Abstract
RECENT SCIENTIFIC ADVANCES HAVE LED to a greater understanding of the neurobiological processes that underlie drug abuse and addiction. These suggest that multiple neurotransmitter systems may play a key role in the development and expression of drug dependence. These advances in our knowledge promise not only to help us identify the underlying cause of drug abuse and dependence, but also to aid the development of effective treatment strategies.
The chemicals that humans abuse are structurally diverse and produce different behavioural effects in the user. Nevertheless, all share the common feature that they can modulate the brain reward system that is fundamental to initiating and maintaining behaviours important for survival (e.g., eating, sexual activity).1 Researchers first postulated that specific neural circuits within the brain were involved in the regulation of reward processes when early studies demonstrated that rats would press a lever in order to obtain electrical stimulation of certain areas of the brain, but not others. The medial forebrain bundle (MFB), which connects the ventral tegmental area (VTA) to the nucleus accumbens (NAcc), was the site first identified in this way (Fig. 1). Other neurotransmitter pathways projecting from the VTA and the NAcc that innervate additional limbic (e.g., the amygdala) and cortical areas of the brain, which are important for the expression of emotions, reactivity to conditioned cues, planning and judgement (Fig. 1), have also been implicated in reward. Although the MFB consists of neurons that contain dopamine, noradrenaline and serotonin (5-HT), it is the dopaminergic projection that has been most closely implicated in reward. Thus, natural and artifical rewards (food, sex, drugs of abuse) have been shown to activate this dopaminergic pathway, also known as the mesolimbic dopamine pathway, causing an increase in dopamine levels within the NAcc. From an evolutionary perspective, this brain reward circuit has ensured survival by giving priority to essential actions such as reproduction. Drugs of abuse are able to exert influence over the brain reward pathway either by directly influencing the action of dopamine within the system, or by altering the activity of other neurotransmitters that exert a modulatory influence over this mesolimbic dopaminergic pathway. γ-Aminobutyric acid (GABA), opioid, serotonergic, cholinergic and noradrenergic neurotransmitter pathways have all been shown to interact at various points along the mesolimbic dopaminergic pathway and to modulate its activity. Some of the major elements in the brain reward circuit are illustrated in Fig. 2.
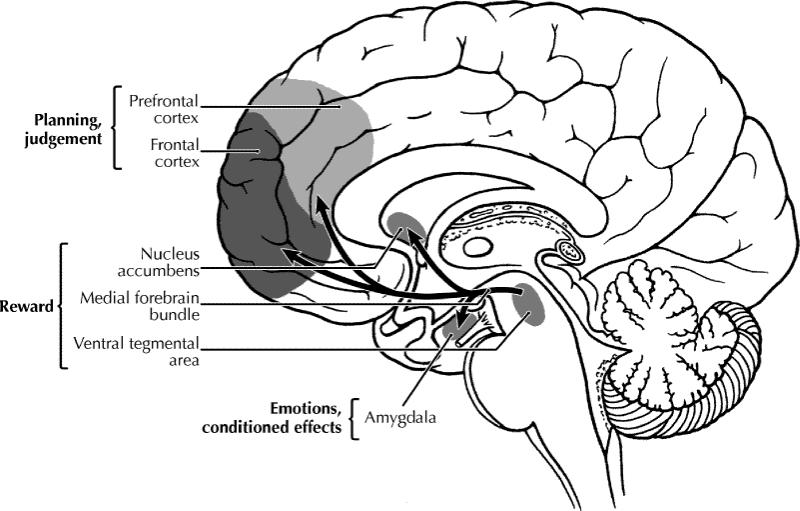
Fig. 1: Schematic diagram of the human brain that highlights some of the main brain areas and neurotransmitter pathways implicated in reward processes. Photo by: Lianne Friesen
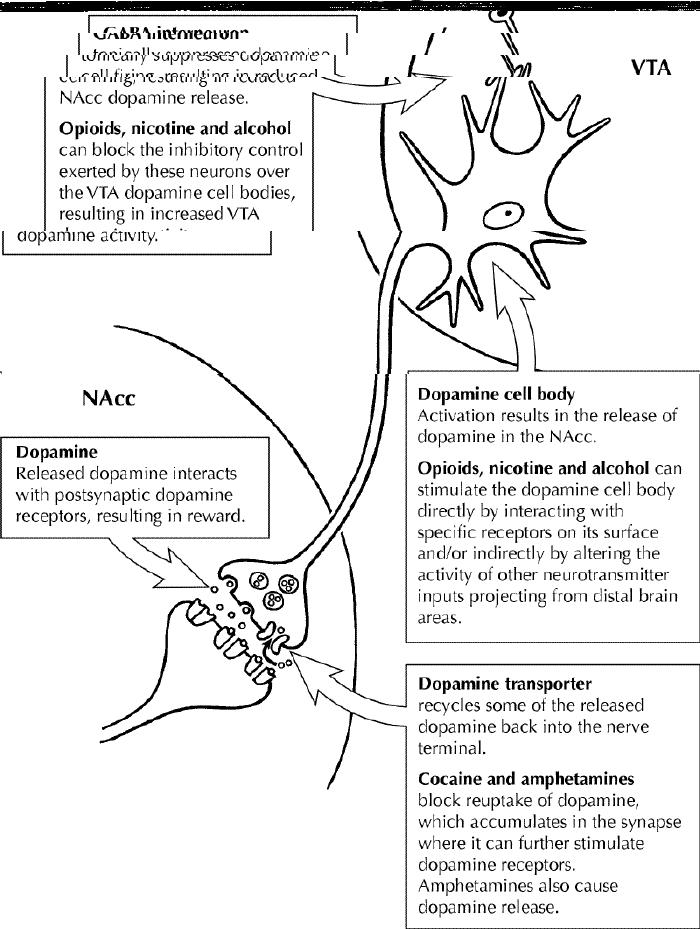
