Abstract
Increasing evidence indicates that inflammation plays a role in PTSD and stress disorder pathophysiology. PTSD is consistently associated with higher circulating inflammatory protein levels. Rodent models demonstrate that inflammation promotes enduring avoidance and arousal behaviors after severe stressors (e.g., predator exposure and social defeat), suggesting that inflammation may play a mechanistic role in trauma disorders. C‐reactive protein (CRP) is an innate acute phase reactant produced by the liver after acute infection and chronic disease. A growing number of investigations report associations with PTSD diagnosis and elevated peripheral CRP, CRP gene mutations, and CRP gene expression changes in immune signaling pathways. CRP is reasonably established as a potential marker of PTSD and trauma exposure, but if and how it may play a mechanistic role is unclear. In this review, we discuss the current understanding of immune mechanisms in PTSD with a particular focus on the innate immune signaling factor, CRP. We found that although there is consistent evidence of an association of CRP with PTSD symptoms and risk, there is a paucity of data on how CRP might contribute to CNS inflammation in PTSD, and consequently, PTSD symptoms. We discuss potential mechanisms through which CRP could modulate enduring peripheral and CNS stress responses, along with future areas of investigation probing the role of CRP and other innate immune signaling factors in modulating trauma responses. Overall, we found that CRP likely contributes to central inflammation, but how it does so is an area for further study.
Keywords: biomarker, C‐reactive protein, inflammation, post‐traumatic stress disorder
This review describes our current understanding of how C‐reactive protein (CRP), potentially contributes mechanistically to PTSD pathology. We discuss the mechanisms through which CRP could modulate enduring peripheral and CNS stress responses, along with future areas of investigation probing the role of CRP and other immune signaling factors in modulating trauma responses. Overall, the evidence suggests that CRP can contribute to central inflammation, but how it does so is an area for further study.
Abbreviations
- BBB
blood–brain barrier
- CNS
central nervous system
- CRP
c‐reactive protein
- CSF
cerebrospinal fluid
- FcγR
Fc gamma receptor
- IFNγ
interferon‐gamma
- IL‐10
interleukin 10
- IL‐1β
interleukin 1 beta
- IL‐2
interleukin 2
- IL‐6
interleukin 6
- mCRP
monomeric CRP
- pCRP
pentameric CRP
- PTSD
post‐traumatic stress disorder
- SNP
single‐nucleotide polymorphism
- TNFα
tumor necrosis factor‐alpha
1. INTRODUCTION
Post‐traumatic stress disorder (PTSD) remains a growing and often debilitating psychiatric disorder resulting from severe trauma. PTSD affects 7%–8% of the general United States population, with even higher rates in Iraq and Afghanistan combating veteran populations (Pace & Heim, 2011). PTSD is a relatively unique disorder in that it requires an environmental event (trauma) to trigger symptom development and maintenance. Symptoms manifest as highly intrusive memories or re‐experiencing in the form of flashbacks or nightmares, avoidance of trauma cues, negative, depressive symptoms, and increased arousal or hypervigilance. PTSD is not limited to behavioral symptoms but is associated with a number of health conditions, including cardiovascular and autoimmune disorders (Coughlin, 2011; O'Donovan et al., 2015), and even premature death (Lohr et al., 2015). Pharmacological treatments for PTSD are limited and are unlikely to be targeting underlying pathology (Krystal et al., 2017). Identifying the biological pathways involved in PTSD pathogenesis will be critical in developing novel pharmacological treatments. In this review, we discuss the evidence for contribution of immune factors as biomarkers and mechanisms of PTSD, focusing in particular on innate immune signaling factor C‐reactive protein (CRP). We then briefly highlight potential future areas of investigation to probe the ability of CRP and other innate immune signaling factors to modulate trauma responses.
2. EVIDENCE FOR IMMUNE DISRUPTION IN PTSD
Increasing evidence suggests a potential role for inflammation in the pathophysiology of PTSD and other stress disorders (for review see Altemus et al., 2006; Baker et al., 2012; Hoge et al., 2009; Michopoulos et al., 2017). PTSD is associated with both increased inflammatory cytokines and innate immune markers (Agorastos et al., 2019; Baker et al., 2001; Bonne et al., 2011; Lerman et al., 2016). Inflammatory cytokines, such as interleukin 6 (IL‐6) and tumor necrosis factor‐alpha (TNFα), serve as signaling proteins and direct downstream immunologic responses, including directing B and T cell antigen‐specific responses as well as activation and proliferation of other leukocytes including macrophages, neutrophils, eosinophils, and basophils (Zhang & An, 2007).
Acute phase reactants are inflammatory factors involved in phagocytosis and tissue repair shortly after injury and include complement proteins, fibrinogen, and CRP (Gabay & Kushner, 1999). Over the last two decades, PTSD and trauma exposure, respectively, have been linked to higher levels of peripheral inflammatory cytokines and acute‐phase inflammatory reactants (Passos et al., 2015; Tursich et al., 2014). In a widely cited meta‐analysis of 20 case–control studies of inflammatory cytokines in PTSD, elevated pro‐inflammatory markers, including interferon‐gamma (IFNγ), interleukin 1 beta (IL‐1β), IL‐6, and TNFα were associated with PTSD diagnosis (Passos et al., 2015). From then, as an update to this work, a more recent meta‐analysis has additionally found associations with increased overall white blood cell counts, as well as interleukin 2 (IL‐2) and CRP in PTSD patients compared to controls (Yang & Jiang, 2020). In studies examining cytokine levels in CSF, PTSD and trauma exposure are associated with inflammatory cytokine abnormalities at baseline or in response to aversive stimuli in some, but not all studies (Agorastos et al., 2019; Baker et al., 2001; Bonne et al., 2011; Lerman et al., 2016). In line with cerebrospinal fluid (CSF) studies, the relationship between central nervous system (CNS) inflammation in PTSD is less clear, likely due to the relative paucity of studies and their relatively small size, as well as potential differences in patient population (e.g., level of alcohol use, age, and level of trauma exposure; Kim et al., 2020). Overall, findings strongly support increased circulating inflammatory signaling factors in PTSD and possibly immune abnormalities in the central nervous system (CNS) in some PTSD patients. However, as we will discuss, it may also be challenging to differentiate immune dysregulation specific to the CNS, given that both cells and signaling factors can translocate through the blood–brain barrier (BBB).
In conjunction with altered inflammatory signaling proteins, PTSD and exposure to trauma are also linked to abnormalities in immune gene expression and methylation patterns, in particular, expression of innate immune genes, in peripheral immune cells (Bam et al., 2016; Breen et al., 2015, 2017; Doostparast Torshizi & Wang, 2017; Guardado et al., 2016; Malan‐Muller et al., 2014; Neylan et al., 2011; Uddin et al., 2010; Yehuda et al., 2009). These transcriptional data support a potential role for abnormal innate immune responses in the development of PTSD symptoms. Furthermore, mounting evidence suggests a higher prevalence of autoimmune disorders in individuals who have experienced trauma or have PTSD symptoms (O'Donovan et al., 2012, 2015; Roberts et al., 2017; Song et al., 2018), suggesting that inflammation resulting from traumatic experiences contributes to co‐morbid medical illness. While there is evidence for both pro‐inflammatory signaling and dysregulated immune function in trauma exposure and PTSD, it is unclear whether inflammation contributes causally to PTSD or is a result of other PTSD pathology (e.g., glucocorticoid hypersensitivity) or lifestyle changes associated with PTSD (e.g., see O'Donovan et al., 2012).
3. ANIMAL MODELS SUPPORT A MECHANISTIC ROLE OF INFLAMMATION IN PTSD
One way to probe the potential causal role of inflammation in stress and trauma disorders is via animal models of enduring anxiety‐like and depressive‐like behaviors after trauma (Deslauriers et al., 2018). Rodent studies confirm an important role for pro‐inflammatory cytokine signaling to promote enduring anxiety‐like and depression‐like behaviors after severe stress, including IL‐6 and IL‐1β (Hodes et al., 2014; Wohleb et al., 2011). These cytokines are necessary for enduring increases in generalized avoidance and arousal after a traumatic stressor (e.g., predator exposure and social defeat; Deslauriers et al., 2017; McKim et al., 2018; Takahashi et al., 2018). IL‐6 and IL‐1β also modulate fear learning and extinction processes, mechanisms that are strongly linked to PTSD. Disruptions in learned fear and extinction are considered a primary mediator of PTSD re‐experiencing and avoidance symptoms (Jones et al., 2018; Young et al., 2018), and PTSD patients consistently exhibit abnormalities in fear conditioning processes (Risbrough et al., 2016). When circulating in the periphery, these cytokines may play a role in the stress‐induced disruption of the blood–brain barrier (BBB) and/or macrophage trafficking across the BBB to drive CNS inflammation (Cathomas et al., 2019; Deslauriers et al., 2017; McKim et al., 2018; Menard et al., 2017; Wohleb et al., 2011). They are also released directly in the brain from astrocytes and microglia after stress (Cathomas et al., 2019). Thus, there is growing evidence from both human and animal studies to support the role of inflammatory cytokine signaling in mechanisms underlying the development of PTSD. Less information is known, however, about a potential mechanistic role for innate immune pathways in PTSD.
4. ACUTE PHASE REACTANTS AND PTSD, IS CRP A MARKER OR A PLAYER?
As discussed above, innate immune pathways, such as complement proteins and acute phase reactants, have been associated with PTSD diagnosis (Speer et al., 2018); however, their functional role in trauma response is not well understood, and it is not clear if associations with innate immune abnormalities are simply a marker of PTSD symptom development or contribute to PTSD risk. Pre‐trauma expression of innate immune genes can predict risk for PTSD and is also associated with pathophysiology post‐trauma (Breen et al., 2015, 2017). One of the most commonly measured innate immune signaling proteins, CRP, is also consistently associated with PTSD symptoms (see above). Previously linked to rheumatologic and cardiovascular disorders, and now more recently related to numerous psychiatric conditions, CRP is an innate acute phase reactant produced by the liver in response to infectious or inflammatory stimuli (Volanakis, 2001). While its precise mechanistic functions are still under investigation (see below), it is known to promote phagocytic clearance of inflamed tissue and pathogens. Below we discuss the evidence for CRP associations with PTSD and trauma exposure, as well as a potential role in trauma mechanisms of PTSD.
