Abstract
We investigated the effects of stent generation on 2-year clinical outcomes between prediabetes and diabetes patients after acute myocardial infarction (AMI). A total of 13,895 AMI patients were classified into normoglycemia (group A: 3673), prediabetes (group B: 5205), and diabetes (group C: 5017). Thereafter, all three groups were further divided into first-generation (1G)-drug-eluting stent (DES) and second-generation (2G)-DES groups. Patient-oriented composite outcomes (POCOs) defined as all-cause death, recurrent myocardial infarction (Re-MI), and any repeat revascularization were the primary outcome. Stent thrombosis (ST) was the secondary outcome. In both prediabetes and diabetes groups, the cumulative incidences of POCOs, any repeat revascularization, and ST were higher in the 1G-DES than that in the 2G-DES. In the diabetes group, all-cause death and cardiac death rates were higher in the 1G-DES than that in the 2G-DES. In both stent generations, the cumulative incidence of POCOs was similar between the prediabetes and diabetes groups. However, in the 2G-DES group, the cumulative incidences of Re-MI and all-cause death or MI were significantly higher in the diabetes group than that in the prediabetes group. To conclude, 2G-DES was more effective than 1G-DES in reducing the primary and secondary outcomes for both prediabetes and diabetes groups.
Subject terms: Cardiology, Endocrinology
Introduction
Diabetes mellitus (DM, diabetes) is regarded as a “coronary artery disease (CAD) risk equivalent”1, conferring an approximately twofold increased risk of acute myocardial infarction (AMI)2. Moreover, almost two thirds of those presenting with CAD have either diabetes or prediabetes2. Coronary vessels in patients with diabetes usually present extensive atherosclerosis with a larger number of significant stenosis, longer lesions, and more diffuse disease3,4. Therefore, despite advances in interventional skill, devices, and antiplatelet agents, outcomes of coronary revascularization in patients with diabetes have been poorer than those without5,6. Percutaneous coronary intervention (PCI) in patients with diabetes is associated with increased incidence of restenosis, repeat revascularization, stent thrombosis (ST), and all-cause mortality than those without3,4. Drug-eluting stents (DES) reduce the risk of restenosis as compared with bare-metal stents (BMS). However, ST remains a major concern after the implantation of first-generation (1G)-DES in patients with diabetes7. Relative superiority between the 1G- and 2G-DESs in patients with diabetes remains controversial8–11. Although recent reports revealed that prediabetes is an intergrade between normoglycemia and diabetes12–14, PCI patients with prediabetes were prone to experience adverse clinical events. Individuals with prediabetes are important and common patients who visit interventional cardiologists. However, the main treatment strategies for hyperglycemia are focused on the patients with diabetes rather those with prediabetes15. Moreover, studies regarding the effects of the 1G-DES and 2G-DES on clinical outcomes between prediabetes and diabetes patients after AMI were limited. To better understand the characteristic of prediabetes, we compared the 2-year clinical outcomes of the 1G-DES and 2G-DES under two different glycemic states (prediabetes and diabetes).
Results
Baseline characteristics
Table 1 summarizes the baseline clinical, laboratory, and procedural characteristics of the study population. The study population consisted of patients who had a relatively well-preserved left ventricular ejection fraction (LVEF; mean: 52.1 ± 11.4%). The mean value of high-sensitivity C-reactive protein (hs-CRP) and number of patients who received clopidogrel and cilostazole as the discharge medications were significantly higher in 1G-DES group than in 2G-DES group in all three different glycemic groups. In contrast, the number of patients requiring cardiopulmonary resuscitation (CPR) on admission; number of patients who received PCI within 24 h; number of patients who received aspirin, ticagrelor, prasugrel, beta-blockers, and lipid lowering agents as the discharge medications; American College of Cardiology/American Heart Association (ACC/AHA) type C lesion; and mean length of deployed stent were significantly higher in 2G-DES group than in 1G-DES group in all three different glycemic groups. However, the mean value of age, LVEF, body mass index, systolic blood pressure; number of ST-segment-elevation myocardial infarction (STEMI) and dyslipidemia; number of patient with previous history of PCI, coronary artery bypass graft, cerebrovascular accident, and heart failure; number of current smoker and treated vessel; mean value of serum creatinine and diameter of deployed stent; and the use of intravascular ultrasound (IVUS) were similar between the 1G-DES and 2G-DES groups in all three different glycemic groups.
Table 1.
Baseline clinical, laboratory, and procedural characteristics.
| Variables | Group A normoglycemia (n = 3673) | Group B prediabetes (n = 5205) | Group C diabetes (n = 5017) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A1 1G-DES (n = 482) |
Group A2 2G-DES (n = 3191) |
p value | Group B1 1G-DES (n = 767) |
Group B2 2G-DES (n = 4438) |
p value | Group C1 1G-DES (n = 779) |
Group C2 2G-DES (n = 4238) |
p value | |
| Male, n (%) | 367 (76.1) | 2558 (80.2) | 0.041 | 555 (72.4) | 3276 (73.8) | 0.398 | 539 (69.2) | 3013 (71.7) | 0.283 |
| Age, years | 61.6 ± 13.4 | 61.4 ± 13.0 | 0.836 | 64.0 ± 12.0 | 64.3 ± 12.4 | 0.488 | 63.4 ± 11.6 | 63.3 ± 11.6 | 0.823 |
| LVEF, % | 53.4 ± 11.9 | 52.9 ± 10.7 | 0.306 | 52.6 ± 12.4 | 52.3 ± 11.2 | 0.578 | 51.3 ± 12.1 | 51.2 ± 11.6 | 0.809 |
| BMI, kg/m2 | 23.9 ± 2.8 | 23.8 ± 3.1 | 0.556 | 24.1 ± 3.1 | 24.1 ± 3.3 | 0.892 | 24.5 ± 3.1 | 24.5 ± 3.2 | 0.699 |
| SBP, mmHg | 129.9 ± 26.9 | 131.2 ± 27.8 | 0.354 | 130.4 ± 27.9 | 129.5 ± 27.7 | 0.421 | 130.5 ± 25.9 | 131.6 ± 28.1 | 0.319 |
| DBP, mmHg | 80.3 ± 16.7 | 80.6 ± 16.7 | 0.791 | 80.2 ± 16.1 | 78.7 ± 16.3 | 0.017 | 78.9 ± 15.5 | 79.1 ± 16.4 | 0.825 |
| Cardiogenic shock, n (%) | 16 (3.3) | 126 (3.9) | 0.612 | 41 (5.3) | 203 (4.6) | 0.351 | 18 (2.3) | 190 (4.5) | 0.004 |
| CPR on admission, n (%) | 6 (1.2) | 154 (4.8) | < 0.001 | 19 (2.5) | 217 (4.9) | 0.002 | 10 (1.3) | 167 (3.9) | < 0.001 |
| STEMI, n (%) | 283 (58.7) | 1894 (59.4) | 0.790 | 437 (57.0) | 2576 (58.0) | 0.580 | 438 (56.2) | 2276 (53.7) | 0.194 |
| Primary PCI, n (%) | 264 (93.3) | 1825 (96.4) | 0.014 | 407 (93.1) | 2477 (96.2) | 0.004 | 415/ (94.7) | 2177/ (95.7) | 0.404 |
| NSTEMI, n (%) | 199 (41.3) | 1298 (40.7) | 0.790 | 330 (43.0) | 1862 (42.0) | 0.580 | 341 (43.8) | 1962 (46.3) | 0.194 |
| PCI within 24 h | 144 (72.4) | 1146 (88.3) | < 0.001 | 255 (77.3) | 1592/ (85.5) | < 0.001 | 247/ (72.4) | 1654/ (84.3) | < 0.001 |
