Abstract
The provenance of oxygen on the Earth and other planets in the Solar System is a fundamental issue. It has been widely accepted that the only prebiotic pathway to produce oxygen in the Earth’s primitive atmosphere was via vacuum ultraviolet (VUV) photodissociation of CO2 and subsequent two O atom recombination. Here, we provide experimental evidence of three-body dissociation (TBD) of H2O to produce O atoms in both 1D and 3P states upon VUV excitation using a tunable VUV free electron laser. Experimental results show that the TBD is the dominant pathway in the VUV H2O photochemistry at wavelengths between 90 and 107.4 nm. The relative abundance of water in the interstellar space with its exposure to the intense VUV radiation suggests that the TBD of H2O and subsequent O atom recombination should be an important prebiotic O2-production, which may need to be incorporated into interstellar photochemical models.
Subject terms: Astronomy and astrophysics, Photochemistry, Excited states
Three-body dissociation of water, producing one oxygen and two hydrogen atoms, has been difficult to investigate due to the lack of intense vacuum ultraviolet sources. Here, using a tunable free-electron laser, the authors obtain quantum yields for this channel showing that it is a possible route to prebiotic oxygen formation in interstellar environments.
Introduction
Oxygen is the third most abundant element in the Universe, but its molecular form (O2) is very rare. Besides on the Earth, molecular oxygen has only been detected in two interstellar clouds1,2, in the moons of Jupiter3 and Saturn4, and on Mars5. Geologically based arguments suggested that Earth’s original atmosphere had no oxygen and was composed mostly of H2O, CO2, and N2, with only small amounts of CO and H26. Therefore, how oxygen is produced in the primitive atmosphere is a fundamentally important issue in the evolution of the early primitive atmosphere. Before the emergence of the oxygen-rich atmosphere due to the “great oxidation event”7,8, about 2.33 billion years ago, which allowed the Earth to evolve into a living planet, a small amount of oxygen was already present, and this was previously attributed to an abiotic formation mechanism involving photodissociation of CO2 by vacuum ultraviolet (VUV) light, followed by three-body recombination processes9:
| 1 |
| 2 |
where M is a third body to carry off the excess energy in the reaction process. Direct O2 production pathways via VUV photodissociation of CO210 and dissociative electron attachment to CO211 have recently been identified. These findings provide new insights into the sources of O2 in Earth’s early atmosphere.
In contrast, photodissociation of H2O, one of the dominant oxygen carriers12, has long been assumed to proceed mainly to produce hydroxyl (OH) and hydrogen (H) atom primary products, and contribute limitedly to the O2 production9. Recently, abundant molecular O2 in the coma of comet 67P/Churyumov–Gerasimenko, which is dominated by H2O, CO, and CO2, has been detected13. Interestingly, a strong correlation between O2 and H2O has been identified, indicating the O2 formation is linked to H2O in the comet. One plausible explanation for the strong O2–H2O correlation would be that the O2 is produced by radiolysis or photolysis of water, or single collisions of energetic H2O+ with surfaces14. However, the existing photochemical reaction mechanisms underestimate the O2 abundance13. Thus, the detailed O2 production mechanism in the coma of comets is still unclear.
The photodissociation of water has been the subject of many experimental studies, which have revealed fascinating dynamics arising from strongly coupled electronic states with strikingly different potential energy surfaces (PESs)15,16. Excitation to the first excited singlet (1B1) state of H2O at wavelengths λ ~160 nm results in direct O−H bond fission yielding an H atom plus a ground state hydroxyl radical, OH(X2Π), with little internal excitation17–19. The absorption cross-section to the second excited singlet (1A1) state is maximal at λ ~128 nm. Excitation at these wavelengths results in a (minor) direct dissociation channel to electronically excited OH(A2Σ+) + H products. The major dissociation process yields ground state OH(X) + H products following non-adiabatic transitions at conical intersections between the and state PESs at linear H–O–H and H–H–O geometries20–23.