5. CRP ASSOCIATIONS WITH PTSD DIAGNOSIS, SYMPTOM CLUSTER, AND TRAUMA TYPE
Baseline CRP elevation is clinically used to assess infection, systemic inflammation, and monitor the progression of chronic disease states, including risk for cardiovascular disease (Wu et al., 2015). Indeed, one of the strengths utilizing CRP as a biomarker is the ease and reliability in measuring it clinically, allowing it to serve as a proxy for other immune mediators, such as cytokines, that are often more difficult and less reliable to detect (Casaletto et al., 2018; Lasseter et al., 2020). Several reports have shown associations between PTSD diagnosis and elevated peripheral serum CRP in both military personnel and the civilian population (Breen et al., 2015; Groer et al., 2015; Spitzer et al., 2010; Sumner et al., 2017; Uddin et al., 2010). In a cross‐sectional study of over 3,000 civilian adults in the general population, those with PTSD had over a twofold increase in the odds ratio for CRP levels greater than 3.0 mg/L, a clinically meaningful level of inflammation typically associated with health risks (Spitzer et al., 2010). Overall, individuals with PTSD had mean CRP levels of 2.81 mg/L (±2.49) compared to 2.14 mg (±2.17; p = 0.027) in those without PTSD. Thus, a relationship with CRP across civilian and military populations is suggestive that CRP is unlikely to be linked to a particular trauma type (e.g., combat versus accidents). However, to our knowledge, whether CRP is associated more with traumas related to physical injury (combat, car accidents, domestic violence) compared to trauma without physical injury has not been directly studied. Immune mechanisms may be more likely to play a role in PTSD symptoms associated with physical harm, which presumably drives a stronger immune response. Mechanistically, physical trauma would trigger the release of damage‐associated molecular pattern (DAMPs) molecules and the inflammasome, or molecular danger signals and inflammatory pathways activated through by cellular injury for tissue repair (Matzinger, 1994; Relja & Land, 2020; Roh & Sohn, 2018), although there is growing evidence, particularly in animal models that these pathways can also be activated by psychological stressors and aversive stimuli, such as foot shock (Maslanik et al., 2013).
An additional question is if CRP is associated with particular symptom domains. PTSD symptoms fall into four clusters, re‐experiencing, avoidance, negative cognitions and mood, and arousal. Studies suggest that elevated CRP is most consistently associated with avoidance, re‐experiencing, and depression. This conclusion is based on several investigations in civilian PTSD populations reporting a positive association between CRP and PTSD re‐experiencing and avoidance symptoms, in addition to overall PTSD symptom severity (Canetti et al., 2014; Miller et al., 2001) and depression symptoms (Rosen et al., 2017), while significant associations with arousal symptoms were not detected. Notably, the associations with avoidance and re‐experiencing/intrusive symptoms were maintained even when controlling for depression, suggesting CRP is not merely associated with depressive symptoms across disorders.
While few studies have investigated how gender specifically affects the relationship between PTSD symptoms and CRP, an investigation of the relationship between trauma disorder symptomatology and inflammation found gender differences between CRP elevation and either fear or depressive symptoms in relation to the threat of terrorism. This study of 1,153 healthy participants found that fear of a terrorist event or trauma was associated with higher CRP elevations in women than in men. In contrast, depressive symptoms after terrorist acts were associated with higher CRP levels in men (Melamed et al., 2004). While investigating the relationship between trauma and metabolic disorders, Powers and colleagues noted a significant association between CRP and emotional dysregulation among urban African American women (n = 40) with Type 2 Diabetes as well as high rates of childhood trauma (Powers et al., 2016). As evidence by this body of research, the relationship between CRP and PTSD symptom domains may be determined by the interplay with trauma type (e.g., childhood trauma), gender, environment, and other lifestyle factors.
6. CRP AS A MARKER FOR PTSD RISK
Because elevated CRP is associated with PTSD diagnosis, one obvious question is whether elevated CRP is a pre‐trauma risk factor or a marker associated with post‐trauma symptom development. Several recent studies have examined the role of CRP as a potential risk factor. The prospective Marine Resiliency Study of ~2,600 active‐duty Marines reported that increased CRP plasma levels in participants before their combat deployment predicted PTSD symptom endorsement after returning from deployment (Eraly et al., 2014). However, CRP levels were not predictive of symptom severity. This result, yet, was not seen by Sumner and colleagues, who instead, found in a group of 525 women, as part of the longitudinal Nurses' Health Study II, that higher levels of the endothelial function and cardiovascular risk biomarkers, tumor necrosis factor‐alpha receptor‐II (TNFRII), and intercellular adhesion molecule‐1 (ICAM‐1), but not CRP, were predictive of women later developing PTSD symptoms (Sumner et al., 2018). The study populations were different in several significant ways; the Marine study was in young, fit (average age 23 years) males in military service, while the Nurses' Health study was in civilian women (average age 43 years). Sex differences would not be surprising given sex differences in innate immune responses and sex differences in CRP associations with other disorders (van Eijk et al., 2007; Grossman, 1985; Lee et al., 2019; Li et al., 2017). Thus, as with findings of sex effects on CRP associations with specific PTSD symptoms, CRP associations with PTSD risk may depend on gender, trauma‐type, or other study population lifestyle differences (e.g., see Fedewa et al., 2017).
A number of questions arise from these findings. Is CRP simply associated with some other mechanism of PTSD risk? For example, childhood adversity is consistently linked to high CRP in adulthood and is a robust environmental risk factor for PTSD (e.g., Bertone‐Johnson et al., 2012; Danese et al., 2007; Lin et al., 2016; Rooks et al., 2012; Taylor et al., 2006). The study by Eraly and colleagues did control for childhood trauma, but other aspects of adversity, such as socioeconomic status and unpredictability of care, were not measured (Eraly et al., 2014). A twin study supports that environmental factors may contribute to the association between CRP and PTSD risk. The Vietnam Era Twin Registry study reported that mean CRP levels were higher in twin veteran pairs where at least one individual had PTSD, compared to pairs where neither twin had a PTSD diagnosis (Plantinga et al., 2013). The strength of the association between PTSD and CRP levels was similar between monozygotic and dizygotic twin pairs, supporting that CRP associations with PTSD were more likely via shared familial environment than genetics. This study also reported that twins with current PTSD were more likely to have elevated CRP compared to those with past PTSD or no PTSD, suggesting CRP could be a marker of risk for symptom state and chronicity. A role for genetics, however, must not be discounted.
Two studies examined the role of CRP genes in PTSD risk. A cross‐sectional investigation of almost 2,700 individuals from an urban population reported that a single‐nucleotide polymorphism (SNP), rs1130864, was associated both with increased amounts of CRP, as well as an elevated risk for developing PTSD symptoms and increased fear learning (Michopoulos et al., 2015). More recently, a study of 286 military veterans of post‐9/11 conflicts investigated CRP genetic SNP variants and DNA methylation in conjunction with trauma exposure and PTSD diagnosis and symptom severity. Here PTSD was not only associated with higher CRP levels, but the relationships between PTSD symptom severity and CRP were mediated by several CRP SNPs and methylation of the CRP gene promotor locus, AIM2 (Miller et al., 2018). Taken together, these studies hint that increased CRP could play a role in risk for PTSD, either through environmental factors or genetic factors that increase CRP levels. Genetic findings on CRP, however, should be taken with caution, given that thus far, no CRP gene mutation has been implicated via genome‐wide association studies for PTSD, although other immune genes have been implicated (Gelernter et al., 2019; Nievergelt et al., 2018).
7. KNOWN PHYSIOLOGIC FUNCTIONS OF CRP
Because CRP is consistently associated with PTSD, it is worth examining what potential mechanisms might exist for CRP to contribute to the enduring effects of trauma. CRP was named after it was first identified for its reactivity with the Streptococcus pneumonia “C” carbohydrate antigen in serum samples from patients with acute inflammation (Pepys & Baltz, 1983). It is an acute‐phase reactant that binds to phosphatidylcholine and phosphatidylethanolamine expressed on surfaces of dead or dying cells, as well as some bacteria (Schwalbe et al., 1992; Thompson et al., 1999). During an acute inflammatory trigger or infectious response, immune cells release inflammatory cytokines, such as IL‐6, that trigger the liver to synthesize CRP (Volanakis, 2001; Figure 1). CRP levels rise within 2 hr of an inflammatory challenge and can increase up to 10,000 fold by its peak at 48 hr (Pepys & Hirschfield, 2003). The precise mechanistic role of CRP in the immune response is still under active investigation, in part because CRP exists in distinct conformational forms, including pentameric CRP (pCRP) and monomeric CRP (mCRP; McFadyen et al., 2018; Wu et al., 2015), and in part because of functional differences between human CRP and CRP in model species such as rodents (Taylor et al., 1984; Torzewski et al., 2014). It is known, however, to deposit in damaged tissues and activate the classical complement pathway (Figure 1) to promote macrophage phagocytosis of either necrotic or apoptotic cells along with bacteria (Mold et al., 1999). CRP also binds histones and chromatin released during cellular necrosis (Abrams et al., 2013; Du Clos et al., 1988).
Figure 1.
