Abstract
Objective:
To examine the impact of autoimmune disease on the composite outcome of intensive care unit admission, intubation, or death, from COVID-19 in hospitalized patients.
Methods:
Retrospective cohort study of 186 patients hospitalized with COVID-19 between March 1st–April 15th, 2020 at NewYork-Presbyterian Hospital/Columbia University Irving Medical Center. The cohort included 62 patients with autoimmune disease and 124 age- and sex-matched controls. The primary outcome was a composite of intensive care unit admission, intubation, and death, with secondary outcome assessing time to in-hospital death. Baseline demographics, comorbidities, medications, vital signs, and laboratory values were collected. Conditional logistic regression and Cox proportional hazards regression were used to assess the association between autoimmune disease and clinical outcomes.
Results:
Patients with autoimmune disease were more likely to have at least one comorbidity (25.8% vs. 12.9%, p=0.03), take chronic immunosuppressive medications (66.1% vs. 4.0%, p<0.01), and have had a solid organ transplant (16.1% vs. 1.6%, p<0.01). There were no significant differences in intensive care unit admission (14.2% vs. 19.4%, p=0.44), intubation (14.2% vs. 17.7%, p=0.62) or death (17.5% vs. 14.5%, p=0.77). On multivariable analysis, patients with autoimmune disease were not at an increased risk for a composite outcome of intensive care unit admission, intubation, or death (adjOR 0.79, 95%CI 0.37-1.67). On Cox regression, autoimmune disease was not associated with in-hospital mortality (adjHR 0.73, 95%CI 0.33-1.63).
Conclusion:
Among patients hospitalized with COVID-19, individuals with autoimmune disease did not have an increased risk of a composite outcome of intensive care unit admission, intubation, or death.
Keywords: SARS-CoV-2, COVID-19, autoimmune disease, immunosuppression
Introduction:
In January 2020, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, was isolated and identified as the novel pathogen causing the coronavirus disease 2019, COVID-19.1 In the following months, SARS-CoV-2 spread rapidly to over 200 countries, and has spanned the clinical spectrum of disease from asymptomatic infection to respiratory failure and death.2-5 Although several cohort studies have presented characteristics and outcomes for patients with COVID-19,2, 5-10 few studies have focused upon the impact in those hospitalized who had autoimmune (AI) disease.11-17
It has been suggested that a hyperinflammatory response to COVID-19 (cytokine storm) may lead to a more severe disease course.18 Accordingly, a retrospective study of 150 COVID-19 cases from Wuhan China found that increased levels of inflammatory markers such as ferritin and IL-6 were associated with an increase in overall mortality.3 As patients with autoimmune disease have underlying immune dysregulation, it is important to examine how COVID-19 affects their risk of adverse outcomes, including those on chronic immunosuppression.
In a recent matched cohort study by D’Silva et al., the authors examined adverse outcomes among COVID-19 patients with AI disease and found similar rates of hospitalization and mortality, but an increased risk of respiratory failure among patients with AI disease.11 However, only a small number of patients with AI disease (23 patients) were hospitalized, which limits evaluation of these outcomes. As such, we aimed to analyze a large cohort of hospitalized AI patients with COVID-19 in order to further assess differences in hospital presentation and clinically relevant outcomes such as intubation, intensive care unit (ICU) admission, and death.
Methods:
Matched Cohort Study Design
We conducted a retrospective cohort study examining patients, both cases and controls, ≥ 18 years-old who presented to NewYork-Presbyterian Hospital/Columbia University Irving Medical Center from March 1st 2020-April 15th 2020. Only patients found to be SARS-CoV-2 positive on reverse transcriptase polymerase chain reaction (RT-PCR) were included for analysis. Autoimmune disease, including immune-mediated inflammatory disease (IMID) such as inflammatory bowel disease and spondyloarthritis, was initially identified based upon International Classification of Diseases from all hospitalized COVID-19 patients during this time period, with records then reviewed manually for confirmation. Patients were then classified based on the presence or absence of AI disease (all included AI diseases captured in Supplemental Table S1).
As part of our matched cohort study, each patient with AI disease was binned with all non-AI age- and sex- matched controls. Two controls were chosen for each AI patient using a random number generator (matched 2:1, control:AI). All data elements were then manually retrieved from the electronic health record to ensure accuracy.
Primary Outcome
The primary outcome was a composite of adverse events comprising of death, intubation, or admission to an ICU. The secondary outcome was time to in-hospital death.
Co-variables & Exposures of Interest
Demographics collected included age, sex, race, and ethnicity. Additional exposure factors such as body mass index (BMI), smoking status, and prior solid organ transplant were ascertained. Comorbidities relevant to COVID-19 (hypertension, chronic obstructive pulmonary disease, asthma, interstitial lung disease, diabetes mellitus, chronic kidney disease, coronary artery disease, heart failure, or active malignancy)2,5,19 were recorded, and then subsequently categorized based on the number of underlying comorbid conditions (0, 1, ≥2 conditions). Home medications were manually reviewed and were only classified as home medications if used for at least one month and continued prior to admission; medications that were initiated for suspected/confirmed COVID-19 were not included as home medications. Presenting symptoms, vital signs, and lab values, as well as inpatient treatments, were also obtained from the electronic health record.