Fig. 2: Schematic diagram that represents the dopamine pathway projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc), indicating how substances of abuse can alter the activity of this pathway to produce their rewarding effects. Photo by: Lianne Friesen
Advances in our knowledge of the mechanisms by which various drugs of abuse alter the activity of this neuroanatomical system have helped our understanding of the neurobiological underpinnings of addiction and in the development of effective treatment strategies. To illustrate some of the advances in research and the application of this knowledge, a brief review of the literature is provided. This has been subdivided by drug class and presented in order of the prevalence of each drug's use in Canada.
Nicotine
Nicotine is the main psychoactive constituent found in tobacco that has been shown to be responsible for its behavioural and physiological effects, which can lead to addiction. Nicotine exerts its effects in the brain by acting on a specific type of receptor for the neurotransmitter acetylcholine, known as the nicotinic receptor. Some nicotinic receptors are located on the cell bodies of dopamine neurons within the VTA, and activation of these receptors increases the activity of these dopamine neurons, leading to an increase in dopamine release in the NAcc, which is thought to mediate reward.2,3 Nicotinic receptors are also located on other neurotransmitter inputs to the VTA and further increase dopamine release by removing the inhibitory influence that these other neurotransmitter inputs exert over the dopamine neurons (Fig. 2). The role of the mesolimbic dopamine system in nicotine reward has been clearly demonstrated in animals, because both the administration of dopamine blockers and the destruction of the mesolimbic dopamine pathway with selective neurotoxins attenuate nicotine self-administration.4
Similarly, research in humans suggests a role for dopaminergic processes in regulating the reinforcing effects of nicotine. In the limited number of studies conducted to date, administration of dopamine blockers has been found to alter smoking behaviour. However, in the case of humans, dopamine blockade leads to a compensatory increase in, and not suppression of, smoking.4 Other neurotransmitter systems have also been implicated, including the opioid and cholinergic systems. Thus, administration of either an opioid or nicotinic antagonist reduced smoking behaviour in some, but not all, studies reported.5,6
The most commonly used pharmacological approach for treating nicotine dependence is the use of nicotine replacement therapies, such as the nicotine patch, which reduce craving by maintaining plasma nicotine levels.7 However, further advances in our understanding of the neurobiological factors underlying nicotine dependence have led to the introduction of new pharmacotherapies for its treatment. The nonnicotine medication, bupropion, which was originally developed as an antidepressant, has recently been approved as an aid to smoking cessation. Its primary mechanisms of action are reported to be via interactions with the noradrenergic and dopaminergic systems implicated in nicotine dependence, however, the nature of its mechanism of action is poorly understood at the present time. Multiple clinical trials have demonstrated that bupropion is an effective aid in facilitating smoking cessation, although, in the majority of cases, the abstinence from smoking was not maintained in the long term.8