| Hypertension, n (%) | 228 (47.3) | 1333 (41.8) | 0.022 | 393 (51.2) | 2188 (49.3) | 0.322 | 432 (55.5) | 2427 (57.3) | 0.348 |
| Dyslipidemia, n (%) | 36 (7.5) | 271 (8.5) | 0.481 | 17 (10.0) | 524 (11.8) | 0.178 | 117 (15.0) | 623 (14.7) | 0.826 |
| Previous MI, n (%) | 14 (2.9) | 98 (3.1) | 0.843 | 20 (2.6) | 137 (3.1) | 0.567 | 21 (2.7) | 196 (4.6) | 0.013 |
| Previous PCI, n (%) | 17 (3.5) | 135 (4.2) | 0.540 | 42 (5.5) | 239 (5.4) | 0.931 | 49 (6.3) | 314 (7.4) | 0.292 |
| Previous CABG, n (%) | 0 (0.0) | 9 (0.3) | 0.616 | 3 (0.4) | 13 (0.3) | 0.720 | 4 (0.5) | 30 (0.7) | 0.811 |
| Previous CVA, n (%) | 18 (3.7) | 153 (4.8) | 0.354 | 47 (6.1) | 268 (6.0) | 0.935 | 49 (6.3) | 311 (7.3) | 0.326 |
| Previous HF, n (%) | 2 (0.4) | 18 (0.6) | 0.678 | 8 (1.0) | 50 (1.1) | 0.839 | 18 (2.3) | 62 (1.5) | 0.087 |
| Current smokers, n (%) | 233 (48.3) | 1441 (45.2) | 0.191 | 309 (40.3) | 1943 (43.8) | 0.076 | 317 (40.7) | 1731 (40.8) | 0.968 |
| Peak CK-MB, mg/dL | 128.9 ± 149.4 | 139.0 ± 203.7 | 0.189 | 126.0 ± 204.3 | 138.3 ± 197.0 | 0.119 | 102.6 ± 152.9 | 106.3 ± 143.3 | 0.534 |
| Peak troponin-I, ng/mL | 39.5 ± 54.9 | 48.3 ± 75.2 | 0.002 | 40.7 ± 80.7 | 46.7 ± 107.5 | 0.072 | 37.7 ± 63.4 | 48.6 ± 94.8 | 0.001 |
| NT-ProBNP, pg/mL | 2307.6 ± 4254.5 | 1879.2 ± 3457.2 | 0.035 | 2194.5 ± 4071.7 | 2070.7 ± 3721.1 | 0.431 | 2549.2 ± 4658.0 | 2414.0 ± 5379.2 | 0.468 |
| Hs-CRP, mg/dL | 15.4 ± 83.9 | 7.9 ± 28.5 | < 0.001 | 12.8 ± 35.4 | 9.8 ± 46.2 | 0.038 | 16.3 ± 62.6 | 11.5 ± 43.7 | 0.039 |
| Serum creatinine, mg/L | 1.08 ± 0.96 | 1.03 ± 0.99 | 0.268 | 1.12 ± 1.02 | 1.11 ± 1.50 | 0.926 | 1.26 ± 2.50 | 1.18 ± 1.66 | 0.352 |
| Total cholesterol, mg/dL | 183.4 ± 40.2 | 180.6 ± 40.8 | 0.161 | 187.7 ± 43.2 | 186.0 ± 44.1 | 0.332 | 186.3 ± 47.6 | 181.1 ± 48.4 | 0.005 |
| Triglyceride, mg/L | 116.4 ± 75.8 | 118.3 ± 88.0 | 0.625 | 118.4 ± 74.4 | 131.5 ± 101.1 | < 0.001 | 153.4 ± 124.4 | 157.1 ± 136.7 | 0.459 |
| HDL cholesterol, mg/L | 44.7 ± 12.7 | 44.4 ± 15.2 | 0.616 | 44.6 ± 12.7 | 43.4 ± 15.1 | 0.022 | 43.3 ± 22.9 | 41.8 ± 14.2 | 0.090 |
| LDL cholesterol, mg/L | 117.2 ± 34.8 | 114.6 ± 36.0 | 0.120 | 120.4 ± 37.4 | 118.8 ± 45.0 | 0.304 | 116.8 ± 42.4 | 112.0 ± 38.5 | 0.003 |
| Diabetes management | |||||||||
| Diet, n (%) | 53 (6.8) | 306 (7.2) | 0.762 | ||||||
| Oral agent, n (%) | 469 (60.2) | 2488 (58.7) | 0.435 | ||||||
| Insulin, n (%) | 49 (6.3) | 252 (5.9) | 0.710 | ||||||
| Untreated, n (%) | 208 (26.7) | 1192 (28.1) | 0.413 | ||||||
| Discharge medications | |||||||||
| Aspirin, n (%) | 454 (94.2) | 3092 (96.9) | 0.002 | 724 (94.4) | 4276 (96.3) | < 0.001 | 726 (93.2) | 4072 (96.1) | < 0.001 |
| Clopidogrel, n (%) | 477 (99.0) | 2573 (80.6) | < 0.001 | 746 (97.3) | 3810 (85.8) | < 0.001 | 757 (97.2) | 3624 (85.5) | < 0.001 |
| Ticagrelor, n (%) | 1 (0.2) | 382 (12.0) | < 0.001 | 4 (0.5) | 382 (8.6) | < 0.001 | 8 (1.0) | 328 (7.7) | < 0.001 |
| Prasugrel, n (%) | 0 (0.0) | 198 (6.2) | < 0.001 | 4 (0.5) | 203 (4.6) | < 0.001 | 2 (0.3) | 204 (4.8) | < 0.001 |
| Cilostazole, n (%) | 137 (28.4) | 449 (14.1) | < 0.001 | 223 (29.1) | 848 (19.1) | < 0.001 | 231 (29.7) | 830 (19.6) | < 0.001 |
| Beta-blockers, n (%) | 380 (78.8) | 2651 (83.1) | 0.022 | 608 (79.3) | 3680 (82.9) | < 0.001 | 594 (76.3) | 3522 (83.1) | < 0.001 |
| ACEIs, n (%) | 308 (63.9) | 1843 (57.8) | 0.011 | 444 (57.9) | 2404 (54.2) | 0.012 | 438 (56.2) | 2176 (51.3) | 0.012 |
| ARBs, n (%) | 91 (18.9) | 765 (24.0) | 0.014 | 185 (24.1) | 1133 (25.5) | 0.004 | 189 (24.3) | 1240 (29.3) | 0.004 |
| CCBs, n (%) | 37 (7.7) | 181 (5.7) | 0.083 | 55 (7.2) | 245 (5.5) | 0.248 | 68 (8.7) | 319 (7.5) | 0.248 |
| Lipid lowering agents | 393 (81.5) | 2876 (90.1) | < 0.001 | 618 (80.6) | 3937 (88.7) | < 0.001 | 601 (77.2) | 3645 (86.0) | < 0.001 |
| IRA | |||||||||
| Left main, n (%) | 7 (1.5) | 54 (1.7) | 0.849 | 19 (2.5) | 77 (1.7) | 0.670 | 16 (2.1) | 79 (1.9) | 0.670 |
| LAD, n (%) | 257 (53.3) | 1603 (50.2) | 0.207 | 373 (48.6) | 2179 (49.1) | 0.275 | 377 (48.4) | 1961 (46.3) | 0.275 |
| LCx, n (%) | 81 (16.8) | 523 (16.4) | 0.819 | 136 (17.7) | 728 (16.4) | 0.174 | 147 (18.9) | 715 (16.9) | 0.174 |
| RCA, n (%) | 136 (28.2) | 1010 (31.7) | 0.140 | 239 (31.2) | 1454 (32.8) | 0.024 | 238 (30.6) | 1474 (34.8) | 0.024 |
| Treated vessel | |||||||||
| Left main, n (%) | 17 (3.5) | 84 (2.6) | 0.293 | 27 (3.5) | 129 (2.9) | 0.237 | 30 (3.9) | 129 (3.0) | 0.237 |
| LAD, n (%) | 291 (60.4) | 1883 (59.0) | 0.570 | 460 (60.0) | 2591 (58.4) | 0.994 | 458 (58.8) | 2491 (58.8) | 0.994 |
| LCx, n (%) | 122 (25.3) | 786 (24.6) | 0.747 | 217 (28.3) | 1144 (25.8) | 0.985 | 219 (28.1) | 1190 (28.1) | 0.985 |
| RCA, n (%) | 169 (35.1) | 1182 (37.0) | 0.418 | 292 (38.1) | 1744 (39.3) | 0.252 | 313 (40.2) | 1799 (42.4) | 0.252 |
| ACC/AHA lesion type | |||||||||
| Type B1, n (%) | 82 (17.0) | 424 (13.3) | 0.027 | 120 (15.6) | 597 (13.5) | 0.009 | 124 (15.9) | 530 (12.5) | 0.009 |
| Type B2, n (%) | 153 (31.7) | 1064 (33.3) | 0.500 | 248 (32.3) | 1425 (32.1) | 0.180 | 231 (29.7) | 1362 (32.1) | 0.180 |
| Type C, n (%) | 168 (34.9) | 1424 (44.6) | < 0.001 | 284 (37.0) | 1957 (44.1) | < 0.001 | 277 (35.6) | 1942 (45.8) | < 0.001 |
| Extent of CAD | |||||||||
| 1-vessel, n (%) | 229 (47.5) | 1744 (54.7) | 0.003 | 327 (42.6) | 2234 (50.3) | < 0.001 | 280 (35.9) | 1807 (42.6) | < 0.001 |
| 2-vessel, n (%) | 166 (34.4) | 962 (30.1) | 0.057 | 251 (32.7) | 1398 (31.5) | 0.847 | 265 (34.0) | 1426 (33.6) | 0.847 |
| ≥ 3-vessel, n (%) | 87 (18.0) | 485 (15.2) | 0.121 | 189 (24.6) | 806 (18.2) | < 0.001 | 234 (30.0) | 1005 (23.7) | < 0.001 |
| DESs | |||||||||
| SES, n (%) | 225 (46.7) | 330 (43.0) | 352 (45.2) | ||||||
| PES, n (%) | 257 (53.3) | 437 (57.0) | 427 (54.8) | ||||||
| ZES, n (%) | 1015 (31.8) | 1529 (34.5) | 1478 (34.9) | ||||||
| EES, n (%) | 1625 (50.9) | 2278 (51.3) | 2194 (51.8) | ||||||
| BES, n (%) | 525 (16.4) | 600 (13.5) | 536 (12.6) | ||||||
| Others, n (%) | 26 (0.8) | 31 (0.7) | 30 (0.7) | ||||||
| IVUS | 119 (24.7) | 682 (21.4) | 0.110 | 185 (24.1) | 1038 (23.4) | 0.533 | 156 (20.0) | 894 (21.1) | 0.533 |
| OCT | 0 (0.0) | 24 (0.8) | 0.064 | 1 (0.1) | 34 (0.8) | 0.010 | 0 (0.0) | 31 (0.7) | 0.010 |
| FFR | 1 (0.2) | 30 (0.9) | 0.114 | 1 (0.1) | 60 (1.4) | 0.026 | 2 (0.3) | 46 (1.1) | 0.026 |
| Stent diameter, mm | 3.16 ± 0.42 | 3.16 ± 0.43 | 0.907 | 3.14 ± 0.42 | 3.14 ± 0.42 | 0.098 | 3.07 ± 0.39 | 3.10 ± 0.42 | 0.098 |
| Stent length, mm | 25.9 ± 7.8 | 27.1 ± 11.4 | 0.004 | 26.0 ± 7.2 | 26.9 ± 11.5 | 0.003 | 26.5 ± 7.9 | 27.5 ± 11.8 | 0.003 |
| Number of stent | 1.50 ± 0.84 | 1.42 ± 0.75 | 0.053 | 1.55 ± 0.84 | 1.48 ± 0.80 | 0.469 | 1.59 ± 0.90 | 1.56 ± 0.84 | 0.469 |
Values are means ± SD or numbers and percentages. The p values for continuous data were obtained from the analysis of variance. The p values for categorical data were obtained from the chi-square or Fisher’s exact test.
