Key Points
Question
What is the prevalence of hypertension and albuminuria in children and adolescents with type 2 diabetes?
Findings
This systematic review and meta-analysis of 60 studies found that 25% of children and adolescents with type 2 diabetes had hypertension and 22% had albuminuria. Pacific Islander and Indigenous youth had a higher risk of these conditions than children from other racial groups.
Meaning
In this study, the burden of hypertension and albuminuria in pediatric type 2 diabetes was substantial, especially among Pacific Islander and Indigenous youth.
This systematic review and meta-analysis measures the prevalence of hypertension and albuminuria in pediatric patients with type 2 diabetes and evaluates the association of sex and race/ethnicity with these conditions.
Abstract
Importance
Hypertension and albuminuria are markers of diabetes-related nephropathy and important factors associated with kidney outcomes in pediatric type 2 diabetes. However, their prevalence in these patients is unknown.
Objective
To measure the prevalence of hypertension and albuminuria in pediatric patients with type 2 diabetes and to evaluate the association of sex and race/ethnicity with these conditions.
Data Sources
MEDLINE, Embase, CINAHL, Cochrane Library, Web of Science, the gray literature, and references of the screened articles were searched for human studies from date of database inception to February 20, 2020.
Study Selection
Observational studies with at least 10 participants reporting the prevalence of hypertension and/or albuminuria in pediatric patients with type 2 diabetes were included. Three teams of 2 independent reviewers screened 7614 papers, of which 60 fulfilled the eligibility criteria.
Data Extraction and Synthesis
Three teams of 2 independent reviewers performed data extraction, risk of bias analysis, and level of evidence analyses. The meta-analysis was conducted using a random-effects model and followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.
Main Outcomes and Measures
The primary outcomes included the pooled prevalence rates (percentages with 95% CI) for hypertension and albuminuria. The secondary outcomes assessed pooled prevalence rates by sex and racial/ethnic group.
Results
Sixty studies were included in the systematic review. Diabetes duration varied from inclusion at diagnosis to 15.0 years after diagnosis, and the reported mean age at diagnosis ranged from 6.5 to 21.0 years. Hypertension prevalence among 3463 participants was 25.33% (95% CI, 19.57%-31.53%). Male participants had higher hypertension risk than female participants (odds ratio [OR], 1.42 [95% CI, 1.10-1.83]), with Pacific Islander and Indigenous youth having the highest prevalence of all racial/ethnic groups (Pacific Islander youth: 26.71% [95% CI, 14.54%-40.72%]; Indigenous youth: 26.48% [95% CI, 17.34%-36.74%]; White youth: 20.95% [95% CI, 12.65%-30.57%]; African American youth: 19.04% [95% CI, 12.01%-27.23%]; Hispanic/Latino youth: 15.11% [95% CI, 6.56%-26.30%]; Asian youth: 18.37% [95% CI, 9.49%-29.23%]). Albuminuria prevalence among 2250 participants was 22.17% (95% CI, 17.34%-27.38%). Pacific Islander youth, Indigenous youth, and Asian youth had higher prevalence rates than White youth (Pacific Islander youth: 31.84% [95% CI, 11.90%-55.47%]; Indigenous youth: 24.27% [95% CI, 14.39%-35.73%]; Asian youth: 23.00% [95% CI, 18.85%-27.41%]; White youth: 12.59% [95% CI, 7.75%-18.33%]), with no sex differences (OR for male vs female participants, 0.68 [95% CI, 0.46-1.01]). Heterogeneity was high among studies, with a low to moderate risk of bias.
Conclusions and Relevance
In this study, markers of diabetes-related nephropathy were commonly detected in pediatric patients with type 2 diabetes, with a disproportionate burden noted among Pacific Islander and Indigenous youth. Personalized management strategies to target kidney outcomes are urgently needed in pediatric patients with type 2 diabetes to alleviate the burden of this condition on the kidneys.
Introduction
The global increase in obesity has driven the emergence of type 2 diabetes in children.1,2 Pediatric type 2 diabetes is an aggressive disease with greater risk of end-organ damage and comorbidities than pediatric type 1 diabetes or adult-onset type 2 diabetes.1,2,3,4,5 The kidneys are notable early targets of type 2 diabetes–associated organ damage, and diabetes-related nephropathy commonly manifests as hypertension and albuminuria.6,7,8 If untreated, hypertension is associated with cardiovascular anomalies, including increased carotid intima-media thickness and left ventricular hypertrophy.9,10 These subclinical adverse outcomes are known risk factors for future cardiovascular disease and mortality.9,10 Similarly, microalbuminuria is the first sign of diabetes-related nephropathy and can progress to chronic kidney disease and end-stage kidney disease if untreated.11
To ensure early detection and treatment of nephropathy in the pediatric type 2 diabetes population, current screening guidelines recommend measuring blood pressure (BP) and urine albumin-to-creatine ratio (ACR) at type 2 diabetes diagnosis and annually thereafter.9,12,13 With adequate glycemic and blood pressure control, the onset of end-stage kidney disease can be delayed, and the risk of microvascular and macrovascular complications can be reduced, making the management of hypertension and albuminuria crucial to improving outcomes in patients with pediatric type 2 diabetes.11,14 However, the full burden of diabetes-related nephropathy in pediatric patients with type 2 diabetes is not well established. There has also been some evidence suggesting that the rate of type 2 diabetes complications differs by sex and race/ethnicity.15,16,17 Determining how sex and race/ethnicity are associated with hypertension and albuminuria prevalence is an important step toward identifying at-risk groups and can inform future personalized screening and treatment strategies.
Thus, this systematic review aimed to determine the prevalence of hypertension and albuminuria in pediatric patients with type 2 diabetes and to explore the association of sex and race/ethnicity with prevalence.
Methods
Systematic Review Protocol and Registration
This systematic review has been registered with PROSPERO (CRD42018091127).18 The study is reported according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline.19
Search Strategies
Search strategies were developed by a senior health sciences librarian and conducted in MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews from database inception to February 20, 2020, without language restrictions (eTables 1-5 in the Supplement). The gray literature, including ClinicalTrials.gov and Web of Science: Conference Proceedings Citation Index–Science, was searched. We combined concepts of pediatrics and type 2 diabetes with terms for hypertension, albuminuria, prevalence, and epidemiologic study design. We also searched the references of included articles. If a conference abstract was deemed eligible, we sought a full-text publication and contacted the corresponding author if a published article could not be located or did not report the relevant data set for this analysis.
Eligibility Criteria
We included studies with observational designs, including retrospective and prospective cohort studies as well as cross-sectional studies. The eligibility criteria included studies involving human participants with a sample size of at least 10 that reported on hypertension and/or albuminuria prevalence in patients with type 2 diabetes who were 18 years of age or younger. For studies with serial reporting of data, we included the report with the largest sample size. We excluded studies reporting participants with gestational diabetes.
To be as comprehensive as possible, we included studies reporting on all definitions of hypertension and albuminuria for our prevalence estimate. In the meta-analysis, we only pooled studies with similar definitions. Hypertension was defined as systolic and/or diastolic BP levels in the 95th percentile or greater for sex, age, and height.20 Most studies used BP reference values based on the National Heart, Lung, and Blood Institute data, whereas some used reference values based on the European guidelines.20,21,22,23,24,25 Urine ACR of 30 mg/g or greater defined albuminuria.9,12,13,26 Microalbuminuria was defined as an ACR of 30 or greater to 300 mg/g, and macroalbuminuria was defined as an ACR of greater than 300 mg/g. Persistent albuminuria, microalbuminuria, or macroalbuminuria were defined as 2 of 3 samples with levels greater than the corresponding ACR threshold over 6 months. If not specified, it was assumed that measurements were taken only once. Studies using other definitions of hypertension or albuminuria were removed in the sensitivity analysis.
Study Selection, Data Abstraction, and Quality Appraisal
Three teams of 2 independent reviewers (M.C., M.H., A.N., Y.Q., S.J.J.C., A.R.) screened titles, abstracts, and full-text articles and completed data abstraction, risk of bias assessments, and level of evidence assessments. Reviewers resolved disagreements through discussion, and a third reviewer (M.C.S.) resolved persistent disagreements.
A data abstraction form was designed and piloted specifically for this study. We extracted data on study design, age at diabetes diagnosis, age at study enrollment, duration of diabetes, sample size, sex, and race. We also extracted hypertension and albuminuria definitions and prevalence with sex-specific and race-specific data, if reported.
For longitudinal studies, we extracted the prevalence values closest to the time of type 2 diabetes diagnosis because we wanted to define the prevalence closest to the time of hypertension or albuminuria diagnosis. For unreported data, we contacted the corresponding authors to retrieve the information specific to our study question. Several studies reported on cohorts that included participants older than 18 years. We contacted the study authors to retrieve pediatric-specific data. When we did not receive the data, we included studies if most participants were 18 years or younger and no participants were older than 25 years.
Risk of bias was evaluated for each study using a validated tool for prevalence studies developed by Hoy et al.27 The tool assesses methodological quality across 10 items addressing studies’ external and internal validity.27 Each criterion was given a score of 0 if unaddressed or unclear and 1 if it was met.27 External validity criteria included whether the target population was representative of the national population, the sampling frame was representative of the target population, random selection or a census was used, and whether there was limited evidence of nonresponse bias.27 Internal validity criteria included whether data were collected directly from participants, an acceptable case definition was used, reliable and valid tools were used to assess prevalence, data were collected using the same method for all participants, the length of the shortest prevalence period for the parameter of interest was appropriate, and, if appropriate, numerators and denominators were used to assess prevalence.27 The overall risk of bias was rated as low (score >8), moderate (score 6-8), or high (score ≤5).27 The overall level of evidence was assessed according to the Oxford Centre for Evidence-Based Medicine criteria (OCEBM).28
Statistical Analysis
A random-effects model meta-analysis was performed when 2 or more studies of similar design, populations, methods, and outcomes were available.29,30 The primary outcomes of this review included the pooled prevalence of hypertension and albuminuria (reported as a percentage with 95% CIs) across all study designs. Because it was expected that some reports would have a small number of events, we transformed all prevalence estimates using the Freeman-Tukey double arcsine method30 and converted the results back to prevalence estimates for reporting.31,32 Inconsistency index (I2) and χ2 test P values were used to quantify heterogeneity among studies, and an I2 greater than 75% and P < .10 defined significant heterogeneity.33 Prespecified subgroup, sensitivity, metaregression, and publication bias assessments were performed if at least 10 studies were included in the meta-analysis for a given outcome.33 Subgroup analyses were performed by sex and race.
We also did a meta-analysis with studies comparing the prevalence between male and female participants and calculated odds ratios with 95% CIs. We used the National Institutes of Health definitions to categorize racial groups.34 We used the term Indigenous to report data from Indigenous populations in North America.
We also performed a random-effects metaregression to determine the association of obesity prevalence with hypertension and albuminuria. We performed sensitivity analyses by removing conference abstracts, studies with a sample size smaller than 50 patients, patients older than 18 years, or studies that used different or unspecified definitions of hypertension or albuminuria. A funnel plot was used to investigate publication bias with the Egger test and visual inspection to assess plot asymmetry.35 The prevalence meta-analyses were conducted using the metafor package in RStudio version 1.1.383, R version 3.4.3 (R Project for Statistical Computing).36,37,38 The sex-based forest plots for ORs were generated using Review Manager Version 5.3 (Cochrane Collaboration).39
Results
Search Results
The searches yielded 7614 unique records, and 60 eligible studies were included in the review (eFigure 1 in the Supplement). Most articles were removed, as they were irrelevant to the research question, reported on adult type 2 diabetes, or did not assess hypertension and/or albuminuria prevalence in children and adolescents with type 2 diabetes.
Hypertension
Study Characteristics
Forty-six studies reported hypertension prevalence (Table 1).4,15,17,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82 The reported age at diagnosis of type 2 diabetes ranged from 7.1 to 20.0 years,46,47,49,72 and the duration of diabetes ranged from inclusion at diagnosis40,41,43,51,52,55,56,60,65,66,75,76,81 to 7.8 years after diagnosis.71 While 26 studies (57%) had a cross-sectional design,15,17,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,75,76,77,78,82 13 (28%) were retrospective cohort studies,59,60,61,62,63,64,65,66,67,68,69,79,80 and 7 (15%) were prospective cohort studies.4,70,71,72,73,74,81
Table 1. Characteristics of Included Studies Reporting on Prevalence of Hypertension in Pediatric Type 2 Diabetes.
