Abstract
The biodegradation and biodetoxification of batik industrial wastewater by laccase enzyme immobilised on light expanded clay aggregate (LECA) were investigated. Laccase from Trametes hirsuta EDN 082 was covalently immobilised by modifying the LECA surface using (3–aminopropyl)trimethoxysilane and glutaraldehyde. The enzymatic characterisation of LECA–laccase showed promising results with an enzyme loading of 6.67 U/g and an immobilisation yield of 66.7% at the initial laccase activity of 10 U/g LECA. LECA-laccase successfully degraded batik industrial wastewater containing indigosol dye up to 98.2%. In addition, the decolorisation extent was more than 95.4% after four cycles. The phytotoxicity assessment of Vigna radiata and the microbial toxicity of two pathogenic bacteria, Bacillus subtilis and Pseudomonas aeruginosa, showed biodetoxification of treated batik dye wastewater. The characterisation using 3D light microscopy, scanning electron microscopy and Fourier transform infrared for LECA–laccase confirmed that laccase was successfully immobilised on LECA, and the decolorisation achieved through the combination of adsorption and enzymatic degradation. This study offers an environmentally friendly, effective and affordable LECA–laccase as a method for batik dye wastewater treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02806-8.
Keywords: Biodegradation, Biodetoxification, Batik wastewater, Immobilisation, Laccase, Trametes hirsuta
Introduction
Indonesia’s textile export commodities ranked 12th worldwide and contributed USD 13.3 billion in 2014 (Hengky 2015). One of the textile industries in Indonesia is the batik industry. In the batik dyeing process, an estimated 1.5–111.1 L of water are required per metre cloth (Susanty et al. 2015). The dyes commonly used in the dyeing process are the reactive, indigosol, naphthol, azo, acid, base and direct dyes (Benkhaya et al. 2020). Indigosol is a market trade-name of solubilised vat dyes. Indigosol dye is commonly used for cotton and polyester/cotton blend with bright colour and does not fade easily. Dyes are available in two physical forms: (1) powder form with low stability but can be increased by adding alkaline salt and (2) paste form that is more stable in the dark and the stabilisation can be improved by adding Na2CO3 and NaOH (Chakraborty 2010). Some indigosol dyes belong to the anthraquinoid and indigoid classes (De Wael and Lepot 2011) as shown in Table S1.
During the dyeing process, the use of dye is ineffective because large amount of the dye (10–50%) is not bonded to the fabric and lost to the wastewater (Rehman et al. 2018; Khatri et al. 2018). The large number of dyes released into the environment causes a serious impact on human health and the ecosystem (Lellis et al. 2019). Moreover, some dyes and/or their intermediate compounds have toxic, mutagenic or carcinogenic effects (Gita et al. 2021; Köktürk et al. 2021; Kishor et al. 2021). Until now, there are various methods for processing textile dye wastewater, such as adsorption (Rashid et al. 2021), oxidation (Alderete et al. 2021), irradiation (Arshad et al. 2020), precipitation (Zhu et al. 2007), membrane bioreactor (Yurtsever et al. 2021), flocculation (Tang et al. 2021), coagulation (Demissie et al. 2021; Mcyotto et al. 2021) and ion exchange (Cseri et al. 2021). However, these methods do not apply to large-scale use, unable to fully degrade the dye compounds and their intermediate metabolites and produce sediments that require further processing (Singh et al. 2019).
Recently, biological methods for wastewater treatment, especially using fungi and enzymes, are widely used because they are environmentally friendly and economically viable and degrade pollutants in situ (Routoula and Patwardhan 2020). Laccase (EC 1.10.3.2) is a multicopper oxidase enzyme produced mainly by white-rot fungi used in biofuel production (Malhotra and Suman 2021; Vaishnavi et al. 2020), antibiotic degradation (Mathur et al. 2021), pharmaceuticals and personal care products (PPCPs) degradation (Asif et al. 2017), diclofenac, trimethoprim, carbamazepine and sulfamethoxazole degradation (Alharbi et al. 2019), and textile dye wastewater treatment, such as azo and anthraquinone dyes (Tavares et al. 2020). However, the use of free enzymes has disadvantages, such as difficulties of separation, repeated use, contamination of the surrounding environment and low stability (Eş et al. 2015). Therefore, the enzyme was immobilised to increase the enzyme stability. Several immobilisation methods include entrapment, encapsulation, adsorption, self-immobilisation and covalent binding (Kashefi et al., 2019). Covalent binding methods have some advantages, such as strong bonds, minimising enzyme leakage from the immobilisation matrix and increasing enzyme stability (Zucca and Sanjust 2014).
The covalent immobilisation of GL–7–ACA acylase enzyme to silica gel via silanisation using (3–aminopropyl)triethoxysilane followed by glutaraldehyde was introduced by Park et al. (2002). With regard to laccase immobilisation, Pezzella et al. (2014) reported the immobilisation of laccase from Pleurotus ostreatus on perlite via silanisation using (3–aminopropyl)trimethoxysilane (APTS) and decolorized the Remazol Brilliant Blue R (RBBR) dye. Kashefi et al. (2019) covalently immobilised laccase from genetically modified Aspergillus onto graphene oxide nanosheets. The covalent bonds were obtained from silanisation using APTS and glutaraldehyde activation, used to decolorize Direct Red 23 and Acid Blue 92 dyes. The average decolorisation extent was more than 75% after six cycles.
Light expanded clay aggregate (LECA) is material from clay heated and burned in a rotary kiln at 1100℃–1300℃. LECA is inert, insoluble in water, non-combustible, and fire-resistant and has properties such as neutral pH, non-decomposition against severe conditions and thermal insulation (Rashad 2018). Granulated LECA can be used for the dye adsorption of Acid Cyanin 5R and Reactive Red 33 (Shokoohi et al. 2016; Moradi 2020), toxic chromium adsorption (Kalhori et al. 2013), PAH removal in water (Nkansah et al. 2012) and tetracycline removal (Sepehr et al. 2019). A previous study has successfully used LECA as an immobilisation matrix for Trametes hirsuta D7 fungal cells (Ardiati et al. 2019) and laccase from T. hirsuta EDN 082 (Anita et al. 2020) for RBBR dye decolorisation. The immobilisation of fungal cells on LECA showed the detoxification of anthraquinone dye degradation products on human dermal fibroblast cells (Alam et al. 2021). However, there has been no report about the application of the laccase-immobilised LECA to the real batik wastewater.
In this study, laccase from T. hirsuta EDN 082 covalently immobilised on LECA was used to decolorize batik dye wastewater containing indigosol dye. The characterizations of LECA–laccase using 3D microscopy, scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) were also investigated. The phytotoxicity assessment on the growth of mung bean (Vigna radiata) and microbial toxicity on two bacteria, Bacillus subtilis and Pseudomonas aeruginosa, was tested on three conditions (i.e., distilled water, untreated water and treated wastewater).
