Abstract
Flowering time is known to be regulated by numerous pathways, such as the autonomous, gibberellin, aging, photoperiod-mediated, and vernalization pathways. These regulatory mechanisms involve both environmental triggers and endogenous hormonal cues. Additional flowering control mechanisms mediated by other phytohormones, such as auxin, are less well understood. We found that in cultivated strawberry (Fragaria × ananassa), the expression of auxin response factor4 (FaARF4) was higher in the flowering stage than in the vegetative stage. Overexpression of FaARF4 in Arabidopsis thaliana and woodland strawberry (Fragaria vesca) resulted in transgenic plants flowering earlier than control plants. In addition, FveARF4-silenced strawberry plants showed delayed flowering compared to control plants, indicating that FaARF4 and FveARF4 function similarly in regulating flowering. Further studies showed that ARF4 can bind to the promoters of the floral meristem identity genes APETALA1 (AP1) and FRUITFULL (FUL), inducing their expression and, consequently, flowering in woodland strawberry. Our studies reveal an auxin-mediated flowering pathway in strawberry involving the induction of ARF4 expression.
Subject terms: RNAi, Gene regulation, Auxin
Introduction
Flowering marks a transition from vegetative to reproductive growth in plants and involves numerous physiological processes, metabolic pathways, and gene regulatory mechanisms1,2. These mechanisms involve intracellular and intercellular signal transduction cascades and the specific spatiotemporal expression of flowering genes3–5. To elucidate the molecular underpinnings of flowering, many studies have been performed on the model species Arabidopsis thaliana, resulting in the identification of genes involved in flowering regulation, as well as multiple signaling pathways: photoperiodic, vernalization, ambient temperature, autonomous, aging, and gibberellin (GA)6–8. However, it is recognized that given the interactions among phytohormones in regulating plant development, other signaling systems may also be involved9. For example, auxin is known to control many processes of plant growth and development and broadly regulates gene expression; however, its role in flowering and the associated molecular mechanisms remain poorly understood10,11.
Central to auxin-regulated transcription are three families of primary auxin-responsive genes: Aux/IAA (AUXIN/INDOLE ACETIC ACID), GH3 (GRETCHEN HAGEN3), and SAUR (SMALL AUXIN UP RNA)12. All of the gene promoters from these families contain the TGTCNC motif (auxin response element, AuxRE), which is bound to AUXIN RESPONSE FACTOR (ARF) proteins to mediate auxin responses13. In A. thaliana, ARF functions have been well studied, and it is known that AtARF1, AtARF2, AtARF6, and AtARF8 are involved in floral organ development14, while AtARF7, AtARF16, and AtARF19 are associated with root development13,15, AtARF12-15 regulates embryogenesis and seed development, and AtARF20-22 has similar functions to AtARF12-1513,16.
AtARF3 and AtARF4 are involved in the transition from vegetative to reproductive growth, and a unique feature of ARF3/4 regulation is that their transcripts are post-transcriptionally cleaved by trans-acting short-interfering RNAs, such as tasiRNA3, an endogenous trans-acting small-interfering RNA13. The production of tasiRNA3 is initiated by cleavage of the non-protein-coding TAS3 RNA by miR390. A complex of the miR390-cleaved transcript bound to ARGONAUTE7 (AGO7) is then used as a template for polymerization by RNA-DEPENDENT RNA POLYMERASE6 (RDR6) and SUPPRESSOR OF GENE SILENCING3 (SGS3)10. The resulting double-stranded RNA is cleaved by DICER-LIKE4 (DCL4) to generate the 21-nucleotide-long tasiRNA317. Thus, AGO7/RDR6/SGS3/DCL4 is required for the production and/or stability of tasiRNA3, which targets both ARF3 and ARF4. Mutations in AGO7/RDR6/SGS3/DCL4 in A. thaliana accelerate the juvenile-to-adult transition, and the expression of ARF3 and ARF4 is upregulated in the ago7/rdr6/sgs3/dcl4 mutant17. This indirectly demonstrates that the ago7/rdr6/sgs3/dcl4 phenotype is attributable to the absence of tasiRNA3-mediated repression of ARF3 and ARF410. However, the specific function of AtARF3 and AtARF4 in the phase transition is unclear.
A potentially useful experimental system to study flowering transition is strawberry (Fragaria)18. Early flowering is a great advantage for fruit crop breeding and cultivation since it shortens the process of generating new cultivars and reduces the time to produce a marketable crop, resulting in economic benefits7,19. Octoploid cultivated strawberry (Fragaria × ananassa) is widely cultivated in all arable regions around the globe and has high economic value20. However, although F. × ananassa is of great agricultural and research importance, genetic transformation and associated functional studies are hindered by its complex genetics, which include a large genome and high degree of heterozygosity21. In contrast, diploid woodland strawberry (Fragaria vesca) is suitable for regeneration, and a transformation system has been established in vitro22. Moreover, F. vesca has a substantial seed set, rapid growth, a small size, a relatively small genome, and existing genetic maps, which makes it a good option as a model plant for the development of genomic tools, enabling us to perform functional studies using forward or reverse genetic approaches23. Notably, F. vesca shares considerable sequence similarity with F. ×ananassa and other rosaceous plants, and the high levels of functional conservation at the genetic level mean that knowledge of diploid strawberry gene function has the potential to be applied to octoploid strawberry or other members of the Rosaceae family7,23.
In this study, we investigated the role and mechanism of auxin-regulated flowering in strawberry and found that the expression level of auxin-induced FaARF4 was upregulated in the flowering stage rather than during vegetative growth, indicating that FaARF4 is related to flowering. Using transgenic plants, we discovered that FaARF4 promoted flowering and that FveARF4-silenced plants showed delayed flowering. Further studies revealed that FaARF4 and FveARF4 bound to the promoters of FveAP1 and FveFUL to induce their expression. Our findings showed that auxin is involved in the flowering pathway in strawberry through its regulation of ARF4 expression.