| Source | Country | Study design | Age at diagnosis, mean (SD), y | Age at enrollment, mean (SD), y | Diabetes duration, mean (SD), y | Cases, No. (%) | Sample size, No. | Sex distribution No. (%) | Racial group distribution, No. (%) | Cases by sex and/or racial group, No. (%) | Hypertension definition | Reference values source | Prevalence of obesity, No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hypertension | |||||||||||||
| Pinhas-Hamiel et al,40 1996 | United States | CS | 13.8 (1.9) | 13.8 (1.9) | 0 | 9 (17) | 54 | M: 20 (37); F: 34 (63) | NHB: 37 (68), NHW: 17 (32) | NR | NR | NR | 50 (92) |
| Scott et al,41 1997 | United States | CS | 13.9 (0.4)a | 13.9 (0.4)a | 0 | 14 (32) | 44 | M: 17 (38); F: 27 (62) | AA: 32 (74); NHW: 11 (24); H: 1 (2) | NR | NR | NR | 42 (85)b |
| Ettinger et al,42 2005 | United States | CS | NR | 15.0 (1.9) | 1.5 (1.0) | 15 (58) | 26 | M: 12 (46); F: 14 (54) | H: 15 (58); NHB: 8 (31); other: 2 (7); multiracial: 1 (4) | NR | BP >95th percentile for sex and height | NHLBI Update on Second Task Force Report23 | NR |
| Reinehr et al,43 2005 | Germany | CS | 14.2 (13.0-15.0)c | 14.2 (13.0-15.0)c | 0 | 8 (50) | 16 | M: 10 (63); F: 6 (37) | White: 16 (100) | White: 8 (50) | BP ≥95th percentile for age, gender, and height | US age-sex-height specific values21 | 14 (88) |
| Eppens et al,44 2006 | Western Pacific | CS | 12.0 (10.7-13.5)b,c | 14.9 (13.2-16.4)b,c | 2.3 (1.4-3.6)b,c | 64 (24.2) | 265 | M: 120 (45.3); F: 145 (54.7) | NR | M: 34 (28.3); F: 30 (20.7) | Systolic and diastolic BP >95th percentile for age, sex, and height | NHLBI Update on Second Task Force Report23 | 106 (32.0)b |
| Unnikrishnan et al,45 2008 | India | CS | 16.2 (2.9) | 18.9 (4.9) | NR | 1 (3) | 36 | M: 21 (58); F: 15 (42) | Indian: 36 (100)d | Indian: 1 (3) | NR | NR | NR |
| Bell et al,46 2009 | United States | CS | range, 10-19 | Age 10-14 y: 41 (38.4%); age ≥15 y: 65 (61.3%) | NR | 17 (16.0) | 106 | NR | NHW: 106 (100.0) | NHW: 17 (16.0) | BP ≥95th percentile for age, sex, and height | NHLBI Fourth Report20 | 83 (79.0)b |
| Dabelea et al,47 2009 | United States | CS | All participants <20 | 18.0 (2.8) | 3.5 (2.2) | 22 (36) | 62 | NR | Navajo: 62 (100) | Navajo: 22 (36) | BP ≥95th percentile for age, sex, and height | NHLBI Fourth Report20 | 42 (68) |
| Lawrence et al,48 2009 | United States | CS | Age 10-14 y: 11.6 (1.5); age ≥15 y: 14.6 (2.1)b | Age 10-14 y: 37 (30.8%); age ≥15 y: 83 (69.2%) | Age 10-14 y: 1.2 (0.9); age ≥15 y: 2.2 (2.0)b | 25 (20.8) | 120 | NR | H: 120 (100.0) | H: 25 (20.8) | Systolic and/or diastolic BP ≥95th percentile for sex, age, and height | NHLBI Fourth Report20 | 93 (73.2)b,e |
| Liu et al,49 2009 | United States | CS | All participants <20 | NR | Asian: 1.6 (1.4); API: 3.4 (3.1); PI: 1.7 (1.7)b | 13 (27) | 48 | NR | Asian: 29 (60); API: 11 (23); PI: 8 (17) | Asian: 8 (28); API: 4 (36); PI: 1 (13) | Systolic or diastolic BP ≥95th percentile based on age, sex, and height | NHLBI Fourth Report20 | 38 (76)b |
| Mayer-Davis et al,50 2009 | United States | CS | Age 10-14 y: 11.7 (1); age ≥15 y: 15.1 (1.9) | Age 10-14 y: 81 (38.2%); age ≥15 y: 131 (61.8%) | Age 10-14 y: 1.2 (0.7); age ≥15 y: 2.6 (2.1) | 49 (23.1) | 212 | NR | AA: 212 (100.0) | AA: 49 (23.1) | Systolic or diastolic BP ≥95th percentile for age, height, and sex | NHLBI Fourth Report20 | NR |
| Urakami et al,51 2009 | Japan | CS | 12.9 (1.5) | 12.9 (1.5) | 0 | 13 (11.6) | 112 | M: 45 (40.2); F: 67 (59.8) | Japanese: 112 (100.0)d | Japanese: 13 (11.6) | Systolic BP ≥130 mm Hg and/or diastolic BP ≥85 mm Hg | NR | 93 (83.0) |
| Rodriguez et al,17 2010 | United States | CS | 12.9 (2.1) | 14.8 (2.0) | 1.6 (1.5) | 97 (23.7) | 410 | M: 152 (37.1); F: 258 (62.9) | AA: 130 (31.7); H: 99 (24.1); NHW: 84 (20.5); AI: 56 (13.7); API: 37 (9.0); other: 4 (1.0) | M: 38 (25.0); F: 59 (22.9); AA: 36 (27.7); H: 22 (22); NHW: 17 (20); AI: 12 (21); API: 10 (27); other: 0 | Systolic or diastolic BP >95th percentile for age, sex, and height | NHLBI Fourth Report20 | 332 (81.0) |
| Copeland et al,15 2011 | United States | CS | range, 10-17 | 14.0 (2.0) | 0.7 (0.5) | 96 (13.6) | 704 | M: 247 (35.1); F: 457 (64.9) | H: 289 (41.1); NHB: 222 (31.5); NHW: 138 (19.6); AI: 43 (6.1); Asian: 12 (1.7) | M: 44 (17.8); F: 52 (11.4); H: 31 (10.7); NHB: 34 (15.3); NHW: 23 (16.7); AI: 7 (16) | BP ≥95th percentile for age, sex and height | NR | NR |
| Amed et al,52 2012 | Canada | CS | Canadian Aboriginal: 12.9 (12.4-13.4)f; White: 14.4 (13.8-15.1)f; other (African/Caribbean, Asian, H, Middle Eastern): 14.3 (13.7-14.9)f | Canadian Aboriginal: 12.9 (12.4-13.4)f, White: 14.4 (13.8-15.1)f, other (African/Caribbean, Asian, H, Middle Eastern): 14.3 (13.7-14.9)f | 0 | 61 (27.8) | 221 | M: 91 (41.2); F: 130 (58.8) | Canadian Aboriginal: 100 (45.2); White: 57 (25.8); other (African/Caribbean, Asian, H, Middle Eastern): 64 (29.0) | Canadian Aboriginal: 27 (27.3); White: 16 (28); other (African/Caribbean, Asian, H, Middle Eastern): 18 (28) | NR | NR | 211 (95.3) |
| Amutha et al,53 2012 | India | CS | NR | 16.1 (2.5) | 22.2 (9.7) | 47 (23.7) | 198 | M: 81 (40.9); F: 117 (59.1) | South Indian: 198 (100.0)d | M: 22 (27); F: 25 (21.4); South Indian: 47 (23.7) | BP ≥130/85 mm Hg | NR | NR |
| Drutel and Paulo,54 2014g | United States | CS | NR | range, 3-18 | NR | 73 (27.8) | 263 | NR | NR | NR | NR | NR | NR |
| Klingensmith et al,55 2016 | United States | CS | 13.1 (2.3)b | 13.1 (2.3)b | 0 | 44 (29.3) | 150 | NR | NR | NR | BP ≥95th percentile for age and height | NR | 315 (62.6)b |
| Zabeen et al,56 2016 | Bangladesh | CS | Age 9-10 y: 11 (14.3%); age 11-14 y: 46 (59.7%); age 15-17 y: 20 (26.0%) | Age 9-10 y: 11 (14.3%); age 11-14 y: 46 (59.7%); age 15-17 y: 20 (26.0%) | 0 | 25 (32) | 77 | M: 26 (34); F: 51 (66) | Bangladeshi: 77 (100)d | Bangladeshi: 25 (32) | BP ≥95th percentile for age and sex | NHLBI Second Task Force Report22 | 45 (58) |
| Aulich et al,57 2019 | Australia | CS | NR | 15.1 (1.9) | 1.8 (0.3-3.3)c | 6 (19) | 31 | NR | NR | NR | BP ≥95th percentile for age and sex for patients <18 y, ≥140/90 mm Hg for patients ≥18 y, or relevant medical therapy | NHLBI Second Task Force Report22 | 24 (75)b |
| Khalil et al,58 2019 | Egypt | CS | 18.0 (2.0) | 19.8 (1.1) | 2.5 (2.0) | 1 (8) | 13 | M: 6 (46); F: 7 (54) | Egyptian: 13 (100)d | M: 1 (17); F: 0; Egyptian: 0 | Systolic BP ≥140 mm Hg or diastolic BP ≥80 mm Hg or taking antihypertensive medication | NR | NR |
| Scott et al,59 2004 | New Zealand | RC | NR | mean, 19.6; range, 14-23 | 1.7 | 5 (39) | 13 | M: 7 (54); F: 6 (46) | Maori: 7 (54); European: 4 (30); PI: 1 (8); Asian Indian: 1 (8) | NR | Systolic BP >130 mm Hg or diastolic BP >80 mm Hg | NR | 13 (100) |
| Zdravkovic et al,60 2004 | Canada | RC | 13.5 (2.2) [range, 8.8-17.5] | 13.5 (2.2) [range, 8.8-17.5] | 0 | 4 (10) | 41 | M: 15 (37); F: 26 (63) | South/East Asian: 19 (46); African Canadian: 11 (27); NHW: 6 (15); H: 4 (10); FN: 1 (2) | NR | BP ≥95th percentile for age and sex | NHLBI Update on Second Task Force Report23 | 33 (80) |
| Pérez-Perdomo et al,61 2005 | Puerto Rico | RC | NR | Age ≤17 y: 35 (80%); age 18-19 y: 9 (20%) | NR | 5 (11) | 44 | NR | NR | NR | NR | NR | 69 (80)b |
| Scott et al,62 2006 | New Zealand | RC | NR | 20.0 (0.4) | 3.0 (0.3) | 21 (20.0) | 105 | NR | Maori/PI/other: 66 (62.9); European: 39 (37.1) | NR | BP >130/85 mm Hg | NR | 105 (100) |
| Balasanthiran et al,63 2012 | United Kingdom | RC | 15.2 (3.3) | 21.2 (3.2) | 5.4 (3.1) | 9 (21) | 44 | M: 17 (39); F: 27 (61) | Bangladeshi: 11 (25); Pakistani: 9 (20); Indian: 7 (16); White British: 6 (14); Black African: 4 (9); Black Caribbean: 4 (9); unclear: 3 (7) | NR | BP persistently >130/85 mm Hg | NR | 23 (59)b,e |
| Osman et al,64 2013 | Sudan | RC | Age <11 y: 3 (8%); age 11-18 y: 35 (92%) | NR | NR | 22 (58) | 38 | M: 17 (45); F: 21 (55) | Arab: 32 (84); multiracial: 4 (11); non-Arab: 2 (5) | NR | BP ≥95th percentile for age and sex on >1 occasion | NR | 29 (76) |
| Dart et al,65 2014 | Canada | RC | 13.5 (2.2) | 13.5 (2.2) | 0 | 34 (9.9) | 342 | M: 129 (37.8); F: 213 (62.2) | NR | NR | Elevated BP for age, sex, and height | NHLBI Fourth Report20 | NR |
| Haynes et al,66 2014g | Australia | RC | 13.3 (2.0)b | 13.3 (2.0)b | 0 | 15 (20) | 75 | NR | NR | NR | NR | NR | 82 (60.7)b |
| Yafi,67 2019g | United States | RC | range, 8-15 | NR | NR | 1 (5) | 25 | M: 11 (44); F: 14 (56) | H: 15 (60); other: 10 (40) | NR | NR | NR | NR |
| Yeow et al,68 2019 | Malaysia | RC | 14.3 (3.5) | 20.7 (3.7) | 6.5 (2.8) | 3 (13) | 24 | M: 10 (42); F: 14 (58) | Malay: 12 (50); Chinese: 11 (46); Asian Indian: 1 (4) | NR | BP ≥95th percentile for age and sex for patients <17 y; BP ≥140/90 mm Hg for patients ≥17 y | NHLBI Fourth Report20; NHLBI Seventh Report24 | 10 (42) |
| Curran et al,69 2020 | Australia | RC | All participants <10 | All participants, <16 | NR | 3 (27) | 11 | NR | PI: 11 (100) | PI: 3 (27) | NR | NR | 11 (100) |
| Eppens et al,4 2006 | Australia | PC | 13.2 (11.6-15.0)b,c | 15.3 (13.6-16.4)b,c | 1.3 (0.6-3.1)b,c | 21 (36) | 58 | NR | NR | NR | Systolic or diastolic BP >95th percentile for age and sex | NHLBI Update on Second Task Force Report23 | 36 (56)b |
| Shield et al,70 2009 | United Kingdom and Republic of Ireland | PC | mean, 13.6; range, 9.9-16.8b | mean, 14.5; range, 10.8-17.8b | mean, 1b | 19 (32) | 59 | M: 24 (41); F: 35 (59) | NR | M: 7 (29); F: 12 (34) | Systolic or diastolic BP ≥98th percentile for age and sex | UK Reference Values25 | 61 (80)b |
| Ruhayel et al,71 2010 | Australia | PC | 13.4 (range, 9.2-17.4)a,c | M: 16.0 (13.6-18.2); F: 15.6 (11.7-19.8)a,c | M: 2.2 (0.0-7.8); F: 2.3 (0.1-7.4)a,c | 9 (30) | 30 | NR | NR | NR | BP >98th percentile for age and sex | UK Reference Values25 | 23 (70)b |
