2010). Interestingly, the Lamanmf223 mutation does not affect the localization of other ILM components such as collagen IV, perlecan, and dystroglycan, nor does it alter Müller cell endfeet attachment to the ILM. These results may indicate that the persistence of hyaloid vasculature is mainly dependent on astrocytes bridging the retinal and vitreal domains through ILM defects.
Laminins also serve as ligands for dystroglycans (Colognato and Yurchenco, 2000), which are found at high density in Müller cell endfeet adjacent to the ILM (Noël et al., 2005). Müller glial-ILM interactions are important in several ways. Genetic deletions of laminin subunits can result in disrupted Müller glial polarity, disarray of Müller cell endfeet, vitreal glial cell process retraction, and decreased Müller cell survival (Pinzón-Duarte et al., 2010). In a reciprocal fashion, absence of Müller cell dystroglycans in development leads to ILM degeneration, defective axon guidance for RGCs, and impaired RGC dendritic stratification in the inner retina (Clements et al., 2017). Glycosylation of Müller cell dystroglycans alters their interactions with laminin. Mutation in the glycosyltransferase Large leads to disrupted ILM and abnormal astrocyte migration in mice (Zhou et al., 2017). Binding between laminin and dystroglycan is impaired in Large mutants, indicated by a weak signal around dystroglycan in laminin overlay assays. In this assay, wildtype or mutant retinal lysate is first bound to PVDF membranes, which is then incubated with a solution containing laminin; after washing the PVDF membranes, any bound laminin is then detected by Western blots. H&E staining of the ILM in mutant mice demonstrate intact gross structure, but electron microscopy exhibits focal breaks. Similar to the findings in Lamanmf223 mutants, Large mutant astrocytes can migrate into the vitreous cavity and ensheathe the hyaloid vessels, and fundus examination with indirect ophthalmoscopy reveals retinal vessel tortuosity, persistent hyaloid vessels, and vitreal fibroplasia reminiscent of PFV.
The regulation of astrocyte migration essential for proper angiogenesis may also depend on ILM proteoglycan expression. Ablation of proteoglycan GAG chains in the neuroretina by knocking out the key synthesis gene Ugdh leads to BM disruption (Tao and Zhang, 2016). In the α-Cre/Ugdhflox/flox mouse mutant, which restricts the loss of GAG synthesis to the peripheral retina (Cai et al., 2011), astrocytes fail to invade the peripheral retina (Tao and Zhang, 2016). Astrocyte migration initiates postnatally, whereas retinal cell differentiation begins embryonically, and interestingly Ugdh mutants show normal retinal cell differentiation. However, clusters of photoreceptors forming rosettes, a hallmark of retinal degeneration, are observed in the peripheral retina. Indeed, extensive cell death, detectable by TUNEL staining in the mutant, results in severely hypoplastic peripheral retina. Immunostaining with laminin and collagen IV in whole mount retinas of α-Cre/Ugdh knockouts reveal many large holes in the peripheral retina, often associated with persistent hyaloid vessels. The ILM defect in Ugdh-deficient retinas resembles that of neonatal wildtype retinas treated with both heparinase and chondroitinase ABC. Interestingly, treating wildtype retina with either enzyme alone only produces partial laminin network disruption, suggesting that heparan sulfate and chondroitin sulfate may compensate for each other during ILM assembly. Therefore, ILM-derived astrocyte migration defects manifest with significant neuroretinal degeneration at the affected eccentricities.
Ex vivo astrocyte migration assays demonstrate the critical role of an intact ILM in astrocyte migration (Tao and Zhang, 2016). Placing PDGF-coated beads near the edge of an ILM void leads astrocytes to migrate toward the beads as efficiently as they would in wildtype retina. Placing the beads inside the ILM void causes astrocytes to move toward but remain aggregated at the boundary of intact retina, unable to migrate into the ILM-deficient region. These findings suggest that, while PDGF is a chemoattractant for astrocyte migration, ILM components regulate cell adhesion during astrocyte migration.
5. Role of the ILM in ocular gene therapy
Hereditary retinal dystrophies cause irreversible blindness and are candidates for gene therapy. Ophthalmic adeno-associated virus (AAV) delivery has emerged as a preferred method for therapeutic gene transfer due to its relatively high transduction efficiency and safety (Ellis et al., 2013). While the limited repertoire of currently approved retinal gene therapies utilize a subretinal approach designed to transduce photoreceptors (Peng et al., 2017), subretinal delivery is associated with photoreceptor stress as it creates a de facto iatrogenic retinal detachment. On the other hand, intravitreal (IVT) injection may have more desirable safety profile and the potential to achieve larger retinal surface coverage (Ochakovski et al., 2017). It may also be more relevant for treating inherited optic neuropathies in which RGCs are the retinal neuron of interest. One critical limitation of IVT AAV delivery at present is relatively poor transduction efficiency due to vector accumulation at the ILM (Dalkara et al., 2009; Mowat et al., 2014). Therefore, methods of improving the efficacy of IVT AAV delivery have been a focus of research.
Viral gene transfer in eyes with ILM disruption or in animal disease models with compromised ILM integrity has been shown to improve retinal transduction (Cehajic-Kapetanovic et al., 2011; Park et al., 2009). Mild enzymatic digestion of the ILM with Pronase E enhances transduction efficiency by several AAV serotypes and produces robust GFP reporter expression in RGCs, Müller cells, photoreceptors and RPE without perturbing retinal electrophysiology (Dalkara et al., 2009).
The effects of IVT-delivered glycosidic (rather than proteolytic) enzymes on viral transduction have also been investigated. When comparing the transduction efficiency of AAV-2 driving GFP reporter expression, mouse eyes treated with chondroitinase ABC or heparinase produce 50- to 150- fold increases in retinal fluorescence compared to treatments with either collagenase or hyaluronan lyase, which individually produce limited change in transduction (Cehajic-Kapetanovic et al., 2011). The modest effect by hyaluronan lyase could be explained by its enzymatic activity limited to the vitreous matrices, where hyaluronic acid is concentrated, thereby facilitating the migration of viral vehicle toward the retina, but not necessarily through the ILM. The greatest enhancement in transduction was achieved with chondroitinase ABC and heparinase III in combination. Furthermore, retinas treated with chondroitinase ABC and heparinase III appear morphologically intact, retain normal ERG response, and exhibit GFP expression into the outer nuclear layer. This improved penetrance beyond the inner retina is likely due to enzymatic break down of retinal ECM high in CSPG and HSPG (Chai and Morris, 1994; Clark et al., 2011), either making the ILM and retinal layers more porous, or modulating the adhesive interactions between the viral capsid and the ECM, thus altering vector migration towards deeper layers of the neural retina (as discussed below).
While proteolytic and glycosidic digestion of ILM components have shown enhancement of IVT viral transduction and encouraging safety profiles in rodent models, translating this approach to larger animals or non-human primates (NHP) may be challenging due to differences in ILM thickness and retinal biology (Mowat et al., 2014; Yin et al., 2011). Larger eyes of NHP also result in greater dilutional effects on viral vectors in the vitreous. Perhaps the most important consideration is the potential humoral immune response elicited in the non-immune-privileged vitreous humor (Kotterman et al., 2015; Ye et al., 2015). For example, NHP develop inflammation after ILM disruption during viral vector delivery (Comander et al., 2016). Another consideration is provided by the recombinant protease ocriplasmin (truncated human plasmin), which holds enzymatic activity against laminin and fibronectin and has been used as a nonsurgical alternative to induce vitreous liquefaction for treating vitreomacular adhesion (Stalmans et al., 2012). In this condition, the posterior vitreous cortex that normally adheres to the ILM by means of proteoglycan linkage persists in a pathogenic configuration with vitreoretinal traction forces that lead to development of macular hole and visual impairment (Schneider and Johnson, 2011). While ocriplasmin can induce a posterior vitreous detachment and relieve macular traction, several studies have reported relatively rare ocular adverse events, including intraocular inflammation, electroretinogram changes, reduction in visual acuity, and other visual phenomena to be significantly higher in patients receiving ocriplasmin compared to placebo (Johnson et al., 2015; Kaiser et al., 2015; Kim, 2014). Thus, ocriplasmin may provide cautionary data relevant to the widespread clinical use of intravitreally administered proteolytic or glycosidic enzymes.