Alcohol
Along with nicotine, alcohol is one of the most commonly used psychoactive substances in Canada. Multiple neurotransmitter systems play a role in mediating the behavioural effects of alcohol that have been linked to its abuse and dependence.9 This undoubtedly reflects the fact that alcohol produces many pharmacological effects within the brain. Nonetheless, the mesolimbic dopamine system has been shown to play a role in the rewarding effects of alcohol. Alcohol is similar to other abused substances in that it increases NAcc dopamine release, and blocking the effects of dopamine reduces alcohol intake by animals.9 Furthermore, innate differences in central dopaminergic neurotransmission have been linked to high levels of alcohol drinking in selectively bred rodent lines.10 However, animals will continue to self-administer alcohol if the mesolimbic dopamine pathway is destroyed using a selective neurotoxin. This suggests that additional mechanisms are involved in regulating the rewarding effects of alcohol. Other neurotransmitter systems that have been implicated include the serotonergic, glutamatergic, GABAergic and opioid systems. Thus, alcohol directly binds to and modulates the activity of various specific receptors of these neurotransmitter systems (e.g., 5-HT3, GABAA and N-methyl-D-aspartate [NMDA] receptors) that are located within the brain reward pathway and can indirectly modulate mesolimbic dopamine activity via feedback mechanisms, for example, by increasing VTA activity through decreasing the suppressant influence that GABAergic inputs exert over them (Fig. 2).11
Altered central dopamine function has also been implicated as influencing the propensity for alcohol consumption in humans, at least in some populations.12 Human genetic studies suggest that an association exists between alcoholism and both the dopamine D2 receptor and the dopamine transporter. This is supported by brain imaging studies that have reported alterations in both D2 receptor and dopamine transporter densities in the brains of alcoholics.13,14,15 These findings could have important implications for treatment, because recent work suggests that increased density of D2 receptors may be a predictor of vulnerability to relapse in alcohol-dependent patients.16 As highlighted by animal studies, other neurotransmitter systems have similarly been implicated as underlying the propensity to consume alcohol in humans, and the newer pharmacotherapies that have been approved for the treatment of alcohol dependence mediate their effects via some of these systems.
