Abbreviations
- b‐HCA
beta‐catenin activated hepatocellular adenoma
- b‐IHCA
beta‐catenin activated inflammatory hepatocellular adenoma
- BMI
body mass index
- CRP
C‐reactive protein
- FNH
focal nodular hyperplasia
- GS
glutamine synthetase
- HCA
hepatocellular adenoma
- H‐HCA
hepatocyte nuclear factor 1α–inactivated hepatocellular adenoma
- HNF1α
hepatocyte nuclear factor 1α
- I‐HCA
inflammatory hepatocellular adenoma
- L‐FABP
liver fatty acid binding protein
- MODY
maturity‐onset diabetes of the young
- MRI
magnetic resonance imaging
- SAA
serum amyloid A
In noncirrhotic livers, benign hepatocellular tumors include focal nodular hyperplasia (FNH) and hepatocellular adenoma (HCA), both of which are most commonly seen in women of reproductive age. HCA is less common than FNH and differs from it by being neoplastic and carrying risk for bleeding or malignant transformation. For this reason, clinical management differs between HCA and FNH. Further, as a result of molecular characterization of HCA over the past decade, a recognized phenotype‐genotype classification of these neoplasms has emerged; this classification has become essential in guiding patient management because different HCA subtypes have distinct clinical and radiological associations and carry different risks for malignancy.
Hepatocellular Adenoma
HCAs are rare, affecting 3 to 4 per 100,000 individuals. 1 By definition, they arise in noncirrhotic livers. Sex, hormones, and genetic predisposition play important roles 2 , 3 , 4 in driving the development of HCA. Approximately 85% of HCAs occur in young to middle‐aged females with a history of oral contraceptive use. 5 Exogenous androgens and anabolic steroids are also a recognized risk factor in the development of HCA. Clinical associations of HCAs are summarized in Table 1.
TABLE 1.
Clinical Associations of HCA
| Associations With Hepatic Adenoma | Conditions |
|---|---|
| Established associations | Oral contraceptives |
| Exogenous androgens/anabolic steroids | |
| Glycogen storage disease | |
| MODY 3 (H‐HCA) | |
| Alcohol use (I‐HCA) | |
| Elevated BMI (I‐HCA) | |
| Metabolic syndrome (I‐HCA) | |
| Isolated reports | Familial adenomatous polyposis |
| Galactosemia | |
| Tyrosinemia | |
| Polycystic ovary syndrome | |
| Peutz‐Jeghers syndrome | |
| Carbamazepine | |
| Valproate | |
| Portal vein disorders (agenesis, shunts, and hepatoportal sclerosis) | |
| Hepatic vein disorders (Budd‐Chiari) |
The clinical presentation of HCA varies, ranging from asymptomatic to acute presentation with tumor rupture. Most present with mild nonspecific abdominal pain or discomfort. 6 Tumors are often solitary, but multifocality is seen in up to 50% of patients, 6 with tumors exceeding 10 labeled as “adenomatosis.” Unlike FNH, HCAs carry a risk for bleeding (20%‐25%) and malignant transformation (4%‐10%). 6 , 7 , 8 , 9 , 10 Both risks increase with tumor size; for instance, in almost 90% of cases of malignant transformation, the adenomas are 5 cm or more in diameter. 6 , 10 Notably, the incidence of malignant transformation is higher in males, reaching 10 times increased risk in one study. 8 , 9 , 10 Histologically, HCA consists of benign hepatocytes of normal plate thickness; they are distinguished from normal liver by the lack of portal tracts. Instead of portal tracts, aberrant naked arteries are scattered throughout the lesion. A reticulin stain shows an intact reticulin meshwork, similar to that of normal livers, although focal loss can be seen in steatotic areas.
Subtypes of HCA
As reflected in the most recent World Health Organization classification, HCA is subdivided into four main groups based on molecular findings, morphology, and immunohistochemical correlates. 3 , 5 , 7 , 11 The main subtypes of HCA are described later, summarized in Table 2, and demonstrated in Fig. 1.