Materials and methods
T. hirsuta EDN 082 (NCBI GenBank accession number MT476912) was newly isolated from Taman Eden 100, Toba-Samosir Regency, North Sumatera, Indonesia. Malt extract powder, glucose, hipolypeptone, 25% glutaraldehyde, 2.2′–azino-bis(3–ethylbenzothiazoline–6–sulphonic acid) (ABTS), APTS, ammonium sulphate and CuSO4 were purchased from Wako Pure Chemical Ltd. (Japan). LECA was obtained from a local company in Ciamis, West Java, Indonesia and activated using steam activation (pyrolysis reactor) at 900 °C for 4 h. Batik wastewater was obtained from Jarum Village, Klaten, Indonesia. The physico-chemical properties of the wastewater are shown in Table 1. The wastewater stock dilution was prepared in milli-Q water.
Table 1.
The physico-chemical properties of the wastewater used in this study
| No | Parameters | Values |
|---|---|---|
| 1 | Synthetic dye used | Indigosol |
| 2 | pH | 8.71 |
| 3 | Colour | green Tosca |
| 4 | Maximum wavelength | 636 nm |
| 5 | Toxiciy (inhibition against V.radiata) | 63% |
Laccase production
Laccase production was conducted according to optimum conditions reported by Ningsih et al. (2020). T. hirsuta EDN 082 was pre-cultured in liquid medium containing 20 g/L malt extract, 20 g/L glucose and 1 g/L hipolypeptone (pH 4.5) and incubated for 7 days at room temperature. Baglog contained 150 g oil palm empty fruit bunches (OPEFB) fibres, 200 mL medium, 2 mM CuSO4 and 100 mL distilled water was sterilised at 121℃ for 15 min, added 10% (w/v) mycelium suspension to baglog and incubated for 30 days at 30 °C. After incubation, a mixture of OPEFB fibres, mycelium fungus and acetate buffer (pH 4.5) was homogenised at 10,000 rpm for 10 min using ACE AM-11 homogeniser (Nissei, Japan). The filtrate was centrifuged using Suprema 21 centrifuge (Tomy Seiko Co., Ltd.) at 10,565 g at 4℃ for 20 min. The supernatant was precipitated using 50% (w/v) ammonium sulphate and then centrifuged at 8000 rpm at 4℃ for 20 min. The precipitated crude enzyme was diluted in acetate buffer pH 4.5 and freeze-dried for 72 h.
Laccase activity assay
Laccase activity was measured using a TECAN Infinite 200 Pro microplate reader (Switzerland). The reaction mixture contained 250 µL of 2 mM ABTS, 200 µL of 0.1 M acetate buffer (pH 4.5) and 50 µL enzyme at 420 nm for 60 s. Laccase activity (U/mL) was calculated according to Eq. (1) with a molar absorptivity () of 36,000 M−1 cm−1 (Yanto et al. 2019):
| 1 |
where Abs.(t) is the final absorbance, Abs.(0) is the initial absorbance and 103 is the correction factor (µmol/mol).
Immobilisation of laccase enzyme on LECA
The activation of LECA was conducted according to Ardiati et al. (2019). LECA was washed with distilled water and then dried at 60 ℃ for 24 h. LECA was soaked in 10% APTS at 80 ℃ for 2 h and then dried for 24 h. LECA-APTS was then soaked in 10% glutaraldehyde at room temperature for 2 h. After drying for 24 h, LECA-glutaraldehyde (120 g) was soaked in 600 mL crude enzyme solution (the initial enzyme activity was 2 U/mL solution or equal to 10 U/g LECA) at 4 ℃ for 24 h. LECA-laccase was freeze-dried for 72 h. The immobilisation yield (IY) was described as the percentage of the total laccase activity from the free enzyme solution that was immobilised (Sheldon and van Pelt 2013). The IY was calculated according to Eq. (2), where Ui is the initial laccase activity (U/mL) and Uf is the laccase activity detected in the remaining solution (U/mL). The enzyme loading was described as the laccase activity immobilised in 1 g LECA and calculated according to Eq. (3) (Brugnari et al. 2018):
| 2 |
| 3 |
Characterisation of LECA
The morphological characteristics of LECA (L), LECA–laccase (LL), LECA–wastewater (LW) and LECA–laccase–wastewater (LLW) were analysed using Digital Microscope Keyence VHX-600 (Japan). Before analysis, all samples were dried in the desiccator for 24 h. The analysis was performed in the outer and inner parts of the samples with 2D and 3D imaging using 250 × magnification of Keyence VH-Z250R lens (Japan). All samples were also analysed using SEM (Thermo Quatro, USA) without gold coating. For functional group analysis, the L, LL, LW and LLW samples were dried in the desiccator for 24 h. All samples were analysed using FTIR Spectrum two (Perkin-Elmer) at 400–4000 nm and 32 scans with KBr preparation. The spectra were displayed in baseline mode.
Wastewater decolorisation under different conditions
Batch experiments were conducted in 100 and 300 mL Erlenmeyer flask on reciprocal Taitec Bio-Shaker BR-300 (Japan) at 80 rpm and 30℃. All experiments were conducted in triplicate. The effect of LECA–laccase weight was investigated using 20 mL wastewater with fivefold dilution mixed with various LECA–laccase dosages (0.4, 0.6, 0.8, 1.2 and 1.8 g). The effect of the initial wastewater dilution was investigated using 20 mL wastewater with various dilutions (0, 2.5-fold and fivefold dilution) mixed with optimum LECA–laccase weight as a previous result. The effect of the initial volume was investigated using various volumes (20, 80 and 140 mL) with various dilutions (0, 2.5-fold and fivefold dilution) mixed with the optimum LECA–laccase weight.
Decolorisation efficiency assay
The absorbance reading was taken at 1 h interval reaction using a TECAN Infinite 200 Pro microplate reader in a wavelength value interval of 400–800 nm. The decolorisation efficiency was calculated according to Eq. (4), where Ao is the initial absorbance and A1 is the absorbance after decolorisation:
| 4 |
Reusability
The reusability test was carried out in 20 mL batik wastewater (fivefold dilution) with 1.8 g LECA-laccase. The test was carried out in triplicate on a reciprocal shaker (80 rpm) at 30℃ for 1 h. LECA-laccase was then taken and then put in 20 mL of the new fivefold dilution of batik dye wastewater. The absorbance was measured every 1 h, and repetition was done for seven cycles.
Analysis of degradation products
Untreated and treated wastewater were extracted by ethyl acetate, and the solvent was removed by a rotatory evaporator and dried under vacuum conditions (Hossen et al. 2019). All samples were analysed using FTIR Spectrum two (Perkin-Elmer) with UATR at 400 to 4000 nm and 32 scans. The spectra were displayed in baseline mode.
Phytotoxicity assay
Mung bean seeds (V. radiata) were soaked in distilled water for 1 h before use, and the submerged seeds were used for phytotoxicity assay. One seed was put in every test tube containing 0.2 g cotton. Watering of the mung bean seeds was conducted using untreated and treated batik wastewater (0, 2.5-fold and fivefold dilution) three times: 1000 µL at the beginning, 500 µL on the second day and 500 µL on the third day. Watering with distilled water was used as a control with the same volume and time. After 4 days, the growth of green beans was observed, and the growth of the mung bean shoot length was measured. The test was done in triplicate. Data obtained were processed using one-way analysis of variance and Tukey’s test.