Alternative strategies to improve transduction nonenzymatically are under development for eventual clinical application of gene therapy. One potential method is to enhance vector localization to the retina by altering AAV capsid constituents that facilitate entry through the ILM or neuronal plasma membranes. For instance, in vitro assays demonstrate that heparin sulfate proteoglycans on HeLa and CHO-K1 cell membranes mediate AAV-2 attachment and transduction, and transgenic deletion or enzymatic removal of cell surface heparans greatly reduces viral infectivity (Summerford and Samulski, 1998). In contrast, applying rational mutagenesis to capsid proteins in AAV-1 and AAV-8, two serotypes lacking HSPG binding sites (Woodard et al., 2016), mutant AAV-1 and AAV-8 with HSPG affinity can dramatically increase retinal accumulation of viral vehicle and achieve higher transduction than wild type vehicle controls.
AAV-5, another serotype lacking HSPG affinity, depends on sialic acid for capsid binding (Kaludov et al., 2001). Because the ILM lacks sialic acid, AAV-5 is unable to dock at the vitreoretinal junction, resulting in poor retinal transduction (Dalkara et al., 2009). However, ILM disruption with Pronase E leads to robust retinal transduction of this sialic acid-dependent serotype (Dalkara et al., 2009).
Further illustrating the barrier effect that the ILM imposes on viral gene therapy, inherited X-linked retinoschisis (XLRS) with compromised ILM is associated with increased retinal transduction efficiency (Park et al., 2009). XLRS is caused by a recessive mutation in the RS1 gene coding for retinoschisin, a soluble protein secreted by photoreceptors and bipolar cells and involved in retinal cell adhesion (Tolun et al., 2016). Mutations in retinoschisin lead to disorganized retinal layers and cystic cavity formation causing retinal layer separations (schisis cavities) (Molday, 2007), particularly involving the macula and decreasing visual acuity (Tantri et al., 2004). The extensive retinal separations result in fragile retina and pathological changes in the ILM permeability (Park et al., 2009). Studies in Rs1-KO mice, which phenocopy those with XLRS, have shown that AAV-8 vectors delivering RS1 gene can successfully transduce all layers of the retina after IVT injection, whereas the same administration performed in wild-type retina fail to transduce (Bush et al., 2016; Park et al., 2009). This approach of delivering RS1 gene has now moved to human trials (ClinicalTrials.gov: NCT02317887) and has demonstrated positive safety profiles (Cukras et al., 2018). Similarly, Müller cell transduction has been demonstrated to be more efficient in Dp71-null mice, which are deficient in dystrophin Dp71 and consequently have much thinner ILM compared to wildtype (Vacca et al., 2014). Surgical ILM peeling also demonstrates enhanced transduction in a variety of animal studies (Takahashi et al., 2017; Teo et al., 2018). This procedure combined with IVT AAV-2 delivery in NHPs results in increased fluorescence intensity and depth of retinal penetration to the outer nuclear layer, whereas transduction is confined to the GCL in sham controls (Teo et al., 2018).
A novel “sub-ILM” injection technique that delivers viral vectors between the ILM and neuroretina has shown efficacy in experimental NHP models (Boye et al., 2016; Gamlin et al., 2019). By circumventing the ILM completely, AAV2-GFP transduction was achieved in RGCs, Müller glia, bipolar cells, and photoreceptors in the region underneath the injection bleb. Additional benefits include sequestration of the vector near the target cells, reduction of exposure to immune surveillance, and maintenance of high concentrations at low delivery volumes. However, efficacy of this approach is localized to the injection site, performance requires concomitant pars plana vitrectomy, and ILM detachment and retinal layer disorganization are important potential drawbacks. Further development of sub-ILM injection is needed to supplant the conventional IVT injection, which boasts good safety profile and is a simple office-based procedure.
Exosome-associated AAV (exo-AAV) has gained interest as an alternative to circumventing the ILM without surgical or enzymatic disruption. Exosomes are lipid vesicles secreted by cells and can package and transfer both proteins or RNA (György et al., 2011). These exo-AAV vectors have demonstrated increased transduction compared to conventional AAV vectors, and have additional advantages of resistance to neutralizing antibodies and the ability to cross the blood brain barrier (György et al., 2014; Yang et al., 2015). IVT injected exo-AAV show increased retinal penetration as well as transduction of more retinal cell types compared to AAV-2 vectors not packaged in exosomes (Wassmer et al., 2017). The exact mechanism explaining increased cellular tropism is not understood, but exosomes are involved in multiple transport pathways, including passive diffusion and receptor mediated endocytosis and phagocytosis (Mulcahy et al., 2014).
In summary, research into ocular AAV delivery has demonstrated myriad approaches to circumventing the ILM. Methods that have increased efficacy in intraocular viral transduction include enzymatic ILM digestion, surgical ILM peeling, modulating molecular interactions with ILM, and sub-ILM injections. All these techniques facilitate intravitreal therapeutic delivery, which may pose lower morbidity compared to subretinal injections. Ultimately, the methodologies employed by gene delivery to overcome the ILM might be repurposed toward addressing an even greater challenge – intraocular cell transplantation for neuronal replacement.
6. Role of the ILM in cell transplantation
RGCs are progressively and irreversibly lost in patients with optic neuropathies, owing to their central nervous system (CNS) lineage and negligible regenerative capacity in mammals. This translates to permanent blindness for a subset of patients with glaucoma, ischemic optic neuropathy, and a number of other inflammatory, traumatic, compressive, mitochondria, toxic, or metabolic causes of RGC loss. Tremendous advancements have been made in identifying the molecular pathways involved in RGC axonal and somal degeneration, and a number of interventions have established that RGCs which survive a primary injury can be induced to regenerate axons beyond the optic chiasm into central visual targets (de Lima et al., 2012; Li et al., 2015; Lim et al., 2016; Yungher et al., 2015). Nonetheless, no neuroprotective or regenerative treatments for optic neuropathy have, as of yet, successfully preserved or restored vision in human clinical trials. A critical limitation of such approaches is that they depend on surviving endogenous RGCs, and therefore will provide limited benefit to patients with very advanced disease and significant vision loss.
Neuronal replacement through transplantation may offer a promising therapeutic approach to functional restoration in neurodegenerative diseases, including optic neuropathy. Differentiation and engineering of donor RGCs for transplantation is an area of active research and beyond the scope of this review (Ji and Tang, 2019; Lee et al., 2018; Sluch et al., 2017). However, it is worth considering that donor cells could be genetically engineered to secrete neuroprotective stimuli and modulate the host microenvironment to promote endogenous cell survival and prevent further structural and functional damage (Flachsbarth et al., 2018). Furthermore, intrinsic molecular pathways in donor cells could be engineered to enhance their own resiliency to disease conditions in the host (Welsbie et al., 2019). Key obstacles to be overcome in order to translate RGC transplantation to patients include promoting and guiding axon regeneration to central brain targets and achieving functional integration into existing neuroretinal circuitries. Though much knowledge about molecular pathways involved in reestablishing efferent connectivity has been elucidated by studying endogenous RGC axon regeneration (de Lima et al., 2012; Li et al., 2015; Lim et al., 2016; Yungher et al., 2015), relatively less is understood about the obstacles preventing donor RGCs from growing dendrites into the inner plexiform layer and synapsing with bipolar and amacrine cells. The ILM may present one such obstacle.
Pioneering studies transplanting hippocampus-derived neural progenitor cells into the retina demonstrated some structural integration, albeit with limited success. Grafting these cells into adult rat eyes that have either undergone ischemia-reperfusion injury, iatrogenic retinal scarring, or were dystrophic, resulted in donor neurite integration and cellular expression of MAP2 and GFAP (Kurimoto et al., 2001; Nishida et al., 2000; Young et al., 2000). These findings provided early proof of principle for the therapeutic concept of cell transplantation within the inner retina. Since then, research has been performed assessing intravitreal transplantation of an array of cell types, including embryonic stem cells (ESC), mesenchymal stem cells (MSC), Müller glial-derived cells, and primary or stem cell-derived retinal ganglion cells (Becker et al., 2016; Bull et al., 2008; Cho et al., 2012; Divya et al., 2017; Hertz et al., 2014; Jagatha et al., 2009; Johnson et al., 2010; Koike-Kiriyama et al., 2007; Singhal et al., 2012; Wang et al., 2019).