Additional fragmentation pathways, named three-body dissociation (TBD), become accessible energetically at shorter photolysis wavelengths, e.g.:
| 3 |
| 4 |
where the threshold energies (Eth) for these fragmentation channels are given in parentheses (on the basis of thermodynamic calculations with the data available from the thermochemical network) (https://atct.anl.gov). Fragmentation channel (3) has been previously detected with small quantum yields24,25 following photoexcitation of H2O at the Lyman-α wavelength (λ = 121.57 nm). Because of the lack of intense tunable VUV laser sources, the quantitative assessment of the importance of the H2O TBD processes in the VUV region and its role in the O2 formation in the interstellar space has not been possible. Recent development of the intense VUV free electron laser, at the Dalian Coherent Light Source (DCLS), has provided an exciting tool for experimental studies of molecular photochemistry throughout the entire VUV region26.
Herein we report the studies of the H2O photochemistry in the VUV region using the DCLS and the H-atom Rydberg tagging time-of-flight (HRTOF) technique. These experiments allow quantitative determination of the relative importance of the binary dissociation and the TBD processes following photoexcitation of H2O in the 90–110 nm region. The present results show conclusively that the H2O TBD process is an important pathway to form oxygen in the interstellar space.
Results and discussion
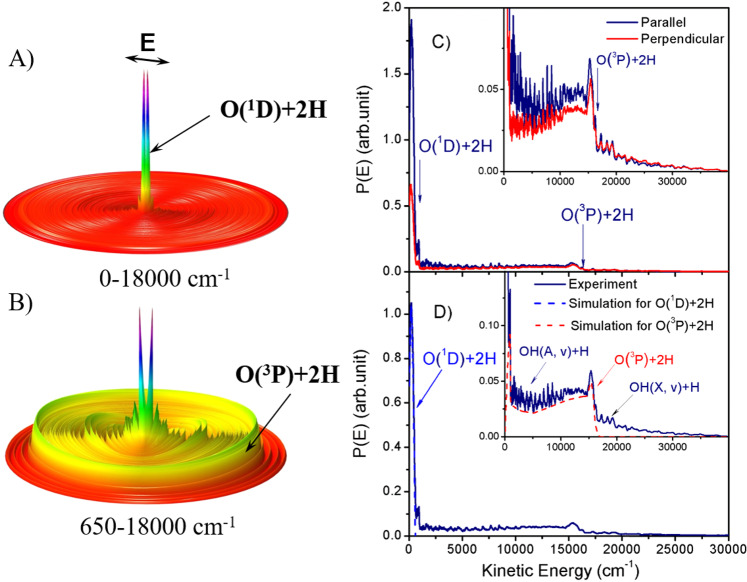
The H2O sample was generated in a supersonic beam, with a rotational temperature estimated to be about 10 K. The H2O molecules were photoexcited to different Rydberg states27 (see Supplementary Fig. 2 and Supplementary Note 2). The dissociated H atom fragments were then detected using the HRTOF technique (see Supplementary Fig. 1 and Supplementary Note 1). TOF spectra of the H atoms resulting from H2O photodissociation at λ = 107.4 nm have been recorded, with the detection axis aligned parallel and perpendicular to the polarization vector of the VUV-FEL radiation. Knowing both the distance traveled by the H atom from the photodissociation area to the detector and its mass, the TOF spectra can be converted into the distributions of total kinetic energy release (TKER), which is derived using the following equation, , where d is the flying path length of H atom (d ≈ 28 cm) from the photodissociation region to the detector, t is the measured time of flight28. Using the TKER distributions obtained in the parallel and perpendicular directions, we can construct a 3-dimensional (3D) flux diagram of the H atom fragments. Figure 1 shows the 3D product flux diagrams in two regions of the kinetic energy with rich structures. The tall feature at low kinetic energy (Fig. 1A) shows a large product angular anisotropy, whereas the product anisotropy in the higher kinetic energy region (Fig. 1B) is rather small.
Fig. 1. The kinetic energy release spectra from H2O photodissociation.
A The 3D product contour diagram from the photodissociation of H2O at 107.4 nm for the total kinetic energy release (TKER) between 0 and 18,000 cm−1; B the 3D product contour diagram for the TKER between 650 and 18,000 cm−1. C The product TKER distributions at λ = 107.4 nm with the detection axis parallel and perpendicular to the polarization vector of the VUV-FEL radiation. The energetic limits of the two TBD channels (EKEmax ~580 and ~16,448 cm−1) are marked, and the inset displays the same spectra on an expanded vertical scale. D The product TKER distribution at λ = 107.4 nm with the detection axis at 54.7° (magic angle) to the polarization direction, along with the simulated TKER distributions for the O(1D) + 2H and O(3P) + 2H TBD channels. Source data are provided as a Source Data file.