TABLE 2.
HCA Subtypes
| H‐HCA | I‐HCA | b‐HCA and b‐IHCA | |
|---|---|---|---|
| Molecular abnormality | HNF1α inactivation | IL‐6/JAK/STAT activating mutations | CTNNB1 activating mutations (exons 3 and 7/8) |
| Frequency | 30%‐35% | 35%‐40% | 25% |
| Clinical associations | MODY 3 | Alcohol use | More common in males |
| Rare in males | Elevated BMI | Male hormones | |
| Metabolic syndrome | Metabolic diseases | ||
| Rare in males | |||
| Risk for malignancy | Low | Low | High (exon 3) |
| Fatty change | Most common | Less common | Less common |
| Faux portal tracts | − | + | − |
| Inflammation within lesion | − | + | − |
| Telangiectasia | Uncommon | Common | Uncommon |
| Atypia | − | − | +/− |
| L‐FABP | Lost | Retained | Retained |
| Beta‐catenin nuclear staining | − | − | +/− |
| GS (diffuse strong staining) | − | − | + |
| CRP/SAA | − | + | − |
FIG 1.
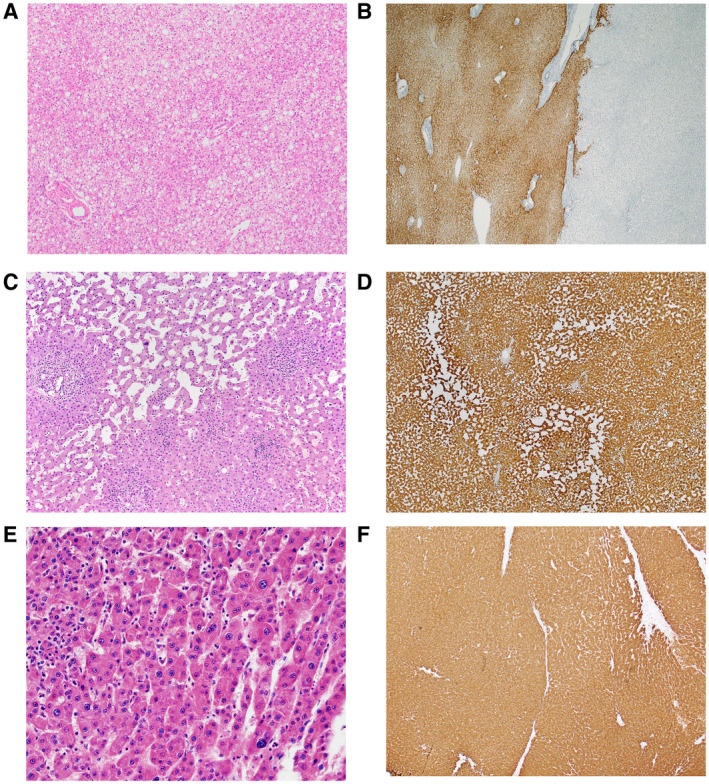
HCA subtypes. (A) H‐HCA: this subtype of HCA often shows steatosis. Scattered naked arteries are present. (B) H‐HCA: the tumor shows aberrant loss of L‐FABP (right) by immunostain, but L‐FABP is preserved in the background liver (left). (C) I‐HCA: this subtype of HCA shows areas of sinusoidal dilatation separated by abortive portal tracts. (D) I‐HCA: the tumor shows strong diffuse immunohistochemical expression of CRP. (E) b‐HCA: mild cytological atypia is present. (F) b‐HCA: strong diffuse immunohistochemical expression of GS indicates beta‐catenin activation.
