Abstract
Inflammation is an essential part of the immune response; it has been found to be central to the disruption of kidney function in acute kidney injury, diabetic nephropathy, hypertension, and other renal conditions. One of the well‐known mediators of the inflammatory response is histamine. Histamine receptors are expressed throughout different tissues, including the kidney, and their inhibition has proven to be a viable strategy for the treatment of many inflammation‐associated diseases. Here, we provide an overview of the current knowledge regarding the role of histamine and its metabolism in the kidney. Establishing the importance of histamine signaling for kidney function will enable new approaches for the treatment of kidney diseases associated with inflammation.
Inflammation is central to renal pathophysiology. Here, we provide an overview of the current knowledge regarding the role of histamine, a well‐known mediator of inflammation, and its metabolism in the kidney.
![]()
1. THE KIDNEY AND INFLAMMATION
Inflammation is a complex adaptive reaction aimed at eliminating external or internal pathogenic stimuli and involves both cellular and humoral factors. The process of inflammation can be triggered by various stimuli. One of the classical pathways that can activate inflammation is recognition of the pathogen‐associated molecular patterns and endogenous damage‐associated molecular patterns (DAMPs), that lead to the production of inflammatory cytokines (Janeway & Medzhitov, 2002; Zindel & Kubes, 2020). On the one hand, inflammation initiates adaptive immunity‐related processes; on the other hand, later stages of the adaptive immune response can enhance inflammation (Cronkite & Strutt, 2018). Allergen‐mediated degranulation of mast cells, which leads to a release of pro‐inflammatory cytokines and vasoactive amines (primarily histamine), is yet another trigger of inflammation (Branco et al., 2018; Thangam et al., 2018). During the inflammatory response, the blood flow to inflamed tissues is enhanced as a result of vasodilation, capillary permeability is increased, leading to recruitment of leukocytes (mainly, neutrophils) to the area of inflammation (Medzhitov, 2008). The main function of phagocytes (including neutrophils) at the site of inflammation is to ingest and destroy foreign bodies and damaged cells (Medzhitov, 2008; Rosales & Uribe‐Querol, 2017). Typically, inflammation is resolved by elimination of the pathogen that initiated it, and tissue regeneration (Headland & Norling, 2015).
Although inflammation is critically important for our body's defense mechanisms and maintenance of homeostasis, it can also lead to a pathophysiological response and tissue damage. Effector cascades employed by phagocytes to eliminate the pathogens are accompanied by the release of proteolytic enzymes and reactive oxygen species (ROS), which can be detrimental for cells and tissues (Hunter, 2012; Medzhitov, 2008). Inflammation is an essential process for a variety of pathologies, including diseases that implicate the kidney, such as diabetes, hypertension, metabolic syndrome, polycystic kidney disease (PKD), chronic kidney disease, and acute kidney injury (AKI; Furman et al., 2019; Karihaloo, 2015; Matoba et al., 2019; Rabb et al., 2016). Stressors such as a high salt diet, high blood pressure, or high glucose levels can trigger renal tissue damage and evoke an inflammatory response (Mattson, 2019). Inflammation will then cause immune cell infiltration into the kidney and production of inflammatory cytokines, which increases oxidative stress and amplifies the damage by affecting the vascular response, producing pathologies of the glomerulus and tubules, and increasing the retention of sodium and water (Mattson, 2014, 2019). Histamine, a well‐known mediator of inflammation, could represent one of the important regulators of the renal tissue function in inflammatory response (Branco et al., 2018), which will be discussed in this review.
2. HISTAMINE METABOLISM, AND ITS POTENTIAL RENAL SOURCES
Histamine is a biogenic amine produced from the amino acid histidine through a removal of a carboxyl group, catalyzed by histidine decarboxylase (HDC; Huang et al., 2018). Figure 1 shows a schematic summary of histamine production and metabolism,and major enzymes involved in these processes. Many types of cells are capable of storing and releasing histamine, including mast cells, basophils, enterochromaffin‐like cells, histaminergic neurons, dendritic cells (DCs), macrophages, and even epithelial cells (Fultz et al., 2019; Huang et al., 2018; Schirmer et al., 2020; Tiligada & Ennis, 2020). When needed, histamine can be released from local stores in the tissue, or its de novo production may be triggered by a stimulus. The stored histamine is located mainly in mast cells and basophils; basophils are rare, short‐lived cells recruited to tissues upon inflammation, whereas mast cells are resident cells widely distributed throughout mucosal and connective tissues (Galli & Tsai, 2012; Hirasawa, 2019). In these cells, following its synthesis, histamine is stored in intracellular granules, in up to millimolar concentrations, until an activating stimulus triggers its release (Hirasawa, 2019; Huang et al., 2018). Histamine synthesis can also be induced in cells such as macrophages and neutrophils, from which it can be released in micromolar concentrations (Hirasawa, 2019). Histamine is metabolized via extracellular oxidative deamination catalyzed by diamine oxidase (DAO) or intracellular methylation by histamine‐N‐methyltransferase (HNMT; Comas‐Baste et al., 2020).
FIGURE 1.
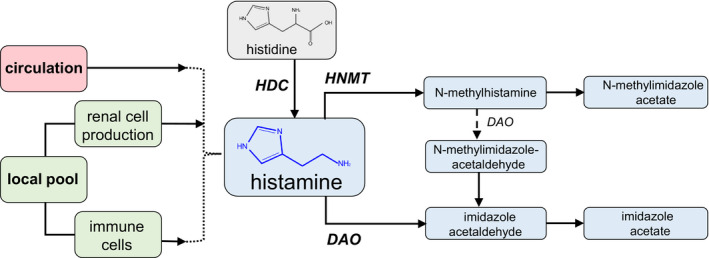
Summary of possible sources of renal histamine, and its metabolism. It is hypothesized that in the renal tissue, histamine can emerge from the circulation, or from a local pool—via intrarenal production by immune cells, such as mast cells, or renal epithelium. Histamine is formed from histidine with the help of HDC (histamine decarboxylase), and further histamine metabolites are formed by HNMT (histamine‐N‐methyltransferase) or DAO (diamine oxidase)
Multiple studies suggest that the main source of histamine in the kidney is local production. The level of the histamine metabolite N‐methylhistamine, resulting from the enzymatic activity of HNMT, is higher in the kidney as compared to most other organs (Zimmermann et al., 2011). In addition, it has been shown that histamine can be produced in isolated human glomeruli, in the absence of mast cells (Sedor & Abboud, 1984). HDC has been reported to be expressed in the human kidney, specifically in the proximal tubule cells, podocytes, distal tubules, collecting duct, and loop of Henle (according to Kidney Interactive Transcriptomics (KIT) data from Humphrey's laboratory (Wu et al., 2018)). In pregnancy, HDC is thought to have a functional role of increasing renal blood flow and recruiting immune cells to renal tissues (Morgan et al., 2006). Other local sources of renal histamine have been hypothesized, for instance, mast cells. Although the number of mast cells is constitutively low in the kidney, they can still release large amounts of histamine locally, whereas the circulatory level of histamine (10 nM) is much smaller than what was reported in the kidney (2 pmol/mg organ weight) (Grange et al., 2020; Krystel‐Whittemore et al., 2015; Zimmermann et al., 2011). Thus, the intrarenal production of histamine is likely, and has the potential to be changed during the implementation of the inflammatory response.
