Abstract
Metabolic syndrome is a condition associated with obesity, diabetes, dyslipidemia, and high blood pressure. Recently, the use of phytochemicals is suggested in the control and treatment of metabolic syndrome. The Azadirachta indica (neem) is an evergreen tree belonging to the family of Meliaceae. Multiple studies have been confirmed the anti-diabetic and anti-hypertension, anti-hyperlipidemia, and anti-obesity effects of neem. In this review, we reported the protective effects of neem against the complications of metabolic syndrome with a special focus on mechanisms that are involved. It has been shown that neem can control hyperglycemia and hypertension through over-expression of transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2) and anti-oxidant effects. Neem also reduced the glucose uptake through up-regulation of glucose transporter 4 (GLUT4) and inhibition of key intestinal enzymes such as glucosidases. Moreover, neem showed anti-hypertensive effects possibility via the block of calcium channels, up-regulation of endothelial nitric oxide synthase (eNOS), and extracellular signal-regulated kinases 1/2 (ERK1/2) signaling pathway. Anti-oxidant effects play an important role in protective mechanisms of neem against metabolic syndrome and its complications.
Key Words: Azadirachta indica, Diabetes, Hyperlipidemia, Hypertension, Metabolic syndrome, Neem, Obesity
Introduction
Metabolic syndrome (MetS) is a common metabolic disorder that is described for more than multiple decades. The MetS is also known as insulin resistance syndrome and syndrome X (1). Physical inactivity, smoking, increasing age, obesity, and positive family history are risk factors associated with its development (2). Epidemiologic data have been suggested that the prevalence of MetS among the population over 60 years is the highest, and it is increasing among children and adolescents (3). People with MetS have a higher risk of type 2 diabetes and cardiovascular disease (CVD) (4, 5). Also, hypertension and an increase in triglyceride (TG)/high-density lipoprotein (HDL) cholesterol ratio are important components of the MetS and are one of the most risk factors for CVD (6, 7).
Moreover, several studies have been shown other side effects of MetS such as fatty liver disease (8), cirrhosis (9), and polycystic ovary syndrome (10). The pathogenesis of MetS has not been clearly defined, but insulin resistance, oxidative stress, and chronic inflammation are key pathogenic factors of it. Insulin resistance has a role in the development of diabetes mellitus. It has been reported that oxidative stress accelerates the development of complications of MetS. The activation of the inflammatory pathway leads to insulin resistance and diabetes (11, 12).
The first-line treatment of MetS is lifestyle modification on diet, weight, and physical activity. Second-line therapy for patients with MetS is drug therapy (13). In line with the treatment of MetS, the use of herbs has been regarded. Medicinal plants contain bioactive compounds with various metabolic effects. In several studies have been reported the protective potential of plants and herbs against MetS such as Capsicum annuum L. (14), Crataegus pinnatifida (15), and green tea (16). Taken together, the management of complications of MetS is the aim of the treatment in these patients, and medicinal plants can play an important role in its treatment.
Neem (Azadirachta indica) is an evergreen tree of southeastern Asia that is widely distributed in the Indian subcontinent. The height of this tree is approximately 15-20 m and sometimes even up to 35-40 m. The word A. indica was derived from the Persian language. The Azad means “free,” and the dirakht is meaning “tree,” and “I” refer to “Indian origin.” (17). Neem is a common name, and also it’s known with the name of Nimbay, Veppai, Ariyaveppu, Vepa in India (18). More than 300 compounds are derived from different parts of neem, such as leaves, flower, seed, fruit, bark, and root. Non-isoprenoids and isoprenoids metabolites are two major groups of these compounds. Some active constituents of neem include nimbidin, sodium nimbidate, nimbin, nimbolide, gallic acid, azadirachtin, and polysaccharides (19). Nimbidin, as a major constituent extracted from neem seeds, demonstrated several biological activities such as anti-inflammatory, anti-pyretic, anti-diabetic, anti-fungal, and anti-ulcer activities. The spermicidal activity of nimbin has been reported in humans. Nimbolide has been shown to exert anti-malaria and antibacterial effects (20). Several studies have been reported the different pharmacologic effects of neem, including hypolipidemic (21), hepatoprotective (22), antimicrobial, anticancer, and anti-diabetes (23) properties. In line with these properties, the US National Academy of Science (NAS) has stated the neem tree as a tree that is solving global problems (24). On the other hand, neem oil has shown vomiting, diarrhea, acidosis, drowsiness, and encephalopathy in human studies. Also, mild to severe changes in the liver, intestine, spleen, kidney, and heart of chick and genotoxicity and anti-fertility in mice and rats by neem leaves and seeds have been reported. Neem leaf extract also decreased sperm count and sperm motility, probably due to androgen deficiency. Nimbolide has induced the kidney, small intestine, liver dysfunction, and blood pressure drop suddenly in animals (20). This review focuses on the effects of neem in treatment of diabetes, high blood pressure, dyslipidemia, and obesity.
Methodology
The databases of PubMed, Scopus, and Google Scholar have been involved in this review. Articles have been collected from the date of inception up to January 2020. The search keywords included metabolic syndrome, hypertension, blood pressure, hypotensive, antihypertensive, dyslipidemia, hyperlipidemia, high cholesterol, high triglyceride, hypercholesterolemia, hypertriglyceridemia, atherogenic, atherosclerosis, obesity, overweight, appetite, anti-obesity, weight loss, diabetes, hyperglycemia, insulin, hypoglycemic, antihyperglycemic, antidiabetic, blood glucose, neem, and Azadirachta indica.
Effects of neem on metabolic syndrome
Effects of neem on high blood pressure
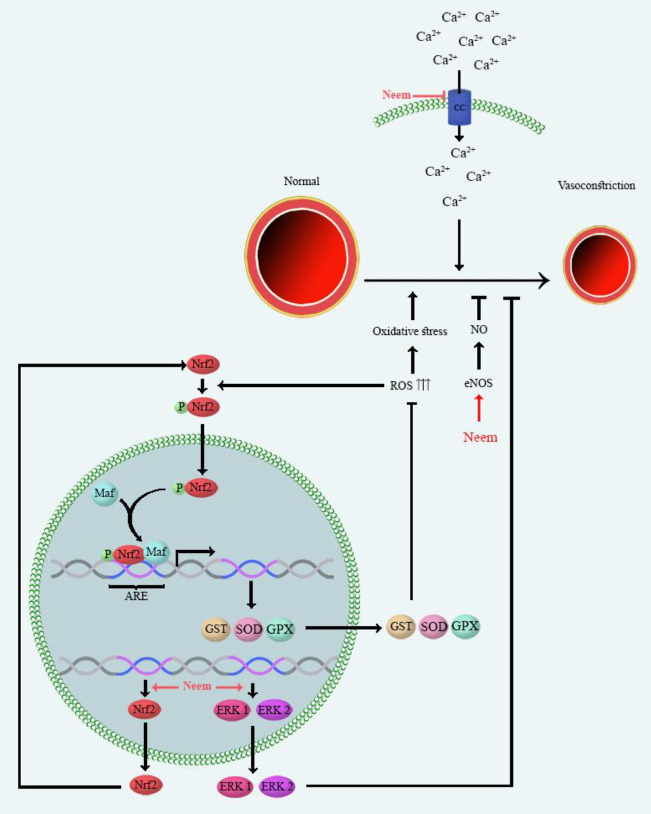
One of the main constituents of MetS is high blood pressure (BP). Effects of several plants investigated on BP such as Aloe vera (25) and Capsicum annuum L. (14). High BP has an increased risk of heart and blood vessel diseases. Multiple mechanisms induce high BP, including: (1) Calcium channels initiate vascular smooth muscle contraction through the release of calcium, which is mediated by Ca2+ influx via L-type and voltage-gated calcium channels. Calcium channels have an important role in the induction of high BP (26); (2) the extracellular signal-regulated kinase (ERK 1 and 2) are from the mitogen-activated protein kinases (MAPK) family. ERK1 and ERK2 play an essential role in the regulation of vascular smooth muscle contraction. Down-regulation of ERK 1 and 2 genes reduces both vascular smooth muscle cell growth and vasoconstriction. Therefore, ERK 1and 2 are a target for the induction of high BP (27); (3) Nitric oxide (NO) is a vasodilator produced by nitric oxide synthase (NOS) enzymes. The NOS isozymes and NO level are candidates for involvement in high BP (28); (4) Nuclear factor erythroid 2–related factor 2 (Nrf2) as a transcription factor involves transcriptional induction of several anti-oxidant genes. Nrf2 regulates signaling pathway functions to reduce reactive oxygen radicals (ROS) production. The down-regulation of Nrf2 expressions induces ROS production and resulting in high BP. The contraction of smooth muscle and depletion of NO are the mechanisms of ROS-induced high BP (29). Several studies have been reported the beneficial effects of different extracts (aqueous, alcoholic) of neem leaves against high BP which have been categorized in Table 1. The mechanisms underlying the protective effect of neem against high BP have been presented in Figure 1. The mechanisms are including the block of calcium channels (30), up-regulation of ERK 1 and 2 (31) and Nrf2 gene expression, reduction of oxidative stress markers, and elevation of the nitric oxide (NO) levels (32). It has been reported that neem exerted the vasodilatation effects possibility through the block of calcium channel in the isolated aorta of rat and rabbit. Also, it has been shown that neem exerted dose-dependent fall in arterial pressure of isolated guinea-pig atrial (30). The down-regulation of ERK1 and 2 have been reported in cardiac and renal tissues of rats treated by sodium fluoride (600 ppm in drinking water). Neem protected hypertensive rats through (100 and 200 mg/kg, p.o.) up-regulation of ERK (31). L-NAME (N ω -Nitro-L-Arginine Methyl Ester) is a NOS inhibitor and reduces NO bioavailability. Polyphenol-rich fraction of neem (100 and 200 mg/kg) restored NO level in rats were treated with L-NAME (orally, 40 mg/kg) (32). The methanol extract of neem (orally, 100 and 200 mg/kg for 7 days) increased NO level in serum of rats exposed to sodium fluoride (NaF) (600 ppm in drinking water) (31). The crude (0.3-3 mg/ml), aqueous (1-5 mg/ml) and ethyl acetate (0.1-1 mg/kg) extracts of neem induced endothelium-dependent vasorelaxation in isolated rat aorta (30).