Microbial toxicity assay
In this assay, Gram-negative (P. aeruginosa) and Gram-positive (B. subtilis) bacteria were used as inocula. Before assay, the bacterial inoculum was inoculated in 20 mL nutrient broth and then incubated in a shaker condition at 100 rpm at 30 ℃ for 24 h. The toxicity assay was applied based on the inhibitory effect of the sample as modified from Mostafa et al. (2018), with the addition of 20 μL microbial inoculum to 20 mL agar plates. A mixture of agar medium and bacterial inoculum was then homogenised in Petri dishes until solidified. Wastewater samples (before and after treatment) were autoclaved at 121 ℃ for 15 min, and sterile filter paper discs with a diameter of 6 mm were loaded with each wastewater for 10 min. The sterile filter paper discs soaked in chloramphenicol (4 mg/mL) for 10 min were used as the positive control. The disc paper was then placed on the top-centre of the agar plates containing bacterial inoculum. The plates were kept in a refrigerator at 5 ℃ for 1 h to permit wastewater diffusion and then incubated at 30 ℃ for 24 h. The presence of inhibition zones (mm) was measured and considered as an indication of toxicity.
Results and discussion
Characteristic of batik wastewater
Wastewater from the batik industry had the green Tosca colour originating from indigosol dyes at pH 8.71 with a maximum wavelength at 636 nm. The toxicity of the wastewater against V. radiata growth showed 63%, 51% and 40% inhibition at dilution factors of 0-, 2.5- and fivefold, respectively. This justified the potency of toxic compound release from wastewater into the environmental ecosystem by disposing this wastewater directly to the body of water. Awang et al. (2017) reported the cytotoxicity effect of batik wastewater on V79 cells. Lellis et al. (2019) reviewed the effects of textile dyes, which significantly produced a high level of toxic and recalcitrant compounds on human health and the environment.
Immobilisation of laccase on LECA
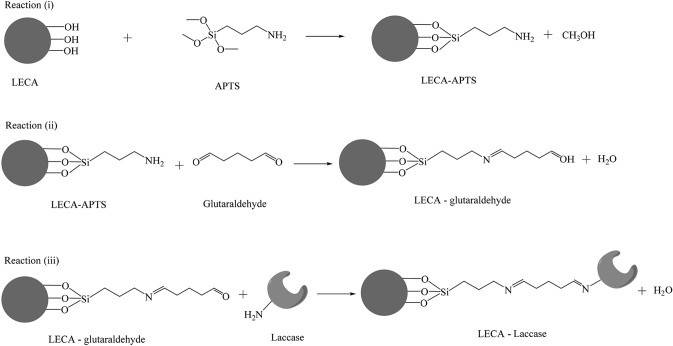
T. hirsuta EDN 082 produced 0.06 U/mg powder of laccase after 1 month incubation in solid-state fermentation of OPEFB fibres. Immobilisation of laccase using the cross-linker APTS and glutaraldehyde in LECA resulted in an IY of 66.7% with an enzyme loading of 6.67 U/g LECA. The proposed mechanism of immobilisation is shown in Fig. 1. The modification of the LECA surface was achieved by three steps of reaction: reaction (1) is silanisation with an organosilane agent that provides the reactive amino groups, reaction (2) is activation with glutaraldehyde (Pezzella et al. 2014), and reaction (3) is immobilisation by covalent bond formation between the reactive amino groups of the enzyme and the aldehyde group of glutaraldehyde. Several studies have reported the IY of laccase in some carrier agents (Daronch et al. 2020; Ramírez et al. 2021). The covalent immobilisation of laccase from Trametes versicolor in green coconut fibre (CF) obtained 60%–98% of IY with an enzyme loading of 6.55 U/5 g CF (Bezerra et al. 2015). Brugnari et al. (2018) reported that the IY of laccase in the ionic support MANAE-agarose was 100%, with an enzyme loading of 120 U/g. Laccase was effectively immobilised 31.4%, 24.3% and 14.58% in pig manure, almond shell and pinewood micro-biochar (Lonappan et al. 2018).
Fig. 1.
Proposed mechanism of laccase immobilization on LECA with modified LECA surface by APTMS and glutaraldehyde (modified from
Bankeeree et al., 2020)
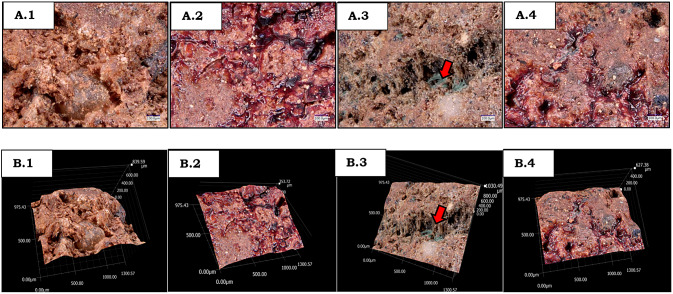
Morphological characteristics of LECA
On the surface of activated LECA, an uneven surface with pores was observed. The colour of raw LECA was light brown (Fig. 2a1, b1). In contrast, the surface become flat after immobilisation of laccase due to covering of the pores by the enzyme (Fig. 2a2, b2). Apart from the pores, enzyme immobilisation also occurs on the surface of the activated LECA. Enzyme immobilisation was seen in the colour difference of LECA, which appeared dark brown, and the colour of the enzyme bonds was seen in contrast to the surroundings. From this image, laccase enzyme was successfully immobilised in LECA on both the surface and the inner part, as shown in Fig. S1A.2 and B.2. Activated LECA has a good pore structure to possibly adsorb the dye wastewater. Fig 2a3, b3 shows the adsorption of indigosol dye to the surface of LECA, and a very small amount of indigosol could be adsorbed in the inner part of LECA. The activation of LECA by APTS and glutaraldehyde improved the surface area generation (Anita et al. 2020), facilitating the adsorption of wastewater. After decolorisation with LECA–laccase, only a very small amount of indigosol dyes remained in the outer surface or inner part of LECA, indicating that the degradation of indigosol dye occurred instead of adsorption in LECA. These results were in line with Antecka et al. (2018) who reported the dye decolorisation mechanism using immobilised enzymes in porous materials through two mechanisms, adsorption and biodegradation, which take place synergistically. Due to the combination of adsorption and biodegradation mechanisms, LECA-laccase was more effective in decolorizing batik dye wastewater, as evidenced by the significant decolorisation yield (99.18%) compared to that of only by LECA (16.36%) or free laccase (80.01%) within 1 h, respectively.
Fig. 2.