PCI percutaneous coronary intervention, BMS bare-metal stents, 1G first-generation, 2G second-generation, DES drug-eluting stents, LVEF left ventricular ejection fraction, BMI body mass index, CPR cardiopulmonary resuscitation, MI myocardial infarction, CABG coronary artery bypass graft, CVA cerebrovascular accidents, HF heart failure, CK-MB creatine kinase myocardial band, NT-ProBNP N-terminal pro-brain natriuretic peptide, Hs-CRP high-sensitivity-C-reactive protein, HDL high-density lipoprotein, LDL low-density lipoprotein, ACEIs angiotensin converting enzyme inhibitors, ARBs angiotensin receptor blockers, CCBs calcium channel blockers, IRA infarct-related artery, ACC/AHA American College of Cardiology/American Heart Association, CAD coronary artery disease, SES sirolimus-eluting stent, PES paclitaxel-eluting stent, ZES zotarolimus-eluting stent, EES everolimus-eluting stent, BES biolimus-eluting stent, IVUS intravascular ultrasound, OCT optical coherence tomography, FFR fractional flow reserve.
Clinical outcomes
Cumulative incidences of major clinical outcomes during the 2-year follow-up period are summarized in Tables 2, 3, and Fig. 1, and Supplementary information.
Table 2.
Clinical outcomes between 1G-DES and 2G-DES at 2 years.
| Outcomes | Normoglycemia | ||||||
|---|---|---|---|---|---|---|---|
| Group A1 1G-DES (n = 482) |
Group A2 2G-DES (n = 3191) |
Log-rank | Unadjusted HR (95% CI) |
p value | Adjusteda HR (95% CI) |
p value | |
| POCOs | 41 (8.6) | 193 (6.7) | 0.098 | 1.328 (0.948–1.861) | 0.099 | 1.216 (0.854–1.730) | 0.278 |
| All-cause death | 22 (4.6) | 80 (2.7) | 0.025 | 1.705 (1.063–2.734) | 0.027 | 1.504 (0.914–2.474) | 0.109 |
| Cardiac death | 17 (3.6) | 59 (1.9) | 0.027 | 1.825 (1.064–3.131) | 0.029 | 1.487 (0.838–2.639) | 0.176 |
| Re-MI | 8 (1.7) | 42 (1.5) | 0.675 | 1.175 (0.551–2.505) | 0.676 | 1.180 (0.532–2.619) | 0.665 |
| All-cause death or MI | 27 (5.6) | 116 (4.0) | 0.088 | 1.438 (0.945–2.187) | 0.090 | 1.307 (0.843–2.026) | 0.232 |
| Any repeat revascularization | 17 (3.6) | 85 (3.1) | 0.444 | 1.225 (0.728–2.063) | 0.444 | 1.118 (0.645–1.938) | 0.692 |
| Stent thrombosis (probable or definite) | 7 (1.5) | 15 (0.5) | 0.009 | 3.015 (1.266–7.616) | 0.013 | 3.262 (1.226–8.678) | 0.018 |
| Outcomes | Prediabetes | ||||||
|---|---|---|---|---|---|---|---|
| Group B1 1G-DES (n = 767) |
Group B2 2G-DES (n = 4438) |
Log-rank | Unadjusted HR (95% CI) |
p value | Adjustedb HR (95% CI) | p value | |
| POCOs | 91 (12.0) | 371 (8.9) | 0.007 | 1.372 (1.091–1.726) | 0.007 | 1.369 (1.044–1.720) | 0.012 |
| All-cause death | 43 (5.6) | 185 (4.4) | 0.121 | 1.299 (0.932–1.810) | 0.122 | 1.350 (0.939–1.845) | 0.110 |
| Cardiac death | 34 (4.5) | 140 (3.3) | 0.098 | 1.370 (0.942–1.993) | 0.100 | 1.364 (0.916–1.963) | 0.132 |
| Re-MI | 19 (2.5) | 80 (2.0) | 0.305 | 1.299 (0.787–2.142) | 0.306 | 1.293 (0.780–2.137) | 0.316 |
| All-cause death or MI | 52 (6.8) | 258 (6.1) | 0.467 | 1.117 (0.829–1.505) | 0.468 | 1.114 (0.824–1.502) | 0.483 |
| Any repeat revascularization | 46 (6.3) | 144 (3.6) | 0.001 | 1.780 (1.277–2.481) | 0.001 | 1.795 (1.280–2.518) | 0.001 |
| Stent thrombosis (probable or definite) | 14 (1.8) | 29 (0.7) | 0.001 | 2.806 (1.483–5.311) | 0.002 | 2.637 (1.370–5.077) | 0.004 |
| Outcomes | Diabetes | ||||||
|---|---|---|---|---|---|---|---|
| Group C1 1G-DES (n = 779) |
Group C2 2G-DES (n = 4238) |
Log-rank | Unadjusted HR (95% CI) |
p value | Adjustedc HR (95% CI) |
p value | |
| POCOs | 107 (13.9) | 410 (10.3) | 0.003 | 1.373 (1.110–1.699) | 0.003 | 1.331 (1.070–1.657) | 0.010 |
| All-cause death | 50 (6.5) | 189 (4.7) | 0.037 | 1.390 (1.018–1.899) | 0.038 | 1.534 (1.115–2.112) | 0.009 |
| Cardiac death | 42 (5.4) | 144 (3.5) | 0.012 | 1.544 (1.095–2.178) | 0.013 | 1.700 (1.195–2.448) | 0.003 |
| Re-MI | 24 (3.2) | 105 (2.8) | 0.474 | 1.176 (0.754–1.832) | 0.475 | 1.318 (0.833–2.085) | 0.237 |
| All-cause death or MI | 64 (8.3) | 296 (7.4) | 0.377 | 1.129 (0.862–1.480) | 0.378 | 1.290 (0.977–1.703) | 0.073 |
| Any repeat revascularization | 51 (6.9) | 160 (4.3) | 0.001 | 1.661 (1.212–2.276) | 0.002 | 1.673 (1.211–2.313) | 0.002 |
| Stent thrombosis (probable or definite) | 16 (2.1) | 40 (0.9) | 0.007 | 2.189 (1.226–3.909) | 0.008 | 2.065 (1.100–3.876) | 0.024 |
POCOs patient-oriented composite outcomes defined as a composite of all-cause deaths, Re-MI or any repeat revascularization, Re-MI recurrent myocardial infarction, LVEF left ventricular ejection fraction, DBP diastolic blood pressure, CPR cardiopulmonary resuscitation, PCI percutaneous coronary intervention, hs-CRP high-sensitivity-C-reactive protein, HDL high-density lipoprotein, LDL low-density lipoprotein, ACEI angiotensin converting enzyme inhibitor, ARB angiotensin receptor blockers, IRA infarct-related artery, RCA right coronary artery, ACC/AHA American College of Cardiology/American Heart Association, IVUS intravascular ultrasound, OCT optical coherence tomography, FFR fractional flow reserve.
aAdjusted by male, age, CPR on admission, primary PCI, PCI within 24hours, hypertension, peak troponin-I, aspirin, clopidogrel, ticagrelor, prasugrel, cilostazole, beta-blocker, ACEI, ARB, lipid lowering agent, ACC/AHA type B1/C lesions, 1-vessel disease, stent length (p vales of these covariates were < 0.05 or having predictive values).
bAdjusted by male, age, DBP, cardiogenic shock, CPR on admission, primary PCI, PCI within 24 h, hs-CRP, triglyceride, HDL-cholesterol, aspirin, clopidogrel, ticagrelor, prasugrel, cilostazole, beta-blocker, lipid lowering agents, ACC/AHA type C lesions, 1-vessel disease, ≥ 3-vessel disease, FFR, stent length, number of stent (p vales of these covariates were < 0.05 or having predictive values).
cAdjusted by male, age, cardiogenic shock, CPR on admission, PCI within 24hours, previous MI, peak troponin-I, hs-CRP, total cholesterol, LDL-cholesterol, aspirin, clopidogrel, ticagrelor, prasugrel, cilostazole, beta-blocker, ACEI, ARB, lipid lowering agent, IRA (RCA), ACC/AHA type B1/C lesions, OCT, FFR, stent length (p vales of these covariates were < 0.05 or having predictive values).
Table 3.