| Jefferies et al,72 2012 | New Zealand | PC | 12.9 (1.8) [range, 7.1-15.5] | NR | NR | 27 (52) | 52 | M: 17 (33); F: 35 (67) | PI/Maori: 47 (90); other: 5 (10) | NR | BP ≥95th percentile for sex and age | NR | NR |
| Schmidt et al,73 2012 | Germany and Austria | PC | 13.5 (3.4) | 15.3 (3.0) | NR | 202 (29.5) | 684 | M: 261 (38.2); F: 423 (61.8) | German/Austrian: 482 (70.5); other: 202 (29.5) | NR | BP >95th percentile for age, sex, and height | NHLBI Fourth Report20 | NR |
| Dart et al,74 2019 | Canada | PC | All participants <18 | 15 (13.3-16.8)c | 2.3 (0.9-4.1)c | 155 (82.9) | 187 | M: 62 (33.2); F: 125 (66.8) | Indigenous: 179 (95.7); other: 8 (4.3) | NR | BP ≥95th percentile for age, sex and height | NHLBI Fourth Report20 | NR |
| Systolic hypertension | |||||||||||||
| Upchurch et al,75 2003 | United States | CS | 13.6 (2.3)b | 13.6 (2.3)b | 0 | 41 (49) | 83 | NR | NR | NR | Systolic BP ≥95th percentile for age, sex, and height | NR | 91 (93)b |
| Wei et al,76 2003 | Taiwan | CS | M: 13.7 (2.5); F: 13.0 (2.5)a,b | M: 13.7 (2.5); F: 13.0 (2.5)a,b | 0 | 49 (39.2) | 125 | M: 47 (37.6); F: 78 (62.4) | NR | M: 23 (49); F: 26 (33) | Systolic BP ≥85th percentile of sex and age based on population in the study | NR | 63 (48.1)b |
| Cruz et al,77 2004 | Mexico | CS | All participants <18 | 13.8 (1.8) | mean, 0.9; range, 0.08-3 | 2 (5) | 44 | M: 20 (46); F: 24 (54) | Mexican: 44 (100)d | Mexican: 2 (5) | NR | NR | NR |
| Hotu et al,78 2004 | New Zealand | CS | mean, 15; range, 11-19 | NR | NR | 5 (28) | 18 | M: 9 (50); F: 9 (50) | Maori/PI: 18 (100) | M: 3 (33); F: 2 (22); Maori/PI: 5 (28) | Systolic BP >95th percentile for age, sex and height | NHLBI Update on Second Task Force Report23 | NR |
| Rodriguez et al,17 2010 | United States | CS | 12.9 (2.1) | 14.8 (2.0) | 1.6 (1.5) | 106 (25.9) | 410 | M: 152 (37.1); F: 258 (62.9) | AA: 130 (31.7); H: 99 (24.1); NHW: 84 (20.5); AI: 56 (13.7); API: 37 (9.0); other: 4 (1.0) | NR | Systolic BP >95th percentile for age, sex and height | NHLBI Fourth Report20 | 332 (81.0) |
| Sellers et al,79 2007 | Canada | RC | mean, 13.1; range, 9-17 | mean, 15.3; range, 9-18 | NR | 13 (13) | 99 | M: 42 (42); F: 57 (58) | FN/Metis: 94 (95); other: 5 (5) | M: 5 (12); F: 8 (14) | Systolic BP >95th percentile for age and gender | NHLBI Fourth Report20 | 38 (38) |
| Pelham et al,80 2018 | United States | RC | NR | 15.2 (2.7) | 2.7 (1.7) | 34 (37) | 93 | M: 27 (29); F: 66 (71) | NR | NR | Systolic BP ≥95th percentile for age, gender, and height | NHLBI Fourth Report20 | NR |
| Shield et al,70 2009 | United Kingdom and Republic of Ireland | PC | mean, 13.6; range, 9.9-16.8b | mean, 14.5; range, 10.8-17.8b | mean, 1b | 4 (7) | 59 | M: 24 (41); F: 35 (59) | NR | M: 1 (4); F: 3 (9) | Systolic BP ≥98th percentile for age and sex | UK Reference Values25 | 61 (80)b |
| Candler et al,81 2018 | United Kingdom and Republic of Ireland | PC | 14.3 (7.9-16.9)h | 14.3 (7.9-16.9)h | 0 | 22 (20.8) | 106 | M: 35 (33.0); F: 71 (67.0) | NHW: 47 (44.3); Asian/Asian British: 36 (34.0); BACBB: 14 (13.2); other: 5 (4.7); uncertain: 4 (3.8) | NR | Systolic BP ≥95th percentile for sex, age and height | NHLBI Fourth Report20 | 86 (81.1) |
| Diastolic hypertension | |||||||||||||
| Upchurch et al,75 2003 | United States | CS | 13.6 (2.3)b | 13.6 (2.3)b | 0 | 9 (11) | 83 | NR | NR | NR | Diastolic BP ≥95th percentile for age, sex, and height | NR | 91 (93)b |
| Wei et al,76 2003 | Taiwan | CS | M: 13.7 (2.5); F: 13.0 (2.5)a,b | M: 13.7 (2.5); F: 13.0 (2.5)a,b | 0 | 53 (42.4) | 125 | M: 47 (37.6); F: 78 (62.4) | NR | M: 24 (51); F: 29 (37) | Diastolic BP ≥85th percentile of sex and age based on population in the study | NR | 63 (48.1)b |
| Cruz et al,77 2004 | Mexico | CS | NR | 13.8 (1.7) | mean, 0.9; range, 0.08-3 | 5 (11) | 44 | M: 20 (46); F: 24 (54) | Mexican: 44 (100)d | Mexican: 5 (11) | NR | NR | NR |
| Rodriguez et al,17 2010 | United States | CS | 12.9 (2.1) | 14.8 (2.0) | 1.6 (1.5) | 75 (18.3) | 410 | M: 152 (37.1); F: 258 (62.9) | AA: 130 (31.7); H: 99 (24.1); NHW: 84 (20.5); AI: 56 (13.7); API: 37 (9.0); other: 4 (1.0) | NR | Diastolic BP >95th percentile for age, sex and height | NHLBI Fourth Report20 | 332 (81.0) |
| Shalitin et al,82 2014 | Israel | CS | NR | 15.9 (3.6) | 3.3 (2.1) | 4 (36) | 11 | M: 5 (45); F: 6 (55) | Israeli: 11 (100)d | Israeli: 4 (36) | NR | NR | 11 (100) |
| Sellers et al,79 2007 | Canada | RC | mean, 13.1; range, 9-17 | mean, 15.3; range, 9-18 | NR | 6 (6) | 99 | M: 42 (42); F: 57 (58) | FN/Metis: 94 (95); Other: 5 (5) | M: 3 (7); F: 3 (5) | Diastolic BP >95th percentile for age and gender | NHLBI Fourth Report20 | 38 (38) |
| Pelham et al,80 2018 | United States | RC | NR | 15.2 (2.7) | 2.7 (1.7) | 7 (8) | 93 | M: 27 (29); F: 66 (71) | NR | NR | Diastolic BP ≥95th percentile for age, gender, and height | NHLBI Fourth Report20 | NR |
| Shield et al,70 2009 | United Kingdom and Republic of Ireland | PC | mean, 13.6; range, 9.9-16.8b | mean, 14.5; range, 10.8-17.8b | mean, 1b | 11 (19) | 59 | M: 24 (41); F: 35 (59) | NR | M: 6 (25); F: 5 (14) | Diastolic BP ≥98th percentile for age and sex | UK Reference Values25 | 61 (80)b |
Abbreviations: AA, African American; AI, American Indian; API, Asian–Pacific Islander; BACBB, Black/African/Caribbean/Black British; BP, blood pressure; CS, cross-sectional; F, female; FN, First Nations; H, Hispanic; M, male; NHB, Non-Hispanic Black; NHLBI, National Heart, Lung, and Blood Institute; NHW, Non-Hispanic White; NR, not reported; PC, prospective cohort; PI, Pacific Islander; RC, retrospective cohort.
Mean (SE).
Based on total study cohort instead of only patients examined for the specific comorbidity.
Median (interquartile range).
Racial group distribution assumed to match country of origin.
Value estimated based on graph.
Mean (CI).
Abstract only.
Median (range).
Pooled Prevalence of Hypertension
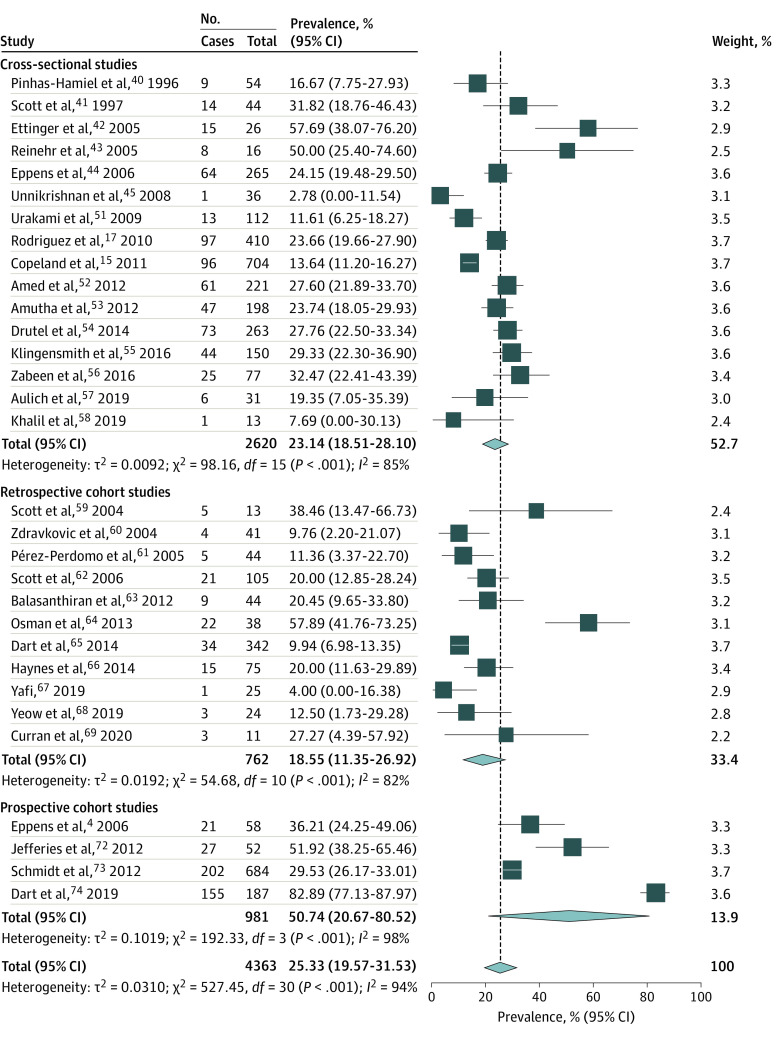
Thirty-one studies including 4363 patients with type 2 diabetes reported on the prevalence of hypertension defined as BP in the 95th percentile or greater for age, sex, and height or systolic BP 130 to 140 mm Hg or greater and diastolic BP of 80 to 90 mm Hg or greater.4,15,17,40,41,42,43,44,45,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,72,73,74 The pooled prevalence of hypertension was 25.33% (95% CI, 19.57%-31.53%) (Figure 1). High heterogeneity was noted across studies (I2 = 94%; P < .001).
Figure 1. Forest Plot Showing Pooled Prevalence of Hypertension in Pediatric Type 2 Diabetes.
Another 15 studies that reported on the prevalence of hypertension were not included in the meta-analysis. Two studies had a higher cutoff for hypertension (BP ≥98th percentile) and reported a prevalence of 30% among 30 participants and 32% among 59 participants, respectively.70,71 Five studies presented racial subgroup data46,47,48,49,50 of another included study.17 Eight studies were only included in the analysis of isolated systolic or diastolic hypertension.75,76,77,78,79,80,81,82
When pooling only the studies with hypertension definition of BP in 95th percentile or greater for age, sex, and height (2763 participants), the prevalence was significantly higher at 34.00% (95% CI, 24.00%-45.00%; I2 = 97%; P < .001) (eTable 6 in the Supplement).4,15,17,42,43,44,55,56,57,60,64,68,72,73,74 The metaregression analysis revealed no significant association between hypertension prevalence and obesity prevalence.
Pooled Prevalence of Systolic and Diastolic Hypertension in Type 2 Diabetes
Isolated systolic hypertension prevalence across 6 studies with 747 participants was 24.79% (95% CI, 14.04%-37.31%; I2 = 90%; P < .001) (eFigure 2 in the Supplement).17,75,77,78,79,80 Two additional studies using different definitions reported a prevalence of 39.2% among 125 participants (hypertension definition, BP ≥85th percentile)76 and 7% among 59 participants (hypertension definition, BP ≥98th percentile).70 Another study determined a prevalence of 20.8% among 106 participants; because it was the only prospective cohort study, it was not included in the meta-analysis.81
Isolated diastolic hypertension prevalence across 6 studies with 740 participants was 11.65% (95% CI, 6.41%-18.04%; I2 = 75%; P = .001) (eFigure 3 in the Supplement).17,75,77,79,80,82 Two additional studies using different definitions reported a prevalence of 42.4% among 125 participants (diastolic hypertension, BP ≥85th percentile)76 and 19% among 59 participants (diastolic hypertension, BP ≥98th percentile).70
Sex and Race Associations With Hypertension
Four studies reported hypertension prevalence in 600 male participants of 23.81% (95% CI, 18.56%-29.47%; I2 = 58%; P = .07) and 977 female participants of 18.56% (95% CI, 12.25%-25.82%; I2 = 85%; P < .001) with an OR of 1.42 (95% CI, 1.10-1.83; I2 = 0%; P for heterogeneity = .65) (eFigure 4 and eFigure 5 in the Supplement).15,17,44,53 In contrast, 1 study with hypertension definition of BP in the 98th percentile or greater reported a prevalence of 29% in 24 male participants and 34% in 35 female participants.70
When assessing the prevalence of hypertension in different racial groups, Indigenous and Pacific Islander youth had the highest rates of hypertension when compared with other groups (Pacific Islander youth17,69: 48 participants; prevalence, 26.71% [95% CI, 14.54%-40.72%]; I2 = 0%; P = .92; Indigenous youth15,47,52: 205 participants; prevalence, 26.48% [95% CI, 17.34%-36.74%]; I2 = 58%, P = .09; White youth15,43,46,52,58: 330 participants; prevalence, 20.95% [95% CI, 12.65%-30.57%]; I2 = 66%; P = .02; African American youth15,50: 434 participants; prevalence, 19.04% [95% CI, 12.01%-27.23%]; I2 = 76%; P = .04; Hispanic/Latino youth15,48: 409 participants; prevalence, 15.11% [95% CI, 6.56%-26.30%]; I2 = 85%; P < .001; Asian youth45,49,51,53,56: 452 participants; prevalence, 18.37% [95% CI, 9.49%-29.23%]; I2 = 84%, P < .001) (eFigure 6 in the Supplement).
