Abstract
Ulcerative colitis (UC) is a frequent type of inflammatory bowel disease, characterized by periods of remission and exacerbation. Gut dysbiosis may influence pathophysiology and clinical response in UC. The purpose of this study was to evaluate whether gut microbiota is related to the active and remission phases of pancolitis in patients with UC as well as in healthy participants. Fecal samples were obtained from 18 patients with UC and clinical‐endoscopic evidenced pancolitis (active phase n = 9 and remission phase n = 9), as well as 15 healthy participants. After fecal DNA extraction, the 16S rRNA gene was amplified and sequenced (Illumina MiSeq), operational taxonomic units were analyzed with the QIIME software. Gut microbiota composition revealed a higher abundance of the phyla Proteobacteria and Fusobacteria in active pancolitis, as compared with remission and healthy participants. Likewise, a marked abundance of the genus Bilophila and Fusobacteria were present in active pancolitis, whereas a higher abundance of Faecalibacterium characterized both remission and healthy participants. LEfSe analysis showed that the genus Roseburia and Faecalibacterium were enriched in remission pancolitis, and genera Bilophila and Fusobacterium were enriched in active pancolitis. The relative abundance of Fecalibacterium and Roseburia showed a higher correlation with fecal calprotectin, while Bilophila and Fusobacterium showed AUCs (area under the curve) of 0.917 and 0.988 for active vs. remission pancolitis. The results of our study highlight the relation of gut dysbiosis with clinically relevant phases of pancolitis in patients with UC. Particularly, Fecalibacterium, Roseburia, Bilophila, and Fusobacterium were identified as genera highly related to the different clinical phases of pancolitis.
Keywords: active and remission phase, gut dysbiosis, gut microbiota, pancolitis
The purpose of this study was to evaluate whether gut microbiota is related to the active and remission phases of pancolitis in patients with ulcerative colitis, as well as in healthy participants.

1. INTRODUCTION
The intestinal tract houses a large and diverse community of microorganisms collectively referred to as the gut microbiota. These microorganisms contribute to human health by promoting both immune and metabolic functions (Burman et al., 2016; Chassaing et al., 2017). It is widely accepted that the gut microbiota has a crucial role in regulating the function of the intestinal epithelium, the immune system, and its homeostasis within the gut (Imhann et al., 2018). The term “dysbiosis” refers to an imbalance in the composition and function of the microbiota (Danilova et al., 2019; Nishida et al., 2017; Vemuri et al., 2017), whereas gut dysbiosis along with the altered host immune response has been observed in clinically relevant immunological and inflammatory diseases, such as Ulcerative Colitis (UC), which is a frequent type of Inflammatory Bowel Disease, characterized by periods of remission and exacerbation (Nishida et al., 2018). UC has been classified according to its extent and severity in the so‐called Montreal classification, defining the extent as E1 indicates ulcerative proctitis; E2 as UC on the left side; and E3 as extensive UC or pancolitis. Likewise, the severity of the disease is classified into clinical remission (S0), mild disease (S1), moderate disease (S2), and severe disease (S3). Pancolitis is considered the most serious clinical phase of UC, and its involvement represents 10%–15% of all UC (Mohammed Vashist et al., 2018).
Although a causal effect has not been evidenced; nowadays, it is widely accepted that altered interactions between gut dysbiosis and the intestinal immune system promote UC (Imhann et al., 2018), while the precise nature of the intestinal microbiota dysfunction in UC remains to be elucidated. In this sense, the gut microbiota has been considered as a “fingerprint” reflecting the natural history of UC, since it associates with the clinical severity, remission, and flare‐up responses (Marchesi et al., 2016). Gut microbiota from patients with UC has been characterized by a reduced number of bacteria with anti‐inflammatory capacities and a higher proportion of bacteria with pro‐inflammatory properties. Microbiota diversity is also reduced; low abundance of microorganisms like Firmicutes and high abundance of Proteobacteria have been found (Manichanh et al., 2012; Yu, 2018). Rapid development and application of culture‐independent, high throughput DNA‐based sequencing technologies have elicited the recognition of such dysbiotic signatures, which may play a role during the early identification of clinical‐therapeutic phases of UC, and particularly useful in severe clinical manifestations like pancolitis (Peterson et al., 2008; Rintala et al., 2017). Despite this notion, the relation of gut dysbiosis with pancolitis has been poorly characterized. Given the increasing UC prevalence worldwide, including Latin American countries (Bosques‐Padilla et al., 2011; Farrukh & Mayberry, 2014), along with the strong interest to understand the relation of dysbiotic gut microbiota with most serious phases of UC like pancolitis, the present study aimed to characterize gut microbiota from patients with UC and different clinical phases of pancolitis.
2. METHODS
2.1. Study population
In this cross‐sectional study, groups of 18 patients with UC and clinical‐endoscopic‐evidenced pancolitis (active phase n = 9 and remission phase n = 9) as well as 15 healthy participants, attended the Department of Gastroenterology, Centro Médico Nacional ‘20 de Noviembre’ ISSSTE, Mexico City, Mexico, between July 2017 and January 2019. Patients with concomitant irritable bowel syndrome, pseudomembranous colitis, and antibiotic treatment during the previous 4 weeks were excluded. Pancolitis was defined according to clinical, radiological, endoscopic, and histological criteria (Van Assche et al., 2010). All the patients had experienced at least one previous episode of pancolitis before their recruitment. The study at remission phase of pancolitis received therapy based on pharmacological treatment, a fiber‐rich diet, and the use of probiotics (Owczarek et al., 2016). Some patients with active pancolitis did not receive treatment due to non‐medical reasons, like the inability to attend their follow‐up appointment. Characteristics like age, time since disease onset, affected gastrointestinal location, frequency of bowel movements, and presence of blood in stools were collected from clinical records. Clinical activity was defined as a value of 4 or higher for colitis activity index (CAI [Clinical activity index], used for ulcerative colitis), and clinical remission was defined with CAI value <2 for at least 3 months (Siegel et al., 2018; Van Assche et al., 2010). Healthy participants were volunteers without previous history of chronic disease, belonging to a different family than those with UC, but with a similar diet, as assessed by a 24‐h recall (R24H) survey (Parks et al., 2018).
2.2. Stool samples
Stool samples were collected either during hospitalization (active pancolitis) or prepared at home and collected during programmed medical consultation (remission phase and healthy participants); samples were stored at home between 4 and 8°C for up to 24 h, before hospital collection. Samples were collected with the help of a stool sampling kit, which consisted of a plastic lining to cover the toilet, two stool sample tubes with spoons, two plastic bags, and a clipping system for safe closure of the outer bag. Samples were labeled upon arrival, and one part was processed for fecal calprotectin assay; while the remaining was aliquoted and frozen directly at −80°C for further microbiota analyses (Tedjo et al., 2015).
2.3. DNA extraction of fecal samples
Frozen stool samples were thawed on ice, and approximately 200 mg were added to dry‐bead tubes with lysis buffer (AllPrep PowerFecal DNA, Qiagen). The stool samples were homogenized followed by a combined chemical and mechanical lysis by using prefilled lysis tubes. Inhibitors commonly present in stool samples were then removed before isolation of nucleic acids. DNA isolation was continued by using the AllPrep DNA MiniElute spin column, according to the manufacturer's instructions. DNA was eluted in 30 μl EB‐buffer. Negative control samples (consisted only of PCR grade water) were handled in the same way as the fecal samples, to rule out contamination during the isolation procedure (Tedjo et al., 2015). A Nanodrop ND‐1000 (NanoDrop Technologies), was used to estimate DNA concentrations. DNA concentration was adjusted to a final concentration of 10 ng/µl (Tedjo et al., 2016).
2.4. Amplification and sequencing of bacterial 16S rRNA gene
The V3 and V6 hypervariable regions of the 16S rRNA gene were PCR amplified from microbial genomic DNA with the forward (TAT GGT AAT TGT GTG CCA GCM GCC GCG GTA A) and reverse (GGA CTA CHV GGG TWT CTA AT) primers. The primers were designed with overhanging adapters (Forward: AATGATA CGGC GACC ACCGA GATCT ACAC), (Reverse: GGA CTA CHV GGG TWT CTA AT) for annealing to Illumina universal index sequencing adaptors that were added in a later PCR (Dubinsky & Braun, 2015; Haas et al., 2011). The PCR products were evaluated by 2% agarose gel electrophoresis and purified. After purification, spectrophotometry was used to quantify the PCR products. Samples were normalized to a final concentration of 2 nM.
2.5. Microbial composition and analysis by Illumina
A two‐steps PCR methodology was used to prepare 16S rRNA libraries. For the first‐step, extracted DNA was quantified and samples were diluted to the amount of the least concentrated sample. Then 2 μL were used for the PCR reaction (quadruplicates) at the following conditions: 98°C for 30 s [98°C for 30 s, 52°C for 30 s, 72°C for 30 s] for 20 cycles, 4°C hold. Then, the 4 resulting reactions were amalgamated. The samples were then cleaned by using AmpureXP beads and eluted in 40 μL final volume. For the second step, a 4 μL of the obtained DNA was mixed with primers PE‐PCR‐III‐F and PE‐PCR‐IV‐barcode, in a 25 μL final volume PCR reaction (quadruplicates), at run cycle conditions of 98°C for 30 s [98°C for 30 s, 83°C for 30 s, 72°C for 30 s] for 7 cycles, 4°C hold. Then, the 4 PCR reactions were pooled and the products were cleaned by using 16S Metagenomic Sequencing Purification beads (Caporaso et al., 2012). The DNA library concentrations were quantified and then multiplexed to provide the same amount of DNA in each sample. A single Illumina MiSeq lane set for paired‐end 300‐basepair reads was used to sequence the libraries. Paired‐end reads of 16S rRNA gene libraries were generated with the Illumina, MiSeq platform. A total of 10,629,314 raw sequences were obtained, with further quality filter and binned resulting in 8,349,697 usable sequences, with a sample average of 378,489 per sequence. Sequences were clustered and singletons removed; the data were rarefied to control for variations in sequencing efforts. The datasets supporting the conclusions of this article are available in https://www.ncbi.nlm.nih.gov/bioproject/596546, under the ID PRJNA596549 repository. The analyses of taxonomy and diversity of the samples were performed taking as a reference the SILVA database (Bokulich et al., 2013; Bolyen et al., 2018).