Surface morphology of 2D and 3D images of L (a1, b1), LL (a2, b2), LW (a3, b3) and LLW (a4, b4)
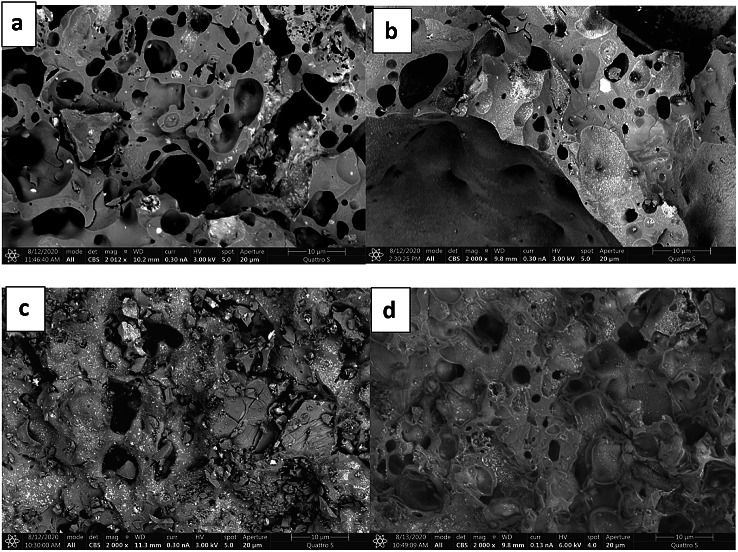
The SEM images of L, LL, LW, and LLW are shown in Fig. 3 and Fig. S2. The results were in line with the 3D microscopy image that demonstrated the success of laccase immobilisation in LECA (Fig. 3b).
Fig. 3.
SEM image of L a, LL b, LW c and LLW
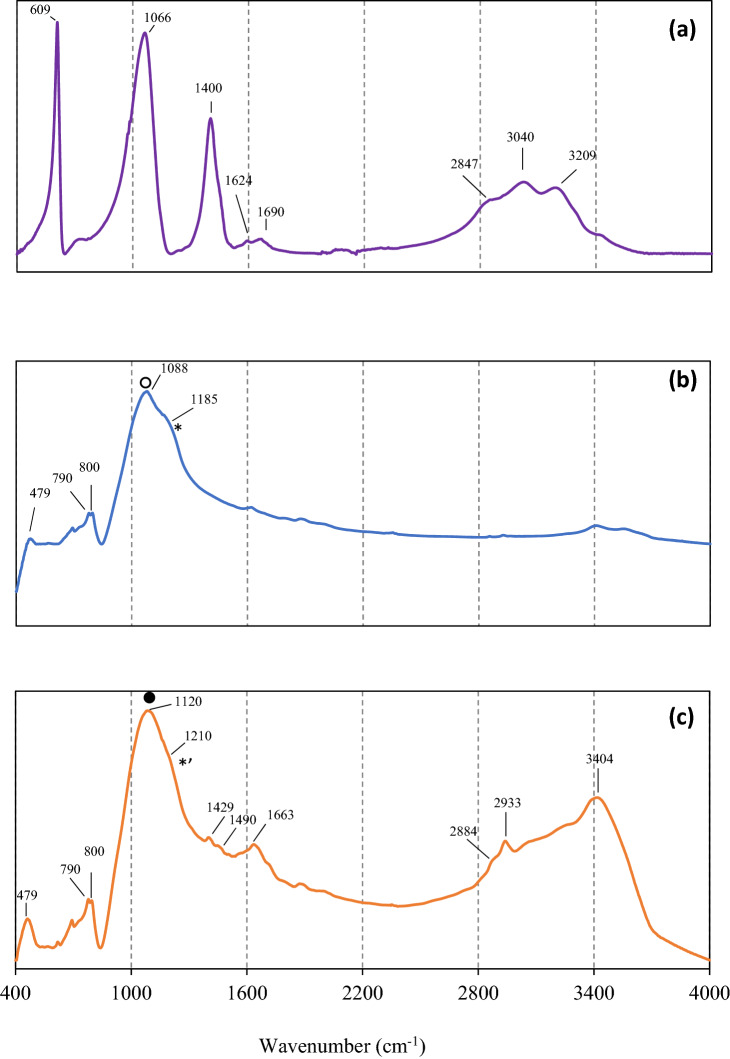
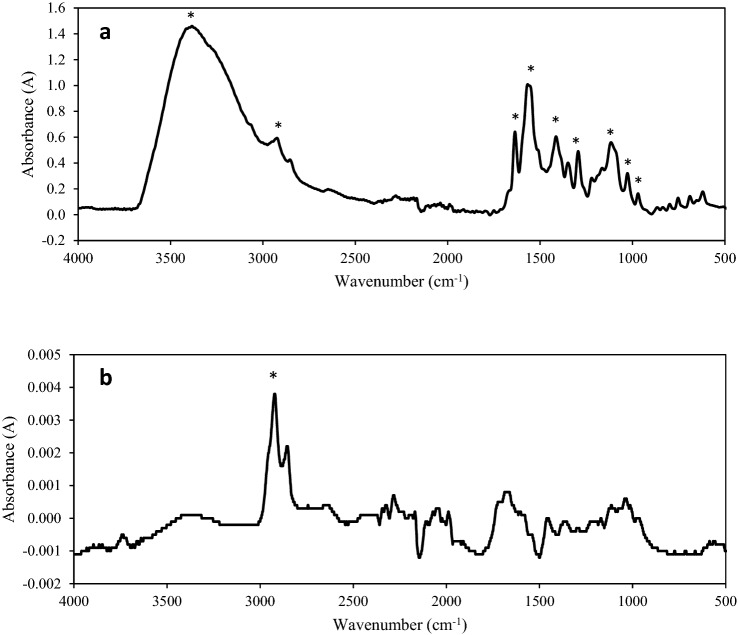
The functional group analysis using FTIR confirmed the immobilisation of laccase covalently in LECA (Fig. 4). In Fig. 4a, the characteristic bands of laccase were observed at double peaks at 1624 and 1690 cm−1, which were ascribed to amide I with a β-sheet structure, a peak at 1400 cm−1 from amide III bands, a peak at 609 cm−1 from amide V and a peak at 1066 cm−1 from the C–O–C bands of proteins (Fortes et al. 2017; Ji et al. 2020). Figure 4b shows the FTIR spectrum of activated raw LECA, which can be recognised by a strong band centered at 1088 cm−1 and ascribed to the stretching vibration of Si–O-Si (Kalhori et al. 2017). The band at 470 cm−1 was ascribed to O-Si–O bending vibration (silicate). Tian et al. (2010) reported that the spectra of initial oxides are characterised by two absorbance features: a transverse optical (TO) band at 1000–1150 cm−1 and a longitudinal optical (LO) band at 1200–1260 cm−1. The spectra of modified LECA covalently immobilised by laccase are provided in Fig. 4c. In this study, LECA was previously modified by silanisation using APTS and followed by cross-linking using glutaraldehyde. The silanisation of LECA was evidenced by the change of the spectra in the TO band (black cycle) and LO band (*′). This is in accordance with the results of Tian et al. (2010) who reported the formation of new Si–O bonds at the silane/SiO2 interface detectable at the LO band. The change of FTIR spectrum after silanisation also showed the new peaks at 2884 and 2933 cm−1, which were attributed to the asymmetric and symmetric C–H vibrations of methoxy groups and the CH2 chains from silane molecules. The scissoring deformation mode due to CH2 groups were found at 1429 and 1490 cm−1. All of these signals absent on the spectrum of raw LECA confirmed the modification of LECA by APTS. For LECA-laccase, the appearance of a medium peak at 1640–1690 cm−1 attributed to imine (–C = N–) bond and peak at 3404 cm−1 ascribed to the (–NH) bond indicated the success of covalent immobilisation of LECA via the bonding of glutaraldehyde and laccase enzyme.