Albuminuria
Study Characteristics
Thirty-nine studies reported on albuminuria prevalence (Table 2).4,8,15,16,42,44,45,46,50,52,53,57,58,59,62,64,65,66,67,68,69,71,72,73,74,78,81,83,84,85,86,87,88,89,90,91,92,93,94 The age at type 2 diabetes diagnosis ranged from 6.5 to 21.0 years,90,93 and type 2 diabetes duration ranged from diagnosis52,65,66,71,81,83,85,89,94 to more than 15.0 years after diagnosis.50,53 Nineteen studies (49%) were cross-sectional studies,15,16,42,44,45,46,50,52,53,57,58,78,83,84,86,87,88,89,90 14 (36%) were retrospective cohort studies,8,59,62,64,65,66,67,68,69,85,91,92,93,94 and 6 (15%) were prospective cohort studies.4,71,72,73,74,81
Table 2. Characteristics of Included Studies Reporting on the Prevalence of Albuminuria in Pediatric Type 2 Diabetes.
| Source | Country | Study design | Age at diagnosis, mean (SD), y | Age at study enrollment or measurement, mean (SD), y | Duration of diabetes, mean (SD), y | Cases, No. (%) | Sample size | Sex distribution, No. (%) | Racial group distribution, No. (%) | Cases by sex or racial group, No. (%) | Albuminuria definition | Prevalence of obesity, No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albuminuria | ||||||||||||
| Hotu et al,78 2004 | New Zealand | CS | mean, 15; range, 11-19 | NR | NR | 7 (58) | 12 | M: 6 (50); F: 6 (50) | Maori/PI: 12 (100) | M: 4 (67); F: 3 (50); Maori/PI: 7 (58) | ACR ≥30mg/g | NR |
| Ettinger et al,42 2005 | United States | CS | NR | 15.0 (1.9) | 1.5 (1.0) | 10 (40) | 25 | M: 12 (46); F: 14 (54)a | H: 15 (58); NHB: 8 (31); other: 2 (7); multiracial: 1 (4)a | NR | AER ≥30mg albumin/24 h | NR |
| Maahs et al,16 2007 | United States | CS | All participants <20 | Age <12 y: 19 (5.1%); age ≥12 y: 355 (94.9%) | 1.9 (0.4-3.2)b | 83 (22.2) | 374 | M: 140 (37.4); F: 234 (62.6) | AA: 110 (29.4); AI: 92 (24.6); NHW: 71 (19.0); H: 64 (17.1); API: 25 (6.8); multiracial or other: 11 (2.9); unknown: 1 (0.2) | M: 29 (20.7); F: 54 (23.1); AA: 18 (16.4); AI: 33 (36); NHW: 9 (13); H: 15 (23); API: 6 (24); multiracial or other: 2 (18); unknown: 0 (0) | ACR ≥30mg/g | 266 (72.3)a |
| Unnikrishnan et al,45 2008 | India | CS | 16.2 (2.9) | 18.9 (4.9) | NR | 0 | 36 | M: 21 (58); F: 15 (42) | Indian: 36 (100)c | Indian: 0 | AER >500mg albumin/24 h | NR |
| Bell et al,46 2009 | United States | CS | All participants <20 | Age 10-14 y: 42 (41.6%); age ≥15 y: 59 (58.4%) | NR | 14 (13.9) | 101 | NR | NHW: 101 (100) | NHW: 14 (13.9) | ACR ≥30mg/g | 83 (79.0)a |
| Mayer-Davis et al,50 2009 | United States | CS | 10-14: 11.7 (1); ≥15: 15.1 (1.9) | 10-14: 81 (38.2%); ≥15: 131 (61.8%) | 10-14: 1.2 (0.7); ≥15: 2.6 (2.1) | 30 (14.1) | 212 | NR | AA: 212 (100) | AA: 30 (14.1) | ACR ≥30mg/g | NR |
| Kim et al,83 2010 | United States | CS | NR | 14.5 (3.0) | mean, 1.3; range, 0.0-2.1 | 22 (21.4) | 103 | M: 40 (38.8); F: 63 (61.2) | Pima Indian: 103 (100) | Pima Indian: 22 (21.4) | ACR ≥30mg/g | NR |
| Amed et al,52 2012 | Canada | CS | Canadian Aboriginal: 12.9 (12.4-13.4)d; White: 14.4 (13.8-15.1)d; other (African/Caribbean, Asian, H, Middle Eastern): 14.3 (13.7-14.9)d | Canadian Aboriginal: 12.9 (12.4-13.4)d; White: 14.4 (13.8-15.1)d; other (African/Caribbean, Asian, H, Middle Eastern): 14.3 (13.7-14.9)d | 0 | 32 (14.4) | 221 | M: 91 (41.2); F: 130 (58.8) | Canadian Aboriginal: 100 (45.2); White: 57 (25.8); other (African/Caribbean, Asian, H, Middle Eastern): 64 (29.0) | Canadian Aboriginal: 16 (16.7); White: 6 (10); other (African/Caribbean, Asian, H, Middle Eastern): 9 (14) | NR | 211 (95.3) |
| Amutha et al,53 2012 | India | CS | 16.1 (2.5) | 22.2 (9.7) | Age ≤5 y: 219 (59.5%); age >5 to ≤10 y: 67 (18.2%); age >10 to ≤15: 21 (5.7%); age >15 y: 61 (16.6%) | 85 (23.1) | 368 | M: 168 (45.7); F: 200 (54.3) | South Indian: 368 (100.0)c | South Indian: 85 (23.1) | ACR ≥30mg/g | NR |
| Holman et al,84 2015 | United Kingdom | CS | All participants >12 | All participants, >12 | NR | NR (23) | NR | NR | NR | NR | NR | NR |
| Sellers et al,85 2009 | Canada | RC | NR | NR | 0 | 26 (29) | 90 | M: 40 (45); F: 50 (55) | FN/Metis: 88 (98); other: 2 (2) | NR | ACR >3 mg/mmol | NR |
| Yafi,67 2019e | United States | RC | range, 8-15 | NR | NR | 3 (12) | 25 | M: 11 (44); F: 14 (56) | H: 15 (60); other: 10 (40) | NR | NR | NR |
| Yeow et al,68 2019 | Malaysia | RC | 14.3 (3.5) | 20.7 (3.7) | 6.5 (2.8) | 7 (29) | 24 | M: 10 (42); F: 14 (58) | Malay: 12 (50); Chinese: 11 (46); Asian Indian: 1 (4) | Asian: 7 (29) | M: ACR >2.5mg/ mmol, F: ACR >3.5mg/ mmol | 10 (42) |
| Curran et al,69 2020 | Australia | RC | All participants <10 | All participants, <16 | NR | 2 (18) | 11 | NR | PI: 11 (100) | PI: 2 (18) | NR | 11 (100) |
| Ruhayel et al,71 2010 | Australia | PC | 13.4 (9.2-17.4)a,d | M: 16.0 (13.6-18.2; F: 15.6 (11.7-19.8)a,d | M: 2.2 (0.0-7.8; F: 2.3 (0.1-7.4)a,d | 9 (45) | 20 | NR | NR | NR | ACR >3.5mg/ mmol | 23 (70)a |
| Schmidt et al,73 2012 | Germany and Austria | PC | 13.5 (3.4) | 15.3 (3.0) | NR | 170 (24.9) | 684 | M: 261 (38.2); F: 423 (61.8) | German/Austrian: 482 (70.5); other: 202 (29.5) | NR | NR | NR |
| Candler et al,81 2018 | United Kingdom and Republic of Ireland | PC | 14.3 (7.9-16.9)d | 14.3 (7.9-16.9)d | 0 | 3 (2.8) | 106 | M: 35 (33.0); F: 71 (67.0) | NHW: 47 (44.3); Asian/Asian-British: 36 (34.0); BACBB: 14 (13.2); other: 5 (4.7); uncertain: 4 (3.8) | NR | NR | 86 (81.1) |
| Dart et al,74 2019 | Canada | PC | All participants <18 | 15 (13.3-16.8)b | 2.3 (0.9-4.1)b | 47 (25.1) | 187 | M: 62 (33.2); F: 125 (66.8) | Indigenous: 179 (95.7); other: 8 (4.3) | NR | ACR >2mg/mmol | NR |
| Persistent albuminuria | ||||||||||||
| Yoo et al,86 2004 | Korea | CS | 12.8 (1.5) | 18.4 (4.3) | 5.5 (3.9) | 5 (23) | 22 | M: 8 (36); M: 14 (64) | Korean: 22 (100) | Korean: 5 (23) | AER >20 μg/min on samples 3 mo apart | NR |
| Farah et al,87 2006 | United States | CS | NR | range, 10-21 | mean, 1.8; range, <2-5 | 9 (27) | 33 | NR | NR | NR | ACR >30 mg/g on 2 samples within 3-6 mo | 29 (73)a |
| Copeland et al,15 2011 | United States | CS | range, 10-17 | 14.0 (2.0) | 0.7 (0.5) | 92 (13.0) | 704 | M: 247 (35.1); F: 457 (64.9) | H: 289 (41.1); NHB: 222 (31.5); NHW: 138 (19.6); AI: 43 (6.1); Asian: 12 (1.7) | M: 26 (10.6); F: 65 (14.3); H: 41 (14.1); NHB: 25 (11.2); NHW: 20 (14.6); AI: 3 (8) | ACR ≥30mg/g on 2 of 3 samples during 3-mo period | NR |
| Sellers et al,88 2016 | Canada | CS | All participants <18 | NR | NR | 50 (5.1) | 976 | NR | NR | NR | M: ACR >2mg/ mmol, F: ACR >2.8mg/ mmol on 2 occasions during 6-mo period | NR |
| Aulich et al,57 2019 | Australia | CS | NR | 15.1 (1.9) | 1.8 (0.3-3.3)b | 6 (30) | 20 | NR | NR | NR | AER ≥20 μg/min in ≥2 of 3 samples or mean ACR, M: ≥3.5 mg/mmol; F: ≥4 mg/mmol from 3 first morning collections | 24 (75)a |
| Khalil et al,58 2019 | Egypt | CS | 18.0 (2.0) | 19.8 (1.1) | 2.5 (2.0) | 0 (0) | 13 | M: 6 (46); F: 7 (54) | Egyptian: 0c | Egyptian: 0 | ACR ≥30mg/g on 2 samples within 3-6 mo | NR |
| Scott et al,59 2004 | New Zealand | RC | NR | mean, 19.6; range, 14-23 | 1.7 | 2 (15) | 13 | M: 7 (54); F: 6 (46) | Maori: 7 (54); European: 4 (30); PI: 1 (8); Asian Indian: 1 (8) | NR | M: ACR >2.5mg/mmol; F: ACR >3.5mg/mmol on ≥2 occasions | 13 (100) |
| Scott et al,62 2006 | New Zealand | RC | NR | 20.0 (0.4) | 3.0 (0.3) | 76 (72.4) | 105 | NR | Maori/PI/other: 66 (62.9); European: 39 (37.1) | NR | M: ACR >2.5mg/ mmol; F: ACR >3.5mg/ mmol on ≥2 occasions | 105 (100) |
| Dart et al,65 2014 | Canada | RC | 13.5 (2.2) | 13.5 (2.2) | 0 | 93 (27.1) | 342 | M: 129 (37.8); F: 213 (62.2) | NR | NR | ACR >3mg/mmol or AER >30mg/24 h on ≥2 of 3 measurements 1 mo apart | NR |
| Eppens et al,4 2006 | Australia | PC | 13.2 (11.6-15.0)a,b | 15.3 (13.6-16.4)a,b | 1.3 (0.6-3.1)a,b | 10 (28) | 36 | NR | NR | NR | AER ≥20 μg/min in ≥2 of 3 samples or ACR ≥2.5 mg/mmol | 36 (56)a |
| Jefferies et al,72 2012 | New Zealand | PC | mean, 12.9; range, 7.1-15.5 | NR | NR | 18 (35) | 52 | M: 17 (33); F: 35 (67) | PI/Maori: 47 (90); other: 5 (10) | NR | ACR ≥2.5 mg/mmol on ≥2 of 3 samples during 6-mo period | NR |