3. HISTAMINE RECEPTORS AND THEIR ASSOCIATED CASCADES
Histamine brings about physiologic changes by binding to its four G‐protein‐oupled receptor (GPCR) subtypes: H1–H4 receptors (H1R–H4R). The basic membrane topology of each receptor includes an extracellular N terminus, an intracellular C terminus, and seven transmembrane helices interconnected by three intracellular loops and three extracellular loops (Panula et al., 2015). The receptors are constitutively active, that is, are able to adopt an active conformation independent of ligand binding (Mehta et al., 2020), but differ in their localization and downstream signaling cascades, which allows for distinctive effects in various tissues and cell types. An overview of histamine receptors and generalized downstream cascades is shown in Figure 2. Each receptor has different binding affinities for histamine, with H1R and H2R having low affinity for histamine (micromolar range), whereas H3R and H4R exhibit high affinity (5–10 nM; Mehta et al., 2020; Panula et al., 2015). The cascades downstream of each receptor are likewise unique. For instance, H1R couples to Gαq/11 proteins and activates phospholipase C‐β (PLC‐β). This catalyzes the hydrolysis of phosphatidylinositol diphosphate (PIP2) into membrane diacylglycerol (DAG) and inositol 1,4,5‐trisphosphate (IP3; Mocking et al., 2016; Panula et al., 2015). DAG activates protein kinase C (PKC), which can increase vasodilation and activate the mitogen‐activated protein kinase (MAPK) pathway. IP3 mobilizes Ca2+ via activation of the receptors on the endoplasmic reticulum (ER). The mobilization of Ca2+ can then produce such diverse effects as further release of histamine, transcription of cytokines IL‐6 and IL‐8, and activation of nuclear factor kappa (Holden et al., 2007; Mocking et al., 2016).
FIGURE 2.
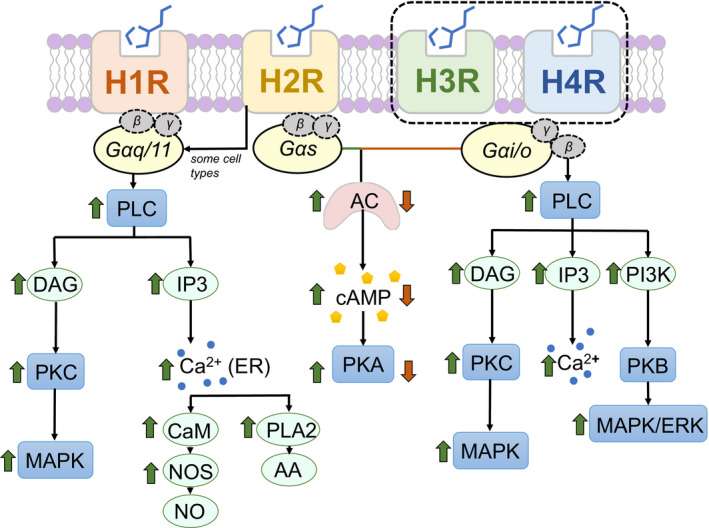
Generalized signaling pathways downstream of histamine receptors (HRs). Histamine binds to its four G‐protein‐coupled receptors: H1R, H2R, H3R, and H4R, and activates downstream signaling cascades depending on the type of G‐protein associated with each receptor. AA, arachidonic acid; AC, adenylyl cyclase; CaM, calmodulin; cAMP, 3′,5′‐cyclic adenosine monophosphate; DAG, diacylglycerol; IP3, inositol 1,4,5‐trisphosphate; MAPK, mitogen‐activated protein kinase; NOS, nitric oxide synthase; PI3K, phosphatidylinositol 3‐kinase; PKA, protein kinase A; PLA2, phospholipase A2; PLC, phospholipase C
In response to histamine, H2R binds to a different G‐protein, Gαs, and, less often, Gαq/11, resulting in activation of adenylyl cyclase (AC) and increased cAMP levels. cAMP then stimulates protein kinase A (PKA) and induces Ca2+ influx to the cell. H2R has been revealed to play a role in the immune response, as inhibition of the MAPK pathway activated by H2R leads to decreased production of pro‐inflammatory tumor necrosis factors (Mocking et al., 2016; Thomas et al., 2012). Contrary to H2R, signaling through the H3R receptor is coupled to pertussis toxin‐sensitive Gαi/o proteins, and results in the inhibition of AC and subsequent reduction in formation of cAMP, and reduced PKA activity. Gβγ subunits reduce Ca2+ entry through voltage‐gated calcium channels and possibly activate pathways involving PLC‐IP3‐Ca2+ and PKC. While the basic pathway mediated by the H4R receptor is similar to H3R, this receptor has additionally been shown to be implicated in intracellular Ca2+ release (Hofstra et al., 2003), cell migration (Ferreira et al., 2012; Mocking et al., 2016), PI3 kinase activation, and inflammatory cytokine and chemokine regulation (Jemima et al., 2014; Mehta et al., 2020).
The HRs receptors show differential expression throughout the body. H1R is expressed in the brain, smooth muscle, endothelial cells, DCs, monocytes, and B/T cells (Panula et al., 2015; Thangam et al., 2018). H2R was shown in the brain, smooth muscle, endothelial and epithelial cells, neutrophils, monocytes, and B/T cells (Monczor et al., 2017; Panula et al., 2015). H3R is mostly expressed throughout the mature brain, but also in salivary glands, respiratory epithelium, gastric mucosa, skin, heart, and kidney at the embryonic stage of development (Grange et al., 2020; Panula et al., 2015). The H4R is most strongly expressed in the bone marrow, but its expression at the mRNA level was detected in the spleen, thymus, lung, heart, kidney, epithelial cells, and peripheral blood. The widespread pattern of HR expression is further supported by functional data in numerous immune cells, including mast cells, neutrophils, DCs, and monocytes (Kay et al., 2018; Mehta et al., 2020; Panula et al., 2015; Thangam et al., 2018).
4. EFFECTS OF HISTAMINE ON RENAL PHYSIOLOGY AND PATHOPHYSIOLOGY
All four histamine receptors have been reported to express in the kidney, with each receptor showing a unique localization pattern. According to the KIT databases (Wu et al., 2018), in human renal tissues all four HRs can be found in the distal tubule, whereas podocytes, proximal tubules, loop of Henle, and collecting ducts express only H1R, H2R, and H4R, respectively (see Figure 3). KIT does not report H3R expression in human samples. In other species, H3R was demonstrated in the apical membrane of the principal cells of the collecting duct of the rat, whereas H4R was found at the apical membrane of the epithelial cells of the ascending loop of Henle and the proximal convoluted tubule (Grange et al., 2020; Rosa et al., 2013; Veglia et al., 2015). H1R and H2R are both expressed in the glomerular capsule, the mesangial cells, the proximal tubule, and the distal convoluted tubule (Grange et al., 2020; Veglia et al., 2015). H1R has also been found to be expressed in podocytes in humans (Grange et al., 2020). The better‐known HR is H1R, the first one to be found and originally known as “the'’ histamine receptor, now widely used for the treatment of allergies (Tiligada & Ennis, 2020). However, distinct avenues of research have been developed for each of the receptors; for instance, H2R is well known to be important for gastric acid secretion, H3R for neurotransmission, and H4R for immunomodulation. Each receptor is constitutively active, and the preferred method of treatment utilizing these receptors has been to block their activity with inverse agonists (Branco et al., 2018; Thangam et al., 2018). A list of some current pharmacology targeting histamine receptors, and specifically their known renal effects, are shown in Table 1.
FIGURE 3.