Fig. 4.
FTIR spectra of a laccase enzyme, b raw LECA and c LECA after modification with APTS, glutaraldehyde and immobilisation of laccase (LECA–laccase)
Decolorisation efficiency
Dosage variation
Laccase immobilised in LECA was applied to decolorise batik dye wastewater. In this study, different dosages of laccase-LECA were used to obtain the effective dosage for decolorisation. The experiment was conducted in a fivefold dilution of dye wastewater with dosages of 0.4, 0.6, 0.8, 1.2 and 1.8 g per 20 mL solution. Decolorisation was measured every time for 7 h. In Fig. 5a, all dosages achieved more than 94% decolorisation. Significant differences in decolorisation were observed after 1 h treatment, which showed that the decolorisation was 45.78%, 48.47%, 60.75%, 79.44% and 100% for 0.4, 0.6, 0.8, 1.2 and 1.8 g, respectively. These data demonstrated that the higher dosage applied to the dye wastewater is, the higher is the decolorisation yield. It can also be explained that increasing the weight of LECA–laccase will increase the pores formed and the number of the active site of the enzyme. Therefore, the frequency of the substrate to encounter the active site of the enzyme will be even greater. This is in line with Kashefi et al. (2019) who reported the immobilisation of laccase on graphene oxide nanosheets to decolorise Direct Red 23 and Acid Blue 92 dyes with mass variations of 30–600 mg/L.
Fig. 5.
Decolorisation efficiency of batik wastewater at a different LECA–laccase weight using dilution fivefold (0.4 g ,0.6 g , 0.8 g ,1.2 g and 1.8 g LECA–laccase); b different dilution of batik wastewater using 1.8 g LECA-laccase at fivefold dilution , dilution 2.5-fold and no dilution ; c wavelength spectra of wastewater (no dilution) during decolorisation for 7 h and d decolorisation of different initial volumes of batik wastewater using 1.8 g LECA–laccase with fivefold dilution after 1 h , 2.5-fold dilution after 1 h and no dilution after 7 h
Dilution and volume variation
The dilution variation aims to determine the effectiveness of immobilised laccase on LECA on the decolorisation of batik wastewater at various dilution levels. Tests were carried out on 20 mL wastewater at variations of 0-, 2.5- and fivefold dilution using 1.8 g LECA-laccase for 7 h with measurement of the remaining absorbance and enzyme activity at every hour.
In Fig. 5b, at the first hour, the decolorisation rate of the wastewater with 0-, 2.5- and fivefold dilution was 39.1%, 99.2% and 100%, respectively. With increasing decolorisation time, the decolorisation rate for batik dye liquid waste with no dilution reached 98.2% at the seventh hour. The ultraviolet–visible spectrum of decolorisation for different times is shown in Fig. 5c. The peak (at 636 nm) gradually decreased before disappearing at the seventh hour, indicating that the dye was completely decolorised by laccase–LECA.
The effect of volume was investigated at various volumes of wastewater (20, 80 and 140 mL). Fig. 5d shows that the decolorisation extent decreased with increase in the volume of batik dye wastewater. This is because indigosol molecules have more space in line with the increase in volume so they can move freely and reduce the probability of encountering the enzyme active site.
Reusability
One of the limitations of free enzyme application is the low ability of enzymes for reuse in continuous decolorisation. Reusability is an important factor for industrial/practical applications of laccase. Herein, the reusability of immobilised laccase was investigated for seven cycles, and the results are shown in Fig. 6. The catalytic activity on immobilised laccase gradually decreased with the increasing number of repeated use. The experimental results demonstrated that the residual activity of immobilised laccase in LECA could be retained at 95.37% of the initial catalytic activity after being reused for four cycles, and such a result could be considered as an excellent on enzyme reusability. Anita et al. (2020) reported that the immobilisation of laccase on LECA could be repeatedly used to decolorise RBBR dye for six cycles. However, this study used batik industrial wastewater that contained complex substances that may reduce the reusability of LECA-laccase. The decrease in the decolorisation extent can be related to the repetitive encounter of the substrate to the active site of the enzyme. Because of repetitive encountering, the binding between the immobilisation matrix and the enzyme is weak, causing the leaching of the enzyme. The leaching of the enzyme will cause loss of activity and decrease the decolorisation effectiveness.
Fig. 6.
Reusability of LECA–laccase for the decolorisation of indigosol wastewater
FTIR analysis of degraded wastewater
Figure 7 shows the FTIR spectra of untreated and treated batik dye wastewater. Differences in the spectra between untreated and treated wastewater confirmed the biodegradation. Spectra from untreated wastewater (Fig. 7a) showed the peak at 3392 cm−1 that can be related to –OH bonds. The adsorptions at 2912 cm−1 may be associated with the stretching vibration of C–H sp3 alkyl. The adsorptions at 1640 and 1572 cm−1 corresponded to the stretching vibration of the C = C aromatic bond. The sample was water-free, so the peaks at 3392 and 1290 cm−1 were related to the –OH group of the indigosol molecule. Peaks at 1290 and 1120 cm−1 were attributed to the ether group. Fig. 7b shows the FTIR spectra of the treated wastewater. Peaks at 2918 and 2848 cm−1 with very low absorbance indicated the stretching vibration of C–H sp3 alkyl, but only a small amount was detected. The absence of some important peaks (3392, 1640, 1572, 1290 and 1120 cm−1) from untreated samples indicated the complete breakdown of dyes in wastewater. The removal of absorption peaks in treated samples has been used as an indicator of dye degradation (Hossen et al. 2019; Sen et al. 2019).
Fig. 7.
FTIR spectra of batik dye wastewater a before and b after treatment
Toxicity assays
One of the important points of wastewater treatment is to reduce the toxicity of wastewater against human health and the environment. In some cases, degraded byproducts may result in more toxicity than the parent wastewater. Therefore, the evaluation of the toxicity of treated wastewater using reliable methods is needed. In this study, two bioassays methods (phytotoxicity and microbial toxicity) were used for the toxicity monitoring of treated and untreated wastewater. Phytotoxicity was performed using the mung bean (V. radiata) assay. Based on the Tukey’s test in Table 2, the growth of mung bean in untreated and treated wastewater was significantly different (α = 0.05). In comparison to control (15.27 ± 1.11 cm), the growth of mung bean in untreated wastewater was shortened (5.70 ± 0.41 cm), whereas mung bean seeds in treated wastewater showed a considerable improvement in shoot lengths (8.63 ± 0.56 cm). The results suggested that batik wastewater treated by LECA–laccase reduced the toxicity level of wastewater. In addition, the increase in wastewater dilution visibly affected the mung bean growth (Fig. S3).