Results
FaARF4 expression analysis
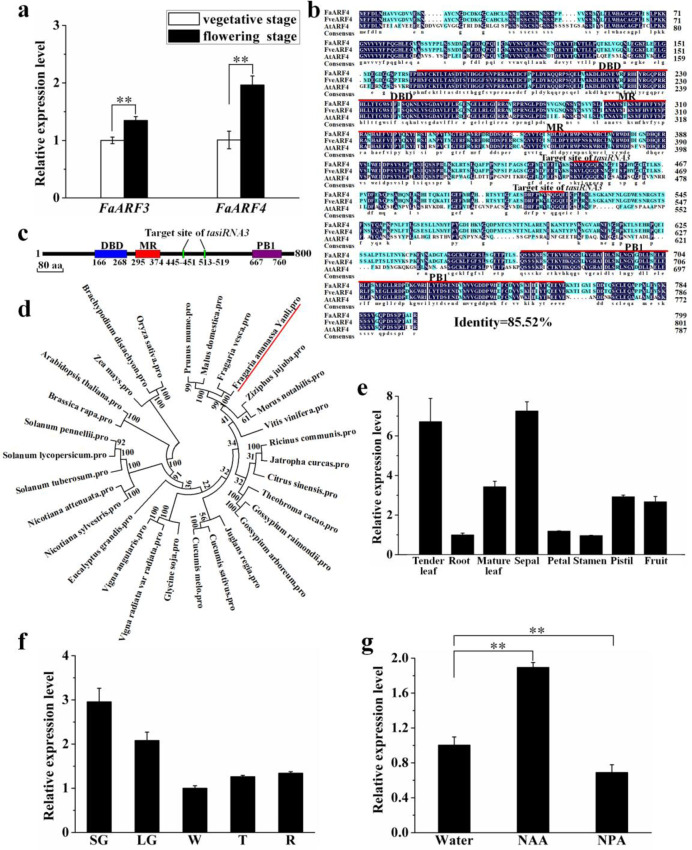
Elite strawberry cultivars, free of plant pathogenic fungi and bacteria, are commonly produced by micropropagation, although several problems, such as epigenetic variation, can be encountered using this approach24. In our previous study, we reported that the flowering characteristics of micropropagated strawberry (F. × ananassa) changed so that it exhibited early flowering, along with a low level of miR390 expression25. Next, we overexpressed miR390 in tobacco and found that the juvenile-to-adult phase transition was significantly delayed26. Since miR390 negatively regulates the expression of ARF3/4, we speculated that ARF3/4 may be related to flowering in cultivated strawberry. To study the expression patterns of FaARF3 and FaARF4 in response to flowering, we determined their expression profiles in stem tips at different developmental stages in the cultivated strawberry ‘Yanli’ by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis. As shown in Fig. 1a, the expression levels of FaARF3 and FaARF4 were higher in the flowering stage than in the vegetative stage, indicating that these two genes are involved in flowering. Since the expression level of FaARF4 changed more than that of FaARF3, FaARF4 was chosen for further study.
Fig. 1. Expression pattern, sequence alignment, and phylogenetic analysis of the FaARF4 gene or cognate protein.
a The expression levels of FaARF3 and FaARF4 during the flowering and vegetative stages. b Amino acid sequence alignment of FaARF4, FvARF4, and AtARF4. c DNA binding domain (DBD), middle region (MR), and protein binding (PB1) domain structure in FaARF4. d Phylogenetic tree of FaARF4 with other ARF4 proteins from different plant species. Expression of FaARF4 in different tissues (e), different stages of fruit development (f), and ‘Yanli’ strawberry treated with α-naphthalene acetic acid (NAA) (g). Vertical bars represent the SDs (n = 3). **p < 0.01
Cloning and phylogenetic analysis of FaARF4
Primers for FaARF4 from the cultivated strawberry ‘Yanli’ were designed based on the FveARF4 sequence in the NCBI database (https://www.ncbi.nlm.nih.gov/). The full-length coding sequence (CDS) of FaARF4 (GenBank No. MG765454) was found to be 2403 bp long and encode an 800-amino-acid protein with many α-helices, β-sheets, and hydrophilic and flexible regions (Fig. S1). An alignment of the amino acid sequences of FaARF4, FveARF4, and AtARF4 revealed 85.52% sequence identity (Fig. 1b). The conserved regions were primarily confined to three domains, the DNA binding domain (DBD), the middle region (MR), and the protein binding (PB1) domain, consistent with FaARF4 being a member of the ARF family. As shown in Fig. 1c, FaARF4 contains a DBD, an MR, a PB1, and two tasiRNA3 target sites.
To study the phylogenetic relationships of ARF4 with different species, the ARF4 amino acid sequences from members of the Rosaceae and other plant species were retrieved from the NCBI database. A phylogenetic analysis (Fig. 1d) showed that the FaARF4 and FveARF4 sequences were the most closely related, followed by other Rosaceae proteins and then proteins from other species, consistent with their phylogenetic placement.
FaARF4 expression profile in strawberry
qRT-PCR analysis of FaARF4 in different strawberry tissues revealed that this gene was universally expressed; the highest expression level was found in sepals, where the expression level was approximately 7-fold higher than that in roots, which had the lowest expression level (Fig. 1e). Next, we measured FaARF4 expression during fruit development and ripening. Strawberry fruit maturity can be divided into five stages: small green (SG), large green (LG), white (W), turning (T), and red (R)27. As shown in Fig. 1f, FaARF4 was strongly expressed in the SG stage, followed by a gradual decline until the W stage, with no change in the T and R stages. FaARF4 expression in the SG stage was ~3-fold higher than that in the W stage.
To test the hypothesis that auxin controls FaARF4 expression, we analyzed its expression in ‘Yanli’ fruit after treatment with water (control), 500 μM NAA (α-naphthalene acetic acid, a synthetic auxin), or 500 μM NPA (N-1-naphthylphthalamic acid, an auxin inhibitor) by qRT-PCR. As shown in Fig. 1g, the expression of FaARF4 was upregulated after NAA treatment and downregulated after NPA treatment, suggesting that auxin induces the expression of FaARF4.
Identification of the transcription factor FaARF4
FaARF4 sequence analysis predicted that it localizes to the nucleus (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc/), and this hypothesis was tested by expressing a green fluorescent protein (GFP):ARF4 fusion (FaARF4-GFP) driven by the constitutive CaMV 35S promoter (Fig. 2a) in A. thaliana protoplasts and Nicotiana benthamiana leaves. A constitutively expressed GFP control was similarly tested. The green fluorescent signal of FaARF4-GFP was only detected in the nucleus, while GFP alone was found throughout the entire cell (Figs. 2b and S2), suggesting that FaARF4 specifically accumulates in the nucleus.
Fig. 2. FaARF4 transcription factor characteristics.
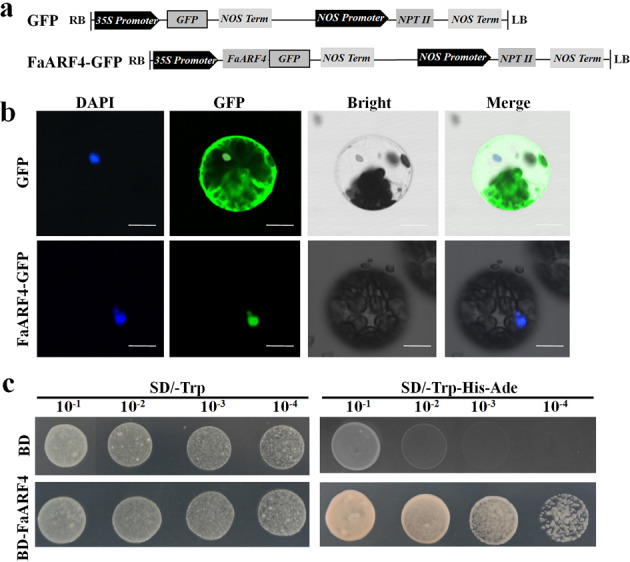
a Structural diagram of the pRI 101-AN-GFP vector and the pRI 101-AN-FaARF4-GFP construct. b Subcellular localization of FaARF4 in Arabidopsis thaliana protoplasts. A. thaliana cell nuclei were identified using 4ʹ,6-diamidino-2-phenylindole (DAPI) staining. Scale bars = 25 μm. c FaARF4 transcriptional activity
To investigate whether FaARF4 has transcriptional activation activity, we fused its CDS with the GAL4 DBD and created the pGBT9-FaARF4 construct (BD-FaARF4). BD-FaARF4 and the empty vector pGBT9 (BD), as a negative control, were then transformed into yeast for transcriptional activity analysis. As shown in Fig. 2c, the yeast strain containing BD-FaARF4 grew well on SD/-Trp-His-Ade media, while the yeast strain containing BD grew only in SD/-Trp, demonstrating that FaARF4 is a transcriptional activator.
