Abstract
ATP‐sensitive K+ channels (KATP) have been implicated in the regulation of resting vascular smooth muscle membrane potential and tone. However, whether KATP channels modulate skeletal muscle microvascular hemodynamics at the capillary level (the primary site for blood‐myocyte O2 exchange) remains unknown. We tested the hypothesis that KATP channel inhibition would reduce the proportion of capillaries supporting continuous red blood cell (RBC) flow and impair RBC hemodynamics and distribution in perfused capillaries within resting skeletal muscle. RBC flux (f RBC), velocity (V RBC), and capillary tube hematocrit (Hctcap) were assessed via intravital microscopy of the rat spinotrapezius muscle (n = 6) under control (CON) and glibenclamide (GLI; KATP channel antagonist; 10 µM) superfusion conditions. There were no differences in mean arterial pressure (CON:120 ± 5, GLI:124 ± 5 mmHg; p > 0.05) or heart rate (CON:322 ± 32, GLI:337 ± 33 beats/min; p > 0.05) between conditions. The %RBC‐flowing capillaries were not altered between conditions (CON:87 ± 2, GLI:85 ± 1%; p > 0.05). In RBC‐perfused capillaries, GLI reduced f RBC (CON:20.1 ± 1.8, GLI:14.6 ± 1.3 cells/s; p < 0.05) and V RBC (CON:240 ± 17, GLI:182 ± 17 µm/s; p < 0.05) but not Hctcap (CON:0.26 ± 0.01, GLI:0.26 ± 0.01; p > 0.05). The absence of GLI effects on the %RBC‐flowing capillaries and Hctcap indicates preserved muscle O2 diffusing capacity (DO2m). In contrast, GLI lowered both f RBC and V RBC thus impairing perfusive microvascular O2 transport (Q̇m) and lengthening RBC capillary transit times, respectively. Given the interdependence between diffusive and perfusive O2 conductances (i.e., %O2 extraction∝DO2m/Q̇m), such GLI alterations are expected to elevate muscle %O2 extraction to sustain a given metabolic rate. These results support that KATP channels regulate capillary hemodynamics and, therefore, microvascular gas exchange in resting skeletal muscle.
Keywords: ATP‐sensitive K+ channel, blood flow, intravital microscopy, microcirculation, red blood cell
Superfusion of the rat spinotrapezius muscle with the sulfonylurea glibenclamide (GLI) was used to locally inhibit KATP channels in vivo. GLI reduced both red blood cell (RBC) flux and velocity thereby impairing perfusive microvascular O2 transport and lengthening RBC capillary transit times, respectively. These results support that KATP channels regulate capillary hemodynamics and microvascular gas exchange in resting skeletal muscle.

1. INTRODUCTION
The skeletal muscle capillary vascular bed provides the largest surface area for gas and substrate exchange within the body (Hirai et al., 2019; Poole et al., 2011; Poole, 2019). Regulation of skeletal muscle capillary hemodynamics is mediated primarily at the arteriolar level (Joyner & Casey, 2015; Kindig & Poole, 2001; Laughlin et al., 2012; Segal, 2005). Several complex and often interacting mechanisms are responsible for alterations in arteriolar resistance and thus vascular conductance. Among these processes, K+ channels constitute the dominant ion conductance of the vascular smooth muscle cell determining membrane potential, contractile activity, and thus vascular tone (Foster & Coetzee, 2016; Jackson, 2017; Tykocki et al., 2017).
ATP‐sensitive K+ (KATP) channels constitute one of the, at least, five distinct classes of K+ channels regulating vascular smooth muscle function (Foster & Coetzee, 2016; Jackson, 2017; Tykocki et al., 2017). Inhibition (closing) of KATP channels decreases K+ efflux leading to depolarization of the smooth muscle cell. Voltage‐gated Ca2+ channels then transduce membrane depolarization into increased Ca2+ influx promoting vascular muscle contraction (i.e., vasoconstriction). In vitro and in vivo studies have thus used pharmacological KATP channel inhibition to evaluate its potential role in setting vascular tone and, consequently, tissue perfusion. Sulfonylureas such as glibenclamide (GLI), which are employed clinically in the treatment of non‐insulin‐dependent diabetes (Montvida et al., 2018), represent the most frequently used class of KATP channel inhibitors. To date, however, there is a lack of agreement regarding the role of KATP channels in the regulation of resting vascular tone and blood flow within skeletal muscle (Foster & Coetzee, 2016; Jackson, 2017; Tykocki et al., 2017). KATP channel inhibition with GLI has produced conflicting effects on resting skeletal muscle arteriolar diameter (Hammer et al., 2001; Hodnett et al., 2008; Jackson, 1993; Lu et al., 2013; Murrant & Sarelius, 2002; Saito et al., 1996; Xiang & Hester, 2009) and bulk blood flow (Bank et al., 2000; Colburn, Holdsworth, et al., 2020; Duncker et al., 2001; Farouque & Meredith, 2003a, 2003b,2003a, 2003b; Holdsworth et al., 2015; Vanelli & Hussain, 1994). Importantly, the functional role of KATP channels within the skeletal muscle capillary network (i.e., the primary site for blood‐myocyte O2 exchange) remains to be determined. Resolution of the mechanisms modulating capillary red blood cell (RBC) hemodynamics has direct relevance to transcapillary O2 flux and cellular energetic status.
The purpose of this study was to evaluate the regulation of capillary RBC hemodynamics by KATP channels in resting skeletal muscle. Using intravital microscopy techniques combined with the rat spinotrapezius preparation, we tested the hypothesis that acute local KATP channel inhibition with GLI would impair key determinants of capillary diffusive and perfusive O2 conductances. Specifically, GLI was anticipated to reduce the proportion of capillaries supporting continuous RBC flow and impair microvascular hemodynamics and distribution (i.e., RBC flux, velocity, and capillary tube hematocrit) in perfused capillaries within resting skeletal muscle. Confirmation of these hypotheses would support a role for KATP channels in the regulation of skeletal muscle capillary hemodynamics (and thus microvascular gas exchange) under resting conditions.
2. METHODS
Intravital microscopy was used to evaluate the in vivo spinotrapezius muscle microcirculation in healthy young male Sprague–Dawley rats (n = 6; ~3–4 months old; 386 ± 26 g). Rats were maintained in accredited facilities (Association for the Assessment and Accreditation of Laboratory and Animal Care) under a 12:12 h light–dark cycle with food and water provided ad libitum. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee of Kansas State University and followed the guidelines established by the National Institutes of Health.
2.1. Surgical procedures
2.1.1. Anesthesia and catheter placement procedures
All rats were anesthetized initially with a 5% isoflurane‐O2 mixture and maintained subsequently on 2–3% isoflurane‐O2 (Butler Animal Health Supply). Anesthetized rats were kept on a heating pad to maintain core temperature at ~37–38°C as measured via a rectal probe. The right carotid artery was cannulated (PE‐10 connected to PE‐50; BD IntraMedic Polyethylene Tubing) for continuous measurements of mean arterial pressure and heart rate (MAP and HR, respectively; PowerLab; ADInstruments). The caudal artery was cannulated (PE‐10 connected to PE‐50) for infusion of anesthetic agents. Isoflurane inhalation was then discontinued progressively and rats were kept under anesthesia with pentobarbital sodium (50 mg/kg i.a.) throughout the remainder of the experiment. Anesthesia level was monitored at frequent and regular intervals via the toe‐pinch and blink reflexes and supplemented as necessary (0.02–0.05 ml of 50 mg/ml pentobarbital sodium diluted in ~0.2 ml of heparinized saline).