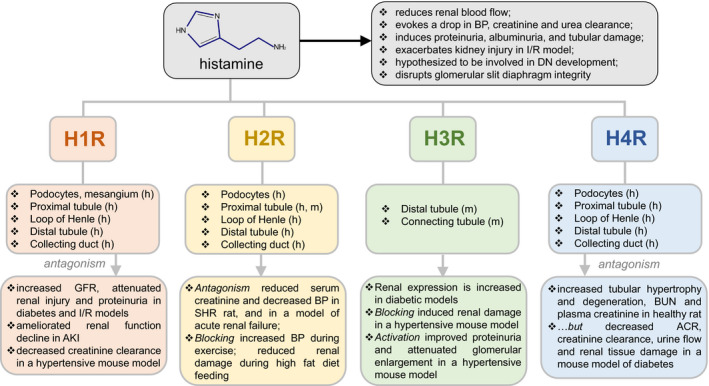
Known renal histamine receptors expression patterns and effects of their activation/antagonism in renal physiology. Shown are the known effects of histamine on renal physiology (gray), as well as the reported expression of the histamine receptors 1–4 (H1R, H2R, H3R, H4R) in various renal segments of mice (m) or humans (h) according to publicly available transcriptomic databases. The bottom row summarizes the effects of histamine receptors’ agonism or antagonism on renal physiology. ACR, albumin‐to‐creatinine ratio; AKI, acute kidney injury; BUN, blood urea nitrogen; DN, diabetic nephropathy; GFR, glomerular filtration rate; I/R, ischemia–reperfusion; BP, blood pressure
TABLE 1.
Pharmacology targeting histamine receptors and their combinations; reported are the drugs known to affect renal system function
| Drug | Mode of action | IC50/EC50/pKi | Delivery | Renal effects |
|---|---|---|---|---|
| H1R | ||||
| Cetirizine | Blocker | 226 nm (IC50) | Osmotic mini‐pump, 10 mg/kg/day | Decreased creatinine clearance in ANS mice model (Noguchi et al., 2020) |
| Desloratidine | Antagonist | 51 nm (IC50), 0.87 nM (Ki) | Oral gavage, 5 mg/kg | Decreased NF‐κB, TNF‐α, IL‐1β, creatinine, BUN, reduced histopathology in renal rat I/R model (Kocaturk et al., 2020) |
| Levocetirizine | Antagonist | 0.13 uM (IC50) | Orally, 0.5 mg/kg/day in 0.5% CMC | Increased GFR, attenuated renal hypertrophy, polyuria, proteinuria, elevation of serum creatinine and urea, kidney to body weight ratio, serum albumin, renal levels of TNF‐α and TGF‐β1, and reduced renal oxidative stress in rat diabetic model (Anbar et al., 2016) |
| Chlorpheniramine | Antagonist | 12 nM (IC50) |
pre‐treatment of immortalized podocytes, 10 μM (Veglia et al., 2016) ICV injection, 50 nmol (Jochem et al., 2016); IP injection, 20 mg/kg (Hattori et al., 2016) |
Protected slit diaphragm integrity in human immortalized podocytes in response to treatment with histamine (Veglia et al., 2016); reversed leptin‐induced rise in mean arterial pressure, heart rate, and renal blood flow in rat model of hemorrhagic shock (Jochem et al., 2016); reduced blood levels of IL‐6, IL‐1β, and TNF‐α, reduced tissue levels of IL‐1β, ILβ6, and TNF‐α mRNAs, reduced levels of NGAL, serum BUN, and creatinine in mouse model of sepsis (Hattori et al., 2016) |
| Rupatidine | Antagonist | 3.8 nM (IC50) | Oral, 3 and 6 mg/kg/day in CMC | Ameliorated diabetic nephropathy, improved histopathology, reduced fibrosis, and senescence markers in a rat model of streptozotocin‐induced diabetes (Hafez et al., 2020) |
| Bilastine | Antagonist | 64 nM (IC50) | Oral gavage in water solution, 1–30 mg/kg | Prevented the increase in ACR, restored creatinine clearance, and preserved junctional integrity in a rat model of streptozotocin‐induced diabetes (Verta et al., 2019) |
| Ketotifen | Antagonist | 1.3 nM (Ki) | IV injection, 1 mg/kg (Tong et al., 2016); IP injection, 1 mg/kg/day in 0.5% CMC (Reena et al., 2016) | Reduced histological injury score, BUN serum creatinine, IL‐6, and TNF‐α, downregulated expression of ICAM‐1, increased activity of SOD, in renal tissues in I/R rat model (Tong et al., 2016); attenuated HFD induced increase in body weight, kidney weight to body weight ratio, and in renal parameters (decrease in serum creatinine, increased levels in BUN, uric acid, K+, and microproteinuria, increase in systolic blood pressure), reversed HFD induced morphological changes in a HFD‐fed model in rats (Reena et al., 2016) |
| Mirtazapine | Antagonist | 9.3 (pKi) | Oral gavage, 20 mg/kg/day dissolved in 2 ml saline (Sahin et al., 2019); Orally via catheter, 20 mg/kg (Tok et al., 2012) | Ameliorated glomerular and tubular histology, decreased kidney expression of caspase‐1 and fraction of TUNEL‐positive cells (apoptosis) and kidney expression of NLRP3 and IL‐1β (inflammation) in a rat model of diabetes (Sahin et al., 2019); reduced levels of lipid peroxidation and oxidative injury products in renal tissue, improved histological appearance in a rat I/R model (Tok et al., 2012) |
| Meclizine | Blocker | 250 nM (Ki) | IP injection, 10, 30, 60, 100 mg/kg | Decreased serum creatinine, BUN, reduced tubular necrosis and kidney oxygen consumption, reduced inflammation, inhibited renal mitochondrial fragmentation in an IRI model in mice, attenuated cytochrome C release in tubular epithelial cells in vitro (Kishi et al., 2015) |
| Promethazine | Antagonist | 0.00091 μM (IC50) | 20 mg/kg in water | Mitigated renal function decline and renal tubular damage, reduced the renal lipid peroxidation, increased survival rate in mouse AKI model (Mishima et al., 2020) |
| H2R | ||||
| Famotidine | Antagonist | 33 nM (IC50) | Intraperitoneal injection (20 mg/kg) | Reduced blood levels of IL‐6, IL‐1β, and TNF‐α, reduced tissue levels of IL‐1β, IL‐6, and TNF‐α mRNAs, reduced levels of NGAL, serum BUN, and creatinine in mouse model of sepsis (Hattori et al., 2016) |
| Ranitidine | Blocker | 36–94 ng/ml (IC50) | Fed at 1.5 mg/30 g body weight | Reduced renal damage and attenuated atherosclerosis in a HFD mouse model (Liu et al., 2020) |
| Cimetidine | Antagonist | 70 nM (Ki) | IP injection of 150 mg/kg (Estaphan et al., 2015); 0.25–1.2 mM addition to cell‐free extract (Minai‐Tehrani et al., 2011) | Decreased creatinine, BUN, K+, Na+, NO, blood pressure creatine kinase, increased GFR, urine volume, and renal glutathione (Estaphan et al., 2015); improved renal function when used in combination with L‐carnitine in a rat model of glycerol induced acute renal failure (Minai‐Tehrani et al., 2011) |
| H3R | ||||
| Carcinine | Blocker | 0.29 μM (Ki) | 20 mg/kg/day via osmotic mini‐pump | Decreased creatinine clearance, LVFS, increased NGAL excretion, resulted in deterioration of cardiac and renal function in ANS mice (Noguchi et al., 2020) |
| Immethridine | Agonist | 0.85 nM (Ki) | 10 mg/kg/day via osmotic mini‐pump | Increased creatinine clearance, decreased urinary albumin and NGAL, kidney NGAL mRNA expression, and urinary β2‐microglubulin, decreased perivascular fibrosis, increased kidney collagen, attenuated glomeruli and protein cast enlargement, and mesangial growth; decreased proinflammatory signal gene expression in ANS mice (Noguchi et al., 2020) |
| H4R | ||||
| JNJ39758979 | Antagonist | 12.5 nm (Ki) | Water solution by oral gavage, 25, 50, 100 mg/kg/day | Decreased ACR, creatinine clearance, 24 h urine volume, and urine acidification, reduced immune infiltration and fibrosis, preserved Na+/H− exchanger 3 function in a mouse model of streptozotocin‐induced diabetes (Pini et al., 2018) |