ESCs have the potential to generate multiple differentiated neuronal cell lineages at large scale, and thus are an attractive source for retinal neuronal transplantation. For example, when induced with FGF2, ESC-derived neural progenitor cells differentiate along the RGC lineage (Jagatha et al., 2009). Intravitreal transplantation of these ESC-derived RGC-like cells in a mouse NMDA-induced glaucoma model led to improved light responsiveness, though this was likely related to neuroprotection of surviving endogenous RGCs (Divya et al., 2017). Although the graft survival rate was not reported, donor cells localized to the retinal surface without somal integration into the parenchyma. Another group recently generated functional RGCs from human induced-pluripotent stem cells and demonstrated their viability four weeks after intravitreal injection in a murine optic nerve crush model. Similar to the previous study, the graft survival rate was not reported, and evidence of cellular integration into the host ganglion cell layer was limited (Rabesandratana et al., 2020).
In culture, mammalian Müller glia exhibit neural stem cell properties (Das et al., 2006), and have been investigated for their potential to integrate into host retinal tissue. Grafted human Müller stem cells into either glaucomatous or normal rat eyes do not migrate spontaneously into the host retinal parenchyma (Bull et al., 2008). However, intravitreal co-administration of chondroitinase ABC leads to increased Müller stem cells migration into the ganglion cell layer and extension of neurites into the host. On the other hand, Müller stem cells transplanted into the subretinal space in rats are capable of migrating into the retina spontaneously without host tissue modification (Lawrence et al., 2007). These results suggest a major obstacle is situated at the vitreoretinal interface.
To identify the potential source of inner retinal blockade to intravitreal graft migration, we previously transplanted MSCs both in vivo and ex vivo into rat retina (Johnson et al., 2010). Building on the hypothesis that transplanted cell migration appears to arrest at the ILM, we mechanically removed the ILM. Whereas the majority of the transplanted MSCs remain outside of intact ILM, regions devoid of ILM had significantly increased integration into the retinal parenchyma. However, high levels of MSC engraftment also coincided with significantly reduced local glial reactivity, possibly from damaging Müller endfeet during ILM peeling. To address this potential confounder, selective gliotoxin α-aminoadipic acid (AAA) was used to transiently suppress Müller cell function without affecting other retinal neurons or ECM architecture. AAA treatment downregulated expression of the glial intermediate filaments GFAP, nestin, and vimentin, while preserving neuronal and ECM protein expression and ILM integrity. Glial suppression was associated with significant enhancement of graft migration into the inner layers of the host retina through the intact ILM, both in retinal explants and in vivo. Moreover, ILM digestion with collagenase did not improve MSC migration into the retina when gliosis was preserved. Thus, in contrast to stem cells differentiated toward the ectodermal lineage (see below), mesenchymal stem cells can migrate through intact ILM if reactive gliosis is suppressed. This finding suggests that cell type specific interactions with the ILM may differentially modulate the ability to achieve retinal integration following transplantation.
Evidence of functional engraftment of purified primary mouse RGCs into live mice may support the possibility that migration through the ILM is cell type dependent. Indeed, some transplanted primary RGCs can migrate through an intact native ILM and localize to the host RGC layer (Venugopalan et al., 2016). However, this is an inefficient process with less than 10% of injected eyes showing successful engraftment, and typically only 1% of injected RGCs surviving. Although the mechanism of cellular migration through the ILM or the rate of localization to the host RGC layer were not specified in that work, the authors of this landmark paper do demonstrate evidence of light responsiveness of these donor cells, confirming functional integration into the neuroretinal circuitry.
Taken together, existing work in cellular transplantation provides evidence of limited graft integration in a variety of donor cell types, including neuronal and mesenchymal lineage, but suggests that the efficiency of intraretinal migration for a variety of transplanted cell types can be improved by either mechanically peeling or enzymatically disrupting the ILM (Johnson and Martin, 2008; Johnson et al., 2011; Singhal et al., 2008; Suzuki et al., 2007). These works provide circumstantial evidence that the ILM is an important physical obstacle for RGCs without directly investigating its barrier role. Recently, we provided the first direct empiric evidence that identifies the ILM as a critical barrier to transplanted human RGC integration (Zhang et al., 2021). Using organotypic mouse retinal explants with transplanted human embryonic stem cell derived RGCs (hES-RGCs) we observed that hES-RGCs encountering an intact ILM are unable to extend neurites into the recipient retinal parenchyma. In contrast, transplanted RGCs are able to integrate neurites into the inner plexiform layer (IPL) in areas where ILM was mechanically broken, such as near cut edges on the explants. In order to enhance RGC neurite ingrowth, we enzymatically digested ECM proteins with Pronase E at a concentration (0.6U/ml) that effectively increased ILM permeability without evidence of toxic effects such as accelerated neurodegeneration or reactive gliosis. Pronase E treatment of ILM resulted in a dramatic increase (~40 fold) in hES-RGC neurite localization to the IPL. Ongoing work is required to translate this approach into a method that facilitates increasing the cellular permeability of the ILM in vivo. Nonetheless, this finding could pave the way for achieving true functional RGC replacement through transplantation for large numbers of donor neurons.
In addition to somal and neurite migration in the radial orientation, any transplantation therapy must also consider the topographical distribution of engrafted donor cells along the retinal lamellae, a previously understudied aspect of transplantation experiments. Lessons from developmental studies have shown that interactions between immature RGCs and the ILM govern cellular mosaicism and spatial patterning (Clements et al., 2017; Riccomagno et al., 2014). In our organotypic mouse retinal explant model, we observe dramatic spatial clustering of transplanted hES-RGCs on intact ILM, a phenomenon not observed on retinas receiving proteolytic enzyme treatment (Zhang et al., 2021). The difference was quantified in a statistically robust manner using spatial analyses previously applied to characterizing endogenous retinal mosaicism, and which is readily repurposed for defining the topography of transplanted RGCs (Keeley et al., 2020). This finding suggests that interactions between hES-RGCs and the ILM promote cell clustering, and dispersion of transplanted RGCs may ultimately be necessary to achieve widespread retinotopic coverage. Whether cell clumping and neurite ingrowth are related will need to be determined by future work.
Molecular pathways governing retinal development inform us that neurons rely on interactions with ECM components for survival, polarization, growth and maturation (as discussed above). Therefore, while mechanical removal or enzymatic disruption of the ILM enhances graft migration and neurite ingrowth into the host retina, ECM signaling may still be required for achieving true functional integration that requires donor cell polarization, dendrite stratification, and axon pathfinding. Thus, rather than elimination of this barrier, manipulating cell-ILM interactions may be a preferred approach moving forward.
Neurons interact with the ILM through cell surface receptors. The integrin family is the main class of receptors on RGCs that are activated by extracellular matrix laminins. Conditional deletion of integrins or their downstream Cas adaptor proteins in RGCs is sufficient to cause ganglion cell layer disruption and ectopic migration of RGCs across the ILM (Riccomagno et al., 2014). Therefore, approaches of genetically modifying the integrin signaling pathways in donor RGCs may promote their migration through the ILM and enhance integration following transplantation.
Rational mutagenesis used to enhance AAV transduction may also be translatable to cellular transplantation. For example, gain-of-function expression of HSPG binding partners on viral capsids dramatically increases accumulation of viral vectors at the ILM and enhances viral transduction of retinal neurons. Repurposing this approach to genetically express HSPG receptors on donor RGCs may improve cellular migration into retinal parenchyma.
6. Conclusions
The ILM and its associated ECM participate in key steps of retinal development by orchestrating embryonic milestones including neuroblast migration, cell body polarization, and axonal growth. These functional roles are mediated through specific structural constituents of the ILM. Studying the structure of the vitreoretinal interface is pertinent to our understanding of the pathophysiology of certain vitreoretinal diseases, and may provide the basis for optimizing innovative retina-targeted therapies. Nonetheless, the ILM appears dispensable in adulthood, as surgical ILM removal has been used for the treatment of vitreoretinal disorders such as macular hole, leading to visual benefit.
Viral gene therapy and stem cell transplantation are two emerging technologies that have significant potential for preserving or restoring vision in a host of retinal neurodegenerative disorders. The ILM appears to be a significant obstacle for the clinical translation of these potential therapies. Experimental evidence suggests that mitigating its barrier effect through enzymatic and physical disruption increases the efficacy of gene and cell therapy in the retina. Importantly, most of our knowledge regarding the roles of the ILM during retinal development and in limiting gene and cell therapy are derived from rodent models, the ultimate therapeutic target is human patients. While surgical methods for removing the ILM within the central macula in human patients exist and are relatively safe, variations in overall eye anatomy, ILM permeability, and ILM thickness between humans and laboratory animals present challenges in translating results from animal models to human intervention. Moving these concepts forward will likely require experimentation in larger pre-clinical models. Moreover, the importance of ILM-retinal signaling during development suggests that overt removal of the ILM may be inferior to transient disruption of donor RGC-ILM interactions for achieving functional engraftment. Resolving these issues represents an important step toward implementing ILM circumvention for the augmentation of innovative emerging ophthalmic therapies.