2.1.2. Intravital microscopy preparation
The rat spinotrapezius muscle was used for the microcirculatory studies herein because a) its exteriorization allows for clear visualization of capillary structure and hemodynamics via light transmission microscopy (Gray, 1973; Poole et al., 1997); b) it can be exteriorized without neural or substantial vascular disruption (Bailey et al., 2000; Gray, 1973; Poole et al., 1997); c) a physiological sarcomere length can be maintained throughout the experimental protocol (thereby preventing muscle overstretching and adverse microcirculatory effects) (Poole et al., 1997; Welsh & Segal, 1996); and d) its mixed fiber‐type composition and oxidative capacity are similar to those of the human quadriceps (Delp & Duan, 1996; Leek et al., 2001), thus representing a useful analog of human locomotor muscle. Following catheter placement procedures, the left spinotrapezius was exposed and exteriorized as described previously (Bailey et al., 2000; Gray, 1973; Poole et al., 1997) with minimal fascial removal to limit tissue damage and microcirculatory disturbances (Mazzoni et al., 1990). Briefly, the caudal end of the muscle was isolated from its origin and sutured at equidistant points to a horseshoe‐shaped manifold. The rat was then placed on a water circulation‐heated (38°C) Lucite platform and the manifold secured with the ventral aspect of the muscle reflected upwards for microscopic observation. The preparation was frequently superfused with Krebs–Henseleit bicarbonate‐buffered solution (2.0 mM CaCl2, 2.4 mM MgSO4, 4.7 mM KCl, 22 mM NaHCO3, 131 mM NaCl; pH 7.4; equilibrated with 5% CO2 and 95% N2 at ~38°C) and exposed surrounding tissue covered with Saran wrap (Dow Brands). As noted earlier, the spinotrapezius was maintained at physiological sarcomere length (2.6 ± 0.1 µm) to prevent stretch‐induced reductions in capillary blood flow (Poole et al., 1997; Welsh & Segal, 1996).
2.2. Experimental protocol
2.2.1. Intravital video microscopy
Microcirculatory fields located midway between arteriolar and venular ends within the mid‐caudal region of the spinotrapezius that provided optimal clarity were selected randomly for the study. Microcirculatory images were obtained via a bright‐field microscope (Nikon Eclipse E600‐FN) equipped with a non‐contact illuminated lens (x40, numerical aperture 0.8) and viewed in real time on a high‐resolution color monitor (Sony Trinitron PVM‐1954Q) under a final magnification of x1,184 (as confirmed by initial calibration of the system with a stage micrometer; MA285; Meiji Techno). Images were recorded by a video camera (JVC KY‐F55B) and stored on a computer for subsequent offline analyses.
2.2.2. Experimental design
Once the spinotrapezius muscle was positioned on the platform, a quiescent period of at least 15 min was allowed before any data were acquired. Intravital microscopy recordings were then made under two separate superfusion conditions: control (Krebs–Henseleit; CON) and KATP channel inhibition (glibenclamide; GLI). GLI was the last treatment due to its long‐lasting effects (Thomas et al., 1997) and the possibility of incomplete washout with Krebs–Henseleit. All superfusion solutions were maintained at approximately 38°C. The spinotrapezius was superfused with each solution for 3 min (average flow rate of 1 ml/min) followed by a 30 min equilibration period as employed by previous microcirculatory studies (Hodnett et al., 2008; Jackson, 1993; Lu et al., 2013; Murrant & Sarelius, 2002; Saito et al., 1996). Microcirculatory fields were chosen randomly from each rat (based on clear visualization of sarcomeres, fibers, and capillaries) and each recorded for ~1–1.5 min. At the end of the experimental protocol, rats were killed with intra‐arterial pentobarbital sodium overdose (100 mg/kg) followed by pneumothorax.
2.2.3. Acute local KATP channel inhibition
The pharmacological sulphonylurea derivative GLI (494 g/mol; 5‐chloro‐N‐[4‐(cyclohexylureidosulfonyl)phenethyl]‐2‐methoxybenzamide; Sigma‐Aldrich; St. Louis, MO) was used to inhibit KATP channels via superfusion (topical application) of the spinotrapezius muscle. GLI stock solutions were made fresh daily with the solvent dimethyl sulfoxide (DMSO) and diluted with control (Krebs–Henseleit) superfusate. The final working superfusate had a GLI concentration of 10 µM and contained <0.01% DMSO. This GLI concentration was based on previous microcirculatory research using similar drug delivery methods with the rat spinotrapezius and hamster cheek pouch and cremaster muscles (Cohen & Sarelius, 2002; Hammer et al., 2001; Hodnett et al., 2008; Jackson, 1993; Lu et al., 2013; Murrant & Sarelius, 2002; Saito et al., 1996; Xiang & Hester, 2009). Previous studies have shown that ≤0.05% DMSO solutions have no significant effects on resting skeletal muscle arteriolar diameter or reactivity (Cohen et al., 2000; Jackson, 1993) or KATP channel currents in the absence or presence of ATP (Mele et al., 2014; Tricarico et al., 2006). Recent reports from our laboratory have shown no differences in arterial blood PO2, PCO2, O2 saturation, hematocrit, pH, [lactate] or [glucose] following superfusion of the rat spinotrapezius muscle with KATP channel agonists (pinacidil; 5 mg/kg) and antagonists (GLI; 5 mg/kg) (Holdsworth et al., 2017).
2.3. Analysis of muscle capillary hemodynamics
Within each preparation, only those microcirculatory fields with the best overall clarity were selected for further examination via frame‐by‐frame techniques (30 frames/s; Dartfish) as described previously (Kindig et al., 1999, 2002; Poole et al., 1997). Sarcomere length was determined from sets of 10 consecutive in‐register sarcomeres (i.e., distance between 11 consecutive A‐bands) measured in parallel to the muscle fiber longitudinal axis. This procedure was repeated 3 times where sarcomeres were visible to obtain a mean sarcomere length for each field. Comparisons between control and GLI conditions were made within the same microcirculatory field for each rat and, whenever possible, between the same capillaries (which occurred in ~47% of cases; i.e., 14 of 30 vessels). The percentage of RBC‐perfused vessels was established as (no. of capillaries supporting RBC flow/total no. of visible capillaries per field) × 100. Vessels demonstrating impeded or stopped flow (i.e., stationary or no visible RBC flow) for ≥10 s were regarded as non‐flowing capillaries. Five capillaries supporting continuous RBC flow were selected randomly for hemodynamics analysis from each microcirculatory field based on clarity. For all capillaries in which hemodynamics was evaluated and where the capillary endothelium was clearly visible on both sides of the lumen, capillary luminal diameter (d cap) was measured at two random sites per capillary (2–3 measurements/site). RBC velocity (V RBC) was determined by following the RBC path length over several frames and for the maximum capillary length over which the cells remained in crisp focus. RBC flux (f RBC) was measured by counting the number of cells in a capillary passing an arbitrary point over several frames. These measurements were performed three times per capillary. For each capillary in which hemodynamics data were measured, capillary tube hematocrit (Hctcap) was calculated as follows:
| (1) |
where volRBC is RBC volume and assumed to be 61 µm3 (Altman & Dittmer, 1974) and capillaries were approximated as circular in cross section (Desjardins & Duling, 1990; Kindig et al., 1998).
2.4. Statistical analyses
Statistical analyses were performed using a commercially available software package (SigmaPlot 11.2; Systat Software). Data distribution was assessed via the Shapiro–Wilk test for normality. Central hemodynamics (MAP and HR) and spinotrapezius muscle capillary structure (d cap) and hemodynamics (%RBC‐flowing capillaries, f RBC, V RBC, and Hctcap) data were compared between control and GLI conditions using paired Student's t‐tests. Coefficients of variation were calculated as (SD/mean) × 100 and compared between control and GLI conditions using the methods described by Forkman (2009). Pearson's product‐moment correlations and linear regression analyses were used to examine relationships between f RBC and V RBC. Linear regression slope comparisons were performed as described by Zar (1984). Significance was accepted at p < 0.05. Data are reported as mean ± SE.
3. RESULTS
3.1. Central hemodynamics
As expected based on the topical drug delivery method employed herein (i.e., local superfusion of the spinotrapezius muscle), no differences in MAP (CON: 120 ± 5, GLI: 124 ± 5 mmHg; p > 0.05) or HR (CON: 322 ± 32, GLI: 337 ± 33 beats/min; p > 0.05) were observed between conditions.