Mutation of FaARF4 tasiRNA3 target sites
tasiRNAs are a group of endogenous non-coding small RNAs that function on their target genes by complementary base pairing. It is well known that ARF4, the target gene of tasiRNA3, is negatively regulated by tasiRNA3 in A. thaliana10,13. To verify whether tasiRNA3 cleaves FaARF4 in strawberry, an RLM-5ʹ rapid RACE assay was performed. As shown in Fig. 3a, FaARF4 contains two tasiRNA3 target sites (site 1 and site 2) located in the coding region that were cleaved by tasiRNA3. To prevent FaARF4 cleavage by endogenous tasiRNA3, we performed rapid PCR site-directed mutagenesis of FaARF4 using the methods of Picard et al. (1994)28. We obtained the nucleotide sequence of a tasiRNA3-insensitive mutant (named FaARF4mut) and confirmed that the amino acid sequence was the same as that of FaARF4 and that only the nucleotide sequence was affected (Fig. 3b). FaARF4mut was used for further functional studies.
Fig. 3. Rapid PCR site-directed mutagenesis of FaARF4.
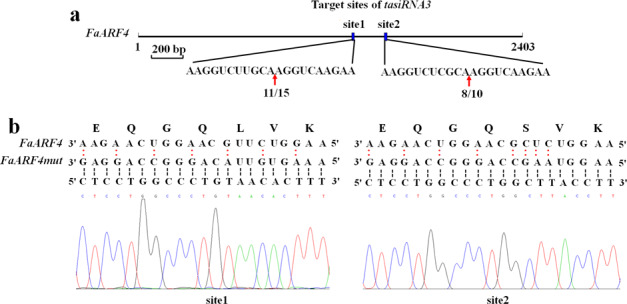
a RLM-5ʹ RACE assay showing that tasiRNA3 cleaves FaARF4. The red arrows represent the cleavage sites. The numbers under the red arrows represent the number of clones that possessed the cleavage site and the total number of clones in the RLM-5ʹ RACE assay. b Nucleotide and amino acid sequence analysis of FaARF4mut and FaARF4. The red colons indicate the nucleotides that differ between FaARF4mut and FaARF4. The dotted lines represent complementary sequences
FaARF4 promotes flowering in A. thaliana and woodland strawberry
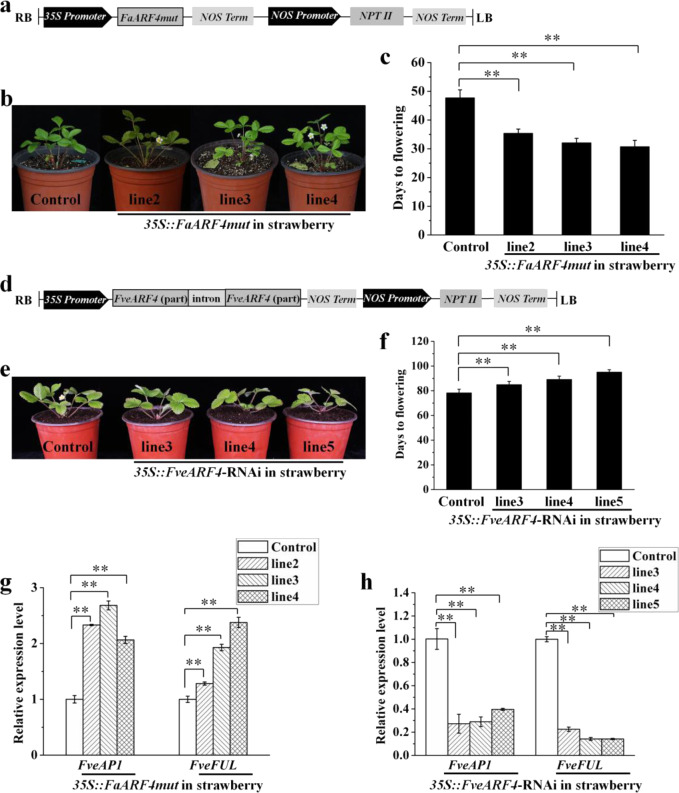
A. thaliana is often used to assess the function of genes from other plant species20, and here we transformed both A. thaliana (Columbia) and ‘Ruegen’ (F. vesca) with the pRI 101-FaARF4mut plasmid (Fig. 4a). All transgenic lines were identified by detection of the FaARF4 gene using the 35S and ARF4-R primers. A 2000–3000 bp band was amplified from the genomic DNA of all transgenic lines, and no corresponding bands were amplified from control plants (Fig. S3a). We obtained four transgenic A. thaliana lines and four transgenic strawberry lines. FaARF4 expression was investigated in the transgenic lines and control plants, and it was clearly higher in the transgenic plants (Fig. S3b, c).
Fig. 4. Phenotypic observation and flowering time analysis of transgenic strawberry plants.
a Structural diagram of the vector used to overexpress FaARF4 driven by the CaMV 35S promoter. FaARF4-overexpressing plants showed early flowering (b), and the number of days until flowering was less than that for the control plants (c). Vertical bars represent the SDs of approximately 10 plants (**p < 0.01). d Structural diagram of the FveARF4 RNAi vector driven by the CaMV 35S promoter. By observing FveARF4 RNAi plants (e), we found that the number of days until flowering was greater than that of the control plants (f). Vertical bars represent the SDs of approximately 10 plants (**p < 0.01). The expression of FveAP1 and FveFUL, which are tissue-specific genes expressed in the flower meristem, increased in FaARF4-overexpressing plants (g) and was reduced in FveARF4 RNAi plants (h). Vertical bars represent the SDs (n = 3). **p < 0.01
The phenotypic differences between the transgenic lines and wild-type A. thaliana plants are shown in Fig. S3d. We found that transgenic plants flowered earlier than the wild-type and that the vegetative growth stage was approximately 52 days for the wild-type and 44 days for the transgenic plants (Fig. S3e). These results indicated that FaARF4 can regulate the flowering process in A. thaliana.
To better observe the phenotype of the transgenic strawberry plants, we transplanted them into a greenhouse. We observed an early flowering phenotype in the transgenic lines (Fig. 4b) and found that the transgenic plants flowered 15 days earlier than the control plants (Fig. 4c). Thus, FaARF4 also regulates flowering in strawberry.
FveARF4 silencing inhibits flowering in woodland strawberry
FaARF4 and FveARF4 have a high degree of sequence identity (99.47%), suggesting similar functions (Fig. 1b, d). To confirm the function of FveARF4, an RNAi vector was created and transformed into ‘Ruegen’ using Agrobacterium-mediated transformation (Fig. 4d). Transgenic lines were confirmed by PCR amplification of a 250–500 bp band that was not amplified from control plants (Fig. S3f). We also analyzed FveARF4 expression by qRT-PCR in the RNAi lines. Compared with the control plants, the expression of FveARF4 in the RNAi lines was lower (Fig. S3g). We observed that the flowering time of the RNAi lines was delayed by 8 days compared to that of the control plants, indicating that FveARF4 regulates the flowering process in strawberry (Fig. 4e, f).