Highlights:
Retinal development is governed by molecular interactions between the internal limiting membrane (ILM) and neurons, glia, and the vasculature.
The ILM is an important obstacle to some emerging ocular treatments, including gene therapy and cell transplantation.
Revisiting molecular interactions that are critical during retinal development may inform methods for circumventing the ILM for cell transplantation
Other Acknowledgements
The authors are grateful to Sarah Quillen for helping to acquire the transmission electron microscopy images shown in Figure 1.
Funding
Received funding from the National Eye Institute (K08-EY031801 and P30-EY001765), Research to Prevent Blindness: Career Development Award (TVJ) and Unrestricted Grant Support (Wilmer Eye Institute)
Financial disclosures
None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
None.
References:
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, Kim SR, Maguire A, Rex TS, Di Vicino U, Cutillo L, Sparrow JR, Williams DS, Bennett J, Auricchio A, 2008. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J. Clin. Invest 118, 1955–1964. 10.1172/JCI34316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpy F, Jivkov I, Sorokin L, Klein A, Arnold C, Huss Y, Kedinger M, Simon-Assmann P, Lefebvre O, 2005. Generation of a conditionally null allele of the laminin α1 gene. genesis 43, 59–70. 10.1002/gene.20154 [DOI] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Cepko CL, 1995. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development 121, 3637–50. [DOI] [PubMed] [Google Scholar]
- Balasubramani M, Schreiber EM, Candiello J, Balasubramani GK, Kurtz J, Halfter W, 2010. Molecular interactions in the retinal basement membrane system: A proteomic approach. Matrix Biol. 29, 471–483. 10.1016/j.matbio.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Becker S, Eastlake K, Jayaram H, Jones MF, Brown RA, McLellan GJ, Charteris DG, Khaw PT, Limb GA, 2016. Allogeneic Transplantation of Müller-Derived Retinal Ganglion Cells Improves Retinal Function in a Feline Model of Ganglion Cell Depletion. Stem Cells Transl. Med 5, 192–205. 10.5966/sctm.2015-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD, 2007. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037. 10.1038/nature05817 [DOI] [PubMed] [Google Scholar]
- Boye SE, Alexander JJ, Witherspoon CD, Boye SL, Peterson JJ, Clark ME, Sandefer KJ, Girkin CA, Hauswirth WW, Gamlin PD, 2016. Highly Efficient Delivery of Adeno-Associated Viral Vectors to the Primate Retina. Hum. Gene Ther 27, 580–597. 10.1089/hum.2016.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DC, Yoshida-Moriguchi T, Zheng T, Venzke D, Anderson ME, Strazzulli A, Moracci M, Yu L, Hohenester E, Campbell KP, 2016. Structural basis of laminin binding to the LARGE glycans on dystroglycan. Nat. Chem. Biol 12, 810–814. 10.1038/nchembio.2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu SC, Kuijer R, van der Worp RJ, Li XR, Hooymans JMM, Los LI, 2015. The Ultrastructural Localization of Type II, IV, and VI Collagens at the Vitreoretinal Interface. PLoS One 10, e0134325. 10.1371/journal.pone.0134325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ND, Limb GA, Martin KR, 2008. Human Müller Stem Cell (MIO-M1) Transplantation in a Rat Model of Glaucoma: Survival, Differentiation, and Integration. Investig. Opthalmology Vis. Sci 49, 3449. 10.1167/iovs.08-1770 [DOI] [PubMed] [Google Scholar]
- Bush RA, Zeng Y, Colosi P, Kjellstrom S, Hiriyanna S, Vijayasarathy C, Santos M, Li J, Wu Z, Sieving PA, 2016. Preclinical Dose-Escalation Study of Intravitreal AAV-RS1 Gene Therapy in a Mouse Model of X-linked Retinoschisis: Dose-Dependent Expression and Improved Retinal Structure and Function. Hum. Gene Ther 27, 376–389. 10.1089/hum.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Simons DL, Fu X-Y, Feng G-S, Wu SM, Zhang X, 2011. Loss of Shp2-Mediated Mitogen-Activated Protein Kinase Signaling in Muller Glial Cells Results in Retinal Degeneration. Mol. Cell. Biol 31, 2973–2983. 10.1128/MCB.05054-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H, 2007. Biomechanical properties of native basement membranes. FEBS J. 274, 2897–2908. 10.1111/j.1742-4658.2007.05823.x [DOI] [PubMed] [Google Scholar]
- Candiello J, Cole GJ, Halfter W, 2010. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 29, 402–410. 10.1016/j.matbio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Cehajic-Kapetanovic J, Le Goff MM, Allen A, Lucas RJ, Bishop PN, 2011. Glycosidic enzymes enhance retinal transduction following intravitreal delivery of AAV2. Mol. Vis 17, 1771–83. [PMC free article] [PubMed] [Google Scholar]
- Chai L, Morris JE, 1999. Heparan Sulfate in the Inner Limiting Membrane of Embryonic Chicken Retina Binds Basic Fibroblast Growth Factor to Promote Axonal Outgrowth. Exp. Neurol 160, 175–185. 10.1006/exnr.1999.7195 [DOI] [PubMed] [Google Scholar]
- Chai L, Morris JE, 1994. Distribution of heparan sulfate proteoglycans in embryonic chicken neural retina and isolated inner limiting membrane. Curr. Eye Res 13, 669–677. 10.3109/02713689408999903 [DOI] [PubMed] [Google Scholar]
- Chatziralli I, Theodossiadis G, Chatzirallis A, Parikakis E, Mitropoulos P, Theodossiadis P, 2018. RANIBIZUMAB FOR RETINAL VEIN OCCLUSION. Retina 38, 559–568. 10.1097/IAE.0000000000001579 [DOI] [PubMed] [Google Scholar]
- Cho J-H, Mao C-A, Klein WH, 2012. Adult mice transplanted with embryonic retinal progenitor cells: new approach for repairing damaged optic nerves. Mol. Vis 18, 2658–72. [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Keenan TDL, Fielder HL, Collinson LJ, Holley RJ, Merry CLR, van Kuppevelt TH, Day AJ, Bishop PN, 2011. Mapping the differential distribution of glycosaminoglycans in the adult human retina, choroid, and sclera. Invest. Ophthalmol. Vis. Sci 52, 6511–21. 10.1167/iovs.11-7909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements R, Turk R, Campbell KP, Wright KM, 2017. Dystroglycan Maintains Inner Limiting Membrane Integrity to Coordinate Retinal Development. J. Neurosci 37, 8559–8574. 10.1523/JNEUROSCI.0946-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI, 1961. Electron microscopic observations of the internal limiting membrane and optic fiber layer of the retina of the rhesus monkey (M. mulatta). Am. J. Anat 108, 179–197. 10.1002/aja.1001080205 [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD, 2000. Form and function: The laminin family of heterotrimers. Dev. Dyn 218, 213–234. [DOI] [PubMed] [Google Scholar]
- Comander J, Carvalho L, Wassmer S, Xiao R, Plovie E, Langsdorf A, Lim L, Hafler B, Wu DM, Eliott D, Kim LA, Vandenberghe LH, 2016. 29. Novel Surgical Method for Intravitreal AAV Administration Overcomes Transduction Barriers in Non-Human Primates. Mol. Ther 24, S13–S14. 10.1016/S1525-0016(16)32838-6 [DOI] [Google Scholar]
- Combs AC, Ervasti JM, 2005. Enhanced laminin binding by α-dystroglycan after enzymatic deglycosylation. Biochem. J 390, 303–309. 10.1042/BJ20050375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukras C, Wiley HE, Jeffrey BG, Sen HN, Turriff A, Zeng Y, Vijayasarathy C, Marangoni D, Ziccardi L, Kjellstrom S, Park TK, Hiriyanna S, Wright JF, Colosi P, Wu Z, Bush RA, Wei LL, Sieving PA, 2018. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther 26, 2282–2294. 10.1016/j.ymthe.2018.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara D, Kolstad KD, Caporale N, Visel M, Klimczak RR, Schaffer DV, Flannery JG, 2009. Inner Limiting Membrane Barriers to AAV-mediated Retinal Transduction From the Vitreous. Mol. Ther 17, 2096–2102. 10.1038/mt.2009.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I, 2006. Neural stem cell properties of Müller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev. Biol 299, 283–302. 10.1016/j.ydbio.2006.07.029 [DOI] [PubMed] [Google Scholar]