Statistical Approach
When comparing baseline characteristics and individual outcomes (intubation, ICU, death) between AI patients and controls, we used the Cochran-Mantel-Haenszel test and univariable conditional logistic regression for categorical and continuous variables respectively. To identify factors associated with our composite outcome (intubation/ICU/death), we used the chi-squared test or Fisher’s exact test if the expected count was < 5. Continuous variables were reported as medians and interquartile ranges, and compared using the Wilcoxon rank-sum test. We then performed multivariable conditional logistic regression to evaluate factors independently associated with our composite outcome, with the model including all variables with a p-value ≤0.2 on univariable analysis. AI disease was included in the multivariable model a priori.
Kaplan-Meier analysis was performed when assessing the outcome of death alone, with log-rank test used to measures differences. Multivariable Cox proportional hazards models were also used to characterize time to death, with adjustment of all included variables. All p-values are two-sided with an α level of 0.05, and statistical analysis was performed with Stata 15.1 (StataCorp., College Station, Texas, USA). The Institutional Review Board at Columbia University Medical Center approved this study (AAAS9860).
Results:
Baseline Characteristics
We identified 62 patients with autoimmune disorders and COVID-19 admitted to the hospital between March 1st and April 15th (Supplemental Figure S1) Of the autoimmune disorders included (Supplemental Table S1), rheumatoid arthritis was the most represented with 16 (25.8%) patients admitted, followed by sarcoidosis with 8 (12.9%).
Among the 186 patients included in the analysis, there were no significant differences in BMI or smoking status between patients with AI disease and their non-AI age- and sex- matched controls (Table 1). Patients with AI disease were more likely to report white race/ethnicity versus patients without autoimmune disease who were more likely to report being Hispanic or black (p=0.01). Patients with AI disease were more likely to have at least one comorbidity, and were more likely to have hypertension (75.8% vs. 60.5%, p=0.03). Among the other comorbidities included, there were no significant differences between the prevalence of underlying respiratory diseases, diabetes mellitus, chronic kidney disease, cardiac disease, or active malignancy.
Table 1.
Univariable analysis of demographic information (age-sex matched) stratified by autoimmune disease in 186 hospitalized patients testing positive for SARS-CoV-2.
| No Autoimmune Disease (n=124) |
Autoimmune Disease (n=62) |
p-value | |
|---|---|---|---|
| N [%] | N [%] | ||
| Age in years | |||
| 18-30 | 6 (4.8%) | 3 (4.8%) | - |
| 1-44 | 6 (4.8%) | 3 (4.8%) | |
| 45-59 | 32 (25.8%) | 16 (25.8%) | |
| 60-74 | 54 (43.6%) | 27 (43.6%) | |
| ≥75 | 26 (21.0%) | 13 (21.0%) | |
| Sex | |||
| Male | 48 (38.7%) | 24 (38.7%) | - |
| Female | 76 (61.3%)> | 38 (61.3%) | |
| Race/Ethnicity | |||
| White | 26 (21.0%) | 24 (38.7%) | 0.01 |
| Hispanic | 61 (49.2%) | 17 (27.4%) | |
| Black | 14 (11.3%) | 5 (8.1%) | |
| Other/Not Listed | 23 (18.6%) | 16 (25.8%) | |
| BMI | |||
| <18.5 | 6 (4.8%) | 1 (1.6%) | 0.29 |
| 18.5-24.9 | 18 (4.5%) | 10 (16.1%) | |
| ≥ 25–29.9 | 36 (29.0% | 25 (40.3%) | |
| 30–39.9 | 38 (30.7%) | 18 (29.0%) | |
| ≥40 | 26 (21.0%) | 8 (12.9%) | |
| Comorbidities | |||
| None | 32 (25.8%) | 8 (12.9%) | 0.03 |
| HTN | 75 (60.5%) | 47 (75.8%) | 0.03 |
| COPD/Asthma/ILD | 20 (16.1%) | 14 (22.6%) | 0.28 |
| DM2 | 38 (30.7%) | 14 (22.6%) | 0.26 |
| CKD | 14 (11.3%) | 11 (17.7%) | 0.23 |
| CAD/HF | 25 (20.2%) | 15 (24.2%) | 0.54 |
| Active Cancer | 6 (4.8%) | 4 (6.5%) | 0.66 |
| Smoking | |||
| Never | 103 (83.1%) | 49 (79.0%) | 0.46 |
| Current | 4 (3.5%) | 1 (1.7%) | |
| Former | 17 (15.0%) | 12 (20.0%) | |
| Solid Organ Transplant | |||
| No | 122 (98.4%) | 52 (83.9%) | <0.01 |
| Yes | 2 (1.6%) | 10 (16.1%) | |
| Home Medications | |||