For detailed analysis and feature assignment, the TKER distributions in parallel and perpendicular directions at λ = 107.4 nm are plotted in Fig. 1C, while the product TKER distribution from photodissociation of H2O at the magic angle (with detection angle of 54.7° relative to the polarization direction) is shown in Fig. 1D. These distributions show both sharp and broad features. Using the energy conservation relationship appropriate for a binary photodissociation process:
| 5 |
where Eint(H2O) and Eint(OH) are the internal energies of H2O and OH, respectively, EKE is the product total kinetic energy and D0(H−OH) is the dissociation energy of H2O29. We can assign all of the sharp structures to specific ro-vibrational levels of the OH product in the X and A states formed via the binary dissociation channel, H + OH (X or A, v, N). In addition to these sharp structures, the TKER spectra show two broad features: one with EKE ≤ 600 cm−1 that has a large angular anisotropy, and an underlying feature that spans the range of 600 ≤ EKE ≤ 16,000 cm−1 which displays a much smaller angular anisotropy. These broad features are obviously not from the binary dissociation channel, H + OH. Based on energy conservation, the maximum kinetic energy for the H atom product from the TBD channels (3) and (4) at 107.4 nm are 16,448 and 580 cm−1, respectively. These limits of the two TBD channels match well with the upper limits of the two broad features in the distributions (Fig. 1). Thus, the intense broad feature at EKE < 600 cm−1 is assigned to the O(1D) + 2H channel, while the broad underlying signal extending to EKE ~16,000 cm−1 is attributed to the O(3P) + 2H products.
Given the above analysis, the product TKER distribution in Fig. 1D can be divided into three components: O(1D) + 2H, O(3P) + 2H, and OH + H. The first two components are broad features, while the third comprises sharp structures attributable to ro-vibrational levels of the OH(A) and OH(X) products. From Fig. 1D, the O(1D) + 2H channel obviously has the highest intensity in the kinetic energy <600 cm−1, yet it has never been reported previously in H2O photodissociation. The O(3P) + 2H channel clearly makes the major contribution in the higher kinetic energy region. This indicates that the TBD is likely the dominant process following VUV photoexcitation of H2O at 107.4 nm.
Branching ratios for the O(1D) + 2H, O(3P) + 2H, and OH + H fragmentation channels have been estimated by simulating the TKER distribution (Fig. 1D) using the three components shown in Fig. 2. Integrating the areas under the respective distributions returns relative H atom yields for the three channels. Recognizing that two H atoms are formed in each TBD process, the relative H atom yields are used to determine the branching ratio, e.g. 67% at 107.4 nm for the TBD channels (Table 1).
Fig. 2. The simulated kinetic energy release spectra from H2O photodissociation.

TKER distributions determined from simulating the (A) O(1D) + 2H, (B) O(3P) + 2H, and (C) OH + H product yields from photodissociation of H2O at λ = 107.4 nm. The sharp features in the H + OH product yield have been assigned to population of ro-vibrational levels of the X and A states of the OH radical. Source data are provided as a Source Data file.
Table 1.
The branching ratios for the binary and TBD channels following the H2O photodissociation at different VUV wavelengths (nm).
| Dissociation channel | Photolysis wavelength (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 109.0 | 107.4 | 106.7 | 105.7 | 101.3 | 98.1 | 96.2 | 94.5 | 92.0 | |
| TBD (O(1D/3P) + 2H) | 0.35 | 0.67 | 0.76 | 0.62 | 0.77 | 0.72 | 0.79 | 0.86 | 0.86 |
| Binary dissociation: (H + OH) | 0.65 | 0.33 | 0.24 | 0.38 | 0.23 | 0.28 | 0.21 | 0.14 | 0.14 |
The maximum uncertainty on the branching ratios is ±10%.
Photodissociation of H2O has also been investigated at eight more VUV wavelengths between 92 and 109 nm, and a similar data analysis procedure is applied at these photolysis wavelengths (Supplementary Fig. 3 and Supplementary Note 3). The branching ratios determined for the binary and TBD channels at each wavelength are listed in Table 1. At 109.0 nm, only one TBD channel (O(3P) + 2H) exists because the O(1D) + 2H channel is energetically not accessible. The results mean that oxygen atoms (1D and 3P), not OH radicals, are the major oxygen-containing products from H2O photolysis at λ < 107.4 nm, in striking contrast to the dominant binary photofragmentation (i.e. H + OH) behavior displayed by H2O photochemistry at longer VUV wavelengths15.