| Dart et al,74 2019 | Canada | PC | All participants <18 | 15 (13.3-16.8b | 2.3 (0.9-4.1)b | 57 (30.5) | 187 | M: 62 (33.2); F: 125 (66.8) | Indigenous: 179 (95.7); other: 8 (4.3) | M: 15 (24); F: 42 (33.6); Indigenous: 56 (31.3) | ACR >2mg/mmol on 2 of 3 samples during a 6-mo period | NR |
| Microalbuminuria | ||||||||||||
| Hotu et al,78 2004 | New Zealand | CS | mean, 15 (11-19 | NR | NR | 5 (42) | 12 | M: 6 (50); F: 6 (50) | Maori/PI: 12 (100) | M: 3 (50); F: 2 (33); Maori/PI: 5 (42) | ACR ≥30-300mg/g | NR |
| Ettinger et al,42 2005 | United States | CS | NR | 15.0 (1.9) | 1.5 (1.0) | 10 (40) | 25 | M: 12 (46); F: 14 (54)a | H: 15 (58); NHB: 8 (31); other: 2 (7); multiracial: 1 (4)a | NR | AER ≥30mg albumin/24 h | NR |
| Eppens et al,44 2006 | Western Pacific | CS | 12.0 (10.7-13.5)a,b | 14.9 (13.2-16.4)a,b | 2.3 (1.4-3.6)a,b | 20 (8.0) | 251 | NR | NR | NR | AER 30-300mg/24 h or >20 μg/min or ACR >2.5mg/mmol | 106 (32.0)a |
| Kim et al,83 2010 | United States | CS | NR | 14.5 (3.0) | mean, 1.3 (0-2.1 | 19 (18.5) | 103 | M: 40 (38.8); F: 63 (61.2) | Pima Indian: 103 (100.0) | Pima Indian: 19 (18.5) | ACR ≥30-300mg/g | NR |
| Amutha et al,53 2012 | India | CS | 16.1 (2.5) | 22.2 (9.7) | Age ≤5 y: 219 (59.5%); age >5 to ≤10 y: 67 (18.2%); age >10 to ≤15 y: 21 (5.7%); age >15 y: 61 (16.6%) | 54 (14.7) | 368 | M: 168 (45.7); F: 200 (54.3) | South Indian: 368 (100)c | South Indian: 54 (14.7) | ACR ≥30-299mg/g | NR |
| Zabeen et al,89 2016e | Bangladesh | CS | 13.0 (11.0-15.0)d | 13.0 (11.0-15.0)d | 0 | 14 (10.0) | 144 | NR | Bangladeshi: 144 (100)c | Bangladeshi: 14 (10.0) | ACR ≥30-300mg/g | NR |
| Nambam et al,90 2017 | United States | CS | All participants <21 | 16.0 (14.0-17.7)b | 2.0 (0.7-4.2)b | 36 (6.0) | 598 | M: 218 (36.5); F: 380 (63.5) | H: 329 (55.0); AA: 179 (30.0); NHW: 48 (8.0); other/multiracial: 42 (7.0) | NR | NR | 472 (85.0)a |
| Le et al,91 2013 | United States | RC | 13.8 (2.4)a | 14.2 (2.4) | NR | 11 (17) | 64 | M: 20 (31); F: 44 (69) | AA: 52 (81); NHW: 12 (19) | AA: 11 (21); NHW: 0f | ACR ≥30-299 mg/g | NR |
| Osman et al,64 2013 | Sudan | RC | Age <11 y: 3 (7.9%); age 11-18 y: 35 (92.1%) | NR | NR | 7 (18) | 38 | M: 17 (45); F: 21 (55) | Arab: 32 (84); multiracial: 4 (11); non-Arab: 2 (5) | NR | NR | 29 (76) |
| Haynes et al,66 2014e | Australia | RC | 13.3 (2.0)a | 13.3 (2.0)a | 0 | 11 (18) | 61 | NR | NR | NR | NR | 82 (60.7)a |
| Calagua Quispe et al,92 2015e | Peru | RC | 12.6 (2.3) | NR | 3.7 (2.4) | 9 (43) | 20 | M: 10 (50); F: 10 (50) | NR | NR | NR | NR |
| Newton et al,93 2015e | New Zealand | RC | range, 6.5-17 | All participants, <17 | NR | 6 (55) | 11 | NR | NR | NR | NR | 22 (96)a |
| Son et al,94 2015 | Korea | RC | NR | 15.4 (12.6-17.4)b | 0.9 (0.0-3.0)b | 8 (44) | 18 | M: 4 (22); F: 14 (78) | Korean: 18 (100)c | Korean: 8 (44) | ACR ≥30-300mg/g | NR |
| Yeow et al.,68 2019 | Malaysia | RC | 14.3 (3.5) | 20.7 (3.7) | 6.5 (2.8) | 7 (29) | 24 | M: 10 (42); F: 14 (58) | Malay: 12 (50); Chinese: 11 (46); Asian Indian: 1 (4) | Asian: 7 (29) | M: ACR >2.5mg/mmol, F: ACR >3.5mg/mmol | 10 (42) |
| Ruhayel et al,71 2010 | Australia | PC | 13.4 (9.2-17.4)a,d | M: 16.0 (13.6-18.2); F: 15.6 (11.7-19.8)a,d | M: 2.2 (0.0-7.8); F: 2.3 (0.1-7.4)a,d | 9 (45) | 20 | NR | NR | NR | ACR >3.5mg/mmol | 23 (70)a |
| Schmidt et al,73 2012 | Germany and Austria | PC | 13.5 (3.3) | 15.3 (3.0) | NR | 154 (22.5) | 684 | M: 261 (38.2); F: 423 (61.8) | German/Austrian: 482 (70.5); other: 202 (29.5) | NR | NR | NR |
| Persistent microalbuminuria | ||||||||||||
| Yoo et al,86 2004 | Korea | CS | 12.8 (1.5) | 18.4 (4.3) | 5.5 (3.9) | 4 (18) | 22 | M: 8 (36); M: 14 (64) | Korean: 22 (100) | Korean: 4 (18) | AER >20 μg/min on samples 3 mo apart | NR |
| Farah et al,87 2006 | United States | CS | NR | range, 10-21 | mean, 1.8; range, <2-5 | 9 (27) | 33 | NR | NR | NR | ACR >30mg/g on 2 samples within 3-6 mo | 29 (73)a |
| Copeland et al,15 2011 | United States | CS | range, 10-17 | 14.0 (2.0) | 0.7 (0.5) | 92 (13.0) | 704 | M: 247 (35.1); F: 457 (64.9) | H: 289 (41.1); NHB: 222 (31.5); NHW: 138 (19.6); AI: 43 (6.1); Asian: 12 (1.7) | M: 35 (14.3); F: 48 (10.6); H: 41 (14.1); NHB: 25 (11.2); NHW: 20 (14.6); AI: 1 (8) | ACR ≥30mg/g on 2 of 3 samples during 3-mo period | NR |
| Aulich et al,57 2019 | Australia | CS | NR | 15.1 (1.9) | 1.8 (0.3-3.3)b | 6 (30) | 20 | NR | NR | NR | AER ≥20 μg/min in ≥2 of 3 samples or mean ACR, M: ≥3.5 mg/mmol; F: ≥4 mg/mmol from 3 first morning collections | 24 (75)a |
| Scott et al,59 2004 | New Zealand | RC | NR | 19.6 range, 14-23 | 1.7 | 2 (15) | 13 | M: 7 (54); F: 6 (46) | Maori: 7 (54); European: 4 (30); PI: 1 (8); Asian Indian: 1 (8) | NR | M: ACR >2.5mg/mmol, F: ACR >3.5mg/mmol on ≥2 occasions | 13 (100) |
| Scott et al,62 2006 | New Zealand | RC | NR | 20.0 (0.4) | 3.0 (0.3) | 76 (72.4) | 105 | NR | Maori/PI/other: 66 (62.9); European: 39 (37.1) | NR | M: ACR >2.5mg/mmol, F: ACR >3.5mg/mmol on ≥2 occasions | 105 (100) |
| Dart et al,8 2012 | Canada | RC | 13.5 (2.2) | 14.9 (2.1) | 1.6 (1.5) | 92 (26.9) | 342 | M: 129 (37.8); F: 213 (62.2) | NR | NR | ACR ≥3mg/mmol or AER 30mg/24 h on 2 of 3 samples 1 mo apart | NR |
| Son et al,94 2015 | Korea | RC | NR | 15.4 (12.6-17.4)b | 0.9 (0.0-3.0)b | 5 (28) | 18 | M: 4 (22); F: 14 (78) | Korean: 18 (100)c | Korean: 5 (28) | ACR ≥30-300 mg/g at baseline and follow-up | NR |
| Eppens et al,4 2006 | Australia | PC | 13.2 (11.6-15.0)a,b | 15.3 (13.6-16.4)a,b | 1.3 (0.6-3.1)a,b | 10 (28) | 36 | NR | NR | NR | AER ≥20 μg/min in at least 2 of 3 samples or ACR ≥2.5 mg/mmol | 36 (56)a |
| Jefferies et al,72 2012 | New Zealand | PC | mean, 12.9; range, 7.1-15.5 | NR | NR | 18 (35) | 52 | M: 17 (33); F: 35 (67) | PI/Maori: 47 (90); other: 5 (10) | NR | ACR ≥2.5 mg/mmol on ≥2 of 3 samples during 6-mo period | NR |
| Macroalbuminuria | ||||||||||||
| Hotu et al,78 2004 | New Zealand | CS | mean, 15; range, 11-19 | NR | NR | 2 (17) | 12 | M: 6 (50); F: 6 (50) | Maori/PI: 12 (100) | M: 1 (17); F: 1 (17); Maori/PI: 2 (17) | ACR >300mg/g | NR |
| Eppens et al,44 2006 | Western Pacific | CS | 12.0 (10.7-13.5)a,b | 14.9 (13.2-16.4)a,b | 2.3 (1.4-3.6)a,b | 1 (0.6) | 247 | NR | NR | NR | AER >300mg/24 h | 106 (32.0)a |
| Kim et al,83 2010 | United States | CS | NR | 14.5 (3.0) | 1.3 (0-2.1)b | 3 (2.9) | 103 | M: 40 (38.8); F: 63 (61.2) | Pima Indian: 103 (100) | Pima Indians: 3 (2.9) | ACR >300mg/g | NR |
| Amutha et al,53 2012 | India | CS | 16.1 (2.5) | 22.2 (9.7) | Age ≤5 y: 219 (59.5%); age >5 to ≤10 y: 67 (18.2%); age >10 to ≤15: 21 (5.7%); age >15 y: 61 (16.6%) | 31 (8.4) | 368 | NR | South Indian: 368 (100)c | South Indian: 31 (8.4) | ACR >300mg/g | NR |
| Schmidt et al,73 2012 | Germany and Austria | PC | 13.5 (3.4) | 15.3 (3.0) | NR | 16 (2.4) | 684 | M: 261 (38.2); F: 423 (61.8) | German/Austrian: 482 (70.5); other: 202 (29.5) | NR | NR | NR |
| Persistent macroalbuminuria | ||||||||||||
| Yoo et al,86 2004 | Korea | CS | 12.8 (1.5) | 18.4 (4.3) | 5.5 (3.9) | 1 (5) | 22 | M: 8 (36); F: 14 (64) | Korean: 22 (100) | Korean: 1 (5) | AER >200 μg/min on samples 3 mo apart | NR |
| Dart et al,8 2012 | Canada | RC | 13.5 (2.2) | 14.9 (2.1) | 1.6 (1.5) | 16 (4.7) | 342 | M: 129 (37.8); F: 213 (62.2) | NR | NR | NR | NR |
Abbreviations: AA, African American; ACR, albumin-to-creatinine ratio; AER, albumin excretion rate; AI, American Indian; API, Asian–Pacific Islander; BACBB, Black/African/Caribbean/Black British; CS, cross-sectional; F, Females; FN, First Nations; H, Hispanic; NR, not reported; M, Males; NHB, Non-Hispanic Black; NHW, Non-Hispanic White; PC, prospective cohort; PI, Pacific Islander; RC, retrospective cohort.
Based on total study cohort instead of only patients examined for the specific comorbidity.
Median (interquartile range).
Racial group distribution assumed to match country of origin.
Median (range).
Abstract only.
Value estimated based on graph.
Pooled Prevalence of Albuminuria and Persistent Albuminuria in Type 2 Diabetes
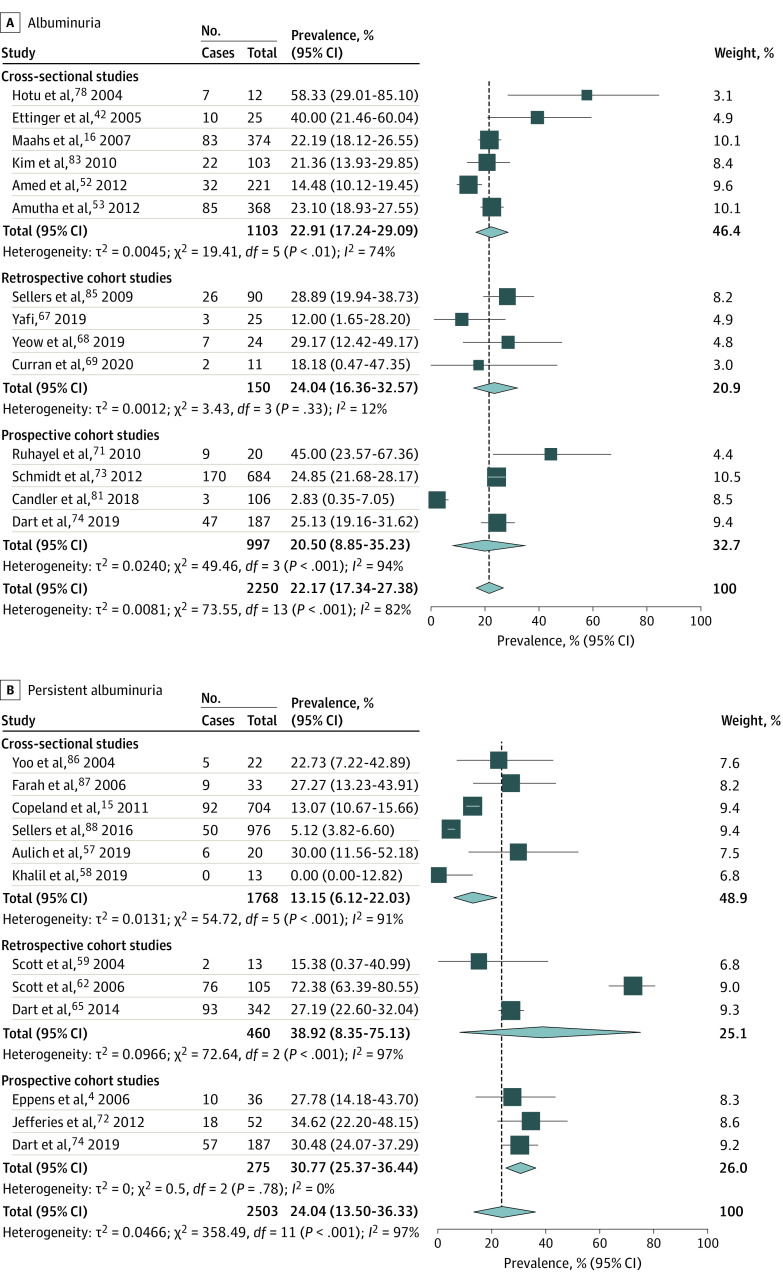
Pooled albuminuria prevalence in 14 studies of 2250 patients with type 2 diabetes was 22.17% (95% CI, 17.34%-27.38%) (Figure 2).16,42,52,53,67,68,69,71,73,74,78,81,83,85 There were high levels of heterogeneity (I2 = 82%; P < .001).