Table 1.
Effects of neem against high blood pressure
| Part (s) of the plant used/ Extract(s) | Neem dose/ route | Study design | Results | Ref |
|---|---|---|---|---|
| Leaves/ methanolic | 100 and 200 (mg/kg), p.o. | Male rats, L-NAM (40 mg/kg), p.o. |
↓ BP ↑ NO level ↓Oxidative stress ↑Expressions of Nrf2 |
(32, 115) |
| Leaves/ methanolic | 100 and 200(mg/kg), p.o. | Male rats, NaF 600 (ppm) | ↓ BP ↓Oxidative stress ↑ Expressions of ERK |
(31, 116) |
| Crude/ aqueous | 1, 3, 10 and 30 (mg/kg), p.o. | Male and female rats, arterial cannula | ↓ BP Blockade Ca++ channel ↑ NO |
(30) |
| Crude/ aqueous and ethylacetate | 0.01- 10 mg/ml, p.o. | Rabbit, isolated rabbit aorta | ↓ BP Blockade Ca++ channel ↑ NO |
(30) |
| Crude/ aqueous and ethylacetate | 0.001- 10 mg/ml, p.o. | Rats, isolated rat aorta | ↓ BP Blockade Ca++ channel ↑ NO |
(30) |
| Crude/ aqueous and ethylacetate | 0.01- 10 mg/ml, p.o. | Guinea pig, isolated guinea pig atrial | ↓ BP Blockade Ca++ channel ↑ NO |
(30) |
| Leaves/ aqueous | 20 (mg/kg), p.o. | Male rats, DOCA-salt 15 (mg/kg), s.c. | ↓ MAP ↓ Alterations of ECG |
(33) |
| Leaves/ alcoholic | 100, 300 and 1000, (mg/kg), i.v. | Male rats, atropine (1mg/kg) and mepyramine (3 mg/kg), i.v. | ↓ BP | (117) |
| Leaves | 5, 10, 20, 40, 80, 100, and 200, (mg/kg), i.v. | Rabbit and guinea pig, ouabain-induced cardiac dysrhythmias | ↓ BP | (118) |
| Leaves/ aqueous | 2 g, p.o. | Male patients (40-60 years) |
↓ BP | (34) |
↑: increase; ↓: decrease; BP: blood pressure; DOCA: deoxycorticosterone acetate; ECG: electrocardiogram; ERK: extracellular signal-regulated kinase; g: gram; i.v.: intravenous; kg: kilogram; L-NAM: N ω -nitro-L-arginine methyl ester; MAP: mitogen-activated protein; mg: milligram; NO: nitric oxide; Nrf2: Nuclear factor erythroid 2–related factor 2; p.o.: per os (orally); ppm: parts per million; s.c.: subcutaneous
Figure 1.
Main mechanisms of neem on high blood pressure. Mechanisms underlying the protective effect of neem against high BP are including the block of calcium channels, up-regulation of ERK 1/2 and Nrf2 gene expression, and normalize serum of NO bioavailability. ARE: Anti-oxidant response element; eNOS: endothelial nitric oxide synthase; ERKs: extracellular-regulated kinases; GPX: glutathione peroxidase; GST: glutathione S-transferases, Nrf2: nuclear factor erythroid-2-related factor 2; NO: nitric oxide; ROS: reactive oxygen species; SOD: superoxide dismutase
Neem restored anti-oxidant enzyme activity, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione S-transferase (GSTs) in animal models of high BP. Neem also improved glutathione (GSH) and reduced malondialdehyde (MDA) and protein carbonyl (markers of oxidative stress) levels (31, 32). The aqueous extract of neem (20 mg/kg) reduced mean arterial pressure in rats which were treated by DOCA-salt (15 mg/kg, s.c.) and drinking water containing 1.0% NaCl and 0.03% KCl (33).
A study has been reported on the effect of neem leaves on high BP of the 90 diabetic patients aged 40-60, which were kept under observation for a month. During the study, patients received 2 g powder of neem daily for three months. A significant reduction was observed in the BP of treated patients (34).
Effects of neem on hyperlipidemia
One of the most components of MetS is hyperlipidemia. Many medicinal plants showed positive effects on hyperlipidemia such as barberry (Berberis vulgaris) (35) and rosemary (Rosmarinus officinalis) (36). Plasma lipid levels elevate in people with diabetes and obesity (37). Hyperlipidemia contributes to impair endothelial function, development of atherosclerosis, and coronary heart disease (CHD) through the enhancement of oxidative stress (38). Anti-oxidant defense system (SOD and GPx) protects plasma lipoproteins against oxidative stress (39). The elevation of ROS generation under stress conditions (diabetes and obesity) causes oxidative damage of lipoproteins in the plasma (40, 41). The oxidation of lipoproteins increases TG, very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL) concentrations in plasma (40). The measure of serum TG, total cholesterol (TC), LDL, HDL, and cholesterol is the reference procedure for the determination of lipid profile (42). As mentioned in Table 2 several studies have been shown the effects of neem in the management of hyperlipidemia.
Table 2.
Effects of neem against hyperlipidemia
| Part (s) of the plant used/ Extract(s) | Neem dose/ route | Study design | Results | Ref |
|---|---|---|---|---|
| Leaves/ aqueous | 250 mg/kg, p.o. | Male and female rats, STZ (60 mg/kg), i.p. |
↓ LDL, TG, and cholesterol ↑ HDL |
(45) |
| Leaves/ aqueous | 400 mg/kg, p.o. | Male rats, STZ (35 mg/kg), i.p. |
Normalized lipid profile | (47) |
| Leaves/ aqueous | 500 mg/kg, p.o. | Rats, STZ (45 mg/kg), i.p. |
↓ Cholesterol, TG | (48) |
| Leaves/ chloroform | 300 mg/kg, p.o. | Male rats, STZ (60 mg/kg), i.p. |
↓ LPO, ↑SOD, CAT activity, ↑ GSH levels, ↓ GSSG levels | (46) |
| Leaves/ alcoholic | 200 mg/kg, p.o. | Male rats, STZ (50 mg/kg), i.v. |
↓TC, ↓ TG, LDL and VLDL | (44) |
| Seeds/ petroleum ether | 0.09 and 2 mg/kg, p.o. | Male rats, STZ (55 mg/kg), i.v. |
↓TC, TG | (119) |
| Allopolyherbal | 500 mg/kg, p.o. | Male and female rats, STZ (60 mg/kg), i.p. |
↓ TC, TG, LDL, VLDL, serum creatinine, SGOT, and SGPT ↑ HDL |
(107) |
| Glucova Active | _ | Rats, STZ (35 and 50 mg/kg), i.p. |
↓ Serum cholesterol, TG, LDL, VLDL ↑ HDL |
(43) |
| Dihar | 10%, p.o. | Male rats, STZ (45 mg/kg), i.v. |
↓ Cholesterol, TG, LDL, Creatinine, Urea and LPO ↑ HDL, SOD and CAT |
(108) |
| Leaves/ chloroform | 20 mg/kg, p.o. | Male mice, STZ (60 mg/kg), i.v. |
↓ TG, TC, ↑ HDL ↓ LPO |
(50) |
| Dianex | 7.5 mg/kg, p.o. | Male and female mice, STZ (60 mg/kg), i.p. |
↓ TG, cholesterol, urea and cratininine | (49) |
| Leaves/ methanolic | 500 mg/kg, p.o. | Male and female rats, alloxan (100 mg/kg), i.p. |
↑ HDL ↓ LDL and TG |
(53) |
| Leaves/ ethanolic | 100 and 250 mg/kg, p.o. | Male rats, alloxan (120 mg/kg), i.p. |
↓ TC, TG, HDL, LDL, VLDL | (52) |
| Leaves/ ethanolic | 100 mg/kg, p.o. | Male rats, alloxan (120 mg/kg), i.p. |
↓ Serum cholesterol, TG, LDL, creatinine, and urea ↑ HDL |
(89) |
| Karnim Plus | 200 and 400 mg/kg, p.o. | Rats, alloxan (120 mg/kg), i.p. |
↓ Serum cholesterol, TG, creatinine, and urea | (51) |
| Ethanolic (leaves) | 50 and 300 mg/kg, p.o. | Male rats, cholesterol |
↓TC, LDL and TG | (55) |
| Aqueous (leaves) | 250, 500 and 1000 mg/kg, p.o. | Male rats, isoprenaline (25 mg/kg), s.c. |
↓TC and TG ↑ HDL |
(54) |
↑: increase; ↓: decrease; CAT: catalase; GSH: glutathione; GSSG: glutathione disulfide; HDL: high-density lipoprotein; i.p.: intraperitoneal; i.v.: intravenous, kg: kilogram; LDL: low-density lipoprotein; LPO: lipid peroxidation; mg: milligram; p.o.: per os (orally); SGOT: serum glutamic oxaloacetic transaminase; SGPT: serum glutamic pyruvic transaminase, SOD: superoxide dismutase; STZ: streptozotocin; TC: total cholesterol; TG: triglyceride; VLDL: very-low-density lipoprotein
Different doses of neem (100, 200, 250, 300, 400, and 500 mg/kg) in streptozotocin (STZ)-diabetic rats decreased serum TC, TG, LDL, VLDL levels and increased serum HDL levels (43-48). Also, two different doses of neem (7.5 and 20 mg/kg) in STZ-diabetic mice normalized lipid profile (49, 50). Neem (200, 250, 400, and 500 mg/kg, p.o.) reduced TC, TG, HDL, LDL, VLDL in rats which were treated with alloxan (120 mg/kg, i.p.) (51-53). Also, neem attenuated hyperglycemia, and hyperlipidemia via induction of SOD, catalase (CAT) levels in diabetic rats (46). The aqueous leaf extract of neem (250, 500, and 1000 mg/kg, p.o.) decreased TC and TG levels and increased HDL levels in rats which were treated with isoprenaline (54). The leaf extract of neem (50 and 300 mg/kg/day orally) prevented the rise of TC, LDL, and TG in cholesterol-fed rats (55). The mechanisms which are important in the effects of neem against hyperlipidemia have been shown in Figure 2.