The dissociation dynamics of the two TBD channels are also quite interesting. Since the two H atoms in the water molecule are equivalent, if they dissociate simultaneously it should yield a narrow H atom kinetic energy distribution, peaking at an EEK value close to half of the available energy (Supplementary Figs. 5 and 6 and Supplementary Note 5). However, the observed distributions are much broader than the narrow distributions for a simultaneous concerted process, implying that both TBD processes are due to mostly a sequential dissociation mechanism. Possible dissociation routes for the two channels are illustrated in Fig. 3 (and more details in Supplementary Fig. 7 and Supplementary Note 6). The water molecule undergoes efficient non-adiabatic coupling from the initial excited nd Rydberg states to the state. Path 1 illustrates the possible direct dissociation route from the state to form O(1D) + 2H products. The overall anisotropy parameter of this channel is about 0.8, according with a fast and direct dissociation process. Paths 2 and 3 illustrate plausible routes for the more complicated O(3P) + 2H dissociation paths: from the state to the state and then to the ground state via the two CIs between the and state PESs, following the initial internal conversion from the excited nd Rydberg states to the state. The averaged anisotropy parameter of this channel is considerably smaller (~0.2). This also accords with the dissociation undergoing several internal conversions, and with the more scrambling that leads to small product anisotropy.
Fig. 3. Illustration of the TBD mechanisms of H2O upon VUV excitation.

Photoexcitation populates the nd Rydberg state (nd RS) which undergo efficient non-adiabatic coupling to the state. The light blue arrow displays the photoexcitation process and the gray oval represents the crossing region between the nd RS and the state. Path 1 illustrates the possible direct dissociation route from the state to form O(1D) + 2H products (the yellow curve). Paths 2 and 3 illustrate plausible routes for the more complicated O(3P) + 2H dissociation paths: from the state to the state and then to the ground state via the two conical intersections (CI #1 or CI #2), following the initial internal conversion from the excited nd RS to the state (the red curves). This picture is consistent with the observed angular anisotropy for the two channels: the O(1D) + 2H channel is a more direct dissociation process with larger angular anisotropy, while the O(3P) + 2H channel is a more complicated dissociation process with smaller angular anisotropy. The PES contour color from blue to red represents the potential energy from 0 to 12 eV.
The conclusion that the TBD is the dominant decay process following excitation of H2O at these VUV wavelengths could have profound implications for our understanding of the source of oxygen production. For quantitative assessment, we have calculated the fragment-dependent photodissociation rate of H2O by using: JH2O = ʃ ΦλΓσλdλ, where Φλ is the solar photon flux, Γ is the fragment quantum yield, and σλ is the photodissociation cross-section30. Figure 4 collects together the wavelength dependences of the solar photon flux in the early period31, the total photoabsorption cross-sections of the parent H2O molecule in the VUV region (90–200 nm)32 and the production yields of O atoms at studied photolysis wavelengths. Convoluting the solar photon flux, the photoabsorption cross-sections, and the production yields implies that ~21% of the photoexcitation events of H2O will result in O atoms. Considering the water abundance in widely interstellar circumstances, like in interstellar clouds2,3 and in the moons of planets in the Solar System (e.g., the comets 67P13,33), oxygen production from the water photolysis should be an important process. The following recombination of oxygen atoms will produce molecular oxygen.
Fig. 4. The wavelength dependences of O atom quantum yields.

Plot showing (A) the wavelength dependences of the reconstructed VUV solar flux (90–200 nm, the black lines) at ~10 My (10 My = 1 × 107 years, reconstructed from ref. 31. The VUV solar flux at modern period or the interstellar radiation field (ISRF)41 also can be used, which may modify the yield of O-production a little, but the final conclusion holds), the total absorption (σtot, the solid blue curve)32 and photoionization (σion, the dotted blue curve) cross-sections42 of H2O, and (B) the quantum yield for forming O-atom photoproducts (O(3P/1D) + 2H), Γ, determined in the present work (the red dot). It is noted that the predissociation rate of H2O is sufficiently fast that the fluorescence quantum yield must be negligible, so the total photodissociation cross-section will be almost the same as the photoabsorption cross-section. The polynomial function through the latter data is used to derive the reported overall O product quantum yield (the black curve in B). The quantum yields at λ = 111.5, 115.2, 121.57 nm are obtained from refs. 15, 24, 26. The error bars represent the standard deviation (1σ) of three times measurements. Source data are provided as a Source Data file.