ARF4 directly binds to the promoters of FveAP1 and FveFUL in strawberry
In A. thaliana, the floral integration genes FLOWERING LOCUS T (FT) and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) initiate flowering by activating tissue-specific flower meristem genes, including LEAFY (LFY), APETALA1 (AP1), and FRUITFULL (FUL); in addition, TERMINAL FLOWER1 (TFL1) inhibits the expression of LFY and AP1, and plays an important role in regulating the flowering process1,7. To explore the cause of early flowering in strawberry, we measured the expression of these flowering-related genes. qRT-PCR analysis showed that the expression levels of FveAP1 and FveFUL were higher in FaARF4-overexpressing plants than in control plants (which was set to 1) (Fig. 4g), while the expression of FveFT, FveSOC1, FveLFY, and FveTFL1 was not significantly different between transgenic plants and control plants (Fig. S4). FveAP1 and FveFUL expression was lower in the RNAi lines than in control plants (Fig. 4h).
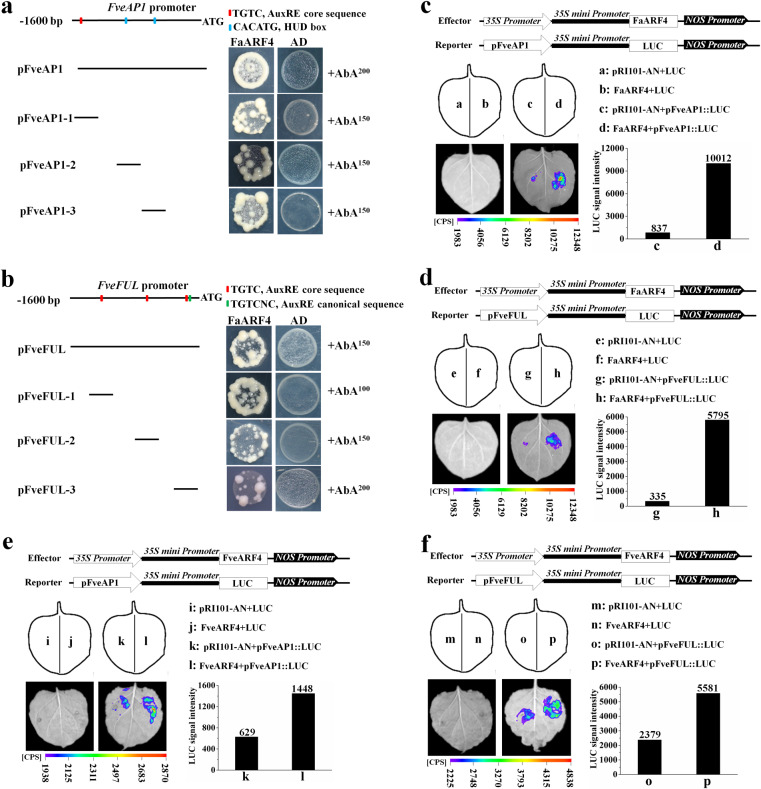
Previous studies have shown that ARF proteins can also bind to G boxes (CACGTG) and HUD boxes (CACATG) in addition to the AuxRE29. As shown in Fig. 5a, the FveAP1 promoter is predicted to have one AuxRE and two HUD boxes distributed across four regions (pFveAP1, pFveAP1-1, pFveAP1-2, and pFveAP1-3). In addition, the FveFUL promoter contains four AuxREs distributed across four regions (pFveFUL, pFveFUL-1, pFveFUL-2, and pFveFUL-3), as shown in Fig. 5b.
Fig. 5. ARF4 positively regulates FveAP1 and FveFUL expression by binding to their promoters.
Yeast one-hybrid (Y1H) assay showing that FaARF4 binds to FveAP1 promoter fragments (pFveAP1, pFveAP1-1, pFveAP1-2, and pFveAP1-3) (a) and FveFUL promoter fragments (pFveFUL, pFveFUL-1, pFveFUL-2, and pFveFUL-3) (b) in vitro. The empty pGAD424 and pAbAi vectors were used as negative controls. In vivo luciferase reporter assay showing that FaARF4 and FveARF4 positively regulate FveAP1 expression (c, e) and FveFUL expression (d, f), respectively. The FaARF4/FveARF4 effector and either the pFveAP1 or the pFveFUL reporter were coinfiltrated into tobacco leaves, and the luciferase signal was measured
To verify whether FaARF4 binds to the FveAP1 and FveFUL promoters, yeast one-hybrid (Y1H) analysis and luciferase reporter assay were carried out. The Y1H assay indicated that FaARF4 bound to the AuxRE and HUD boxes in the FveAP1 and FveFUL promoters in vitro (Fig. 5a, b). The FveAP1 and FveFUL promoters were then individually inserted into the pRI-mini35S-LUC vector (luciferase reporter vector) as reporters, and FaARF4 driven by the 35S promoter was used as the effector (Fig. 5c, d) in a transient tobacco leaf expression assay. We observed that FaARF4 also bound to the FveAP1 and FveFUL promoters in vivo (Fig. 5c, d). The Y1H and luciferase reporter assays suggested that FaARF4 directly binds to the FveAP1 and FveFUL promoters, and activates the transcription of the corresponding genes. Since the expression of FveAP1 and FveFUL was reduced in the RNAi lines, we speculated that FveARF4 and FaARF4 had the same mechanism. Supporting this hypothesis were the results of the transactivation activity assay demonstrating that FveARF4 is also a transcriptional activator (Fig. S5) and those of the luciferase reporter assay indicating that FveARF4 can bind to the promoters of both FveAP1 and FveFUL (Fig. 5e, f).
Discussion
ARF4 is involved in the IAA-mediated flowering pathway in strawberry
In species such as sweet cherry (Prunus avium)30 and longan (Dimocarpus longan)7, shortening the life cycle can allow multiple generations to be grown commercially in a single season, and the breeding process can be accelerated. It is also possible to increase the yield of plants by prolonging the number of days of vegetative growth in species such as sugar beet (Beta vulgaris)4 and perennial sugarcane (Saccharum officinarum)3. Moreover, flowering genes can also be used to adjust the flowering period, which greatly improves the economic value of ornamental plants31. In cultivated strawberry, the regulation of flowering is used to provide fresh berries throughout the year32. Most strawberry cultivars are June-bearing, and their flower formation is affected by environmental conditions, especially light and temperature18. Growth studies have revealed that short days and/or cool temperatures can promote flowering, and short days greatly promote and enhance flower initiation19,33. At 15 °C or during crown-cooling treatments, flowering time was hastened, and the number of flowers increased34. It was also reported that light quality affected flowering time and that strawberry could be induced to flower when exposed to far red light33. The role of GA in strawberry flowering has also been studied, and it has been shown to inhibit flowering and promote the formation of runners32. Additionally, it was reported that both auxin IAA and cytokinin affect the type of first bud initiation, and exogenously applied IBA (indole-3-butytric acid, an IAA analog) +6-BA (6-benzylaminopurine, a synthetic cytokinin) leads to the production of inflorescences instead of runners35. However, it has not been reported how IAA regulates flowering at the molecular level.