| Immunosuppression | 5 (4.0%) | 41 (66.1%) | <0.01 |
| Long-term Hydroxychloroquine | 0 (0.0%) | 7 (11.3%) | <0.01 |
| ACEi/ARB | 33 (26.6%) | 21 (33.9%) | 0.31 |
| Statin | 41 (33.1%) | 28 (45.2%) | 0.07 |
| NSAID | 34 (27.4%) | 21 (33.9%) | 0.30 |
| Inpatient Medications | |||
| Hydroxychloroquine | 61 (49.2%) | 37 (59.7%) | 0.17 |
| Azithromycin | 43 (34.7%) | 22 (35.5%) | 0.92 |
| Tocilizumab | 5 (4.0%) | 2 (3.2%) | 0.79 |
| Corticosteroids | 13 (10.5%) | 17 (27.4%) | <0.01 |
| Remdesivir | 2 (1.6%) | 0 (0.0%) | - |
| Symptoms at Admission | |||
| Constitutional | 96 (89.7%) | 46 (86.8%) | 0.50 |
| Neurologic | 29 (27.1%) | 9 (17.0%) | 0.09 |
| Pulmonary | 90 (84.1%) | 43 (81.1%) | 0.55 |
| GI | 51 (47.7%) | 29 (54.7%) | 0.66 |
| Initial Vital Signs | |||
| Tmax in 24 hours | |||
| <38°C | 47 (37.9%) | 30 (48.4%) | 0.16 |
| ≥38°C | 77 (62.1%) | 32 (51.6%) | |
| Lowest BP in 24 hours | |||
| <100 systolic | 33 (26.6%) | 16 (25.8%) | 0.90 |
| 100-130 systolic | 80 (64.5%) | 37 (59.7%) | |
| >130 systolic | 11 (8.9%) | 9 (14.5%) | |
| Admission HR | |||
| <100 | 73 (58.9%) | 28 (45.2%) | 0.07 |
| ≥100 | 51 (41.1%) | 34 (54.8%) | |
| Admission RR | |||
| ≤20 | 96 (77.4%) | 41 (66.1%) | 0.12 |
| >20 | 28 (22.6%) | 21 (33.9%) | |
| O2 support on ER presentation | |||
| Room Air | 105 (84.7%) | 48 (77.4%) | 0.21 |
| Nasal Cannula | 11 (8.9%) | 10 (16.1%) | |
| Non-Rebreabreakther | 7 (5.7%) | 3 (4.8%) | |
| Intubation | 1 (0.8%) | 1 (1.6%) | |
| Initial Lab Values | |||
| White Blood Cell [/uL] | 7.0 (5.2-9.3) | 7.3 (5.5-10.4) | 0.18 |
| Lymphocyte Count [%] | 15.9 (8.7-22.0) | 13.2 (8.7-22.1) | 0.32 |
| ESR [mm/hr] | 74 (44.0-97.0) | 62 (48.5-100) | 0.94 |
| CRP [mg/L] | 99.1 (50.5-181.5) | 93.8 (43.9-197.1) | 0.81 |
| Procal [ng/mL] | 0.2 (0.1-.5) | 0.2 (0.12-0.52) | 0.41 |
| IL-6 [pg/mL] | 26.8 (5.0-96.9) | 16.0 (5.0-68.9) | 0.17 |
| Ferritin [ng/mL] | 646.5 (234.7-1039.0) | 526.8 (233.3-807.5) | 0.14 |
| D-dimer [ug/mL] | 1.2 (0.8-1.9) | 1.3 (0.8-2.9) | 0.17 |
| LDH [U/L] | 398 (285.0-556.0) | 395 (257.0-516.0) | 0.30 |
| CK [U/L] | 117.0 (71.0-300.0) | 119.5 (70.0-294.0) | 0.36 |
| LOS | |||
| Median LOS in days | 5.0 (1.0-10.0) | 7.0 (3.0-12.0) | 0.26 |
| Intubation | |||
| No | 103 (85.8%) | 51 (82.3%) | 0.62 |
| Yes | 17 (14.2%) | 11 (17.7%) | |
| ICU Admission | |||
| No | 103 (85.8%) | 50 (80.6%) | 0.44 |
| Yes | 17 (14.2%) | 12 (19.4%) | |
| Death | |||
| No | 94 (82.5%) | 53 (85.5%) | 0.77 |
| Yes | 20 (17.5%) | 9 (14.5%) |
Abbreviations: Body Mass Index (BMI), Hypertension (HTN), Chronic Obstructive Pulmonary Disease (COPD), Interstitial Lung Disease (ILD), Type 2 Diabetes Mellitus (DM2), Chronic Kidney Disease (CKD), Coronary Artery Disease (CAD), Heart Failure (HF), Angiotensin-Converting Enzyme Inhibitor (ACEi), Angiotensin II Receptor Blocker (ARB), Nonsteroidal Anti-inflammatory Drug (NSAID), Gastrointestinal (GI), Maximum Temperature (Tmax), Blood Pressure (BP), Heart Rate (HR), Respiratory Rate (RR), Emergency Room (ER), Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), Procalcitonin (Procal), Interleukin-6 (IL-6), Lactate Dehydrogenase (LDH), Creatine Kinase (CK), Length Of Stay (LOS), Intensive Care Unit (ICU)
When comparing transplantation history between the two groups, AI patients had a significantly higher proportion of patients with a history of solid organ transplant compared to controls (16.1% vs. 1.6%, p<0.01), with the majority of transplant patients (75%) continuing home immunosuppressive medications as inpatients. As expected, a higher proportion of individuals with AI disease compared to controls were on chronic immunosuppression prior to admission (66.1% vs 4.0%, p<0.01), with a complete list of the medications noted in Supplemental Table S2. For those that were on chronic corticosteroids, the majority (73.3%) were on low dose corticosteroids (budesonide, <10mg prednisone). Of note, patients with AI disease were also more likely to be on long-term hydroxychloroquine therapy than their matched controls (11.3% vs. 0.0%, p<0.01).