In addition, it is well known that the water photolysis has nothing to do with oxygen production in the Earth’s atmosphere under equilibrium conditions due to VUV photon screening by the thick atmosphere10,34. However, in the earliest period of Earth, i.e., the period approaching to clement conditions on the earliest Earth followed by the current Earth–Moon system formed, the surface of Earth remained quite hot (>1000 K)34, all of the water on the Earth was vaporized to the atmosphere and part of water clouds (emitted from volcanos or delivered by carbonaceous chondrite meteorites6) populated at the top of the atmosphere could absorb the VUV photons and dissociate. Given [H2O] is ten times abundant than [CO2] in the atmosphere during this early, chaotic period of Earth6 (see Supplementary Fig. 4 and Supplementary Note 4), the O production rate from H2O VUV photochemistry could be three times larger than that of CO2 in the same VUV wavelength region, via TBD processes: NH2O(O)/NCO2(O) = (JH2O(O) × [H2O])/(JCO2(O) × [CO2]) = ~3, where JH2O(O) = ~5.2 × 10−5 s−1 (Fig. 4), JCO2(O) = ~1.8 × 10−4 s−1 (Supplementary Fig. 8 and Supplementary Note 7), [H2O] and [CO2] are the densities of H2O and CO2, respectively. Since the molecular oxygen generation process should be the same in the three-body recombination process (Eq. (2)), this analysis implies that the H2O photochemistry might be an important prebiotic source of O2 in Earth’s early atmosphere.
From the experimental results, it seems that more than one-third of O atoms produced from H2O TBD process populate in the metastable 1D state. The generation of O(1D) atoms from photodissociation of H2O in a significant amount is also very interesting because the metastable O(1D) atom is highly reactive35. It can react with almost all the gases emitted into the atmosphere. For instance, the reaction of O(1D) with methane could be a significant source of formaldehyde in the earth’s primitive atmosphere36,37. Thus, the production of O(1D) atoms from the exposure of water to VUV radiation, and the subsequent reactions of these atoms, could have been important drivers in the evolution of the earliest atmosphere.
In the existing interstellar photochemical model, reactions (1) and (2) are the major pathways to produce prebiotic O2. In this work, we propose an alternative prebiotic O2 pathway: atomic oxygen production from the TBD of water, followed by oxygen recombination reactions. Recent International Ultraviolet Explorer satellite observation of pre-main-sequence stars suggested that the nascent Sun has emitted more than ten times VUV radiation than it does today38. This implies that oxygen formation by VUV photoinduced TBD of H2O is likely an important process in the coma of comets, in the interstellar clouds and even in Earth’s primitive atmosphere, and thus needs to be incorporated into interstellar photochemical models. Furthermore, the TBD of H2O may well be important for the oxygen evolution in the atmospheres of all water-rich terrestrial planets39.
Methods
Vacuum ultraviolet free electron laser (VUV-FEL) radiation
The experiments employ a recently constructed apparatus for molecular photochemistry, which is centered on the VUV-FEL beam line at the DCLS26. The VUV-FEL facility runs in the high gain harmonic generation mode, in which the seed laser is injected to interact with the electron beam in the modulator (Supplementary Fig. 1). The seeding pulse, in the wavelength range (λseed) 240–360 nm, can be generated from a picosecond Ti:sapphire laser pulse. The electron beam is generated from a photocathode RF gun, and accelerated to the beam energy of ~300 MeV by seven S-band accelerator structures, with a bunch charge of 500 pC. The micro-bunched beam is then sent through the radiator, which is tuned to the 2nd/3rd/4th harmonic of the seed wavelength, and coherent FEL radiation with wavelength λseed/2, λseed/3, or λseed/4 is emitted. Optimization of the linear accelerator yields a high quality electron beam with emittance of ~1.5 mm mrad, energy spread of ~1‰, and pulse duration of ~1.5 ps. In this work, the VUV-FEL operates at 10 Hz, and the maximum pulse energy is >100 μJ/pulse. The output wavelength is continuously tunable in the range 50–150 nm and the typical spectral bandwidth of the VUV-FEL output is 30–50 cm−1.