Figure 2. Forest Plot Showing Pooled Prevalence of Albuminuria and Persistent Albuminuria in Pediatric Type 2 Diabetes.
Four studies were not included in the meta-analysis. One used a definition of albuminuria of 24-hour urine protein excretion of greater than 500 mg, and found no patients with this outcome.45 Another study did not report the sample size or the definition of albuminuria but reported a prevalence of 23%.84 Two other studies46,50 reported the prevalence in specific racial groups, and the data were captured by another included study.16 Removing studies with 678 patients older than 18 years lowered the estimate to 17.00% (95% CI, 9.00%-27.00%; I2 = 86%; P < .001),42,52,67,69,74,81,83 suggesting that albuminuria worsens with age in this population (eTable 7 in the Supplement).
Pooled prevalence of persistent albuminuria across 12 studies with 2503 participants was 24.04% (95% CI, 13.50%-36.33%; I2 = 97%; P < .001) (Figure 2).4,15,57,58,59,62,65,72,74,86,87,88 Removing studies using different definitions of albuminuria lowered the pooled estimate to 17.00% (95% CI, 7.00%-29.00%; I2 = 92%; P < .001) (eTable 8 in the Supplement).
Microalbuminuria pooled prevalence across 16 studies with 2441 participants was 21.57% (95% CI, 15.59%-28.16%; I2 = 90%; P < .001) (eFigure 7 in the Supplement).42,44,53,64,66,68,71,73,78,83,89,90,91,92,93,94 Removing studies with 50 participants or fewer changed the estimate to 14.00% (95% CI, 9.00%-20.00%; I2 = 92%; P < .001; 2273 participants),44,53,66,73,83,89,90,91 as this resulted in the elimination of Pacific Islander group from the analysis; this group had the highest microalbuminuria prevalence (eTable 9 in the Supplement). Ten studies with 1345 participants reported persistent microalbuminuria pooled prevalence of 29.19% (95% CI, 16.85%-43.21%; I2 = 95%; P < .001) (eFigure 8 in the Supplement).4,8,15,57,59,62,72,86,87,94 Similarly, removing studies with patients older than 18 years or those using different definitions of microalbuminuria reduced the prevalence and heterogeneity estimates to 23.00% (95% CI, 14.00%-34.00%; I2 = 88%; P < .001) and 24.00% (95% CI, 11.00%-39.00%; I2 = 84%; P < .001), respectively, because this resulted in the exclusion of a study reporting a very high microalbuminuria prevalence of 72% among 105 participants in a sample with a high proportion of Pacific Islander and Indigenous patients (eTable 10 in the Supplement).62
Macroalbuminuria pooled prevalence from 4 studies with 730 participants was 3.85% (95% CI, 0.02%-11.63%; P < .001) (eFigure 9 in the Supplement).44,53,78,83 Another study reported a prevalence of 2.4% among 684 participants,73 but it was the only prospective cohort study, so it was not included in the meta-analysis. In addition, the prevalence of persistent macroalbuminuria was 5% among 22 participants in 1 cross-sectional study86 and 4.7% among 342 participants in another retrospective cohort study.8 Metaregression analysis revealed no statistically significant correlation between obesity prevalence and albuminuria, persistent albuminuria, microalbuminuria, or persistent microalbuminuria prevalence.
Sex and Racial Group Associations With Albuminuria
One study reported that albuminuria in 140 male participants (20.7%) was lower than in 234 female participants (23.1%).16 Similarly, persistent microalbuminuria prevalence was higher in 247 male participants (14.3%) than in 457 female participants (10.6%) in another study.15 Persistent albuminuria prevalence across 2 studies in 309 male participants was 16.14% (95% CI, 5.05%-31.65%; I2 = 86%; P < .001) and 22.90% (95% CI, 7.08%-44.22%; I2 = 95%; P < .001) in 582 female participants (OR, 0.68 [95% CI, 0.46-1.01]; I2 = 0%; P for heterogeneity = .78) (eFigure 10 and eFigure 11 in the Supplement).15,74
Albuminuria prevalence was assessed by racial group, and White youth had lower rates of albuminuria than other groups. The pooled prevalence among 158 White participants was 12.59% (95% CI, 7.75%-18.33%; I2 = 0%; P = .58)46,52 compared to 23.00% (95% CI, 18.85%-27.41%; I2 = 0%; P = .46) in 392 Asian participants,53,68 24.27% (95% CI, 14.39%-35.73%; I2 = 79%; P < .01) in 295 Indigenous participants,16,52,83 and 31.84% (95% CI, 11.90%-55.47%; I2 = 58%; P = .09) in 48 Pacific Islander participants16,69,78 (eFigure 12 in the Supplement). Single studies found an albuminuria prevalence of 14.1% in 212 African American participants50 and 23% in 64 Hispanic/Latino participants.16
Individual studies reported a prevalence of persistent albuminuria of 11.2% in 222 African American participants,15 14.1% in 289 Hispanic/Latino participants,15 and 23% in 22 Korean participants.86 Two studies reported a prevalence of 6.96% (95% CI, 0.00%-25.91%; I2 = 70%; P = .07) in 150 White participants15,58 and 19.06% (95% CI, 2.27%-45.67%; I2 = 91%; P < .001) in 217 Indigenous participants (eFigure 13 in the Supplement).15,74
For microalbuminuria, the pooled prevalence was 18.98% (95% CI, 9.98%-29.87%; I2 = 80%; P = .002) in 554 Asian participants (eFigure 14 in the Supplement).53,68,89,94 Individual studies found a prevalence of microalbuminuria of 0% in 12 White participants,91 21% in 52 African American participants,91 18.5% in 103 Indigenous participants,83 and 42% in 12 Pacific Islander participants.78
Finally, for persistent microalbuminuria, the pooled prevalence 22.31% (95% CI, 10.22%-37.01%; I2 = 0%; P = .48) in 40 Asian participants (eFigure 15 in the Supplement).86,94 One study reported a prevalence of 14.1% in 289 Hispanic/Latino participants, 11.2% in 222 African American participants, 14.6% in 138 White participants, and 8% in 43 Indigenous participants.15
Publication Bias
Publication bias was found for the prevalence of microalbuminuria based on the funnel plot and Egger test. It was not found for hypertension, albuminuria, persistent albuminuria, or persistent microalbuminuria (eFigures 16-20 in the Supplement).
Risk of Bias and Overall Quality of Evidence
The included studies had either a low (n = 27)8,15,16,17,40,44,45,46,47,48,50,53,55,56,60,64,65,69,70,72,76,78,79,81,85,89,90 or moderate (n = 33)4,41,42,43,49,51,52,54,57,58,59,61,62,63,66,67,68,71,73,74,75,77,80,82,83,84,86,87,88,91,92,93,94 risk of bias (eTable 11 and eFigure 21 in the Supplement). Some studies did not have a nationally representative sample, which limits their generalizability.4,8,40,41,42,43,46,47,48,49,50,51,53,54,56,57,58,59,60,63,64,65,66,67,68,71,72,74,75,77,78,79,80,82,83,85,86,87,89,91,92,93,94 The sampling frame of some studies was not representative of their target population,41,42,44,51,52,54,61,63,67,74,75,77,80,82,86,87,88,91,92,94 and some did not take a random or census sample.15,41,42,51,52,54,57,67,75,82,86,87,92,94 Some studies also had missing data of greater than 25%, potentially leading to nonresponse bias.4,43,49,57,58,61,62,66,68,71,73,75,83,84,91,93 In 3 studies, the definition used to diagnose hypertension or albuminuria was unspecified,67,69,92 and in some studies it was unclear that all participants were examined using the same methods.45,52,55,59,61,62,70,73,81,84,88,90
Based on the OCEBM criteria,28 29 studies (48%)8,16,17,40,44,46,47,48,50,53,55,56,62,65,66,70,72,73,74,76,79,80,81,83,85,88,89,90,91 had an evidence level of 1; 17 studies (28%)4,43,45,49,58,59,60,61,63,64,68,69,71,77,78,84,93, 2; and 14 studies (23%),15,41,42,51,52,54,57,67,75,82,86,87,92,94 3 (eTable 11 in the Supplement). Nearly half of the studies thus provide the highest level of evidence to answer the prevalence question we posed, although a significant portion of studies did not use a random sample or census to estimate prevalence.
Discussion
The rates of type 2 diabetes in children and adolescents are increasing globally, and this rise is associated with the obesity epidemic.95 Type 2 diabetes is associated with rapid progression of kidney complications, and early detection and treatment are crucial to avoid end-stage kidney disease, cardiovascular morbidities, and mortality.7,8,11,14 Current clinical guidelines on the management of pediatric type 2 diabetes are informed by independent studies with variable sample sizes.9,12,13
This systematic review investigated the prevalence of hypertension and albuminuria, markers of diabetes-related nephropathy and important predictors of kidney outcomes, in pediatric type 2 diabetes. Approximately 1 in 4 pediatric patients with type 2 diabetes had hypertension. Although more female patients had type 2 diabetes than male patients,9 male patients appeared to be more likely to develop hypertension than female patients. Pacific Islander and Indigenous youth had a higher burden of hypertension than other racial groups.
While most studies followed the National Heart, Lung, and Blood Institute guidelines for assessing hypertension in children and adolescents4,17,42,44,46,47,48,49,50,56,57,60,68,73,74 (ie, BP ≥95th percentile for age, sex, and height20), 6 studies used the adult definition of hypertension of systolic BP level of 130 to 140 mm Hg or greater and diastolic BP level of 80 to 90 mm Hg or greater.51,53,58,59,62,63 The different definitions of hypertension across studies were partly responsible for the noted heterogeneity, and using adult definitions might underestimate hypertension prevalence in children and adolescents.
Updated National Heart, Lung, and Blood Institute guidelines were released in 2017 that lowered BP thresholds for hypertension, as they were based on only children with weight in the reference range, whereas older guidelines also included children with overweight and obesity.96 While no studies reported using the 2017 guidelines, it is possible that hypertension prevalence will be higher if assessing data from existing studies against the 2017 guidelines.
Obesity is an important contributor to hypertension risk, with an estimated 6% increased risk of hypertension per unit of body mass index increase.6 Conversely, the metaregression analysis revealed that obesity was not associated with the prevalence of hypertension. However, obesity prevalence and severity were not available in all studies, and it is probable that obesity may contribute indirectly to the risk of hypertension in pediatric type 2 diabetes. On a mechanistic level, obesity-driven insulin resistance and hyperinsulinemia increases sodium reabsorption from the renal tubules.97 In addition, hyperglycemia can lead to hypervolemia, increased sympathetic activity,97 and the activation of the renin-angiotensin-aldosterone system, which increases cardiac output and peripheral vascular resistance, leading to hypertension.97 The associations between obesity and hypertension in pediatric type 2 diabetes require further study.
This review also demonstrated that between 1 in 5 and 1 in 4 pediatric patients with type 2 diabetes had albuminuria. While no sex differences were identified, Pacific Islander, Indigenous, and Asian youth had higher rates of albuminuria than White youth. While macroalbuminuria occurred in 4% of participants, fewer studies reported the persistence of albuminuria, despite the need for confirmation of persistence being a key criterion for albuminuria diagnosis.9,12,13 Persistent albuminuria is associated with macrovascular disease98 and predicts the progression to end-stage kidney disease.9,99 Prospective studies are needed to assess persistent albuminuria in pediatric type 2 diabetes. When studies of adult patients with type 2 diabetes were excluded, the albuminuria pooled prevalence estimate decreased from 22.17% (95% CI, 17.34%-27.38%) to 17.00% (95% CI, 9.00%-27.00%), and these results corroborate current evidence that albuminuria increases with age and duration of diabetes.6,53
These data have several important implications. Type 2 diabetes–related nephropathy exerts a much higher burden than that seen in children with type 1 diabetes.16,100 For example, the SEARCH for Diabetes in Youth study reported elevated urine ACR of 9.2% in children with type 1 diabetes vs 22.2% in those with type 2 diabetes,16 and youth with type 2 diabetes had 4-fold higher rates of kidney failure compared with youth with type 1 diabetes.8 In addition, a study including Pima Indians, an Indigenous group with high rates of type 2 diabetes, found that those who developed type 2 diabetes before 20 years of age had a 5-fold increased risk of end-stage kidney disease by middle age and higher mortality rates compared with patients with adult-onset type 2 diabetes.7 Ongoing intensive screening and intervention strategies are warranted to reduce mortality and end-stage kidney disease in pediatric patients with type 2 diabetes.
The specific renal pathology that drives proteinuria and hypertension in pediatric type 2 diabetes is unknown. While studies of kidney biopsies in youth with type 2 diabetes are limited, most anomalies found on kidney ultrasounds have been classified as congenital.88 Kidney biopsies from patients describe immune complex disease and glomerulosclerosis, findings that are not characteristic of typical diabetes-related nephropathy, which are considered non–diabetes-driven pathologies.85 However, these observations are based on a small sample of Indigenous youth and may not be generalizable to all children and adolescents with type 2 diabetes. Further studies are urgently needed to assess renal histopathology in type 2 diabetes across different sexes and racial groups to define the exact mechanisms of nephropathy in this population.
Limitations
This study has limitations, including the high heterogeneity among studies. Some studies did not achieve a high quality rating (n = 31) because of small sample sizes (n = 17) or the lack of clarity as to whether the results were based on a randomized sample or census (n = 14). Moreover, a large proportion of the studies did not have a nationally representative sample, as they were based in a single center or clinic. As such, larger studies across multiple centers are needed to assess prevalence. In addition, obesity severity data, which could confound hypertension and proteinuria prevalence, were also not available. While the results should be interpreted with this information in mind, this report presents all current data available to assess hypertension and albuminuria in pediatric patients with type 2 diabetes.