2.6. Bioinformatic analysis
The Illumina Real‐Time Analysis software (version 1.17.28) was used for base calling, image analysis, and error estimation. Sequencing provided read lengths of 300 bp, which were demultiplexed, verifying that the paired ends provided a clear overlap. The paired ends were then linked together with the fastq‐join program (http://code.google.com/p/ea‐utils/). Separate files of each sample (R1 and R2) were entered in fastq format by using the split_libraries_fastq.py pipelines. Sequences that had quality value (QV) scores of ≥20 (Phred score of 20) for no‐less than 99% of the sequence were selected for further study. All sequences with ambiguous base calls were discarded. Subsequently, the sequences were grouped in Operational Taxonomic Units (OTU), where the pick_closed_reference_otus.py pipelines were used. QIIME, which uses the BIOM format, was used to represent OTU tables (Bolyen et al., 2018; Dubinsky & Braun, 2015; Edgar et al., 2011). Analyses of sequence reads were performed by using SILVA multiclassifier tools with a 97% confidence threshold (Navas‐Molina et al., 2013). Subsequent analyses of diversity index were all performed based on this output normalized data (Allali et al., 2017; Aßhauer et al., 2015). To perform the diversity analyses, the core_diversity_analyses.py pipelines were executed with the pipeline alpha_diversity.py. Alpha diversity metrics were calculated with QIIME, that is, the observed OTUs (observed species) and the phylogenetic diversity or complete tree PD (PD_whole_tree) (Bolyen et al., 2018); whereas the weighted distances of UniFrac of the beta diversity were determined with beta_diversity.py pipelines, and the R software v.2.15.3 was used to display the results (Barwell et al., 2015; Chao et al., 2006; Hass et al., 2011). The “Linear discriminant analysis (LDA) effect size (LEfSe)” algorithm was performed with the Galaxy online platform to determine the different relative abundances of bacterial communities among the different groups of patients. The significance thresholds used were those recommended in the program. LEfSe considered statistical significance between the different biological classes with a Kruskal–Wallis test and subsequently analyzed the biological significance with a Wilcoxon test (Segata et al., 2011).
2.7. Fecal calprotectin test
Fecal calprotectin (FC) was measured as a marker of intestinal inflammation by using a commercial ELISA (MyBioSource), following the manufacturer's instructions. Optical densities were read at 405 nm with a microplate ELISA reader. Samples were tested in duplicate, and results were calculated from a standard curve and expressed as μg/g stool (Chang & Cheon, 2018).
2.8. Statistical analysis
Data normality was evaluated with the Shapiro–Wilk test. Quantitative data were compared by non‐paired, two‐tail, t test, or U‐Mann Whitney, as appropriate. Analyses of the sequences were carried out in the QIIME and R software. Multivariate nonparametric ANOVA was used to determine the differences in the abundance of the microbial community between groups, whereas Unifrac was used to compare the abundance of the specific microbiota and the concentration of fecal calprotectin, and it was visualized by principal coordinate analysis. To test whether the clusters of microbiota from the study conditions were different between them, Unifrac p‐values, based on principal coordinate analysis applied to the matrix distance, were performed to allow pairwise comparison of microbiota from clinical phases of pancolitis and healthy controls (Caporaso et al., 2010; Lawley & Tannock, 2017). Finally, the Area Under the Curve (AUC) was calculated to explore whether the relative abundance of the bacterial genus most frequently observed (cutoff value according to ROC analysis) may predict UC severity. The Statistical Package for Social Sciences SPSS v.18.0. was used, and p‐values of ≤0.05 (2‐tailed) were considered to be statistically significant.
3. RESULTS
3.1. Study population
Eighteen patients diagnosed with UC and pancolitis, mean aged 37‐years‐old constituted the study population, who were further divided according to the disease activity, as demonstrated by the CAI and fecal calprotectin values. A cohort of sex‐ and age‐matched, healthy volunteers were included for comparison. Baseline clinical‐demographic characteristics are shown in Table 1. Stools from patients with active pancolitis were characterized by being watery, corresponding to Bristol type 7, and bloody (two points in rectal bleeding of Mayo Clinical Score) in all cases.
TABLE 1.
Demographic and clinical characteristics of the study population (n = 33)
|
AP (n = 9) |
RP (n = 9) |
HS (n = 15) |
p‐value | |
|---|---|---|---|---|
| Age (years old) | 36.9 ± 1.4 | 37.9 ± 1.1 | 36. 4 ± 1.6 | NS |
| Male | 7 (77.7) | 6 (66.6) | 6 (40) | NS |
| Index CAI | 11.0 ± 1.3 | 1.7 ± 0.6 | N/A | <0.05 |
| Montreal A (age at onset) | ||||
| A1 (16) | None | None | N/A | NS |
| A2 (17–40) | 7 (77.7) | 6 (66.6) | ||
| A3 (41) | 2 (22.2) | 3 (33.3) | ||
| Montreal Score Extensive | ||||
| E1 ulcerative proctitis | None | None | N/A | NS |
| E2 left‐sided UC | None | None | ||
| E3 extensive UC | 9 (100) | 9 (100) | ||
| Montreal Score Severity | ||||
| S0 silent colitis | None | 9 (100) | N/A | NS |
| S1 mild colitis | None | None | ||
| S2 moderate colitis | None | None | ||
| S3 severe colitis | 9 (100) | None | ||
| Endoscopy Mayo Score | ||||
| 0 | NONE | N/A | N/A | N/A |
| 1 | NONE | |||
| 2 | NONE | |||
| 3 | 9 (100) | |||
| Frequency of bowel movements | ≥ 10 | 2 to 4 | 1 to 2 | NS |
| Presence of blood in stools | 9 (100) | None | None | NS |
| Time (years) from diagnosis | ||||
| ≥10 | 8 (88.8) | 6 (66.6) | N/A | NS |
| ≤10 | 1 (11.1) | 3 (33.3) | ||
| Currently smoking | 2 (22.2) | None | None | N/A |
| Medication use | ||||
| Mesalazine | None | 6 (66.6) | N/A | NS |
| Corticosteroids | 2 (22.2) | 2 (22.2) | ||
| Infliximab | none | 1 (11.1) | ||
| No treatment | 7 (77.7) | None | ||
| Fecal calprotectin (μg/g) | 480.1 ± 13.7 | 99.6 ± 8.9 | 21.6 ± 4.3 | p < 0.05 |
Quantitative data were resumed as mean ± SD and qualitative data as n (%). Statistical analysis was performed with a two‐way U‐Mann Whitney and Fisher's test, as appropriate.
Abbreviations: AP, active pancolitis; HS, healthy subjects; N/A, not applicable; NS, non‐significant; RP Remission pancolitis.
3.2. Microbial composition and diversity
The analysis of microbiome from fecal samples showed the relative abundance of OTUs at different taxonomic levels (Figure 1a, b, Table 2). OTUs were created out of the filtered tags and were grouped at a similarity of 97%. This gave a total of 1533 OTUs for the 33 samples used in this study. Taxonomic composition at the level of phyla is summarized in Figure 1a. The bacterial phyla Firmicutes, Bacteroidetes, Proteobacteria, and Fusobacteria were the most common sequences showing 97% of similarity. For remission pancolitis and healthy participants, Firmicutes was the most abundant bacterial phylum. Microbiota abundance in remission pancolitis was very similar to that observed in healthy participants, at the phyla level; whereas, active pancolitis showed phylum Proteobacteria as the most abundant. Genus distribution provided a subjective perception of the difference between the relative abundance of patients with active vs. remission pancolitis and healthy participants (Figure 1b) The most abundant genera in active pancolitis were Fusobacterium and Bilophila. For the group of remission pancolitis and healthy participants, the most abundant genera were Faecalibacterium, Roseburia, and Bacteroides (Appendix: Table A1, A2, and A3). Regarding bacterial alpha diversity comparison, pancolitis activity was related to the lowest community richness (Chao index) and diversity (Shannon index) (Figure 1c, d), whereas community richness and diversity were similar between remission pancolitis and healthy participants. Likewise, significant differences in species dominance of microbiota (Simpson index) (Figure 1e) were found between active vs. remission pancolitis and healthy participants (Appendix: Table A4).
FIGURE 1.
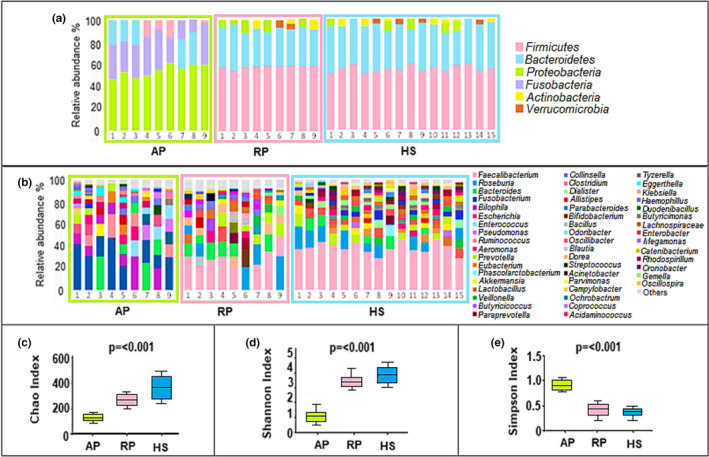
Characteristics of the microbial community in pancolitis, remission, and healthy participants. (a) Taxonomic composition distribution in samples of phylum level. (b) The taxonomic composition distribution in samples of genus level. (c) Alpha diversity index boxplot, including community richness (Chao), (d) diversity (Shannon), and (e) Dominance (Simpson). the p‐value indicates the statistical significance of two‐way ANOVA. Abbreviations: AP, active pancolitis; HS, healthy subjects; RP, Remission pancolitis
TABLE 2.
Comparison of gut dysbiosis in fecal samples from the pancolitis population
| Most abundant gut microbiota |
AP (n = 9) |
RP (n = 9) |
HS (n = 15) |
|
|---|---|---|---|---|
| Phylum | Firmicutes | 4.0 ± 1.5*/** | 50.0 ± 5.2 | 54.6 ± 6.4 |
| Bacteroidetes | 15.0 ± 0.2*/** | 46.0 ± 4.2 | 45.0 ± 3.4 | |
| Proteobacteria | 52.5 ± 5.6*/** | 0.0 ± 0.0 | 2.5 ± 1.0 | |
| Fusobacteria | 30.0 ± 2.5*/** | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Actinobacteria | 1.5 ± 0.5 | 2.5 ± 1.0 | 2.5 ± 1.0 | |
| Verrucomicrobia | 0.0 ± 0.0 | 1.5 ± 0.3 | 1.5 ± 0.5 | |
| Genus | Lactobacillus | 0.0 ± 0.0*/** | 5.6 ± 4.2 | 8.5 ± 2.4 |
| Faecalibacterium | 0.5 ± 1.5*/** | 21.0 ± 8.7*** | 40.2 ± 4.9 | |
| Roseburia | 0.0 ± 0.0*/** | 5.4 ± 7.2*** | 7.3 ± 7.4 | |
| Bacteroides | 7.6 ± 4.1 | 11.5 ± 10.8*** | 3.5 ± 2.1 | |
| Bilophila | 12.0 ± 9.1*/** | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Fusobacterium | 35.6 ± 15.4*/** | 0.0 ± 0.0 | 0.0 ± 0.0 |
Relative abundance is shown as mean ± SD and (†) percentage of the relative abundance in relation to that observed in healthy participants. Statistical analysis was performed with two‐way ANOVA. Significant difference (p < 0.01) between: (*) AP vs. RP; (**) AP vs. HS; (***) RP vs. HS.