The high-n HRTOF technique used in this work was pioneered by Welge et al.40. The key point of this technique is the 1 + 1′ (VUV + UV) excitation of the H atom. The first step involves VUV laser excitation of the H atom from its n = 1 ground state to the n = 2 state by absorbing one λ = 121.57 nm photon. In the second step, the H (n = 2) atom is excited with a UV (λ ~365 nm) photon to a high-n (n = 30–80) Rydberg state. Charged species formed in the interaction region are extracted from the TOF axis by a small electric field (~20 V/cm) placed across this region. Rydberg tagged neutral H atoms fly a known distance (d ≈280 mm) from the interaction region to a rotatable microchannel plate (MCP) Z-stack detector located close behind a grounded fine metal grid. After passing through the grid, the Rydberg atoms are immediately field-ionized by the electric field (~2000 V/cm) applied between the grid and the front plate of the Z-stack MCP detector. The signal detected by the MCP is amplified by a fast preamplifier and counted by a multichannel scaler.
Supplementary information
Acknowledgements
The experimental work was supported by the National Natural Science Foundation of China (NSFC Center for Chemical Dynamics (Grant No. 21688102)), the National Natural Science Foundation of China (Grant Nos. 21873099, 21922306), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB17000000), the Key Technology Team of the Chinese Academy of Sciences (Grant No. GJJSTD20190002), the Liaoning Revitalization Talents Program (XLYC1907154), and the international partnership program of Chinese Academy of Sciences (No. 121421KYSB20170012). The theoretical work was supported by the National Natural Science Foundation of China (Grant Nos. 21733006, 22073042, and U1932147). M.N.R.A. is grateful for funding from the Engineering and Physical Sciences Research Council (EPSRC, EP/L005913) and to the NFSC Center for Chemical Dynamics for the award of a Visiting Fellowship.
Source data
Author contributions
K.Y. and X.Y. designed the experiments. Y.C., Y.Y., Z.L., Q.L., J.Y., and Z.C. performed the experiments. X.Z., D.Q., K.Y., M.N.R.A., L.C., W.Z., G.W., and X.Y. discussed the experimental results. F.A., X.H., and D.Q. performed the theoretical calculations. K.Y., M.N.R.A., and X.Y. prepared the manuscript.
Data availability
All other data supporting this study are available from the authors upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Chaya Weeraratna, Piergiorgio Casavecchia and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yao Chang, Yong Yu.
Contributor Information
Xixi Hu, Email: xxhu@nju.edu.cn.
Kaijun Yuan, Email: kjyuan@dicp.ac.cn.
Xueming Yang, Email: xmyang@dicp.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-22824-7.
References
- 1.Goldsmith PF, et al. Herschel measurements of molecular oxygen in Orion. Astrophys. J. 2011;737:96. doi: 10.1088/0004-637X/737/2/96. [DOI] [Google Scholar]
- 2.Liseau R, et al. Multi-line detection of O2 toward ρ Ophiuchi A. Astron. Astrophys. 2012;541:A73. doi: 10.1051/0004-6361/201118575. [DOI] [Google Scholar]
- 3.Hall DT, et al. Detection of an oxygen atmosphere on Jupiter’s moon Europa. Nature. 1995;373:677–679. doi: 10.1038/373677a0. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RE, et al. Production, ionization and redistribution of O2 in Saturn’s ring atmosphere. Icarus. 2006;180:393–402. doi: 10.1016/j.icarus.2005.08.021. [DOI] [Google Scholar]
- 5.Barker ES. Detection of molecular oxygen in the martian atmosphere. Nature. 1972;238:447–448. doi: 10.1038/238447a0. [DOI] [Google Scholar]