Two-year clinical outcomes according to the different glycemic status.
| Outcomes | 1G-DES | ||||||
|---|---|---|---|---|---|---|---|
| Group A1 normoglycemia (n = 482) | Group B1 prediabetes (n = 767) | Log-rank | Unadjusted | Adjusteda | |||
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| POCOs | 41 (8.6) | 91 (12.0) | 0.072 | 1.400 (1.968–2.024) | 0.074 | 1.483 (0.985–2.232) | 0.059 |
| All-cause death | 22 (4.6) | 43 (5.6) | 0.422 | 1.234 (0.738–2.062) | 0.423 | 1.227 (0.675–2.033) | 0.502 |
| Cardiac death | 17 (3.6) | 34 (4.5) | 0.433 | 1.261 (0.705–2.257) | 0.435 | 1.455 (0.721–2.935) | 0.295 |
| Re-MI | 8 (1.7) | 19 (2.5) | 0.334 | 1.498 (0.656–3.422) | 0.337 | 1.748 (0.685–4.464) | 0.243 |
| All-cause death or MI | 27 (5.6) | 52 (6.8) | 0.412 | 1.215 (0.763–1.933) | 0.413 | 1.194 (0.707–1.919) | 0.507 |
| Any repeat revascularization | 17 (3.6) | 46 (6.3) | 0.053 | 1.719 (0.986–2.999) | 0.056 | 1.858 (1.027–3.359) | 0.040 |
| ST (probable or definite) | 7 (1.5) | 14 (1.8) | 0.642 | 1.259 (0.508–3.119) | 0.619 | 1.346 (0.511–3.547) | 0.548 |
| Outcomes | Group A1 Normoglycemia (n = 482) | Group C1 diabetes (n = 779) | Log-rank | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| POCOs | 41 (8.6) | 107 (13.9) | 0.007 | 1.630 (1.137–2.336) | 0.008 | 1.667 (1.105–2.515) | 0.015 |
| All-cause death | 22 (4.6) | 50 (6.5) | 0.168 | 1.420 (0.860–2.345) | 0.170 | 1.451 (0.797–2.639) | 0.164 |
| Cardiac death | 17 (3.6) | 42 (5.4) | 0.130 | 1.540 (0.877–2.705) | 0.133 | 1.652 (0.815–3.349) | 0.129 |
| Re-MI | 8 (1.7) | 24 (3.2) | 0.118 | 1.873 (0.841–4.169) | 0.124 | 2.500 (0.971–6.441) | 0.058 |
| All-cause death or MI | 27 (5.6) | 64 (8.3) | 0.084 | 1.483 (0.946–2.325) | 0.086 | 1.496 (0.885–2.531) | 0.072 |
| Any repeat revascularization | 17 (3.6) | 51 (6.9) | 0.022 | 1.878 (1.084–3.251) | 0.024 | 1.875 (1.029–3.215) | 0.038 |
| ST (probable or definite) | 7 (1.5) | 16 (2.1) | 0.642 | 1.240 (0.500–3.071) | 0.643 | 1.539 (0.580–4.084) | 0.386 |
| Outcomes | Group B1 Prediabetes (n = 767) |
Group C1 Diabetes (n = 779) |
Log-rank | Unadjusted | Adjusteda | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| POCOs | 91 (12.0) | 107 (13.9) | 0.285 | 1.165 (0.881–1.540) | 0.285 | 1.135 (0.836–1.535) | 0.417 |
| All-cause death | 43 (5.6) | 50 (6.5) | 0.500 | 1.151 (0.765–1.730) | 0.500 | 1.166 (0.731–1.860) | 0.488 |
| Cardiac death | 34 (4.5) | 42 (5.4) | 0.385 | 1.221 (0.777–1.919) | 0.386 | 1.137 (0.678–1.909) | 0.627 |
| Re-MI | 19 (2.5) | 24 (3.2) | 0.462 | 1.253 (0.686–2.287) | 0.463 | 1.148 (0.599–2.199) | 0.678 |
| All-cause death or MI | 52 (6.8) | 64 (8.3) | 0.283 | 1.221 (0.847–1.761) | 0.284 | 1.189 (0.788–1.757) | 0.410 |
| Any repeat revascularization | 46 (6.3) | 51 (6.9) | 0.663 | 1.093 (0.733–1.627) | 0.663 | 1.035 (0.681–1.574) | 0.872 |
| ST (probable or definite) | 14 (1.8) | 16 (2.1) | 0.969 | 1.015 (0.484–2.128) | 0.969 | 1.175 (0.551–2.507) | 0.677 |
| Outcomes | 2G-DES | ||||||
|---|---|---|---|---|---|---|---|
| Group A2 Normoglycemia (n = 3191) |
Group B2 Prediabetes (n = 4438) |
Log-rank | Unadjusted | Adjustedb | |||
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| POCOs | 193 (6.7) | 371 (8.9) | < 0.001 | 1.388 (1.167–1.650) | < 0.001 | 1.294 (1.078–1.553) | 0.006 |
| All-cause death | 80 (2.7) | 185 (4.4) | < 0.001 | 1.642 (1.263–2.134) | < 0.001 | 1.353 (1.021–1.793) | 0.035 |
| Cardiac death | 59 (1.9) | 140 (3.3) | 0.001 | 1.693 (1.249–2.295) | 0.001 | 1.392 (1.004–1.930) | 0.047 |
| Re-MI | 42 (1.5) | 80 (2.0) | 0.121 | 1.342 (0.924–1.950) | 0.122 | 1.288 (0.876–1.894) | 0.198 |
| All-cause death or MI | 116 (4.0) | 258 (6.1) | < 0.001 | 1.578 (1.268–1.965) | < 0.001 | 1.425 (1.132–1.794) | 0.003 |
| Any repeat revascularization | 85 (3.1) | 144 (3.6) | 0.206 | 1.189 (0.909–1.554) | 0.206 | 1.223 (0.923–1.619) | 0.161 |
| ST (probable or definite) | 15 (0.5) | 29 (0.7) | 0.296 | 1.392 (0.746–2.597) | 0.298 | 1.520 (0.787–2.937) | 0.213 |
| Outcomes | Group A2 Normoglycemia (n = 3191) |
Group C2 Diabetes (n = 4238) |
Log-rank | Unadjusted | Adjustedb | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| POCOs | 193 (6.7) | 410 (10.3) | < 0.001 | 1.566 (1.320–1.859) | < 0.001 | 1.400 (1.165–1.683) | < 0.001 |
| All-cause death | 80 (2.7) | 189 (4.7) | < 0.001 | 1.748 (1.346–2.271) | < 0.001 | 1.430 (1.074–1.095) | 0.014 |
| Cardiac death | 59 (1.9) | 144 (3.5) | < 0.001 | 1.815 (1.341–2.457) | < 0.001 | 1.471 (1.055–2.052) | 0.023 |
| Re-MI | 42 (1.5) | 105 (2.8) | 0.001 | 1.829 (1.278–2.616) | 0.001 | 1.694 (1.161–2.472) | 0.006 |
| All-cause death or MI | 116 (4.0) | 296 (7.4) | < 0.001 | 1.885 (1.521–2.336) | < 0.001 | 1.684 (1.338–2.120) | < 0.001 |
| Any repeat revascularization | 85 (3.1) | 160 (4.3) | 0.018 | 1.370 (1.053–1.783) | 0.019 | 1.362 (1.031–1.769) | 0.030 |
| ST (probable or definite) | 15 (0.5) | 40 (0.9) | 0.018 | 2.012 (1.112–3.642) | 0.021 | 2.068 (1.125–3.869) | 0.014 |
| Outcomes | Group B2 Prediabetes (n = 4438) |
Group C2 Diabetes (n = 4238) |
Log-rank | Unadjusted | Adjustedb | ||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||||
| POCOs | 371 (8.9) | 410 (10.3) | 0.046 | 1.153 (1.002–1.327) | 0.046 | 1.116 (0.962–1.294) | 0.148 |
| All-cause death | 185 (4.4) | 189 (4.7) | 0.531 | 1.067 (0.871–1.307) | 0.531 | 1.109 (0.887–1.386) | 0.365 |
| Cardiac death | 140 (3.3) | 144 (3.5) | 0.543 | 1.075 (0.852–1.356) | 0.544 | 1.062 (0.822–1.334) | 0.644 |
| Re-MI | 80 (2.0) | 105 (2.8) | 0.036 | 1.368 (1.022–1.829) | 0.035 | 1.393 (1.135–2.043) | 0.032 |
| All-cause death or MI | 258 (6.1) | 296 (7.4) | 0.033 | 1.197 (1.013–1.415) | 0.034 | 1.224 (1.023–1.524) | 0.029 |
| Any repeat revascularization | 144 (3.6) | 160 (4.3) | 0.203 | 1.157 (0.924–1.449) | 0.204 | 1.088 (0.863–1.373) | 0.474 |
| ST (definite or probable) | 29 (0.7) | 40 (0.9) | 0.129 | 1.445 (0.896–2.331) | 0.131 | 1.546 (0.942–2.538) | 0.085 |
POCOs patient-oriented composite outcomes defined as a composite of all-cause deaths, Re-MI or any repeat revascularization, Re-MI recurrent myocardial infarction, LVEF left ventricular ejection fraction, BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, MI myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, HF heart failure, CVA cerebrovascular accidents, CK-MB creatine kinase myocardial band, NT-ProBNP N-terminal pro-brain natriuretic peptide, HDL high-density lipoprotein, LDL low-density lipoprotein, ACEIs angiotensin converting enzyme inhibitors, ARBs angiotensin receptor blockers, CCBs calcium channel blockers, IRA infarct-related artery, RCA right coronary artery, ACC/AHA American College of Cardiology/American Heart Association, FFR fractional flow reserve.
aAdjusted by male, age, LVEF, BMI, cardiogenic shock, hypertension, dyslipidemia, previous HF, current smoker, CK-MB, ACEIs, 1-vessel, ≥ 3-vessel disease, triglyceride, stent diameter (p vales of these covariates were < 0.005 or having predictive values).
bAdjusted by male, age, LVEF, BMI, DBP, STEMI, hypertension, dyslipidemia, previous MI, previous PCI, previous CVA, CK-MB, serum creatinine, total cholesterol, triglyceride, HDL-cholesterol, LDL-cholesterol, clopidogrel, ticagrelor, cilostazole, ACEIs, ARBs, CCB, lipid lowering agents, RCA (treated vessel), 1-vessel disease, ≥ 3-vessel disease, stent diameter, number of stent (p vales of these covariates were < 0.001 or having predictive values).