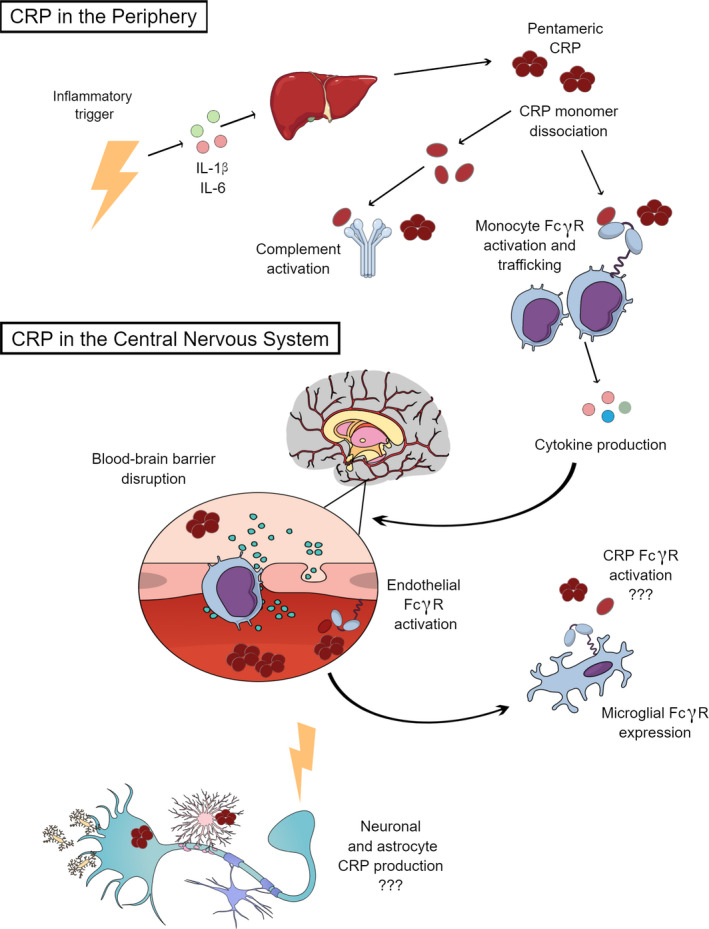
The potential mechanistic role CRP in trauma response in the periphery and central nervous system. A stressor or inflammatory trigger primes the immune response and the release of inflammatory cytokines, such as IL‐1β or IL‐6, triggering the liver to synthesize CRP. CRP exists in distinct conformational forms, including pCRP and mCRP forms, where fully dissociated mCRP is generally thought to be more proinflammatory towards activating macrophages. Within the peripheral vasculature, CRP activates the complement system and activates monocyte Fcγ receptors to promote macrophage activation, trafficking, and induce the inflammatory signaling cascade. Inflammatory cytokines within this cascade may increase BBB permeability, enhancing macrophage and CRP trafficking into the CNS. CRP can also induce BBB disruption by activating endothelial Fcγ receptors. Microglia and astrocytes also express Fcγ receptors that can be activated by any CRP within the CNS. Both neurons and astrocytes can upregulate CRP expression with acute and chronic inflammatory states. Figure created in the Mind the Graph platform www.mindthegraph.com
CRP may also have anti‐inflammatory properties. Once CRP is cleaved by neutrophil surface proteolytic enzymes, the derived peptides limit both neutrophil chemotaxis (Heuertz et al., 1993; Kew et al., 1990) as well as neutrophil reactive oxygen species production (Dobrinich & Spagnuolo, 1991; Filep & Foldes‐Filep, 1989; Ling et al., 2014). As circulating cellular and nuclear breakdown products likely contribute to autoimmune pathology (Szalai, 2004), CRP has been trialed as a disease therapeutic in autoimmune mouse models (Du Clos et al., 1994; Rodriguez et al., 2005, 2007). In this context, CRP has been under investigation as an immunoregulatory treatment in patients after an acute physical trauma (West et al., 2012).
8. POTENTIAL MECHANISMS OF CRP RISK—MODULATION OF THE COMPLEMENT SYSTEM
There are several mechanisms by which CRP may play a role in psychiatric disorders, including PTSD, for example, via indirect effects through peripheral signaling, or through more direct central effects. Within the peripheral vasculature, CRP activates the complement system or macrophages to induce an inflammatory signaling cascade (Figure 1; Mold et al., 1999). Because there is growing evidence that peripheral complement activation may play a role directly in risk for other psychiatric diseases, including schizophrenia and depression (Canetta et al., 2014; Mayilyan et al., 2008; Morgan, 2018), it is possible that CRP could play a mechanistic role in PTSD development by activating the classical complement system in peripheral blood. Upon immune challenge, rising circulating levels of CRP will bind to complement ligand, C1q, to initiate the classical complement cascade (Agrawal et al., 2001). In both the periphery and CNS, this cascade triggers C3 convertase and later C5 convertase as part of the terminal pathway to ultimately form the Membrane Attack Complex (MAC; Lucas et al., 2006; Merle et al., 2015). The MAC has been found to create pores in target cells, while the C3 complement protein has been shown to simultaneously increase leukocyte recruitment and opsonization, which enhances phagocytosis (Nesargikar et al., 2012). This signaling process may also affect neural function. Altered CRP and complement levels are associated with neuropsychiatric illnesses, including schizophrenia and depression (Canetta et al., 2014; Horn et al., 2018). Increased maternal levels of CRP and C1q both increase the likelihood of schizophrenia in offspring, and many patients with schizophrenia have elevated complement system activity (Canetta et al., 2014; Mayilyan et al., 2008; Severance et al., 2014). More recently, genetic C4 complement gene allelic variation with increased C4A protein expression has also been found in schizophrenia, which has been implicated in possible excessive synaptic pruning (Sekar et al., 2016). Patients with depression express higher levels of CRP in addition to higher levels of C4 protein (Berk et al., 1997).
While little has been published regarding complement and its relationship with CRP in PTSD subjects to date, one investigation involving 31 patients with PTSD compared to age‐ and sex‐matched healthy controls found hyperactivation of the classical complement cascade pathway, hypoactivation of the alternative complement pathway, and overactivation of the terminal complement pathway (Hovhannisyan et al., 2010). The study authors postulated that alternative pathway hypoactivation could be due to C3 component depletion from terminal pathway overactivation in which C3 is overutilized. While alterations in the complement cascade are a further indicator that inflammation is associated with PTSD etiology or progression, we must await replication of these results with larger studies and investigation of whether a direct correlation is detectable between CRP levels and classical complement cascade hyperactivation in trauma‐disorder populations.
9. POTENTIAL ROLE OF CRP IN MODULATING CNS INFLAMMATION
Aside from its activity in the complement system, CRP is thought to also have pro‐inflammatory effects by decreasing macrophage secretion of interleukin 10 (IL‐10), an anti‐inflammatory cytokine (Singh et al., 2006), and by promoting monocyte proliferation and transformation to activated macrophages (Figure 1; Devaraj & Jialal, 2011; Devaraj et al., 2009). Activated macrophages release inflammatory cytokines, recruit other pro‐inflammatory cells, and can phagocytose bacteria and cellular debris. It is now becoming increasingly clear that peripheral macrophages are trafficked across the BBB during stress or trauma to drive CNS inflammation further through microglia and astrocyte activation (Figure 1; Jones et al., 2018; McKim et al., 2018; Menard et al., 2017). Moreover, reduced circulating IL‐10 is associated with PTSD risk (Teche et al., 2017). Thus it is possible that CRP modulates peripheral immune contributions to PTSD through interactions with other immune cytokines and signaling factors, as well as stimulating macrophage proliferation/activation.
CRP could additionally impact CNS function by accessing the CNS directly. CRP levels are increased in both periphery and CSF in depressed patients, suggesting that CRP is a marker for both peripheral and central inflammation that could potentially modulate affect and cognition in psychiatric illness (Felger et al., 2016; Haroon et al., 2016). CSF CRP levels also correlate with depression symptom severity in other patient populations (e.g., Parkinson's Disease; Lindqvist et al., 2013), and CRP can be found deposited in Alzheimer's disease brain tissue senile plaques (Iwamoto et al., 1994). How CRP accesses the central nervous system in patients with psychiatric illness and its functional role in the CNS is not clear.
10. CRP ACCESS TO CNS AND POTENTIAL FUNCTIONS
CRP could access the CSF during periods of BBB permeability, including after traumatic events such as brain injury, severe stress, or during high inflammation (Figure 1; Prakash & Carmichael, 2015; Welcome & Mastorakis, 2020; Yurgil et al., 2014). CSF protects the CNS by maintaining buoyancy, clearing waste, and distributing electrolytes and small molecule neuroendocrine factors (Johanson et al., 2011; Strazielle & Ghersi‐Egea, 2013), and with normally much lower concentrations of inflammatory cells and proteins (de Graaf et al., 2011; Ohtori et al., 2011). The BBB, therefore, has been postulated to assist in maintaining the CNS as an immunologically privileged site and limit inflammatory destruction in order to protect the CNS from immunological destruction (Engelhardt & Sorokin, 2009; Muldoon et al., 2013). An increasing body of evidence suggests that peripheral CRP may cross the BBB to enter the CSF during such pathologic states (i.e., infectionBoje, 1996; Gerdes et al., 1998; Sindic et al., 1984). As discussed earlier, with regard to animal models demonstrating a role for inflammatory cytokine signaling in promoting stress‐induced psychopathology, severe stress has been shown to induce BBB permeability (Menard et al., 2017). Moreover, traumatic brain injury, one of the most substantial risk factors for PTSD (Prakash & Carmichael, 2015; Yurgil et al., 2014), is also associated with BBB permeability, allowing immune signaling factors, including CRP, to access the CNS. CRP itself can also induce BBB disruption by binding and activating the Fc gamma receptors (FcγR), CD16, and CD32, on endothelial cells (Kuhlmann et al., 2009). Intravenous injections of mice with radioactively labeled CRP have shown that elevated doses of CRP mimicking inflammatory states increases both BBB permeability as well as CNS CRP levels (Hsuchou et al., 2012). Thus, by increasing BBB permeability, CRP could further contribute to PTSD risk not only by directly infiltrating the CNS but also by allowing other cytokines (i.e., IL‐1β and IL‐6) or immune cells to enter the CNS at the time of trauma (Figure 1). Thus, it is possible that CRP and other immune factors contribute to PTSD in specific trauma cases where BBB permeability is highest (e.g., TBI, trauma involving severe physical injury and infection).
11. CRP PRESENCE AND FUNCTION WITHIN THE BRAIN
CRP is primarily produced by the liver in response to macrophage secreted IL‐6. Gene expression mapping through the Allen Brain Atlas demonstrates little, if any, expression in healthy tissue in both mouse and human brain. CRP expression, however, may be upregulated in glutamate neurons during specific disease states, such as Alzheimer's dementia (Figure 1; Yasojima et al., 2000). Furthermore, Slevin and colleagues reported that mCRP resides in human brain tissue after ischemic stroke within both the endothelial microvasculature as well as within neuronal cytoplasm in stroke‐affected neurons (Slevin et al., 2010). The study authors also demonstrate that both human fetal neurons, as well as human brain microvessel endothelial cells, will express CRP in vitro when subjected to oxygen and glucose deprivation (Slevin et al., 2010). Additionally, ex vivo astrocytes express CRP when presented with an immune challenge (lipopolysaccharide), suggesting astrocytes may be a source of CRP in the CNS under pathological conditions (Wight et al., 2012). Like endothelial cells, microglia and astrocytes also express Fcγ receptors (Figure 1; Fuller et al., 2014; Li et al., 2008), although there have not yet been direct studies demonstrating that these glial receptors are activated by CRP. Human post‐mortem tissue studies and direct injection of CRP into mouse hippocampus indicate that mCRP colocalizes with α‐amyloid plaques and with phosphorylated‐tau protein (Slevin et al., 2015). Furthermore, in vitro studies demonstrate neuronal uptake of exogenously administered CRP in human‐derived neuronal cell culture and that CRP was toxic to these cultures when at concentrations that would occur in an inflammatory state (Duong et al., 1998). Overall, these studies indicate that CRP is produced in the CNS, either in neurons, glia, and/or microvessel endothelial cells, during immune and homeostatic challenges with some indication that CRP may be neurotoxic. The next step is to understand if and how CRP exposure is linked to the functional effects of trauma, such as changes in brain morphology and behaviors relevant to psychiatric symptoms. Alternatively, CRP's primary effects could be further contributing to increasing BBB permeability through the endothelial modifications described above and thus allowing other inflammatory signaling factors to enter the CNS.