3.2. Spinotrapezius muscle capillary structure and hemodynamics
The percentage of capillaries supporting continuous RBC flow was not altered between conditions (Figure 1; p > 0.05). Capillaries subjected to hemodynamics analysis supported RBC flow prior to, and continued to do so, following KATP inhibition (i.e., during CON and GLI, respectively). In these RBC‐flowing capillaries, GLI reduced both f RBC and V RBC (Figures 2 and 3; p < 0.05 for both) but not Hctcap (CON: 0.26 ± 0.01, GLI: 0.26 ± 0.01; p > 0.05). GLI‐induced alterations in muscle capillary hemodynamics occurred in the absence of changes in d cap between conditions (CON: 4.9 ± 0.1, GLI: 4.9 ± 0.1 µm; p > 0.05). Frequency histograms of capillary f RBC and V RBC are shown in Figure 3. Microvascular blood flow heterogeneity, as evaluated by the individual capillary coefficient of variation, was not different between conditions with respect to f RBC (CON: 61.8, GLI: 57.4%; p > 0.05), V RBC (CON: 49.4, GLI: 47.8%; p > 0.05) or Hctcap (CON: 23.6, GLI: 22.3%; p > 0.05). Similar results were found when comparing heterogeneity between microvascular fields in terms of f RBC (CON: 21.4, GLI: 21.6%; p > 0.05), V RBC (CON: 17.4, GLI: 22.9%; p > 0.05), and Hctcap (CON: 13.5, GLI: 11.1%; p > 0.05).
FIGURE 1.
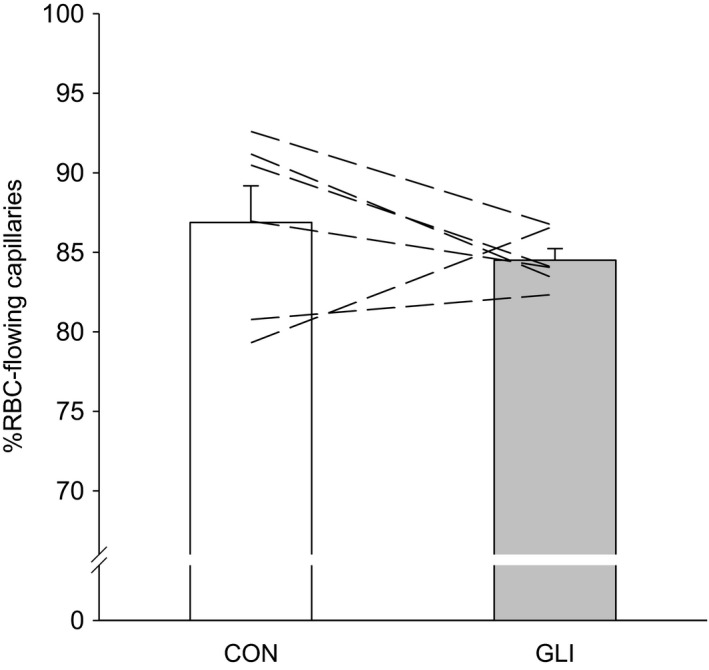
Percentage of capillaries supporting red blood cell (RBC) flow during control (CON; n = 6) and KATP channel inhibition (glibenclamide, GLI; n = 6) conditions. Dashed lines represent individual muscle data
FIGURE 2.
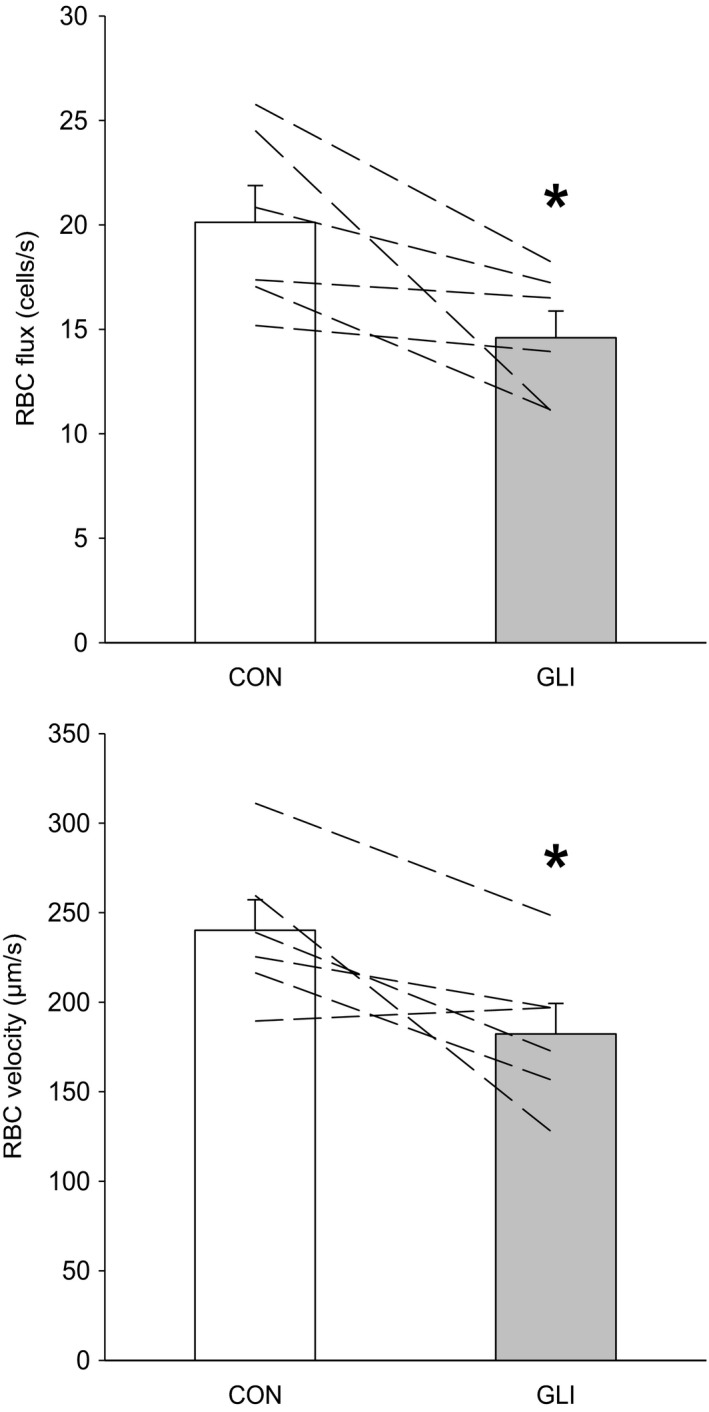
Capillary red blood cell flux (top panel) and velocity (bottom panel) during control (CON; n = 6) and KATP channel inhibition (glibenclamide, GLI; n = 6) conditions. Dashed lines represent individual muscle data. *p < 0.05 vs. CON
FIGURE 3.