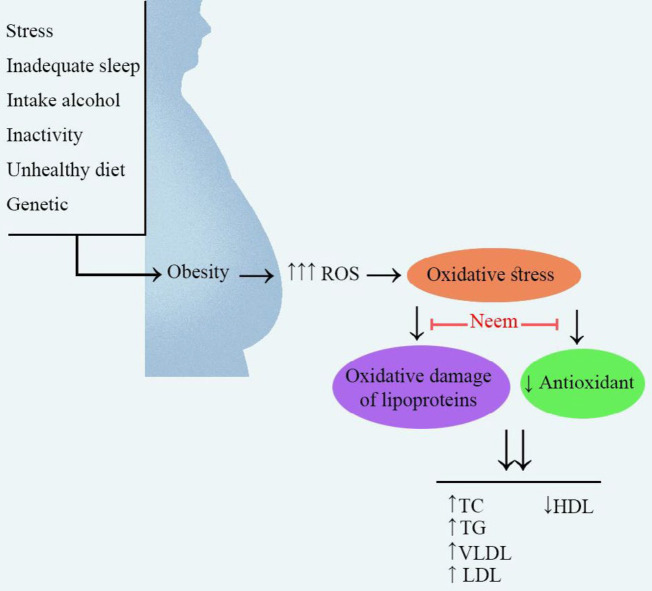
Figure 2.
Main mechanisms of neem on hyperlipidemia. Neem has been shown protective effects against hyperlipidemia via improvement of the function of anti-oxidant markers and inhibition of oxidative damage of lipoproteins
HDL: high-density lipoprotein; LDL: low-density lipoprotein; ROS: reactive oxygen species; TC: total cholesterol; TG: triglyceride; VLDL: very-low-density lipoprotein
Effects of neem on obesity
Obesity and overweight are a serious health problem that is increasing worldwide. Obesity is associated with a life expectancy decrease and a significant increase in mortality (56). Stress, inadequate sleep, intake of alcohol, inactivity, unhealthy diet, age, genetic are some of the risk factors for obesity Figure 2 (57). Diabetes, heart disease, high blood pressure, hyperlipidemia, and atherosclerosis are obesity-related complications (58). Lipase and α-glucosidase are two types of obesity agents that use of their inhibitors can be the ideal therapy for obesity control (59, 60).
The aqueous and methanolic extract of stem bark and roots of neem (520 µg/ml) inhibited pancreatic lipase and α- glucosidase in an in vitro system (59). But, the leaf extract of neem as a medicinal plant (500 mg/kg, orally) is not decreased the body weight in rats were treated for 28 days (60). There are a few studies available on the protective effects of neem against obesity which has been included in Table 3. Therefore, the effects of neem on obesity cannot be appropriately explained, and further studies are needed.
Table 3.
Effects of neem against obesity
| Part (s) of the plant used/ Extract(s) | Neem dose/ route | Study design | Results | Ref |
|---|---|---|---|---|
| Leaves/ aqueous | 500 mg/kg, p.o. | Male and female rats | No effect | (60) |
| Stem-bark and roots/ aqueous and methanolic | IC50: 520 µg/ml | In vitro | Inhibited pancreatic lipase and α- glucosidase | (59) |
IC50: inhibitory concentration; kg: kilogram; µg: microgram; mg: milligram; ml: milliliter; p.o.: per os (orally)
Effects of neem on diabetes
Diabetes, as a growing public health problem, is characterized by impairment of systemic insulin secretion, reduction of insulin action, and resulting in hyperglycemia (61). Diabetes-associated main complications are nerve damage (62), myocardial infarction (63), atherosclerosis (64), renal failure (65), blindness (66), and limb amputation (67). The microvascular disease has been known as the foremost cause of these complications (68). Glucose-mediated vascular damage occurs as a result of the overproduction of ROS and oxidative stress (69). Enzymatic and nonenzymatic anti-oxidants are defense mechanisms against oxidative stress. Common enzymatic anti-oxidants include SOD, CAT, GPx, and glutaredoxin (GRx). Vitamins A, C, E, and glutathione are common nonenzymatic anti-oxidants (70). Pancreatic beta cells are more susceptible to oxidative stress than other cells because they have relatively low levels of anti-oxidants (71). Therefore, the reduction of ROS and induction of anti-oxidant activity are therapeutic approaches to decrease hyperglycemia and diabetes (72). On the other hand, salivary α-amylase and intestinal glucosidases play an essential role in the digestion of starch to produce glucose in the small intestine (73). Also, the inhibition of these enzymes could be effective in the control of diabetes (74). Several reports have been shown that medicinal plants useful for the management and treatment of diabetes. Some of these plants include Vernonia amygdalina (75), Nigella sativa L. (76), grapes (Vitis vinifera) (77), and Allium sativum (garlic) (78) which are useful in the remedy of diabetes. The use of the neem is most popular to control diabetes in different regions of the world, such as India (79), Pakistan (80), Bengal (81), Indonesia (82), and Northwest Nigeria (83). Glucagon-like peptide-1 (GLP-1) is a hormone that plays an essential role in the release of insulin and is inactivated by dipeptidyl peptidase IV (DPP-IV) (84, 85). Inhibition of DPP-IV (a peptidase) is a method for diabetes treatment (86). In this method, substrate Gly-Pro-p-Nitroanilide (GPPN) was cleaved to paranitroanilide (a yellow-colored product) by DPP-IV and the absorbance was measured at 380 nm. Inhibitory activity of neem leaves (35 μl with varying concentrations) was determined on DPP-IV activity via this method, and neem exhibited a weak inhibitory activity (17%) on DPP-IV (82).
• Effects of neem in diabetic human
Neem is available as a dietary supplement in an herbal mixture in North America. Treatment with this dietary supplement (2 capsules 3 times per day) for 3 months in type 2 diabetic patients (the ages of 18 and 70) improved glucose control and HbA1c levels (87). The study of Kochhar has been investigated the antidiabetic effect of neem in 90 diabetic men 40 to 60 years of age. Subjects received 2 g of neem leaf powder daily for three months. The results of this study showed that neem reduces sweating, headache, burning feet, itching, polydipsia, and polyphagia in diabetic humans (34).
• Effects of neem in alloxan/streptozotocin-induced diabetic animals
•• Effects of neem in alloxan-induced diabetic rats
Alloxan is a toxic glucose analog that accumulates in pancreatic beta cells via glucose transporter 2 (GLUT2) and inhibits its function. The intraperitoneal injection of alloxan (at doses of 100, 120, and 150 mg/kg) is a conventional method for the induction of diabetes in rat models (88). The oral administration of ethanolic extract of neem in different doses (100 to 800 mg/kg for 14 or 28 days) reduced blood glucose levels in rats which were treated with alloxan (52, 89, 90). The combination of neem (50 mg/kg) with Gynura procumbens ethanolic (112.5 mg/kg) extracts (2 times a day for 15 days) increased insulin expression, decreased blood glucose concentration, and improved the morphology of the islets of Langerhans and beta-cells in rats (91). The aqueous extract of neem leaf and bark was effective in reducing oxidative stress markers and lipid peroxidation of the blood sample, liver, and kidney tissues in diabetic rats (92, 93). Polyherbal formulation (PHF) is containing more than one herb that is used all around the world to treat diseases (94). PHFs used in the treatment of diabetes are including Karnim Plus and DIA7. The antidiabetic activity of Karnim Plus and DIA7 is investigated in rats treated with alloxan. Karnim Plus and DIA7 contain neem extract and decrease blood glucose levels in diabetic rats (51, 95). In Table 4, different studies on the effect of neem on diabetes have been summarized.
Table 4.