| Toreforant | Antagonist | 8.4 nM (Ki) | Oral gavage, 3, 10, 100 mg/kg/day in 0.5% methylcellulose | Increased renal tubular epithelial cell vacuolation, hypertrophy, degeneration, luminal dilation in the outer medulla, increased kidney weight, BUN, and serum creatinine in a 100 mg/kg/day of toreforant‐treated rat model (Ma et al., 2017) |
| Conessine | Antagonist | 25 nM (Ki) | Oral administration, 10, 20, 50 mg/kg in water solution with combination with 7% Tween and 3% Ethanol | Increased alkaline phosphatase activity, bilirubin, urea, and creatinine concentration in liver and kidney tissues of a mouse model (Dua et al., 2013) |
| H3R/H4R | ||||
| Thioperamide | H3/H4 Antagonist | 25 nM [H3] and 27 nM [H4] | Lateral cerebral ventricular injection,20 μg/10 μl | Reversed the effects of anserine on renal sympathetic nerve activity, blood pressure, and heart rate (Tanida et al., 2010) |
Abbreviations: ACR, albumin‐to‐creatinine ratio; AKI, acute kidney injury; ANS, mice co‐treated with angiotensin II, nephrectomy, and salt; BUN, blood urea nitrogen; CMC, carboxymethyl cellulose; GFR, glomerular filtration rate; HFD, high‐fat diet; I/R, ischemia–reperfusion; ICAM‐1, intracellular adhesion molecule‐1; ICV, intracerebroventricular; IL‐6, IL‐1β, interleukin‐6, interleukin‐1 beta; IP, intraperitoneal; IRI, ischemia–reperfusion injury; IV, intravenous; LVFS, left ventricular fractional shortening; NF‐κB, nuclear factor kappa; NGAL, neutrophil gelatinase‐associated lipocalin; NO, nitric oxide; SOD, superoxide dismutase; TNF‐α, tumor necrosis factor‐α; TGF‐β1, transforming growth factor‐β1.
As a signaling molecule with a widespread receptor expression, histamine has effects throughout many systems (Mocking et al., 2016). In the skin, it can both amplify inflammation and participate in wound healing (Rossbach et al., 2016; Wolak et al., 2017). In the nervous system, histamine can promote the production of ROS and the loss of the mitochondrial membrane potential, and to both increase and dampen neuroinflammation (Chen et al., 2020; Kim & Song, 2017; Zhu et al., 2014). In the respiratory system, histamine can enhance the degree of fibrosis (Lucarini et al., 2016). It is the vasoactive and inflammation‐related functions of histamine that likely have the greatest relevance to renal function (Branco et al., 2018; Li et al., 2020).
Plasma histamine levels are increased in nephrotic syndrome, end‐stage renal failure, renal insufficiency, uremic pruritus, and in patients undergoing hemodialysis or peritoneal dialysis (Cao et al., 2018; Grange et al., 2020; Reszke & Szepietowski, 2018). The presence of mast cells has been correlated with loss of renal function and development of nephropathy, allograft rejection, fibrosis, and PKD (Grange et al., 2020; Holdsworth & Summers, 2008). Initial studies into the effects of histamine in the kidney were done in the 1930s with histamine challenge experiments. A low dose (0.3–0.5 mg) of histamine delivered by a subcutaneous injection resulted in an acute reduction of renal plasma flow, thought to be indicative of efferent arteriolar constriction (Bjering, 1937). A higher dose of histamine (1 mg) caused a fall in blood pressure and a drop in creatinine and urea clearance. The effect of histamine on glomerular filtration rate has been theorized to be a consequence of histamine‐mediated regulation of renal arteriolar constriction (Grange et al., 2020). Histamine has also been found to induce proteinuria, albuminuria, and degenerative tubular damage (Bjering, 1937). Furthermore, histamine was demonstrated to affect renal sympathetic nerve activity, with as low as 0.1 pM having a suppressive effect, and a 100 nM concentration having a stimulating effect (Tanida et al., 2007). In glomeruli, histamine not only induced the loss of foot processes but also disrupted cell‐to‐cell contacts and the slit diaphragm, which increased albumin excretion (Gurgen et al., 2013; Veglia et al., 2016). Histamine has also been shown to depolarize neurons that contain vasopressin, thus releasing it; vasopressin in turn stimulates water transport through the aquaporin channels (Ranieri et al., 2019). Therefore, high doses of histamine (25–500 μg i.c.v) elicit a dose‐dependent antidiuretic response with a concurrent rise in blood vasopressin in dogs (Bhargava et al., 1973).
The effect of histamine on the kidney seems to be particularly important for the development of ischemic AKI, diabetes, and hypertension. In models of AKI, administrations of DAO or HR antagonists resulted in a decrease in vascular permeability, and preservation of renal function and structural integrity (Kaneko et al., 1998). The use of cromoglicic acid, which inhibits the release of mediators by mast cells, likewise resulted in beneficial effects in this model (Tong et al., 2016). However, the use of compound 48/80, which induces histamine release, exacerbated the degree of kidney injury in the ischemic reperfusion model (Tong et al., 2016). Some studies of AKI, however, have shown a protective effect of histidine‐containing carnosine (dipeptide, beta‐alanyl‐L‐histidine), which was reversible with an H3R antagonist thioperamide (Kurata et al., 2006).
One of the leading causes of end‐stage renal disease is renal injury induced by diabetes. Lower expression or knockout of major inflammatory genes have been linked to protection against diabetes, and inhibition of inflammation has been suggested as a diabetes treatment, whereas pro‐inflammatory mediators are thought to induce diabetes progression (Hojs et al., 2016; Matoba et al., 2019; Thomas & Cherney, 2018). Histamine was also proposed to be involved in the pathogenesis of diabetic nephropathy. In earlier studies, a significant increase in the activity of HDC was reported in renal diabetic tissues (Gill et al., 1990; Markle et al., 1986). Both HDC and histamine deficiency delayed the onset of autoimmune diabetes, and decreased its incidence in the non‐obese diabetic mouse model (Alkan et al., 2015). H4R antagonism showed a protective effect in diabetic nephropathy, as indicated by reduced urine volume, pH, creatinine clearance, fibrosis, and fewer infiltrated immune cells (Pini et al., 2018; Rosa et al., 2013; Veglia et al., 2015). Treatment with H1R antagonists in a diabetic model resulted in a reduction of proteinuria and albuminuria (Anbar et al., 2016).