12. CONCLUSION AND FUTURE WORKS
PTSD remains a growing and often debilitating psychiatric disorder that affects military personnel, first responders, and civilians after severe trauma. Numerous studies in the last decade have found associations between PTSD symptomatology and chronic inflammation. However, it is yet to be determined if CRP, or any immune marker, will be a useful biomarker for PTSD, as they have been shown to be for depression (Ironside et al., 2019). It is important to note that immune dysfunction cuts across neuropsychiatric disorder diagnoses (Pinto et al., 2017). Increased inflammation, such as with high circulating CRP, may reflect particular symptom clusters that cross diagnostic boundaries (e.g., endorse high anhedonia symptoms; Bekhbat et al., 2020; Haroon et al., 2018; Loas et al., 2016; Mehta et al., 2020) or occur in populations with specific risk factors for mood and anxiety symptoms, such as those with childhood trauma (McIntyre et al., 2019). Identifying robust biomarkers of such populations is a critical step in personalized medicine, in particular in predicting treatment response or prognosis (Heinzelmann et al., 2014; McIntyre et al., 2019; Raison et al., 2013). At the mechanistic level, however, there is also much work to be done. High plasma CRP is linked to increased central inflammation, as well as alterations in threat circuit activation and morphology relevant to mood and anxiety disorders such as PTSD (Mehta et al., 2018; Yin et al., 2019). CRP is also associated with circuit and behavioral disruptions in neuropsychiatric disorders like PTSD, but whether it is actually contributing to the disease is unclear. We have highlighted potential ways in which CRP could affect enduring trauma responses via both direct and indirect signaling routes and described limited studies of possible CNS effects of CRP signaling. More studies in model systems will be needed to understand the potential causal contributions of CRP and other innate immune signaling factors in PTSD risk and symptom development. Such studies include both in vitro and in vivo model systems to understand if CRP is expressed within the brain after trauma, how and where it signals in the brain, as well as its potential effects in boosting peripheral immune access to CNS sites through BBB disruption. There are now tissue‐specific mouse model (Wright et al., 2015) and tools to distinguish between the functionally distinct isoforms (Sproston & Ashworth, 2018) to begin to interrogate the role of CRP in trauma response and recovery.
CONFLICTS OF INTEREST
The authors do not have a conflict of interest, financial, or otherwise. No primary data have been reported.
AUTHORS' CONTRIBUTIONS
S.F.F, R.N., and V.B.R. drafted the manuscript. S.B.P. critically reviewed and revised the manuscript.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15031.
ACKNOWLEDGEMENTS
We would like to acknowledge that work was funded by the National Institute of Health (AA026560, MH096889, VBR), Veterans Affairs Center of Excellence for Stress and Mental Health (VBR, SFF) and VISN22 Mental Illness Research, Education and Clinical Center (SBP), VA Merit Award BX004312 (VBR), the National Institute of Mental Health R25 MH101072 Training grant (SFF), and Cohen Veterans Biosciences (VBR).
Friend SF, Nachnani R, Powell SB, Risbrough VB. C‐Reactive Protein: Marker of risk for post‐traumatic stress disorder and its potential for a mechanistic role in trauma response and recovery. Eur J Neurosci. 2022;55(9):2297–2310. 10.1111/ejn.15031
Handling Editor: Dr. Vidita Vaidya
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this review.
REFERENCES
- Abrams, S. T. , Zhang, N. , Dart, C. , Wang, S. S. , Thachil, J. , Guan, Y. , Wang, G. , & Toh, C. H. (2013). Human CRP defends against the toxicity of circulating histones. Journal of Immunology, 191, 2495–2502. 10.4049/jimmunol.1203181 [DOI] [PubMed] [Google Scholar]
- Agorastos, A. , Hauger, R. L. , Barkauskas, D. A. , Lerman, I. R. , Moeller‐Bertram, T. , Snijders, C. , Haji, U. , Patel, P. M. , Geracioti, T. D. , Chrousos, G. P. , & Baker, D. G. (2019). Relations of combat stress and post‐traumatic stress disorder to 24‐h plasma and cerebrospinal fluid interleukin‐6 levels and circadian rhythmicity. Psychoneuroendocrinology, 100, 237–245. 10.1016/j.psyneuen.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Agrawal, A. , Shrive, A. K. , Greenhough, T. J. , & Volanakis, J. E. (2001). Topology and structure of the C1q‐binding site on C‐reactive protein. Journal of Immunology, 166, 3998–4004. 10.4049/jimmunol.166.6.3998 [DOI] [PubMed] [Google Scholar]
- Altemus, M. , Dhabhar, F. S. , & Yang, R. (2006). Immune function in PTSD. Annals of the New York Academy of Sciences, 1071, 167–183. 10.1196/annals.1364.013 [DOI] [PubMed] [Google Scholar]
- Baker, D. G. , Ekhator, N. N. , Kasckow, J. W. , Hill, K. K. , Zoumakis, E. , Dashevsky, B. A. , Chrousos, G. P. , & Geracioti, T. D. Jr (2001). Plasma and cerebrospinal fluid interleukin‐6 concentrations in post‐traumatic stress disorder. NeuroImmunoModulation, 9, 209–217. 10.1159/000049028 [DOI] [PubMed] [Google Scholar]
- Baker, D. G. , Nievergelt, C. M. , & O'Connor, D. T. (2012). Biomarkers of PTSD: Neuropeptides and immune signaling. Neuropharmacology, 62, 663–673. 10.1016/j.neuropharm.2011.02.027 [DOI] [PubMed] [Google Scholar]
- Bam, M. , Yang, X. , Zhou, J. , Ginsberg, J. P. , Leyden, Q. , Nagarkatti, P. S. , & Nagarkatti, M. (2016). Evidence for epigenetic regulation of pro‐inflammatory cytokines, interleukin‐12 and interferon gamma, in peripheral blood mononuclear cells from PTSD patients. Journal of Neuroimmune Pharmacology, 11, 168–181. 10.1007/s11481-015-9643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat, M. , Treadway, M. T. , Goldsmith, D. R. , Woolwine, B. J. , Haroon, E. , Miller, A. H. , & Felger, J. C. (2020). Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub‐type of depression with high CRP and anhedonia. Brain, Behavior, and Immunity, 88, 161–165. 10.1016/j.bbi.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk, M. , Wadee, A. A. , Kuschke, R. H. , & O'Neill‐Kerr, A. (1997). Acute phase proteins in major depression. Journal of Psychosomatic Research, 43, 529–534. 10.1016/S0022-3999(97)00139-6 [DOI] [PubMed] [Google Scholar]
- Bertone‐Johnson, E. R. , Whitcomb, B. W. , Missmer, S. A. , Karlson, E. W. , & Rich‐Edwards, J. W. (2012). Inflammation and early‐life abuse in women. American Journal of Preventive Medicine, 43, 611–620. 10.1016/j.amepre.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boje, K. M. (1996). Inhibition of nitric oxide synthase attenuates blood‐brain barrier disruption during experimental meningitis. Brain Research, 720, 75–83. 10.1016/0006-8993(96)00142-4 [DOI] [PubMed] [Google Scholar]
- Bonne, O. , Gill, J. M. , Luckenbaugh, D. A. , Collins, C. , Owens, M. J. , Alesci, S. , Neumeister, A. , Yuan, P. , Kinkead, B. , Manji, H. K. , Charney, D. S. , & Vythilingam, M. (2011). Corticotropin‐releasing factor, interleukin‐6, brain‐derived neurotrophic factor, insulin‐like growth factor‐1, and substance P in the cerebrospinal fluid of civilians with post‐traumatic stress disorder before and after treatment with paroxetine. Journal of Clinical Psychiatry, 72, 1124–1128. 10.4088/JCP.09m05106blu [DOI] [PubMed] [Google Scholar]
- Breen, M. S. , Maihofer, A. X. , Glatt, S. J. , Tylee, D. S. , Chandler, S. D. , Tsuang, M. T. , Risbrough, V. B. , Baker, D. G. , O'Connor, D. T. , Nievergelt, C. M. , & Woelk, C. H. (2015). Gene networks specific for innate immunity define post‐traumatic stress disorder. Molecular Psychiatry, 20, 1538–1545. 10.1038/mp.2015.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, M. S. , Tylee, D. S. , Maihofer, A. X. , Neylan, T. C. , Mehta, D. , Binder, E. , Chandler, S. D. , Hess, J. L. , Kremen, W. S. , Risbrough, V. B. , Woelk, C. H. , Baker, D. G. , Nievergelt, C. M. , Tsuang, M. T. , Buxbaum, J. D. , & Glatt, S. J. (2017). PTSD blood transcriptome mega‐analysis: Shared inflammatory pathways across biological sex and modes of trauma. Neuropsychopharmacology, 43(3):469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta, S. , Sourander, A. , Surcel, H. M. , Hinkka‐Yli‐Salomaki, S. , Leiviska, J. , Kellendonk, C. , McKeague, I. W. , & Brown, A. S. (2014). Elevated maternal C‐reactive protein and increased risk of schizophrenia in a national birth cohort. The American Journal of Psychiatry, 171, 960–968. 10.1176/appi.ajp.2014.13121579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetti, D. , Russ, E. , Luborsky, J. , Gerhart, J. I. , & Hobfoll, S. E. (2014). Inflamed by the flames? The impact of terrorism and war on immunity. Journal of Traumatic Stress, 27, 345–352. 10.1002/jts.21920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto, K. B. , Elahi, F. M. , Fitch, R. , Walters, S. , Fox, E. , Staffaroni, A. M. , Bettcher, B. M. , Zetterberg, H. , Karydas, A. , Rojas, J. C. , Boxer, A. L. , & Kramer, J. H. (2018). A comparison of biofluid cytokine markers across platform technologies: Correspondence or divergence? Cytokine, 111, 481–489. 10.1016/j.cyto.2018.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomas, F. , Murrough, J. W. , Nestler, E. J. , Han, M. H. , & Russo, S. J. (2019). Neurobiology of resilience: Interface between mind and body. Biological Psychiatry, 86(6), 410–420. 10.1016/j.biopsych.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin, S. S. (2011). Post‐traumatic stress disorder and cardiovascular disease. Open Cardiovascular Medicine Journal, 5, 164–170. 10.2174/1874192401105010164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese, A. , Pariante, C. M. , Caspi, A. , Taylor, A. , & Poulton, R. (2007). Childhood maltreatment predicts adult inflammation in a life‐course study. Proceedings of the National Academy of Sciences of the United States of America, 104, 1319–1324. 10.1073/pnas.0610362104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, M. T. , Smitt, P. A. E. S. , Luitwieler, R. L. , van Velzen, C. , van den Broek, P. D. M. , Kraan, J. , & Gratama, J. W. (2011). Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry Part B: Clinical Cytometry, 80B, 43–50. 10.1002/cyto.b.20542 [DOI] [PubMed] [Google Scholar]