- de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert H-Y, Fagiolini M, Martinez AMB, Benowitz L, 2012. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci 109, 9149–9154. 10.1073/pnas.1119449109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya MS, Rasheed VA, Schmidt T, Lalitha S, Hattar S, James J, 2017. Intraocular Injection of ES Cell-Derived Neural Progenitors Improve Visual Function in Retinal Ganglion Cell-Depleted Mouse Models. Front. Cell. Neurosci 11. 10.3389/fncel.2017.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Landfair J, Balasubramani M, Bier ME, Cole G, Halfter W, 2002. Expression of basal lamina protein mRNAs in the early embryonic chick eye. J. Comp. Neurol 447, 261–273. 10.1002/cne.10245 [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M, 2002. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest. Ophthalmol. Vis. Sci 43, 3500–10. [PubMed] [Google Scholar]
- Edwards MM, Mammadova-Bach E, Alpy F, Klein A, Hicks WL, Roux M, Simon-Assmann P, Smith RS, Orend G, Wu J, Peachey NS, Naggert JK, Lefebvre O, Nishina PM, 2010. Mutations in Lama1 Disrupt Retinal Vascular Development and Inner Limiting Membrane Formation. J. Biol. Chem 285, 7697–7711. 10.1074/jbc.M109.069575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MM, McLeod DS, Grebe R, Heng C, Lefebvre O, Lutty GA, 2011. Lama1 mutations lead to vitreoretinal blood vessel formation, persistence of fetal vasculature, and epiretinal membrane formation in mice. BMC Dev. Biol 11, 60. 10.1186/1471-213X-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BL, Hirsch ML, Barker JC, Connelly JP, Steininger RJ, Porteus MH, 2013. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1–9) and one engineered adeno-associated virus serotype. Virol. J 10, 74. 10.1186/1743-422X-10-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fässler R, Meyer M, 1995. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9, 1896–908. 10.1101/gad.9.15.1896 [DOI] [PubMed] [Google Scholar]
- Flachsbarth K, Jankowiak W, Kruszewski K, Helbing S, Bartsch S, Bartsch U, 2018. Pronounced synergistic neuroprotective effect of GDNF and CNTF on axotomized retinal ganglion cells in the adult mouse. Exp. Eye Res 176, 258–265. 10.1016/j.exer.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J, 1991. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 10, 3137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M, 2007. Development of the retinal vasculature. Angiogenesis 10, 77–88. 10.1007/s10456-007-9065-1 [DOI] [PubMed] [Google Scholar]
- Fukai N, 2002. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 21, 1535–1544. 10.1093/emboj/21.7.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Alexander JJ, Boye SL, Witherspoon CD, Boye SE, 2019. SubILM Injection of AAV for Gene Delivery to the Retina. pp. 249–262. 10.1007/978-1-4939-9139-6_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmüller A, 1998. Essential role of α6 integrins in cortical and retinal lamination. Curr. Biol 8, 983–S1. 10.1016/S0960-9822(98)70402-6 [DOI] [PubMed] [Google Scholar]
- Guidry C, 2005. The role of Müller cells in fibrocontractive retinal disorders. Prog. Retin. Eye Res 24, 75–86. 10.1016/j.preteyeres.2004.07.001 [DOI] [PubMed] [Google Scholar]
- György B, Fitzpatrick Z, Crommentuijn MHW, Mu D, Maguire CA, 2014. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 35, 7598–7609. 10.1016/j.biomaterials.2014.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI, 2011. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci 68, 2667–88. 10.1007/s00018-011-0689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Candiello J, Hu H, Zhang P, Schreiber E, Balasubramani M, 2013. Protein composition and biomechanical properties of in vivo-derived basement membranes. Cell Adh. Migr 7, 64–71. 10.4161/cam.22479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Dong A, Eller AW, Nischt R, 2008. Origin and turnover of ECM proteins from the inner limiting membrane and vitreous body. Eye 22, 1207–1213. 10.1038/eye.2008.19 [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Schurer B, Ring C, Cole GJ, Eller A, 2005. Embryonic Synthesis of the Inner Limiting Membrane and Vitreous Body. Investig. Opthalmology Vis. Sci 46, 2202. 10.1167/iovs.04-1419 [DOI] [PubMed] [Google Scholar]
- Halfter W, Oertle P, Monnier CA, Camenzind L, Reyes‐Lua M, Hu H, Candiello J, Labilloy A, Balasubramani M, Henrich PB, Plodinec M, 2015. New concepts in basement membrane biology. FEBS J. 282, 4466–4479. 10.1111/febs.13495 [DOI] [PubMed] [Google Scholar]
- Halfter W, Sebag J, Cunningham ET, 2014. II.E. Vitreoretinal Interface and Inner Limiting Membrane, in: Vitreous. Springer New York, New York, NY, pp. 165–191. 10.1007/978-1-4939-1086-1_11 [DOI] [Google Scholar]
- Hardingham TE, Fosang AJ, 1992. Proteoglycans: many forms and many functions. FASEB J. 6, 861–870. 10.1096/fasebj.6.3.1740236 [DOI] [PubMed] [Google Scholar]
- Harris TJC, Tepass U, 2010. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol 11, 502–514. 10.1038/nrm2927 [DOI] [PubMed] [Google Scholar]
- Heegaard S, Jensen OA, Prause JU, 1986. Structure and composition of the inner limiting membrane of the retina. Graefe’s Arch. Clin. Exp. Ophthalmol 224, 355–360. 10.1007/BF02150029 [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP, 1998. A Role for Dystroglycan in Basement Membrane Assembly. Cell 95, 859–870. 10.1016/S0092-8674(00)81708-0 [DOI] [PubMed] [Google Scholar]
- Hertz J, Qu B, Hu Y, Patel RD, Valenzuela DA, Goldberg JL, 2014. Survival and Integration of Developing and Progenitor-Derived Retinal Ganglion Cells following Transplantation. Cell Transplant. 23, 855–872. 10.3727/096368913X667024 [DOI] [PubMed] [Google Scholar]
- Hogan MJ, 1963. THE VITREOUS, ITS STRUCTURE, AND RELATION TO THE CILIARY BODY AND RETINA. PROCTOR AWARD LECTURE. Invest. Ophthalmol 2, 418–45. [PubMed] [Google Scholar]
- Hurskainen M, Eklund L, Hägg PO, Fruttiger M, Sormunen R, Ilves M, Pihlajaniemi T, 2005. Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 19, 1564–1566. 10.1096/fj.04-3101fje [DOI] [PubMed] [Google Scholar]
- Jackson TL, Antcliff RJ, Hillenkamp J, Marshall J, 2003. Human Retinal Molecular Weight Exclusion Limit and Estimate of Species Variation. Investig. Opthalmology Vis. Sci 44, 2141. 10.1167/iovs.02-1027 [DOI] [PubMed] [Google Scholar]
- Jagatha B, Divya MS, Sanalkumar R, Indulekha CL, Vidyanand S, Divya TS, Das AV, James J, 2009. In vitro differentiation of retinal ganglion-like cells from embryonic stem cell derived neural progenitors. Biochem. Biophys. Res. Commun 380, 230–235. 10.1016/j.bbrc.2009.01.038 [DOI] [PubMed] [Google Scholar]