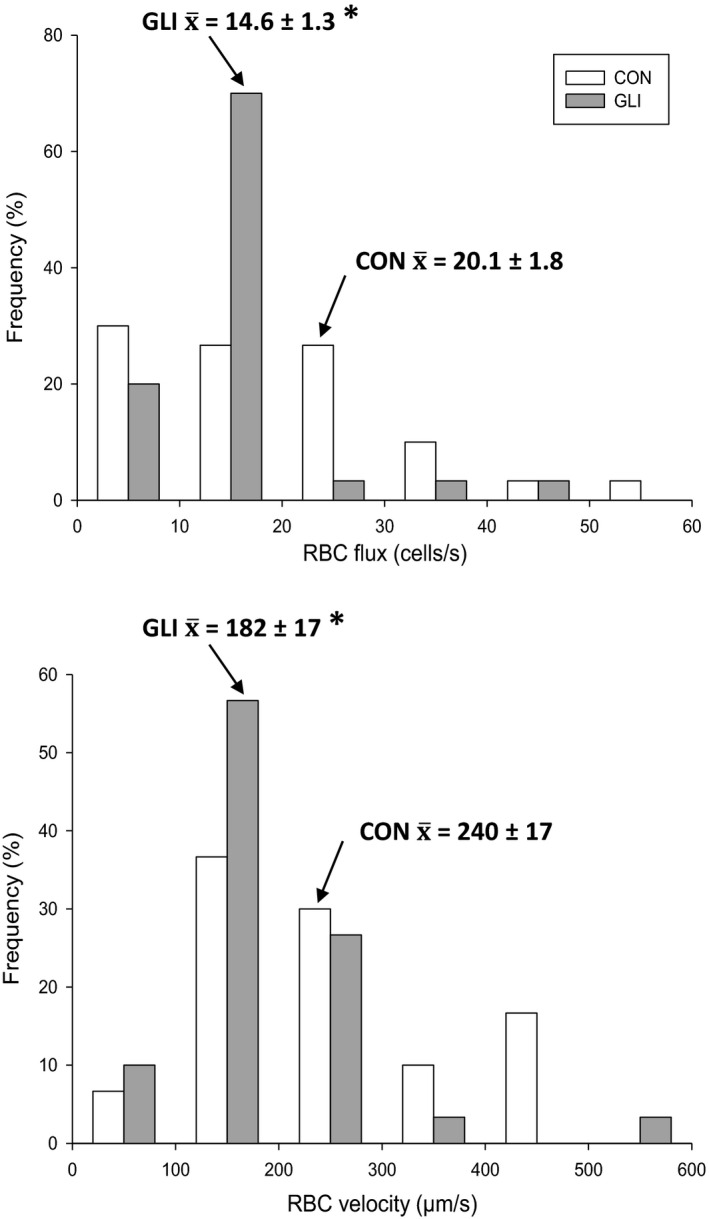
Relative frequency histograms of capillary red blood cell flux (top panel) and velocity (bottom panel) during control (CON; n = 6) and KATP channel inhibition (glibenclamide, GLI; n = 6) conditions. Arrows show mean values. *p < 0.05 vs. CON
Consistent with the lack of changes in Hctcap with GLI (and the relationship described in Eq. 1 above), no significant differences were observed in the slope of the f RBC/V RBC relationship between conditions (individual capillaries; CON: 8.75, GLI: 9.44; individual muscles; CON: 7.46, GLI: 12.25; p > 0.05 for both). Therefore, Figure 4 presents linear correlations and regression analyses for the combined CON and GLI data. Capillary f RBC and V RBC were significantly correlated in both individual capillaries and individual muscles (Figure 4, top and bottom panels, respectively) thus illustrating their proportional reduction with GLI.
FIGURE 4.
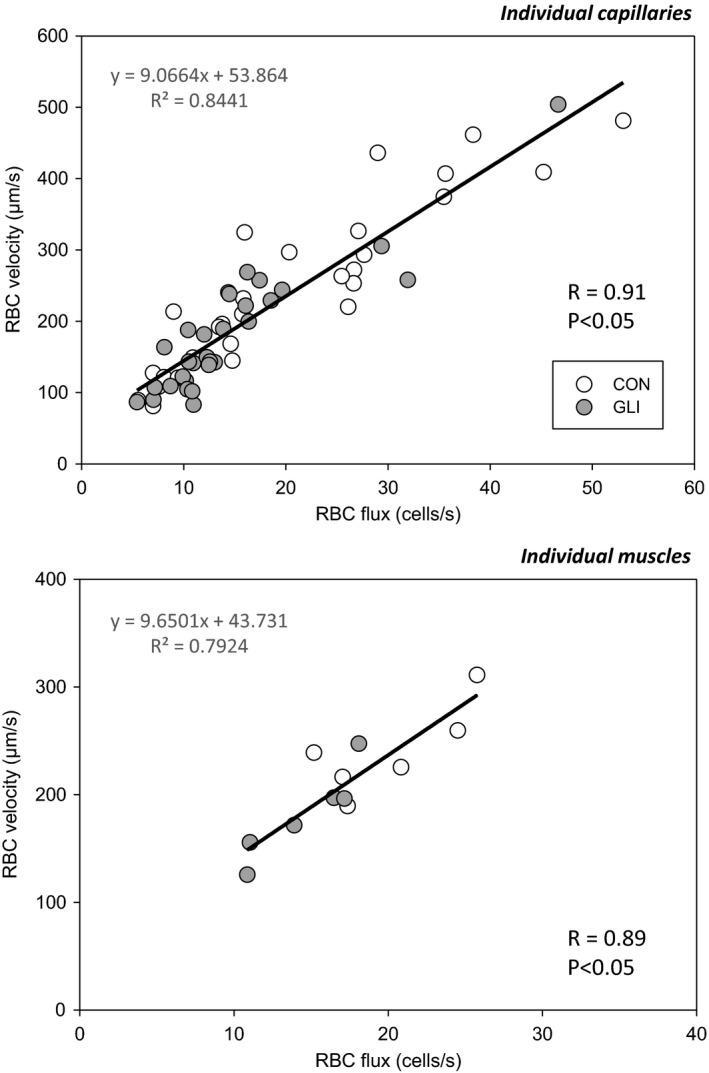
Individual and mean capillary relationships (top and bottom panels, respectively) between red blood cell flux and velocity during control (CON) and KATP channel inhibition (glibenclamide, GLI) conditions. Each data point represents a single capillary in the top panel (n = 30) and the average value for all capillaries within a single muscle (n = 6) in the bottom panel
4. DISCUSSION
This investigation examined, for the first time, the regulation of capillary hemodynamics by KATP channels in resting skeletal muscle. Superfusion of the rat spinotrapezius with the sulfonylurea GLI was used to locally inhibit KATP channels in vivo. The principal novel findings are as follows: a) inconsistent with our hypothesis, GLI did not reduce the proportion of capillaries supporting continuous RBC flow or Hctcap; and b) consistent with our hypothesis, GLI lowered microvascular blood flow (i.e., both f RBC and V RBC) in perfused capillaries. These data suggest that KATP channels, via modulation of arteriolar vascular conductance, regulate microvascular hemodynamics at the capillary level (the primary site for blood‐myocyte O2 diffusion) (Hirai et al., 2019; Poole et al., 2011; Poole, 2019) in resting skeletal muscle.
4.1. Intravital microscopy preparation
Surgical exteriorization of the rat spinotrapezius muscle was performed as described previously (Bailey et al., 2000; Gray, 1973; Poole et al., 1997) with minimal fascial disturbance to curtail tissue damage and related microcirculatory consequences. Accordingly, previous studies from our laboratory indicate that the surgical exteriorization requisite for transmission intravital microscopy as performed herein does not impair the microvascular integrity or responsiveness of the spinotrapezius muscle (Bailey et al., 2000).
In the present investigation, spinotrapezius sarcomere length was set at physiological values (2.6 ± 0.1 µm) to prevent stretch‐induced capillary luminal diameter reductions (Poole et al., 1997) and/or sympathetically mediated decreases in arteriolar blood flow (Welsh & Segal, 1996; cf. Kindig & Poole, 2001). Capillary structure (d cap) and hemodynamics (%RBC‐flowing vessels, f RBC, V RBC, and Hctcap) data obtained herein during the control condition are consistent with those published previously in the resting rat spinotrapezius and hamster cheek pouch and cremaster muscles (Copp et al., 2009; Kano et al., 2005; Kindig et al., 1998, 1999, 2002; Kindig & Poole, 2001; Poole et al., 1997; Richardson et al., 2003; Russell et al., 2003; Sarelius & Duling, 1982). Our data are also consistent with the well‐documented lower than systemic Hctcap values found in resting skeletal muscle (Poole et al., 2011; Poole, 2019). Based on both theoretical and empirical studies, potential mechanisms for the systemic versus capillary tube hematocrit difference include a) the presence of the endothelial surface layer (i.e., the often termed “glycocalyx”); b) the Fahraeus effect; and c) plasma skimming at arteriolar bifurcations (Desjardins & Duling, 1990; Ellsworth et al., 2009; Pries et al., 1986; Secomb et al., 1998).
As reviewed recently (Poole et al., 2011; Poole, 2019), a compelling body of evidence demonstrates that the vast majority of skeletal muscle capillaries support continuous RBC perfusion at rest. Our data are consistent with this notion showing that ~85% of capillaries supported continuous f RBC during both CON and GLI conditions (Figure 1).