- Deslauriers, J. , Powell, S. , & Risbrough, V. B. (2017). Immune signaling mechanisms of PTSD risk and symptom development: Insights from animal models. Current Opinion in Behavioral Sciences, 14, 123–132. 10.1016/j.cobeha.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers, J. , Toth, M. , Der‐Avakian, A. , & Risbrough, V. B. (2018). Current status of animal models of posttraumatic stress disorder: Behavioral and biological phenotypes, and future challenges in improving translation. Biological Psychiatry, 83, 895–907. 10.1016/j.biopsych.2017.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj, S. , & Jialal, I. (2011). C‐reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arteriosclerosis, Thrombosis, and Vascular Biology, 31, 1397–1402. 10.1161/ATVBAHA.111.225508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj, S. , Yun, J. M. , Duncan‐Staley, C. , & Jialal, I. (2009). C‐reactive protein induces M‐CSF release and macrophage proliferation. Journal of Leukocyte Biology, 85, 262–267. 10.1189/jlb.0808458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinich, R. , & Spagnuolo, P. J. (1991). Binding of C‐reactive protein to human neutrophils. Inhibition of respiratory burst activity. Arthritis and Rheumatism, 34, 1031–1038. 10.1002/art.1780340813 [DOI] [PubMed] [Google Scholar]
- Doostparast Torshizi, A. , & Wang, K. (2017). Deconvolution of transcriptional networks in post‐traumatic stress disorder uncovers master regulators driving innate immune system function. Scientific Reports, 7, 14486. 10.1038/s41598-017-15221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Clos, T. W. , Zlock, L. T. , Hicks, P. S. , & Mold, C. (1994). Decreased autoantibody levels and enhanced survival of (NZB x NZW) F1 mice treated with C‐reactive protein. Clinical Immunology and Immunopathology, 70, 22–27. [DOI] [PubMed] [Google Scholar]
- Du Clos, T. W. , Zlock, L. T. , & Rubin, R. L. (1988). Analysis of the binding of C‐reactive protein to histones and chromatin. Journal of Immunology, 141, 4266–4270. [PubMed] [Google Scholar]
- Duong, T. , Acton, P. J. , & Johnson, R. A. (1998). The in vitro neuronal toxicity of pentraxins associated with Alzheimer's disease brain lesions. Brain Research, 813, 303–312. 10.1016/S0006-8993(98)00966-4 [DOI] [PubMed] [Google Scholar]
- Engelhardt, B. , & Sorokin, L. (2009). The blood‐brain and the blood‐cerebrospinal fluid barriers: Function and dysfunction. Seminars in Immunopathology, 31, 497–511. 10.1007/s00281-009-0177-0 [DOI] [PubMed] [Google Scholar]
- Eraly, S. A. , Nievergelt, C. M. , Maihofer, A. X. , Barkauskas, D. A. , Biswas, N. , Agorastos, A. , O'Connor, D. T. , & Baker, D. G. ; Marine Resiliency Study Team . (2014). Assessment of plasma C‐reactive protein as a biomarker of post‐traumatic stress disorder risk. JAMA Psychiatry, 71, 423–431. 10.1001/jamapsychiatry.2013.4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedewa, M. V. , Hathaway, E. D. , & Ward‐Ritacco, C. L. (2017). Effect of exercise training on C reactive protein: A systematic review and meta‐analysis of randomised and non‐randomised controlled trials. British Journal of Sports Medicine, 51, 670–676. 10.1136/bjsports-2016-095999 [DOI] [PubMed] [Google Scholar]
- Felger, J. C. , Li, Z. , Haroon, E. , Woolwine, B. J. , Jung, M. Y. , Hu, X. , & Miller, A. H. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21, 1358–1365. 10.1038/mp.2015.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep, J. , & Foldes‐Filep, E. (1989). Effects of C‐reactive protein on human neutrophil granulocytes challenged with N‐formyl‐methionyl‐leucyl‐phenylalanine and platelet‐activating factor. Life Sciences, 44, 517–524. 10.1016/0024-3205(89)90613-9 [DOI] [PubMed] [Google Scholar]
- Fuller, J. P. , Stavenhagen, J. B. , & Teeling, J. L. (2014). New roles for Fc receptors in neurodegeneration‐the impact on Immunotherapy for Alzheimer's Disease. Frontiers in Neuroscience, 8, 235. 10.3389/fnins.2014.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay, C. , & Kushner, I. (1999). Acute‐phase proteins and other systemic responses to inflammation. The New England Journal of Medicine, 340, 448–454. 10.1056/NEJM199902113400607 [DOI] [PubMed] [Google Scholar]
- Gelernter, J. , Sun, N. , Polimanti, R. , Pietrzak, R. , Levey, D. F. , Bryois, J. , Lu, Q. , Hu, Y. , Li, B. , Radhakrishnan, K. , Aslan, M. , Cheung, K.‐H. , Li, Y. , Rajeevan, N. , Sayward, F. , Harrington, K. , Chen, Q. , Cho, K. , Pyarajan, S. , … Stein, M. B. ; Department of Veterans Affairs Cooperative Studies Program & Million Veteran Program . (2019). Genome‐wide association study of post‐traumatic stress disorder re‐experiencing symptoms in >165,000 US veterans. Nature Neuroscience, 22, 1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, L. U. , Jorgensen, P. E. , Nexo, E. , & Wang, P. (1998). C‐reactive protein and bacterial meningitis: A meta‐analysis. Scandinavian Journal of Clinical and Laboratory Investigation, 58, 383–393. 10.1080/00365519850186364 [DOI] [PubMed] [Google Scholar]
- Groer, M. W. , Kane, B. , Williams, S. N. , & Duffy, A. (2015). Relationship of PTSD symptoms with combat exposure, stress, and inflammation in American soldiers. Biological Research for Nursing, 17, 303–310. 10.1177/1099800414544949 [DOI] [PubMed] [Google Scholar]
- Grossman, C. J. (1985). Interactions between the gonadal steroids and the immune system. Science, 227, 257–261. 10.1126/science.3871252 [DOI] [PubMed] [Google Scholar]
- Guardado, P. , Olivera, A. , Rusch, H. L. , Roy, M. , Martin, C. , Lejbman, N. , Lee, H. , & Gill, J. M. (2016). Altered gene expression of the innate immune, neuroendocrine, and nuclear factor‐kappa B (NF‐kappaB) systems is associated with post‐traumatic stress disorder in military personnel. Journal of Anxiety Disorders, 38, 9–20. [DOI] [PubMed] [Google Scholar]
- Haroon, E. , Chen, X. , Li, Z. , Patel, T. , Woolwine, B. J. , Hu, X. P. , Felger, J. C. , & Miller, A. H. (2018). Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Translational Psychiatry, 8, 189. 10.1038/s41398-018-0241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon, E. , Fleischer, C. C. , Felger, J. C. , Chen, X. , Woolwine, B. J. , Patel, T. , Hu, X. P. , & Miller, A. H. (2016). Conceptual convergence: Increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Molecular Psychiatry, 21, 1351–1357. 10.1038/mp.2015.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzelmann, M. , Lee, H. , Rak, H. , Livingston, W. , Barr, T. , Baxter, T. , Scattergood‐Keepper, L. , Mysliwiec, V. , & Gill, J. (2014). Sleep restoration is associated with reduced plasma C‐reactive protein and depression symptoms in military personnel with sleep disturbance after deployment. Sleep Medicine, 15, 1565–1570. 10.1016/j.sleep.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Heuertz, R. M. , Piquette, C. A. , & Webster, R. O. (1993). Rabbits with elevated serum C‐reactive protein exhibit diminished neutrophil infiltration and vascular permeability in C5a‐induced alveolitis. American Journal of Pathology, 142, 319–328. [PMC free article] [PubMed] [Google Scholar]
- Hodes, G. E. , Pfau, M. L. , Leboeuf, M. , Golden, S. A. , Christoffel, D. J. , Bregman, D. , Rebusi, N. , Heshmati, M. , Aleyasin, H. , Warren, B. L. , Labonté, B. , Horn, S. , Lapidus, K. A. , Stelzhammer, V. , Wong, E. H. F. , Bahn, S. , Krishnan, V. , Bolaños‐Guzman, C. A. , Murrough, J. W. , … Russo, S. J. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America, 111, 16136–16141. 10.1073/pnas.1415191111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge, E. A. , Brandstetter, K. , Moshier, S. , Pollack, M. H. , Wong, K. K. , & Simon, N. M. (2009). Broad spectrum of cytokine abnormalities in panic disorder and post‐traumatic stress disorder. Depress Anxiety, 26, 447–455. 10.1002/da.20564 [DOI] [PubMed] [Google Scholar]
- Horn, S. R. , Long, M. M. , Nelson, B. W. , Allen, N. B. , Fisher, P. A. , & Byrne, M. L. (2018). Replication and reproducibility issues in the relationship between C‐reactive protein and depression: A systematic review and focused meta‐analysis. Brain, Behavior, and Immunity, 73, 85–114. 10.1016/j.bbi.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovhannisyan, L. P. , Mkrtchyan, G. M. , Sukiasian, S. H. , & Boyajyan, A. S. (2010). Alterations in the complement cascade in post‐traumatic stress disorder. Allergy, Asthma, and Clinical Immunology, 6, 3. 10.1186/1710-1492-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou, H. , Kastin, A. J. , Mishra, P. K. , & Pan, W. (2012). C‐reactive protein increases BBB permeability: Implications for obesity and neuroinflammation. Cellular Physiology and Biochemistry, 30, 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside, M. , Admon, R. , Maddox, S. A. , Mehta, M. , Douglas, S. , Olson, D. P. , & Pizzagalli, D. A. (2019). Inflammation and depressive phenotypes: Evidence from medical records from over 12 000 patients and brain morphology. Psychological Medicine, 1–9. 10.1017/S0033291719002940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto, N. , Nishiyama, E. , Ohwada, J. , & Arai, H. (1994). Demonstration of CRP immunoreactivity in brains of Alzheimer's disease: Immunohistochemical study using formic acid pretreatment of tissue sections. Neuroscience Letters, 177, 23–26. 10.1016/0304-3940(94)90035-3 [DOI] [PubMed] [Google Scholar]
- Johanson, C. E. , Stopa, E. G. , & McMillan, P. N. (2011). The blood‐cerebrospinal fluid barrier: Structure and functional significance. Methods in Molecular Biology, 686, 101–131. [DOI] [PubMed] [Google Scholar]