- Ji S-L, Tang S-B, 2019. Differentiation of retinal ganglion cells from induced pluripotent stem cells: a review. Int. J. Ophthalmol 10.18240/ijo.2019.01.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Fahim AT, Rao RC, 2015. Acute Ocriplasmin Retinopathy. Retina 35, 1055–1058. 10.1097/IAE.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR, 2010. Neuroprotective Effects of Intravitreal Mesenchymal Stem Cell Transplantation in Experimental Glaucoma. Investig. Opthalmology Vis. Sci 51, 2051. 10.1167/iovs.09-4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Martin KR, 2008. Development and Characterization of an Adult Retinal Explant Organotypic Tissue Culture System as an In Vitro Intraocular Stem Cell Transplantation Model. Investig. Opthalmology Vis. Sci 49, 3503. 10.1167/iovs.07-1601 [DOI] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Martin KR, 2011. Stem cell therapy for glaucoma: possibilities and practicalities. Expert Rev. Ophthalmol 6, 165–174. 10.1586/eop.11.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser PK, Kampik A, Kuppermann BD, Girach A, Rizzo S, Sergott RC, 2015. SAFETY PROFILE OF OCRIPLASMIN FOR THE PHARMACOLOGIC TREATMENT OF SYMPTOMATIC VITREOMACULAR ADHESION/TRACTION. Retina 35, 1111–1127. 10.1097/IAE.0000000000000448 [DOI] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA, 2001. Adeno-Associated Virus Serotype 4 (AAV4) and AAV5 Both Require Sialic Acid Binding for Hemagglutination and Efficient Transduction but Differ in Sialic Acid Linkage Specificity. J. Virol 75, 6884–6893. 10.1128/JVI.75.15.6884-6893.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Eglen SJ, Reese BE, 2020. From random to regular: Variation in the patterning of retinal mosaics*. J. Comp. Neurol 528, 2135–2160. 10.1002/cne.24880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, 2014. Safety and complications of ocriplasmin: ocriplasmin, ocriplasmin; oh, how safe art thou? JAMA Ophthalmol. 132, 379–80. 10.1001/jamaophthalmol.2014.278 [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR, 1982. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21, 6188–93. 10.1021/bi00267a025 [DOI] [PubMed] [Google Scholar]
- Koike-Kiriyama N, Adachi Y, Minamino K, Iwasaki M, Nakano K, Koike Y, Yamada H, Mukaide H, Shigematsu A, Mizokami T, Matsumura M, Ikehara S, 2007. Human cord blood cells can differentiate into retinal nerve cells. Acta Neurobiol. Exp. (Wars) 67, 359–65. [DOI] [PubMed] [Google Scholar]
- Kotterman MA, Chalberg TW, Schaffer DV, 2015. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng 17, 63–89. 10.1146/annurev-bioeng-071813-104938 [DOI] [PubMed] [Google Scholar]
- Kröger S, Mann S, 1996. Biochemical and Functional Characterization of Basal Laminabound Agrin in the Chick Central Nervous System. Eur. J. Neurosci 8, 500–509. 10.1111/j.1460-9568.1996.tb01234.x [DOI] [PubMed] [Google Scholar]
- Kunze A, Abari E, Semkova I, Paulsson M, Hartmann U, 2010. Deposition of Nidogens and Other Basement Membrane Proteins in the Young and Aging Mouse Retina. Ophthalmic Res. 43, 108–112. 10.1159/000247595 [DOI] [PubMed] [Google Scholar]
- Kurimoto Y, Shibuki H, Kaneko Y, Ichikawa M, Kurokawa T, Takahashi M, Yoshimura N, 2001. Transplantation of adult rat hippocampus-derived neural stem cells into retina injured by transient ischemia. Neurosci. Lett 306, 57–60. 10.1016/S0304-3940(01)01857-2 [DOI] [PubMed] [Google Scholar]
- Labelle-Dumais C, Dilworth DJ, Harrington EP, de Leau M, Lyons D, Kabaeva Z, Manzini MC, Dobyns WB, Walsh CA, Michele DE, Gould DB, 2011. COL4A1 Mutations Cause Ocular Dysgenesis, Neuronal Localization Defects, and Myopathy in Mice and Walker-Warburg Syndrome in Humans. PLoS Genet. 7, e1002062. 10.1371/journal.pgen.1002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT, Limb GA, 2007. MIO-M1 Cells and Similar Müller Glial Cell Lines Derived from Adult Human Retina Exhibit Neural Stem Cell Characteristics. Stem Cells 25, 2033–2043. 10.1634/stemcells.2006-0724 [DOI] [PubMed] [Google Scholar]
- Lee J, Choi S-H, Kim Y-B, Jun I, Sung JJ, Lee DR, Kim YI, Cho MS, Byeon SH, Kim D-S, Kim D-W, 2018. Defined Conditions for Differentiation of Functional Retinal Ganglion Cells From Human Pluripotent Stem Cells. Investig. Opthalmology Vis. Sci 59, 3531. 10.1167/iovs.17-23439 [DOI] [PubMed] [Google Scholar]
- Lee Y, Kameya S, Cox GA, Hsu J, Hicks W, Maddatu TP, Smith RS, Naggert JK, Peachey NS, Nishina PM, 2005. Ocular abnormalities in Largemyd and Largevls mice, spontaneous models for muscle, eye, and brain diseases. Mol. Cell. Neurosci 30, 160–172. 10.1016/j.mcn.2005.07.009 [DOI] [PubMed] [Google Scholar]
- Lei W-L, Xing S-G, Deng C-Y, Ju X-C, Jiang X-Y, Luo Z-G, 2012. Laminin/β1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell Res. 22, 954–972. 10.1038/cr.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LA, 2011. Adler’s Physiology of the Eye.
- Li S, He Q, Wang H, Tang X, Ho KW, Gao X, Zhang Q, Shen Y, Cheung A, Wong F, Wong YH, Ip NY, Jiang L, Yung WH, Liu K, 2015. Injured adult retinal axons with Pten and Socs3 co-deletion reform active synapses with suprachiasmatic neurons. Neurobiol. Dis 73, 366–376. 10.1016/j.nbd.2014.09.019 [DOI] [PubMed] [Google Scholar]
- Li YN, Radner S, French MM, Pinzón-Duarte G, Daly GH, Burgeson RE, Koch M, Brunken WJ, 2012. The γ3 chain of laminin is widely but differentially expressed in murine basement membranes: Expression and functional studies. Matrix Biol. 31, 120–134. 10.1016/j.matbio.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby RT, Lavallee CR, Balkema GW, Brunken WJ, Hunter DD, 1999. Disruption of Laminin β2 Chain Production Causes Alterations in Morphology and Function in the CNS. J. Neurosci 19, 9399–9411. 10.1523/JNEUROSCI.19-21-09399.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J-HA, Stafford BK, Nguyen PL, Lien BV, Wang C, Zukor K, He Z, Huberman AD, 2016. Neural activity promotes long-distance, target-specific regeneration of adult retinal axons. Nat. Neurosci 19, 1073–1084. 10.1038/nn.4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lössl P, Kölbel K, Tänzler D, Nannemann D, Ihling CH, Keller MV, Schneider M, Zaucke F, Meiler J, Sinz A, 2014. Analysis of Nidogen-1/Laminin γ1 Interaction by Cross-Linking, Mass Spectrometry, and Computational Modeling Reveals Multiple Binding Modes. PLoS One 9, e112886. 10.1371/journal.pone.0112886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S, Kröger S, 1996. Agrin Is Synthesized by Retinal Cells and Colocalizes with Gephyrin [corrected title]. Mol. Cell. Neurosci 8, 1–13. 10.1006/mcne.1996.0039 [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P, 2001. Pax6 Is Required for the Multipotent State of Retinal Progenitor Cells. Cell 105, 43–55. 10.1016/S0092-8674(01)00295-1 [DOI] [PubMed] [Google Scholar]
- Matsuo N, Kitajima S, Hasegawa E, 1963. [ELECTRON MICROSCOPICAL STUDIES ON THE INTERNAL LIMITING MEMBRANE OF THE HUMAN RETINA]. Nihon Ganka Kiyo 14, 428–41. [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA, 1999. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development 126, 5713–24. [DOI] [PubMed] [Google Scholar]
- Mets MB, 1999. Childhood blindness and visual loss: an assessment at two institutions including a “new” cause. Trans. Am. Ophthalmol. Soc 97, 653–96. [PMC free article] [PubMed] [Google Scholar]
- Molday RS, 2007. Focus on Molecules: Retinoschisin (RS1). Exp. Eye Res 84, 227–228. 10.1016/j.exer.2005.12.013 [DOI] [PubMed] [Google Scholar]
- Mowat FM, Gornik KR, Dinculescu A, Boye SL, Hauswirth WW, Petersen-Jones SM, Bartoe JT, 2014. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 21, 96–105. 10.1038/gt.2013.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy LA, Pink RC, Carter DRF, 2014. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641. 10.3402/jev.v3.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Takahashi M, Tanihara H, Nakano I, Takahashi JB, Mizoguchi A, Ide C, Honda Y, 2000. Incorporation and differentiation of hippocampus-derived neural stem cells transplanted in injured adult rat retina. Invest. Ophthalmol. Vis. Sci 41, 4268–74. [PubMed] [Google Scholar]