Effects of neem against diabetes
| Part (s) of the plant used/ Extract (s) | Neem dose/ route | Study design | Results | Ref |
|---|---|---|---|---|
| Bark root/ ethanolic | 200, 400, 800 mg/kg, p.o. | Rats, alloxan (100 mg/kg), i.p. | ↓BG | (90) |
| Leaves and seeds/ ethanolic | 500 mg/kg, p.o. | Males rats, alloxan (120 mg/kg), i.p. | ↓BG | (120) |
| Leaves/ ethanolic | 200 mg/kg, p.o. | Rats, alloxan (150 mg/kg), i.p. | ↓BG | (121) |
| Leaves/ ethanolic | 100 and 250 mg/kg, p.o. | Males rats, alloxan (120 mg/kg), i.p. | ↓BG | (52) |
| Leaves/ ethanolic | 250 mg/kg, p.o. | Males rats, alloxan (100 mg/kg), i.p. | ↓BG | (122) |
| Leaves/ ethanolic | 100 mg/kg, p.o. | Males rats, alloxan (120 mg/kg), i.p. | ↓BG | (89) |
| Leaves/ ethanolic | 200 mg/kg, p.o. | Males rats, alloxan (150 mg/kg), i.p. | ↓BG, ↑Insulin Protected beta cells and islets Langerhans |
(91) |
| Leaves and bark/ aqueous | 100 and 500 mg/kg, p.o. | Rats, alloxan (150 mg/kg), s.c. | ↓BG ↓ Oxidative stress markers and LPO and DNA fragmentation and PKC beta II |
(92) |
| Leaves and bark/ aqueous | 100 and 500 mg/kg, p.o. | Rats, alloxan | ↓BG ↓ Oxidative stress markers and LPO |
(93) |
| Leaves and bark/ aqueous | 75 mg/kg, p.o. | Rats, alloxan (150 mg/kg), i.p. | ↓BG | (123) |
| Leaves/ aqueous | 25, 50 and 100 mg/kg, p.o. | Rats, alloxan (150 mg/kg), i.p. | ↓BG ↓ Oxidative stress markers and LPO |
(124) |
| Leaves/ aqueous | 400 mg/kg, p.o. | Males and females rats, alloxan (150 mg/kg), i.p. | ↓BG Improved liver function |
(125) |
| Seeds/ aqueous | 500 mg/kg, p.o. | Males and females rats, alloxan (150 mg/kg), i.p. | ↓BG | (126) |
| Leaves/ polyherbal | 200 and 400 mg/kg, p.o. | Rats, alloxan (120 mg/kg), i.p. | ↓BG | (51) |
| Leaves/ polyherbal | 14.28% | Rats, alloxan (150 mg/kg), i.p. | ↓BG | (95) |
| Leaves/ ethanolic | 200 g, p.o. | Rabbits, alloxan (150 mg/kg), i.v. | ↓BG | (96) |
| Leaves/ aqueous | 500 ml/kg, p.o. | Males and female rabbits, alloxan | ↓BG | (97) |
| Seeds/ aqueous | 5 mg/kg, p.o. | Males and females rabbits, alloxan | ↓BG | (97) |
| Leaves/ chloroform | 200 mg/kg, p.o. | Males rats, STZ (50 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(104) |
| Leaves/ chloroform | 300 mg/kg, p.o. | Males rats, STZ (65 mg/kg), i.v. | ↓BG, ↑ Insulin ↑ Antioxidant markers ↓ LPO |
(46) |
| Leaves/ aqueous | 600 mg/kg, p.o. | Males rats, STZ (60 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers protected beta cells and islets langerhans ↑ Pain threshold |
(99) |
| Leaves/ aqueous | 600 mg/kg, p.o. | Males rats, STZ (60 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers protected beta cells and islets langerhans |
(105) |
| Leaves/ aqueous | 400 mg/kg, p.o. | Males rat, STZ (35 mg/kg), i.p. | ↓BG, ↑ Insulin Normalized GluT4 |
(47) |
| Leaves/ aqueous | 500 mg/kg, p.o. | Rats, STZ (45 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers protected beta cells and islets langerhans |
(48) |
| Leaves/ aqueous | 500 mg/kg, p.o. | Males and females rat, STZ (55 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(98) |
| Leaves/ aqueous | 250 mg/kg, p.o. | Males rat, STZ (60 mg/kg), i.p. | ↓BG | (45) |
| Leaves/ aqueous | 100 mg/kg, p.o. | Males rat, STZ (65 mg/kg), i.p. | ↓BG | (100) |
| Leaves/ aqueous | 10 ml/kg, p.o. | Males rat, STZ (65 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(127) |
| Leaves/ aqueous | 50, 100, 200 and 400 mg/kg, p.o. | Males and females rat, STZ (50 mg/kg), i.p. | ↓BG ↑ Antioxidant markers ↓ LPO |
(128) |
| Leaves/ ethanolic | 200 mg/kg, p.o. | Males rat, STZ (65 mg/kg), i.p. | ↓BG | (103) |
| Leaves/ ethanolic | 200 mg/kg, p.o. | Males rat, STZ (50 mg/kg), i.v. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(44) |
| Leaves/ ethanolic | 500 mg/kg, p.o. | Males and females rat, STZ (70 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers protected beta cells and islets langerhans |
(101) |
| Leaves/ ethanolic | 500 mg/kg, p.o. | Males rat, STZ (70 mg/kg), i.v. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(129) |
| Leaves/ ethanolic | 500 mg/kg, p.o. | Males rat, STZ (70 mg/kg), i.p. | ↓BG protected beta cells and islets langerhans |
(130) |
| Seeds / ethanolic | 1.2 ml, p.o. | Females rat, STZ (100 mg/kg), s.c. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(102) |
| Leaves/ ethanolic | 200 mg/kg, p.o. | Males rat, STZ (65 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(103) |
| Leaves/ ethanolic | 500 mg/kg, p.o. | Males and females rat, STZ (70 mg/kg), i.p. | ↓BG | (128) |
| Seeds/ petroleum ether | 0.9 and 2 mg/kg, p.o. | Males rat, STZ (55 mg/kg), i.p. | ↓BG, ↓ LPO ↑ Antioxidant markers |
(131) |
| Allopolyherbal | 500 mg/kg, p.o. | Males and females rat, STZ (60 mg/kg), i.p. | ↓BG, ↑ Insulin | (107) |
| Glucova Active | _ | Rat, STZ (35 and 50 mg/kg), i.p. | ↓BG, ↑ Insulin protected beta cells |
(43) |
| Dihar | 10%, p.o. | Males rat, STZ (45 mg/kg), i.v. | ↓BG, ↑ Insulin protected beta cells |
(108) |
| MAC-ST/001 | 20 g/100 g, p.o. | Males and females rat, STZ (55 mg/kg), i.p. | ↓BG, ↑ Insulin protected beta cells ↓ G6Pase |
(109) |
| Herbo-mineral | 25 mg/kg, p.o. | Males rat, STZ (60 mg/kg), i.p. | ↓BG, ↑ Insulin | (132) |
| _ | 100 g, p.o. | Females rat, STZ (65 mg/kg), i.p. | ↓BG, ↑ Insulin protected beta cells ↓ G6Pase |
(133) |
| Leaves/ aqueous | 100 μg/ 200 μL, p.o. | Mice, STZ (3 mg/25 g), i.p. | ↓BG, ↓ G6Pase | (110) |
| Seeds/ aqueous | 1mg/ml, p.o. | Females mice, STZ (100 mg/kg), s.c. | ↓BG | (102) |
| Leaves and seeds/ aqueous | 100, 200, 300 µl, p.o. | Males mice, STZ (55 mg/kg), i.p. | ↓BG ↑ Antioxidant markers |
(111) |
| Leaves/ chloroform | 20 and 30 mg/kg, p.o. | Males mice, STZ (60-120 mg/kg), i.p. | ↓BG, ↑ Insulin ↑ Antioxidant markers ↓ LPO, G6Pase, GK, α-amylase and α-glucosidase activity ↑ HK activity |
(50) |
| Dianex | 7.5 mg/kg, p.o. | Males and females mice, STZ (60 mg/kg), i.p. | ↓BG | (49) |
| Leaves/ aqueous | 200 mg/kg, p.o. | Males rabbit, STZ (50 mg/kg), i.p. | ↓BG, ↑Insulin | (113) |
| Leaves/ aqueous | 400 mg/kg, p.o. | Males and females rat, glucose (3 g/kg), p.o. | ↓BG | (112) |
| Leaves/ ethanolic | _ | Rat, glucose (3 mg/ml), p.o. | ↓BG | (113) |
| Rhizome / ethanolic | 300 mg/kg, p.o. | Males mice, glucose (1 g/kg), p.o. | ↓BG | (114) |
| Leaves/ aqueous | 10 mg/kg, p.o. | Males and females rat | ↓BG | (134) |
| Seeds, stems, flowers, and bark/ aqueous | 0.1, 0.092, 0.084, 0.071 and 0.05 g/ml, p.o. | Males rat | ↓BG | (135) |
| Stem bark/ ethanolic | 15, 30, 60, 120 and 240 (µg/ml), p.o. | Males rat | ↑ Antioxidant markers ↓ LPO |
(136) |
| Plant/ aqueous | 25–1000 µg/ml | INS-1 b-cells, glucose (5.6 mM) g/kg | ↑ Insulin release ↑ Glucose consumption |
(137) |
| Plant/ aqueous | 25–1000 µg/ml | 3T3-L1 adipocytes, glucose 5.6 mM) g/kg | ↑ Insulin release ↑ Glucose consumption |
(137) |
↑: increase; ↓: decrease; BG: blood glucose; GK: glucokinase; G6Pase: glucose-6-phosphatase; GluT4: glucose transporter 4; g: gram; HK: hexokinases; INS-1 b-cells: insulin-secreting cells; i.p.: intraperitoneal; I.V.: intravenous; kg: kilogram; LPO: lipid peroxidation; µg: microgram; µl: microliter; mg: milligram; ml:milliliter; PKC: protein kinase C; p.o.: per os (orally); s.c.: subcutaneous; STZ: streptozotocin
•• Effects of neem in alloxan-induced diabetic rabbits
The hypoglycemic effect of neem (ethanolic extract of leaves, 200 mg/kg) was observed in rabbits that were treated with alloxan (150 mg/kg, i.v.) (96). Also, leaf extract (500 mg/kg, p.o. daily for six weeks) and seed oil (5 mg/kg, p.o. daily for six weeks) of neem decreased blood glucose in diabetic rabbits (alloxan in a single dose, 140 mg/kg, i.v.) (97).
