Abstract
The global pandemic of COVID-19 has been lasting for more than one year and there is little known about the long-term health effects of the disease. Long-COVID is a new term that is used to describe the enduring symptoms of COVID-19 survivors. Huang et al. reported that fatigue, muscle weakness, sleep disturbances, anxiety, and depression were the most common complaints in COVID-19 survivors after 6 months of the infection. A recent meta-analysis showed that 80% of COVID-19 survivors have developed at least one long-term symptom and the most common five were fatigue, headache, attention deficit disorder, hair loss, and dyspnea. In this paper, we discuss the hypothesis that altered tryptophan absorption and metabolism could be the main contributor to the long-term symptoms in COVID-19 survivors.
Keywords: Covid-19, Tryptophan, Malabsorption, Altered, Metabolism, Long-covid
Introduction
The global coronavirus disease 2019 (COVID-19) pandemic has lasted for more than 1 y and little is known about the long-term health effects of the disease. “Long COVID” is a new term that is used to describe the enduring symptoms of people who have had COVID-19 [1]. Huang et al. report that fatigue, muscle weakness, sleep disturbances, anxiety, and depression are the most common complaints in people who have had COVID-19 after 6 mo of infection [2]. A recent meta-analysis showed that 80% of people who have had COVID-19 developed at least one long-term symptom; the five most common were fatigue, headache, attention disorder, hair loss, and dyspnea [3]. In this article, we discuss the hypothesis that altered tryptophan absorption and metabolism could be the main contributor to the long-term symptoms in people who have had COVID-19.
l-Tryptophan metabolism
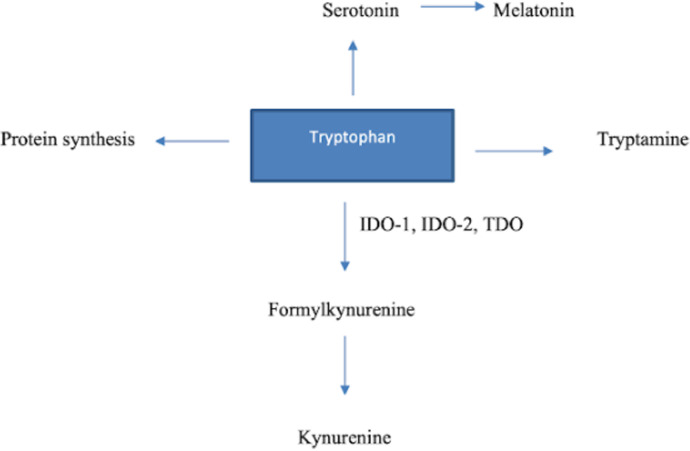
l-Tryptophan (Try) is an essential amino acid that is obtained primarily through dietary intake in humans. Besides being a building block for protein synthesis, it serves as a precursor for some important biological molecules such as serotonin, melatonin, and tryptamine [4]. Only about 5% of free Try is used in the production of proteins, neurotransmitters, and neuromodulators; the kynurenine (Kyn) pathway (KP) degrades the remaining more than 95% (Fig. 1.). The conversion of Try to N-formylkynurenine is the rate-limiting step in the KP, catalyzed by three enzymes: indoleamine 2,3-dioxygenase (IDO) 1 and 2 and tryptophan 2,3-dioxygenase (TDO). Although the latter is found primarily in the liver, IDO is the main extrahepatic enzyme of the KP and can be found in various cells, including macrophages. Kynurenines (metabolites generated in the KP) have some central roles in inflammatory and immunologic responses and also been linked to psychiatric disorders such as depression [5,6].
Fig. 1.
Overview of tryptophan metabolism. IDO-1, indoleamine 2,3-dioxygenase 1; IDO-2, indoleamine 2,3-dioxygenase 2; TDO, tryptophan 2,3-dioxygenase.
Tryptophan metabolism during SARS-CoV-2 infection
It is known that IDO-1 is the primary extrahepatic enzyme of the KP and can be induced by several proinflammatory cytokines, such as tumor necrosis factor-α, interferons, and prostaglandins. Interferon-γ is the most potent stimulator of IDO's enzymatic activity. That is, why IDO activity increases with inflammation like in chronic inflammatory diseases, infections, and cancers [7]. A number of studies suggest that IDO-1 activation is essential for the inhibition of intracellular pathogens and tumor cells. The activated KP in IDO-competent cells reduces inflammation and promotes long-term immune tolerance by inducing the proliferation of regulatory T cells. Along with the immunoregulation effect, IDO also works as an intracellular pathogen repressor by removing environmental tryptophan, which is required for replication of microorganisms during infection [8], [9], [10].