- Nishihara H, 1991. Studies on the ultrastructure of the inner limiting membrane of the retina--distribution of anionic sites in the inner limiting membrane of the retina. Nihon. Ganka Gakkai Zasshi 95, 951–8. [PubMed] [Google Scholar]
- Noël G, Belda M, Guadagno E, Micoud J, Klöcker N, Moukhles H, 2005. Dystroglycan and Kir4.1 coclustering in retinal Müller glia is regulated by laminin-1 and requires the PDZ-ligand domain of Kir4.1. J. Neurochem 94, 691–702. 10.1111/j.1471-4159.2005.03191.x [DOI] [PubMed] [Google Scholar]
- Ochakovski GA, Bartz-Schmidt KU, Fischer MD, 2017. Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials. Front. Neurosci 11. 10.3389/fnins.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri S, Omri B, Savoldelli M, Jonet L, Thillaye-Goldenberg B, Thuret G, Gain P, Jeanny JC, Crisanti P, Behar-Cohen F, 2010. The outer limiting membrane (OLM) revisited: clinical implications. Clin. Ophthalmol 4, 183–95. 10.2147/opth.s5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TK, Wu Z, Kjellstrom S, Zeng Y, Bush RA, Sieving PA, Colosi P, 2009. Intravitreal delivery of AAV8 retinoschisin results in cell type-specific gene expression and retinal rescue in the Rs1-KO mouse. Gene Ther. 16, 916–926. 10.1038/gt.2009.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor JC, Rojas J, Pastor-Idoate S, Di Lauro S, Gonzalez-Buendia L, Delgado-Tirado S, 2016. Proliferative vitreoretinopathy: A new concept of disease pathogenesis and practical consequences. Prog. Retin. Eye Res 51, 125–155. 10.1016/j.preteyeres.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Patel TR, Bernards C, Meier M, McEleney K, Winzor DJ, Koch M, Stetefeld J, 2014. Structural elucidation of full-length nidogen and the laminin–nidogen complex in solution. Matrix Biol. 33, 60–67. 10.1016/j.matbio.2013.07.009 [DOI] [PubMed] [Google Scholar]
- Pedler C, 1961. THE INNER LIMITING MEMBRANE OF THE RETINA. Br. J. Ophthalmol 45, 423–38. 10.1136/bjo.45.6.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Tang L, Zhou Y, 2017. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 58, 217–226. 10.1159/000479157 [DOI] [PubMed] [Google Scholar]
- Peynshaert K, Devoldere J, De Smedt SC, Remaut K, 2018. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv. Drug Deliv. Rev 126, 44–57. 10.1016/j.addr.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Peynshaert K, Devoldere J, Minnaert A-K, De Smedt SC, Remaut K, 2019. Morphology and Composition of the Inner Limiting Membrane: Species-Specific Variations and Relevance toward Drug Delivery Research. Curr. Eye Res 44, 465–475. 10.1080/02713683.2019.1565890 [DOI] [PubMed] [Google Scholar]
- Pinzón-Duarte G, Daly G, Li YN, Koch M, Brunken WJ, 2010. Defective Formation of the Inner Limiting Membrane in Laminin β2- and γ3-Null Mice Produces Retinal Dysplasia. Investig. Opthalmology Vis. Sci 51, 1773. 10.1167/iovs.09-4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven I, Moisseiev J, 2010. THE INNER LIMITING MEMBRANE DOES NOT REGROW AFTER INDOCYANINE GREEN-GUIDED PEELING: PHOTO-REPORT OF TWO CASES. Retin. Cases Brief Rep 4, 284–286. 10.1097/ICB.0b013e3181ae7193 [DOI] [PubMed] [Google Scholar]
- Pournaras CJ, Emarah A, Petropoulos IK, 2011. Idiopathic Macular Epiretinal Membrane Surgery and ILM Peeling: Anatomical and Functional Outcomes. Semin. Ophthalmol 26, 42–46. 10.3109/08820538.2010.544237 [DOI] [PubMed] [Google Scholar]
- Rabesandratana O, Chaffiol A, Mialot A, Slembrouck-Brec A, Joffrois C, Nanteau C, Rodrigues A, Gagliardi G, Reichman S, Sahel J-A, Chédotal A, Duebel J, Goureau O, Orieux G, 2020. Generation of a Transplantable Population of Human iPSC-Derived Retinal Ganglion Cells. Front. Cell Dev. Biol 8. 10.3389/fcell.2020.585675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randlett O, Poggi L, Zolessi FR, Harris WA, 2011. The Oriented Emergence of Axons from Retinal Ganglion Cells Is Directed by Laminin Contact In Vivo. Neuron 70, 266–280. 10.1016/j.neuron.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wölfel J, Lüscher D, Zurlinden A, Stephan A, Ahmed S, Baici A, Ledermann B, Kunz B, Sonderegger P, 2007. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 21, 3468–3478. 10.1096/fj.07-8800com [DOI] [PubMed] [Google Scholar]
- Retzius G, 1871. V.: Bidrag till kännedomen om de inre lagren i ögats näthinna. Nord. Med. Ark 3, 23–31. 10.1111/j.0954-6820.1871.tb00806.x [DOI] [Google Scholar]
- Rhodes RH, 1982. An ultrastructural study of the complex carbohydrates of the mouse posterior vitreoretinal juncture. Invest. Ophthalmol. Vis. Sci 22, 460–77. [PubMed] [Google Scholar]
- Riccomagno MM, Sun LO, Brady CM, Alexandropoulos K, Seo S, Kurokawa M, Kolodkin AL, 2014. Cas Adaptor Proteins Organize the Retinal Ganglion Cell Layer Downstream of Integrin Signaling. Neuron 81, 779–786. 10.1016/j.neuron.2014.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechet SA, DeVience E, Thompson JT, 2017. THE EFFECT OF INTERNAL LIMITING MEMBRANE PEELING ON IDIOPATHIC EPIRETINAL MEMBRANE SURGERY, WITH A REVIEW OF THE LITERATURE. Retina 37, 873–880. 10.1097/IAE.0000000000001263 [DOI] [PubMed] [Google Scholar]
- Schneider E, Johnson, 2011. Emerging nonsurgical methods for the treatment of vitreomacular adhesion: a review. Clin. Ophthalmol 1151. 10.2147/OPTH.S14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Bosenko DV, Zinkevich NC, Soules KA, Hyde DR, Vihtelic TS, Willer GB, Gregg RG, Link BA, 2006. Mutations in laminin alpha 1 result in complex, lens-independent ocular phenotypes in zebrafish. Dev. Biol 299, 63–77. 10.1016/j.ydbio.2006.07.005 [DOI] [PubMed] [Google Scholar]
- Singhal S, Bhatia B, Jayaram H, Becker S, Jones MF, Cottrill PB, Khaw PT, Salt TE, Limb GA, 2012. Human Müller Glia with Stem Cell Characteristics Differentiate into Retinal Ganglion Cell (RGC) Precursors In Vitro and Partially Restore RGC Function In Vivo Following Transplantation. Stem Cells Transl. Med 1, 188–199. 10.5966/sctm.2011-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S, Lawrence JM, Bhatia B, Ellis JS, Kwan AS, MacNeil A, Luthert PJ, Fawcett JW, Perez M-T, Khaw PT, Limb GA, 2008. Chondroitin Sulfate Proteoglycans and Microglia Prevent Migration and Integration of Grafted Müller Stem Cells into Degenerating Retina. Stem Cells 26, 1074–1082. 10.1634/stemcells.2007-0898 [DOI] [PubMed] [Google Scholar]
- Sluch VM, Chamling X, Liu MM, Berlinicke CA, Cheng J, Mitchell KL, Welsbie DS, Zack DJ, 2017. Enhanced Stem Cell Differentiation and Immunopurification of Genome Engineered Human Retinal Ganglion Cells. Stem Cells Transl. Med 6, 1972–1986. 10.1002/sctm.17-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, Haller JA, 2012. Enzymatic Vitreolysis with Ocriplasmin for Vitreomacular Traction and Macular Holes. N. Engl. J. Med 367, 606–615. 10.1056/NEJMoa1110823 [DOI] [PubMed] [Google Scholar]