Abbreviations: AP, active pancolitis; HS, healthy subjects; RP, Remission pancolitis.
Interestingly, the relative abundance of the most frequent bacterial genus observed in active pancolitis was significantly different from those corresponding to remission pancolitis and healthy participants (Table 2; Figure 2).
FIGURE 2.
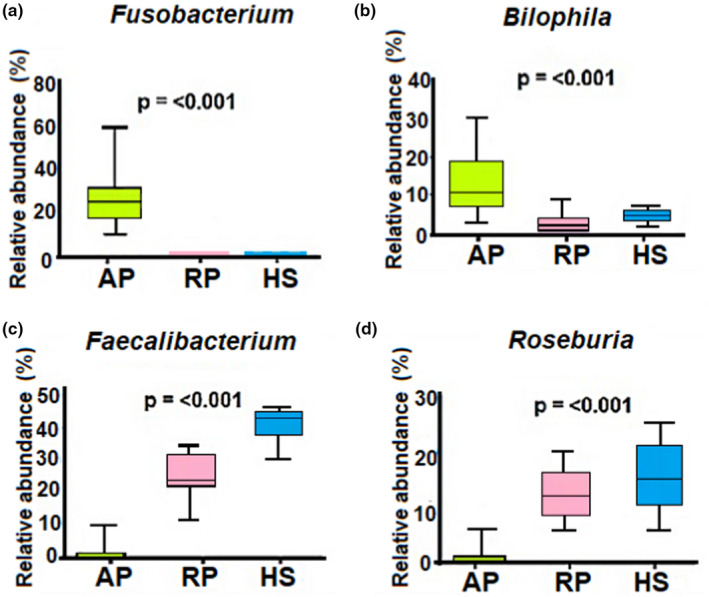
Abundance analyses. Whisker‐box plots comparing bacterial genera in the fecal microbiota of pancolitis, remission, and healthy participants. Only the four most relevant bacterial genera, according to abundant taxonomic composition, were analyzed: (a) Fusobacterium; (b) Bilophila; (c) Faecalibacterium, and (d) Roseburia. the p‐value indicates the statistical significance of two‐way ANOVA. Abbreviations: AP, active pancolitis; HS, healthy subjects; RP, Remission pancolitis
The structure of the most abundant microbial species was explored with the biomarker of UC severity, fecal calprotectin; where the clusters of the different phases are significantly separated based on fecal calprotectin (Figure 3).
FIGURE 3.
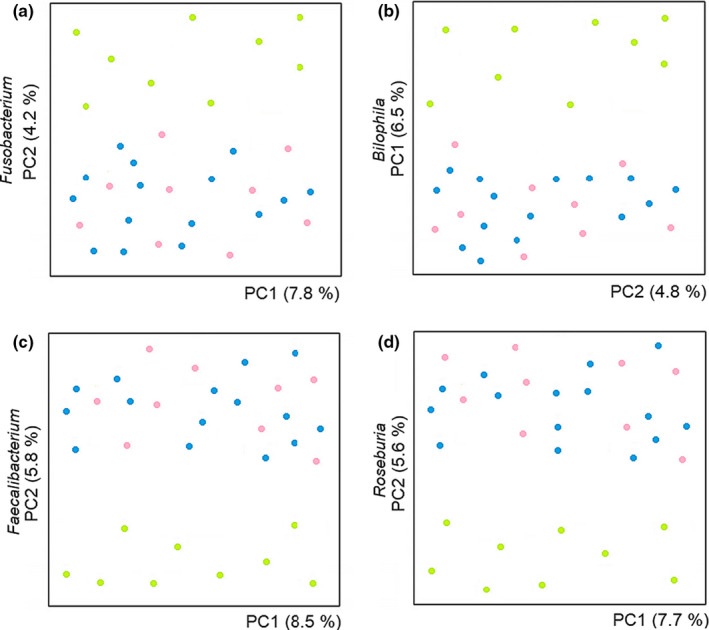
Gut microbiota abundance and UC severity marker. The plots show the clusters between the bacterial genera in the fecal microbiota with calprotectin, a biomarker of UC severity. The four most abundant bacterial genera, according to abundant taxonomic composition: (a) Fusobacterium; (b) Bilophila; (c) Faecalibacterium, and (d) Roseburia; were analyzed in the subgroups of active pancolitis (green), remission pancolitis (pink), and healthy participants (blue)
The microbial community structure was investigated by using the principal component analysis, which allows splitting particular microbial communities according to their potential relation with clinical scenarios. Highly defined microbiota clusters were distributed according to clinical phases of pancolitis, where the distribution of active pancolitis clusters was significantly separated from those of remission pancolitis and healthy participants, showing these two last clusters a closer distribution between them (Figure 4).
FIGURE 4.
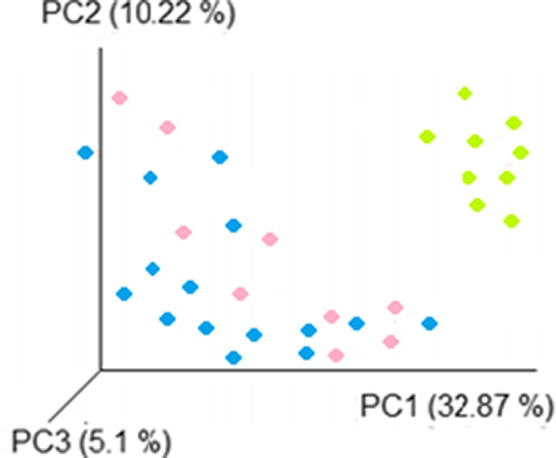
Principal component analysis. The overall structure of the fecal microbiota was plotted according to the different clinical scenarios (active pancolitis [green], remission pancolitis [pink], and healthy participants [blue]). Each data point represents an individual sample
Linear discriminant analysis (LDA) effect size (LEfSe) was used to determine differentially abundant bacterial taxa between active and remission pancolitis. Patients with active pancolitis were related to the phylum Proteobacteria and Fusobacteria, while patients in remission pancolitis were related to the phylum Firmicutes and Bacteroidetes (α = 0.01, LDA score >3.0) (Figure 5; Appendix: Table A5).
FIGURE 5.
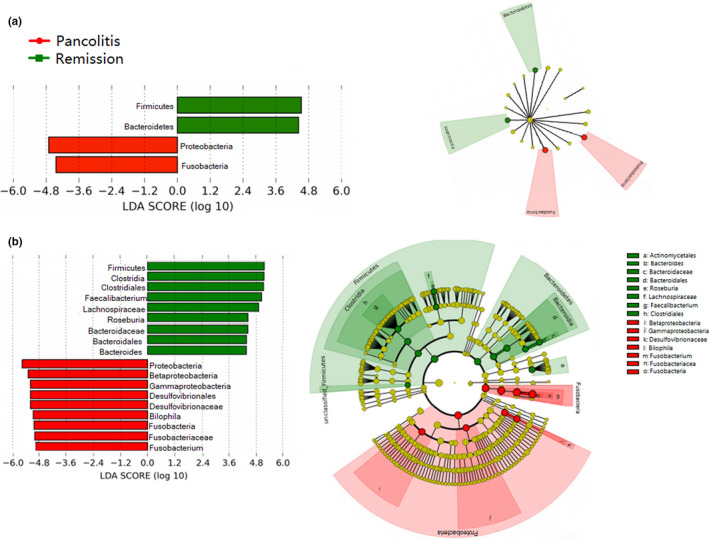
The linear discriminant analysis effect size (LEfSe) analysis of fecal microbiota with active pancolitis vs. remission pancolitis. (A) Bar graph showing LDA scores of phylum and cladogram generated by LEfSe indicating differences at phylum among active pancolitis and remission pancolitis. (b) Bar graph showing LDA scores of genus and cladogram generated by LEfSe indicating differences at genus among active pancolitis and remission pancolitis. Each successive circle represents a phylogenetic level. Regions in red indicate taxa enriched in active pancolitis, while regions in green indicate taxa enriched in remission pancolitis. Differing genera are listed on the right side of the cladogram
Finally, we explored whether the relative abundance of the bacterial genus most frequently observed (cutoff values according to ROC analysis: active vs. remission pancolitis Bilophila 10%, Faecalibacterium 40%; pancolitis vs. healthy participants Bilophila 10%, Fusobacterium 10%) were related with active pancolitis. Bilophila and Fusobacterium showed an AUC of 0.917 and 0.988 for active vs. remission pancolitis, while similar AUCs were observed for active pancolitis vs. healthy participants, but not for remission pancolitis vs. healthy participants (Appendix: Table A6).
4. DISCUSSION
Our main finding was the significant differences of fecal microbiota composition from patients with active vs. remission pancolitis, with potential clinical application. Our study population was constituted of young aged patients with UC and severe stage of pancolitis. Scarce studies have explored gut microbiota in such population, probably due to the low prevalence of pancolitis between cases with UC. However, gut dysbiosis observed in patients with active vs. remission pancolitis in the present study is comparable with other reports (Alam et al., 2020; Danilova et al., 2019; Franzosa et al., 2019; Halfvarson et al., 2017; Imhann et al., 2018; Kumari et al., 2013; Sha et al., 2013). Our results were further validated by comparison with gut microbiota from healthy participant controls from a family who shares a similar diet and they are expected to exert a lower influence on the gut microbiota composition.