Conclusions
In this study, hypertension and albuminuria were frequent comorbidities of pediatric type 2 diabetes, and Pacific Islander and Indigenous youth had a disproportionately higher burden of these conditions than youth from other racial groups. There is a critical need for personalized screening and treatment strategies to provide renoprotection from prolonged hyperglycemia and obesity to prevent end-stage kidney disease, future cardiovascular disease, and improve life expectancy. The exact etiopathogenetic mechanisms driving nephropathy in youth with type 2 diabetes need to be elucidated. These data are relevant for health care professionals and policy makers, as clinical services treating pediatric patients with type 2 diabetes need to be resourced to track kidney screening and treatments to improve outcomes.
eTable 1. Search Strategy, MEDLINE
eTable 2. Search Strategy, Embase
eTable 3. Search Strategy, CINAHL
eTable 4. Search Strategy, Cochrane Library: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews
eTable 5. Search Strategy, Web of Science: Conference Proceedings Citation Index-Science
eTable 6. Results of Sensitivity Analysis for Prevalence of Hypertension in Pediatric Type 2 Diabetes Meta-analysis
eTable 7. Results of Sensitivity Analysis for Prevalence of Albuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 8. Results of Sensitivity Analysis for Prevalence of Persistent Albuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 9. Results of Sensitivity Analysis for Prevalence of Microalbuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 10. Results of Sensitivity Analysis for Prevalence of Persistent Microalbuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 11. Risk of Bias and OCEBM Level of Evidence of Included Studies
eFigure 1. Study Flow Diagram
eFigure 2. Forest Plot Showing Pooled Prevalence of Systolic Hypertension in Pediatric Type 2 Diabetes
eFigure 3. Forest Plot Showing Pooled Prevalence of Diastolic Hypertension in Pediatric Type 2 Diabetes
eFigure 4. Forest Plot Showing Pooled Prevalence of Hypertension in Pediatric Type 2 Diabetes by Sex
eFigure 5. Forest Plot Showing Pooled Odds Ratio of Hypertension in Male vs Female Participants with Pediatric Type 2 Diabetes
eFigure 6. Forest Plot Showing Pooled Prevalence of Hypertension Across Different Racial Groups with Pediatric Type 2 Diabetes
eFigure 7. Forest Plot Showing Pooled Prevalence of Microalbuminuria in Pediatric Type 2 Diabetes
eFigure 8. Forest Plot Showing Pooled Prevalence of Persistent Microalbuminuria in Pediatric Type 2 Diabetes
eFigure 9. Forest Plot Showing Pooled Prevalence of Macroalbuminuria in Pediatric Type 2 Diabetes
eFigure 10. Forest Plot Showing Pooled Prevalence of Persistent Albuminuria in Pediatric Type 2 Diabetes by Sex
eFigure 11. Forest Plot Showing Pooled Odds Ratio of Persistent Albuminuria in Male vs Female Participants with Pediatric Type 2 Diabetes
eFigure 12. Forest Plot Showing Pooled Prevalence of Albuminuria across Different Racial Groups with Pediatric Type 2 Diabetes
eFigure 13. Forest Plot Showing Pooled Prevalence of Persistent Albuminuria across Different Racial Groups with Pediatric Type 2 Diabetes
eFigure 14. Forest Plot Showing Pooled Prevalence of Microalbuminuria in Asian Patients with Pediatric Type 2 Diabetes
eFigure 15. Forest Plot Showing Pooled Prevalence of Persistent Microalbuminuria in Asian Patients with Pediatric Type 2 Diabetes
eFigure 16. Funnel Plot Examining Publication Bias for Pooled Prevalence of Hypertension Outcome
eFigure 17. Funnel Plot Examining Publication Bias for Pooled Prevalence of Albuminuria Outcome
eFigure 18. Funnel Plot Examining Publication Bias for Pooled Prevalence of Persistent Albuminuria Outcome
eFigure 19. Funnel Plot Examining Publication Bias for Pooled Prevalence of Microalbuminuria Outcome
eFigure 20. Funnel Plot Examining Publication Bias for Pooled Prevalence of Persistent Microalbuminuria Outcome
eFigure 21. Distribution of Risk of Bias Sources in the Included Studies
eAppendix. List of Studies Excluded at the Full-Text Screening Stage
eReferences.
References
- 1.Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4(6):270-281. doi: 10.4239/wjd.v4.i6.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228-236. doi: 10.1038/nrendo.2011.183 [DOI] [PubMed] [Google Scholar]
- 3.Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26(11):2999-3005. doi: 10.2337/diacare.26.11.2999 [DOI] [PubMed] [Google Scholar]
- 4.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300-1306. doi: 10.2337/dc05-2470 [DOI] [PubMed] [Google Scholar]
- 5.Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years—clinical observation from a secondary care cohort. QJM. 2009;102(11):799-806. doi: 10.1093/qjmed/hcp121 [DOI] [PubMed] [Google Scholar]
- 6.TODAY Study Group . Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735-1741. doi: 10.2337/dc12-2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296(4):421-426. doi: 10.1001/jama.296.4.421 [DOI] [PubMed] [Google Scholar]
- 8.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265-1271. doi: 10.2337/dc11-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(S27)(suppl 27):28-46. doi: 10.1111/pedi.12719 [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346(15):1145-1151. doi: 10.1056/NEJMcp011773 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association . 13. Children and adolescents: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S148-S164. doi: 10.2337/dc19-S013 [DOI] [PubMed] [Google Scholar]
- 13.Panagiotopoulos C, Hadjiyannakis S, Henderson M; Diabetes Canada Clinical Practice Guidelines Expert Committee . Type 2 diabetes in children and adolescents. Can J Diabetes. 2018;42(suppl 1):S247-S254. doi: 10.1016/j.jcjd.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 14.UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703-713. doi: 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copeland KC, Zeitler P, Geffner M, et al. ; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159-167. doi: 10.1210/jc.2010-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maahs DM, Snively BM, Bell RA, et al. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30(10):2593-2598. doi: 10.2337/dc07-0450 [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez BL, Dabelea D, Liese AD, et al. ; SEARCH Study Group . Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157(2):245-251.e1. doi: 10.1016/j.jpeds.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 18.Samaan MC, Cioana M, Banfield L, et al. The prevalence of comorbidities in pediatric type 2 diabetes mellitus: a systematic review. PROSPERO. Published March 19, 2018. Accessed April 5, 2021. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=91127
- 19.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2)(Suppl 4th Report):555-576. [PubMed] [Google Scholar]
- 21.Rosner B, Prineas RJ, Loggie JM, Daniels SR. Blood pressure nomograms for children and adolescents, by height, sex, and age, in the United States. J Pediatr. 1993;123(6):871-886. doi: 10.1016/S0022-3476(05)80382-8 [DOI] [PubMed] [Google Scholar]
- 22.Report of the Second Task Force on Blood Pressure Control in Children—1987: Task Force on Blood Pressure Control in Children, National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79(1):1-25. [PubMed] [Google Scholar]
- 23.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents . Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996;98(4 Pt 1):649-658. [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 25.Jackson LV, Thalange NKS, Cole TJ. Blood pressure centiles for Great Britain. Arch Dis Child. 2007;92(4):298-303. doi: 10.1136/adc.2005.081216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49-61. doi: 10.1038/ki.2013.444 [DOI] [PubMed] [Google Scholar]
- 27.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 28.OCEBM Levels of Evidence Working Group . The Oxford 2011. Levels of Evidence. Oxford Center for Evidence-Based Medicine. Accessed July 20, 2020. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 29.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 30.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974-978. doi: 10.1136/jech-2013-203104 [DOI] [PubMed] [Google Scholar]
- 31.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950:607-611. doi: 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 32.Miller JJ. The inverse of the Freeman–Tukey double arcsine transformation. Am Stat. 1978;32(4):138. doi: 10.1080/00031305.1978.10479283 [DOI] [Google Scholar]
- 33.Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2019. doi: 10.1002/9781119536604.ch10 [DOI] [Google Scholar]
- 34.National Institutes of Health . Racial and ethnic categories and definitions for NIH diversity programs and for other reporting purposes. Published April 8, 2015. Accessed March 17, 2021. https://grants.nih.gov/grants/guide/notice-files/not-od-15-089.html [Google Scholar]
- 35.Page MJ, Higgins JPT, Sterne JAC. Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2019. doi: 10.1002/9781119536604.ch13 [DOI] [Google Scholar]
- 36.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 37.RStudio . Accessed March 17, 2021. https://www.rstudio.com/
- 38.The R Project for Statistical Computing. Accessed March 17, 2021. https://www.r-project.org/
- 39.The Cochrane Collaboration . Review Manager version 5. Accessed March 17, 2021. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download
- 40.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608-615. doi: 10.1016/S0022-3476(96)80124-7 [DOI] [PubMed] [Google Scholar]
- 41.Scott CR, Smith JM, Cradock MM, Pihoker C. Characteristics of youth-onset noninsulin-dependent diabetes mellitus and insulin-dependent diabetes mellitus at diagnosis. Pediatrics. 1997;100(1):84-91. doi: 10.1542/peds.100.1.84 [DOI] [PubMed] [Google Scholar]
- 42.Ettinger LM, Freeman K, DiMartino-Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr. 2005;147(1):67-73. doi: 10.1016/j.jpeds.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 43.Reinehr T, Andler W, Kapellen T, et al. Clinical characteristics of type 2 diabetes mellitus in overweight European Caucasian adolescents. Exp Clin Endocrinol Diabetes. 2005;113(3):167-170. doi: 10.1055/s-2005-837522 [DOI] [PubMed] [Google Scholar]
- 44.Eppens MC, Craig ME, Jones TW, Silink M, Ong S, Ping YJ; International Diabetes Federation Western Pacific Region Steering Committee . Type 2 diabetes in youth from the Western Pacific region: glycaemic control, diabetes care and complications. Curr Med Res Opin. 2006;22(5):1013-1020. doi: 10.1185/030079906X104795 [DOI] [PubMed] [Google Scholar]
- 45.Unnikrishnan AG, Bhatia E, Bhatia V, et al. Type 1 diabetes versus type 2 diabetes with onset in persons younger than 20 years of age. Ann N Y Acad Sci. 2008;1150(1):239-244. doi: 10.1196/annals.1447.056 [DOI] [PubMed] [Google Scholar]
- 46.Bell RA, Mayer-Davis EJ, Beyer JW, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetes in non-Hispanic White youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S102-S111. doi: 10.2337/dc09-S202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dabelea D, DeGroat J, Sorrelman C, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetes in Navajo youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S141-S147. doi: 10.2337/dc09-S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S123-S132. doi: 10.2337/dc09-S204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu LL, Yi JP, Beyer J, et al. ; SEARCH for Diabetes in Youth Study Group . Type 1 and type 2 diabetes in Asian and Pacific Islander U.S. youth: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S133-S140. doi: 10.2337/dc09-S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer-Davis EJ, Beyer J, Bell RA, et al. ; SEARCH for Diabetes in Youth Study Group . Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S112-S122. doi: 10.2337/dc09-S203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urakami T, Suzuki J, Yoshida A, et al. Prevalence of components of the metabolic syndrome in schoolchildren with newly diagnosed type 2 diabetes mellitus. Pediatr Diabetes. 2009;10(8):508-512. doi: 10.1111/j.1399-5448.2009.00533.x [DOI] [PubMed] [Google Scholar]
- 52.Amed S, Hamilton JK, Sellers EAC, et al. Differing clinical features in Aboriginal vs. non-Aboriginal children presenting with type 2 diabetes. Pediatr Diabetes. 2012;13(6):470-475. doi: 10.1111/j.1399-5448.2012.00859.x [DOI] [PubMed] [Google Scholar]
- 53.Amutha A, Datta M, Unnikrishnan R, Anjana RM, Mohan V. Clinical profile and complications of childhood- and adolescent-onset type 2 diabetes seen at a diabetes center in south India. Diabetes Technol Ther. 2012;14(6):497-504. doi: 10.1089/dia.2011.0283 [DOI] [PubMed] [Google Scholar]
- 54.Drutel RO, Paulo R. Abstract 660: prevalence of hypertension among children with diabetes mellitus. Hypertension. 2014;64(suppl_1):A660. Accessed April 5, 2020. https://www.ahajournals.org/doi/10.1161/hyp.64.suppl_1.660 [Google Scholar]
- 55.Klingensmith GJ, Connor CG, Ruedy KJ, et al. ; Pediatric Diabetes Consortium . Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. 2016;17(4):266-273. doi: 10.1111/pedi.12281 [DOI] [PubMed] [Google Scholar]