- Steel DHW, Lotery AJ, 2013. Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye 27, S1–S21. 10.1038/eye.2013.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel D, Franco CA, Estrach S, Mettouchi A, Sauvaget D, Rosewell I, Schertel A, Armer H, Domogatskaya A, Rodin S, Tryggvason K, Collinson L, Sorokin L, Gerhardt H, 2011. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 12, 1135–1143. 10.1038/embor.2011.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ, 1998. Membrane-Associated Heparan Sulfate Proteoglycan Is a Receptor for Adeno-Associated Virus Type 2 Virions. J. Virol 72, 1438–1445. 10.1128/JVI.72.2.1438-1445.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Akimoto M, Imai H, Ueda Y, Mandai M, Yoshimura N, Swaroop A, Takahashi M, 2007. Chondroitinase ABC Treatment Enhances Synaptogenesis between Transplant and Host Neurons in Model of Retinal Degeneration. Cell Transplant. 16, 493–503. 10.3727/000000007783464966 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Igarashi T, Miyake K, Kobayashi M, Yaguchi C, Iijima O, Yamazaki Y, Katakai Y, Miyake N, Kameya S, Shimada T, Takahashi H, Okada T, 2017. Improved Intravitreal AAV-Mediated Inner Retinal Gene Transduction after Surgical Internal Limiting Membrane Peeling in Cynomolgus Monkeys. Mol. Ther 25, 296–302. 10.1016/j.ymthe.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantri A, Vrabec TR, Cu-Unjieng A, Frost A, Annesley WH, Donoso LA, 2004. X-linked retinoschisis: A clinical and molecular genetic review. Surv. Ophthalmol 49, 214–230. 10.1016/j.survophthal.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Tao C, Zhang X, 2016. Retinal Proteoglycans Act as Cellular Receptors for Basement Membrane Assembly to Control Astrocyte Migration and Angiogenesis. Cell Rep. 17, 1832–1844. 10.1016/j.celrep.2016.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo KYC, Lee SY, Barathi AV, Tun SBB, Tan L, Constable IJ, 2018. Surgical Removal of Internal Limiting Membrane and Layering of AAV Vector on the Retina Under Air Enhances Gene Transfection in a Nonhuman Primate. Investig. Opthalmology Vis. Sci 59, 3574. 10.1167/iovs.18-24333 [DOI] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K, 2002. Deletion of the Laminin α4 Chain Leads to Impaired Microvessel Maturation. Mol. Cell. Biol 22, 1194–1202. 10.1128/MCB.22.4.1194-1202.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R, Brown JC, 1996. Supramolecular assembly of basement membranes. BioEssays 18, 123–132. 10.1002/bies.950180208 [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR, 1979. Laminin--a glycoprotein from basement membranes. J. Biol. Chem 254, 9933–7. [PubMed] [Google Scholar]
- Tolun G, Vijayasarathy C, Huang R, Zeng Y, Li Y, Steven AC, Sieving PA, Heymann JB, 2016. Paired octamer rings of retinoschisin suggest a junctional model for cell–cell adhesion in the retina. Proc. Natl. Acad. Sci 113, 5287–5292. 10.1073/pnas.1519048113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca O, Darche M, Schaffer DV, Flannery JG, Sahel J-A, Rendon A, Dalkara D, 2014. AAV-mediated gene delivery in Dp71-null mouse model with compromised barriers. Glia 62, 468–476. 10.1002/glia.22617 [DOI] [PubMed] [Google Scholar]
- Venugopalan P, Wang Y, Nguyen T, Huang A, Muller KJ, Goldberg JL, 2016. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun 7, 10472. 10.1038/ncomms10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vracko R, 1974. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am. J. Pathol 77, 314–46. [PMC free article] [PubMed] [Google Scholar]
- Wang S-T, Chen L, Zhang P, Wang X-B, Sun Y, Ma L-X, Liu Q, Zhou G-M, 2019. Transplantation of Retinal Progenitor Cells from Optic Cup-Like Structures Differentiated from Human Embryonic Stem Cells In Vitro and In Vivo Generation of Retinal Ganglion-Like Cells. Stem Cells Dev. 28, 258–267. 10.1089/scd.2018.0076 [DOI] [PubMed] [Google Scholar]
- Wassmer SJ, Carvalho LS, György B, Vandenberghe LH, Maguire CA, 2017. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep 7, 45329. 10.1038/srep45329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS, Ziogas NK, Xu L, Kim B-J, Ge Y, Patel AK, Ryu J, Lehar M, Alexandris AS, Stewart N, Zack DJ, Koliatsos VE, 2019. Targeted disruption of dual leucine zipper kinase and leucine zipper kinase promotes neuronal survival in a model of diffuse traumatic brain injury. Mol. Neurodegener 14, 44. 10.1186/s13024-019-0345-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelock JM, Murdoch AD, Iozzo RV, Underwood PA, 1996. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem 271, 10079–86. 10.1074/jbc.271.17.10079 [DOI] [PubMed] [Google Scholar]
- Wolff E, 1937. Internal limiting membrane of the retina. Trans Ophthalmol Soc UK 186–195. [Google Scholar]
- Woodard KT, Liang KJ, Bennett WC, Samulski RJ, 2016. Heparan Sulfate Binding Promotes Accumulation of Intravitreally Delivered Adeno-associated Viral Vectors at the Retina for Enhanced Transduction but Weakly Influences Tropism. J. Virol 90, 9878–9888. 10.1128/JVI.01568-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Lyon KA, Leung H, Leahy DJ, Ma L, Ginty DD, 2012. Dystroglycan Organizes Axon Guidance Cue Localization and Axonal Pathfinding. Neuron 76, 931–944. 10.1016/j.neuron.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Boylan NJ, Suk JS, Wang Y-Y, Nance EA, Yang J-C, McDonnell PJ, Cone RA, Duh EJ, Hanes J, 2013. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J. Control. Release 167, 76–84. 10.1016/j.jconrel.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S, 2015. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res 32, 2003–14. 10.1007/s11095-014-1593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye G-J, Budzynski E, Sonnentag P, Miller PE, Sharma AK, Ver Hoeve JN, Howard K, Knop DR, Neuringer M, McGill T, Stoddard J, Chulay JD, 2015. Safety and Biodistribution Evaluation in Cynomolgus Macaques of rAAV2tYF-CB-hRS1, a Recombinant Adeno-Associated Virus Vector Expressing Retinoschisin. Hum. Gene Ther. Clin. Dev 26, 165–176. 10.1089/humc.2015.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Greenberg K, Hunter JJ, Dalkara D, Kolstad KD, Masella BD, Wolfe R, Visel M, Stone D, Libby RT, Diloreto D, Schaffer D, Flannery J, Williams DR, Merigan WH, 2011. Intravitreal injection of AAV2 transduces macaque inner retina. Invest. Ophthalmol. Vis. Sci 52, 2775–83. 10.1167/iovs.10-6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Ray J, Whiteley SJO, Klassen H, Gage FH, 2000. Neuronal Differentiation and Morphological Integration of Hippocampal Progenitor Cells Transplanted to the Retina of Immature and Mature Dystrophic Rats. Mol. Cell. Neurosci 16, 197–205. 10.1006/mcne.2000.0869 [DOI] [PubMed] [Google Scholar]
- Yungher BJ, Luo X, Salgueiro Y, Blackmore MG, Park KK, 2015. Viral vector-based improvement of optic nerve regeneration: characterization of individual axons’ growth patterns and synaptogenesis in a visual target. Gene Ther. 22, 811–821. 10.1038/gt.2015.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL, 2009. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des 15, 1277–94. 10.2174/138161209787846766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A, 2004. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet 13, 2625–2632. 10.1093/hmg/ddh284 [DOI] [PubMed] [Google Scholar]
- Zhang KY, Tuffy C, Mertz JL, Quillen S, Wechsler L, Quigley HA, Zack DJ, Johnson TV, 2021. Role of the Internal Limiting Membrane in Structural Engraftment and Topographic Spacing of Transplanted Human Stem Cell-Derived Retinal Ganglion Cells. Stem Cell Reports 16, 149–167. 10.1016/j.stemcr.2020.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Wang H, Ren H, Jiang R, Zhang C, Wu X, Xu G, 2017. Large is required for normal astrocyte migration and retinal vasculature development. Cell Biosci. 7, 18. 10.1186/s13578-017-0143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolessi FR, Poggi L, Wilkinson CJ, Chien C-B, Harris WA, 2006. Polarization and orientation of retinal ganglion cells in vivo. Neural Dev. 1, 2. 10.1186/1749-8104-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