Our results showed an increased proportion of the phylum Proteobacteria and the genera Fusobacterium and Bilophila in active pancolitis, which was significantly different from the group of remission pancolitis and healthy participants, who shared a microbiota profile of higher proportion of phylum Firmicutes, and genera Faecalibacterium and Roseburia (Vester‐Andersen et al., 2019). These results are similar to those obtained by Franzosa et al., 2019, Kumari et al., 2013; Sha et al., 2013. Particularly, the findings of a reduced proportion of the genera Faecalibacterium and Roseburia in active pancolitis, and their restoration in remission pancolitis, has also been observed in previous reports (Khan et al., 2019; Man et al., 2011; Palmela et al., 2018; Vigsnæs et al., 2012). Such characterization is relevant due to scanty information regarding microbiota abundance in the remission phase of pancolitis, whereas consistent identification of specific genus in the remission phase may be useful to design more efficient therapeutic strategies, prompted to reduce UC severity. Interestingly, a particular bacterial composition like Faecalibacterium was shared by remission pancolitis and healthy participants. These bacteria have been reported to metabolize dietary components that promote colonic motility, maintain the intestinal immune system, and anti‐inflammatory properties (Dicks et al., 2018). Consistently, reduced abundance of these microorganisms has been associated with a higher rate of recurrence of UC (Alam et al., 2020; Al‐Bayati et al., 2018; Ferreira‐Halder et al., 2017; Kinross et al., 2011; Lopez‐Siles et al., 2017; Machiels et al., 2014) although increased levels of Faecalibacterium in stool samples have been associated with a lower activity index, supporting their role as potential biomarkers of disease severity and outcome, as suggested in other studies (Paramsothy et al., 2019; Wang et al., 2018).
Other findings were the higher abundance of the phylum Proteobacteria, and particularly the expansion of the genus Bilophila in active pancolitis. It is known that the relative abundance of Bilophila is promoted by diets enriched in saturated fats, which increase bacterial resistance to bile elimination. Furthermore, a change in the type of fat consumed affects the composition of gut microbiota, which may modify the onset and severity of UC (Devkota & Chang, 2015; Pittayanon et al., 2020; Torres et al., 2018). Dietary modifications involving excessive consumption of fried food, dairy products, and wheat flour are associated with the development of severe diarrhea in patients with active pancolitis (Keshteli et al., 2019). In the present study, we consider that there is no significant effect derived from the modification of the diet, since the population consumed a soft diet with abundant hydration; without a specific recommendation for dietary restrictions, even during active pancolitis.
Certain species of Fusobacterium show pro‐inflammatory, invasive, and adherent capacity to the intestinal mucosa, while the increased proportion of Bilophila in the gut promotes an immune response mediated by Th1, resulting in the development of colitis in experimental mice models (Bashir et al., 2016; Chen et al., 2020; Hirano et al., 2018; Liu et al., 2019; Ohkusa et al., 2002; Tahara et al., 2015; Wright et al., 2015). Although a direct pathophysiological mechanism is not possible to elucidate from the present study, we can propose that the relative abundance of some species is associated with the degree of inflammation and pancolitis, derived from the inverse relationship observed between the abundance of Fecalibacterium and Roseburia with calprotectin, a biomarker of severity of UC, which was consistent with a recent report (Björkqvist et al., 2019; Yu et al., 2019). Likewise, differences in bacterial richness, diversity, and dominance were highly related to the clinical scenarios studied. Remarkably, remission pancolitis and healthy participants showed the highest relative abundance of the phylum Firmicutes, which contributed to most of the bacterial diversity and richness (Björkqvist et al., 2019; Ganji‐Arjenaki & Rafieian‐Kopaei, 2018; Jandhyala et al., 2015). Further analyses of cluster distribution of bacterial communities showed differences in active pancolitis, as compared to remission pancolitis and healthy participants, which was consistent with previous studies showing a difference in the structure of microbiota between active pancolitis and healthy participants (Forbes et al., 2016; Havenaar, 2011; Louis & Flint, 2017).
Furthermore, studies characterizing gut microbiota composition and its modification during pancolitis are relevant, since (a) pancolitis provides a higher risk for colorectal cancer, whereas gut dysbiosis is thought to facilitate colorectal cancer development; (b) the study of gut microbial communities during clinical phases of pancolitis contributes to a better understanding of potential interactions with the host immune response; (c) characterization of a specific genus of gut microbial communities may own potential clinical application derived from their association with pancolitis or remission phases; and (d) specific microbial manipulation, concomitant to antibiotic use, is currently used as a therapeutic approach for UC (Alard et al., 2018; Devkota & Chang, 2015; Galazzo et al., 2019).
Finally, gut dysbiosis has been proposed as an important contributing factor to the increasing prevalence of pancolitis, with a potential role for the related clinical‐therapeutic phases (Halfvarson et al., 2017; Miyoshi et al., 2018; Petrof et al., 2013). Consistently, we found a significant ability of the genus Bilophila and Fusobacterium to selectively associate with cases of activity/remission pancolitis (Fukuda & Fujita, 2014; Guo et al., 2019).
To our knowledge, this is the first study that investigated the composition of fecal microbiota in Mexican patients with active and remission pancolitis. Our study faces some limitations. First, 16S rRNA analysis provides the taxonomic composition of the microbes present in the community and does not provide an analysis of the role of the microbiota in the disease. Second, data analysis may show limitations regarding the specific characterization of microbiota composition as an isolated endpoint; however, we think that the analysis performed yields an adequate interpretation within a translational context, highlighting the role of microbiota diversity in the clinical phases of pancolitis. Third, larger sample size may be required to confirm our data and further research is required to better characterize the role of gut microbiota in patients with pancolitis.
Here, we provide a broad investigation of the fecal microbial community in Mexican patients presenting pancolitis. We demonstrate differences in the microbiota communities in patients with active pancolitis, remission pancolitis, and healthy participants. Selective association of gut dysbiosis with active/remission pancolitis may set the basis for further applications of non‐invasive methods, clinically useful for early identification of disease severity.
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Brenda Maldonado Arriaga: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Sergio Sandoval‐Jimenez: Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Juan Rodriguez ‐Silverio: Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Sofía Lizeth Alcaráz‐Estrada: Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Tomás Cortés‐Espinosa: Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Rebeca Pérez‐Cabeza de Vaca: Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Cuauhtémoc Licona‐Cassani: Data curation (equal); Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). July Stephany Gámez‐Valdez: Data curation (equal); Formal analysis (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Jonathan Shaw: Formal analysis (equal); Investigation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Paul Mondragón‐Terán: Investigation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Cecilia Hernández‐Cortez: Writing‐original draft (equal); Writing‐review & editing (equal). Juan Antonio Suárez‐Cuenca: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). GRACIELA CASTRO‐ESCARPULLI: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
ETHICS STATEMENT
The study was carried out according to the 1975 ethical guidelines of the Declaration of Helsinki. All participants provided written informed consent. The study was approved by the Local Committees of Research, Ethics in Research and Biosafety of the Centro Médico Nacional ‘20 de Noviembre’ ISSSTE, Mexico City (Protocol ID No. 358.2017).
ACKNOWLEDGMENTS
This study was funded by the E‐015 institutional program and Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP 20200675 and 20194936) (IPN). BMA held a scholarship from CONACyT. GCE received support from Estímulos al Desempeño en Investigación, Comisión y Fomento de Actividades Académicas (Instituto Politécnico Nacional), and Sistema Nacional de Investigadores (SNI, CONACyT), CHC received support from Sistema Nacional de Investigadores (SNI, CONACyT). We would like to thank Sofia Mulia for kindly correcting the style of the manuscript.
1.
TABLE A1.
List of bacterial taxa with their OTUs ID in active pancolitis
| No. | OTUs ID | Bacteria | No. | OTUs ID | Bacteria |
|---|---|---|---|---|---|
| 1 | 925 | Brenneria | 49 | 471278 | Buttiauxella |
| 2 | 1006 | Curtobacterium | 50 | 479863 | Shimwellia |
| 3 | 1163 | Microbacteriaceae | 51 | 484145 | Enterobacteriaceae |
| 4 | 4981 | Rothia | 52 | 489853 | Cronobacter |
| 5 | 5294 | Micrococcaceae | 53 | 498961 | Klebsiella |
| 6 | 5605 | Trueperella | 54 | 501249 | Enterobacteriaceae |
| 7 | 6509 | Actinomycetaceae | 55 | 504890 | Phocoenobacter |
| 8 | 7967 | Actinomyces | 56 | 526378 | Pasteurellaceae |
| 9 | 8584 | Actinomycetaceae | 57 | 528975 | Pasteurellaceae |
| 10 | 9376 | Anaerofilum | 58 | 542569 | Mannheimia |
| 11 | 10096 | Ruminococcaceae | 59 | 554567 | Actinobacillus |
| 12 | 10256 | Sporosarcina | 60 | 561469 | Aggregatibacter |
| 13 | 10458 | Planococcaceae | 61 | 566893 | Pasteurellaceae |
| 14 | 10725 | Gemella | 62 | 576178 | Haemophilus |
| 15 | 11203 | Bacillales | 63 | 580361 | Pasteurellaceae |
| 16 | 14506 | Pediococcus | 64 | 603577 | Fusobacteriaceae |
| 17 | 14625 | Lactobacillaceae | 65 | 640658 | Fusobacteriaceae |
| 18 | 15213 | Tetragenococcus | 66 | 648973 | Ilyobacter |
| 19 | 16721 | Enterococcaceae | 67 | 689746 | Propionigenium |
| 20 | 19563 | Delftia | 68 | 704128 | Fusobacteriaceae |
| 21 | 125300 | Comamonadaceae | 69 | 728910 | Bacteroides |
| 22 | 128695 | Burkholderiales | 70 | 731897 | Bacteroidaceae |
| 23 | 132065 | Neisseriaceae | 71 | 735981 | Blautia |
| 24 | 136219 | Rhodospirillum | 72 | 765640 | Lachnospiraceae |
| 25 | 139865 | Rhodospirillaceae | 73 | 777038 | Aeromonas |
| 26 | 174563 | Rhodospirillales | 74 | 796328 | Aeromonadaceae |
| 27 | 176398 | Gemmiger | 75 | 857896 | Coprococcus |
| 28 | 182397 | Hyphomicrobiaceae | 76 | 871259 | Lachnospiraceae |
| 29 | 187569 | Hyphomicrobiaceae | 77 | 901257 | Ruminococcaceae |
| 30 | 195637 | Rhizobiales | 78 | 943078 | Ruminococcus |
| 31 | 198759 | Campylobacterales | 79 | 963365 | Ruminococcaceae |
| 32 | 201289 | Campylobacter | 80 | 1005973 | Pseudomonas |
| 33 | 206986 | Campylobacteraceae | 81 | 1019639 | Pseudomonadaceae |
| 34 | 217893 | Campylobacterales | 82 | 1025697 | Enterococcus |
| 35 | 241479 | Pseudomonadales | 83 | 1056986 | Enterococcaceae |
| 36 | 274893 | Aeromonadaceae | 84 | 1078963 | Bacilli |
| 37 | 289963 | Shewanella | 85 | 1086935 | Escherichia |
| 38 | 301329 | Shewanellaceae | 86 | 1087789 | Fusobacterium |
| 39 | 325698 | Alteromonadales | 87 | 1096348 | Fusobacteriaceae |
| 40 | 341449 | Enterobacteriaceae | 88 | 1098986 | Fuobacteria |
| 41 | 371893 | Yersinia | 89 | 1118963 | Bilophila |
| 42 | 378963 | Providencia | 90 | 1116035 | Desulfovibrionaceae |
| 43 | 390132 | Pantoea | 91 | 1131789 | Desulfovibrionales |
| 44 | 405698 | Enterobacteriales | 92 | 1135348 | Deltaproteobacteria |
| 45 | 424147 | Citrobacter | 93 | 1137986 | Enterobacteriaceae |
| 46 | 444893 | Raoultella | 94 | 1142963 | Gammaproteobacteria |
| 47 | 459993 | Kluyvera | 95 | 1145350 | Enterobacteriaceae |
| 48 | 461329 | Enterobacteriaceae | 96 | 1147789 | Betaproteobacteria |
TABLE A2.