Hepatocyte Nuclear Factor 1α–Inactivated HCA
Hepatocyte nuclear factor 1α (HNF1α)‐inactivated HCA (H‐HCA) is defined by HNF1α inactivation and represents 30% to 35% of all HCAs. Histologically, it often shows steatosis and aberrant loss of liver fatty acid binding protein (L‐FABP) by immunohistochemistry (as a result of HNF1α inactivation). H‐HCAs occur as sporadic somatic mutations or as germline mutations in the HNF1α gene, which occurs in the setting of maturity‐onset diabetes of the young (MODY) 3. This subtype carries a low risk for malignant transformation. Magnetic resonance imaging (MRI) in typical fat‐rich lesions shows diffuse and homogeneous signal dropout on T1‐weighted images and moderate arterial enhancement that does not persist during the delayed phase.
Inflammatory HCA
Inflammatory HCA (I‐HCA) represents 35% to 40% of HCAs and is associated with alcohol intake and elevated body mass index (BMI). The background liver often shows fatty liver disease. Histologically, I‐HCAs often have distinctive portal tract‐like structures (faux portal tracts) that contain arteries and bile ductular proliferation embedded in a sleeve of stroma that resembles a portal tract. I‐HCAs also frequently have distinctly dilated/telangiectatic sinusoids. The diagnosis requires immunohistochemical demonstration of an inflammatory profile, defined by expression of C‐reactive protein (CRP) and/or serum amyloid A (SAA). MRI is typically hyperintense on T2, either diffusely or at the periphery of the tumor (atoll sign), usually with strong arterial enhancement that persists during the delayed phase.
Beta‐Catenin Activated HCA and Beta‐Catenin Activated I‐HCA
The presence of beta‐catenin activating mutation defines the last two subtypes, with the beta‐catenin activated I‐HCA (b‐IHCA) also exhibiting an inflammatory phenotype. beta‐Catenin activated HCA (b‐HCA) and b‐IHCA represent 10% and 10% to 15% of all HCAs, respectively. beta‐Catenin mutations, particularly affecting exon 3, are associated with an increased risk for malignant transformation. In fact, two‐thirds of HCAs with malignant transformation show beta‐catenin activation. 10 b‐HCAs occur more frequently in men (up to 38% of lesions are found in males) and are only rarely multiple. Histologically, these two subtypes exhibit mild cytological and architectural atypia, in contrast with other HCA subtypes. beta‐Catenin activation is characterized by either diffuse strong cytoplasmic staining for glutamine synthetase (GS) or by aberrant nuclear expression of beta‐catenin. However, some exon 3 mutations can produce other GS staining patterns (diffuse heterogeneous or starry sky). More sensitive and specific detection of beta‐catenin mutation can be accomplished by molecular testing.
Other Variants
HCAs not demonstrating any of the specific earlier phenotypes are considered “unclassified.” These represent 5% to 10% of HCAs. Although some found immunohistochemical expression of Argininosuccinate Synthase 1 (ASS1) to identify in unclassified HCA a subgroup with a high risk for bleeding, others found no such correlation. 12 A more recently described subtype is sonic hedgehog (sh) activated HCA (5% of HCAs), which is frequently identified in obese patients and may be associated with increased risk for bleeding. 13 Other variants include pigmented HCAs, characterized by prominent lipofuscin pigment deposition; this variant has an increased risk (27%) for a malignancy, especially in males. 14 Finally, rare HCAs show abundant sinusoidal accumulation of myxoid material. 15 These adenomas can be seen in men and women and are often multiple.
Management of HCA
A diagnosis of HCA dictates discontinuation of exogenous hormones, including oral contraceptives. Additional management of HCA is based mainly on tumor size, patient sex, and tumor subtype. Women with an HCA lacking beta‐catenin activation and smaller than 5 cm may be managed by follow‐up imaging every 6 months for 2 years to establish growth patterns and monitor for malignant transformation. Surgical resection is generally recommended for HCAs larger than 5 cm, in men, in HCAs that grow at least 20% on surveillance, b‐HCA or b‐IHCA subtypes, and HCAs associated with glycogen storage disease. Less invasive interventions, such as transarterial embolization and radiofrequency ablation, may also be offered for poor surgical candidates, for small unresectable tumors, in pregnant women, or as a bridge to surgical resection by decreasing the size and bleeding potential of larger tumors. 16
Focal Nodular Hyperplasia
FNH is the second most common hepatic tumor in adults, second to hemangioma 17 ; it is 3 to 10 times more common than HCAs. It represents a nonneoplastic lesion, featuring hyperplastic parenchymal response to increased blood flow secondary to localized shunting of arterial blood flow, as a result of vascular malformations. 18 FNH arises in noncirrhotic liver by definition. Most FNHs develop in younger women, with 75% of cases occurring between the ages of 20 and 50 years (median, 41 years). FNH is most frequently asymptomatic, often discovered by chance. Unlike HCAs, FNHs do not carry any significant risk for bleeding or for malignant transformation.