•• Effects of neem in streptozotocin-induced diabetic rats
Streptozotocin (STZ) is one of the most diabetogenic agents using in diabetes research. Its mechanisms for the induction of diabetes are inhibition of insulin secretion and the death of the beta-cells (88). In rat models, the injection of STZ (at doses of 35, 45, 55, 60, 65, 70, and 100 mg/kg) is a standard method for the induction of diabetes (47, 48, 98-102). The ethanolic extract of neem leaves (at doses of 200 and 500 mg/kg, p.o.) reduced blood glucose levels in rats were treated with STZ (44). Moreover, the oral administration of neem (leaf ethanolic extract) induced markers of the anti-oxidant system (SOD, CAT, GPx, and GSH levels) and reduced lipid peroxidation in diabetic rats (103) (Figure 3). The aqueous extract of neem leaves (at doses of 100, 200, 250, 400, 500 and 600 mg/kg, p.o.) decreased blood glucose levels and improved serum insulin levels in rats were treated with STZ (45, 47, 100, 101, 104, 105). Moreover, neem (400 mg/kg, p.o. for 30 days) increased insulin receptor protein expression in diabetic rats (STZ: 35 mg/kg, i.p.). It also up-regulated cytosolic and plasma membrane glucose transporter 4 (GLUT4) in the gastrocnemius muscle of diabetic rats (47). Insulin enhances glucose uptake into muscle tissues through GLUT4, and therefore it controls glucose homeostasis (106). Several studies investigated the effects of PHFs on diabetes in rats which were treated with STZ. Allopolyherbal (neem at the dose of 500 mg/kg) (107), Glucova Active (43), Dihar (10 % of neem) (108), MAC-ST/001 (20 g/100 g of neem) (109), and Herbo-mineral (25 mg of neem) (109) are PHFs that decrease blood glucose levels and increase insulin level in STZ-diabetic rat.
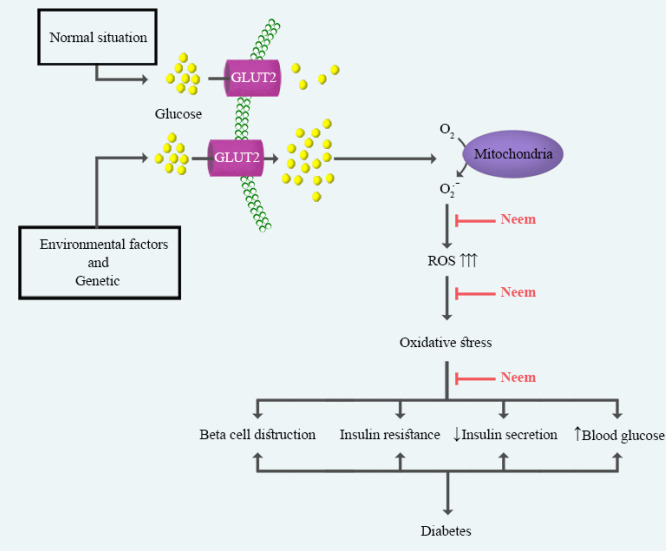
Figure 3.
Main mechanisms of neem on diabetes. Neem has been shown protective effects against diabetes via inhibition of the mitochondrial/oxidative stress pathways
GLUT2: glucose transporter 2; ROS: reactive oxygen species
•• Effects of neem in streptozotocin-induced diabetic mice
The decrease of serum glucose levels and increase of glycogen content, plasma insulin, and c-peptide levels with aqueous extract of neem have been shown in mice treated with STZ (110, 111). Also, the increase of glucose-6-phosphate dehydrogenase (G6PD) activity with neem has been shown in diabetic mice (110). The chloroform extract of neem in addition to reducing glucose level and induction of insulin level decreased oxidative stress markers and LPO in mice treated with STZ-nicotinamide. Also, it reduced glucose-6-phosphatase-α (G6Pase), glucokinase (GK), α-amylase, and α-glucosidase activities, and induced HK activity (50). Dianex, an herbal formulation is containing neem at a dose of 7.5 mg/kg. Dianex has been shown hypoglycemic effects in STZ-diabetic mice (49).
• Effects of neem in glucose-induced diabetic animals
The aqueous and ethanolic extracts of neem leaves decreased glucose level and increased insulin secretion in rats were treated with glucose (3 mg/ml, p.o.) (112, 113). Also, the potential use of ethanolic extract rhizome of neem (300 mg/kg, p.o.) was investigated in mice that were treated with glucose (1 g/kg). In this study, neem reduced blood glucose (114).
Conclusion
In summary, A. indica (neem) is effective in MetS and anti-oxidant effects appear to play an important role in protective mechanisms of neem against MetS and the complications associated with it. Neem increases the expression of Nrf2-mediated anti-oxidant enzymes and can regulate blood pressure and lipid profile. Also, neem inhibits vascular smooth muscle contraction through the block of calcium channels and decreases high blood pressure. Neem up-regulates eNOS expression as a vasodilator and increases NO level. Moreover, neem reduces vasoconstriction through the regulation of the ERK1/2 signaling pathway. In the diabetic condition, neem up-regulates GLUT4 and reduces the glucose uptake. Neem also inhibits intestinal enzymes such as glucosidases. Understanding the signaling pathways help to expand the use of neem in the treatment of the MetS. However, few studies have been conducted to investigate the anti-diabetic, anti-hypertension, anti-hyperlipidemia, and anti-obesity activities of neem in humans. Therefore, further clinical studies are needed to assess the protective effects of neem.
Acknowledgment
The authors are thankful to Mashhad University of Medical Sciences, Mashhad, Iran.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Hu G, Lindstrom J, Jousilahti P, Peltonen M, Sjoberg L, Kaaja R, etal The increasing prevalence of metabolic syndrome among Finnish men and women over a decade. J Clin Endocrinol Metab. 2008;93:832–836. doi: 10.1210/jc.2007-1883. [DOI] [PubMed] [Google Scholar]
- 2.Lee W-Y, Jung C-H, Park J-S, Rhee E-J, Kim S-W. Effects of smoking, alcohol, exercise, education, and family history on the metabolic syndrome as defined by the ATP III. Diabetes Res Clin Pract. 2005;67:70–77. doi: 10.1016/j.diabres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Toms TE, Panoulas VF, John H, Douglas KM, Kitas GD. Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60-more than just an anti-inflammatory effect? A cross sectional study. Arthritis Res Ther. 2009;11:R110. doi: 10.1186/ar2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 5.Mahdian D, Abbaszadeh-Goudarzi K, Raoofi A, Dadashizadeh G, Abroudi M, Zarepour E, etal Effect of Boswellia species on the metabolic syndrome: A review. Iran J Basic Med Sci. 2020;23:1374–1381. doi: 10.22038/ijbms.2020.42115.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeed A, Feofanova EV, Yu B, Sun W, Virani SS, Nambi V, etal Remnant-like particle cholesterol, low-density lipoprotein triglycerides, and incident cardiovascular disease. J Am Coll Cardiol. 2018;72:156–169. doi: 10.1016/j.jacc.2018.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galavi A, Hosseinzadeh H, Razavi BM. The effects of Alliumcepa (onion) and its active constituents on metabolic syndrome: A review. Iran J Basic Med Sci. 2020;23:1–14. doi: 10.22038/ijbms.2020.46956.10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181–190. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Floreani A, Cazzagon N, Franceschet I, Canesso F, Salmaso L, Baldo V. Metabolic syndrome associated with primary biliary cirrhosis. J Clin Gastroenterol. 2015;49:57–60. doi: 10.1097/MCG.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 10.Lim S, Kakoly N, Tan J, Fitzgerald G, Bahri Khomami M, Joham A, etal Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obe Rev. 2019;20:339–352. doi: 10.1111/obr.12762. [DOI] [PubMed] [Google Scholar]
- 11.Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol. 2015;2015:508409. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe M, Yokotsuka M, Yamaoka K, Adachi M, Nemoto A, Tango T. Effects of a lifestyle modification programme to reduce the number of risk factors for metabolic syndrome: a randomised controlled trial. Public Health Nut. 2017;20:142–153. doi: 10.1017/S1368980016001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanati S, Razavi BM, Hosseinzadeh H. A review of the effects of Capsicumannuum L and its constituent, capsaicin, in metabolic syndrome. Iran J Basic Med Sci. 2018;21:439–448. doi: 10.22038/IJBMS.2018.25200.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dehghani S, Mehri S, Hosseinzadeh H. The effects of Crataeguspinnatifida (Chinese hawthorn) on metabolic syndrome: A review. Iran J Basic Med Sci. 2019;22:460–468. doi: 10.22038/IJBMS.2019.31964.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razavi BM, Lookian F, Hosseinzadeh H. Protective effects of green tea on olanzapine-induced-metabolic syndrome in rats. Biomed Pharmacother. 2017;92:726–731. doi: 10.1016/j.biopha.2017.05.113. [DOI] [PubMed] [Google Scholar]
- 17.Gupta SC, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB. Neem (Azadirachtaindica): An indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. doi: 10.1016/j.phymed.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Selvaraj B, Periyasamy S. Indian medicinal plants for diabetes: text data mining the literature of different electronic databases for future therapeutics. Biomed Res. 2016;27:430–436. [Google Scholar]
- 19.Patel SM, Venkata KCN, Bhattacharyya P, Sethi G, Bishayee A. Potential of neem (Azadirachtaindica for prevention and treatment of oncologic diseases. Semin Cancer Biol. 2016;41:100–115. doi: 10.1016/j.semcancer.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachtaindica) Curr Sci. 2002;82:1336–1345. [Google Scholar]