- Jones, M. E. , Lebonville, C. L. , Paniccia, J. E. , Balentine, M. E. , Reissner, K. J. , & Lysle, D. T. (2018). Hippocampal interleukin‐1 mediates stress‐enhanced fear learning: A potential role for astrocyte‐derived interleukin‐1beta. Brain, Behavior, and Immunity, 67, 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew, R. R. , Hyers, T. M. , & Webster, R. O. (1990). Human C‐reactive protein inhibits neutrophil chemotaxis in vitro: Possible implications for the adult respiratory distress syndrome. Journal of Laboratory and Clinical Medicine, 115, 339–345. [PubMed] [Google Scholar]
- Kim, T. D. , Lee, S. , & Yoon, S. (2020). Inflammation in post‐traumatic stress disorder (PTSD): A review of potential correlates of PTSD with a neurological perspective. Antioxidants (Basel), 9, 107. 10.3390/antiox9020107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal, J. H. , Davis, L. L. , Neylan, T. C. , A. Raskind, M. , Schnurr, P. P. , Stein, M. B. , Vessicchio, J. , Shiner, B. , Gleason, T. D. , & Huang, G. D. (2017). It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: A consensus statement of the PTSD Psychopharmacology Working Group. Biological Psychiatry, 82, e51–e59. 10.1016/j.biopsych.2017.03.007 [DOI] [PubMed] [Google Scholar]
- Kuhlmann, C. R. , Librizzi, L. , Closhen, D. , Pflanzner, T. , Lessmann, V. , Pietrzik, C. U. , de Curtis, M. , & Luhmann, H. J. (2009). Mechanisms of C‐reactive protein‐induced blood‐brain barrier disruption. Stroke, 40, 1458–1466. 10.1161/STROKEAHA.108.535930 [DOI] [PubMed] [Google Scholar]
- Lasseter, H. C. , Provost, A. C. , Chaby, L. E. , Daskalakis, N. P. , Haas, M. , & Jeromin, A. (2020). Cross‐platform comparison of highly sensitive immunoassay technologies for cytokine markers: Platform performance in post‐traumatic stress disorder and Parkinson's disease. Cytokine: X, 2, 100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Oh, S. S. , Jang, S. I. , & Park, E. C. (2019). Sex difference in the association between high‐sensitivity C‐reactive protein and depression: The 2016 Korea National Health and Nutrition Examination survey. Scientific Reports, 9, 1918. 10.1038/s41598-018-36402-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman, I. , Davis, B. A. , Bertram, T. M. , Proudfoot, J. , Hauger, R. L. , Coe, C. L. , Patel, P. M. , & Baker, D. G. (2016). Post‐traumatic stress disorder influences the nociceptive and intrathecal cytokine response to a painful stimulus in combat veterans. Psychoneuroendocrinology, 73, 99–108. 10.1016/j.psyneuen.2016.07.202 [DOI] [PubMed] [Google Scholar]
- Li, Y. N. , Qin, X. J. , Kuang, F. , Wu, R. , Duan, X. L. , Ju, G. , & Wang, B. R. (2008). Alterations of Fc gamma receptor I and Toll‐like receptor 4 mediate the anti‐inflammatory actions of microglia and astrocytes after adrenaline‐induced blood‐brain barrier opening in rats. Journal of Neuroscience Research, 86, 3556–3565. 10.1002/jnr.21810 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zhong, X. , Cheng, G. , Zhao, C. , Zhang, L. , Hong, Y. , Wan, Q. , He, R. , & Wang, Z. (2017). Hs‐CRP and all‐cause, cardiovascular, and cancer mortality risk: A meta‐analysis. Atherosclerosis, 259, 75–82. 10.1016/j.atherosclerosis.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Lin, J. E. , Neylan, T. C. , Epel, E. , & O'Donovan, A. (2016). Associations of childhood adversity and adulthood trauma with C‐reactive protein: A cross‐sectional population‐based study. Brain, Behavior, and Immunity, 53, 105–112. 10.1016/j.bbi.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist, D. , Hall, S. , Surova, Y. , Nielsen, H. M. , Janelidze, S. , Brundin, L. , & Hansson, O. (2013). Cerebrospinal fluid inflammatory markers in Parkinson's disease–Associations with depression, fatigue, and cognitive impairment. Brain, Behavior, and Immunity, 33, 183–189. 10.1016/j.bbi.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Ling, M. R. , Chapple, I. L. , Creese, A. J. , & Matthews, J. B. (2014). Effects of C‐reactive protein on the neutrophil respiratory burst in vitro. Innate Immunity, 20, 339–349. [DOI] [PubMed] [Google Scholar]
- Loas, G. , Dalleau, E. , Lecointe, H. , & Yon, V. (2016). Relationships between anhedonia, alexithymia, impulsivity, suicidal ideation, recent suicide attempt, C‐reactive protein and serum lipid levels among 122 inpatients with mood or anxious disorders. Psychiatry Research, 246, 296–302. 10.1016/j.psychres.2016.09.056 [DOI] [PubMed] [Google Scholar]
- Lohr, J. B. , Palmer, B. W. , Eidt, C. A. , Aailaboyina, S. , Mausbach, B. T. , Wolkowitz, O. M. , Thorp, S. R. , & Jeste, D. V. (2015). Is post‐traumatic stress disorder associated with premature senescence? A review of the literature. American Journal of Geriatric Psychiatry, 23, 709–725. 10.1016/j.jagp.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas, S. M. , Rothwell, N. J. , & Gibson, R. M. (2006). The role of inflammation in CNS injury and disease. British Journal of Pharmacology, 147(Suppl 1), S232–S240. 10.1038/sj.bjp.0706400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan‐Muller, S. , Seedat, S. , & Hemmings, S. M. (2014). Understanding post‐traumatic stress disorder: Insights from the methylome. Genes, Brain, and Behavior, 13, 52–68. 10.1111/gbb.12102 [DOI] [PubMed] [Google Scholar]
- Maslanik, T. , Mahaffey, L. , Tannura, K. , Beninson, L. , Greenwood, B. N. , & Fleshner, M. (2013). The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain, Behavior, and Immunity, 28, 54–62. 10.1016/j.bbi.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Matzinger, P. (1994). Tolerance, danger, and the extended family. Annual Review of Immunology, 12, 991–1045. 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- Mayilyan, K. R. , Dodds, A. W. , Boyajyan, A. S. , Soghoyan, A. F. , & Sim, R. B. (2008). Complement C4B protein in schizophrenia. The World Journal of Biological Psychiatry, 9, 225–230. 10.1080/15622970701227803 [DOI] [PubMed] [Google Scholar]
- Mayilyan, K. R. , Weinberger, D. R. , & Sim, R. B. (2008). The complement system in schizophrenia. Drug News & Perspectives, 21, 200–210. 10.1358/dnp.2008.21.4.1213349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen, J. D. , Kiefer, J. , Braig, D. , Loseff‐Silver, J. , Potempa, L. A. , Eisenhardt, S. U. , & Peter, K. (2018). Dissociation of C‐reactive protein localizes and amplifies inflammation: Evidence for a direct biological role of C‐reactive protein and its conformational changes. Frontiers in Immunology, 9, 1351. 10.3389/fimmu.2018.01351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, R. S. , Subramaniapillai, M. , Lee, Y. , Pan, Z. , Carmona, N. E. , Shekotikhina, M. , Rosenblat, J. D. , Brietzke, E. , Soczynska, J. K. , Cosgrove, V. E. , Miller, S. , Fischer, E. G. , Kramer, N. E. , Dunlap, K. , Suppes, T. , & Mansur, R. B. (2019). Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: A randomized clinical trial. JAMA Psychiatry, 76, 783–790. 10.1001/jamapsychiatry.2019.0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim, D. B. , Weber, M. D. , Niraula, A. , Sawicki, C. M. , Liu, X. , Jarrett, B. L. , Ramirez‐Chan, K. , Wang, Y. , Roeth, R. M. , Sucaldito, A. D. , Sobol, C. G. , Quan, N. , Sheridan, J. F. , & Godbout, J. P. (2018). Microglial recruitment of IL‐1beta‐producing monocytes to brain endothelium causes stress‐induced anxiety. Molecular Psychiatry, 23, 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, N. D. , Haroon, E. , Xu, X. , Woolwine, B. J. , Li, Z. , & Felger, J. C. (2018). Inflammation negatively correlates with amygdala‐ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: Preliminary results. Brain, Behavior, and Immunity, 73, 725–730. 10.1016/j.bbi.2018.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, N. D. , Stevens, J. S. , Li, Z. , Gillespie, C. F. , Fani, N. , Michopoulos, V. , & Felger, J. C. (2020). Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma‐exposed women. Social Cognitive and Affective Neuroscience, 15(10), 1046–1055. 10.1093/scan/nsz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed, S. , Shirom, A. , Toker, S. , Berliner, S. , & Shapira, I. (2004). Association of fear of terror with low‐grade inflammation among apparently healthy employed adults. Psychosomatic Medicine, 66, 484–491. 10.1097/01.psy.0000130963.52755.b9 [DOI] [PubMed] [Google Scholar]
- Menard, C. , Pfau, M. L. , Hodes, G. E. , Kana, V. , Wang, V. X. , Bouchard, S. , Takahashi, A. , Flanigan, M. E. , Aleyasin, H. , LeClair, K. B. , Janssen, W. G. , Labonte, B. , Parise, E. M. , Lorsch, Z. S. , Golden, S. A. , Heshmati, M. , Tamminga, C. , Turecki, G. , Campbell, M. , … Russo, S. J. (2017). Social stress induces neurovascular pathology promoting depression. Nature Neuroscience, 20, 1752–1760. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle, N. S. , Church, S. E. , Fremeaux‐Bacchi, V. , & Roumenina, L. T. (2015). Complement system part I – Molecular mechanisms of activation and regulation. Frontiers in Immunology, 6, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos, V. , Powers, A. , Gillespie, C. F. , Ressler, K. J. , & Jovanovic, T. (2017). Inflammation in fear‐ and anxiety‐based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology, 42, 254–270. 10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos, V. , Rothbaum, A. O. , Jovanovic, T. , Almli, L. M. , Bradley, B. , Rothbaum, B. O. , Gillespie, C. F. , & Ressler, K. J. (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. American Journal of Psychiatry, 172, 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W. , Maniates, H. , Wolf, E. J. , Logue, M. W. , Schichman, S. A. , Stone, A. , Milberg, W. , & McGlinchey, R. (2018). CRP polymorphisms and DNA methylation of the AIM2 gene influence associations between trauma exposure, PTSD, and C‐reactive protein. Brain, Behavior, and Immunity, 67, 194–202. 10.1016/j.bbi.2017.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R. J. , Sutherland, A. G. , Hutchison, J. D. , & Alexander, D. A. (2001). C‐reactive protein and interleukin 6 receptor in post‐traumatic stress disorder: A pilot study. Cytokine, 13, 253–255. 10.1006/cyto.2000.0825 [DOI] [PubMed] [Google Scholar]