4.2. Local KATP channel inhibition with GLI superfusion
As expected, GLI‐induced alterations in microvascular hemodynamics (f RBC and V RBC; Figure 2) occurred in the absence of changes in d cap between conditions. This suggests increased vascular resistance at sites upstream of the capillary bed consistent with the well‐established arteriolar control of skeletal muscle blood flow (Joyner & Casey, 2015; Kindig & Poole, 2001; Laughlin et al., 2012; Segal, 2005). Our data are thus in agreement with some, but not all, reports of decreased arteriolar diameter (Hammer et al., 2001; Hodnett et al., 2008; Jackson, 1993; Lu et al., 2013; Murrant & Sarelius, 2002; Saito et al., 1996; Xiang & Hester, 2009) and bulk blood flow (Bank et al., 2000; Colburn, Weber, et al., 2020; Duncker et al., 2001; Farouque & Meredith, 2003a, 2003b,2003a, 2003b; Holdsworth et al., 2015; Vanelli & Hussain, 1994) following KATP channel inhibition in the resting skeletal muscle. Potential reasons for this discrepancy include species differences, experimental models, GLI doses, KATP channel density distribution, compensatory vasodilation by redundant pathways, muscle fiber type, and arteriolar size and branch order. Although providing an invaluable framework for understanding the control of vascular tone and tissue perfusion, previous measurements of arteriolar diameter and bulk blood flow responses with GLI presented no information concerning the distribution of microvascular flow within the capillary network. Moreover, it was not known whether KATP channel inhibition would impact uniformly RBC‐perfused capillaries or whether the proportion of RBC‐perfused capillaries could be reduced. Evaluation of capillary hemodynamics within those vessels supporting continuous RBC flow is critical to identifying how KATP channels may modulate blood‐myocyte O2 and substrate exchange. This is especially true when considering that the capillary network provides the largest surface area for gas and substrate exchange within the skeletal muscle microcirculation (Hirai et al., 2019; Poole et al., 2011; Poole, 2019).
The potential for KATP channels to modulate skeletal muscle O2 exchange must be considered in light of the interdependence between diffusive and perfusive conductances setting fractional O2 extraction (Roca et al., 1992):
| (2) |
where DO2m is muscle O2 diffusing capacity, β is the slope of the O2 dissociation curve in the physiologically relevant range, and Q̇m is muscle blood flow. Theoretical models of skeletal muscle O2 exchange indicate that DO2m is determined by structural (capillary‐to‐fiber ratio, capillary length, and d cap) and functional (Hctcap at constant arterial O2 saturation) elements (Federspiel & Popel, 1986; Groebe & Thews, 1990). Capillary‐to‐fiber ratio, capillary length, and β are not expected to be affected by acute GLI treatment as employed herein. Moreover, we observed no differences in d cap or Hctcap between CON and GLI. It thus seems that KATP channels do not modulate significantly DO2m in resting skeletal muscle. On the other hand, the current GLI‐induced reductions in f RBC (which largely dictates Q̇m within the microcirculation) (Berg & Sarelius, 1996) are anticipated to elevate the DO2m/Q̇m ratio within RBC‐perfused capillaries and, therefore, necessitate higher muscle fractional O2 extraction for a given metabolic rate according to Eq. 2 above. Importantly, the functional consequence of lowered f RBC with GLI (Figure 2, top panel) is impaired O2 delivery per capillary (Q̇O2cap). Disregarding the small amount of O2 dissolved in plasma (and, therefore, its relevance to capillary gas exchange), Q̇O2cap can be estimated as the product of f RBC, the hemoglobin content per RBC (17 × 10−12 g per RBC) (Altman & Dittmer, 1974) and the O2 carrying capacity of hemoglobin (at 89% saturation, assuming arterial PO2 = 80 mmHg in anesthetized rats) as described previously (Kindig et al., 1998):
| (3) |
Using the equation above, KATP inhibition with GLI superfusion of the resting spinotrapezius herein reduced mean Q̇O2cap by approximately 27% from 4.08 to 2.96 × 10−10 ml O2/s. Interestingly, this estimation is strikingly similar to the ~28% reduction in total hindlimb skeletal muscle blood flow of conscious resting rats following GLI administration (Colburn, Holdsworth, et al., 2020).
GLI‐induced alterations in V RBC (Figure 2, bottom panel) underscore the potential of KATP channels to further modulate capillary gas exchange via changes in RBC transit time. Given the ~25% decrease in V RBC with GLI observed herein and the mean capillary length within the rat spinotrapezius (i.e., 430 µm based on previous morphometric analyses by Gray, 1984), total capillary RBC residence time is calculated to increase from ~1.8 s during control to ~2.4 s following KATP channel inhibition. While it is acknowledged that these calculations may underestimate true residence time due to the actual RBC path length being somewhat longer than the anatomical path length (Sarelius, 1986), the amount of error introduced should not preferentially bias either superfusion condition. Longer RBC transit times provide the mechanistic basis for the elevated fractional O2 extraction expected with GLI as discussed above.
The proportional reductions in f RBC and V RBC with GLI were such that Hctcap remained unchanged between conditions (vide supra its mathematical description; Eq. 1). It is interesting that the relationship between f RBC and V RBC in the resting skeletal muscle may also be preserved with aging and some disease states. Accordingly, previous studies from our laboratory have shown that the microvascular dysregulation characteristic of aging (Copp et al., 2009; Russell et al., 2003), type I diabetes (Kindig et al., 1998), and chronic heart failure (Kindig et al., 1999; Richardson et al., 2003) does not appear to affect resting Hctcap. As noted above, Hctcap is a primary determinant of DO2m and, therefore, diffusive O2 conductance within the microcirculation (Federspiel & Popel, 1986; Groebe & Thews, 1990). This derives from the low O2 diffusivity in plasma and the particulate nature of blood, which render only the capillary surface area in close proximity to the RBC functional for diffusion at any given time. The current Hctcap data thus indicate preserved capillary surface area available for diffusive O2 exchange within continuously RBC‐perfused vessels following KATP channel inhibition.
An important question for future studies is whether the impairments in resting capillary hemodynamics seen here after KATP inhibition are also present during muscle contractions and, if so, their potential implications for exercise tolerance. In the resting spinotrapezius, GLI lowered f RBC and V RBC (i.e., reduced Q̇m; Figure 2) whilst not affecting Hctcap or the %RBC‐flowing capillaries (i.e., unaltered DO2m as noted above), thereby predisposing to higher fractional O2 extraction (i.e., higher DO2m/Q̇m ratio; Eq. 2). While there has been no prior evaluation of KATP channel inhibition on contracting muscle capillary blood flow per se, studies on arteriolar diameter and bulk blood flow have produced contrasting evidence regarding the role of KATP channels in functional hyperemia (Tykocki et al., 2017). Nonetheless, recent reports from our laboratory indicate that GLI lowers microvascular O2 partial pressures (PO2) as well as estimated DO2m in the contracting spinotrapezius and mixed gastrocnemius muscles (Colburn, Weber, et al., 2020; Holdsworth et al., 2016). Under these circumstances, intramyocyte PO2 is expected to fall to generate the driving pressure (ΔPO2) and possibly elevate intramyocyte O2 diffusing capacity by and by further deoxygenating myoglobin as necessary to maintain a given metabolic rate as described by Fick's law of diffusion:
| (4) |
where V̇O2 corresponds to the rate of O2 flux, DO2m is the diffusing capacity as defined above and ΔPO2 the O2 partial pressure difference between the microvascular and intramyocyte spaces. Reduction in intramyocyte PO2 can be problematic because it promotes depletion of finite muscle phosphocreatine and glycogen stores, intracellular accumulation of metabolites (including ADP, Pi, and H+) and acid‐base perturbations associated with fatigue (Hogan et al., 1992; Wilson et al., 1977). Therefore, based on the above observations, potential impairments in contracting muscle capillary hemodynamics with GLI could result in pernicious consequences for oxidative metabolism and exercise tolerance (Colburn, Weber, et al., 2020; Lu et al., 2013).