- 21.Nwobodo EI. Evaluation of antilipid peroxidation and hypolipidemic potentials of AzadirachtaIndica leaf aqueous extract in paracetamol-induced hepatotoxicity in wistar rats. Int J Inform Res Rev. 2017;4:3615–3619. [Google Scholar]
- 22.Igwenyi I, Eze A, Aja P, Elom S, Uraku A, Awoke J, etal Cholesterol-lowering and hepatoprotective effect of fruit juice extract of Azadirachtaindica on Plasmodium berghei infected mice. Int J Curr Microbiol App Sci. 2017;6:3367–3375. [Google Scholar]
- 23.Moga MA, Bălan A, Anastasiu CV, Dimienescu OG, Neculoiu CD, Gavriș C. An overview on the anticancer activity of Azadirachtaindica (Neem) in gynecological cancers. Int J Mol Sci. 2018;19:3898–3924. doi: 10.3390/ijms19123898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singaravelu S, Sankarapillai J, Chandrakumari AS, Sinha P. Effect of Azadirachtaindica crude bark extracts concentrations against gram-positive and gram-negative bacterial pathogens. J Pharm Bioallied Sci. 2019;11:33–37. doi: 10.4103/jpbs.JPBS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakib Z, Shahraki N, Razavi BM, Hosseinzadeh H. Aloe vera as an herbal medicine in the treatment of metabolic syndrome: A review. Phytother Res. 2019;33:2649–2660. doi: 10.1002/ptr.6465. [DOI] [PubMed] [Google Scholar]
- 26.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vas Pharmacol. 2006;44:131–142. doi: 10.1016/j.vph.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouallegue A, Bou Daou G, Srivastava AK. Endothelin-1-induced signaling pathways in vascular smooth muscle cells. Curr Vasc Pharmacol. 2007;5:45–52. doi: 10.2174/157016107779317161. [DOI] [PubMed] [Google Scholar]
- 28.Ignarro LJ, Kadowitz PJ. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annual Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- 29.Howden R. Nrf2 and cardiovascular defense. Oxid Med Cell Longev. 2013;2013:104308. doi: 10.1155/2013/104308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah AJ, Gilani A-H, Hanif HM. Neem (Azadirachta indica) lowers blood pressure through a combination of Ca+ channel blocking and endothelium-dependent muscarinic receptors activation. Int J Pharmacol. 2014;10:418–428. [Google Scholar]
- 31.Omóbòwálé TO, Oyagbemi AA, Alaba BA, Ola-Davies OE, Adejumobi OA, Asenuga ER, etal Ameliorative effect of Azadirachtaindica on sodium fluoride-induced hypertension through improvement of antioxidant defence system and upregulation of extracellular signal regulated kinase 1/2 signaling. J Basic Clin Physiol Pharmacol. 2018;29:155–164. doi: 10.1515/jbcpp-2017-0029. [DOI] [PubMed] [Google Scholar]
- 32.Omóbòwálé TO, Oyagbemi AA, Ogunpolu BS, Ola-Davies OE, Olukunle JO, Asenuga ER, et al. Antihypertensive effect of polyphenol-rich fraction of Azadirachtaindica on Nω-Nitro-L-arginine methyl ester-induced hypertension and cardiorenal dysfunction. Drug Res. 2019;69:12–22. doi: 10.1055/a-0635-0638. [DOI] [PubMed] [Google Scholar]
- 33.Obiefuna I, Young R. Concurrent administration of aqueous Azadirachtaindica (neem) leaf extract with DOCA-salt prevents the development of hypertension and accompanying electrocardiogram changes in the rat. Phytother Res. 2005;19:792–795. doi: 10.1002/ptr.1739. [DOI] [PubMed] [Google Scholar]
- 34.Kochhar A, Sharma N, Sachdeva R. Effect of supplementation of Tulsi (Ocimumsanctum) and Neem (Azadirachtaindica) leaf powder on diabetic symptoms, anthropometric parameters and blood pressure of non insulin dependent male diabetics. Stud EthnoMed. 2009;3:5–9. [Google Scholar]
- 35.Tabeshpour J, Imenshahidi M, Hosseinzadeh H. A review of the effects of Berberisvulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci. 2017;20:557–568. doi: 10.22038/IJBMS.2017.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassani FV, Shirani K, Hosseinzadeh H. Rosemary (Rosmarinusofficinalis) as a potential therapeutic plant in metabolic syndrome: a review. Naunyn-Schmiedeberg’s Arch Pharmacol. 2016;389:931–949. doi: 10.1007/s00210-016-1256-0. [DOI] [PubMed] [Google Scholar]
- 37.Yoshino G, Hirano T, Kazumi T. Dyslipidemia in diabetes mellitus. Diabetes Res Clin Pract. 1996;33:1–14. doi: 10.1016/0168-8227(96)01263-6. [DOI] [PubMed] [Google Scholar]
- 38.Pirinccioglu AG, Gökalp D, Pirinccioglu M, Kizil G, Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin Biochem. 2010;43:1220–1224. doi: 10.1016/j.clinbiochem.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 39.El-Demerdash FM, Nasr HM. Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Biol. 2014;28:89–93. doi: 10.1016/j.jtemb.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Morel DW, Chisolm GM. Antioxidant treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J Lipid Res. 1989;30:1827–1834. [PubMed] [Google Scholar]
- 41.Araujo FB, Barbosa DS, Hsin CY, Maranhão RC, Abdalla DS. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis. 1995;117:61–71. doi: 10.1016/0021-9150(94)05558-z. [DOI] [PubMed] [Google Scholar]
- 42.Davignon J, Cohn JS. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis. 1996;124:57–64. doi: 10.1016/0021-9150(96)05858-3. [DOI] [PubMed] [Google Scholar]
- 43.Soni H, Patel S, Patel G, Paranjape A. Evaluation of anti-diabetic activity of Glucova Active Tablet on Type I and Type II diabetic model in rats. J Ayurveda Integr Med. 2014;5:97–103. doi: 10.4103/0975-9476.133806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bisht S, Sisodia S. Anti-hyperglycemic and antidyslipidemic potential of Azadirachtaindica leaf extract in STZ-induced diabetes mellitus. J Pharm Sci Res. 2010;2:622–627. [Google Scholar]
- 45.Hussain HEMA. Reversal of diabetic retinopathy in streptozotocin induced diabetic rats using traditional Indian anti-diabetic plant, Azadirachtaindica (L. ). Indian J Clin Biochem. 2002;17:115–123. doi: 10.1007/BF02867983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez RMP, Gómez YGY, Guzman MD. Attenuation of nonenzymatic glycation, hyperglycemia, and hyperlipidemia in streptozotocin-induced diabetic rats by chloroform leaf extract of Azadirachtaindica. Pharmacogn Mag. 2011;7:254–259. doi: 10.4103/0973-1296.84243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satyanarayana K, Sravanthi K, Shaker IA, Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachtaindica. J Ayurveda Integr Med. 2015;6:165–174. doi: 10.4103/0975-9476.157950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gautam MK, Gangwar M, Singh SK, Goel RK. Effects of Azardirachtaindica on vascular endothelial growth factor and cytokines in diabetic deep wound. Planta Med. 2015;81:713–721. doi: 10.1055/s-0035-1545917. [DOI] [PubMed] [Google Scholar]
- 49.Mutalik S, Chetana M, Sulochana B, Devi PU, Udupa N. Effect of Dianex, a herbal formulation on experimentally induced diabetes mellitus. Phytother Res. 2005;19:409–415. doi: 10.1002/ptr.1570. [DOI] [PubMed] [Google Scholar]
- 50.Perez-Gutierrez RM, Damian-Guzman M. Meliacinolin: a potent α-glucosidase and α-amylase inhibitor isolated from Azadirachtaindica leaves and invivo antidiabetic property in streptozotocin-nicotinamide-induced type 2 diabetes in mice. Biol Pharm Bull. 2012;35:1516–1524. doi: 10.1248/bpb.b12-00246. [DOI] [PubMed] [Google Scholar]
- 51.Bangar OP, Jarald EE, Asghar S, Ahmad S. Antidiabetic activity of a polyherbal formulation (Karnim Plus) Int J Green Pharm. 2009;3:211–214. [Google Scholar]
- 52.Dholi SK, Raparla R, Mankala SK, Nagappan K. Invivo Antidiabetic evaluation of Neem leaf extract in alloxan induced rats. J App Pharm Sci. 2011;1:100–105. [Google Scholar]
- 53.Mgbeje BAI, Essien NA, Iwara IA, Egbung GE, Igile GO, Ebong PE. Lipid profile and hepatoprotective effects of combined leaf extracts of AzadirachtaIndica (Neem) and Peristrophebicalyculata in Alloxan-induced diabetic rats. Int J Phytomedicine. 2013;5:159–162. [Google Scholar]
- 54.Peer PA, Trivedi PC, Nigade PB, Ghaisas MM, Deshpande AD. Cardioprotective effect of Azadirachtaindica A Juss on isoprenaline induced myocardial infarction in rats. Int J Cardiol. 2008;126:123–126. doi: 10.1016/j.ijcard.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 55.Zuraini A, Vadiveloo T, Hidayat MT, Arifah A, Sulaiman M, Somchit M. Effects of neem (Azadirachtaindica) leaf extracts on lipid and C-reactive protein concentrations in cholesterol-fed rats. J Nat Remedies. 2006;6:109–114. [Google Scholar]