In hypertension, increased mean arterial pressure (MAP) causes damage to cardiac and renal tissues, which results in the release of endogenous DAMPs. In turn, DAMPs, via activation of TLRs, may induce de novo histamine production or histamine release by infiltrating or resident immune cells, and epithelial cells equipped with histamine synthesis machinery. Thus, antagonism of histamine receptors has been proposed for the management of cardiac damage in hypertensive diseases (Potnuri et al., 2018). H2R blocker famotidine reduced ventricular hypertrophy and improved cardiac function in a model of spontaneously hypertensive rats compared to beta‐blockers (Potnuri et al., 2018). H1R antagonist mepyramine and H2R antagonist cimetidine also inhibited a hypertensive response induced by acute restraint stress (de Almeida et al., 2015). An injection of ranitidine in embryos of the red‐footed tortoise resulted in a transient decrease in MAP and an increase in heart rate, whereas the introduction of histamine following ranitidine injection caused a transient increase in MAP and a decrease in heart rate (Crossley et al., 2013). The hypertensive effect of central injections of histamine is likely mediated by activation of the sympathetic nervous system and the release of hormones, including vasopressin, catecholamines, and angiotensin II (de Almeida et al., 2015). However, very little has been reported regarding the effects of HR antagonism or activation in renal tissues during hypertension. We can speculate about the potential effects of histamine in salt‐sensitive hypertension (SSH), a subset of hypertension affecting 30%–50% of hypertensive patients, in which a change in salt intake can produce meaningful differences in blood pressure. Salt‐sensitive (SS) subjects experience double the rate of fatal and nonfatal cardiovascular events versus non‐SS patients over the same time period (Elijovich et al., 2016; Mattson, 2014); renal inflammation is a crucial pathological feature for SSH. The latest studies suggest that a high‐salt diet produces neoantigens that lead to a release of pro‐hypertensive cytokines by the immune cells. These can trigger further cytokine release, which increases sodium retention and worsens vascular dysfunction, exacerbating the blood pressure increase (Lu & Crowley, 2018). An inflammatory response observed in SSH points to the potential importance of histamine in the orchestration of the events leading to renal damage; however, there is a gap in knowledge regarding the specific effects of histamine in this pathology.
5. CONCLUSION AND FUTURE DIRECTIONS
The latest research suggests that the role of histamine in the inflammatory response extends into modulation of kidney function. However, the overarching role of histamine in renal physiology, especially in renal epithelial function, has not yet been fully defined. In this review, we discussed the role of inflammation in kidney disease, as well as the importance of histamine and its receptors in renal physiology and pathology. Current literature lacks a consensus on the pattern of histamine receptors’ expression in the kidney, thus, rigorous studies with a variety of methodology and models are needed to fill this gap. Some future avenues of research may include selective in vivo blocking of each receptor, and subsequent examination of the affected pathways and related physiological functions, as well as an exploration of the exact effects of histamine in certain segments of the nephron. A determination of the precise role of histamine in renal physiology could potentially lead to novel therapies for renal inflammation‐accompanied diseases, including AKI, SSH, and diabetic nephropathy.
DISCLOSURES
The authors have no competing interests to disclose.
AUTHOR CONTRIBUTION
All authors participated in writing of the manuscript; the text is approved by all co‐authors. AVS and MVF contributed equally to this manuscript.
ACKNOWLEDGMENTS
This work was supported by NHLBI R01HL148115, the APS Lazaro J Mandel award (to DVI), and the RFBR 19‐015‐00211 (to AVS).
Anastasia V. Sudarikova and Mikhail V. Fomin have contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alkan, M. , Machavoine, F. , Rignault, R. , Dam, J. , Dy, M. , & Thieblemont, N. (2015). Histidine decarboxylase deficiency prevents autoimmune diabetes in NOD mice. Journal of Diabetes Research, 2015, 965056. 10.1155/2015/965056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anbar, H. S. , Shehatou, G. S. , Suddek, G. M. , & Gameil, N. M. (2016). Comparison of the effects of levocetirizine and losartan on diabetic nephropathy and vascular dysfunction in streptozotocin‐induced diabetic rats. European Journal of Pharmacology, 780, 82–92. 10.1016/j.ejphar.2016.03.035 [DOI] [PubMed] [Google Scholar]
- Bhargava, K. P. , Kulshrestha, V. K. , Santhakumari, G. , & Srivastava, Y. P. (1973). Mechanism of histamine‐induced antidiuretic response. British Journal of Pharmacology, 47, 700–706. 10.1111/j.1476-5381.1973.tb08196.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjering, T. (1937). The influence of histamine on renal function. Acta Medica Scandinavica, 91, 268–278. [Google Scholar]
- Branco, A. , Yoshikawa, F. S. Y. , Pietrobon, A. J. , & Sato, M. N. (2018). Role of histamine in modulating the immune response and inflammation. Mediators of Inflammation, 2018, 9524075. 10.1155/2018/9524075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, T. , Tey, H. L. , & Yosipovitch, G. (2018). Chronic pruritus in the geriatric population. Dermatologic Clinics, 36, 199–211. 10.1016/j.det.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Chen, Y. N. , Sha, H. H. , Wang, Y. W. , Zhou, Q. , Bhuiyan, P. , Li, N. N. , Qian, Y. N. , & Dong, H. Q. (2020). Histamine 2/3 receptor agonists alleviate perioperative neurocognitive disorders by inhibiting microglia activation through the PI3K/AKT/FoxO1 pathway in aged rats. Journal of Neuroinflammation, 17, 217. 10.1186/s12974-020-01886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas‐Baste, O. , Sanchez‐Perez, S. , Veciana‐Nogues, M. T. , Latorre‐Moratalla, M. , & Vidal‐Carou, M. D. C. (2020). Histamine intolerance: The current state of the art. Biomolecules, 10. 10.3390/biom10081181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronkite, D. A. , & Strutt, T. M. (2018). The regulation of inflammation by innate and adaptive lymphocytes. Journal of Immunology Research, 2018, 1467538. 10.1155/2018/1467538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley 2nd, D. A. , Sartori, M. R. , Abe, A. S. , & Taylor, E. W. (2013). A role for histamine in cardiovascular regulation in late stage embryos of the red‐footed tortoise, Chelonoidis carbonaria Spix, 1824. Journal of Comparative Physiology B, 183, 811–820. 10.1007/s00360-013-0746-3 [DOI] [PubMed] [Google Scholar]
- de Almeida, D. O. , Ferreira, H. S. , Pereira, L. B. , & Fregoneze, J. B. (2015). Hypertensive response to stress: The role of histaminergic H1 and H2 receptors in the medial amygdala. Physiology & Behavior, 144, 95–102. 10.1016/j.physbeh.2015.03.009 [DOI] [PubMed] [Google Scholar]