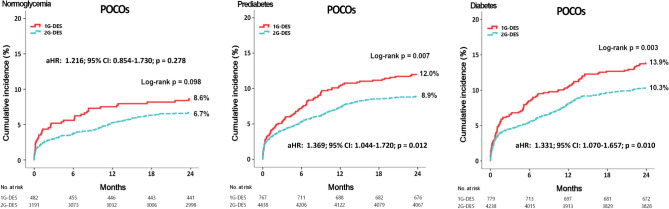
Figure 1.
Kaplan–Meier analysis of the incidence of POCOs.
Prediabetes group
After the adjustment, the cumulative incidences of POCOs (adjusted hazard ratio [aHR]: 1.369; 95% confidence interval [CI] 1.044–1.720; p = 0.012), any repeat revascularization (aHR: 1.795; 95% CI 1.280–2.518; p = 0.001), and ST (aHR: 2.637; 95% CI 1.370–5.077; p = 0.004) were significantly higher in the 1G-DES than that in the 2G-DES group.
Diabetes group
After the adjustment, the cumulative incidences of POCOs (aHR: 1.331; 95% CI 1.070–1.657; p = 0.010), all-cause death (aHR: 1.534; 95% CI 1.115–2.112; p = 0.009), CD (aHR: 1.700; 95% CI 1.195–2.448; p = 0.003), any repeat revascularization (aHR: 1.673; 95% CI 1.211–2.313; p = 0.002), and ST (aHR: 2.065; 95% CI 1.100–3.876; p = 0.024) were significantly higher in the 1G-DES group than that in the 2G-DES group.
Normoglycemia group
After the adjustment, the cumulative incidences of POCOs, all-cause death, CD, Re-MI, all-cause death or MI, and any repeat revascularization were similar between the 1G-DES and 2G-DES groups. However, the cumulative incidence of ST (aHR: 3.262; 95% CI 1.226–8.678; p = 0.018) was significantly higher in the 1G-DES than that in the 2G-DES group.
1G-DES group
Cumulative incidences of POCOs (aHR: 1.135; 95% CI 0.836–1.535; p = 0.417) and ST (aHR: 1.175; 95% CI 0.551–2.507; p = 0.677) were similar between prediabetes and diabetes groups. The cumulative incidence of any repeat revascularization was significantly higher in the prediabetes than that in the normoglycemia group (aHR: 1.858; 95% CI 1.027–3.359; p = 0.040). Cumulative incidences of POCOs (aHR: 1.667; 95% CI 1.105–2.515; p = 0.015) and any repeat revascularization (aHR: 1.875; 95% CI 1.029–3.215; p = 0.038) were significantly higher in the diabetes than that in the normoglycemia group.
2G-DES group
Cumulative incidences of POCOs (aHR: 1.116; 95% CI 0.962–1.294; p = 0.148) and ST (aHR: 1.546; 95% CI 0.942–2.538; p = 0.085) were similar between prediabetes and diabetes groups. However, cumulative incidences of Re-MI (aHR: 1.393; 95% CI 1.135–2.043; p = 0.032) and all-cause death or MI (aHR: 1.224; 95% CI 1.023–1.524; p = 0.029) in the diabetes group were significantly higher than that in the prediabetes group. Cumulative incidences of POCOs (aHR: 1.294; 95% CI 1.078–1.553; p = 0.006), all-cause death (aHR: 1.353; 95% CI 1.021–1.793; p = 0.035), CD (aHR: 1.392; 95% CI 1.004–1.930; p = 0.047), and all-cause death or MI (aHR: 1.425; 95% CI 1.132–1.794; p = 0.003) were significantly higher in the prediabetes than that in the normoglycemia group. Cumulative incidences of POCOs (aHR: 1.400; 95% CI 1.165–1.683; p < 0.001), all-cause death (aHR: 1.430; 95% CI 1.074–1.095; p = 0.014), CD (aHR: 1.471; 95% CI 1.055–2.052; p = 0.023), Re-MI (aHR: 1.694; 95% CI 1.161–2.472; p = 0.006), all-cause death or MI (aHR: 1.684; 95% CI 1.338–2.120; p < 0.001), any repeat revascularization (aHR: 1.362; 95% CI 1.031–1.769; p = 0.030), and ST (aHR: 2.068; 95% CI 1.125–3.869; p = 0.014) were significantly higher in the diabetes than that in the normoglycemia group.
Table 4 shows independent predictors for POCOs and ST at the 2-year follow-up. Old age (≥ 65 years), male sex, low LVEF (< 40%), cardiogenic shock, cardiopulmonary resuscitation on admission, and multivessel disease were significant independent predictors for POCOs. Low LVEF and < 3 mm diameter of the deployed stent were independent predictors for ST in this study.
Table 4.
Independent predictors for POCOs and stent thrombosis at 2 years.
| Variables | POCOs | Stent thrombosis | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |
| 1G-DES vs. 2G-DES | 1.382 (1.200–1.592) | < 0.001 | 1.415 (1.226–1.633) | < 0.001 | 2.451 (1.652–3.636) | < 0.001 | 2.668 (1.786–3.988) | < 0.001 |
| Age (≥ 65 years) | 1.631 (1.455–1.828) | < 0.001 | 1.356 (1.196–1.537) | < 0.001 | 1.131 (0.787–1.624) | 0.506 | 1.325 (0.890–1.974) | 0.166 |
| Male | 1.452 (1.289–1.637) | < 0.001 | 1.178 (1.034–1.342) | 0.014 | 1.151 (0.773–1.713) | 0.488 | 1.084 (0.701–1.676) | 0.717 |
| LVEF (< 40%) | 2.487 (2.186–2.831) | < 0.001 | 2.133 (1.870–2.432) | < 0.001 | 1.846 (1.188–2.870) | 0.006 | 1.775 (1.133–2.782) | 0.012 |
| Hypertension | 1.291 (1.153–1.447) | < 0.001 | 1.116 (0.991–1.257) | 0.071 | 1.033 (0.721–1.480) | 0.860 | 1.102 (0.756–1.605) | 0.613 |
| Dyslipidemia | 1.051 (0.885–1.247) | 0.572 | 1.052 (0.884–1.251) | 0.568 | 1.502 (0.929–2.429) | 0.097 | 1.482 (0.911–2.411) | 0.113 |
| Cardiogenic shock | 1.673 (1.335–2.096) | < 0.001 | 1.284 (1.020–1.617) | 0.033 | 1.400 (0.652–3.004) | 0.388 | 1.350 (0.620–2.938) | 0.450 |
| CPR on admission | 3.668 (3.079–4.370) | < 0.001 | 3.251 (2.714–3.894) | < 0.001 | 1.687 (0.823–3.457) | 0.153 | 1.479 (0.708–3.088) | 0.298 |
| Multivessel disease | 1.630 (1.449–1.833) | < 0.001 | 1.489 (1.321–1.678) | < 0.001 | 1.256 (0.873–1.808) | 0.219 | 1.128 (0.779–1.633) | 0.523 |
| ACC/AHA type B2/C lesion | 1.171 (1.023–1.341) | 0.022 | 1.078 (0.939–1.237) | 0.287 | 1.582 (0.979–2.558) | 0.061 | 1.531 (0.940–2.493) | 0.087 |
| Stent diameter < 3.0 mm | 1.195 (1.060–1.347) | 0.004 | 1.098 (0.972–1.241) | 0.132 | 2.537 (1.771–3.634) | < 0.001 | 2.518 (1.745–3.635) | < 0.001 |
| Stent length ≥ 28 mm | 1.202 (1.073–1.345) | 0.001 | 1.071 (0.954–1.202) | 0.247 | 1.335 (0.932–1.913) | 0.115 | 1.145 (0.793–1.653) | 0.470 |
1G first-generation, 2G second-generation, DES drug-eluting stent, POCOs patient-oriented composite outcomes, HR hazard ratio, LVEF left ventricular ejection fraction, CPR cardiopulmonary resuscitation, ACC/AHA American College of Cardiology/American Heart Association.