When assessing symptoms present on admission (list included in Supplemental Table S3), there did not appear to be any significant differences in constitutional, neurologic, pulmonary or gastrointestinal symptoms between the two populations. Additionally, there was no statistical difference in clinical presentation between cases and controls, as initial vital signs and lab values were similar (Table 1). However, during their hospital course, a greater proportion of patients with AI disease were given corticosteroids as compared to controls (27.4% vs. 10.5%, p<0.01). The majority (76.5%) of AI patients receiving inpatient corticosteroids were continuing their home dose, in comparison to the non-AI group in which the majority (92.3%) were newly initiating corticosteroids for COVID-19. There were no significant differences in any other administered inpatient treatments including hydroxychloroquine, azithromycin, or tocilizumab.
Median length of stay was not noted to be significantly longer in the AI group as compared to the control group (7 vs. 5 days, p=0.26), even when assessing only the 141 patients alive and discharged during the study period (5 vs. 4 days, p=0.22). A total of 29 deaths out of 186 patients were noted (15.6%), with similar outcomes between the AI group and the controls; no differences in intubation rates (17.74% vs. 14.2%; p=0.62), ICU admissions (19.4% vs. 14.2%, p=0.44), or rates of death (14.5% vs. 17.5%; p=0.77). Of note, only one patient was admitted to the ICU but not intubated, and all patients placed on hospice care during their admission died within the hospital.
Univariable and Multivariable Analysis of Composite Outcome (Intubation/ICU/Death)
On univariable analysis, the presence of autoimmune disease among patients hospitalized with COVID-19 was not associated with our composite outcome of intubation, ICU, or death (p=0.63, see Table 2). Similarly, we found that patients on long-term immunosuppression, including biologics, were also not at increased risk for adverse outcomes (25.0% vs. 23.9%, p=0.88). Predictors of adverse outcomes included older age (p<0.01) and the presence of hypertension (p=0.04) or cardiovascular disease (p=0.04). Conversely, having no comorbidities was associated with a lower risk of an adverse event (p=0.04). Of note, inpatient treatment with hydroxychloroquine (p=0.02) or new initiation of a corticosteroid (p=0.04) was associated with adverse outcomes.
Table 2.
Univariable analysis of adverse outcome (intubation/ICU/death) in all admitted SARS-CoV-2 positive patients.
| No Adverse Outcome | Adverse Outcome | p-value | |
|---|---|---|---|
| (n=140) | (n=46) | ||
| N [%] | N [%] | ||
| Age in years | |||
| 18-30 | 9 (6.4%) | 0 (0.0%) | <0.01 |
| 31-44 | 8 (5.7%) | 1 (2.2%) | |
| 45-59 | 41 (29.3%) | 7 (15.2%) | |
| 60-74 | 62 (44.3%) | 19 (41.3%) | |
| ≥75 | 20 (14.3%) | 19 (41.3%) | |
| Sex | |||
| Male | 53 (37.9%) | 19 (41.3%) | 0.68 |
| Female | 87 (62.1%) | 27 (58.7%) | |
| Race/Ethnicity | |||
| White | 35 (25.0%) | 15 (32.6%) | 0.33 |
| Hispanic | 64 (45.7%) | 14 (30.4%) | |
| Black | 13 (9.3%) | 6 (13.0%) | |
| Other/Not Listed | 28 (20.0%) | 11 (23.9%) | |
| BMI | |||
| <18.5 | 4 (2.9%) | 3 (6.5%) | 0.28 |
| 18.5-24.9 | 18 (12.9%) | 10 (21.7%) | |
| ≥ 25–29.9 | 49 (35.0%) | 12 (26.1%) | |
| 30–39.9 | 45 (32.1%) | 11 (23.9%) | |
| ≥40 | 24 (17.1%) | 10 (21.7%) | |
| Autoimmune Disease | |||
| No | 92 (65.7%) | 32 (69.6%) | 0.63 |
| Yes | 48 (34.3%) | 14 (30.4%) | |
| Comorbidities | |||
| None | 35 (25.0%) | 5 (10.9%) | 0.04 |
| HTN | 86 (61.4%) | 36 (78.3%) | 0.04 |
| COPD/Asthma/ILD | 27 (19.3%) | 7 (15.2%) | 0.54 |
| DM2 | 37 (26.4%) | 15 (32.6%) | 0.42 |
| CKD | 19 (13.6%) | 6 (13.0%) | 0.93 |