IAA regulates plant growth and development by employing signal transduction, and as the center of the auxin signaling pathway, the expression of ARF proteins is known to be regulated by IAA13. The expression of ARF4 is also affected by IAA, and it has been shown to be upregulated by IAA treatment in model plants, such as A. thaliana36, Medicago truncatula37, and Brachypodium distachyon38. In a previous study, we also demonstrated that the expression of FveARF4 was induced by IAA in woodland strawberry39, and here, we found that the expression of FaARF4 was increased by IAA and reduced by NPA (Fig. 1g). We analyzed the function of both FaARF4 and FveARF4 and found them to be involved in the regulation of flowering time, which helps to elucidate the IAA-mediated flowering pathway in strawberry (Fig. 4a–f).
ARF4 promotes flowering by directly activating the expression of FveAP1 and FveFUL in strawberry
In A. thaliana, flowering pathways involving endogenous cues, exogenous cues, and intrinsic genetic programs govern flowering time7. Based on the analysis of loss-of-function mutants and transgenic plants, approximately 180 genes have been shown to be involved in the regulation of flowering time, including AtARF3 and AtARF410,13. It has been suggested that AtARF3 and AtARF4 indirectly activate AtLFY, AtAP1, and AtFUL by enhancing the expression of AtSPL3, the target gene of miR156, which promotes flowering in A. thaliana, but this has not been experimentally confirmed6. However, we observed no FveSPL3 transcriptional activation activity in yeast (Li, 2020; unpublished data). To explore how ARF4 regulates flowering in woodland strawberry, we analyzed the expression of flowering-related genes in transgenic and control strawberry and observed significant differences in the expression levels of FveFUL and FveAP1 (Fig. 4g, h). We also showed that FaARF4 and FveARF4 have transcriptional activation activity (Figs. 2b and S5), and Y1H and luciferase reporter assays further confirmed that ARF4 can bind to the AuxRE and HUD-box motifs in the FveFUL and FveAP1 promoters and activate their expression (Fig. 5). FUL and AP1 belong to the AP1/SQUA gene subfamily and are key genes in flowering regulation, and it has been reported that they promote flowering in various species, such as A. thaliana7, soybean (Glycine max)40, tomato (Solanum lycopersicum)41, maize (Zea mays)42, apple (Malus domestica)43, and others. Notably, the expression of FUL and AP1 is important in controlling flowering in woodland strawberry: high expression of FUL and AP1 promotes flowering, and if they are not highly expressed, vegetative growth continues18. In addition, we found that flowering time correlated with the expression levels of FveFUL and FveAP1 in transgenic strawberry lines (Fig. 4g, h). Taken together, we propose an IAA-mediated flowering pathway in strawberry (Fig. 6), which suggests a target for altering flowering time for horticultural purposes.
Fig. 6. Model of how auxin induces ARF4 to promote the flowering pathway.
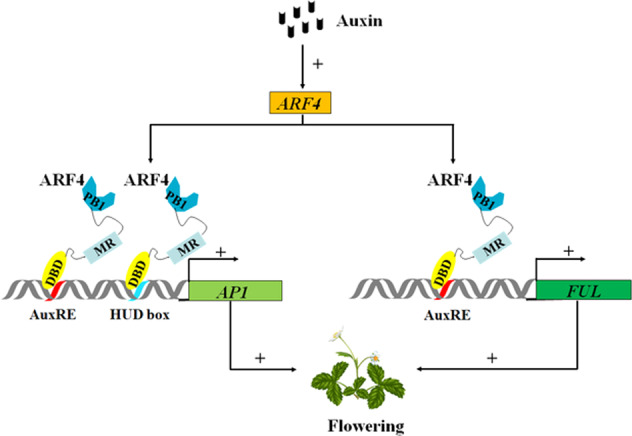
In woodland strawberry, auxin induces the expression of ARF4, and the ARF4 protein binds to and activates the AP1 and FUL promoters. The expression of AP1 and FUL initiates flowering. Symbols/abbreviations: ‘+’, promotion; AuxRE and HUD box, auxin-responsive element (ARF4 binding sites)
Flowering is an important factor in the production of fruit trees, and the existence of a long juvenile phase in fruit trees has been suggested to limit breeding and genetic improvement32. Furthermore, most economically important phenotypes related to fruits of hybrid cultivars cannot be identified in this phase, and one of the major goals of fruit breeding programs is to reduce the juvenile phase and accelerate floral production18. Genetic engineering has been used to successfully shorten the juvenile phase and promote flowering in several fruit species7. In our study, we found that the auxin-induced FaARF4 and FveARF4 genes were involved in the regulation of flowering. ARF4 has a sequence similar to those of homologs from fruit tree species, especially members of the Rosaceae, indicating analogous functions. Therefore, it may be possible to break the long juvenile phase of fruit trees using overexpression of the ARF4 gene.
Materials and methods
Plant materials and treatments
The cultivated strawberry (F. × ananassa) cultivar ‘Yanli’ was grown under greenhouse conditions at Shenyang Agricultural University, China. Stem tips from the 4-leaf and bud stages were collected as vegetative stage and flowering stage samples, respectively. Different organs and fruits at different developmental stages were harvested individually for gene expression analysis, frozen in liquid nitrogen, and stored at –80 °C. In the white ripening stage, the fruits were picked and transported to the laboratory, where they were divided into three groups: fruits from one group were submerged in water (as the control); fruits from the second group were submerged in 500 μM NAA (Solarbio, Beijing, China); and fruits from the third group were submerged in 500 μM NPA (a polar auxin transport inhibitor; Shanghai Yuanye Bio-Technology Company Limited, Shanghai, China). All treatments lasted for 30 s. After 1 h, total RNA samples were extracted using the cetyltrimethyl ammonium bromide (CTAB)-based method for the analysis of FaARF4 expression44. Three biological replicates were prepared.
The strawberry leaf transformation experiment was performed using ‘Ruegen’ (F. vesca), which was grown in tissue culture. Agrobacterium-mediated leaf transformation was carried out after plants had grown 4–5 leaves45.
Seeds of wild-type Arabidopsis thaliana Columbia were sown on peat:vermiculite:perlite::3:3:1 substrate after 3 days of vernalization at 4 °C and were placed in climate-controlled boxes. Transformation was carried out using the floral dip method20.
Gene isolation
cDNA samples were synthesized from the total RNA described above using the RNA PCR Kit (Takara, Dalian, China) following the manufacturer’s instructions. The full-length FaARF4 CDS was cloned by RT-PCR using the FaARF4-F and FaARF4-R primers (Table S1) with Nde I and Sal I restriction sites added at the 5ʹ and 3ʹ ends, respectively.