Table 2.
Toxicity assay of indigosol dye wastewater from batik industry before and after treatment
| Wastewater | Dilution factor | Shoots length (cm) | Inhibition zone (mm) | |
|---|---|---|---|---|
| B. subtilis | P. aeruginosa | |||
| Untreated wastewater | No dilution | 5.70 ± 0.41 | 9.17 ± 0.29 | 8.00 ± 0.00 |
| 2.5-fold | 7.47 ± 0.40 | 7.83 ± 0.29 | 0.00 ± 0.00 | |
| Fivefold | 9.20 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Treated wastewater | No dilution | 8.63 ± 0.56* | 0.00 ± 0.00* | 0.00 ± 0.00* |
| 2.5-fold | 11.83 ± 0.24** | 0.00 ± 0.00** | 0.00 ± 0.00 | |
| Fivefold | 12.80 ± 0.41*** | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Control (water) | 15.27 ± 1.11 | |||
| Control (4 mg/mL chloramphenicol) | 28.3 ± 1.53 | 30.6 ± 0.58 | ||
Data are mean ± SD of three replicates. Values after treatment followed by *, ** and *** are significantly different from the same dilution factor in values before treatment
Another method to monitor the toxicity of wastewater is using microbial toxicity based on the inhibition zone against two bacteria, B. subtilis and P aeruginosa. Table 2 shows that untreated wastewater inhibited the growth of B. subtilis and P. aeruginosa with inhibition zones of 9.17 ± 0.29 and 8.00 ± 0.00 mm, respectively. However, after treatment by LECA-laccase, no inhibition zone on the growth of B. subtilis and P. aeruginosa was observed.
Based on the obtained data, the decolorisation of batik dye wastewater by LECA-laccase reduced the toxicity level of batik dye wastewater. To the authors’ knowledge, this is the first study that presented concise and first-hand information on the immobilisation of crude laccase on LECA that can be applied to the efficient treatment of dye wastewater from batik industrial effluent. Thus, LECA–laccase is ecofriendly and affordable biotechnology that can be used for real wastewater treatment in the field.
Conclusions
Laccase from T. hirsuta EDN 082 was successfully immobilised in LECA covalently using APTS and glutaraldehyde with an immobilisation efficiency of 66.7%. Immobilised laccase on LECA demonstrated the effective degradation of batik wastewater (98.2%) with four times reusability. The degraded wastewater showed much less toxicity than untreated wastewater, indicating that detoxification occurred. This study suggested that immobilisation of laccase from T. hirsuta EDN 082 on LECA has high potential for practical use in the field experiments of wastewater treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Part of this research was supported by the Indonesian Institute of Sciences (LIPI) through DIPA 2020 of the Deputy for Life Sciences, by the Japan ASEAN Science, Technology and Innovation Platform (JASTIP) and by the Humanosphere Asia Research Node (ARN) program of the Research Institute for Sustainable Humanosphere, Kyoto University. The authors also acknowledge the facilities and the scientific and technical assistance of the Integrated Laboratory of Bioproducts at the LIPI.
Author contributions
DHYY: Conceptualisation, supervision, funding acquisition, formal analysis, original draft. MAG: Experiment, original draft. ODN: Formal analysis. SHA: Formal analysis. MO: Formal analysis. KPR: Formal analysis. MFP: Supervision. TW: Supervision, funding acquisition, review draft.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alam R, Ardiati FC, Solihat NN, Alam MB, Lee SH, Yanto DHY, Watanabe T, Kim S. Biodegradation and metabolic pathway of anthraquinone dyes by Trametes hirsuta D7 immobilized in light expanded clay aggregate and cytotoxicity assessment. J Hazard Mater. 2021;405:124176. doi: 10.1016/j.jhazmat.2020.124176. [DOI] [PubMed] [Google Scholar]
- Alderete BL, da Silva J, Godoi R, da Silva FR, Taffarel SR, da Silva LP, Garcia ALH, Junior HM, de Amorim HLN, Picada JN. Evaluation of toxicity and mutagenicity of a synthetic effluent containing azo dye after advanced oxidation process treatment. Chemosphere. 2021;263:128291. doi: 10.1016/j.chemosphere.2020.128291. [DOI] [PubMed] [Google Scholar]
- Alharbi SK, Nghiem LD, van de Merwe JP, Leusch FDL, Asif MB, Hai FI, Price WE. Degradation of diclofenac, trimethoprim, carbamazepine, and sulfamethoxazole by laccase from Trametes versicolor: transformation products and toxicity of treated effluent. Biocatal Biotransformation. 2019 doi: 10.1080/10242422.2019.1580268. [DOI] [Google Scholar]
- Anita SH, Ardiati FC, Oktaviani M, Sari FP, Nurhayat OD, Ramadhan KP, Yanto DHY. Immobilization of laccase from Trametes hirsuta EDN 082 in light expanded clay aggregate for decolorization of Remazol Brilliant Blue R dye. Bioresour Technol Rep. 2020 doi: 10.1016/j.biteb.2020.100602. [DOI] [Google Scholar]
- Antecka K, Zdarta J, Siwi K. Synergistic degradation of dye wastewaters using binary or ternary oxide systems with immobilized laccase. Catalysts. 2018;8:1–18. doi: 10.3390/catal8090402. [DOI] [Google Scholar]
- Ardiati FC, Yanto DHY, Anita SH, Watanabe T. Immobilization of Trametes hirsuta D7 in light expanded clay aggregate for decolorization of synthetic dye. IOP Conf Ser Earth Environ Sci. 2019;308:012002. doi: 10.1088/1755-1315/308/1/012002. [DOI] [Google Scholar]
- Arshad R, Bokhari TH, Khosa KK, Bhatti IA, Munir M, Iqbal M, Iqbal DN, Khan MI, Iqbar M, Nazir A. Gamma radiation induced degradation of anthraquinone Reactive Blue-19 dye using hydrogen peroxide as oxidizing agent. Radiat Phys Chem. 2020;168:108637. doi: 10.1016/j.radphyschem.2019.108637. [DOI] [Google Scholar]
- Asif MB, Hai FI, Singh L, Price WE, Nghiem LD. Degradation of pharmaceuticals and personal care products by white-rot fungi-a critical review. Curr Pollut Rep. 2017;3:88–103. doi: 10.1007/s40726-017-0049-5. [DOI] [Google Scholar]
- Awang N, Farahin SN, Chan KM. Cytotoxicity and genotoxicity assessments of batik industrial wastewater on V79 cells. Asian J Appl Sci. 2017;5:109–117. [Google Scholar]
- Bankeeree W, Watanabe T, Punnapayak H, Lotrakul P, Prasongsuk S, Li R, Yanto DHY. Alkyl β-D-xyloside synthesis from black liquor xylan using Aureobasidium pullulans CBS 135684 β-xylosidases immobilized on spent expanded perlite. Biomass Convers Biorefin. 2020 doi: 10.1007/s13399-020-00755-5. [DOI] [Google Scholar]