Several studies have looked into changes in tryptophan metabolism in people infected with SARS-CoV-2 and found augmented activation of the KP. Thomas et al. [11] conducted a metabolomic study with the plasma of 33 participants who were positive for SARS-CoV-2 positive and 16 negative, and determined that tryptophan metabolism was the leading pathway affected by COVID-19. In those who were infected, they discovered lower levels of tryptophan, serotonin, and indolepyruvate, as well as higher levels of kynurenine, kynurenic acid, picolinic acid, and nicotinic acid, all of which were positively correlated with interleukin-6 levels. Despite the fact that the infection group was younger than the control group, this finding was significant because it demonstrated disturbed tryptophan metabolism in people infected with SARS-CoV-2. In a separate study, Lionetto et al. [9] compared the serum Kyn:Try ratio—which reflects KP activation—in three groups: positive for SARS-CoV-2, negative for SARS-CoV-2 and admitted to the emergency department with illnesses other than COVID-19, and a healthy control group. The SARS-CoV-2-positive group had the highest Kyn:Try ratios. In the subgroup analysis, the SARS-CoV-2-positive participants with the most severe outcomes had the highest Kyn:Try ratios. The Kyn:Try ratio was also higher in those with severe lymphopenia, which is an ominous prognostic predictor in COVID-19, and in males, who are thought to be more vulnerable to infection [9]. Altered tryptophan metabolism in acute COVID-19 infection is also supported by several other studies [12,13]. It is still unclear whether KP activation is a defense mechanism or a mechanism that causes infection to flare up by inducing immune tolerance. Although several studies have indicated that acute COVID-19 infection causes increased KP activation, there is no evidence of long-term effects of disturbed tryptophan metabolism in these populations. As discussed later, kynurenines could underlie long-COVID symptoms.
KP and long-COVID symptoms
As discussed before, the most commonly seen long-term symptoms in people who have had COVID-19 are depression, fatigue, sleep disturbances, attention disorders, anxiety, muscle weakness, and dyspnea. When taken together, KP activation may also contribute these symptoms.
The “kynurenine shunt” refers to the increased degradation of tryptophan toward kynurenine and away from serotonin production [14]. Increased IDO activity has been related to depression in studies, owing to both serotonin depletion and neurotoxic effects of KP metabolites [15]. Fatigue, the most common long-term symptom in people who have had COVID-19, is divided into central and peripheral fatigue, with central fatigue causing complex weakness and making recovery difficult [16]. Several studies have revealed that increased metabolites of the KP in the brain trigger central fatigue and memory issues by inducing neurotoxicity [17], [18], [19], [20]. The long-term symptoms seen in COVID-19 infection could be related to the kynurenine shunt. Although there has been evidence of increased KP activation in acute COVID-19, there is no evidence in long-term COVID-19 survivors. Studies in this area can aid in our understanding of the pathophysiology underlying COVID-19’s long-term symptoms.
Angiotensin-converting enzyme 2 and tryptophan absorption
COVID-19 has the ability to cause Try malabsorption in addition to disrupting Try metabolism. It is known that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as a receptor and that ACE2 expression is abundant in the intestines. When SARS-CoV-2 infects the intestines, it disrupts the expression of ACE2 in the gastrointestinal system [21]. ACE2 is required for the expression of intestinal B0AT1, which is a neutral amino acid transporter in the intestinal lumen. Therefore, there could be a relatively neutral amino acid malabsorption in case of diminished ACE2 expression in the intestines. It has been evidenced that plasma tryptophan levels significantly decline in ACE2-lacking mice [22].
l-Tryptophan is the main precursor of serotonin and other neurotransmitters which have a key role in the pathogenesis of depression and anxiety. Previous studies have shown that brain serotonin levels are low in ACE2-deficient mice [23]. Acute tryptophan depletion in rodents decreased tryptophan levels in the brain by up to 70%, resulting in lower central serotonin levels in the brain and causing inhibition of serotonin synthesis in humans. Acute tryptophan depletion has also been linked to mood disturbances, especially in people who are prone to depression [24], [25], [26]. Melatonin, one of the end products of Try, plays an important role in sleep control as well as immune response. Try deficiency has been shown to reduce rapid-eye-movement latency and lengthen rapid-eye-movement sleep [27,28]. On the other hand, tryptophan also plays a major role in skeletal muscle mass regulation. Ninomiya et al. found that skeletal muscle mass in people with lymphoma was closely associated with serum tryptophan levels. Moreover, they revealed that there was a reversible muscle loss in mice fed a tryptophan-deficient diet [29]. In addition to lung damage, long-term dyspnea from COVID-19 may be caused by weakness of the diaphragm muscle due to relative tryptophan deficiency.
Conclusion
In conclusion, SARS-CoV-2 infection causes long-term dysregulation of Try absorption from the intestines due to an ACE2 imbalance in the gastrointestinal system. Furthermore, Try metabolism is disturbed in favor of the KP. Low serum and muscle tryptophan levels, as well as elevated kynurenine levels, may be to blame for COVID-19’s most common long-term symptoms, such as depression, sleep disturbances, fatigue, and muscle weakness—which are similar to the symptoms of tryptophan deficiency. It is unknown whether the severity of gastrointestinal symptoms during acute infection or tryptophan supplementation have an influence on the long-term health effects of COVID-19.