List of bacterial taxa with their OTUs ID in remission pancolitis
| No. | OTUs ID | Bacteria | No. | OTUs ID | Bacteria |
|---|---|---|---|---|---|
| 1 | 9012 | Peptococcus | 49 | 502589 | Pediococcus |
| 2 | 9378 | Trueperella | 50 | 527810 | Lactobacillus |
| 3 | 9646 | Actinomycetaceae | 51 | 530182 | Pilibacter |
| 4 | 9899 | Peptostreptococcaceae | 52 | 537891 | Enterococcaceae |
| 5 | 10463 | Dialister | 53 | 540128 | Lactobacillales |
| 6 | 10896 | Adlercreutzia | 54 | 562189 | Anaerococcus |
| 7 | 11302 | Bacteroides | 55 | 567812 | Peptoniphilus |
| 8 | 14567 | Bacteroidaceae | 56 | 572143 | Parvimonas |
| 9 | 14996 | Clostridiales | 57 | 577810 | Clostridiales |
| 10 | 15027 | Ruminococcaceae | 58 | 583071 | Pseudoramibacter |
| 11 | 15201 | Atopobium | 59 | 594777 | Eubacterium |
| 12 | 16457 | Lactobacillus | 60 | 617802 | Eubacteriaceae |
| 13 | 16830 | Collinsella | 61 | 631492 | Clostridiales |
| 14 | 18473 | Bradyrhizobium | 62 | 637490 | Peptococcus |
| 15 | 18990 | Rikenellaceae | 63 | 658714 | Peptococcaceae |
| 16 | 117963 | Clostridiaceae | 64 | 669748 | Acetanaerobacterium |
| 17 | 118634 | Bifidobacterium | 65 | 689816 | Ruminococcus |
| 18 | 119012 | Bifidobacteriaceae | 66 | 705933 | Butyricicoccus |
| 19 | 119986 | Peptostreptococcaceae | 67 | 729801 | Flavonifractor |
| 20 | 122479 | Catenibacterium | 68 | 742137 | Clostridium IV |
| 21 | 126239 | Bacteroidales | 69 | 775910 | Ruminococcaceae |
| 22 | 129301 | Barnesiellaceae | 70 | 778426 | Parasporobacterium |
| 23 | 134203 | Erysipelotrichaceae | 71 | 798763 | Lachnospiraceae |
| 24 | 137748 | Prevotella | 72 | 825479 | Dorea |
| 25 | 150193 | Phascolarctobacterium | 73 | 876900 | Coprococcus |
| 26 | 189760 | Parabacteroides | 74 | 889861 | Lachnospiraceae |
| 27 | 199127 | Porphyromonas | 75 | 918733 | Ruminococcaceae |
| 28 | 215079 | Odoribacter | 76 | 934759 | Peptostreptococcus |
| 29 | 215630 | Butyricimonas | 77 | 989744 | Peptostreptococcaceae |
| 30 | 217998 | Barnesiella | 78 | 1012989 | Anaerostipes |
| 31 | 260327 | Porphyromonadaceae | 79 | 1026597 | Lachnospiraceae |
| 32 | 269160 | Prevotella | 80 | 1044390 | Pediococcus |
| 33 | 285496 | Prevotellaceae | 81 | 1073619 | Lactobacillaceae |
| 34 | 290112 | Alistipes | 82 | 1079801 | Enterococcus |
| 35 | 301028 | Rikenellaceae | 83 | 1093607 | Bacteroides |
| 36 | 303241 | Staphylococcus | 84 | 1107218 | Bacteroidaceae |
| 37 | 339875 | Staphylococcaceae | 85 | 1128970 | Bacteroidales |
| 38 | 340178 | Bacillales | 86 | 1140126 | Enterococcaceae |
| 39 | 346920 | Aerococcus | 87 | 1149566 | Clostridiales |
| 40 | 375984 | Aerococcaceae | 88 | 1151490 | Lachnospiraceae |
| 41 | 379617 | Granulicatella | 89 | 1156301 | Blautia |
| 42 | 398863 | Carnobacteriaceae | 90 | 1189612 | Ruminococcaceae |
| 43 | 420159 | Weissella | 91 | 1211437 | Roseburia |
| 44 | 426713 | Leuconostocaceae | 92 | 1237019 | Lachnospiraceae |
| 45 | 456906 | Enterococcaceae | 93 | 1240789 | Clostridiales |
| 46 | 479802 | Streptococcus | 94 | 1246772 | Faecalibacterium |
| 47 | 481590 | Streptococcaceae | 95 | 1250116 | Ruminococcaceae |
| 48 | 499301 | Lactobacillales | 96 | 1257301 | Clostridiales |
TABLE A3.
List of bacterial taxa with their OTUs ID in healthy participants
| No. | OTUs ID | Bacteria | No. | OTUs ID | Bacteria |
|---|---|---|---|---|---|
| 1 | 11456 | Rhodospirillum | 49 | 583010 | Dorea |
| 2 | 11895 | Duodenibacillus | 50 | 588322 | Roseburia |
| 3 | 11634 | Lactobacillales | 51 | 593701 | Lachnospiraceae |
| 4 | 12581 | Collinsella | 52 | 601086 | Faecalibacterium |
| 5 | 12988 | Atopobium | 53 | 605865 | Clostridiales |
| 6 | 16217 | Ruminococcaceae | 54 | 612789 | Bifidobacterium |
| 7 | 18759 | Bacteroidales | 55 | 645277 | Lachnospiraceae |
| 8 | 24663 | Enterococcus | 56 | 648712 | Blautia |
| 9 | 169384 | Leuconostocaceae | 57 | 657899 | Ruminococcaceae |
| 10 | 183691 | Bacteroidaceae | 58 | 678970 | Sutterella |
| 11 | 183968 | Enterococcaceae | 59 | 698782 | Rikenellaceae |
| 12 | 214569 | Streptococcus | 60 | 719017 | Prevotella |
| 13 | 221447 | Bacteroides | 61 | 733265 | Lactobacillales |
| 14 | 225896 | Streptococcaceae | 62 | 739100 | Staphylococcus |
| 15 | 237562 | Enterococcaceae | 63 | 753101 | Staphylococcaceae |
| 16 | 268741 | Porphyromonas | 64 | 772368 | Odoribacter |
| 17 | 271458 | Ruminococcaceae | 65 | 794890 | Porphyromonadaceae |
| 18 | 276398 | Phascolarctobacterium | 66 | 827745 | Eubacteriaceae |
| 19 | 281145 | Butyricimonas | 67 | 875214 | Eubacterium |
| 20 | 287482 | Parvimonas | 68 | 889803 | Clostridiales |
| 21 | 301189 | Pseudoramibacter | 69 | 914732 | Prevotella |
| 22 | 308756 | Clostridiales | 70 | 963365 | Prevotellaceae |
| 23 | 324470 | Peptococcus | 71 | 980217 | Butyricicoccus |
| 24 | 328621 | Peptococcaceae | 72 | 998510 | Ruminococcaceae |
| 25 | 331398 | Butyricicoccus | 73 | 1038960 | Clostridium |
| 26 | 335563 | Parabacteroides | 74 | 1041517 | Parasporobacterium |
| 27 | 346308 | Barnesiella | 75 | 1048796 | Dorea |
| 28 | 362391 | Flavonifractor | 76 | 1057893 | Lachnospiraceae |
| 29 | 367522 | Peptostreptococcus | 77 | 1096780 | Clostridiales |
| 30 | 371145 | Peptostreptococcaceae | 78 | 1101203 | Mobiluncus |
| 31 | 376598 | Acetanaerobacterium | 79 | 1106891 | Actinomyces |
| 32 | 379856 | Lachnospiraceae | 80 | 1110289 | Actinomycetaceae |
| 33 | 392350 | Pediococcus | 81 | 1115981 | Prevotella |
| 34 | 398571 | Anaerostipes | 82 | 1145780 | Dialister |
| 35 | 402265 | Enterococcus | 83 | 1148765 | Clostridiales |
| 36 | 428796 | Lactobacillaceae | 84 | 1150127 | Lachnospiraceae |
| 37 | 441278 | Ruminococcaceae | 85 | 1159812 | Bacteroides |
| 38 | 449127 | Ruminococcaceae | 86 | 1163970 | Bacteroidales |
| 39 | 451281 | Ruminococcus | 87 | 1167095 | Ruminococcus |
| 40 | 456580 | Clostridiaceae | 88 | 1208976 | Bacteroidaceae |
| 41 | 459102 | Bifidobacterium | 89 | 1220178 | Blautia |
| 42 | 467458 | Bifidobacteriaceae | 90 | 1227141 | Ruminococcaceae |
| 43 | 481097 | Peptostreptococcaceae | 91 | 1240139 | Roseburia |
| 44 | 489810 | Catenibacterium | 92 | 1247890 | Lachnospiraceae |
| 45 | 501433 | Bacteroidales | 93 | 1261372 | Faecalibacterium |
| 46 | 510129 | Bacteroides | 94 | 1266716 | Ruminococcaceae |
| 47 | 514780 | Barnesiellaceae | 95 | 1284820 | Clostridiales |
| 48 | 568894 | Erysipelotrichaceae | 96 | 1289362 | Clostridiales |
TABLE A4.