| CAD/HF | 25 (17.9%) | 15 (32.6%) | 0.04 |
| Active Cancer | 8 (5.7%) | 2 (4.4%) | 0.72 |
| Smoking | |||
| Never | 119 (85.0%) | 33 (71.7%) | 0.09 |
| Current | 3 (2.3%) | 2 (4.8%) | |
| Former | 18 (13.7%) | 11 (26.2%) | |
| Solid Organ Transplant | |||
| No | 130 (92.9%) | 44 (95.7%) | 0.50 |
| Yes | 10 (7.1%) | 2 (4.4%) | |
| Home Medications | |||
| Immunosuppression | 35 (25.0%) | 11 (23.9%) | 0.88 |
| -Biologic | 10 (7.1%) | 1 (2.2%) | 0.22 |
| -Corticosteroids | 21 (15.0%) | 9 (19.6%) | 0.45 |
| Long-term Hydroxychloroquine | 6 (4.3%) | 1 (2.2%) | 0.45 |
| ACEi/ARB | 38 (27.1%) | 16 (34.8%) | 0.32 |
| Statin | 51 (36.4%) | 18 (39.1%) | 0.74 |
| NSAID | 40 (28.6%) | 15 (32.6%) | 0.60 |
| Inpatient Medications | |||
| Hydroxychloroquine | 67 (47.9%) | 31 (67.4%) | 0.02 |
| Azithromycin | 46 (32.9%) | 19 (41.3%) | 0.30 |
| Tocilizumab | 3 (2.1%) | 4 (8.7%) | 0.06 |
| Corticosteroids* | 6 (4.3%) | 6 (13.0%) | 0.04 |
| Remdesivir | 0 (0.0%) | 2 (4.4%) | 0.06 |
| Symptoms at Admission | |||
| Constitutional | 115 (89.8%) | 27 (84.4%) | 0.38 |
| Neurologic | 34 (26.6%) | 4 (12.5%) | 0.07 |
| Pulmonary | 108 (84.4%) | 25 (78.1%) | 0.40 |
| GI | 68 (53.1%) | 12 (37.5%) | 0.11 |
| Initial Vital Signs | |||
| Tmax in 24 hours | |||
| <38°C | 55 (39.3%) | 22 (47.8%) | 0.31 |
| ≥38°C | 85 (60.7%) | 24 (52.2%) | |
| Lowest BP in 24 horns | |||
| <100 systolic | 29 (20.7%) | 20 (43.5%) | <0.01 |
| 100-130 systolic | 94 (67.1%) | 23 (50.0%) | |
| >130 systolic | 17 (12.1%) | 3 (6.5%) | |
| Admission HR | |||
| <100 | 77 (55.0%) | 24 (52.2%) | 0.74 |
| ≥100 | 63 (45.0%) | 22 (47.8%) | |
| Admission RR | |||
| ≤20 | 113 (80.7%) | 24 (52.2%) | <0.01 |
| >20 | 27 (19.3%) | 22 (47.8%) | |
| O2 support on ER presentation | |||
| Room Air | 127 (90.7%) | 26 (56.5%) | <0.01 |
| Nasal Cannula | 12 (8.6%) | 9 (19.6%) | |
| Non-Rebreather | 1 (0.7%) | 9 (19.6%) | |
| Intubation | 0 (0.0%) | 2 (4.4%) | |
| Initial Lab Values | |||
| White Blood Cell [/uL] | 6.7 (5.1-9.1) | 8.4 (5.8-13.9) | <0.01 |
| Lymphocyte Count [%] | 16.6 (10.0-23.1) | 10.8 (7.0-18.6) | <0.01 |
| ESR [mm/hr] | 65.0 (43.0-96.5) | 30.7 (55.5-102.5) | 0.06 |
| CRP [mg/L] | 88.9 (41.7-173.3) | 152.7 (92.4-222.2) | <0.01 |
| Procal [ng/mL] | 0.14 (0.1-0.3) | 0.48 (0.2-1.2) | <0.01 |
| IL-6 [pg/mL] | 15.0 (5.0-41.0) | 75.2 (16.0-164.7) | <0.01 |
| Ferritin [ng/mL] | 589.0 (217.3-972.0) | 625.8 (374.3-1117.0) | 0.31 |
| D-dimer [ug/mL] | 1.06 (0.8-1.7) | 1.88 (0.9-3.2) | <0.01 |
| LDH [U/L] | 373.5 (274.0-514.0) | 458.0 (333.0-661.0) | 0.02 |
| CK [U/L] | 110.5 (62.0-285.0) | 146.0 (77.0-403.0) | 0.17 |
Abbreviations: Body Mass Index (BMI), Hypertension (HTN), Chronic Obstructive Pulmonary Disease (COPD), Interstitial Lung Disease (ILD), Type 2 Diabetes Mellitus (DM2), Chronic Kidney Disease (CKD), Coronary Artery Disease (CAD), Heart Failure (HF), Angiotensin-Converting Enzyme Inhibitor (ACEi), Angiotensin II Receptor Blocker (Arbs), Nonsteroidal Anti-inflammatory Drug (NSAID), Gastrointestinal (GI), Maximum Temperature (Tmax), Blood Pressure (BP), Heart Rate (HR), Respiratory Rate (RR), Emergency Room (ER), Erythrocyte Sedimentation Rate (ESR), C-Reactive Protein (CRP), Procalcitonin (Procal), Interleukin-6 (IL-6), Lactate Dehydrogenase (LDH), Creatine Kinase (CK), Intensive Care Unit (ICU)
Excluding patients continued on home corticosteroid dosing
Patients with a higher incidence of adverse outcomes also had higher median white blood cell count, procalcitonin level, and pro-inflammatory laboratory profiles including higher C-reactive protein, interleukin-6 levels, and D-dimer, but were noted to have a lower lymphocyte count on admission (p<0.01 for all mentioned lab values, Table 2).