Imaging techniques, such as MRI and contrast‐enhanced sonography, are diagnostic in up to 90% of cases. FNH is isointense or slightly hypointense on T1‐weighted MRIs and slightly hyperintense or isointense on T2. If a central scar is present, it is usually hypointense on T1 and hyperintense on T2. By gadolinium‐enhanced imaging, the arterial phase shows intense homogeneous enhancement, but returns to isointensity during the portal and delayed phase. In contrast, the central scar, if present, shows delayed enhancement. Contrast‐enhanced sonography shows a distinctive pattern of hypervascularity in the arterial phase, centrifugal filling, and stellate arteries producing a “spoke on a wheel” sign. Because imaging features are usually characteristic, a biopsy is needed only in tumors with unusual imaging features. 5
Pathologically, FNHs are usually solitary, but they can be multiple in about 20% to 30% of cases. 17 A single or multiple central scars are a characteristic feature of FNHs and are seen in approximately 62% of lesions. 17 The histology (Fig. 2) features nodules of benign hepatocytes without atypia, separated by fibrous septa, resembling a localized area of cirrhosis. The fibrous bands may coalesce into a central scar in most lesions. The septa contain a bile ductular proliferation, inflammation, and abnormal blood vessels. Like HCA, FNH has an intact reticulin meshwork. GS shows a characteristic “map‐like” staining pattern in most (90%) FNHs, reflecting zonal activation of beta‐catenin. 19 Immunohistochemistry for LFABP shows retained normal expression, beta‐catenin is negative for nuclear expression, and SSA and CRP are usually negative. 19
FIG 2.
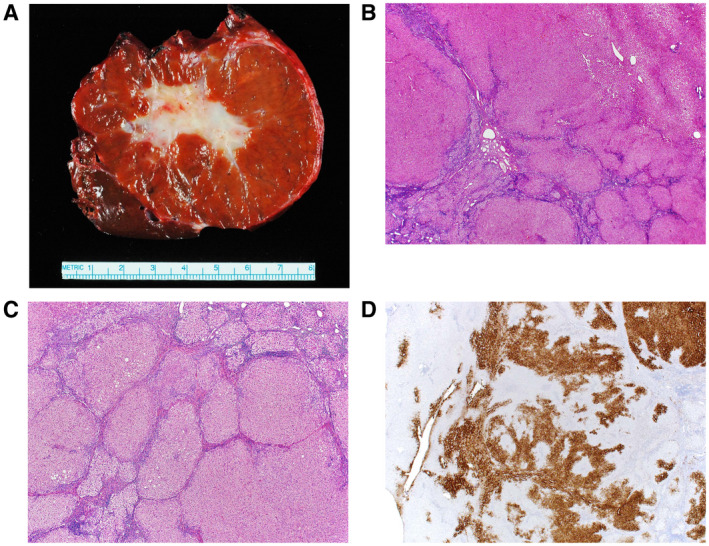
FNH. (A) Gross image of a typical FNH demonstrating a central scar. (B) The lesion (lower left) is distinct from the background liver (upper right) but is not encapsulated. (C) The lesion is composed of nodules of benign hepatocytes separated by fibrous septa that contain inflammation and ductular proliferation. (D) A GS immunostain shows a characteristic map‐like staining pattern, with interconnecting broad bands of strongly staining hepatocytes, but with no staining of hepatocytes adjacent to the fibrous bands. A small portion of normal liver, with normal zone 3 perivenular staining of hepatocytes, is seen on the left side of the image.