- Dua, V. K. , Verma, G. , Singh, B. , Rajan, A. , Bagai, U. , Agarwal, D. D. , Gupta, N. C. , Kumar, S. , & Rastogi, A. (2013). Anti‐malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malaria Journal, 12, 194. 10.1186/1475-2875-12-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elijovich, F. , Weinberger, M. H. , Anderson, C. A. , Appel, L. J. , Bursztyn, M. , Cook, N. R. , Dart, R. A. , Newton‐Cheh, C. H. , Sacks, F. M. , Laffer, C. L. , American Heart Association Professional and Public Education Committee of the Council on Hypertension , Council on Functional Genomics and Translational Biology , & Stroke Council . (2016). Salt sensitivity of blood pressure: A scientific statement from the American Heart Association. Hypertension, 68, e7–e46. 10.1161/HYP.0000000000000047 [DOI] [PubMed] [Google Scholar]
- Estaphan, S. , Eissa, H. , Elattar, S. , Rashed, L. , & Farouk, M. (2015). A study on the effect of cimetidine and L‐carnitine on myoglobinuric acute kidney injury in male rats. Injury, 46, 1223–1230. 10.1016/j.injury.2015.03.037 [DOI] [PubMed] [Google Scholar]
- Ferreira, R. , Santos, T. , Goncalves, J. , Baltazar, G. , Ferreira, L. , Agasse, F. , & Bernardino, L. (2012). Histamine modulates microglia function. Journal of Neuroinflammation, 9, 90. 10.1186/1742-2094-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz, R. , Engevik, M. A. , Shi, Z. , Hall, A. , Herrmann, B. , Ganesh, B. P. , Major, A. , Haag, A. , Mori‐Akiyama, Y. , & Versalovic, J. (2019). Phagocytosis by macrophages depends on histamine H2 receptor signaling and scavenger receptor 1. Microbiologyopen, 8, e908. 10.1002/mbo3.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman, D. , Campisi, J. , Verdin, E. , Carrera‐Bastos, P. , Targ, S. , Franceschi, C. , Ferrucci, L. , Gilroy, D. W. , Fasano, A. , Miller, G. W. , Miller, A. H. , Mantovani, A. , Weyand, C. M. , Barzilai, N. , Goronzy, J. J. , Rando, T. A. , Effros, R. B. , Lucia, A. , Kleinstreuer, N. , & Slavich, G. M. (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine, 25, 1822–1832. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli, S. J. , & Tsai, M. (2012). IgE and mast cells in allergic disease. Nature Medicine, 18, 693–704. 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, D. S. , Thompson, C. S. , & Dandona, P. (1990). Histamine synthesis and catabolism in various tissues in diabetic rats. Metabolism, 39, 815–818. 10.1016/0026-0495(90)90124-u [DOI] [PubMed] [Google Scholar]
- Grange, C. , Gurrieri, M. , Verta, R. , Fantozzi, R. , Pini, A. , & Rosa, A. C. (2020). Histamine in the kidneys: What is its role in renal pathophysiology? British Journal of Pharmacology, 177, 503–515. 10.1111/bph.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgen, S. G. , Erdogan, D. , & Take‐Kaplanoglu, G. (2013). The effect of histamine on kidney by fasting in rats. Bratislavske Lekarske Listy, 114, 251–257. 10.4149/bll_2013_052 [DOI] [PubMed] [Google Scholar]
- Hafez, H. M. , Abdel‐Hakeem, E. A. , & Hassanein, H. (2020). Rupatadine, a dual antagonist of histamine and platelet‐activating factor (PAF), attenuates experimentally induced diabetic nephropathy in rats. Naunyn‐Schmiedeberg's Archives of Pharmacology, 393, 1487–1500. 10.1007/s00210-020-01856-8 [DOI] [PubMed] [Google Scholar]
- Hattori, M. , Yamazaki, M. , Ohashi, W. , Tanaka, S. , Hattori, K. , Todoroki, K. , Fujimori, T. , Ohtsu, H. , Matsuda, N. , & Hattori, Y. (2016). Critical role of endogenous histamine in promoting end‐organ tissue injury in sepsis. Intensive Care Medicine Experimental, 4, 36. 10.1186/s40635-016-0109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headland, S. E. , & Norling, L. V. (2015). The resolution of inflammation: Principles and challenges. Seminars in Immunology, 27, 149–160. 10.1016/j.smim.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Hirasawa, N. (2019). Expression of histidine decarboxylase and its roles in inflammation. International Journal of Molecular Sciences, 20. 10.3390/ijms20020376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra, C. L. , Desai, P. J. , Thurmond, R. L. , & Fung‐Leung, W. P. (2003). Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. Journal of Pharmacology and Experimental Therapeutics, 305, 1212–1221. 10.1124/jpet.102.046581 [DOI] [PubMed] [Google Scholar]
- Hojs, R. , Ekart, R. , Bevc, S. , & Hojs, N. (2016). Markers of inflammation and oxidative stress in the development and progression of renal disease in diabetic patients. Nephron, 133, 159–162. 10.1159/000447434 [DOI] [PubMed] [Google Scholar]
- Holden, N. S. , Gong, W. , King, E. M. , Kaur, M. , Giembycz, M. A. , & Newton, R. (2007). Potentiation of NF‐κB‐dependent transcription and inflammatory mediator release by histamine in human airway epithelial cells. British Journal of Pharmacology, 152, 891–902. 10.1038/sj.bjp.0707457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth, S. R. , & Summers, S. A. (2008). Role of mast cells in progressive renal diseases. Journal of the American Society of Nephrology, 19, 2254–2261. 10.1681/ASN.2008010015 [DOI] [PubMed] [Google Scholar]
- Huang, H. , Li, Y. , Liang, J. , & Finkelman, F. D. (2018). Molecular regulation of histamine synthesis. Frontiers in Immunology, 9, 1392. 10.3389/fimmu.2018.01392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, P. (2012). The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Reports, 13, 968–970. 10.1038/embor.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway Jr., C. A. , & Medzhitov, R. (2002). Innate immune recognition. Annual Review of Immunology, 20, 197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Jemima, E. A. , Prema, A. , & Thangam, E. B. (2014). Functional characterization of histamine H4 receptor on human mast cells. Molecular Immunology, 62, 19–28. 10.1016/j.molimm.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Jochem, J. , Altinbas, B. , Yalcin, M. , Ottani, A. , Giuliani, D. , Savci, V. , Kasperska‐Zajac, A. , & Guarini, S. (2016). Involvement of the histaminergic system in the resuscitating effect of centrally acting leptin in haemorrhagic shock in rats. Journal of Physiology and Pharmacology, 67, 67–74. [PubMed] [Google Scholar]
- Kaneko, H. , Koshi, S. , Hiraoka, T. , Miyauchi, Y. , Kitamura, N. , & Inoue, M. (1998). Inhibition of post‐ischemic reperfusion injury of the kidney by diamine oxidase. Biochimica Et Biophysica Acta, 1407, 193–199. 10.1016/s0925-4439(98)00039-8 [DOI] [PubMed] [Google Scholar]
- Karihaloo, A. (2015). Role of inflammation in polycystic kidney disease. In Li X. (Ed.), Polycystic kidney disease, Chapter 14. Codon Publications. Available from https://www.ncbi.nlm.nih.gov/books/NBK373373/ [PubMed] [Google Scholar]
- Kay, L. J. , Suvarna, S. K. , & Peachell, P. T. (2018). Histamine H4 receptor mediates chemotaxis of human lung mast cells. European Journal of Pharmacology, 837, 38–44. 10.1016/j.ejphar.2018.08.028 [DOI] [PubMed] [Google Scholar]
- Kim, J. , & Song, J. H. (2017). Inhibitory effects of antihistamines, diphenhydramine and chlorpheniramine, on proton currents in BV2 microglial cells. European Journal of Pharmacology, 798, 122–128. 10.1016/j.ejphar.2017.01.032 [DOI] [PubMed] [Google Scholar]