PCR was performed under the following conditions: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 57 °C for 30 s, and 72 °C for 3 min, with a final extension at 72 °C for 5 min. The PCR products were ligated into the pMD18-T vector (Takara, Dalian, China) and subsequently transformed into Escherichia coli strain DH5α. Subsequently, positive colonies were selected, the gene sequence was amplified by PCR, and the product was sequenced at BGI-Shenzhen, China. The ExPasy ProtParam tool (http://web.expasy.org/compute_pi/) and DNASTAR program (DNASTAR Inc., USA) were used to predict the structure of the encoded proteins. The amino acid sequences of FaARF4, FveARF4, and AtARF4 were aligned using DNAMAN 6.0 software (Lynnon Biosoft, USA).
Phylogenetic analysis
The ARF4 amino acid sequences from A. thaliana (NP_200853), B. distachyon (XP_003564986), Brassica rapa (XP_009120578), Citrus sinensis (XP_006488135), Cucumis melo (XP_008463923), Cucumis sativus (XP_030503928), Eucalyptus grandis (XP_010043718), F. vesca (XP_004309870), Glycine soja (XP_028190393), Gossypium arboretum (XP_017611878), Gossypium raimondii (XP_012443345), Jatropha curcas (XP_012064855), Juglans regia (XP_018819532), M. domestica (XP_008385290), Morus notabilis (XP_010104118), Nicotiana attenuata (XP_019229596), Nicotiana sylvestris (XP_009779766), Oryza sativa (XP_015650953), Prunus mume (XP_008225336), Ricinus communis (XP_015579153), S. lycopersicum (NP_001233771), Solanum pennellii (XP_015058398), Solanum tuberosum (XP_006340145), Theobroma cacao (XP_007015441), Vigna angularis (XP_017425709), Vigna radiata var. radiata (XP_014498033), Vitis vinifera (XP_002285019), Z. mays (XP_008656904), and Ziziphus jujuba (XP_024928576) were obtained from the NCBI nucleotide database (http://www.ncbi.nlm.nih.gov/nucleotide/). A phylogenetic tree was constructed using the neighbor-joining method using MEGA 6.0 software with 1000 bootstrap replicates to evaluate the reliability of the phylogenetic grouping. The tree files were viewed and edited using MEGA 6.046.
Quantitative RT-PCR analysis
Quantitative RT-PCR (qRT-PCR) was performed using the 7500 system (Applied Biosystems, Foster City, USA) according to the manufacturer’s instructions with SYBR Green II (Takara, Dalian, China) and the FaARF3 and FaARF4 qRT-PCR primers listed in Table S1 (FaARF3-QF/R and FaARF4-QF/R). The experiments were conducted on three biological replicates, and the results were normalized using strawberry 26S rRNA as the housekeeping gene45. The qRT-PCR analysis of Fa26S was conducted with the following primers: Fa26S-F and Fa26S-R (Table S1). Tender leaves from transgenic strawberry plants and transgenic A. thaliana plants were harvested for gene expression analysis. Fve26S was used as the housekeeping gene for woodland strawberry, and AtACTIN8 was selected as the housekeeping gene for A. thaliana20 (Table S1).
The nucleic acid sequences of flowering-related genes were obtained from the Genome Database for Rosaceae (GDR) (https://www.rosaceae.org/species/fragaria/all). The qRT-PCR analysis of FveSOC1 (FvH4_7g127000), FveFT (FvH4_6g00090), FveLFY (FvH4_5g09660), FveAP1 (FvH4_4g29600), FveFUL (FvH4_5g13500), and FveTFL1 (FvH4_6g18480) was conducted using six pairs of primers: FveSOC1-QF/R, FveFT-QF/R, FveLFY-QF/R, FveAP1-QF/R, FveFUL-QF/R, and FveTFL1-QF/R (Table S1). Stem tips of transgenic strawberry plants were used for gene expression analysis.
PCR was performed using the following thermal cycling conditions: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 1 min. The transcription levels were calculated using the 2–ΔΔCT method47.
Subcellular localization
The full-length FaARF4 CDS without the termination codon was amplified using LA Taq polymerase (Takara, Dalian, China) with gene-specific primers FaARF4-GFP-F and FaARF4-GFP-R (Table S1) harboring Xba I and Xho I sites. PCR was performed under the following conditions: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 3 min, with a final extension at 72 °C for 5 min. The PCR products were inserted into the pGPTVII-GFP vector between the Xba I and Xho I sites to create the 35S::FaARF4-GFP (FaARF4-GFP) construct. Using pGPTVII-GFP containing 35S::GFP (GFP) as a control, vectors were injected into A. thaliana protoplasts and N. benthamiana leaves45. Two days after agroinfiltration, GFP fluorescence was imaged using a laser confocal fluorescence microscope (TCS SP8-SE; Leica, Wetzlar, Germany) with an excitation wavelength of 488 nm and a 505–530 nm bandpass emission filter.
Transcriptional activation analysis
The FaARF4 and FveARF4 CDSs were individually inserted into the pGBT9 vector to create the pGBT9-FaARF4 (BD-FaARF4) and pGBT9-FveARF4 (BD-FveARF4) constructs, respectively. The primers used are listed in Table S1. BD-FaARF4, BD-FveARF4, and the empty vector pGBT9 (BD) were then transformed into yeast (Saccharomyces cerevisiae) for transcriptional activity analysis using the Yeast Transformation Kit (Shaanxi Protein Interaction Biotechnology Company Limited, Shaanxi, China). The transformed yeast strains were grown on SD/−Trp medium and selected on SD/−Trp–His–Ade medium. The transcriptional activity of FaARF4 was determined by observing yeast growth according to previously described methods48. X-α-gal (5-bromo-4-chloro-3-indolyl-α-D-galactoside) was added to the SD/−Trp–His–Ade medium, and the resulting blue coloration was used as an indication of FveARF4 transcriptional activation activity49.
Identification of the tasiRNA3 target by RLM-5ʹ RACE
To identify FaARF4 as a tasiRNA3 target gene, 5ʹ-RNA ligase-mediated rapid amplification of cDNA ends (RLM-5ʹ RACE) was performed with the 5ʹ-Full RACE Kit (Takara, Dalian, China). According to the manufacturer’s instructions, PCR products were ligated into the pMD18-T vector, and the recombinant vectors were verified by sequencing. All primers used for RLM-5ʹ RACE are listed in Table S1.
Rapid PCR site-directed mutagenesis of FaARF4
Rapid PCR site-directed mutagenesis was used to study FaARF4 expression as previously described28. We mutated FaARF4 with two pairs of mutant primers: FaARF4mut-F1/R1 and FaARF4mut-F2/R2 (Table S1).
Transformation of A. thaliana and strawberry
The full-length FaARF4-coding region with added Nde I and Sal I restriction sites was amplified by PCR and inserted into the polylinker site of the plant overexpression vector pRI 101-AN. An RNAi vector, pART27, was constructed as previously described45. Briefly, a 333 bp fragment of FveARF4 was inserted into the left (EcoR I and Xho I) and right (Hind III and Xba I) multiple cloning sites of the pART27 vector. All primers used are listed in Table S1.
A. thaliana was transformed using the Agrobacterium tumefaciens strain GV3101 and the floral dip method20. Transgenic plants were selected on 1/2 Murashige and Skoog (MS) medium with 30 mg/L kanamycin. Transgenic A. thaliana plants were grown in climate-controlled boxes at 24 °C under a 12/12 h light/dark cycle. A. tumefaciens strain EHA105 was used for ‘Ruegen’ transformation following the leaf-disk procedure45. Transgenic strawberry plants were planted in November in a greenhouse under sunlight, and the day and night temperatures were 15–25 °C and 5–10 °C, respectively.