Discussion
The primary findings of this study are as follows: (1) in both prediabetes and diabetes groups, the cumulative incidences of POCOs, any repeat revascularization, and ST were higher in the 1G-DES than that in the 2G-DES; (2) in the diabetes group, the cumulative incidences of all-cause death and CD were higher in the 1G-DES than that in the 2G-DES; (3) in the normoglycemia group, the cumulative incidence of ST was higher in the 1G-DES than that in the 2G-DES; and (4) in two different stent generations, the cumulative incidence of POCOs was similar between the prediabetes and diabetes groups. However, in the 2G-DES group, the cumulative incidences of Re-MI and all-cause death or MI were higher in the diabetes group than that in the prediabetes group.
Hyperglycemia, elevated free fatty acid level, and increased amount of circulating glucosylated serum products can accelerate atherosclerosis and vascular injury in patients with diabetes by inducing endothelial dysfunction and vascular inflammation16. Although previous reports demonstrated that the higher rates of repeat revascularizations and mortality after PCI in patients with diabetes are caused by restenosis and disease progression4–6, comparative clinical outcomes between prediabetes and diabetes were not well illuminated especially, between 1G-DES and 2G-DES. Some recent reports showed that prediabetes is associated with poorer clinical outcomes including cardiovascular mortality and patients with prediabetes and diabetes have similar higher risk profiles compared with normoglycemia13,14,17.
Although DES improved outcomes of high-risk patients by reducing the rate of restenosis as compared with BMS18,19, ST remains a major concern after the DES implantation, especially in diabetes4. Relative superiority between the 1G-DES and 2G-DES in patients with AMI and diabetes remains controversial, and most previous studies were not performed during the prediabetes stage10,20,21. In our study, the cumulative incidence of POCOs was significantly higher in the 1G-DES than that in the 2G-DES in both prediabetes and diabetes groups. Moreover, in two different stent generations, the cumulative incidence of POCOs was similar between the prediabetes and diabetes groups (Table 3). In a substudy of the multicenter BIO-RESORT (BIOdegradable Polymer and DuRable Polymer Drug-eluting Stents in an All COmeRs PopulaTion) trial13, comparative clinical outcomes were similar between prediabetes and diabetes (11.1% vs. 10.5%). Von Birgelen et al.22 reported the results of the BIO-RESORT Silent Diabetes Study. In their study, the cumulative incidence of major adverse cardiac events was different between patients with prediabetes (5.5%) and normoglycemia (3.0%) (Log-rank, p = 0.07). As mentioned, despite the combination of new platforms, more biocompatible polymers were utilized in 2G-DES, the relative superiority between 1G- and 2G-DESs in patients with diabetes remains controversial8–11. In the SPIRIT V Diabetic Study10, everolimus-eluting stent (EES) was superior to paclitaxel-eluting stent (PES) for in-stent late loss at 9 months. The composite death, MI, and TVR rates were the same in the two groups at 1 year. Bavishi et al.9 reported that EES showed significantly lower incidence rates of MACEs by 18% and ST by 46% as compared with the 1G-DES. Moreover, the EES showed a trend toward reduced incidence rates of target lesion revascularization (TLR) and TVR (p = 0.05). In this study, based on the cumulative incidences of POCOs, any revascularization rate was significantly higher in the 1G-DES than that in the 2G-DES group in both prediabetes and diabetes group. Therefore, the major clinical outcomes of our study could reflect the meta-analysis results of Bavishi et al.’s study9.
The overall rate of ST was also higher in the 1G-DES than in the 2G-DES in all three different glycemic groups (prediabetes [1.8% vs. 0.7%, log-rang p = 0.001], diabetes [2.1% vs. 0.9%, log-rank p = 0.007], and normoglycemia [1.5% vs. 0.5%, log-rank p = 0.009]). With regard to prediabetes, follow-up data on the comparative long-term effects of 1G-DES and 2G-DES implantation were limited. According to Bavishi et al.’s report9, EES reduced the incidence of ST by 46% (RR: 0.54, 95% CI 0.35–0.82) as compared with the 1G-DES in patients with diabetes. The cumulative incidence of ST also higher in the 1G-DES than that in the 2G-DES in patients with normoglycemia. Our result is consistent with the result of Nakatsuma et al. study23. This low cumulative incidence of 2G-DES may be related with relatively thin stent struts (50–90 μm) and improved ability for deliverability while maintaining an adequate radial strength24 and more compatible and thromboresistant than those in the 1G-DES25. However, in our study, the occurrence of ST was high within 6 months after index PCI (Supplementary Fig. 1). Therefore, we cannot completely exclude the possibility that ST was associated with PCI procedure26. Even though IVUS-guided27 or functional flow reserve (FFR)-guided PCI28 could reduce MACE rate, the number of PCI base on these intracoronary image- or functional study-based PCI were less than 30% in our study. Unfortunately, currently under the Korea’s health insurance system, the reimbursement program for the use of IVUS, optical coherence of tomography, or fractional flow reserve during the PCI is very limited or absent29.
Interestingly, comparative clinical outcomes of the two different stent generations according to glycemic status showed some different results (Table 3). Different clinical outcomes among three different glycemic states (normoglycemia, prediabetes, and diabetes) were more prominent in the 2G-DES rather 1G-DES. According to advances in interventional skill, devices, and antiplatelet agents5,6, 2G-DES showed decreased incidences of all-cause death (aHR: 1.534; 95% CI 1.115–2.112; p = 0.009) and CD (aHR: 1.700; 95% CI 1.195–2.448; p = 0.003) compared with 1G-DES in diabetes group after adjustment (Table 2). Bavishi et al.9 showed that there was a trend towards reduction in all-cause mortality with zotarolimus compared to 1G-DES (6.3% vs. 7.2%, relative risk: 0.74; 95% CI 0.55–1.00; p = 0.05) in their meta-analysis. However, the cumulative incidences of all clinical outcomes were significantly higher in the diabetes than that in the normoglycemia group. These results may reflect hazardous effects of diabetes are sustained even in the era of 2G-DES.
In our study, in the 1G-DES, the primary and secondary end-points were similar between the prediabetes and diabetes groups. However, in the 2G-DES, the cumulative incidences of Re-MI (aHR: 1.393; 95% CI 1.135-2.043; p = 0.032) and all-cause death or MI (aHR: 1.224; 95% CI 1.023–1.524; p = 0.029) were significantly higher in the diabetes group than that in the prediabetes group. Although the precise mechanisms of the higher incidence of Re-MI in diabetes group are not fully known, one report30 suggested that the association between diabetes and Re-MI may be related with a direct effect of diabetes. According to recent reports14,31, the cumulative incidence of Re-MI of the diabetes group was significantly higher than that of the prediabetes group (aHR: 1.884; 95% CI 1.201–2.954; p = 0.006 or aHR: 1.660; 95% CI 1.000–2.755; p = 0.020).
More than 50 high-volume university or community hospitals in South Korea participated in this study. The limited reports on the impact of stent generation on long-term clinical outcomes in AMI patients with prediabetes or diabetes were the motivation for the current study. Thus, we believe that our study may provide significant information to interventional cardiologists who perform PCI in patients with AMI with prediabetes or diabetes.
This study has several limitations. First, because the study population was obtained from the Korea AMI registry data, some data might be under-reported and/or missed. Second, it is necessary for diagnosing diabetes to check an HbA1c level ≥ 6.5%, FPG ≥ 126 mg/dL (7.0 mmol/L), and/or RPG ≥ 200 mg/dL (11.1 mmol/L) by repeat testing. If first glycemic status was diabetes and second was prediabetes, or first glycemic status was prediabetes and second was normoglycemia, and this is particularly important in patients with AMI, because such patients reveal hyperglycemia in acute phase. However, in this study, the definitions of prediabetes and diabetes were not based on repeat testing. Moreover, considering the limitation of HbA1c, any other diagnostic tests for diabetes including oral glucose tolerance test are needed for a finer classification. However, detailed information on this variable was not included in the KAMIR. Hence, the results of this study can be altered based on other diagnostic tests and which directly influences the assignment of participants, and this factor may have served as an important bias in this study32. Third, the duration and types of antidiabetic treatment are major determinants after PCI in patents with prediabetes or diabetes. However, this study was conducted based on discharge medications, and owing to limitation of registry study, we did not precisely know the adherence or non-adherence of enrolled patients to antidiabetic drugs during the follow-up period. Therefore, this may act as an important bias in this study. Fourth, 2G-DES consisted of durable-polymer-coated DES and biodegradable-polymer-coated DES. The number of biodegradable-polymer DES (BES) was highest in the normoglycemia group (prediabetes: 600/4438 (13.5%); diabetes: 536/4238 (12.6%); normoglycemia: 525/3191 (16.5%); p < 0.001) (Table 1). Although this number was not significantly different between prediabetes and diabetes (p = 0.226), this division may be not reasonable and the composition of 2G-DES could be changed according to other types of utilized newer-generation DES. Therefore, other types of newer-generation DES could influence the outcome of our study. Fifth, although multivariate analysis was performed to strengthen our results, variables not included in the KAMIR may have affected the study outcomes. Sixth, the 2-year follow-up period in this study was relatively short in order to determine the long-term major clinical outcomes; therefore, data from studies with longer follow-up periods are required. Seventh, this study retrospectively enrolled the patients who underwent PCI from 2005–2015. The development of stent platform, potent antiplatelet drugs, and use of intracoronary imaging and improvement of procedural skills, all these factors substantially affect the clinical outcomes. Therefore, these factors could be also important bias of this study. Finally, although 2G-DES are considered the safest in the general population, this study confirms that in a select and growing population.