Diversity Indexes for pancolitis phases and healthy participants
| Diversity index | HS | AP | RP | p‐value |
|---|---|---|---|---|
| Observed | 7122.82 | 2321.01 | 4138.14 | 0.001 |
| Chao1 | 4215.58 | 1112.32 | 3241.56 | 0.001 |
| Shannon | 4.1 | 1.0 | 3.6 | 0.001 |
| Simpson | 0.48 | 1.2 | 0.52 | 0.001 |
p‐value indicates the statistical significance of 2‐way ANOVA.
Abbreviations: AP, active pancolitis; HS, healthy participants; RP Remission pancolitis.
TABLE A5.
Linear discriminant analysis (LDA) effect size (LEfSe) analysis for active pancolitis and remission pancolitis
| Taxa | Group | LDA score | p‐value |
|---|---|---|---|
| p_Proteobacteria;c_Betaproteobacteria | Active Pancolitis | 4.8999 | 0.042 |
| p_Proteobacteria;c_Gammaproteobacteria | Active Pancolitis | 4.8963 | 0.0210 |
| p_Proteobacteria;c_Deltaproteobacteria;o_Desulfovibrionales | Active Pancolitis | 4.8912 | 0.0160 |
| p_Proteobacteria;c_Deltaproteobacteria;o_Desulfovibrionales;f_Desulfovibrionaceae | Active Pancolitis | 4.8836 | 0.0309 |
| p_Proteobacteria;c_Deltaproteobacteria;o_Desulfovibrionales;f_Desulfovibrionaceae;g_Bilophila | Active Pancolitis | 4.8792 | 0.0436 |
| p_Fusobacteria;c_Fusobacteria | Active Pancolitis | 4.8569 | 0.0122 |
| p_Fusobacteria;c_Fusobacteria;o_Fusobacteriales;f_Fusobacteriaceae | Active Pancolitis | 4.8498 | 0.0132 |
| p_Fusobacteria;c_Fusobacteria;o_Fusobacteriales;f_Fusobacteriaceae;g_Fusobacterium | Active Pancolitis | 4.8475 | 0.0145 |
| p_Firmicutes;c_Clostridia | Remission Pancolitis | 4.8961 | 0.0394 |
| p_Firmicutes;c_Clostridia;o_Clostridiales | Remission Pancolitis | 4.8926 | 0.0058 |
| p_Firmicutes;c_Clostridia;o_Clostridiales;f_Ruminococcaceae;g_Faecalibacterium | Remission Pancolitis | 4.8912 | 0.0132 |
| p_Firmicutes;c_Clostridia;o_Clostridiales;f_Lachnospiraceae | Remission Pancolitis | 4.8859 | 0.0246 |
| p_Firmicutes;c_Clostridia;o_Clostridiales;f_Lachnospiraceae;g_Roseburia | Remission Pancolitis | 4.6898 | 0.0322 |
| p_Bacteroidetes;c_Bacteroidia;o_Bacteroidales;f_Bacteroidaceae | Remission Pancolitis | 4.6889 | 0.0125 |
| p_Bacteroidetes;c_Bacteroidia;o_Bacteroidales | Remission Pancolitis | 4.6885 | 0.0203 |
| p_Bacteroidetes;c_Bacteroidia;o_Bacteroidales;f_Bacteroidaceae;g_Bacteroides | Remission Pancolitis | 4.6880 | 0.0209 |
The threshold on the logarithmic LDA score for discriminative features was set to 2.0. The name of a higher taxon level was added before its taxon abbreviation. “p”, phylum; “c”, class; “o”, order; “f”, family; “g”, genus. “LDA” Linear discriminant analysis. p < 0.05 are considered statistically significant.
TABLE A6.
Diagnostic performance of Bilophila, Fusobacterium, Faecalibacterium, and Roseburia in discriminating Pancolitis‐related conditions
| AUC | Se (%) | Sp (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
| AP vs. HS | |||||
| Bilophila | 0.917 | 80 | 100 | 100 | 83 |
| Fusobacterium | 0.955 | 90 | 100 | 100 | 90 |
| Faecalibacterium | 0.222 | 0 | 80 | 0 | 44 |
| Roseburia | 0.237 | 0 | 90 | 0 | 47 |
| AP vs. RP | |||||
| Bilophila | 0.917 | 80 | 100 | 100 | 83 |
| Fusobacterium | 0.955 | 90 | 100 | 100 | 90 |
| Faecalibacterium | 0.206 | 10 | 10 | 10 | 10 |
| Roseburia | 0.237 | 0 | 90 | 0 | 47 |
| RP vs. HS | |||||
| Bilophila | 0.000 | N/A | 100 | N/A | 50 |
| Fusobacterium | 0.000 | N/A | 100 | N/A | 50 |
| Faecalibacterium | 0.237 | 90 | 0 | 47 | 0 |
| Roseburia | 0.400 | 40 | 60 | 50 | 50 |
Cutoffs. HS vs. AP discrimination: Bilophila (10%), Fusobacterium (10%), Faecalibacterium (45%), Roseburia (20%). AP vs RP discrimination: Bilophila (10%), Faecalibacterium (40%), Roseburia (20%). HS vs RP discrimination: Bilophila (5%), Fusobacterium (5%), Faecalibacterium (20%), Roseburia (10%).
Abbreviatures: AP, Active Pancolitis; AUC, Area Under the Curve; HS, Healthy Subjects; N/A, non‐applicable; NPV, Negative Predictive Value; PPV, Positive Predictive Value; RP, Remission Pancolitis; Se, Sensitivity; Sp, Specificity. Bold letters and values indicates the most abundant and have statistical significance.
Contributor Information
Juan Antonio Suárez‐Cuenca, Email: suarej05@gmail.com.
Graciela Castro‐Escarpulli, Email: chelacastro@hotmail.com.
DATA AVAILABILITY STATEMENT
Sequence data are available in the NCBI repository under BioProject accession number PRJNA596546: https://www.ncbi.nlm.nih.gov/bioproject/596546
REFERENCES
- Alam, M. T., Amos, G., Murphy, A., Murch, S., Wellington, E., & Arasaradnam, R. P. (2020). Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathogens, 12, 1. 10.1186/s13099-019-0341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alard, J., Peucelle, V., Boutillier, D., Breton, J., Kuylle, S., Pot, B., Holowacz, S., & Grangette, C. (2018). New probiotic strains for inflammatory bowel disease management identified by combining in vitro and in vivo approaches. Beneficial Microbes, 9(2), 317–331. 10.3920/BM2017.0097 [DOI] [PubMed] [Google Scholar]
- Al‐Bayati, L., Nayeri Fasaei, B., Merat, S., & Bahonar, A. (2018). Longitudinal analyses of gut‐associated bacterial microbiota in ulcerative colitis patients. Archives of Iranian Medicine, 21(12), 578–584. [PubMed] [Google Scholar]
- Allali, I., Arnold, J. W., Roach, J., Cadenas, M. B., Butz, N., Hassan, H. M., Koci, M., Ballou, A., Mendoza, M., Ali, R., & Azcarate‐Peril, M. A. (2017). A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiology, 17(1), 194. 10.1186/s12866-017-1101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aßhauer, K. P., Wemheuer, B., Daniel, R., & Meinicke, P. (2015). Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics (Oxford, England), 31(17), 2882–2884. 10.1093/bioinformatics/btv287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwell, L. J., Isaac, N. J., & Kunin, W. E. (2015). Measuring β‐diversity with species abundance data. The Journal of Animal Ecology, 84(4), 1112–1122. 10.1111/1365-2656.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir, A., Miskeen, A. Y., Hazari, Y. M., Asrafuzzaman, S., & Fazili, K. M. (2016). Fusobacterium nucleatum, inflammation, and immunity: The fire within human gut. Tumour Biology: the Journal of the International Society for Oncodevelopmental Biology and Medicine, 37(3), 2805–2810. 10.1007/s13277-015-4724-0 [DOI] [PubMed] [Google Scholar]
- Björkqvist, O., Repsilber, D., Seifert, M., Brislawn, C., Jansson, J., Engstrand, L., Rangel, I., & Halfvarson, J. (2019). Alterations in the relative abundance of Faecalibacterium prausnitzii correlate with changes in fecal calprotectin in patients with ileal Crohn's disease: A longitudinal study. Scandinavian Journal of Gastroenterology, 54(5), 577–585. 10.1080/00365521.2019.1599417 [DOI] [PubMed] [Google Scholar]
- Bokulich, N. A., Subramanian, S., Faith, J. J., Gevers, D., Gordon, J. I., Knight, R., Mills, D. A., & Caporaso, J. G. (2013). Quality‐filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature Methods, 10(1), 57–59. 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen, E., Rideout, J. R., Chase, J., Pitman, T. A., Shiffer, A., Mercurio, W., Dillon, M. R., & Caporaso, J. G. (2018). An Introduction to Applied Bioinformatics: A free, open, and interactive text. The Journal of Open Source Education, 1(5), 27. 10.21105/jose.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosques‐Padilla, J., Sandoval‐Garcia, R., Martinez‐Vázquez, A., Garza‐González, E., & Maldonado‐Garza, J. (2011). Epidemiología y características clínicas de la colitis ulcerosa crónica idiopática en el noreste de México. Revista Gastroenterología., 76, 34–38. [PubMed] [Google Scholar]
- Burman, S., Hoedt, E. C., Pottenger, S., Mohd‐Najman, N. S., Ó Cuív, P., & Morrison, M. (2016). An (Anti)‐inflammatory microbiota: Defining the role in inflammatory bowel disease? Digestive Diseases (Basel, Switzerland), 34(1–2), 64–71. 10.1159/000443759 [DOI] [PubMed] [Google Scholar]
- Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., Fierer, N., Peña, A. G., Goodrich, J. K., Gordon, J. I., Huttley, G. A., Kelley, S. T., Knights, D., Koenig, J. E., Ley, R. E., Lozupone, C. A., McDonald, D., Muegge, B. D., Pirrung, M., … Knight, R. (2010). QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7(5), 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg‐Lyons, D., Huntley, J., Fierer, N., Owens, S. M., Betley, J., Fraser, L., Bauer, M., Gormley, N., Gilbert, J. A., Smith, G., & Knight, R. (2012). Ultra‐high‐throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal, 6(8), 1621–1624. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. Y., & Cheon, J. H. (2018). Fecal Immunochemical test and fecal calprotectin measurement are noninvasive monitoring tools for predicting endoscopic activity in patients with ulcerative colitis. Gut and Liver, 12(2), 117–118. 10.5009/gnl17445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, A., Chazdon, R. L., Colwell, R. K., & Shen, T. J. (2006). Abundance‐based similarity indices and their estimation when there are unseen species in samples. Biometrics, 62(2), 361–371. 10.1111/j.1541-0420.2005.00489.x [DOI] [PubMed] [Google Scholar]
- Chassaing, B., Vijay‐Kumar, M., & Gewirtz, A. T. (2017). How diet can impact gut microbiota to promote or endanger health. Current Opinion in Gastroenterology, 33(6), 417–421. 10.1097/MOG.0000000000000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Chen, Y., Cao, P., Su, W., Zhan, N., & Dong, W. (2020). Fusobacterium nucleatum facilitates ulcerative colitis through activating IL‐17F signaling to NF‐κB via the upregulation of CARD3 expression. The Journal of Pathology, 250(2), 170–182. 10.1002/path.5358 [DOI] [PubMed] [Google Scholar]