Identification of transgenic A. thaliana and strawberry
To identify transgenic A. thaliana and strawberry plants, genomic DNA was extracted using the CTAB method44. Transgenic plants were confirmed using the 35S-F/FaARF4-R primer set, targeting the pRI 101-AN vector and the transgene sequence. The following PCR conditions were used: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 3 min, with a final extension at 72 °C for an additional 5 min. The expression of ARF4 in the leaves of transgenic and control plants was analyzed using qRT-PCR with the FaARF4 and Fve26S/AtACTIN8 primers (Table S1).
Yeast one-hybrid assay
The FaARF4 sequence was ligated into the pGAD424 vector. The promoter sequences (1600 base pairs) of FveAP1 and FveFUL were cloned and analyzed to determine whether AuxREs were present. Four FveAP1 promoter fragments and four FveFUL promoter fragments were then amplified and individually inserted into the pAbAi vector. Primers are listed in Table S1. The vectors containing FaARF4 and a fragment of the FveAP1 or FveFUL promoter were cotransformed into the Y1H yeast strain, and the Y1H assay was performed as previously described50.
Luciferase reporter assay
The 35S::FaARF4 and 35S::FveARF4 vectors were used as effectors, and the FveAP1 and FveFUL promoters were inserted into the pRI-mini35S-LUC vector and used as reporters. Primers are listed in Table S1. The luciferase reporter assay was carried out following previously described methods49. Finally, the luciferase fluorescence and luciferase signal intensity were imaged and measured using a living fluorescence imager (Lb985, Berthold, Germany).
Statistical analysis
The significance of the differences between the means was analyzed using Duncan’s test with DPS 7.05 software48. The mean values marked with * and ** indicate significant differences at the 5% and 1% levels, respectively.
Supplementary information
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2019YFD1000200), the National Natural Science Foundation of China (31872069), the Shenyang Young and Middle-Aged Science and Technology Innovation Talents Support Plan (RC190446), the Liaoning Key R&D Program (2020JH2/10200032), the Liaoning Revitalization Talents Program (XLYC1902069) and the Liaoning BaiQianWan Talents Program (2016921067). We thank PlantScribe (www.plantscribe.com) for editing this manuscript.
Author contributions
H. Li and Z.Z. designed this project. H. Li, Z.Z., and X.D. wrote the manuscript. X.D. and Y.L. performed most of the experiments. Y.G. and S.W. extracted the RNA and performed qRT-PCR. H. L. and X.L. provided the plant materials. H. Li, Z.Z., and X.D. analyzed the data.
Conflict of interest
The authors declare no competing interests.
Contributor Information
He Li, Email: lihe@syau.edu.cn.
Zhihong Zhang, Email: zhangz@syau.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41438-021-00550-x.
References
- 1.Kinmonth-Schultz HA, et al. Cool night-time temperatures induce the expression of CONSTANS and FLOWERING LOCUS T to regulate flowering in Arabidopsis. New Phytol. 2016;211:208–224. doi: 10.1111/nph.13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin T, et al. VcRR2 regulates chilling-mediated flowering through expression of hormone genes in a transgenic blueberry mutant. Hortic. Res. 2019;6:96. doi: 10.1038/s41438-019-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berding N, Hurney AP. Flowering and lodging, physiological-based traits affecting cane and sugar yield: what do we know of their control mechanisms and how do we manage them? Field Crops Res. 2005;92:261–275. doi: 10.1016/j.fcr.2005.01.015. [DOI] [Google Scholar]
- 4.Shojaei E, Mirzaie-Asl A, Mahmoudi SB, Nazeri S. Identification of sugar beet flowering genes based on Arabidopsis homologous genes. J. Agr. Sci. Tech. 2017;19:719–729. [Google Scholar]
- 5.Ma ZB, Li W, Wang HP, Yu DQ. WRKY transcription factors WRKY12 and WRKY13 interact with SPL10 to modulate age-mediated flowering. J. Integr. Plant Biol. 2020;62:1659–1673. doi: 10.1111/jipb.12946. [DOI] [PubMed] [Google Scholar]
- 6.Teotia S, Tang G. To bloom or not to bloom, role of microRNAs in plant flowering. Mol. Plant. 2015;8:359–377. doi: 10.1016/j.molp.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Liu YX, et al. Over-expression of EjLFY1 leads to an early flowering habit in strawberry (Fragaria × ananassa) and its asexual progeny. Front. Plant Sci. 2017;8:496. doi: 10.3389/fpls.2017.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, et al. Alternate expression of CONSTANS-LIKE 4 in short days and CONSTANS in long days facilitates day-neutral response in Rosa chinensis. J. Exp. Bot. 2020;71:4057–4068. doi: 10.1093/jxb/eraa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanctot A, Nemhauser JL. It’s Morphin’ time: how multiple signals converge on ARF transcription factors to direct development. Curr. Opin. Plant Biol. 2020;57:1–7. doi: 10.1016/j.pbi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahlgren N, et al. Regulation of auxin response factor3 by TAS3 ta–siRNA affects developmental timing and patterning in Arabidopsis. Curr. Biol. 2006;16:939–944. doi: 10.1016/j.cub.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 11.Kalve S, et al. Osmotic stress inhibits leaf growth of Arabidopsis thaliana by enhancing ARF-mediated auxin responses. New Phytol. 2020;226:1766–1780. doi: 10.1111/nph.16490. [DOI] [PubMed] [Google Scholar]
- 12.Fan SH, et al. Molecular functional analysis of auxin/indole-3-acetic acid proteins (Aux/IAAs) in plant disease resistance in cassava. Physiol. Plant. 2020;168:88–97. doi: 10.1111/ppl.12970. [DOI] [PubMed] [Google Scholar]
- 13.John WC. Auxin response factors. Plant Cell Environ. 2016;39:1014–1028. doi: 10.1111/pce.12662. [DOI] [PubMed] [Google Scholar]
- 14.Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM. AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell. 2006;18:1873–1886. doi: 10.1105/tpc.105.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narise T, et al. Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Mol. Biol. 2010;72:533–544. doi: 10.1007/s11103-009-9589-4. [DOI] [PubMed] [Google Scholar]
- 16.Hardtke CS, et al. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL4. Development. 2004;131:1089–1100. doi: 10.1242/dev.00925. [DOI] [PubMed] [Google Scholar]
- 17.Gasciolli V, Mallory AC, Bartel DP, Vaucheret H. Partially redundant functions of Arabidopsis DICER-like enzymes and a role for DCL4 in producing trans-acting siRNAs. Curr. Biol. 2005;15:1494–1500. doi: 10.1016/j.cub.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Koskela EA, et al. Mutation in TERMINAL FLOWER1 reverses the photoperiodic requirement for flowering in the wild strawberry Fragaria vesca. Plant Physiol. 2012;159:1043–1054. doi: 10.1104/pp.112.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CJ, Yamagishi N, Kasajima I, Yoshikawa N. Virus-induced gene silencing and virus-induced flowering in strawberry (Fragaria × ananassa) using apple latent spherical virus vectors. Hortic. Res. 2019;6:18. doi: 10.1038/s41438-018-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao F, et al. Expression and functional analysis of FaPHO1;H9 gene of strawberry (Fragaria × ananassa) J. Integr. Agr. 2017;15:60345–60357. [Google Scholar]