- Kishi, S. , Campanholle, G. , Gohil, V. M. , Perocchi, F. , Brooks, C. R. , Morizane, R. , Sabbisetti, V. , Ichimura, T. , Mootha, V. K. , & Bonventre, J. V. (2015). Meclizine preconditioning protects the kidney against ischemia‐reperfusion injury. EBioMedicine, 2, 1090–1101. 10.1016/j.ebiom.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaturk, H. , Bedir, F. , Altay, M. S. , Bakan, E. , Suleyman, B. , Yazici, G. N. , Sunar, M. , Suleyman, Z. , & Suleyman, H. (2020). The effect of desloratadine on ischemia reperfusion induced oxidative and inflammatory renal injury in rats. Renal Failure, 42, 531–538. 10.1080/0886022X.2020.1769656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystel‐Whittemore, M. , Dileepan, K. N. , & Wood, J. G. (2015). Mast cell: A multi‐functional master cell. Frontiers in Immunology, 6, 620. 10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata, H. , Fujii, T. , Tsutsui, H. , Katayama, T. , Ohkita, M. , Takaoka, M. , Tsuruoka, N. , Kiso, Y. , Ohno, Y. , Fujisawa, Y. , Shokoji, T. , Nishiyama, A. , Abe, Y. , & Matsumura, Y. (2006). Renoprotective effects of l‐carnosine on ischemia/reperfusion‐induced renal injury in rats. Journal of Pharmacology and Experimental Therapeutics, 319, 640–647. 10.1124/jpet.106.110122 [DOI] [PubMed] [Google Scholar]
- Li, H. , Tang, C. , Zhu, X. , Zhang, W. , Abudupataer, M. , Ding, S. , Duan, C. , Yang, X. , & Ge, J. (2020). Histamine deficiency facilitates coronary microthrombosis after myocardial infarction by increasing neutrophil‐platelet interactions. Journal of Cellular and Molecular Medicine, 24, 3504–3520. 10.1111/jcmm.15037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Lai, L. , Lin, J. , Zheng, J. , Nie, X. , Zhu, X. , Xue, J. , & Liu, T. (2020). Ranitidine and finasteride inhibit the synthesis and release of trimethylamine N‐oxide and mitigates its cardiovascular and renal damage through modulating gut microbiota. International Journal of Biological Sciences, 16, 790–802. 10.7150/ijbs.40934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, X. , & Crowley, S. D. (2018). Inflammation in salt‐sensitive hypertension and renal damage. Current Hypertension Reports, 20, 103. 10.1007/s11906-018-0903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarini, L. , Pini, A. , Rosa, A. C. , Lanzi, C. , Durante, M. , Chazot, P. L. , Krief, S. , Schreeb, A. , Stark, H. , & Masini, E. (2016). Role of histamine H4 receptor ligands in bleomycin‐induced pulmonary fibrosis. Pharmacological Research, 111, 740–748. 10.1016/j.phrs.2016.07.037 [DOI] [PubMed] [Google Scholar]
- Ma, J. Y. , Snook, S. , Garrovillo, S. , Johnson, C. , & La, D. (2017). An immunohistochemical investigation of renal phospholipidosis and toxicity in rats. International Journal of Toxicology, 36, 386–394. 10.1177/1091581817726040 [DOI] [PubMed] [Google Scholar]
- Markle, R. A. , Hollis, T. M. , & Cosgarea, A. J. (1986). Renal histamine increases in the streptozotocin‐diabetic rat. Experimental and Molecular Pathology, 44, 21–28. 10.1016/0014-4800(86)90030-4 [DOI] [PubMed] [Google Scholar]
- Matoba, K. , Takeda, Y. , Nagai, Y. , Kawanami, D. , Utsunomiya, K. , & Nishimura, R. (2019). Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. International Journal of Molecular Science, 20. 10.3390/ijms20143393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, D. L. (2014). Infiltrating immune cells in the kidney in salt‐sensitive hypertension and renal injury. American Journal of Physiology. Renal Physiology, 307, F499–508. 10.1152/ajprenal.00258.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, D. L. (2019). Immune mechanisms of salt‐sensitive hypertension and renal end‐organ damage. Nature Reviews Nephrology, 15, 290–300. 10.1038/s41581-019-0121-z [DOI] [PubMed] [Google Scholar]
- Medzhitov, R. (2008). Origin and physiological roles of inflammation. Nature, 454, 428–435. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- Mehta, P. , Miszta, P. , Rzodkiewicz, P. , Michalak, O. , Krzeczynski, P. , & Filipek, S. (2020). Enigmatic histamine receptor H4 for potential treatment of multiple inflammatory, autoimmune, and related diseases. Life, 10. 10.3390/life10040050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minai‐Tehrani, D. , Khodai, S. , Aminnaseri, S. , Minoui, S. , Sobhani‐Damavadifar, Z. , Alavi, S. , Osmani, R. , & Ahmadi, S. (2011). Inhibition of renal alkaline phosphatase by cimetidine. Drug Metabolism Letters, 5, 197–201. 10.2174/187231211796904982 [DOI] [PubMed] [Google Scholar]
- Mishima, E. , Sato, E. , Ito, J. , Yamada, K. I. , Suzuki, C. , Oikawa, Y. , Matsuhashi, T. , Kikuchi, K. , Toyohara, T. , Suzuki, T. , Ito, S. , Nakagawa, K. , & Abe, T. (2020). Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. Journal of the American Society of Nephrology, 31, 280–296. 10.1681/ASN.2019060570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocking, T. A. M. , Bosma, R. , Rahman, S. N. , Verweij, E. W. E. , McNaught‐Flores, D. A. , Vischer, H. F. , & Leurs, R. (2016). Molecular aspects of histamine receptors. In Blandina, P. & Passani M. B. (Ed.), Histamine receptors: Preclinical and clinical aspects (pp. 1–49). Springer International Publishing. [Google Scholar]
- Monczor, F. , Copsel, S. , Fernandez, N. , Davio, C. , & Shayo, C. (2017). Histamine H2 receptor in blood cells: A suitable target for the treatment of acute myeloid leukemia. Handbook of Experimental Pharmacology, 241, 141–160. 10.1007/164_2016_8 [DOI] [PubMed] [Google Scholar]
- Morgan, T. K. , Montgomery, K. , Mason, V. , West, R. B. , Wang, L. , van de Rijn, M. , & Higgins, J. P. (2006). Upregulation of histidine decarboxylase expression in superficial cortical nephrons during pregnancy in mice and women. Kidney International, 70, 306–314. 10.1038/sj.ki.5001553 [DOI] [PubMed] [Google Scholar]
- Noguchi, K. , Ishida, J. , Kim, J. D. , Muromachi, N. , Kako, K. , Mizukami, H. , Lu, W. , Ishimaru, T. , Kawasaki, S. , Kaneko, S. , Usui, J. , Ohtsu, H. , Yamagata, K. , & Fukamizu, A. (2020). Histamine receptor agonist alleviates severe cardiorenal damages by eliciting anti‐inflammatory programming. Proceedings of the National Academy of Sciences of the United States of America, 117, 3150–3156. 10.1073/pnas.1909124117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula, P. , Chazot, P. L. , Cowart, M. , Gutzmer, R. , Leurs, R. , Liu, W. L. , Stark, H. , Thurmond, R. L. , & Haas, H. L. (2015). International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacological Reviews, 67, 601–655. 10.1124/pr.114.010249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini, A. , Grange, C. , Veglia, E. , Argenziano, M. , Cavalli, R. , Guasti, D. , Calosi, L. , Ghe, C. , Solarino, R. , Thurmond, R. L. , Camussi, G. , Chazot, P. L. , & Rosa, A. C. (2018). Histamine H4 receptor antagonism prevents the progression of diabetic nephropathy in male DBA2/J mice. Pharmacological Research, 128, 18–28. 10.1016/j.phrs.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Potnuri, A. G. , Allakonda, L. , Appavoo, A. , Saheera, S. , & Nair, R. R. (2018). Association of histamine with hypertension‐induced cardiac remodeling and reduction of hypertrophy with the histamine‐2‐receptor antagonist famotidine compared with the beta‐blocker metoprolol. Hypertension Research, 41, 1023–1035. 10.1038/s41440-018-0109-2 [DOI] [PubMed] [Google Scholar]