- Mold, C. , Gewurz, H. , & Du Clos, T. W. (1999). Regulation of complement activation by C‐reactive protein. Immunopharmacology, 42, 23–30. 10.1016/S0162-3109(99)00007-7 [DOI] [PubMed] [Google Scholar]
- Morgan, B. P. (2018). Complement in the pathogenesis of Alzheimer's disease. Seminars in Immunopathology, 40, 113–124. 10.1007/s00281-017-0662-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon, L. L. , Alvarez, J. I. , Begley, D. J. , Boado, R. J. , Del Zoppo, G. J. , Doolittle, N. D. , Engelhardt, B. , Hallenbeck, J. M. , Lonser, R. R. , Ohlfest, J. R. , Prat, A. , Scarpa, M. , Smeyne, R. J. , Drewes, L. R. , & Neuwelt, E. A. (2013). Immunologic privilege in the central nervous system and the blood‐brain barrier. Journal of Cerebral Blood Flow and Metabolism, 33, 13–21. 10.1038/jcbfm.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesargikar, P. N. , Spiller, B. , & Chavez, R. (2012). The complement system: History, pathways, cascade and inhibitors. European Journal of Microbiology and Immunology, 2, 103–111. 10.1556/EuJMI.2.2012.2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan, T. C. , Sun, B. , Rempel, H. , Ross, J. , Lenoci, M. , O'Donovan, A. , & Pulliam, L. (2011). Suppressed monocyte gene expression profile in men versus women with PTSD. Brain, Behavior, and Immunity, 25, 524–531. 10.1016/j.bbi.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt, C. M. , Maihofer, A. X. , Klengel, T. , Atkinson, E. G. , Chen, C.‐Y. , Choi, K. W. , Coleman, J. R. I. , Dalvie, S. , Duncan, L. E. , Logue, M. W. , Provost, A. C. , Ratanatharathorn, A. , Stein, M. B. , Torres, K. , Aiello, A. E. , Almli, L. M. , Amstadter, A. B. , Andersen, S. B. , Andreassen, O. A. , … Koenen, K. C. (2018). Largest genome‐wide association study for PTSD identifies genetic risk loci in European and African ancestries and implicates novel biological pathways. bioRxiv, 458562. [Google Scholar]
- O'Donovan, A. , Cohen, B. E. , Seal, K. H. , Bertenthal, D. , Margaretten, M. , Nishimi, K. , & Neylan, T. C. (2015). Elevated risk for autoimmune disorders in iraq and afghanistan veterans with post‐traumatic stress disorder. Biological Psychiatry, 77, 365–374. 10.1016/j.biopsych.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan, A. , Neylan, T. C. , Metzler, T. , & Cohen, B. E. (2012). Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain, Behavior, and Immunity, 26, 642–649. 10.1016/j.bbi.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtori, S. , Suzuki, M. , Koshi, T. , Takaso, M. , Yamashita, M. , Inoue, G. , Yamauchi, K. , Orita, S. , Eguchi, Y. , Kuniyoshi, K. , Ochiai, N. , Kishida, S. , Nakamura, J. , Aoki, Y. , Ishikawa, T. , Arai, G. , Miyagi, M. , Kamoda, H. , Suzuki, M. , … Takahashi, K. (2011). Pro‐inflammatory cytokines in the cerebrospinal fluid of patients with lumbar radiculopathy. European Spine Journal, 20, 942–946. 10.1007/s00586-010-1595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace, T. W. , & Heim, C. M. (2011). A short review on the psychoneuroimmunology of post‐traumatic stress disorder: From risk factors to medical comorbidities. Brain, Behavior, and Immunity, 25, 6–13. 10.1016/j.bbi.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Passos, I. C. , Vasconcelos‐Moreno, M. P. , Costa, L. G. , Kunz, M. , Brietzke, E. , Quevedo, J. , Salum, G. , Magalhaes, P. V. , Kapczinski, F. , & Kauer‐Sant'Anna, M. (2015). Inflammatory markers in post‐traumatic stress disorder: A systematic review, meta‐analysis, and meta‐regression. Lancet Psychiatry, 2, 1002–1012. 10.1016/S2215-0366(15)00309-0 [DOI] [PubMed] [Google Scholar]
- Pepys, M. B. , & Baltz, M. L. (1983). Acute phase proteins with special reference to C‐reactive protein and related proteins (pentaxins) and serum amyloid A protein. Advances in Immunology, 34, 141–212. [DOI] [PubMed] [Google Scholar]
- Pepys, M. B. , & Hirschfield, G. M. (2003). C‐reactive protein: A critical update. The Journal of Clinical Investigation, 111, 1805–1812. 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, J. V. , Moulin, T. C. , & Amaral, O. B. (2017). On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: A systematic review. Neuroscience and Biobehavioral Reviews, 83, 97–108. 10.1016/j.neubiorev.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Plantinga, L. , Bremner, J. D. , Miller, A. H. , Jones, D. P. , Veledar, E. , Goldberg, J. , & Vaccarino, V. (2013). Association between post‐traumatic stress disorder and inflammation: A twin study. Brain, Behavior, and Immunity, 30, 125–132. 10.1016/j.bbi.2013.01.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, A. , Michopoulos, V. , Conneely, K. , Gluck, R. , Dixon, H. , Wilson, J. , Jovanovic, T. , Pace, T. W. , Umpierrez, G. E. , Ressler, K. J. , Bradley, B. , & Gillespie, C. F. (2016). Emotion dysregulation and inflammation in African‐American women with type 2 diabetes. Neural Plasticity, 2016, 8926840. 10.1155/2016/8926840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash, R. , & Carmichael, S. T. (2015). Blood‐brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Current Opinion in Neurology, 28, 556–564. 10.1097/WCO.0000000000000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison, C. L. , Rutherford, R. E. , Woolwine, B. J. , Shuo, C. , Schettler, P. , Drake, D. F. , Haroon, E. , & Miller, A. H. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment‐resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry, 70, 31–41. 10.1001/2013.jamapsychiatry.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relja, B. , & Land, W. G. (2020). Damage‐associated molecular patterns in trauma. European Journal of Trauma and Emergency Surgery, 46, 751–775. 10.1007/s00068-019-01235-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough, V. B. , Glenn, D. E. , & Baker, D. G. (2016). On the road to translation for PTSD treatment: Theoretical and practical considerations of the use of human models of conditioned fear for drug development. Current Topics in Behavioral Neurosciences, 28, 173–196. [DOI] [PubMed] [Google Scholar]
- Roberts, A. L. , Malspeis, S. , Kubzansky, L. D. , Feldman, C. H. , Chang, S. C. , Koenen, K. C. , & Costenbader, K. H. (2017). Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women. Arthritis & Rheumatology, 69, 2162–2169. 10.1002/art.40222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, W. , Mold, C. , Kataranovski, M. , Hutt, J. , Marnell, L. L. , & Du Clos, T. W. (2005). Reversal of ongoing proteinuria in autoimmune mice by treatment with C‐reactive protein. Arthritis and Rheumatism, 52, 642–650. 10.1002/art.20846 [DOI] [PubMed] [Google Scholar]
- Rodriguez, W. , Mold, C. , Kataranovski, M. , Hutt, J. A. , Marnell, L. L. , Verbeek, J. S. , & Du Clos, T. W. (2007). C‐reactive protein‐mediated suppression of nephrotoxic nephritis: Role of macrophages, complement, and Fcgamma receptors. Journal of Immunology, 178, 530–538. [DOI] [PubMed] [Google Scholar]
- Roh, J. S. , & Sohn, D. H. (2018). Damage‐associated molecular patterns in inflammatory diseases. Immune Network, 18, e27. 10.4110/in.2018.18.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks, C. , Veledar, E. , Goldberg, J. , Bremner, J. D. , & Vaccarino, V. (2012). Early trauma and inflammation: Role of familial factors in a study of twins. Psychosomatic Medicine, 74, 146–152. 10.1097/PSY.0b013e318240a7d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, R. L. , Levy‐Carrick, N. , Reibman, J. , Xu, N. , Shao, Y. , Liu, M. , Ferri, L. , Kazeros, A. , Caplan‐Shaw, C. E. , Pradhan, D. R. , Marmor, M. , & Galatzer‐Levy, I. R. (2017). Elevated C‐reactive protein and post‐traumatic stress pathology among survivors of the 9/11 World Trade Center attacks. Journal of Psychiatric Research, 89, 14–21. 10.1016/j.jpsychires.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Schwalbe, R. A. , Dahlback, B. , Coe, J. E. , & Nelsestuen, G. L. (1992). Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphorylethanolamine. Biochemistry, 31, 4907–4915. 10.1021/bi00135a023 [DOI] [PubMed] [Google Scholar]
- Sekar, A. , Bialas, A. R. , de Rivera, H. , Davis, A. , Hammond, T. R. , Kamitaki, N. , Tooley, K. , Presumey, J. , Baum, M. , Van Doren, V. , Genovese, G. , Rose, S. A. , Handsaker, R. E. , Schizophrenia Working Group of the Psychiatric Genomics Consortium , Daly, M. J. , Carroll, M. C. , Stevens, B. , & McCarroll, S. A. (2016). Schizophrenia risk from complex variation of complement component 4. Nature, 530, 177–183. 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance, E. G. , Gressitt, K. L. , Buka, S. L. , Cannon, T. D. , & Yolken, R. H. (2014). Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophrenia Research, 159, 14–19. 10.1016/j.schres.2014.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindic, C. J. , Collet‐Cassart, D. , Depre, A. , Laterre, E. C. , & Masson, P. L. (1984). C‐reactive protein in serum and cerebrospinal fluid in various neurological disorders. Apparent local consumption during bacterial meningitis. Journal of the Neurological Sciences, 63, 339–344. 10.1016/0022-510X(84)90157-6 [DOI] [PubMed] [Google Scholar]
- Singh, U. , Devaraj, S. , Dasu, M. R. , Ciobanu, D. , Reusch, J. , & Jialal, I. (2006). C‐reactive protein decreases interleukin‐10 secretion in activated human monocyte‐derived macrophages via inhibition of cyclic AMP production. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 2469–2475. 10.1161/01.ATV.0000241572.05292.fb [DOI] [PubMed] [Google Scholar]