- 56.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331:166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Boden-Albala B, Sacco RL. Lifestyle factors and stroke risk: exercise, alcohol, diet, obesity, smoking, drug use, and stress. Curr Atheroscler Rep. 2000;2:160–166. doi: 10.1007/s11883-000-0111-3. [DOI] [PubMed] [Google Scholar]
- 58.Malnick SD, Knobler H. The medical complications of obesity. J Assoc Physicians. 2006;99:565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 59.Mukherjee A, Sengupta S. Indian medicinal plants known to contain intestinal glucosidase inhibitors also inhibit pancreatic lipase activity—An ideal situation for obesity control by herbal drugs. Indian J Biotechnol. 2013;12:32–39. [Google Scholar]
- 60.Jayakumar K, Srinivasan M, Ramesh N, Sachan A, Umesh M, Narayana K. Effect of neem leaf extract on feed intake and body weight in rats. Indian Vet J. 2002;79:732–733. [Google Scholar]
- 61.Bogardus C, Lillioja S, Howard B, Reaven G, Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Inves. 1984;74:1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dixit S, Maiya A. Diabetic peripheral neuropathy and its evaluation in a clinical scenario: a review. J Postgrad Med. 2014;60:33–40. doi: 10.4103/0022-3859.128805. [DOI] [PubMed] [Google Scholar]
- 63.Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, etal A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637–642. doi: 10.2337/diabetes.50.3.637. [DOI] [PubMed] [Google Scholar]
- 64.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 65.Ritz E, Rychlík I, Locatelli F, Halimi S. End-stage renal failure in type 2 diabetes: a medical catastrophe of worldwide dimensions. Am J Kidney Dis. 1999;34:795–808. doi: 10.1016/S0272-6386(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 66.Hörle S, Grüner F, Kroll P. Epidemiology of diabetes-induced blindness-a review. Klin Monbl Augenheilkd. 2002;219:777–784. doi: 10.1055/s-2002-36318. [DOI] [PubMed] [Google Scholar]
- 67.Coffey L, Gallagher P, Horgan O, Desmond D, MacLachlan M. Psychosocial adjustment to diabetes-related lower limb amputation. Diabetic Med. 2009;26:1063–1067. doi: 10.1111/j.1464-5491.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 68.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther Assoc. 2008;88:1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matough FA, Budin SB, Hamid ZA, Alwahaibi N, Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ Med J. 2012;12:5–18. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka Y, Tran POT, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic β cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tadera K, Minami Y, Takamatsu K, Matsuoka T. Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52:149–153. doi: 10.3177/jnsv.52.149. [DOI] [PubMed] [Google Scholar]
- 74.Tundis R, Loizzo M, Menichini F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Rev Med Chem. 2010;10:315–331. doi: 10.2174/138955710791331007. [DOI] [PubMed] [Google Scholar]
- 75.St Augustines T. Effects of Vernoniaamygdalina on biochemical and hematological parameters in diabetic rats. Asian J Med Sci. 2009;1:108–113. [Google Scholar]
- 76.Razavi B, Hosseinzadeh H. A review of the effects of Nigellasativa and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 77.Akaberi M, Hosseinzadeh H. Grapes (Vitisvinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 78.Hosseini A, Hosseinzadeh H. A review on the effects of Alliumsativum (Garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 79.Joseph B, Jini D. Insight into the hypoglycaemic effect of traditional Indian herbs used in the treatment of diabetes. Res J Med Plant. 2011;5:352–376. [Google Scholar]
- 80.Yaseen G, Ahmad M, Zafar M, Sultana S, Kayani S, Cetto AA, et al. Traditional management of diabetes in Pakistan: ethnobotanical investigation from traditional health practitioners. J Ethnopharmacol. 2015;174:91–117. doi: 10.1016/j.jep.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 81.Dineshkumar B, Analava M, Manjunatha M. Antidiabetic and hypolipidaemic effects of few common plants extract in type 2 diabetic patients at Bengal. Int J Diabetes Metabo. 2010;18:59–65. [Google Scholar]
- 82.Riyanti S, Suganda AG, Sukandar EY. Dipeptidyl peptidase-IV inhibitory activity of some Indonesian medicinal plants. Asian J Pharm Clin Res. 2016;9:375–377. [Google Scholar]
- 83.Ezuruike U, Prieto JM. Assessment of potential herb-drug interactions among Nigerian adults with Type-2 diabetes. Front Pharmacol. 2016;7:248–255. doi: 10.3389/fphar.2016.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rameshrad M, Razavi BM, Ferns GA, Hosseinzadeh H. Pharmacology of dipeptidyl peptidase-4 inhibitors and its use in the management of metabolic syndrome: a comprehensive review on drug repositioning. DARU J Pharm Sci. 2019;27:341–360. doi: 10.1007/s40199-019-00238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rameshrad M, Razavi BM, Lalau J-D, De Broe ME, Hosseinzadeh H. An overview of glucagon-like peptide-1 receptor agonists for the treatment of metabolic syndrome: A drug repositioning. Iran J Basic Med Sci. 2020;23:556–568. doi: 10.22038/ijbms.2020.41638.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc Dis Res. 2006;3:159–165. doi: 10.3132/dvdr.2006.024. [DOI] [PubMed] [Google Scholar]
- 87.Hsia SH, Bazargan M, Davidson MB. Effect of Pancreas Tonic (an ayurvedic herbal supplement) in type 2 diabetes mellitus. Metabolism. 2004;53:1166–1173. doi: 10.1016/j.metabol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 89.Dholi SK, Raparla R, Kannappan S. Synergic activity of fenugreek seeds and neem leaf extracts against alloxan induced diabetic rats. Int J PharmTech Res. 2011;3:1963–1970. [Google Scholar]
- 90.Patil P, Patil S, Mane A, Verma S. Antidiabetic activity of alcoholic extract of Neem (Azadirachtaindica) root bark. Nat J Physiol Pharm Pharmacol. 2013;3:142–146. [Google Scholar]
- 91.Sunarwidhi AL, Sudarsono S, Nugroho AE. Hypoglycemic effect of combination of Azadirachtaindica and Gynuraprocumbens (Lour ) Mer ethanolic extracts standardized by rutin and quercetin in alloxan-induced hyperglycemic rats. Adv Pharm Bull. 2014;4:613–618. doi: 10.5681/apb.2014.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shailey S, Basir SF. Protective role of Azadirachtaindica against oxidative damage in skeletal and cardiac muscle of alloxan diabetic rats. Int J Pharm Sci. 2012;4:471–477. [Google Scholar]
- 93.Shailey S, Basir SF. Strengthening of anti-oxidant defense by Azadirachtaindica in alloxan-diabetic rat tissues. J Ayurveda Integ Med. 2012;3:130–135. doi: 10.4103/0975-9476.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karole S, Shrivastava S, Thomas S, Soni B, Khan S, Dubey J, et al. Polyherbal Formulation Concept for Synergic Action: A Review. J Drug Deliv Ther. 2019;9:453–466. [Google Scholar]
- 95.TK MM, TE FH, Musambil M, Mirshad P, OM FR, Vasudevan M. The effect of polyherbal formulation DIA7 on fasting blood glucose level in alloxan induced diabetic rats. Der Pharm Lett. 2014;6:215–221. [Google Scholar]
- 96.Akhtar N, Khan BA, Majid A, Khan S, Mahmood T, Gulfishan ST. Pharmaceutical and biopharmaceutical evaluation of extracts from different plant parts of indigenous origin for their hypoglycemic responses in rabbits. Acta Pol Pharm. 2011;68:919–925. [PubMed] [Google Scholar]
- 97.Khosla P, Bhanwra S, Singh J, Seth S, Srivastava R. A study of hypoglycaemic effects of Azadirachtaindica (Neem) in normal and alloxan diabetic rabbits. Indian J Physiol Pharmacol. 2000;44:69–74. [PubMed] [Google Scholar]
- 98.Upreti J, Ali S, Basir SF. Effect of lower doses of vanadate in combination with Azadirachtaindica leaf extract on hepatic and renal antioxidant enzymes in streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2013;156:202–209. doi: 10.1007/s12011-013-9827-0. [DOI] [PubMed] [Google Scholar]
- 99.Gupta NK, Srivastva N, Puri S, Bubber P, Puri V. Neuroprotective potential of Azadirachtaindica leaves in diabetic rats. Asian J Pharm Clin Res. 2017;10:243–248. [Google Scholar]
- 100.Atangwho IJ, Ebong PE, Egbung GE, Ani IF. Effects of co-administration of extracts of Vernoniaamygdalina and Azadirachtaindica on serum electrolyte profile of diabetic and non diabetic rats. Australian J Basic Appl Sci. 2009;3:2974–2978. [Google Scholar]
- 101.Akinola OB, Caxton-Martins EA, Dini L. Chronic treatment with ethanolic extract of the leaves of Azadirachtaindica ameliorates lesions of pancreatic islets in streptozotocin diabetes. Int J Morphol. 2010:28;291–302. [Google Scholar]