On multivariable analysis using conditional logistic regression, the presence of autoimmune disease was not a significant risk factor for adverse outcomes among hospitalized COVID-19 patients, when adjusting for smoking and comorbidities, as compared to age- and sex- matched controls (adjOR 0.79, 95% CI 0.37-1.67, p=0.53, Table 3). On sensitivity analysis, when including BMI and race/ethnicity in our model, we similarly found that AI disease was not associated with adverse outcomes (adjOR 0.67, 95% CI 0.25-1.80, p=0.43).
Table 3.
Conditional logistic regression of adverse outcome (intubation/ICU/death) in all patients admitted with COVID-19.
| Adjusted Odds Ratio (95% CI)* | |
|---|---|
| Autoimmune Disease | |
| No | (Ref.) |
| Yes | 0.79 (0.37-1.67) |
| Comorbidities | |
| None | (Ref.) |
| 1 | 1.44 (0.37-5.62) |
| ≥2 | 1.74 (0.51-6.00) |
| Smoking | |
| Never | (Ref.) |
| Current | 3.06 (0.22-43.61) |
| Former | 5.21 (0.87-31.40) |
Adjusted for all variables in the table
Cox Proportional Hazards Assessing Time to Death
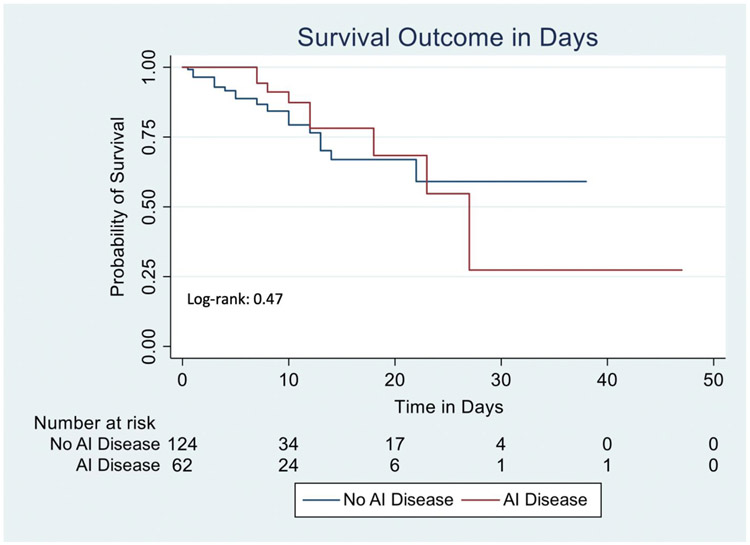
On Kaplan-Meier analysis, although there was increased time to death among AI patients, these results were not statistically significant (Log-rank = 0.47, Figure 1), with a hazard ratio of 0.73 (95% CI 0.33-1.63, p=0.45 Table 4) when adjusting for smoking and comorbidities. On sensitivity analysis, when including BMI and race/ethnicity in our model, we found similar results (adjHR 0.45, 95% CI 0.18-1.13, p=0.09). Additionally, when only including the 170 patients discharged or deceased, we observed similar findings (Log-rank = 0.08, Supplemental Figure S2).
Figure 1: Kaplan-Meier curve examining death, stratified by the presence or absence of autoimmune disease in all 186 patients, with 16 patients censored as of 4/29/2020.
AI: Autoimmune disease
Table 4.