- Slevin, M. , Matou, S. , Zeinolabediny, Y. , Corpas, R. , Weston, R. , Liu, D. , Boras, E. , Di Napoli, M. , Petcu, E. , Sarroca, S. , Popa‐Wagner, A. , Love, S. , Font, M. A. , Potempa, L. A. , Al‐Baradie, R. , Sanfeliu, C. , Revilla, S. , Badimon, L. , & Krupinski, J. (2015). Monomeric C‐reactive protein – A key molecule driving development of Alzheimer's disease associated with brain ischaemia? Scientific Reports, 5, 13281. 10.1038/srep13281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin, M. , Matou‐Nasri, S. , Turu, M. , Luque, A. , Rovira, N. , Badimon, L. , Boluda, S. , Potempa, L. , Sanfeliu, C. , de Vera, N. , & Krupinski, J. (2010). Modified C‐reactive protein is expressed by stroke neovessels and is a potent activator of angiogenesis in vitro. Brain Pathology (Zurich, Switzerland), 20, 151–165. 10.1111/j.1750-3639.2008.00256.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H. , Fang, F. , Tomasson, G. , Arnberg, F. K. , Mataix‐Cols, D. , Fernandez de la Cruz, L. , Almqvist, C. , Fall, K. , & Valdimarsdottir, U. A. (2018). Association of stress‐related disorders with subsequent autoimmune disease. JAMA, 319, 2388–2400. 10.1001/jama.2018.7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer, K. , Upton, D. , Semple, S. , & McKune, A. (2018). Systemic low‐grade inflammation in post‐traumatic stress disorder: A systematic review. Journal of Inflammation Research, 11, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, C. , Barnow, S. , Volzke, H. , Wallaschofski, H. , John, U. , Freyberger, H. J. , Lowe, B. , & Grabe, H. J. (2010). Association of post‐traumatic stress disorder with low‐grade elevation of C‐reactive protein: Evidence from the general population. Journal of Psychiatric Research, 44, 15–21. 10.1016/j.jpsychires.2009.06.002 [DOI] [PubMed] [Google Scholar]
- Sproston, N. R. , & Ashworth, J. J. (2018). Role of C‐reactive protein at sites of inflammation and infection. Frontiers in Immunology, 9, 754. 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazielle, N. , & Ghersi‐Egea, J. F. (2013). Physiology of blood‐brain interfaces in relation to brain disposition of small compounds and macromolecules. Molecular Pharmaceutics, 10, 1473–1491. 10.1021/mp300518e [DOI] [PubMed] [Google Scholar]
- Sumner, J. A. , Chen, Q. , Roberts, A. L. , Winning, A. , Rimm, E. B. , Gilsanz, P. , Glymour, M. M. , Tworoger, S. S. , Koenen, K. C. , & Kubzansky, L. D. (2017). Cross‐sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biological Psychiatry, 82, 875–884. 10.1016/j.biopsych.2017.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner, J. A. , Chen, Q. , Roberts, A. L. , Winning, A. , Rimm, E. B. , Gilsanz, P. , Glymour, M. M. , Tworoger, S. S. , Koenen, K. C. , & Kubzansky, L. D. (2018). Post‐traumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain, Behavior, and Immunity, 69, 203–209. 10.1016/j.bbi.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai, A. J. (2004). C‐reactive protein (CRP) and autoimmune disease: Facts and conjectures. Clinical and Developmental Immunology, 11, 221–226. 10.1080/17402520400001751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, A. , Flanigan, M. E. , McEwen, B. S. , & Russo, S. J. (2018). Aggression, social stress, and the immune system in humans and animal models. Frontiers in Behavioral Neuroscience, 12, 56. 10.3389/fnbeh.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. A. , Bruton, C. J. , Anderson, J. K. , Mole, J. E. , De Beer, F. C. , Baltz, M. L. , & Pepys, M. B. (1984). Amino acid sequence homology between rat and human C‐reactive protein. The Biochemical Journal, 221, 903–906. 10.1042/bj2210903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. E. , Lehman, B. J. , Kiefe, C. I. , & Seeman, T. E. (2006). Relationship of early life stress and psychological functioning to adult C‐reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry, 60, 819–824. 10.1016/j.biopsych.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Teche, S. P. , Rovaris, D. L. , Aguiar, B. W. , Hauck, S. , Vitola, E. S. , Bau, C. H. D. , Freitas, L. H. , & Grevet, E. H. (2017). Resilience to traumatic events related to urban violence and increased IL10 serum levels. Psychiatry Research, 250, 136–140. 10.1016/j.psychres.2017.01.072 [DOI] [PubMed] [Google Scholar]
- Thompson, D. , Pepys, M. B. , & Wood, S. P. (1999). The physiological structure of human C‐reactive protein and its complex with phosphocholine. Structure, 7, 169–177. 10.1016/S0969-2126(99)80023-9 [DOI] [PubMed] [Google Scholar]
- Torzewski, M. , Waqar, A. B. , & Fan, J. (2014). Animal models of C‐reactive protein. Mediators of Inflammation, 2014, 683598. 10.1155/2014/683598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich, M. , Neufeld, R. W. J. , Frewen, P. A. , Harricharan, S. , Kibler, J. L. , Rhind, S. G. , & Lanius, R. A. (2014). Association of trauma exposure with pro‐inflammatory activity: A transdiagnostic meta‐analysis. Translational Psychiatry, 4, e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin, M. , Aiello, A. E. , Wildman, D. E. , Koenen, K. C. , Pawelec, G. , de Los Santos, R. , Goldmann, E. , & Galea, S. (2010). Epigenetic and immune function profiles associated with post‐traumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America, 107, 9470–9475. 10.1073/pnas.0910794107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk, L. T. , Dorresteijn, M. J. , Smits, P. , van der Hoeven, J. G. , Netea, M. G. , & Pickkers, P. (2007). Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Critical Care Medicine, 35, 1464–1469. 10.1097/01.CCM.0000266534.14262.E8 [DOI] [PubMed] [Google Scholar]
- Volanakis, J. E. (2001). Human C‐reactive protein: Expression, structure, and function. Molecular Immunology, 38, 189–197. 10.1016/S0161-5890(01)00042-6 [DOI] [PubMed] [Google Scholar]
- Welcome, M. O. , & Mastorakis, N. E. (2020). Stress‐induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacological Research, 157, 104769. 10.1016/j.phrs.2020.104769 [DOI] [PubMed] [Google Scholar]
- West, S. D. , Goldberg, D. , Ziegler, A. , Krencicki, M. , Du Clos, T. W. , & Mold, C. (2012). Transforming growth factor‐beta, macrophage colony‐stimulating factor and C‐reactive protein levels correlate with CD14(high)CD16+ monocyte induction and activation in trauma patients. PLoS One, 7, e52406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight, R. D. , Tull, C. A. , Deel, M. W. , Stroope, B. L. , Eubanks, A. G. , Chavis, J. A. , Drew, P. D. , & Hensley, L. L. (2012). Resveratrol effects on astrocyte function: Relevance to neurodegenerative diseases. Biochemical and Biophysical Research Communications, 426, 112–115. 10.1016/j.bbrc.2012.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb, E. S. , Hanke, M. L. , Corona, A. W. , Powell, N. D. , Stiner, L. T. M. , Bailey, M. T. , Nelson, R. J. , Godbout, J. P. , & Sheridan, J. F. (2011). Î2‐adrenergic receptor antagonism prevents anxiety‐like behavior and microglial reactivity induced by repeated social defeat. The Journal of Neuroscience, 31, 6277–6288. 10.1523/JNEUROSCI.0450-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, T. T. , Jimenez, R. V. , Morgan, T. E. , Bali, N. , Hou, X. , McCrory, M. A. , Finch, C. E. , & Szalai, A. J. (2015). Hepatic but not CNS‐expressed human C‐reactive protein inhibits experimental autoimmune encephalomyelitis in transgenic mice. Autoimmune Diseases, 2015, 640171. 10.1155/2015/640171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Potempa, L. A. , El Kebir, D. , & Filep, J. G. (2015). C‐reactive protein and inflammation: Conformational changes affect function. Biological Chemistry, 396, 1181–1197. 10.1515/hsz-2015-0149 [DOI] [PubMed] [Google Scholar]
- Yang, J. J. , & Jiang, W. (2020). Immune biomarkers alterations in post‐traumatic stress disorder: A systematic review and meta‐analysis. Journal of Affective Disorders, 268, 39–46. 10.1016/j.jad.2020.02.044 [DOI] [PubMed] [Google Scholar]
- Yasojima, K. , Schwab, C. , McGeer, E. G. , & McGeer, P. L. (2000). Human neurons generate C‐reactive protein and amyloid P: Upregulation in Alzheimer's disease. Brain Research, 887, 80–89. 10.1016/S0006-8993(00)02970-X [DOI] [PubMed] [Google Scholar]
- Yehuda, R. , Cai, G. , Golier, J. A. , Sarapas, C. , Galea, S. , Ising, M. , Rein, T. , Schmeidler, J. , Muller‐Myhsok, B. , Holsboer, F. , & Buxbaum, J. D. (2009). Gene expression patterns associated with post‐traumatic stress disorder following exposure to the World Trade Center attacks. Biological Psychiatry, 66, 708–711. 10.1016/j.biopsych.2009.02.034 [DOI] [PubMed] [Google Scholar]
- Yin, L. , Xu, X. , Chen, G. , Mehta, N. D. , Haroon, E. , Miller, A. H. , Luo, Y. , Li, Z. , & Felger, J. C. (2019). Inflammation and decreased functional connectivity in a widely‐distributed network in depression: Centralized effects in the ventral medial prefrontal cortex. Brain, Behavior, and Immunity, 80, 657–666. 10.1016/j.bbi.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M. B. , Howell, L. L. , Hopkins, L. , Moshfegh, C. , Yu, Z. , Clubb, L. , Seidenberg, J. , Park, J. , Swiercz, A. P. , & Marvar, P. J. (2018). A peripheral immune response to remembering trauma contributes to the maintenance of fear memory in mice. Psychoneuroendocrinology, 94, 143–151. 10.1016/j.psyneuen.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgil, K. A. , Barkauskas, D. A. , Vasterling, J. J. , Nievergelt, C. M. , Larson, G. E. , Schork, N. J. , Litz, B. T. , Nash, W. P. , & Baker, D. G. (2014). Association between traumatic brain injury and risk of post‐traumatic stress disorder in active‐duty Marines. JAMA Psychiatry, 71, 149–157. 10.1001/jamapsychiatry.2013.3080 [DOI] [PubMed] [Google Scholar]
- Zhang, J. M. , & An, J. (2007). Cytokines, inflammation, and pain. International Anesthesiology Clinics, 45, 27–37. 10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this review.