- 6.Zahnle K, Schaefer L, Fegley B. Earth’s earliest atmospheres. Cold Spring Harb. Perspect. Biol. 2010;2:a004895. doi: 10.1101/cshperspect.a004895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo GM, et al. Rapid oxygenation of Earth’s atmosphere 2.33 billion years ago. Sci. Adv. 2016;2:e1600134. doi: 10.1126/sciadv.1600134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland HD. The oxygenation of the atmosphere and oceans. Philos. T. R. Soc. B. 2006;361:903–915. doi: 10.1098/rstb.2006.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasting JF, Liu SC, Donahue TM. Oxygen levels in the prebiological atmosphere. J. Geophys. Res. Oc. Atm. 1979;84:3097–3107. doi: 10.1029/JC084iC06p03097. [DOI] [Google Scholar]
- 10.Lu Z, Chang YC, Yin QZ, Ng CY, Jackson WM. Evidence for direct molecular oxygen production in CO2 photodissociation. Science. 2014;346:61–64. doi: 10.1126/science.1257156. [DOI] [PubMed] [Google Scholar]
- 11.Wang XD, Gao XF, Xuan CJ, Tian SX. Dissociative electron attachment to CO2 produces molecular oxygen. Nat. Chem. 2016;8:258–263. doi: 10.1038/nchem.2427. [DOI] [PubMed] [Google Scholar]
- 12.Young ED. Strange water in the solar system. Science. 2007;317:211–212. doi: 10.1126/science.1145055. [DOI] [PubMed] [Google Scholar]
- 13.Bieler A, et al. Abundant molecular oxygen in the coma of comet 67P/Churyumov—Gerasimenko. Nature. 2015;526:678–681. doi: 10.1038/nature15707. [DOI] [PubMed] [Google Scholar]
- 14.Yao YX, Giapis KP. Dynamic molecular oxygen production in cometary comae. Nat. Commun. 2017;8:15298. doi: 10.1038/ncomms15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan KJ, Dixon RN, Yang XM. Photochemistry of the water molecule: adiabatic versus nonadiabatic dynamics. Acc. Chem. Res. 2011;44:369–378. doi: 10.1021/ar100153g. [DOI] [PubMed] [Google Scholar]
- 16.Yuan KJ, et al. Nonadiabatic dissociation dynamics in H2O: competition between rotationally and nonrotationally mediated pathways. Proc. Natl Acad. Sci. USA. 2008;105:19148–19153. doi: 10.1073/pnas.0807719105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engel V, Schinke R, Staemmler V. Photodissociation dynamics of H2O and D2O in the 1st absorption-band—a complete ab initio treatment. J. Chem. Phys. 1988;88:129–148. doi: 10.1063/1.454645. [DOI] [Google Scholar]
- 18.Brouard M, Langford SR, Manolopoulos DE. New trends in the state-to-state photodissociation dynamics of H2O(A) J. Chem. Phys. 1994;101:7458–7467. doi: 10.1063/1.468268. [DOI] [Google Scholar]
- 19.Yang XF, Hwang DW, Lin JJ, Yang X. Dissociation dynamics of the water molecule on the A1B1 electronic surface. J. Chem. Phys. 2000;113:10597–10604. doi: 10.1063/1.1285899. [DOI] [Google Scholar]
- 20.van Harrevelt R, van Hemert MC. Photodissociation of water. I. electronic structure calculations for the excited states. J. Chem. Phys. 2000;112:5777–5786. doi: 10.1063/1.481153. [DOI] [Google Scholar]
- 21.Dixon RN, et al. Chemical “double slits”: dynamical interference of photodissociation pathways in water. Science. 1999;285:1249–1253. doi: 10.1126/science.285.5431.1249. [DOI] [PubMed] [Google Scholar]
- 22.Fillion JH, et al. Photodissociation of H2O and D2O in B, C, and D states (134-119 nm). Comparison between experiment and ab initio calculations. J. Phys. Chem. A. 2001;105:11414–11424. doi: 10.1021/jp013032x. [DOI] [Google Scholar]
- 23.Mordaunt DH, Ashfold MNR, Dixon RN. Dissociation dynamics of H2O (D2O) following photoexcitation at the lyman-alpha wavelength (121.6 nm) J. Chem. Phys. 1994;100:7360–7375. doi: 10.1063/1.466880. [DOI] [Google Scholar]
- 24.Harich SA, et al. Photodissociation of H2O at 121.6 nm: a state-to-state dynamical picture. J. Chem. Phys. 2000;113:10073–10090. doi: 10.1063/1.1322059. [DOI] [Google Scholar]