- 56.Zabeen B, Nahar J, Tayyeb S, Mohsin F, Nahar N, Azad K. Characteristics of children and adolescents at onset of type 2 diabetes in a tertiary hospital in Bangladesh. Indian J Endocrinol Metab. 2016;20(5):638-642. doi: 10.4103/2230-8210.190544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aulich J, Cho YH, Januszewski AS, et al. Associations between circulating inflammatory markers, diabetes type and complications in youth. Pediatr Diabetes. 2019;20(8):1118-1127. doi: 10.1111/pedi.12913 [DOI] [PubMed] [Google Scholar]
- 58.Khalil SA, Megallaa MH, Rohoma KH, et al. Prevalence of chronic diabetic complications in newly diagnosed versus known type 2 diabetic subjects in a sample of Alexandria population, Egypt. Curr Diabetes Rev. 2019;15(1):74-83. doi: 10.2174/1573399814666180125100917 [DOI] [PubMed] [Google Scholar]
- 59.Scott A, Whitcombe S, Bouchier D, Dunn P. Diabetes in children and young adults in Waikato Province, New Zealand: outcomes of care. N Z Med J. 2004;117(1207):U1219. [PubMed] [Google Scholar]
- 60.Zdravkovic V, Daneman D, Hamilton J. Presentation and course of Type 2 diabetes in youth in a large multi-ethnic city. Diabet Med. 2004;21(10):1144-1148. doi: 10.1111/j.1464-5491.2004.01297.x [DOI] [PubMed] [Google Scholar]
- 61.Pérez-Perdomo R, Pérez-Cardona CM, Allende-Vigo M, Rivera-Rodríguez MI, Rodríguez-Lugo LA. Type 2 diabetes mellitus among youth in Puerto Rico, 2003. P R Health Sci J. 2005;24(2):111-117. [PubMed] [Google Scholar]
- 62.Scott A, Toomath R, Bouchier D, et al. First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. N Z Med J. 2006;119(1235):U2015. [PubMed] [Google Scholar]
- 63.Balasanthiran A, O'Shea T, Moodambail A, et al. Type 2 diabetes in children and young adults in East London: an alarmingly high prevalence. Practical Diabetes. 2012;29(5):193-198a. doi: 10.1002/pdi.1689 [DOI] [Google Scholar]
- 64.Osman HAM, Elsadek N, Abdullah MA. Type 2 diabetes in Sudanese children and adolescents. Sudan J Paediatr. 2013;13(2):17-23. [PMC free article] [PubMed] [Google Scholar]
- 65.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37(2):436-443. doi: 10.2337/dc13-0954 [DOI] [PubMed] [Google Scholar]
- 66.Haynes A, Kalic R, Curran J, et al. Type 2 diabetes and associated complications in Western Australian children: a population-based study (1990-2012). Diabetologia. 2014;57(Suppl 1):S510-S510. [Google Scholar]
- 67.Yafi M. The prevalence of microalbuminuria in children with type 2 diabetes mellitus (T2DM). Arch Dis Child. 2019;104:A250. doi: 10.1136/archdischild-2019-epa.585 [DOI] [Google Scholar]
- 68.Yeow TP, Aun ES-Y, Hor CP, Lim SL, Khaw CH, Aziz NA. Challenges in the classification and management of Asian youth-onset diabetes mellitus—lessons learned from a single centre study. PLoS One. 2019;14(1):e0211210. doi: 10.1371/journal.pone.0211210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curran JA, Haynes A, Davis EA. Clinical characteristics of Western Australian children diagnosed with type 2 diabetes before 10 years of age. Med J Aust. 2020;212(2):95-95.e1. doi: 10.5694/mja2.50451 [DOI] [PubMed] [Google Scholar]
- 70.Shield JPH, Lynn R, Wan KC, Haines L, Barrett TG. Management and 1 year outcome for UK children with type 2 diabetes. Arch Dis Child. 2009;94(3):206-209. doi: 10.1136/adc.2008.143313 [DOI] [PubMed] [Google Scholar]
- 71.Ruhayel SD, James RA, Ehtisham S, Cameron FJ, Werther GA, Sabin MA. An observational study of type 2 diabetes within a large Australian tertiary hospital pediatric diabetes service. Pediatr Diabetes. 2010;11(8):544-551. doi: 10.1111/j.1399-5448.2010.00647.x [DOI] [PubMed] [Google Scholar]
- 72.Jefferies C, Carter P, Reed PW, et al. The incidence, clinical features, and treatment of type 2 diabetes in children <15 yr in a population-based cohort from Auckland, New Zealand, 1995–2007. Pediatr Diabetes. 2012;13(4):294-300. doi: 10.1111/j.1399-5448.2011.00851.x [DOI] [PubMed] [Google Scholar]
- 73.Schmidt F, Kapellen TM, Wiegand S, et al. ; DPV-Wiss Study Group; BMBF Competence Network Diabetes . Diabetes mellitus in children and adolescents with genetic syndromes. Exp Clin Endocrinol Diabetes. 2012;120(10):579-585. doi: 10.1055/s-0032-1306330 [DOI] [PubMed] [Google Scholar]
- 74.Dart AB, Wicklow B, Blydt-Hansen TD, et al. A holistic approach to risk for early kidney injury in Indigenous youth with type 2 diabetes: a proof of concept paper from the iCARE Cohort. Can J Kidney Health Dis. 2019;6:2054358119838836. doi: 10.1177/2054358119838836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Upchurch SL, Brosnan CA, Meininger JC, et al. Characteristics of 98 children and adolescents diagnosed with type 2 diabetes by their health care provider at initial presentation. Diabetes Care. 2003;26(7):2209-2209. doi: 10.2337/diacare.26.7.2209 [DOI] [PubMed] [Google Scholar]
- 76.Wei J-N, Sung F-C, Lin C-C, Lin R-S, Chiang C-C, Chuang L-M. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA. 2003;290(10):1345-1350. doi: 10.1001/jama.290.10.1345 [DOI] [PubMed] [Google Scholar]
- 77.Cruz M, Torres M, Aguilar-Herrera B, et al. Type 2 diabetes mellitus in children—an increasing health problem in Mexico. J Pediatr Endocrinol Metab. 2004;17(2):183-190. doi: 10.1515/JPEM.2004.17.2.183 [DOI] [PubMed] [Google Scholar]
- 78.Hotu S, Carter B, Watson PD, Cutfield WS, Cundy T. Increasing prevalence of type 2 diabetes in adolescents. J Paediatr Child Health. 2004;40(4):201-204. doi: 10.1111/j.1440-1754.2004.00337.x [DOI] [PubMed] [Google Scholar]
- 79.Sellers EAC, Yung G, Dean HJ. Dyslipidemia and other cardiovascular risk factors in a Canadian First Nation pediatric population with type 2 diabetes mellitus. Pediatr Diabetes. 2007;8(6):384-390. doi: 10.1111/j.1399-5448.2007.00284.x [DOI] [PubMed] [Google Scholar]
- 80.Pelham JH, Hanks L, Aslibekyan S, Dowla S, Ashraf AP. Higher hemoglobin A1c and atherogenic lipoprotein profiles in children and adolescents with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2018;15:30-34. doi: 10.1016/j.jcte.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Candler TP, Mahmoud O, Lynn RM, Majbar AA, Barrett TG, Shield JPH. Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabet Med. 2018;35(6):737-744. doi: 10.1111/dme.13609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shalitin S, Tauman R, Meyerovitch J, Sivan Y. Are frequency and severity of sleep-disordered breathing in obese children and youth with and without type 2 diabetes mellitus different? Acta Diabetol. 2014;51(5):757-764. doi: 10.1007/s00592-014-0583-1 [DOI] [PubMed] [Google Scholar]
- 83.Kim NH, Pavkov ME, Knowler WC, et al. Predictive value of albuminuria in American Indian youth with or without type 2 diabetes. Pediatrics. 2010;125(4):e844-e851. doi: 10.1542/peds.2009-1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holman N, Campbell F, Sattar N, Warner J. Microvascular disease among young people with diabetes. Diabetic Medicine. 2015;32(Supplement 1):16. doi: 10.1111/dme.12665_8 [DOI] [Google Scholar]
- 85.Sellers EAC, Blydt-Hansen TD, Dean HJ, Gibson IW, Birk PE, Ogborn M. Macroalbuminuria and renal pathology in First Nation youth with type 2 diabetes. Diabetes Care. 2009;32(5):786-790. doi: 10.2337/dc08-1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoo EG, Choi IK, Kim DH. Prevalence of microalbuminuria in young patients with type 1 and type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17(10):1423-1427. doi: 10.1515/JPEM.2004.17.10.1423 [DOI] [PubMed] [Google Scholar]
- 87.Farah SE, Wals KT, Friedman IB, Pisacano MA, DiMartino-Nardi J. Prevalence of retinopathy and microalbuminuria in pediatric type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2006;19(7):937-942. doi: 10.1515/JPEM.2006.19.7.937 [DOI] [PubMed] [Google Scholar]
- 88.Sellers EAC, Hadjiyannakis S, Amed S, et al. Persistent albuminuria in children with type 2 diabetes: a Canadian Paediatric Surveillance Program Study. J Pediatr. 2016;168:112-117. doi: 10.1016/j.jpeds.2015.09.042 [DOI] [PubMed] [Google Scholar]
- 89.Zabeen B, Nahar J, Tayyeb S, Nhar N, Azad K. Type 2 diabetes in Bangladeshi children and adolescents—an emerging problem. Pediatr Diabetes. 2016;17(Suppl 24):36-164.25524404 [Google Scholar]
- 90.Nambam B, Silverstein J, Cheng P, et al. ; Pediatric Diabetes Consortium . A cross-sectional view of the current state of treatment of youth with type 2 diabetes in the USA: enrollment data from the Pediatric Diabetes Consortium Type 2 Diabetes Registry. Pediatr Diabetes. 2017;18(3):222-229. doi: 10.1111/pedi.12377 [DOI] [PubMed] [Google Scholar]
- 91.Le PT, Huisingh CE, Ashraf AP. Glycemic control and diabetic dyslipidemia in adolescents with type 2 diabetes. Endocr Pract. 2013;19(6):972-979. doi: 10.4158/EP13016.OR [DOI] [PubMed] [Google Scholar]
- 92.Calagua Quispe M, Del Aguila Villar C, Nuñez Almache O, et al. Clinical features and course of pediatric patients with type 1 and type 2 diabetes mellitus. Horm Res Paed. 2015;84(Suppl 2):39. [Google Scholar]
- 93.Newton K, Stanley J, Wiltshire E. Audit of type 2 diabetes in youth in Wellington, New Zealand 2001–2013. Pediatric Diabetes. 2015;16:147. doi: 10.1111/pedi.12309 [DOI] [Google Scholar]
- 94.Son MK, Yoo HY, Kwak BO, et al. Regression and progression of microalbuminuria in adolescents with childhood onset diabetes mellitus. Ann Pediatr Endocrinol Metab. 2015;20(1):13-20. doi: 10.6065/apem.2015.20.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fazeli Farsani S, van der Aa MP, van der Vorst MMJ, Knibbe CAJ, de Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56(7):1471-1488. doi: 10.1007/s00125-013-2915-z [DOI] [PubMed] [Google Scholar]
- 96.Flynn JT, Kaelber DC, Baker-Smith CM, et al. ; Subcommittee on Screening and Management of High Blood Pressure in Children . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 97.Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. 2018;41(6):389-393. doi: 10.1038/s41440-018-0034-4 [DOI] [PubMed] [Google Scholar]
- 98.Fox CS, Matsushita K, Woodward M, et al. ; Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662-1673. doi: 10.1016/S0140-6736(12)61350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310(6):356-360. doi: 10.1056/NEJM198402093100605 [DOI] [PubMed] [Google Scholar]
- 100.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36(12):3863-3869. doi: 10.2337/dc12-2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search Strategy, MEDLINE
eTable 2. Search Strategy, Embase
eTable 3. Search Strategy, CINAHL
eTable 4. Search Strategy, Cochrane Library: Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews
eTable 5. Search Strategy, Web of Science: Conference Proceedings Citation Index-Science
eTable 6. Results of Sensitivity Analysis for Prevalence of Hypertension in Pediatric Type 2 Diabetes Meta-analysis
eTable 7. Results of Sensitivity Analysis for Prevalence of Albuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 8. Results of Sensitivity Analysis for Prevalence of Persistent Albuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 9. Results of Sensitivity Analysis for Prevalence of Microalbuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 10. Results of Sensitivity Analysis for Prevalence of Persistent Microalbuminuria in Pediatric Type 2 Diabetes Meta-analysis
eTable 11. Risk of Bias and OCEBM Level of Evidence of Included Studies
eFigure 1. Study Flow Diagram
eFigure 2. Forest Plot Showing Pooled Prevalence of Systolic Hypertension in Pediatric Type 2 Diabetes
eFigure 3. Forest Plot Showing Pooled Prevalence of Diastolic Hypertension in Pediatric Type 2 Diabetes
eFigure 4. Forest Plot Showing Pooled Prevalence of Hypertension in Pediatric Type 2 Diabetes by Sex
eFigure 5. Forest Plot Showing Pooled Odds Ratio of Hypertension in Male vs Female Participants with Pediatric Type 2 Diabetes
eFigure 6. Forest Plot Showing Pooled Prevalence of Hypertension Across Different Racial Groups with Pediatric Type 2 Diabetes
eFigure 7. Forest Plot Showing Pooled Prevalence of Microalbuminuria in Pediatric Type 2 Diabetes
eFigure 8. Forest Plot Showing Pooled Prevalence of Persistent Microalbuminuria in Pediatric Type 2 Diabetes
eFigure 9. Forest Plot Showing Pooled Prevalence of Macroalbuminuria in Pediatric Type 2 Diabetes
eFigure 10. Forest Plot Showing Pooled Prevalence of Persistent Albuminuria in Pediatric Type 2 Diabetes by Sex
eFigure 11. Forest Plot Showing Pooled Odds Ratio of Persistent Albuminuria in Male vs Female Participants with Pediatric Type 2 Diabetes
eFigure 12. Forest Plot Showing Pooled Prevalence of Albuminuria across Different Racial Groups with Pediatric Type 2 Diabetes
eFigure 13. Forest Plot Showing Pooled Prevalence of Persistent Albuminuria across Different Racial Groups with Pediatric Type 2 Diabetes
eFigure 14. Forest Plot Showing Pooled Prevalence of Microalbuminuria in Asian Patients with Pediatric Type 2 Diabetes
eFigure 15. Forest Plot Showing Pooled Prevalence of Persistent Microalbuminuria in Asian Patients with Pediatric Type 2 Diabetes
eFigure 16. Funnel Plot Examining Publication Bias for Pooled Prevalence of Hypertension Outcome
eFigure 17. Funnel Plot Examining Publication Bias for Pooled Prevalence of Albuminuria Outcome
eFigure 18. Funnel Plot Examining Publication Bias for Pooled Prevalence of Persistent Albuminuria Outcome
eFigure 19. Funnel Plot Examining Publication Bias for Pooled Prevalence of Microalbuminuria Outcome
eFigure 20. Funnel Plot Examining Publication Bias for Pooled Prevalence of Persistent Microalbuminuria Outcome
eFigure 21. Distribution of Risk of Bias Sources in the Included Studies
eAppendix. List of Studies Excluded at the Full-Text Screening Stage
eReferences.