- Benkhaya S, M’Rabet S, El Harfi A. A review on classifications, recent synthesis and application of textile dyes. Inorg Chem Commun. 2020;115:107891. doi: 10.1016/j.inoche.2020.107891. [DOI] [Google Scholar]
- Bezerra TMdS, Bassan JC, Santos VTdO, Ferraz A, Monti R. Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Proc Biochem. 2015;50:417–423. doi: 10.1016/j.procbio.2014.12.009. [DOI] [Google Scholar]
- Brugnari T, Pereira MG, Bubna GA, de Freitas EN, Contato AG, Correa RCG, Castoldi R, de Souza CGM, Polizelli MLTM, Bracht A, Peralta RM. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci Tot Environ. 2018;634:1346–1351. doi: 10.1016/j.scitotenv.2018.04.051. [DOI] [PubMed] [Google Scholar]
- Chakraborty JN. Fundamentals and practices in colouration of textile. New Delhi: Woodhead Publishing India Pvt Ltd; 2010. [Google Scholar]
- Cseri L, Topuz F, Abdulhamid MA, Alammar A, Budd PM, Szekely G. Electrospun adsorptive nanofibrous membranes from ion exchange polymers to snare textile dyes from wastewaters. Adv Mater Technol. 2021 doi: 10.1002/admt.202000955. [DOI] [Google Scholar]
- Daronch NA, Kelbert M, Pereira CS, de Araújo PHH, de Oliveira D. Elucidating the choice for a precise matrix for laccase immobilization: a review. Chem Eng J. 2020;397:125506. doi: 10.1016/j.cej.2020.125506. [DOI] [Google Scholar]
- De Wael K, Lepot L. Dichroism measurements in forensic fibre examination part 3–Dyed cotton and viscose fibres. Sci Justice. 2011;51:173–186. doi: 10.1016/j.scijus.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Demissie H, An G, Jiao R, Ritigala T, Lu S, Wang D. Modification of high content nanocluster-based coagulation for rapid removal of dye from water and the mechanism. Sep Purif Technol. 2021;259:117845. doi: 10.1016/j.seppur.2020.117845. [DOI] [Google Scholar]
- Eş I, Vieira DJG, Amaral AC. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl Microbiol Biotechnol. 2015;99:2065–2082. doi: 10.1007/s00253-015-6390-y. [DOI] [PubMed] [Google Scholar]
- Fortes GCS, Daniel-da-Silva AL, Xavier AMRB, Tavares APM. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions. Chem Eng Proces. 2017;117:1–8. doi: 10.1016/j.cep.2017.03.009. [DOI] [Google Scholar]
- Gita S, Shukla SP, Deshmukhe G, Choudhury TG, Saharan N, Singh AK. Toxicity evaluation of six textile dyes on growth, metabolism and elemental composition (C, H, N, S) of microalgae Spirulina platensis: the environmental consequences. Bull Environ Contam Toxicol. 2021;106:302–309. doi: 10.1007/s00128-020-03074-7. [DOI] [PubMed] [Google Scholar]
- Henky SH. Image analysis: textile industry in Indonesia. World J Bus Manag. 2015;1:42–56. doi: 10.5296/wjbm.v1i1.7883. [DOI] [Google Scholar]
- Hossen MZ, Hussain ME, Hakim Al, Islam K, Uddin MN, Azad AK. Biodegradation of reactive textile dye Novacron Super Black G by free cells of newly isolated Alcaligenes faecalis AZ26 and Bacillus spp obtained from textile effluents. Heliyon. 2019;5:e02068. doi: 10.1016/j.heliyon.2019.e02068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Yang X, Ji YZ, Zhu L, Ma N, Chen D, Jia X, Tang J, Cao Y. DFT-calculated IR spectrum amide I, II, and III band contributions of N-methylacetamide find components. ACS Omega. 2020;5:8572–8578. doi: 10.1021/acsomega.9b04421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhori EM, Yetilmezsoy K, Uygur N, Zarrabi M, Shmeis RMA. Modeling of adsorption of toxic chromium on natural and surface modified lightweight expanded clay aggregate (LECA) Appl Surf Sci. 2013;287:428–442. doi: 10.1016/j.apsusc.2013.09.175. [DOI] [Google Scholar]
- Kalhori EM, Al-Musawi TJ, Ghahramani E, Kazemian H, Zarrabi M. Enhancement of the adsorption capacity of the light-weight expanded clay aggregate surface for the metronidazole antibiotic by coating with MgO nanoparticles: Studies on the kinetic, isotherm, and effects of environmental parameters. Chemosphere. 2017;175:8–20. doi: 10.1016/j.chemosphere.2017.02.043. [DOI] [PubMed] [Google Scholar]
- Kashefi S, Borghei SM, Mahmoodi NM. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J Mol Liq. 2019;276:153–162. doi: 10.1016/j.molliq.2018.11.156. [DOI] [Google Scholar]
- Khatri J, Nidheesh PV, Singh TSA, Kumar MS. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem Eng J. 2018;348:67–73. doi: 10.1016/j.cej.2018.04.074. [DOI] [Google Scholar]
- Kishor R, Purchase D, Saratale GD, Saratale RG, Ferreira LFR, Bilal M, Chandra R, Bharagava RN. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J Environ Chem Eng. 2021;9(2):105012. doi: 10.1016/j.jece.2020.105012. [DOI] [Google Scholar]
- Köktürk M, Altindag F, Ozhan G, Çalimli MH, Nas MS. Textile dyes Maxilon blue 5G and Reactive blue 203 induce acute toxicity and DNA damage during embryonic development of Danio rerio. Comp Biochem Physiol C Toxicol Pharmacol. 2021;242:108947. doi: 10.1016/j.cbpc.2020.108947. [DOI] [PubMed] [Google Scholar]
- Lellis B, Fávaro-Polonio CZ, Pamphile JA, Polonio JC. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov. 2019;3:275–290. doi: 10.1016/j.biori.2019.09.001. [DOI] [Google Scholar]
- Lonappan L, Liu Y, Rouissi T, Brar SK, Verma M, Surampalli RY. Adsorptive immobilization of agro-industrially produced crude laccase on various micro-biochars and degradation of diclofenac. Sci Tot Environ. 2018;640–641:1251–1258. doi: 10.1016/j.scitotenv.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Malhotra M, Suman SK. Laccase-mediated delignification and detoxification of lignocellulosic biomass: removing obstacles in energy generation. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-13283-0. [DOI] [PubMed] [Google Scholar]
- Mathur P, Sanyal D, Dey P. Optimization of growth conditions for enhancing the production of microbial laccase and its application in treating antibiotic contamination in wastewater. 3 Biotech. 2021 doi: 10.1007/s13205-020-02627-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcyotto F, Wei Q, Macharia DK, Huang M, Shen C, Chow CWK. Effect of dye structure on color removal efficiency by coagulation. Chem Eng J. 2021;405:126674. doi: 10.1016/j.cej.2020.126674. [DOI] [Google Scholar]