Cox proportional hazards examining time to death among the 186 included patients.
| Adjusted Hazard Ratio (95% CI)* | |
|---|---|
| Autoimmune Disease | |
| No | (Ref.) |
| Yes | 0.73 (0.33-1.63) |
| Comorbidities | |
| None | (Ref.) |
| 1 | 1.08 (0.31-3.75) |
| ≥2 | 0.93 (0.31-2.84) |
| Smoking | |
| Never | (Ref.) |
| Current | 1.40 (0.18-10.6) |
| Former | 1.86 (0.83-4.19) |
Adjusted for all variables in the table
Discussion:
In this study, we found that patients with autoimmune disease hospitalized with COVID- 19 had a similar constellation of presenting symptoms, vital signs, and lab values, and had similar lengths of stay as compared to matched controls. Furthermore, autoimmune disease did not increase the risk of adverse outcomes and was not associated with an increased hazard of death when adjusting for relevant clinical variables.
In an initial study by Haberman et al., 86 COVID-19 positive (59 confirmed and 27 suspected) patients with immune-mediated inflammatory diseases were found to have a similar incidence of hospitalization as compared to the general New York City population, though no comparator arm was used to make inferences specific to AI disease and only 14 patients were hospitalized.20 More recently, a study by D’Silva et al. built upon this by assessing 52 patients with rheumatic diseases and compared them to 104 matched controls, though only a small number were hospitalized (23 patients) which limited the assessment of in-hospital outcomes.11
In the present study, we specifically looked to assess the outcomes of hospitalized COVID-19 patients with and without AI disease. As such, we analyzed 62 hospitalized AI disease COVID positive cases, and compared them to 124 hospitalized non-AI COVID positive age- and sex- matched controls (all RT-PCR confirmed). On baseline characteristics, we found that individuals with autoimmune disease tended to have more comorbidities than their matched controls, particularly hypertension and a history of solid organ transplant. Furthermore, a high proportion of AI patients were taking chronic immunosuppressive medications (66.1%). Although immunosuppression is currently being used as a potential therapy for COVID-19,21 data looking at outcomes among patients on chronic immunosuppression remains sparse. In a recent study of 90 solid organ transplant patients on chronic immunosuppression, Pereira et al. found that transplant recipients who developed COVID-19 overall had a severe disease course.22 However, in a small study by Cavagna et al., they found that calcineurin inhibitors were associated with a mild course of disease.23 Within our study, we also reassuringly found that long-term immunosuppression, including in AI patients, was not significantly associated with adverse outcomes. When long-term immunosuppression was stratified by high and low dose immunosuppression, there was also no difference in adverse outcomes.24 Additionally, we found that outpatient corticosteroid use was not associated with adverse outcomes. This is in line with prior studies showing that among patients with COVID-19 ill enough to become hospitalized, home corticosteroid use may no longer be a risk factor for severe outcomes.25 When only examining patients on biologics, similar results were seen. This is in accordance with recently published data from international registries of patients with rheumatic and inflammatory bowel diseases, which found that biologics were not associated with increased severity of disease.26
On admission, individuals with autoimmune disorders had similar symptoms, vital signs, and lab values, including those suggestive of cytokine storm, as compared to their matched controls, which is analogous to findings observed by D’Silva et al.11 Patients with AI disease however, were more likely to receive corticosteroids during their hospital course, which was largely driven by the continuation of their home corticosteroid regimens. Of note, we did find that inpatient initiation of corticosteroids or hydroxychloroquine was associated with adverse outcomes, though this is likely a marker of disease severity (higher acuity patients were more likely to receive these medications).
When assessing disease outcomes, recent reports have been conflicting, with some studies noting a higher risk of respiratory failure among rheumatic disease patients hospitalized with COVID-19.11,14,27 In our study, which represents one of the largest inpatient samples of autoimmune disease patients to date, we observed no differences in the risk of intubation, ICU admission, or death between AI patients and matched controls. Furthermore, after adjusting for smoking and comorbidities, as well clinically relevant variables of race/ethnicity and BMI,8,11,28-32 we found that patients with autoimmune disease were not at higher risk for the composite outcome of intubation, ICU admission, or death as compared to controls.
Although some studies have suggested a higher risk of respiratory failure in patients with autoimmune disease, most have not observed an increase in overall mortality.11,14 In accordance with this, when assessing time to death, we found that AI patients did not have an increased hazard ratio. Additionally, we observed no significant difference in length of stay between patients with autoimmune disease and controls, which has been similarly reported in these prior studies. As such, our findings provide reassurance that hospitalized COVID-19 patients with AI disorders, including those on immunosuppressive therapy, do not appear to be at significantly higher risk for adverse clinical outcomes, as compared to hospitalized non-AI COVID-19 controls. In addition, we did not observe significant differences in clinical presentation, suggesting that hospitalized AI patients with COVID-19 may not be at increased risk for cytokine storm. While this may be a result of chronic immunosuppressive therapy, further investigation is needed.
This study has limitations. Given our available sample size, we had sufficient power to detect an increased risk of adverse outcomes with an odds ratio of 1.72 or greater. It is possible that a more modest risk could be present, though our findings suggest a non-significant trend towards a lower overall risk. Although we included a broad range of autoimmune disorders, we were unable to perform stratified analyses by each disorder due to the limited number of AI patients hospitalized with COVID-19. As such, the heterogeneity of these disorders may mask differential risks between them. Nonetheless, given the rarity of these disorders in the hospitalized COVID-19 population, and the critical need for data to help guide clinical practice in the context of this pandemic, we believe these analyses provide valuable insights into the management of individuals with autoimmune conditions diagnosed with COVID-19. Additionally, as many patients did not have race/ethnicity listed in the electronic health record, we were unable to accurately match on this variable. On univariable analysis however, race/ethnicity did not appear to be an independent predictor of our primary outcome. Finally, this is a single center study, which may limit the generalizability of our results.