Management of FNH
Because they lack significant risk for malignant transformation and/or hemorrhage, most FNHs are managed without surgery, unless there are additional findings, such as protracted symptoms, atypical imaging, or evident tumor enlargement. No follow‐up is usually indicated, but it has been recommended for females who continue to use oral contraceptives to undergo an annual ultrasound for a 2‐ to 3‐year period. 16
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 1979;242:644‐648. [PubMed] [Google Scholar]
- 2. Mays ET, Christopherson W. Hepatic tumors induced by sex steroids. Semin Liver Dis 1984;4:147‐157. [DOI] [PubMed] [Google Scholar]
- 3. Bioulac‐Sage P, Blanc JF, Rebouissou S, et al. Genotype phenotype classification of hepatocellular adenoma. World J Gastroenterol 2007;13:2649‐2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bioulac‐Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology 2007;46:740‐748. [DOI] [PubMed] [Google Scholar]
- 5. Nagtegaal ID, Odze RD, Klimstra D, et al.;WHO Classification of Tumours Editorial Board . The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dokmak S, Paradis V, Vilgrain V, et al. A single‐center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 2009;137:1698‐1705. [DOI] [PubMed] [Google Scholar]
- 7. Rebouissou S, Bioulac‐Sage P, Zucman‐Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol 2008;48:163‐170. [DOI] [PubMed] [Google Scholar]
- 8. Micchelli ST, Vivekanandan P, Boitnott JK, et al. Malignant transformation of hepatic adenomas. Mod Pathol 2008;21:491‐497. [DOI] [PubMed] [Google Scholar]
- 9. Stoot JH, Coelen RJ, De Jong MC, et al. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010;12:509‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farges O, Ferreira N, Dokmak S, et al. Changing trends in malignant transformation of hepatocellular adenoma. Gut 2011;60:85‐89. [DOI] [PubMed] [Google Scholar]
- 11. Bioulac‐Sage P, Balabaud C, Zucman‐Rossi J. Subtype classification of hepatocellular adenoma. Dig Surg 2010;27:39‐45. [DOI] [PubMed] [Google Scholar]
- 12. Lehrke HD, Van Treeck BJ, Allende D, et al. Does Argininosuccinate Synthase 1 (ASS1) immunohistochemistry predict an increased risk of hemorrhage for hepatocellular adenomas? Appl Immunohistochem Mol Morphol 2020;28:464‐470. [DOI] [PubMed] [Google Scholar]
- 13. Nault JC, Paradis V, Cherqui D, et al. Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol 2017;67:1074‐1083. [DOI] [PubMed] [Google Scholar]
- 14. Mounajjed T, Yasir S, Aleff PA, et al. Pigmented hepatocellular adenomas have a high risk of atypia and malignancy. Mod Pathol 2015;28:1265‐1274. [DOI] [PubMed] [Google Scholar]
- 15. Young JT, Kurup AN, Graham RP, et al. Myxoid hepatocellular neoplasms: imaging appearance of a unique mucinous tumor variant. Abdom Radiol (NY) 2016;41:2115‐2122. [DOI] [PubMed] [Google Scholar]
- 16. Myers L, Ahn J. Focal nodular hyperplasia and hepatic adenoma: evaluation and management. Clin Liver Dis 2020;24:389‐403. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen BN, Flejou JF, Terris B, et al. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol 1999;23:1441‐1454. [DOI] [PubMed] [Google Scholar]
- 18. Paradis V, Benzekri A, Dargere D, et al. Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology 2004;126:1323‐1329. [DOI] [PubMed] [Google Scholar]
- 19. Bioulac‐Sage P, Cubel G, Balabaud C, et al. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis 2011;31:91‐103. [DOI] [PubMed] [Google Scholar]