Despite marked reductions in RBC hemodynamics (i.e., decreased f RBC and V RBC; Figure 2), GLI did not change microvascular blood flow heterogeneity (as evaluated by the coefficient of variation of f RBC, V RBC, and Hctcap) in the resting spinotrapezius. Due to the nature of our experimental protocol (i.e., assessment of the same microcirculatory fields, and whenever possible, capillaries between conditions), these data indicate that any spatial vascular/metabolic mismatch present at rest was not exacerbated with GLI. Whether the same holds true for the contracting skeletal muscle (amid dynamic temporal and spatial changes in O2 delivery‐utilization) remains to be determined. Importantly, the consequences of microvascular blood flow heterogeneity assume greater significance during exercise when high O2 fluxes (which can rise >100‐fold compared to rest) are required to support oxidative metabolism.
4.3. Clinical implications
Sulfonylureas are the most popular second‐line treatment prescribed for type 2 diabetes mellitus (Montvida et al., 2018), promoting insulin release via inhibition of pancreatic KATP channels. However, systemic inhibition of KATP channels with these oral medications may also compromise key components of capillary gas exchange within skeletal muscle as revealed herein (i.e., lowered f RBC and V RBC; Figure 2). The latter microvascular impairments compound with those described previously in the diabetic skeletal muscle (Kindig et al., 1998; Padilla et al., 2006) thus questioning the therapeutic value of sulfonylurea medications for diabetic patients. Systemic sulfonylurea administration has also been reported to reduce contracting muscle bulk blood flow (Holdsworth et al., 2015; Keller et al., 2004; Thomas et al., 1997) and submaximal and maximal exercise tolerance (Colburn, Weber, et al., 2020; Lu et al., 2013). Although no decrements in intracapillary DO2m with GLI were found herein at rest, potential reductions in the proportion of capillaries supporting continuous RBC flow with sulfonylurea medications during contractions would be expected to impair muscle glucose uptake (via reductions in exchange surface area). As such, exercise intolerance may be exacerbated in diabetes by systemic sulfonylurea medications impairing KATP channel regulation of skeletal muscle capillary hemodynamics.
4.4. Experimental considerations
Superfusion of the spinotrapezius muscle with GLI was employed herein to locally inhibit KATP channels in vivo. This topical drug delivery method was chosen to prevent the potential confound of alterations in central hemodynamics (e.g., MAP, HR, systemic vascular resistance), myocardial hemodynamics and function (e.g., coronary vascular conductance and O2 delivery, left ventricular relaxation rate), sympathetic nerve activity, visceral organ blood flow, blood insulin and glucose concentration, among others with systemic drug administration as reported previously (Colburn, Holdsworth, et al., 2020; Colburn, Weber, et al., 2020; Duncker et al., 2001; Farouque & Meredith, 2003a; Holdsworth et al., 2015, 2016; Rocha et al., 2020; Thomas et al., 1997). Nonetheless, it is not possible to pinpoint the site of GLI action (e.g., across the vascular network and/or among distinct cell types) with the current experimental protocol. Although our GLI concentration and superfusion protocol were based on previous microcirculatory studies (Cohen & Sarelius, 2002; Hammer et al., 2001; Hodnett et al., 2008; Jackson, 1993; Lu et al., 2013; Murrant & Sarelius, 2002; Saito et al., 1996; Xiang & Hester, 2009) as described earlier, this approach could lead to non‐specific vasodilation (Jiang et al., 2007; Tykocki et al., 2017) and potentially underestimate the contribution of KATP channels to microvascular control. It is noteworthy that, despite the latter considerations and the modest sample size (n = 6), significant GLI‐induced reductions in f RBC and V RBC were observed in the resting spinotrapezius muscle (Figures 2 and 3). Post hoc power calculations indicate that a total of 48 animals would be needed to detect significant changes in the %RBC‐flowing capillaries between conditions.
Previous evidence indicates that muscle capillaries increase in diameter by ~0.5–1.0 µm from their arteriolar to venular ends (Smaje et al., 1970). Although d cap measurements herein were not normalized for the distance from arteriolar or venular ends, our d cap assessment at random sites along the capillary length is not anticipated to introduce systematic errors. Moreover, capillary structural and hemodynamics analyses of the same microcirculatory fields within a given animal (and, in 47% of the cases, within the same capillary between CON and GLI conditions) likely minimized the potential for biases (including spatial vascular/metabolic heterogeneity, and heterogeneous f RBC, V RBC and Hctcap distributions) to be expressed in the present results. The lack of changes in d cap in the face of reduced f RBC and V RBC with GLI (Figure 2) is consistent with upstream arteriolar regulation of skeletal muscle blood flow as mentioned above (Joyner & Casey, 2015; Kindig & Poole, 2001; Laughlin et al., 2012; Segal, 2005).
4.5. Summary and conclusions
Local inhibition of KATP channels with GLI did not reduce the proportion of capillaries supporting continuous RBC flow or Hctcap in the resting rat spinotrapezius muscle. These data are indicative of preserved muscle O2 diffusing capacity (DO2m) with GLI. In contrast, GLI lowered both f RBC and V RBC thus impairing perfusive microvascular O2 transport (Q̇m) and lengthening RBC capillary transit times, respectively. Considering the interdependence between diffusive and perfusive O2 conductances (i.e., %O2 extraction determined largely by the local DO2m/Q̇m; vide Eq. 2 above) (Roca et al., 1992), such GLI‐induced alterations in capillary hemodynamics are expected to elevate muscle fractional O2 extraction to sustain a given metabolic rate. Taken together, the current results suggest that KATP channels regulate capillary hemodynamics (and, therefore, microvascular gas exchange) in resting skeletal muscle.
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
DMH, TIM, and DCP conceived and designed research; DMH, AT, JCC, and TDC performed experiments; DMH analyzed data; DMH, TIM, and DCP interpreted results of experiments; DMH prepared figures and drafted manuscript; DMH, AT, JCC, TDC, TIM, and DCP edited and revised manuscript; DMH, AT, JCC, TDC, TIM, and DCP approved final version of the manuscript.
ACKNOWLEDGMENTS
We thank Ms. K. Sue Hageman for expert technical assistance and Dr. Clark T. Holdsworth for helpful discussion and guidance regarding glibenclamide superfusion in this study.
Funding information
This work was supported in part by a Post‐Doctoral Fellowship from the College of Human Ecology, Kansas State University; PRF Summer Faculty Grant, Purdue University; and National Heart, Lung and Blood Institute Grant HL‐2‐108328.