- 21.Eikemo H, Brurberg MB. Resistance to Phytophthora cactorum in diploid Fragaria species. Hort. Science. 2010;45:193–197. [Google Scholar]
- 22.Slovin JP, Schmitt K, Folta KM. An inbred line of the diploid strawberry Fragaria vesca f. semperflorens for genomic and molecular genetic studies in the Rosaceae. Plant Methods. 2009;5:15. doi: 10.1186/1746-4811-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YP, Pi MT, Gao Q, Liu ZC, Kang CY. Updated annotation of the wild strawberry Fragaria vesca V4 genome. Hortic. Res. 2019;6:61. doi: 10.1038/s41438-019-0142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, et al. Tissue culture responsive MicroRNAs in strawberry. Plant Mol. Biol. Rep. 2012;30:1047–1054. doi: 10.1007/s11105-011-0406-2. [DOI] [Google Scholar]
- 25.Li H, Zhang ZH, Huang FF, Chang LL, Ma Y. MicroRNA expression profiles in conventional and micropropagated strawberry (Fragaria × ananassa Duch.) plants. Plant Cell Rep. 2009;28:891–902. doi: 10.1007/s00299-009-0693-3. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Dong XX, Mao WJ, Guan YH, Zhang ZH. An effective artificial microRNA vector based on Fv-miR166 precursor from strawberry. Sci. Hortic. 2019;256:108643. doi: 10.1016/j.scienta.2019.108643. [DOI] [Google Scholar]
- 27.Fait A, et al. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 2008;148:730–750. doi: 10.1104/pp.108.120691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard V, Ersdal-Badju E, Lu A, Bock SC. A rapid and efficient one-tube PCR-based mutagenesis technique using Pfu DNA polymerase. Nucleic Acids Res. 1994;22:2587–2591. doi: 10.1093/nar/22.13.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghelli R, et al. A newly identified flower-specific splice variant of AUXIN RESPONSE FACTOR8 regulates stamen elongation and endothecium lignification in Arabidopsis. Plant Cell. 2018;303:620–637. doi: 10.1105/tpc.17.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meland M, Frøynes O, Coop L, Kaiser C. Modeling of sweet cherry flowering based on temperature and phenology in a mesic Nordic climate. Acta Hortic. 2017;1162:19–22. doi: 10.17660/ActaHortic.2017.1162.4. [DOI] [Google Scholar]
- 31.Cardoso JC, Martinelli AP, Silva J. A novel approach for the selection of Cattleya hybrids for precocious and season-independent flowering. Euphytica. 2016;210:143–150. doi: 10.1007/s10681-016-1714-2. [DOI] [Google Scholar]
- 32.Tenreira T, et al. A specific gibberellin 20-oxidase dictates the flowering-runnering decision in diploid strawberry. Plant Cell. 2017;29:2168–2182. doi: 10.1105/tpc.16.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zahedi SM, Sarikhani H. The effect of end of day far-red light on regulating flowering of short-day strawberry (Fragaria × ananassa Duch. сv. Paros) in a long-day situation. Russ. J. Plant Physiol. 2017;64:83–90. doi: 10.1134/S1021443717010198. [DOI] [Google Scholar]
- 34.Hidaka K, Dan K, Imamura H, Takayama T. Crown-cooling treatment induces earlier flower bud differentiation of strawberry under high air temperatures. Environ. Control Biol. 2017;55:21–27. doi: 10.2525/ecb.55.21. [DOI] [Google Scholar]
- 35.Al-madhagi IAH, Hasan SMZ, bin Ahmad A, Zain AM, bin Yusoff WA. The influence of exogenous hormone on the flowering and fruiting of strawberry (Fragaria × ananassa Duch.) J. Biol. Agric. Health. 2012;2:46–53. [Google Scholar]
- 36.Li SB, et al. Genome-wide identification, isolation and expression analysis of auxin response factor (ARF) gene family in sweet orange (Citrus sinensis) Front. Plant Sci. 2015;6:119. doi: 10.3389/fpls.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen CJ, et al. Genome-wide identification and expression analysis of auxin response factor gene family in Medicago truncatula. Front. Plant Sci. 2015;6:73. doi: 10.3389/fpls.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu NN, et al. Genome-wide identification, molecular evolution, and expression analysis of auxin response factor (ARF) gene family in Brachypodium distachyon L. BMC Plant Biol. 2018;18:336. doi: 10.1186/s12870-018-1559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SX, et al. Genome-wide identification and expression analysis of auxin response factor (ARF) gene family in strawberry (Fragaria vesca) J. Integr. Agr. 2019;18:1587–1603. doi: 10.1016/S2095-3119(19)62556-6. [DOI] [Google Scholar]
- 40.Chen L, et al. Soybean AP1 homologs control flowering time and plant height. J. Integr. Plant Biol. 2020;62:1868–1879. doi: 10.1111/jipb.12988. [DOI] [PubMed] [Google Scholar]
- 41.Leseberg CH, et al. Interaction study of MADS-domain proteins in tomato. J. Exp. Bot. 2008;59:2253–2265. doi: 10.1093/jxb/ern094. [DOI] [PubMed] [Google Scholar]
- 42.Danilevskaya ON, et al. Involvement of the MADS-box gene ZMM4 in floral induction and inflorescence development in maize. Plant Physiol. 2008;147:2054–2069. doi: 10.1104/pp.107.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cevik V, et al. A FRUITFULL-like gene is associated with genetic variation for fruit flesh firmness in apple (Malus domestica Borkh.) Tree Genet. Genomes. 2010;6:271–279. doi: 10.1007/s11295-009-0247-4. [DOI] [Google Scholar]
- 44.Chang LL, Zhang ZH, Yang H, Li H, Dai HY. Detection of strawberry RNA and DNA viruses by RT–PCR using total nucleic acid as a template. J. Phytopathol. 2007;155:431–436. doi: 10.1111/j.1439-0434.2007.01254.x. [DOI] [Google Scholar]
- 45.Li WJ, et al. FveRGA1, encoding a DELLA protein, negatively regulates runner production in Fragaria vesca. Planta. 2018;247:941–951. doi: 10.1007/s00425-017-2839-9. [DOI] [PubMed] [Google Scholar]
- 46.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real–time quantitative PCR and the 2–ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F, et al. MdWRKY100 encodes a group I WRKY transcription factor in Malus domestica that positively regulates resistance to Colletotrichum gloeosporioides infection. Plant Sci. 2019;286:68–77. doi: 10.1016/j.plantsci.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Chen KQ, et al. MdMYB46 could enhance salt and osmotic stress tolerance in apple by directly activating stress-responsive signals. Plant Biotechnol. J. 2019;17:2341–2355. doi: 10.1111/pbi.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li T, et al. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell. 2017;29:1316–1334. doi: 10.1105/tpc.17.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.