- Danilova, N. A., Abdulkhakov, S. R., Grigoryeva, T. V., Markelova, M. I., Vasilyev, I. Y., Boulygina, E. A., Ardatskaya, M. D., Pavlenko, A. V., Tyakht, A. V., Odintsova, A. K., & Abdulkhakov, R. A. (2019). Markers of dysbiosis in patients with ulcerative colitis and Crohn's disease. Terapevticheskii Arkhiv, 91(4), 17–24. 10.26442/00403660.2019.04.000211 [DOI] [PubMed] [Google Scholar]
- Devkota, S., & Chang, E. B. (2015). Interactions between diet, bile acid metabolism, gut microbiota, and inflammatory bowel diseases. Digestive Diseases (Basel, Switzerland), 33(3), 351–356. 10.1159/000371687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicks, L., Geldenhuys, J., Mikkelsen, L. S., Brandsborg, E., & Marcotte, H. (2018). Our gut microbiota: A long walk to homeostasis. Beneficial Microbes, 9(1), 3–20. 10.3920/BM2017.0066 [DOI] [PubMed] [Google Scholar]
- Dubinsky, M., & Braun, J. (2015). Diagnostic and prognostic microbial biomarkers in inflammatory bowel diseases. Gastroenterology, 149(5), 1265–1274.e3. 10.1053/j.gastro.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., & Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics (Oxford, England), 27(16), 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrukh, A., & Mayberry, J. F. (2014). Inflammatory bowel disease in Hispanic communities: A concerted South American approach could identify the aetiology of Crohn's disease and ulcerative colitis. Arquivos De Gastroenterologia, 51(4), 271–275. 10.1590/S0004-28032014000400002 [DOI] [PubMed] [Google Scholar]
- Ferreira‐Halder, C. V., Faria, A., & Andrade, S. S. (2017). Action and function of Faecalibacterium prausnitzii in health and disease. Best Practice & Research. Clinical Gastroenterology, 31(6), 643–648. 10.1016/j.bpg.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Forbes, J. D., Van Domselaar, G., & Bernstein, C. N. (2016). Microbiome survey of the inflamed and noninflamed gut at different compartments within the gastrointestinal tract of inflammatory bowel disease patients. Inflammatory Bowel Diseases, 22(4), 817–825. 10.1097/MIB.0000000000000684 [DOI] [PubMed] [Google Scholar]
- Franzosa, E. A., Sirota‐Madi, A., Avila‐Pacheco, J., Fornelos, N., Haiser, H. J., Reinker, S., Vatanen, T., Hall, A. B., Mallick, H., McIver, L. J., Sauk, J. S., Wilson, R. G., Stevens, B. W., Scott, J. M., Pierce, K., Deik, A. A., Bullock, K., Imhann, F., Porter, J. A., … Xavier, R. J. (2019). Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nature Microbiology, 4(2), 293–305. 10.1038/s41564-018-0306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda, K., & Fujita, Y. (2014). Determination of the discriminant score of intestinal microbiota as a biomarker of disease activity in patients with ulcerative colitis. BMC Gastroenterology, 14, 49. 10.1186/1471-230X-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazzo, G., Tedjo, D. I., Wintjens, D., Savelkoul, P., Masclee, A., Bodelier, A., Pierik, M. J., Jonkers, D., & Penders, J. (2019). Faecal microbiota dynamics and their relation to disease course in Crohn's disease. Journal of Crohn's & Colitis, 13(10), 1273–1282. 10.1093/ecco-jcc/jjz049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji‐Arjenaki, M., & Rafieian‐Kopaei, M. (2018). Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. Journal of Cellular Physiology, 233(3), 2091–2103. 10.1002/jcp.25911 [DOI] [PubMed] [Google Scholar]
- Guo, S., Lu, Y., Xu, B., Wang, W., Xu, J., & Zhang, G. (2019). A simple fecal bacterial marker panel for the diagnosis of Crohn's disease. Frontiers in Microbiology, 10, 1306. 10.3389/fmicb.2019.01306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, B. J., Gevers, D., Earl, A. M., Feldgarden, M., Ward, D. V., Giannoukos, G., Ciulla, D., Tabbaa, D., Highlander, S. K., Sodergren, E., Methe, B., DeSantis, T. Z., Petrosino, J. F., Knight, R., & Birren, B. W. (2011). Chimeric 16S rRNA sequence formation and detection in Sanger and 454‐pyrosequenced PCR amplicons. Genome Research, 21(3), 494–504. 10.1101/gr.112730.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfvarson, J., Brislawn, C. J., Lamendella, R., Vázquez‐Baeza, Y., Walters, W. A., Bramer, L. M., D'Amato, M., Bonfiglio, F., McDonald, D., Gonzalez, A., McClure, E. E., Dunklebarger, M. F., Knight, R., & Jansson, J. K. (2017). Dynamics of the human gut microbiome in inflammatory bowel disease. Nature Microbiology, 2, 17004. 10.1038/nmicrobiol.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenaar, R. (2011). Intestinal health functions of colonic microbial metabolites: A review. Beneficial Microbes, 2(2), 103–114. 10.3920/BM2011.0003 [DOI] [PubMed] [Google Scholar]
- Hirano, A., Umeno, J., Okamoto, Y., Shibata, H., Ogura, Y., Moriyama, T., Torisu, T., Fujioka, S., Fuyuno, Y., Kawarabayasi, Y., Matsumoto, T., Kitazono, T., & Esaki, M. (2018). Comparison of the microbial community structure between inflamed and non‐inflamed sites in patients with ulcerative colitis. Journal of Gastroenterology and Hepatology, 10.1111/jgh.14129 [DOI] [PubMed] [Google Scholar]
- Imhann, F., Vich Vila, A., Bonder, M. J., Fu, J., Gevers, D., Visschedijk, M. C., Spekhorst, L. M., Alberts, R., Franke, L., van Dullemen, H. M., Ter Steege, R., Huttenhower, C., Dijkstra, G., Xavier, R. J., Festen, E., Wijmenga, C., Zhernakova, A., & Weersma, R. K. (2018). Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut, 67(1), 108–119. 10.1136/gutjnl-2016-312135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., & Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World Journal of Gastroenterology, 21(29), 8787–8803. 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshteli, A. H., Madsen, K. L., & Dieleman, L. A. (2019). Diet in the pathogenesis and management of ulcerative colitis; A review of randomized controlled dietary interventions. Nutrients, 11(7), 1498. 10.3390/nu11071498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, I., Ullah, N., Zha, L., Bai, Y., Khan, A., Zhao, T., Che, T., & Zhang, C. (2019). Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens (Basel, Switzerland), 8(3), 126. 10.3390/pathogens8030126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross, J. M., Darzi, A. W., & Nicholson, J. K. (2011). Gut microbiome‐host interactions in health and disease. Genome Medicine, 3(3), 14. 10.1186/gm228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, R., Ahuja, V., & Paul, J. (2013). Fluctuations in butyrate‐producing bacteria in ulcerative colitis patients of North India. World Journal of Gastroenterology, 19(22), 3404–3414. 10.3748/wjg.v19.i22.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley, B., & Tannock, G. W. (2017). Analysis of 16S rRNA gene amplicon sequences using the QIIME software package. Methods in Molecular Biology (Clifton, N.J.), 1537, 153–163. 10.1007/978-1-4939-6685-1_9 [DOI] [PubMed] [Google Scholar]
- Liu, L., Liang, L., Liang, H., Wang, M., Lu, B., Xue, M., Deng, J., & Chen, Y. (2019). Fusobacterium nucleatum aggravates the progression of colitis by regulating M1 macrophage polarization via AKT2 pathway. Frontiers in Immunology, 10, 1324. 10.3389/fimmu.2019.01324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Siles, M., Duncan, S. H., Garcia‐Gil, L. J., & Martinez‐Medina, M. (2017). Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. The ISME Journal, 11(4), 841–852. 10.1038/ismej.2016.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, P., & Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environmental Microbiology, 19(1), 29–41. 10.1111/1462-2920.13589 [DOI] [PubMed] [Google Scholar]
- Machiels, K., Joossens, M., Sabino, J., De Preter, V., Arijs, I., Eeckhaut, V., Ballet, V., Claes, K., Van Immerseel, F., Verbeke, K., Ferrante, M., Verhaegen, J., Rutgeerts, P., & Vermeire, S. (2014). A decrease of the butyrate‐producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut, 63(8), 1275–1283. 10.1136/gutjnl-2013-304833 [DOI] [PubMed] [Google Scholar]
- Man, S. M., Kaakoush, N. O., & Mitchell, H. M. (2011). The role of bacteria and pattern‐recognition receptors in Crohn's disease. Nature Reviews. Gastroenterology & Hepatology, 8(3), 152–168. 10.1038/nrgastro.2011.3 [DOI] [PubMed] [Google Scholar]
- Manichanh, C., Borruel, N., Casellas, F., & Guarner, F. (2012). The gut microbiota in IBD. Nature Reviews Gastroenterology & Hepatology, 9(10), 599–608. 10.1038/nrgastro.2012.152 [DOI] [PubMed] [Google Scholar]
- Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D., Hirschfield, G. M., Hold, G., Quraishi, M. N., Kinross, J., Smidt, H., Tuohy, K. M., Thomas, L. V., Zoetendal, E. G., & Hart, A. (2016). The gut microbiota and host health: A new clinical frontier. Gut, 65(2), 330–339. 10.1136/gutjnl-2015-309990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi, J., Nobutani, K., Musch, M. W., Ringus, D. L., Hubert, N. A., Yamamoto, M., Kase, Y., Nishiyama, M., & Chang, E. B. (2018). Time‐, sex‐, and dose‐dependent alterations of the gut microbiota by consumption of dietary daikenchuto (TU‐100). Evidence‐based Complementary and Alternative Medicine, 2018, 7415975. 10.1155/2018/7415975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Vashist, N., Samaan, M., Mosli, M. H., Parker, C. E., MacDonald, J. K., Nelson, S. A., Zou, G. Y., Feagan, B. G., Khanna, R., & Jairath, V. (2018). Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. The Cochrane Database of Systematic Reviews, 1(1), CD011450. 10.1002/14651858.CD011450.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas‐Molina, J. A., Peralta‐Sánchez, J. M., González, A., McMurdie, P. J., Vázquez‐Baeza, Y., Xu, Z., Ursell, L. K., Lauber, C., Zhou, H., Song, S. J., Huntley, J., Ackermann, G. L., Berg‐Lyons, D., Holmes, S., Caporaso, J. G., & Knight, R. (2013). Advancing our understanding of the human microbiome using QIIME. Methods in Enzymology, 531, 371–444. 10.1016/B978-0-12-407863-5.00019-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, A., Inoue, R., Inatomi, O., Bamba, S., Naito, Y., & Andoh, A. (2018). Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical Journal of Gastroenterology, 11(1), 1–10. 10.1007/s12328-017-0813-5 [DOI] [PubMed] [Google Scholar]
- Nishida, Y., Hosomi, S., Yamagami, H., Yukawa, T., Otani, K., Nagami, Y., Tanaka, F., Taira, K., Kamata, N., Tanigawa, T., Shiba, M., Watanabe, K., Watanabe, T., Tominaga, K., & Fujiwara, Y. (2017). Neutrophil‐to‐lymphocyte ratio for predicting loss of response to infliximab in ulcerative colitis. PLoS One, 12(1), e0169845. 10.1371/journal.pone.0169845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkusa, T., Sato, N., Ogihara, T., Morita, K., Ogawa, M., & Okayasu, I. (2002). Fusobacterium varium localized in the colonic mucosa of patients with ulcerative colitis stimulates species‐specific antibody. Journal of Gastroenterology and Hepatology, 17(8), 849–853. 10.1046/j.1440-1746.2002.02834.x [DOI] [PubMed] [Google Scholar]
- Owczarek, D., Rodacki, T., Domagała‐Rodacka, R., Cibor, D., & Mach, T. (2016). Diet and nutritional factors in inflammatory bowel diseases. World Journal of Gastroenterology, 22(3), 895–905. 10.3748/wjg.v22.i3.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmela, C., Chevarin, C., Xu, Z., Torres, J., Sevrin, G., Hirten, R., Barnich, N., Ng, S. C., & Colombel, J. F. (2018). Adherent‐invasive Escherichia coli in inflammatory bowel disease. Gut, 67(3), 574–587. 10.1136/gutjnl-2017-314903 [DOI] [PubMed] [Google Scholar]
- Paramsothy, S., Nielsen, S., Kamm, M. A., Deshpande, N. P., Faith, J. J., Clemente, J. C., Paramsothy, R., Walsh, A. J., van den Bogaerde, J., Samuel, D., Leong, R., Connor, S., Ng, W., Lin, E., Borody, T. J., Wilkins, M. R., Colombel, J. F., Mitchell, H. M., & Kaakoush, N. O. (2019). Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology, 156(5), 1440–1454.e2. 10.1053/j.gastro.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Parks, C. A., Blaser, C., Smith, T. M., Calloway, E. E., Oh, A. Y., Dwyer, L. A., Liu, B., Nebeling, L. C., & Yaroch, A. L. (2018). Correlates of fruit and vegetable intake among parents and adolescents: findings from the Family Life, Activity, Sun, Health, and Eating (FLASHE) study. Public Health Nutrition, 21(11), 2079–2087. 10.1017/S1368980018000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, D. A., Frank, D. N., Pace, N. R., & Gordon, J. I. (2008). Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host & Microbe, 3(6), 417–427. 10.1016/j.chom.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof, E. O., Claud, E. C., Gloor, G. B., & Allen‐Vercoe, E. (2013). Microbial ecosystems therapeutics: A new paradigm in medicine? Beneficial Microbes, 4(1), 53–65. 10.3920/BM2012.0039 [DOI] [PubMed] [Google Scholar]
- Pittayanon, R., Lau, J. T., Leontiadis, G. I., Tse, F., Yuan, Y., Surette, M., & Moayyedi, P. (2020). Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology, 158(4), 930–946.e1. 10.1053/j.gastro.2019.11.294 [DOI] [PubMed] [Google Scholar]
- Rintala, A., Pietilä, S., Munukka, E., Eerola, E., Pursiheimo, J. P., Laiho, A., Pekkala, S., & Huovinen, P. (2017). Gut microbiota analysis results are highly dependent on the 16S rRNA gene target region, whereas the impact of DNA extraction is minor. Journal of Biomolecular Techniques, 28(1), 19–30. 10.7171/jbt.17-2801-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., & Huttenhower, C. (2011). Metagenomic biomarker discovery and explanation. Genome Biology, 12(6), R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha, S., Xu, B., Wang, X., Zhang, Y., Wang, H., Kong, X., Zhu, H., & Wu, K. (2013). The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagnostic Microbiology and Infectious Disease, 75(3), 245–251. 10.1016/j.diagmicrobio.2012.11.022 [DOI] [PubMed] [Google Scholar]
- Siegel, C. A., Whitman, C. B., Spiegel, B. M. R., Feagan, B., Sands, B., Loftus, E. V., Panaccione, R., D'Haens, G., Bernstein, C. N., Gearry, R., Ng, S. C., Mantzaris, G. J., Sartor, B., Silverberg, M. S., Riddell, R., Koutroubakis, I. E., O'Morain, C., Lakatos, P. L., McGovern, D. P. B., … Peyrin‐Biroulet, L. (2018). Development of an index to define overall disease severity in IBD. Gut, 67(2), 244–254. 10.1136/gutjnl-2016-312648 [DOI] [PubMed] [Google Scholar]
- Tahara, T., Shibata, T., Kawamura, T., Okubo, M., Ichikawa, Y., Sumi, K., Miyata, M., Ishizuka, T., Nakamura, M., Nagasaka, M., Nakagawa, Y., Ohmiya, N., Arisawa, T., & Hirata, I. (2015). Fusobacterium detected in colonic biopsy and clinicopathological features of ulcerative colitis in Japan. Digestive Diseases and Sciences, 60(1), 205–210. 10.1007/s10620-014-3316-y [DOI] [PubMed] [Google Scholar]
- Tedjo, D. I., Jonkers, D. M., Savelkoul, P. H., Masclee, A. A., van Best, N., Pierik, M. J., & Penders, J. (2015). The effect of sampling and storage on the fecal microbiota composition in healthy and diseased subjects. PLoS One, 10(5), e0126685. 10.1371/journal.pone.0126685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedjo, D. I., Smolinska, A., Savelkoul, P. H., Masclee, A. A., van Schooten, F. J., Pierik, M. J., Penders, J., & Jonkers, D. M. (2016). The fecal microbiota as a biomarker for disease activity in Crohn's disease. Scientific Reports, 6, 35216. 10.1038/srep35216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, J., Palmela, C., Brito, H., Bao, X., Ruiqi, H., Moura‐Santos, P., Pereira da Silva, J., Oliveira, A., Vieira, C., Perez, K., Itzkowitz, S. H., Colombel, J. F., Humbert, L., Rainteau, D., Cravo, M., Rodrigues, C. M., & Hu, J. (2018). The gut microbiota, bile acids and their correlation in primary sclerosing cholangitis associated with inflammatory bowel disease. United European Gastroenterology Journal, 6(1), 112–122. 10.1177/2050640617708953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Assche, G., Dignass, A., Panes, J., Beaugerie, L., Karagiannis, J., Allez, M., Ochsenkühn, T., Orchard, T., Rogler, G., Louis, E., Kupcinskas, L., Mantzaris, G., Travis, S., & Stange, E. (2010). The second European evidence‐based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. Journal of Crohn's and Colitis, 4(1), 7–27. 10.1016/j.crohns.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Vemuri, R. C., Gundamaraju, R., Shinde, T., & Eri, R. (2017). Therapeutic interventions for gut dysbiosis and related disorders in the elderly: Antibiotics, probiotics or faecal microbiota transplantation? Beneficial Microbes, 8(2), 179–192. 10.3920/BM2016.0115 [DOI] [PubMed] [Google Scholar]
- Vester‐Andersen, M. K., Mirsepasi‐Lauridsen, H. C., Prosberg, M. V., Mortensen, C. O., Träger, C., Skovsen, K., Thorkilgaard, T., Nøjgaard, C., Vind, I., Krogfelt, K. A., Sørensen, N., Bendtsen, F., & Petersen, A. M. (2019). Increased abundance of proteobacteria in aggressive Crohn's disease seven years after diagnosis. Scientific Reports, 9(1), 13473. 10.1038/s41598-019-49833-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigsnæs, L. K., Brynskov, J., Steenholdt, C., Wilcks, A., & Licht, T. R. (2012). Gram‐negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Beneficial Microbes, 3(4), 287–297. 10.3920/BM2012.0018 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Gao, X., Ghozlane, A., Hu, H., Li, X., Xiao, Y., Li, D., Yu, G., & Zhang, T. (2018). Characteristics of faecal microbiota in paediatric Crohn's disease and their dynamic changes during infliximab therapy. Journal of Crohn's & Colitis, 12(3), 337–346. 10.1093/ecco-jcc/jjx153 [DOI] [PubMed] [Google Scholar]
- Wright, E. K., Kamm, M. A., Teo, S. M., Inouye, M., Wagner, J., & Kirkwood, C. D. (2015). Recent advances in characterizing the gastrointestinal microbiome in Crohn's disease: A systematic review. Inflammatory Bowel Diseases, 21(6), 1219–1228. 10.1097/MIB.0000000000000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L. C. (2018). Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. Journal of Biomedical Science, 25(1), 79. 10.1186/s12929-018-0483-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W., Su, X., Chen, W., Tian, X., Zhang, K., Guo, G., Zhou, L., Zeng, T., & Han, B. (2019). Three types of gut bacteria collaborating to improve Kui Jie'an enema treat DSS‐induced colitis in mice. Biomedicine & Pharmacotherapy, 113, 108751. 10.1016/j.biopha.2019.108751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequence data are available in the NCBI repository under BioProject accession number PRJNA596546: https://www.ncbi.nlm.nih.gov/bioproject/596546