In conclusion, in this study, we observed that 2G-DES was more effective than 1G-DES in reducing POCOs, any repeat revascularization and ST in both prediabetes and diabetes group. Moreover, in two different stent generations, and the cumulative incidence of POCOs was similar between the prediabetes and diabetes groups. However, further studies regarding the most advanced DES technology joined with the most advanced anti-thrombotic regimen are needed to confirm these results.
Methods
Study population
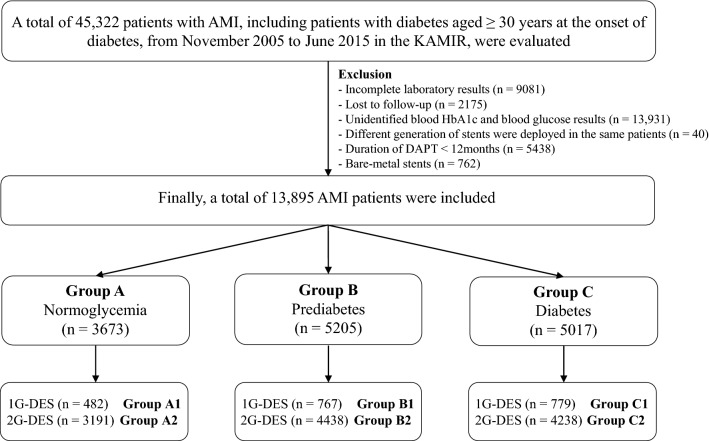
A total of 45,322 patients with AMI who underwent successful stent implantation, including patients with DM aged ≥ 30 years at the onset of diabetes, from November 2005 to June 2015 in the KAMIR, were evaluated. Details of the registry can be found at the KAMIR website (http://www.kamir.or.kr) 33. Among them, patients with incomplete laboratory results (n = 9081, 20.0%), those who were lost to follow-up (n = 2175, 4.8%), those with unidentified blood hemoglobin (Hb) A1c and blood glucose level results (n = 13,931, 30.7%), those with different generations of stents were deployed in the same patients (n = 40, 0.1%), those who received dual antiplatelet therapy (DAPT) less than 12 months (n = 5438, 12.0%), and those who received BMS (n = 762, 1.7%). Finally, a total of 13,895 patients with AMI who underwent successful implantation were considered for inclusion. Patients were classified into normoglycemia (group A: 3673; 26.4%), prediabetes (group B: 5205; 37.5%), and diabetes (group C: 5017; 36.1%) (Table 1). Subsequently, all three groups were further divided into 1G-DES (group A1, group B1, and group C1) and 2G-DES groups (group A2, group B2, and group C2) (Fig. 2). The study protocol was approved by the institutional review board of each participating center and the Chonnam National University Hospital Institutional Review Board ethics committee approved (approval number: CNUH-2011-172) the study protocol. The study has been performed in accordance with the ethical standards laid down in the 1975 Declaration of Helsinki. All persons gave their written informed consent prior to their inclusion in the study. All 13,895 patients completed the 2-year clinical follow-up by face-to-face interviews, phone calls, or medical chart review. All clinical events were evaluated by an independent event adjudicating committee. The event adjudication processes have been described in a previous publication of KAMIR investigators34.
Figure 2.
Study flow chart. AMI, acute myocardial infarction; KAMIR, Korea AMI Registry; HbA1c, hemoglobin A1c; 1G, first-generation; 2G, second-generation; DES, drug-eluting stent; DAPT, dual antiplatelet therapy.
Percutaneous coronary intervention and medical treatment
Before PCI, all patients were administered loading doses of aspirin 200–300 mg and clopidogrel 300–600 mg; alternatively, ticagrelor 180 mg or prasugrel 60 mg was administered. PCI was performed via the femoral or radial approach after an intravenous bolus dose of heparin (50–100 U/kg) to achieve an activated clotting time of > 250 s. DAPT (a combination of aspirin 100 mg/day with clopidogrel 75 mg/day or ticagrelor 90 mg twice daily or prasugrel 5–10 mg/day) was recommended for > 12 months for patients who underwent PCI. Triple antiplatelet therapy (TAPT: cilostazol 100 mg twice daily in addition to DAPT) was left to the discretion of the individual operators. Diagnostic coronary angiography and PCI were performed using standard guideline35.
Study definitions and clinical outcomes
Glycemic status was determined based on medical history and glycated hemoglobin (HbA1c), fasting plasma glucose (FPG), and random plasma glucose (RPG) levels at the index hospitalization. According to the American Diabetes Association clinical practice recommendation32, prediabetes was defined as an HbA1c of 5.7–6.4% and an FPG of 100–125 mg/dL (5.6–6.9 mmol/L). Diabetes was categorized as either known diabetes defined as ongoing medical treatment for diabetes (insulin or antidiabetics), or newly diagnosed diabetes, defined as an HbA1c level ≥ 6.5%, FPG ≥ 126 mg/dL (7.0 mmol/L), and/or RPG ≥ 200 mg/dL (11.1 mmol/L). If the admission electrocardiogram of patients who complained of chest pain showed ST-segment elevations in at least two contiguous leads of ≥ 2 mm (0.2 mV) in men, or ≥ 1.5 mm (0.15 mV) in women in leads V2–V3 and/or ≥ 1 mm (0.1 mV) in other contiguous chest leads or limb leads or new-onset left bundle branch block, the patients were considered to have STEMI36, whereas patients who did not show persistent ST-segment elevation with increased cardiac biomarkers and with appropriate clinical context were considered to have non-STEMI (NSTEMI)37. In cases of NSTEMI, an early invasive treatment strategy was defined as PCI within 24 h after admission37. A successful PCI was defined as a residual stenosis of < 30% and more than grade 3 flow in Thrombolysis In Myocardial Infarction flow for the infarct-related artery (IRA) after the procedure. The primary outcome of this study was the occurrence of POCOs, defined as all-cause death, Re-MI, or any coronary repeat revascularization38. The secondary outcome was definite or probable ST during the 2-year follow-up period. All-cause death was classified as CD or non-CD. Any repeat revascularization comprised target lesion revascularization, target vessel revascularization, and non-TVR. Re-MI, TLR, TVR, and non-TVR definitions have already been published previously39,40. The cumulative incidence of ST was defined by the current consensus41.
Statistical analyses
For continuous variables, differences between the two groups were evaluated with the unpaired t-test. Additionally, differences among the three glycemic groups were evaluated using analysis of variance or the Jonckheere–Terpstra test, whereas a post-hoc analysis of the two groups was performed using the Hochberg test or Dunnett T3 test14; data were expressed as mean ± standard deviation. For categorical variables, intergroup differences were analyzed using chi-squared test or Fisher’s exact test, as appropriate. Data were expressed as numbers and percentages14. The Kaplan–Meier method was used to estimate various clinical outcomes, and the log-rank test was used to compare intergroup differences (Fig. 1 and Supplementary information). Variables with a p value of < 0.00114 or < 0.0542 in the univariate analysis and conventional risk factors of poor outcomes in the AMI population were considered potential confounding factors and were entered into the multivariate analysis. These included variables shown in Tables 2 and 3. For all analyses, two-sided values of p < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS software version 20 (IBM; Armonk, NY, USA)14.
Supplementary Information
Acknowledgements
This research was supported by a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention. Korea Acute Myocardial infarction Registry (KAMIR) investigators Myung Ho Jeong, MD, Youngkeun Ahn, MD, Sung Chul Chae, MD, Jong Hyun Kim, MD, Seung-Ho Hur, MD, Young Jo Kim, MD, In Whan Seong, MD, Donghoon Choi, MD, Jei Keon Chae, MD, Taek Jong Hong, MD, Jae Young Rhew, MD, Doo-Il Kim, MD, In-Ho Chae, MD, Junghan Yoon, MD, Bon-Kwon Koo, MD, Byung-Ok Kim, MD, Myoung Yong Lee, MD, Kee-Sik Kim, MD, Jin-Yong Hwang, MD, Myeong Chan Cho, MD, Seok Kyu Oh, MD, Nae-Hee Lee, MD, Kyoung Tae Jeong, MD, Seung-Jea Tahk, MD, Jang-Ho Bae, MD, Seung-Woon Rha, MD, Keum-Soo Park, MD, Chong Jin Kim, MD, Kyoo-Rok Han, MD, Tae Hoon Ahn, MD, Moo-Hyun Kim, MD, Ki Bae Seung, MD, Wook Sung Chung, MD, Ju-Young Yang, MD, Chong Yun Rhim, MD, Hyeon-Cheol Gwon, MD, Seong-Wook Park, MD, Young-Youp Koh, MD, Seung Jae Joo, MD, Soo-Joong Kim, MD, Dong Kyu Jin, MD, Jin Man Cho, MD, Sang-Wook Kim, MD, Jeong Kyung Kim, MD, Tae Ik Kim, MD, Deug Young Nah, MD, Si Hoon Park, MD, Sang Hyun Lee, MD, Seung Uk Lee, MD, Hang-Jae Chung, MD, Jang-Hyun Cho, MD, Seung Won Jin, MD, Myeong-Ki Hong, MD, Yangsoo Jang, MD, Jeong Gwan Cho, MD, Hyo-Soo Kim, MD and Seung-Jung Park, MD.