- Moradi A, Rahimpour F, Salehi MA, Shojaeimehr T. Impact of operating conditions for the continuous-flow removal of dye effluents in a fixed-bed reactor using light expanded clay aggregate as a green adsorbent with ultrasound-assisted desorption. Asia-Pasific J Chem Eng. 2020 doi: 10.1002/apj.2508. [DOI] [Google Scholar]
- Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018;25:361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ningsih F, Yanto DHY, Mangunwardoyo W, Anita SH, Watanabe T. Optimization of laccase production from a newly isolated Trametes sp. EDN134. IOP Conf. Series: Earth Environ Sci. 2020 doi: 10.1088/1755-1315/572/1/012024. [DOI] [Google Scholar]
- Nkansah MA, Christy AA, Barth T, Francis GW. The use of lightweight expanded clay aggregate (LECA) as sorbent for PAHs removal from water. J Hazard Mater. 2012;217–218:360–365. doi: 10.1016/j.jhazmat.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Park SW, Kim YI, Chung KH, Hong SI, Kim SW. Covalent immobilization of GL-7-ACA acylase on silica gel through silanization. React Funct Polym. 2002;51:79–92. doi: 10.1016/S1381-5148(02)00028-7. [DOI] [Google Scholar]
- Pezzella C, Russo ME, Marzocchella A, Salatino P, Sannia G. Immobilization of a Pleurotus ostreatus laccase mixture on perlite and its application to dye decolourisation. Biomed Res Int. 2014 doi: 10.1155/2014/308613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez LA, Alanis MR, Rodríguez JR, Zacarícas CC, Hernández JES, Barceló D, Iqbal HMN, Saldívar RP. Exploring current tendencies in techniques and materials for immobilization of laccases–A review. Int J Biol Macromol. 2021;181:683–696. doi: 10.1016/j.ijbiomac.2021.03.175. [DOI] [PubMed] [Google Scholar]
- Rashad AM. Lightweight expanded clay aggregate as a building material–an overview. Constr Build Mater. 2018;170:757–775. doi: 10.1016/j.conbuildmat.2018.03.009. [DOI] [Google Scholar]
- Rashid R, Shafiq I, Akhter P, Iqbal MJ, Hussain M. A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ Sci Pollut Res. 2021;28:9050–9066. doi: 10.1007/s11356-021-12395-x. [DOI] [PubMed] [Google Scholar]
- Rehman K, Shahzad T, Sahar A, Hussain S, Mahmood F, Siddique MH, Rashid SMA, MI, Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling. PeerJ. 2018 doi: 10.7717/peerj.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routoula E, Patwardhan SV. Degradation of anthraquinone dyes from effluents: a review focusing on enzymatic dye degradation with industrial potential. Environ Sci Technol. 2020;54:647–664. doi: 10.1021/acs.est.9b03737. [DOI] [PubMed] [Google Scholar]
- Sen SK, Patra P, Das CR, Raut S, Raut S. Pilot-scale evaluation of bio-decolorization and biodegradation of reactive textile wastewater: an impact on its use in irrigation of wheat crop. Water Resour Ind. 2019;21:100106. doi: 10.1016/j.wri.2019.100106. [DOI] [Google Scholar]
- Sepehr MN, Allani F, Zarrabi M, Darvishmotevalli M, Vasseghian Y, Fadaei S, Fazli MM. Dataset for adsorptive removal of tetracycline (TC) from aqueous solution via natural light weight expanded clay aggregate (LECA) and LECA coated with manganese oxide nanoparticles in the presence of H2O2. Data Brief. 2019;22:676–686. doi: 10.1016/j.dib.2018.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RA, van Pelt S. Enzyme immobilization in biocatalysis: why, what, and how. Chem Soc Rev. 2013;42:6223–6235. doi: 10.1039/c3cs60075k. [DOI] [PubMed] [Google Scholar]
- Shokoohi R, Samadi MT, Samarghandi MR, Ahmadian M, Karimaian K, Poormohammadi A. Comparing the performance of granular coral limestone and leca in adsorbing acid cyanine 5 R from aqueous solution. Saudi J Biol Sci. 2016;24:749–759. doi: 10.1016/j.sjbs.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RP, Singh PK, Gupta R, Singh RL. Treatment and recycling of wastewater from textile industry. In: Singh RL, Singh RP, editors. Advances in biological treatment of industrial waste water and their recycling for a sustainable future. Singapore: Springer; 2019. pp. 225–266. [Google Scholar]
- Susanty A, Hartini S, Puspitasari D, Arsiwi P. Measuring efficiency of using resource in the production process of making stamped-batik: a DEA approach. Mediterr J Soc Sci. 2015;6(5):318–327. doi: 10.5901/mjss.2015.v6n5s2p318. [DOI] [Google Scholar]
- Tang X, Wang T, Zhang S, Fang L, Zheng H. Enhanced performance of a novel flocculant containing rich fluorine groups in refractory dyeing wastewater treatment: removal mechanisms. Sep Purif Technol. 2021;263:118411. doi: 10.1016/j.seppur.2021.118411. [DOI] [Google Scholar]
- Tavares MF, Avelino KV, Araújo NL, Marim RA, Linde GA, Colauto NB, do Valle JS, Decolorization of azo and anthraquinone dyes by crude laccase produced by Lentinus crinitus in solid state cultivation. Braz J Microbiol. 2020;51:99–106. doi: 10.1007/s42770-019-00189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Seitz O, Li M, Hu WW, Chabal YJ, Gao J. Infrared characterization of interfacial Si–O bond formation on silanized flat SiO2/Si surfaces. Langmuir. 2010;26:4563–4566. doi: 10.1021/la904597c. [DOI] [PubMed] [Google Scholar]
- Vaishnavi J, Arulprakash A, Selvi A, Rajasekar A. Marine biomass toward biofuel production. Refin Biomass Residues Sustain Energy Bioprod. 2020 doi: 10.1016/B978-0-12-818996-2.00020-X. [DOI] [Google Scholar]
- Yanto DHY, Auliana N, Anita SH, Watanabe T. Decolorization of synthetic textile dyes by laccase from newly isolated Trametes hirsuta EDN084 mediated by violuric acid. IOP Conf Ser Earth Environ Sci. 2019;374:012005. doi: 10.1088/1755-1315/374/1/012005. [DOI] [Google Scholar]
- Yurtsever A, Basaran E, Ucar B, Sahinkaya E. Self-forming dynamic membrane bioreactor for textile industry wastewater treatment. Sci Tot Environ. 2021;751:141572. doi: 10.1016/j.scitotenv.2020.141572. [DOI] [PubMed] [Google Scholar]
- Zhu MX, Lee L, Wang HH, Wang Z. Removal of an anionic dye by adsorption/precipitation processes using alkaline white mud. J Hazard Mater. 2007;149(3):735–741. doi: 10.1016/j.jhazmat.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Zucca P, Sanjust E. Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules. 2014;19:14139–14194. doi: 10.3390/molecules190914139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.