- 25.Slanger TG, Black G. Photodissociative channels at 1216 Å for H2O, NH3, and CH4. J. Chem. Phys. 1982;77:2432–2437. doi: 10.1063/1.444111. [DOI] [Google Scholar]
- 26.Wang HL, et al. Photodissociation dynamics of H2O at 111.5 nm by a vacuum ultraviolet free electron laser. J. Chem. Phys. 2018;148:124301. doi: 10.1063/1.5022108. [DOI] [PubMed] [Google Scholar]
- 27.Fillion JH, et al. High resolution photoabsorption and photofragment fluorescence spectroscopy of water between 10.9 and 12 eV. J. Chem. Phys. 2004;120:6531–6541. doi: 10.1063/1.1652566. [DOI] [PubMed] [Google Scholar]
- 28.Chang Y, et al. Water photolysis and its contributions to the hydroxyl dayglow emissions in the atmospheres of Earth and Mars. J. Phys. Chem. Lett. 2020;11:9086–9092. doi: 10.1021/acs.jpclett.0c02803. [DOI] [PubMed] [Google Scholar]
- 29.Boyarkin OV, et al. Accurate bond dissociation energy of water determined by triple-resonance vibrational spectroscopy and ab initio calculations. Chem. Phys. Lett. 2013;568:14–20. doi: 10.1016/j.cplett.2013.03.007. [DOI] [Google Scholar]
- 30.Heays AN, Bosman AD, van Dishoeck EF. Photodissociation and photoionization of atoms and molecules of astrophysical interest. Astron. Astrophys. 2017;602:A105. doi: 10.1051/0004-6361/201628742. [DOI] [Google Scholar]
- 31.Zahnle KJ, Walker JCG. The evolution of solar ultraviolet luminosity. Rev. Geophys. Space Phys. 1982;20:280–292. doi: 10.1029/RG020i002p00280. [DOI] [Google Scholar]
- 32.Lee LC, Suto M. Quantitative photoabsorption and fluorescence study of H2O and D2O at 50-190 nm. Chem. Phys. 1986;110:161–169. doi: 10.1016/0301-0104(86)85154-0. [DOI] [Google Scholar]
- 33.Hassig M, et al. Time variability and heterogeneity in the coma of 67P/Churyumov-Gerasimenko. Science. 2015;347:aaa0276. doi: 10.1126/science.aaa0276. [DOI] [PubMed] [Google Scholar]
- 34.Sleep NH. The Hadean-Archaean environment. Cold Spring Harb. Perspec. Biol. 2010;2:a002527. doi: 10.1101/cshperspect.a002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tachiev GI, Fischer CF. Breit-Pauli energy levels and transition rates for nitrogen-like and oxygen-like sequences. Astron. Astrophys. 2002;385:716–723. doi: 10.1051/0004-6361:20011816. [DOI] [Google Scholar]
- 36.Lin JJ, Shu J, Lee YT, Yang X. Multiple dynamical pathways in the O(1D) + CH4 reaction: a comprehensive crossed beam study. J. Chem. Phys. 2000;113:5287–5301. doi: 10.1063/1.1289462. [DOI] [Google Scholar]
- 37.Yung YL, DeMore WB. Photochemistry of Planetary Atmospheres. Oxford University Press; 1999. [Google Scholar]
- 38.Zahnle KJ, Gacesa M, Catling DC. Strange messenger: a new history of hydrogen on Earth, as told by Xenon. Geochim. Cosmochim. Ac. 2019;244:56–85. doi: 10.1016/j.gca.2018.09.017. [DOI] [Google Scholar]
- 39.Zeng L, Sasselov D. The effect of temperature evolution on the interior structure of H2O-rich planets. Astrophys. J. 2014;784:96. doi: 10.1088/0004-637X/784/2/96. [DOI] [Google Scholar]
- 40.Schnieder L, et al. Photodissociation dynamics of H2S at 121.6 nm and a determination of the potential energy function of SH(A2Σ+) J. Chem. Phys. 1990;92:7027–7037. doi: 10.1063/1.458243. [DOI] [Google Scholar]
- 41.van Dishoeck EF, Herbst E, Neufeld DA. Interstellar water chemistry: from laboratory to observations. Chem. Rev. 2013;113:9043–9085. doi: 10.1021/cr4003177. [DOI] [PubMed] [Google Scholar]
- 42.Fillion JH, et al. Ionization yield and absorption spectra reveal superexcited Rydberg state relaxation processes in H2O and D2O. J. Phys. B. Mol. Opt. Phys. 2003;36:2767–2776. doi: 10.1088/0953-4075/36/13/308. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All other data supporting this study are available from the authors upon request. Source data are provided with this paper.