We propose that COVID-19-related alteration in Try absorption and metabolism could be the underlying pathophysiology of long-COVID symptoms. People who have had COVID-19 should be evaluated for nutritional status and levels of tryptophan and its metabolites in the long term.
References
- 1.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, et al. Attributes and predictors of long COVID. Nat Med. 2021;NN:1–6. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021 doi: 10.1016/S0140-6736(20)32656-8. NN:PPP–PPP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Preprint. medRxiv. 2021;2021.01.27.21250617. Published 2021 Jan 30. doi:10.1101/2021.01.27.21250617 [DOI] [PMC free article] [PubMed]
- 4.Platten M, Nollen EAA, Röhrig UF, Fallarino F, Opitz CA. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 5.Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan's metabolites in exercise, inflammation, and mental health. Science. 2017;357:PPP–PPP. doi: 10.1126/science.aaf9794. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;NN:PPP–PPP. doi: 10.4137/ijtr.s2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boergeling Y, Ludwig S. Targeting a metabolic pathway to fight the flu. FEBS J. 2017;284:218–221. doi: 10.1111/febs.13997. [DOI] [PubMed] [Google Scholar]
- 8.Hwang SL, Nancy Chung NP-Y, Chan JK-Y, Lin C-L S. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005;15:167–175. doi: 10.1038/sj.cr.7290282. [DOI] [PubMed] [Google Scholar]
- 9.Lionetto L, Ulivieri M, Capi M, Bernardini DD, Fazio F, Petrucca A, et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: an observational cohort study. Biochim Biophys Acta Mol Basis Dis. 2021;1867 doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Mellor AL. Indoleamine 2, 3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5 doi: 10.1172/jci.insight.140327. PPP–PPP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barberis E, Timo S, Amede E, Vanella VV, Puricelli C, Cappellano G, et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int J Mol Sci. 2020;21:8623. doi: 10.3390/ijms21228623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson J, Gostner JM, Nilsson S, Andersson L-M, Fuchs D, Gisslen M. Serum neopterin levels in relation to mild and severe COVID-19. BMC Infect Dis. 2020;20:1–6. doi: 10.1186/s12879-020-05671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangoni A. The “kynurenine shunt” and depression. Adv Biochem Psychopharmacol. 1974;11(0):293-298. [PubMed]
- 15.Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res. 2015;68:316–328. doi: 10.1016/j.jpsychires.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita M. Potential role of neuroactive tryptophan metabolites in central fatigue: establishment of the fatigue circuit. Int J Tryptophan Res. 2020;13 doi: 10.1177/1178646920936279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Németh H, Toldi J, Vécsei L. Role of kynurenines in the central and peripherial nervous systems. Curr Neurovasc Res. 2005;2:249–260. doi: 10.2174/1567202054368326. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Azechi H, Board M. Essential role of excessive tryptophan and its neurometabolites in fatigue. Can J Neurol Sci. 2012;39:40–47. doi: 10.1017/s031716710001266x. [DOI] [PubMed] [Google Scholar]
- 19.Carpenedo R, Pittaluga A, Cozzi A, Attucci S, Galli A, Raiteri M, Moroni F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur J Neurosci. 2001;13:2141–2147. doi: 10.1046/j.0953-816x.2001.01592.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu HQ, Pereira EF, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R. The astrocyte-derived alpha7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci. 2010;40(1-2):204–210. doi: 10.1007/s12031-009-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viana SD, Nunes S, Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities—role of gut microbiota dysbiosis. Ageing Res Rev. 2020 doi: 10.1016/j.arr.2020.101123. NN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlot T, Penninger JM. ACE2—from the renin–angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013;15:866–873. doi: 10.1016/j.micinf.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klempin F, Valentina Mosienko V, Matthes S, Villela DC, Todiras M, Penninger JM, et al. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell Mol Life Sci. 2018;75:3625–3634. doi: 10.1007/s00018-018-2815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieben C, Blokland A, Westerink B, Deutz nep. Acute tryptophan and serotonin depletion using an optimized tryptophan-free protein–carbohydrate mixture in the adult rat. Neurochem Int. 2004;44:9–16. doi: 10.1016/s0197-0186(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 25.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94(10):5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins TA, Nguyen JCD, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8:56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatti T, Gillin JC, Seifritz E, Moore P, Clark C, Golshan S, Stahl S, et al. Effects of a tryptophan-free amino acid drink challenge on normal human sleep electroencephalogram and mood. Biol Psychiatry. 1998;43:52–59. doi: 10.1016/s0006-3223(97)80252-1. [DOI] [PubMed] [Google Scholar]
- 28.Essa MM, Hamdan H, Chidambaram SB, Al-Balushi B, Guillemin GJ, Ojcius DM, et al. Sage UK; London: 2020. Possible role of tryptophan and melatonin in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ninomiya S, Nakamura N, Nakamura H, Mizutani T, Kaneda Y, Yamaguchi K, et al. Low levels of serum tryptophan underlie skeletal muscle atrophy. Nutrients. 2020;12:978. doi: 10.3390/nu12040978. [DOI] [PMC free article] [PubMed] [Google Scholar]