- 102.Dallaqua B, Saito FH, Rodrigues T, Calderon IMP, Rudge MVC, Herrera E, et al. Treatment with Azadirachtaindica in diabetic pregnant rats: negative effects on maternal outcome. J Ethnopharmacol. 2012;143:805–811. doi: 10.1016/j.jep.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 103.Atangwho IJ, Ebong PE, Eyong EU, Asmawi MZ, Ahmad M. Synergistic antidiabetic activity of Vernoniaamygdalina and Azadirachtaindica: Biochemical effects and possible mechanism. J Ethnopharmacol. 2012;141:878–887. doi: 10.1016/j.jep.2012.03.041. [DOI] [PubMed] [Google Scholar]
- 104.Perez Gutierrez RM, de Jesus Martinez Ortiz M. Beneficial effect of Azadirachtaindica on advanced glycation end-product in streptozotocin-diabetic rat. Pharm Biol. 2014;52:1435–1444. doi: 10.3109/13880209.2014.895389. [DOI] [PubMed] [Google Scholar]
- 105.Gupta NK, Srivastva N, Bubber P, Puri S. The antioxidant potential of Azadirachta indica ameliorates cardioprotection following diabetic mellitus-induced microangiopathy. Pharmacogn Mag. 2016;12:371. doi: 10.4103/0973-1296.185772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J Biol Chem. 2003;278:33609–33612. doi: 10.1074/jbc.R300019200. [DOI] [PubMed] [Google Scholar]
- 107.Kumar R, Arora V, Ram V, Bhandari A, Vyas P. Hypoglycemic and hypolipidemic effect of Allopolyherbal formulations in streptozotocin induced diabetes mellitus in rats. Int J Diabetes Mellit. 2015;3:45–50. [Google Scholar]
- 108.Patel SS, Shah RS, Goyal RK. Antihyperglycemic, antihyperlipidemic and anti-oxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J Exp Biol. 2009;47:564–570. [PubMed] [Google Scholar]
- 109.Yadav D, Chaudhary AA, Garg V, Anwar MF, Rahman MM-u, Jamil SS, et al. In vitro toxicity and antidiabetic activity of a newly developed polyherbal formulation (MAC-ST/001) in streptozotocin-induced diabetic Wistar rats. Protoplasma. 2013;250:741–749. doi: 10.1007/s00709-012-0458-7. [DOI] [PubMed] [Google Scholar]
- 110.Bhat M, Kothiwale SK, Tirmale AR, Bhargava SY, Joshi BN. Antidiabetic properties of Azardiracta indica and Bougainvilleaspectabilis: in vivo studies in murine diabetes model. Evid Based Complementary Altern Med. 2011;2011:561625. doi: 10.1093/ecam/nep033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kosta S, Tiwari A. Screening and assessment of anti-diabetic and reactive oxygen scavenging (ros), effects of herbs in streptozotacin induced mice. Pharmacol online. 2009;3:695–704. [Google Scholar]
- 112.Jani DK, Goswami S. Screening of the herbal extracts to compare and investigate effect on glucose lowering and anorexic activity in wistar rats. Asian J Pharm Clin Res. 2017;10:160–165. [Google Scholar]
- 113.Chattopadhyay R. Possible mechanism of antihyperglycemic effect of Azadirachtaindica leaf extract: part V. J Ethnopharmacol. 1999;67:373–376. doi: 10.1016/s0378-8741(99)00094-x. [DOI] [PubMed] [Google Scholar]
- 114.Muhtadi A, Irenka Y, Ayu WC, Hendriani R, Zuhrotun A. Hypoglycemic activity of 10 medicinal plants extract in glucose induced mice. Asian J Pharm Clin Res. 2017;4:2455–3891. [Google Scholar]
- 115.Omobowale TO, Oyagbemi A, Ugbor F, Adejumobi O, Adedapo A, Yakubu M. Nω-Nitro-L-Arginine Methyl Ester (L-NAME) induced hypertension and cardiorenal oxidative stress: Modulatory effect of the methanolic extract of Azadirachta indica. FASEB J. 2017;31:1011.10–1011. [Google Scholar]
- 116.Adejumobi O, Omobowale T, Oyagbemi A, Ayenuro O, Ola-Davies O, Adedapo A, et al. Amelioration of sodium fluoride-induced hypertension, cardio-renal oxidative stress and genotoxicity by Azadirachta indica through antioxidant and extracellular signal-regulated kinase (ERK) 1/2 signalling. FASEB J. 2017;31:843.12–843. [Google Scholar]
- 117.Koley K, Lal J. Pharmacological effects of Azadirachta indica (neem) leaf extract on the ECG and blood pressure of rat. Indian J Physiol Pharmacol. 1994;38:223–225. [PubMed] [Google Scholar]
- 118.Thompson EB, Anderson CC. Cardiovascular effects of Azadirachta indica extract. J Pharm Sci. 1978;67:1476–1478. doi: 10.1002/jps.2600671044. [DOI] [PubMed] [Google Scholar]
- 119.Kataria M, Gupta P, Gupta S. Hypolipidaemic and anti-atherogenic activity of petroleum ether extract of neem(Azadirachta indica) seed husk and kernel in streptozotocin-induced diabetic rats. Toxicol Int. 2006;13:105–110. [Google Scholar]
- 120.Saleem T, Mumtaz U, Bashir MU, Qureshi HJ, Saleem A. Comparison of hypoglycemic effects of Azadirachta indica seeds and leaves on alloxan induced diabetes in male albino rats. Pak J Med Health Sci. 2018;12:753–756. [Google Scholar]
- 121.Nugroho AE, Sari KRP, Sunarwidhi AL. Blood glucose reduction by combination of Andrographis paniculata (Burm ) Ness herbs and Azadirachta indica A Juss leaves in alloxan-induced diabetic rats. J App Pharm Sci. 2014;4:30–35. [Google Scholar]
- 122.Kar A, Choudhary B, Bandyopadhyay N. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003;84:105–108. doi: 10.1016/s0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 123.Maithani A, Parcha V, Pant G, Kumar D, Dhulia I. Anti-hyperglycemic activity guided fractionation of aqueous extract of Azadirachta indica on alloxan induced diabetic rats. Int J Res Pharm Sci. 2011;2:488–491. [Google Scholar]
- 124.Tiwari BK, Pandey KB, Jaiswal N, Abidi A, Rizvi SI. Anti-diabetic and anti-oxidative effect of composite extract of leaves of some Indian plants on alloxan induced diabetic Wistar rats. J Pharm Invest. 2014;44:205–211. [Google Scholar]
- 125.Ebong PE, Atangwho IJ, Eyong EU, Egbung GE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica Juss)(Neem) and Vernonia amygdalina (De )(African bitter leaf) Am J Biochem Biotechnol. 2008;4:239–244. [Google Scholar]
- 126.Nagashayana G, Jagadeesh K, Shreenivas PR. Evaluation of hypoglycemic activity of neem (Azadirachta indica) in albino rats. J Dent Med Sci. 2014;13:4–11. [Google Scholar]
- 127.Chandra A, Mahdi AA, Ahmad S, Singh RK. Indian herbs result in hypoglycemic responses in streptozotocin-induced diabetic rats. Nutr Res. 2007;27:161–168. [Google Scholar]
- 128.Chattopadhyay R. A comparative evaluation of some blood sugar lowering agents of plant origin. J Ethnopharmacol. 1999;67:367–372. doi: 10.1016/s0378-8741(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 129.Akinola OB, Dosumu OO, Akinola OS, Zatta L, Dini L, Caxton-Martins EA. Azadirachtaindica leaf extract ameliorates hyperglycemia and hepatic glycogenosis in streptozotocin-induced diabetic wistar rats. Int J Phytomed. 2010;2:320–331. [Google Scholar]
- 130.Akinola OB, Zatta L, Dosumu OO, Akinola OS, Adelaja AA, Dini L, et al. Intestinal lesions of streptozotocin-induced diabetes and the effects of Azadirachta indica treatment. Pharmacology. 2009;3:872–881. [Google Scholar]
- 131.Gupta S, Kataria M, Gupta P, Murganandan S, Yashroy R. Protective role of extracts of neem seeds in diabetes caused by streptozotocin in rats. J Ethnopharmacol. 2004;90:185–189. doi: 10.1016/j.jep.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 132.Mishra A, Srivsatava R, Srivastava AK. Comparative antidiabetic profile of ayurvedic herbo-mineral formulation and its constituents on normal and streptozotocin-induced diabetic rats. Int J Pharm Sci Rev Res. 2013;22:252–263. [Google Scholar]
- 133.Ansarullah BB, Patel V, Ramachandran A. Improved glucoregulation, insulin resistance and leptin levels by a polyherbal drug in high fat diet and low dose streptozotocin type 2 diabetes model. Diabetol Croat. 2012;41:3–15. [Google Scholar]
- 134.Neeraja Kamakshi U, Srinivasa Rao D, Yamini Suvarchala K, Anusha K, Venkateswara Rao B. Comparative hypoglycemic study of Aloe vera, Murraya koenigii and Azadirachta indica. Int J Pharmacog Phytochem Res. 2015;7:923–927. [Google Scholar]
- 135.Bakr A. Changes of hemoglobin content and glucose levels in the blood of Rattus norvegicus by water extracts of Azadirachta indica. Chin J Nat Med. 2012;10:135–137. [Google Scholar]
- 136.Sanni O, Erukainure OL, Chukwuma CI, Koorbanally NA, Ibeji CU, Islam MS. Azadirachta indica inhibits key enzyme linked to type 2 diabetes invitro, abates oxidative hepatic injury and enhances muscle glucose uptake ex vivo. Biomed Pharmacother. 2019;109:734–743. doi: 10.1016/j.biopha.2018.10.171. [DOI] [PubMed] [Google Scholar]
- 137.Kaur L, Han K-S, Bains K, Singh H. Indian culinary plants enhance glucose-induced insulin secretion and glucose consumption in INS-1 β-cells and 3T3-L1 adipocytes. Food Chem. 2011;129:1120–1125. doi: 10.1016/j.foodchem.2011.05.089. [DOI] [PubMed] [Google Scholar]