Author contributions
Y.H.K. and A.-Y.H. researched data and wrote the manuscript. Y.H.K., A.-Y.H., M.H.J., B.-K.K., J.-S.K., and M.-K.H. contributed to study design. M.H.J., S.-J.H., S.K., C.-M.A., J.-S.K., Y.-G.K., D.C., M.-K.H., and Y.J. contributed to the collection research data. M.H.J., B.-.KK., J.-S.K., Y.-G.K., D.C., M.-K.H., and Y.J. contributed to provide intellectual inputs for the discussion. Y.H.K., A.-Y.H., S.-J.H., S.K. contributed to data analysis and edited the manuscript. M.H.J., D.C., M.-K.H., and Y.J. contributed to provide supervisor role during the full processes of manuscript submitting and editing. All authors take full responsibility for this work.
Data availability
Data is contained with the article or supplementary information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yong Hoon Kim and Ae-Young Her.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88593-x.
References
- 1.Hajar R. Diabetes as "coronary artery disease risk equivalent": A historical perspective. Heart Views. 2017;18:34–37. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_37_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rydén L, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur. Heart J. 2013;34:3035–3087. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 3.Cutlip DE, et al. Beyond restenosis: Five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–1230. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 4.Iakovou I, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 5.Konigstein M, et al. Outcomes among diabetic patients undergoing percutaneous coronary intervention with contemporary drug-eluting stents: Analysis from the BIONICS randomized trial. JACC Cardiovasc. Interv. 2018;11:2467–2476. doi: 10.1016/j.jcin.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo X, et al. In-hospital, short-term and long-term adverse clinical outcomes observed in patients with type 2 diabetes mellitus vs non-diabetes mellitus following percutaneous coronary intervention: A meta-analysis including 139,774 patients. Medicine. 2019;98:e14669. doi: 10.1097/MD.0000000000014669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Luca G, et al. Meta-analysis comparing efficacy and safety of first generation drug-eluting stents to bare-metal stents in patients with diabetes mellitus undergoing primary percutaneous coronary intervention. Am. J. Cardiol. 2013;111:1295–1304. doi: 10.1016/j.amjcard.2013.01.281. [DOI] [PubMed] [Google Scholar]
- 8.Nakatsuma K, et al. Second-generation versus first-generation drug-eluting stents in patients with and without diabetes mellitus: Pooled analysis from the RESET and NEXT trials. Cardiovasc. Interv. Ther. 2018;33:125–134. doi: 10.1007/s12928-017-0458-9. [DOI] [PubMed] [Google Scholar]
- 9.Bavishi C, et al. Efficacy and safety of everolimus and zotarolimus-eluting stents versus first-generation drug-eluting stents in patients with diabetes: A meta-analysis of randomized trials. Int. J. Cardiol. 2017;230:310–318. doi: 10.1016/j.ijcard.2016.12.116. [DOI] [PubMed] [Google Scholar]
- 10.Grube E, et al. The SPIRIT V diabetic study: A randomized clinical evaluation of the XIENCE V everolimus-eluting stent vs the TAXUS Liberte paclitaxel-eluting stent in diabetic patients with de novo coronary artery lesions. Am. Heart J. 2012;163:867–875. doi: 10.1016/j.ahj.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh MJ, et al. Complete and incomplete revascularization in non-ST segment myocardial infarction with multivessel disease: Long-term outcomes of first- and second-generation drug-eluting stents. Heart Vessls. 2019;34:251–258. doi: 10.1007/s00380-018-1252-z. [DOI] [PubMed] [Google Scholar]
- 12.Arnold SV, et al. Prevalence of glucose abnormalities among patients presenting with an acute myocardial infarction. Am. Heart J. 2014;168:466–470. doi: 10.1016/j.ahj.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kok MM, et al. Prediabetes and its impact on clinical outcome after coronary intervention in a broad patient population. EuroIntervention. 2018;14:e1049–e1056. doi: 10.4244/EIJ-D-17-01067. [DOI] [PubMed] [Google Scholar]
- 14.Kim YH, et al. Effects of prediabetes on long-term clinical outcomes of patients with acute myocardial infarction who underwent PCI using new-generation drug-eluting stents. Diabetes Res. Clin. Pract. 2020;160:107994. doi: 10.1016/j.diabres.2019.107994. [DOI] [PubMed] [Google Scholar]
- 15.Carris NW, et al. Prevention of diabetes mellitus in patients with prediabetes. Am. J. Cardiol. 2019;123:507–512. doi: 10.1016/j.amjcard.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creager MA, et al. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone GW, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 19.Weisz G, et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) trial. J. Am. Coll. Cardiol. 2009;53:1488–1497. doi: 10.1016/j.jacc.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, et al. Differential clinical responses to everolimus-eluting and Paclitaxel-eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. doi: 10.1161/CIRCULATIONAHA.111.031070. [DOI] [PubMed] [Google Scholar]
- 21.Jensen LO, et al. Comparison of outcomes in patients with versus without diabetes mellitus after revascularization with everolimus- and sirolimus-eluting stents (from the SORT OUT IV trial) Am. J. Cardiol. 2012;110:1585–1591. doi: 10.1016/j.amjcard.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 22.von Birgelen C, et al. "Silent" diabetes and clinical outcome after treatment with contemporary drug-eluting stents: The BIO-RESORT silent diabetes study. JACC Cardiovasc. Interv. 2018;11:448–459. doi: 10.1016/j.jcin.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Nakatsuma, et al. Second-generation versus first-generation drug-eluting stents in patients with and without diabetes mellitus: Pooled analysis from the RESET and NEXT trials. Cardiovasc Interv. Ther. 2018;33:125–134. doi: 10.1007/s12928-017-0458-9. [DOI] [PubMed] [Google Scholar]
- 24.Torii S, et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat. Rev. Cardiol. 2020;17:37–51. doi: 10.1038/s41569-019-0234-x. [DOI] [PubMed] [Google Scholar]
- 25.Sarno G, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of 'new-generation' drug-eluting stents: A report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR) Eur. Heart J. 2012;33:606–613. doi: 10.1093/eurheartj/ehr479. [DOI] [PubMed] [Google Scholar]
- 26.van Werkum JW, et al. Predictors of coronary stent thrombosis: The Dutch stent thrombosis registry. J. Am. Coll. Cardiol. 2009;53:1399–1409. doi: 10.1016/j.jacc.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 27.Hong SJ, et al. Effect of intravascular ultrasound-guided drug-eluting stent implantation: 5-year follow-up of the IVUS-XPL randomized trial. JACC Cardiovasc. Interv. 2020;13:62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Tonino PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 29.Kim YH, et al. Routine angiographic follow-up versus clinical follow-up after percutaneous coronary intervention in acute myocardial infarction. Yonsei Med. J. 2017;58:720–730. doi: 10.3349/ymj.2017.58.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deedwania PC, et al. Impact of diabetes mellitus on outcomes in patients with acute myocardial infarction and systolic heart failure. Eur. J. Heart Fail. 2011;13:551–559. doi: 10.1093/eurjhf/hfr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YH, et al. Effect of renin-angiotensin system inhibitors on major clinical outcomes in patients with acute myocardial infarction and prediabetes or diabetes after successful implantation of newer-generation drug-eluting stents. J. Diabetes Complications. 2020;34:107574. doi: 10.1016/j.jdiacomp.2020.107574. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim DS, Jeong MH, Kang JC. Current management of acute myocardial infarction: Experience from the Korea Acute Myocardial Infarction Registry. J. Cardiol. 2010;56:1–7. doi: 10.1016/j.jjcc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, et al. Multicenter cohort study of acute myocardial infarction in Korea—Interim analysis of the korea acute myocardial infarction registry-national institutes of health registry. Circ. J. 2016;80:1427–1436. doi: 10.1253/circj.CJ-16-0061. [DOI] [PubMed] [Google Scholar]
- 35.Grech ED. ABC of interventional cardiology: Percutaneous coronary intervention. II: The procedure. BMJ. 2003;326:1137–1140. doi: 10.1136/bmj.326.7399.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Gara PT, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Amsterdam EA, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the American College of Cardiology/American Heart Association task force on practice guidelines. J. Am. Coll. Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Garcia HM, et al. Standardized end point definitions for coronary intervention trials: The academic research consortium-2 consensus document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 39.Kim YH, et al. Two-year outcomes of statin therapy in patients with acute myocardial infarction with or without dyslipidemia after percutaneous coronary intervention in the era of new-generation drug-eluting stents within Korean population: Data from the Korea Acute Myocardial Infarction Registry. Catheter. Cardiovasc. Interv. 2019;93:1264–1275. doi: 10.1002/ccd.27985. [DOI] [PubMed] [Google Scholar]
- 40.Kim YH, et al. Two-year clinical outcomes of Zotarolimus- And Everolimus-Eluting Durable-Polymer-Coated Stents Versus Biolimus-Eluting Biodegradable-Polymer-Coated Stent in Patients With Acute Myocardial Infarction With Dyslipidemia After Percutaneous Coronary Intervention: Data From the KAMIR. Heart Vessls. 2019;34:237–250. doi: 10.1007/s00380-018-1251-0. [DOI] [PubMed] [Google Scholar]
- 41.Bundhun PK, Wu ZJ, Chen MH. Is there any significant difference in stent thrombosis between sirolimus and paclitaxel eluting stents?: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e2651. doi: 10.1097/MD.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song PS, et al. Association of baseline platelet count with all-cause mortality after acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care. 2020 doi: 10.1177/2048872620925257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained with the article or supplementary information.