REFERENCES
- Altman, P. , & Dittmer, D. (1974). Biology data book, 2nd ed. Federation of American Societies for Experimental Biology. [Google Scholar]
- Bailey, J. K. , Kindig, C. A. , Behnke, B. J. , Musch, T. I. , Schmid‐Schoenbein, G. W. , & Poole, D. C. (2000). Spinotrapezius muscle microcirculatory function: Effects of surgical exteriorization. American Journal of Physiology. Heart and Circulatory Physiology, 279, H3131–3137. 10.1152/ajpheart.2000.279.6.H3131. [DOI] [PubMed] [Google Scholar]
- Bank, A. J. , Sih, R. , Mullen, K. , Osayamwen, M. , & Lee, P. C. (2000). Vascular ATP‐dependent potassium channels, nitric oxide, and human forearm reactive hyperemia. Cardiovascular Drugs and Therapy, 14, 23–29. 10.1023/a:1007835003493. [DOI] [PubMed] [Google Scholar]
- Berg, B. R. , & Sarelius, I. H. (1996). Erythrocyte flux in capillary networks during maturation: Implications for oxygen delivery. American Journal of Physiology, 271, H2263–2273. 10.1152/ajpheart.1996.271.6.H2263. [DOI] [PubMed] [Google Scholar]
- Cohen, K. D. , Berg, B. R. , & Sarelius, I. H. (2000). Remote arteriolar dilations in response to muscle contraction under capillaries. American Journal of Physiology. Heart and Circulatory Physiology, 278, H1916–1923. 10.1152/ajpheart.2000.278.6.H1916. [DOI] [PubMed] [Google Scholar]
- Cohen, K. D. , & Sarelius, I. H. (2002). Muscle contraction under capillaries in hamster muscle induces arteriolar dilatation via KATP channels and nitric oxide. Journal of Physiology, 539, 547–555. 10.1113/jphysiol.2001.013388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn, T. D. , Holdsworth, C. T. , Craig, J. C. , Hirai, D. M. , Montgomery, S. , Poole, D. C. , Musch, T. I. , & Kenney, M. J. (2020). ATP‐sensitive K+ channel inhibition in rats decreases kidney and skeletal muscle blood flow without increasing sympathetic nerve discharge. Respiratory Physiology & Neurobiology, 278, 103444. 10.1016/j.resp.2020.103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn, T. D. , Weber, R. E. , Hageman, K. S. , Caldwell, J. T. , Schulze, K. M. , Ade, C. J. , Behnke, B. J. , Poole, D. C. , & Musch, T. I. (2020). Vascular ATP‐sensitive K+ channels support maximal aerobic capacity and critical speed via convective and diffusive O2 transport. The Journal of Physiology, 598, 4843–4858. 10.1113/JP280232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp, S. W. , Ferreira, L. F. , Herspring, K. F. , Musch, T. I. , & Poole, D. C. (2009). The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvascular Research, 77, 113–119. 10.1016/j.mvr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Delp, M. D. , & Duan, C. (1996). Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. Journal of Applied Physiology, 80, 261–270. [DOI] [PubMed] [Google Scholar]
- Desjardins, C. , & Duling, B. R. (1990). Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. American Journal of Physiology, 258, H647–654. 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- Duncker, D. J. , Oei, H. H. , Hu, F. , Stubenitsky, R. , & Verdouw, P. D. (2001). Role of K+ ATP channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. American Journal of Physiology. Heart and Circulatory Physiology, 280, H22–33. 10.1152/ajpheart.2001.280.1.H22. [DOI] [PubMed] [Google Scholar]
- Ellsworth, M. L. , Ellis, C. G. , Goldman, D. , Stephenson, A. H. , Dietrich, H. H. , & Sprague, R. S. (2009). Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology, 24, 107–116. 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farouque, H. M. O. , & Meredith, I. T. (2003a). Effects of inhibition of ATP‐sensitive potassium channels on metabolic vasodilation in the human forearm. Clinical Science, 104, 39–46. [DOI] [PubMed] [Google Scholar]
- Farouque, H. M. O. , & Meredith, I. T. (2003b). Relative contribution of vasodilator prostanoids, NO, and KATP channels to human forearm metabolic vasodilation. American Journal of Physiology. Heart and Circulatory Physiology, 284, H2405–2411. 10.1152/ajpheart.00879.2002. [DOI] [PubMed] [Google Scholar]
- Federspiel, W. J. , & Popel, A. S. (1986). A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvascular Research, 32, 164–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkman, J. (2009). Estimator and tests for common coefficients of variation in normal distributions. Communications in Statistics—Theory and Methods, 38, 233–251. [Google Scholar]
- Foster, M. N. , & Coetzee, W. A. (2016). KATP channels in the cardiovascular system. Physiological Reviews, 96, 177–252. 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, S. D. (1973). Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvascular Research, 5, 395–400. [DOI] [PubMed] [Google Scholar]
- Gray, S. D. (1984). Morphometric analysis of skeletal muscle capillaries in early spontaneous hypertension. Microvascular Research, 27, 39–50. 10.1016/0026-2862(84)90040-2. [DOI] [PubMed] [Google Scholar]
- Groebe, K. , & Thews, G. (1990). Calculated intra‐ and extracellular PO2 gradients in heavily working red muscle. American Journal of Physiology, 259, H84–92. [DOI] [PubMed] [Google Scholar]
- Hammer, L. W. , Ligon, A. L. , & Hester, R. L. (2001). Differential inhibition of functional dilation of small arterioles by indomethacin and glibenclamide. Hypertension, 37, 599–603. 10.1161/01.hyp.37.2.599. [DOI] [PubMed] [Google Scholar]
- Hirai, D. M. , Colburn, T. D. , Craig, J. C. , Hotta, K. , Kano, Y. , Musch, T. I. , & Poole, D. C. (2019). Skeletal muscle interstitial O2 pressures: bridging the gap between the capillary and myocyte. Microcirculation, 26, e12497. 10.1111/micc.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodnett, B. L. , Xiang, L. , Dearman, J. A. , Carter, C. B. , & Hester, R. L. (2008). KATP‐mediated vasodilation is impaired in obese Zucker rats. Microcirculation, 15, 485–494. 10.1080/10739680801942240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, M. C. , Arthur, P. G. , Bebout, D. E. , Hochachka, P. W. , & Wagner, P. D. (1992). Role of O2 in regulating tissue respiration in dog muscle working in situ. Journal of Applied Physiology, 73, 728–736. [DOI] [PubMed] [Google Scholar]
- Holdsworth, C. T. , Copp, S. W. , Ferguson, S. K. , Sims, G. E. , Poole, D. C. , & Musch, T. I. (2015). Acute inhibition of ATP‐sensitive K+ channels impairs skeletal muscle vascular control in rats during treadmill exercise. American Journal of Physiology. Heart and Circulatory Physiology, 308, H1434–1442. 10.1152/ajpheart.00772.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth, C. T. , Ferguson, S. K. , Colburn, T. D. , Fees, A. J. , Craig, J. C. , Hirai, D. M. , Poole, D. C. , & Musch, T. I. (2017). Vascular KATP channels mitigate severe muscle O2 delivery‐utilization mismatch during contractions in chronic heart failure rats. Respiratory Physiology & Neurobiology, 238, 33–40. 10.1016/j.resp.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth, C. T. , Ferguson, S. K. , Poole, D. C. , & Musch, T. I. (2016). Modulation of rat skeletal muscle microvascular O2 pressure via KATP channel inhibition following the onset of contractions. Respiratory Physiology & Neurobiology, 222, 48–54. 10.1016/j.resp.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Jackson, W. F. (1993). Arteriolar tone is determined by activity of ATP‐sensitive potassium channels. American Journal of Physiology, 265, H1797–1803. 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- Jackson, W. F. (2017). Potassium channels in regulation of vascular smooth muscle contraction and growth. Advances in Pharmacology, 78, 89–144. 10.1016/bs.apha.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, B. , Wu, L. , & Wang, R. (2007). Sulphonylureas induced vasorelaxation of mouse arteries. European Journal of Pharmacology, 577, 124–128. 10.1016/j.ejphar.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Joyner, M. J. , & Casey, D. P. (2015). Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiological Reviews, 95, 549–601. 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano, Y. , Padilla, D. J. , Behnke, B. J. , Hageman, K. S. , Musch, T. I. , & Poole, D. C. (2005). Effects of eccentric exercise on microcirculation and microvascular oxygen pressures in rat spinotrapezius muscle. Journal of Applied Physiology, 99, 1516–1522. 10.1152/japplphysiol.00069.2005. [DOI] [PubMed] [Google Scholar]