- Rabb, H. , Griffin, M. D. , McKay, D. B. , Swaminathan, S. , Pickkers, P. , Rosner, M. H. , Kellum, J. A. , Ronco, C. , & Acute Dialysis Quality Initiative Consensus XIII Work Group . (2016). Inflammation in AKI: Current understanding, key questions, and knowledge gaps. Journal of the American Society of Nephrology, 27, 371–379. 10.1681/ASN.2015030261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri, M. , di Mise, A. , Tamma, G. , & Valenti, G. (2019). Vasopressin–aquaporin‐2 pathway: Recent advances in understanding water balance disorders. F1000Research, 8, 149. 10.12688/f1000research.16654.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reena, , Kaur, T. , Kaur, A. , Singh, M. , Buttar, H. S. , Pathak, D. , & Singh, A. P. (2016). Mast cell stabilizers obviate high fat diet‐induced renal dysfunction in rats. European Journal of Pharmacology, 777, 96–103. 10.1016/j.ejphar.2016.02.066 [DOI] [PubMed] [Google Scholar]
- Reszke, R. , & Szepietowski, J. C. (2018). End‐stage renal disease chronic itch and its management. Dermatologic Clinics, 36, 277–292. 10.1016/j.det.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Rosa, A. C. , Grange, C. , Pini, A. , Katebe, M. A. , Benetti, E. , Collino, M. , Miglio, G. , Bani, D. , Camussi, G. , Chazot, P. L. , & Fantozzi, R. (2013). Overexpression of histamine H(4) receptors in the kidney of diabetic rat. Inflammation Research, 62, 357–365. [DOI] [PubMed] [Google Scholar]
- Rosales, C. , & Uribe‐Querol, E. (2017). Phagocytosis: A fundamental process in immunity. BioMed Research International, 2017, 9042851. 10.1155/2017/9042851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach, K. , Schaper, K. , Kloth, C. , Gutzmer, R. , Werfel, T. , Kietzmann, M. , & Baumer, W. (2016). Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy, 71, 189–197. 10.1111/all.12779 [DOI] [PubMed] [Google Scholar]
- Sahin, E. , Bektur, E. , Burukoglu Donmez, D. , Baycu, C. , Can, O. D. , & Sahinturk, V. (2019). Mirtazapine suppresses sterile inflammation through NLRP3‐inflammasome in diabetic rat kidney. Acta Histochemica, 121, 289–296. 10.1016/j.acthis.2019.01.007 [DOI] [PubMed] [Google Scholar]
- Schirmer, B. , Lindemann, L. , Bittkau, K. S. , Isaev, R. , Bosche, D. , Juchem, M. , Seifert, R. , & Neumann, D. (2020). Mouse colonic epithelial cells functionally express the histamine H4 receptor. Journal of Pharmacology and Experimental Therapeutics, 373, 167–174. 10.1124/jpet.119.264408 [DOI] [PubMed] [Google Scholar]
- Sedor, J. R. , & Abboud, H. E. (1984). Actions and metabolism of histamine in glomeruli and tubules of the human kidney. Kidney International, 26, 144–152. 10.1038/ki.1984.148 [DOI] [PubMed] [Google Scholar]
- Tanida, M. , Kaneko, H. , Shen, J. , & Nagai, K. (2007). Involvement of the histaminergic system in renal sympathetic and cardiovascular responses to leptin and ghrelin. Neuroscience Letters, 413, 88–92. 10.1016/j.neulet.2006.11.035 [DOI] [PubMed] [Google Scholar]
- Tanida, M. , Shen, J. , Kubomura, D. , & Nagai, K. (2010). Effects of anserine on the renal sympathetic nerve activity and blood pressure in urethane‐anesthetized rats. Physiological Research, 59, 177–185. 10.33549/physiolres.931623 [DOI] [PubMed] [Google Scholar]
- Thangam, E. B. , Jemima, E. A. , Singh, H. , Baig, M. S. , Khan, M. , Mathias, C. B. , Church, M. K. , & Saluja, R. (2018). The role of histamine and histamine receptors in mast cell‐mediated allergy and inflammation: The hunt for new therapeutic targets. Frontiers in Immunology, 9, 1873. 10.3389/fimmu.2018.01873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, C. M. , Hong, T. , van Pijkeren, J. P. , Hemarajata, P. , Trinh, D. V. , Hu, W. , Britton, R. A. , Kalkum, M. , & Versalovic, J. (2012). Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One, 7, e31951. 10.1371/journal.pone.0031951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, M. C. , & Cherney, D. Z. I. (2018). The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia, 61, 2098–2107. 10.1007/s00125-018-4669-0 [DOI] [PubMed] [Google Scholar]
- Tiligada, E. , & Ennis, M. (2020). Histamine pharmacology: From Sir Henry Dale to the 21st century. British Journal of Pharmacology, 177, 469–489. 10.1111/bph.14524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tok, A. , Sener, E. , Albayrak, A. , Cetin, N. , Polat, B. , Suleyman, B. , Akcay, F. , & Suleyman, H. (2012). Effect of mirtazapine on oxidative stress created in rat kidneys by ischemia‐reperfusion. Renal Failure, 34, 103–110. 10.3109/0886022X.2011.623499 [DOI] [PubMed] [Google Scholar]
- Tong, F. , Luo, L. , & Liu, D. (2016). Effect of intervention in mast cell function before reperfusion on renal ischemia‐reperfusion injury in rats. Kidney and Blood Pressure Research, 41, 335–344. 10.1159/000443437 [DOI] [PubMed] [Google Scholar]
- Veglia, E. , Grange, C. , Pini, A. , Moggio, A. , Lanzi, C. , Camussi, G. , Chazot, P. L. , & Rosa, A. C. (2015). Histamine receptor expression in human renal tubules: A comparative pharmacological evaluation. Inflammation Research, 64, 261–270. 10.1007/s00011-015-0807-z [DOI] [PubMed] [Google Scholar]
- Veglia, E. , Pini, A. , Moggio, A. , Grange, C. , Premoselli, F. , Miglio, G. , Tiligada, K. , Fantozzi, R. , Chazot, P. L. , & Rosa, A. C. (2016). Histamine type 1‐receptor activation by low dose of histamine undermines human glomerular slit diaphragm integrity. Pharmacological Research, 114, 27–38. 10.1016/j.phrs.2016.10.011 [DOI] [PubMed] [Google Scholar]
- Verta, R. , Grange, C. , Gurrieri, M. , Borga, S. , Nardini, P. , Argenziano, M. , Ghe, C. , Cavalli, R. , Benetti, E. , Miglio, G. , Bussolati, B. , Pini, A. , & Rosa, A. C. (2019). Effect of bilastine on diabetic nephropathy in DBA2/J mice. International Journal of Molecular Science, 20. 10.3390/ijms20102554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolak, M. , Bojanowska, E. , Staszewska, T. , Ciosek, J. , Juszczak, M. , & Drobnik, J. (2017). The role of histamine in the regulation of the viability, proliferation and transforming growth factor beta1 secretion of rat wound fibroblasts. Pharmacological Reports, 69, 314–321. 10.1016/j.pharep.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Uchimura, K. , Donnelly, E. L. , Kirita, Y. , Morris, S. A. , & Humphreys, B. D. (2018). Comparative analysis and refinement of human PSC‐derived kidney organoid differentiation with single‐cell transcriptomics. Cell Stem Cell, 23, 869–881.e8. 10.1016/j.stem.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, J. , Qu, C. , Lu, X. , & Zhang, S. (2014). Activation of microglia by histamine and substance P. Cellular Physiology and Biochemistry, 34, 768–780. 10.1159/000363041 [DOI] [PubMed] [Google Scholar]
- Zimmermann, A. S. , Burhenne, H. , Kaever, V. , Seifert, R. , & Neumann, D. (2011). Systematic analysis of histamine and N‐methylhistamine concentrations in organs from two common laboratory mouse strains: C57Bl/6 and Balb/c. Inflammation Research, 60, 1153–1159. 10.1007/s00011-011-0379-5 [DOI] [PubMed] [Google Scholar]
- Zindel, J. , & Kubes, P. (2020). DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annual Review of Pathology: Mechanisms of Disease, 15, 493–518. 10.1146/annurev-pathmechdis-012419-032847 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