This is one of the largest inpatient cohorts to date that examines the association of AI disorders with clinical outcomes of COVID-19. A particular strength is the use of a matched cohort design for the execution of this study. This age- and sex- matched controlled design reduces bias, given the important roles that age and sex play in the development of many autoimmune disorders, as well as the outcomes related to COVID-19. Thus, by ensuring an equal distribution of these variables among both groups, we were able to mitigate their potential effects as confounders. In addition, we included a secondary analysis which allowed us to further evaluate the association between AI disease and time to death. Finally, all data for this analysis were manually extracted and reviewed for accuracy (including the distinction between outpatient hydroxychloroquine initiation for COVID-19 vs. long-term use as a treatment agent for underlying autoimmune disorders), which helps ensure validity of this large dataset. Multivariable analysis was then performed in order to adjust for factors associated with our composite outcome.
In conclusion, we found that among patients hospitalized with COVID-19, individuals with autoimmune disease and those on chronic immunosuppressive therapy did not have an increased risk of adverse events, such as ICU admission, intubation, and death. Additional research is needed to better characterize the management of individuals with autoimmune conditions in the context of COVID-19, particularly with regard to the use of additional immunosuppression in the inpatient setting.
Supplementary Material
Acknowledgements
The authors would like to thank the many employees of NewYork-Presbyterian Hospital/Columbia University Medical Center for their thoughtful care of these patients, as well as the patients themselves for their trust and participation in their care.
Funding: This work was supported by the National Institutes of Health [T32 DK083256 to [ASF], K23AR075112 to [EJB]] and The Louis and Gloria Flanzer Philanthropic Trust to [BL].
Abbreviations:
- AI
Autoimmune
- BMI
Body Mass Index
- COVID-19
Coronavirus Disease 2019
- IMID
Immune-Mediated Inflammatory Disease
- ICU
Intensive Care Unit
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
Footnotes
Conflicts of Interest: None
Writing Assistance: None
References
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of V. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis (Berl). 2020;7:91–6. [DOI] [PubMed] [Google Scholar]
- 10.Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, Colombo S, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Silva KM, Serling-Boyd N, Wallwork R, Hsu T, Fu X, Gravallese EM, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis. 2020;79:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianfrancesco MA, Hyrich KL, Gossec L, Strangfeld A, Carmona L, Mateus EF, et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye C, Cai S, Shen G, Guan H, Zhou L, Hu Y, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis. 2020;79:1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberman R, Axelrad J, Chen A, Castillo R, Yan D, Izmirly P, et al. Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med. 2020;383:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freites Nunez DD, Leon L, Mucientes A, Rodriguez-Rodriguez L, Font Urgelles J, Madrid Garcia A, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020. August 7 (E-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 17.Pablos JL, Galindo M, Carmona L, Lledo A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020. August 12 (E-pub ahead of print). [DOI] [PubMed] [Google Scholar]
- 18.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberman R, Axelrad J, Chen A, Castillo R, Yan D, Izmirly P, et al. Covid-19 in Immune-Mediated Inflammatory Diseases - Case Series from New York. N Engl J Med. 2020;383:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in Solid Organ Transplant Recipients: Initial Report from the US Epicenter. Am J Transplant. 2020;20:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavagna L, Seminari E, Zanframundo G, Gregorini M, Di Matteo A, Rampino T, et al. Calcineurin Inhibitor-Based Immunosuppression and COVID-19: Results from a Multidisciplinary Cohort of Patients in Northern Italy. Microorganisms. 2020;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pileggi GS, Da Mota LMH, Kakehasi AM, De Souza AW, Rocha A, de Melo AKG, et al. Brazilian recommendations on the safety and effectiveness of the yellow fever vaccination in patients with chronic immune-mediated inflammatory diseases. Adv Rheumatol. 2019;59:17. [DOI] [PubMed] [Google Scholar]
- 25.Pablos JL, Galindo M, Carmona L, Lledo A, Retuerto M, Blanco R, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;0:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481–491.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Lago I, Ramirez de la Piscina P, Elorza A, Merino O, Ortiz de Zarate J, Cabriada JL. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain). Gastroenterology. 2020;159:781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71:896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raifman MA, Raifman JR. Disparities in the Population at Risk of Severe Illness From COVID-19 by Race/Ethnicity and Income. Am J Prev Med. 2020;59:137–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laurencin CT, McClinton A. The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities. J Racial Ethn Health Disparities. 2020;7:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakkar DN, Dunphy DJ, Raza DM. Ethnicity profiles of COVID-19 admissions and outcomes. J Infect. 2020; 81:e110–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.