- Keller, D. M. , Ogoh, S. , Greene, S. , Olivencia‐Yurvati, A. , & Raven, P. B. (2004). Inhibition of KATP channel activity augments baroreflex‐mediated vasoconstriction in exercising human skeletal muscle. Journal of Physiology, 561, 273–282. 10.1113/jphysiol.2004.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig, C. A. , Musch, T. I. , Basaraba, R. J. , & Poole, D. C. (1999). Impaired capillary hemodynamics in skeletal muscle of rats in chronic heart failure. Journal of Applied Physiology, 87, 652–660. [DOI] [PubMed] [Google Scholar]
- Kindig, C. A. , & Poole, D. C. (2001). Sarcomere length‐induced alterations of capillary hemodynamics in rat spinotrapezius muscle: vasoactive vs passive control. Microvascular Research, 61, 64–74. 10.1006/mvre.2000.2284. [DOI] [PubMed] [Google Scholar]
- Kindig, C. A. , Richardson, T. E. , & Poole, D. C. (2002). Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. Journal of Applied Physiology, 92, 2513–2520. 10.1152/japplphysiol.01222.2001. [DOI] [PubMed] [Google Scholar]
- Kindig, C. A. , Sexton, W. L. , Fedde, M. R. , & Poole, D. C. (1998). Skeletal muscle microcirculatory structure and hemodynamics in diabetes. Respiration Physiology, 111, 163–175. [DOI] [PubMed] [Google Scholar]
- Laughlin, M. H. , Davis, M. J. , Secher, N. H. , van Lieshout, J. J. , Arce‐Esquivel, A. A. , Simmons, G. H. , Bender, S. B. , Padilla, J. , Bache, R. J. , Merkus, D. , & Duncker, D. J. (2012). Peripheral circulation. Comprehensive Physiology, 2, 321–447. 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- Leek, B. T. , Mudaliar, S. R. , Henry, R. , Mathieu‐Costello, O. , & Richardson, R. S. (2001). Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 280, R441–447. [DOI] [PubMed] [Google Scholar]
- Lu, S. , Xiang, L. , Clemmer, J. S. , Gowdey, A. R. , Mittwede, P. N. , & Hester, R. L. (2013). Impaired vascular KATP function attenuates exercise capacity in obese zucker rats. Microcirculation, 20, 662–669. 10.1111/micc.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni, M. C. , Skalak, T. C. , & Schmid‐Schönbein, G. W. (1990). Effects of skeletal muscle fiber deformation on lymphatic volumes. American Journal of Physiology, 259, H1860–1868. 10.1152/ajpheart.1990.259.6.H1860. [DOI] [PubMed] [Google Scholar]
- Mele, A. , Calzolaro, S. , Cannone, G. , Cetrone, M. , Conte, D. , & Tricarico, D. (2014). Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides‐induced atrophy in skeletal muscle. Pharmacology Research & Perspectives, 2, e00028. 10.1002/prp2.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montvida, O. , Shaw, J. , Atherton, J. J. , Stringer, F. , & Paul, S. K. (2018). Long‐term trends in antidiabetes drug usage in the U.S.: Real‐world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care, 41, 69–78. 10.2337/dc17-1414. [DOI] [PubMed] [Google Scholar]
- Murrant, C. L. , & Sarelius, I. H. (2002). Multiple dilator pathways in skeletal muscle contraction‐induced arteriolar dilations. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 282, R969–978. 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- Padilla, D. J. , McDonough, P. , Behnke, B. J. , Kano, Y. , Hageman, K. S. , Musch, T. I. , & Poole, D. C. (2006). Effects of Type II diabetes on capillary hemodynamics in skeletal muscle. American Journal of Physiology. Heart and Circulatory Physiology, 291, H2439–2444. 10.1152/ajpheart.00290.2006. [DOI] [PubMed] [Google Scholar]
- Poole, D. C. , Copp, S. W. , Hirai, D. M. , & Musch, T. I. (2011). Dynamics of muscle microcirculatory and blood‐myocyte O2 flux during contractions. Acta Physiologica, 202, 293–310. 10.1111/j.1748-1716.2010.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, D. C. (2019). Edward F. Adolph distinguished lecture. Contemporary model of muscle microcirculation. Journal of Applied Physiology, 127, 1012–1033. 10.1152/japplphysiol.00013.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole, D. C. , Musch, T. I. , & Kindig, C. A. (1997). In vivo microvascular structural and functional consequences of muscle length changes. American Journal of Physiology, 272, H2107–2114. 10.1152/ajpheart.1997.272.5.H2107. [DOI] [PubMed] [Google Scholar]
- Pries, A. R. , Ley, K. , & Gaehtgens, P. (1986). Generalization of the Fahraeus principle for microvessel networks. American Journal of Physiology, 251, H1324–1332. 10.1152/ajpheart.1986.251.6.H1324. [DOI] [PubMed] [Google Scholar]
- Richardson, T. E. , Kindig, C. A. , Musch, T. I. , & Poole, D. C. (2003). Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. Journal of Applied Physiology, 95, 1055–1062. 10.1152/japplphysiol.00308.2003. [DOI] [PubMed] [Google Scholar]
- Roca, J. , Agusti, A. G. , Alonso, A. , Poole, D. C. , Viegas, C. , Barbera, J. A. , Rodriguez‐Roisin, R. , Ferrer, A. , & Wagner, P. D. (1992). Effects of training on muscle O2 transport at VO2max. Journal of Applied Physiology, 73, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Rocha, M. P. , Campos, M. O. , Mattos, J. D. , Mansur, D. E. , Rocha, H. N. M. , Secher, N. H. , Nóbrega, A. C. L. , & Fernandes, I. A. (2020). KATP channels modulate cerebral blood flow and oxygen delivery during isocapnic hypoxia in humans. Journal of Physiology, 598, 3343–3356. 10.1113/JP279751. [DOI] [PubMed] [Google Scholar]
- Russell, J. A. , Kindig, C. A. , Behnke, B. J. , Poole, D. C. , & Musch, T. I. (2003). Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. American Journal of Physiology. Heart and Circulatory Physiology, 285, H251–258. 10.1152/ajpheart.01086.2002. [DOI] [PubMed] [Google Scholar]
- Saito, Y. , McKay, M. , Eraslan, A. , & Hester, R. L. (1996). Functional hyperemia in striated muscle is reduced following blockade of ATP‐sensitive potassium channels. American Journal of Physiology, 270, H1649–1654. 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- Sarelius, I. H. (1986). Cell flow path influences transit time through striated muscle capillaries. American Journal of Physiology, 250, H899–907. 10.1152/ajpheart.1986.250.6.H899. [DOI] [PubMed] [Google Scholar]
- Sarelius, I. H. , & Duling, B. R. (1982). Direct measurement of microvessel hematocrit, red cell flux, velocity, and transit time. American Journal of Physiology, 243, H1018–1026. 10.1152/ajpheart.1982.243.6.H1018. [DOI] [PubMed] [Google Scholar]
- Secomb, T. W. , Hsu, R. , & Pries, A. R. (1998). A model for red blood cell motion in glycocalyx‐lined capillaries. American Journal of Physiology, 274, H1016–1022. 10.1152/ajpheart.1998.274.3.H1016. [DOI] [PubMed] [Google Scholar]
- Segal, S. S. (2005). Regulation of blood flow in the microcirculation. Microcirculation, 12, 33–45. 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- Smaje, L. , Zweifach, B. W. , & Intaglietta, M. (1970). Micropressures and capillary filtration coefficients in single vessels of the cremaster muscle of the rat. Microvascular Research, 2, 96–110. 10.1016/0026-2862(70)90055-5. [DOI] [PubMed] [Google Scholar]
- Thomas, G. D. , Hansen, J. , & Victor, R. G. (1997). ATP‐sensitive potassium channels mediate contraction‐induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. Journal of Clinical Investigation, 99, 2602–2609. 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricarico, D. , Mele, A. , Lundquist, A. L. , Desai, R. R. , George, A. L. , & Conte, C. D. (2006). Hybrid assemblies of ATP‐sensitive K+ channels determine their muscle‐type‐dependent biophysical and pharmacological properties. Proceedings of the National Academy of Sciences, 103, 1118–1123. 10.1073/pnas.0505974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykocki, N. R. , Boerman, E. M. , & Jackson, W. F. (2017). Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Comprehensive Physiology, 7, 485–581. 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanelli, G. , & Hussain, S. N. (1994). Effects of potassium channel blockers on basal vascular tone and reactive hyperemia of canine diaphragm. American Journal of Physiology, 266, H43–51. 10.1152/ajpheart.1994.266.1.H43. [DOI] [PubMed] [Google Scholar]
- Welsh, D. G. , & Segal, S. S. (1996). Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circulation Research, 79, 551–559. 10.1161/01.res.79.3.551. [DOI] [PubMed] [Google Scholar]
- Wilson, D. F. , Erecińska, M. , Drown, C. , & Silver, I. A. (1977). Effect of oxygen tension on cellular energetics. American Journal of Physiology, 233, C135–140. [DOI] [PubMed] [Google Scholar]
- Xiang, L. , & Hester, R. L. (2009). Adipocyte‐derived factor reduces vasodilatory capability in ob‐/ob‐ mice. American Journal of Physiology. Heart and Circulatory Physiology, 297, H689–695. 10.1152/ajpheart.01327.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar, J. H. (1984). Biostatistical analysis, 2nd ed. Prentice‐Hall. [Google Scholar]