The most common treatment strategies for alcoholism that are currently employed are psychosocial interventions and self-help groups (e.g., Alcoholics Anonymous), because the use of pharmacotherapies has been limited until recently by the lack of effective therapeutic agents. In the past, pharmacological agents were used mainly to alleviate the symptoms of acute withdrawal (e.g., benzodiazepines and β-blockers to reduce anxiety), to prevent the complications of withdrawal (e.g., anticonvulsant agents to prevent seizures) or to provide an aversive experience when alcohol was ingested (e.g., disulfiram).17 More recently, 2 drugs have been approved for clinical use that are aimed specifically at reducing alcohol consumption. Naltrexone, an opioid receptor antagonist, reliably reduces alcohol intake in preclinical animal studies, which is consistent with the importance of the opioid system in modulating the rewarding effects of alcohol. Within North America, naltrexone is the most widely used pharmacotherapy for the treatment of alcoholism. Initial clinical trials reported that naltrexone significantly improved abstinence rates and reduced relapse rates in recently abstinent alcoholics.18,19 Self-reports indicated that subjects had a reduced desire to drink and, in cases where alcohol was consumed, subjects reported a dampening in the hedonic properties of alcohol. More recent clinical trials, however, have suggested that compliance issues are important in determining treatment efficacy with naltrexone, and strategies for improving treatment outcomes are the subject of current research.20
Acamprosate is the drug of choice for the treatment of alcoholism in Europe. It is a structural analogue of the neurotransmitter GABA, which is reported to exert its effects mainly via an interaction with the NMDA receptor. As noted earlier, the NMDA receptor is one of the specific receptors located within the brain reward pathway to which alcohol directly binds, and it can indirectly modulate mesolimbic dopamine activity. This is relevant to alcoholism because hyperexcitability in the NMDA system has been shown to occur following long-term alcohol use and has been linked with the expression of withdrawal on cessation of drinking. Clinical testing of the efficacy of acamprosate in the treatment of alcoholism has been extensive.21,22,23,24,25 The outcomes of these studies have consistently demonstrated that the use of acamprosate significantly improves abstinence rates and reduces the number of relapses. In contrast to naltrexone, compliance issues do not appear to be as much of a concern with acamprosate. A large multicentre trial with acamprosate is currently underway in the United States.26
In contrast to many other abused substances, the effects of alcohol on the brain reward system are quite complex, as highlighted earlier, with many neurotransmitter systems being implicated. This presents unique challenges for the development of effective pharmacotherapies for the treatment of alcohol abuse and dependence. It is likely that pharmacological interventions directed at altering the influence of one neurotransmitter system on the rewarding effects of alcohol may have limited utility because of compensatory changes in the other regulatory neurotransmitter systems. This is supported by the experiences reported with various pharmacotherapeutic agents used singly in the clinical setting, in which small but significant improvements in alcohol-drinking indices were noted.17 Thus, a broader pharmacotherapeutic approach using a combination of drugs with different mechanisms may prove to be more effective. A recent study supports the notion that such a treatment strategy can improve patient outcomes27 and further improve compliance by reducing the reported frequency of side effects. Although there has been limited research in this area, these initial findings support the need for further work.
Stimulants
Stimulant drugs, such as cocaine and amphetamines, are substances that typically cause heightened feelings of well-being and euphoria, and an increased state of arousal. As with most drugs abused by humans, the psychostimulants are similarly self-administered by animals, and most of the information regarding their mechanism of action has been derived from animal research. From a biochemical perspective, the major mechanism by which the amphetamines and cocaine potentiate the actions of dopamine within the mesolimbic system is by inhibiting dopamine reuptake into the nerve terminals via the dopamine transporter.1,2 The importance of these pharmacodynamic effects within the brain with respect to drug-taking behaviour has been clearly demonstrated in animal models. For example, psychostimulants are self-administered to a lesser extent in animals in which lesions of the mesolimbic dopamine pathway have been produced by the application of a selective neurotoxin or when pretreated with dopamine receptor blockers.2 Furthermore, mice that have been bred to have no dopamine transporter (known as DAT knockout mice) are unresponsive to the stimulant effects of amphetamine and cocaine.28 These mice also fail to show elevations in extracellular dopamine levels following psychostimulant administration, clearly demonstrating the importance of the dopamine transporter in mediating the effects of the psychostimulants.
Although research in humans is more limited, the findings confirm that similar pharmacodynamic effects occur following psychostimulant administration in humans.29 Imaging studies have demonstrated that subcortical regions within the extended amygdala are activated both following cocaine infusion30 and in response to cue-induced cocaine craving.31 Research using positron emission tomography imaging techniques has shown that the reinforcing effects of psychostimulants are correlated with increases in dopamine concentrations in limbic regions due to blockade of the dopamine transporter and occupancy of dopamine D2 receptors.32,33 Chronic psychostimulant use has been shown to cause long-term changes in the function of the dopamine transporter34,35 and in D2 receptor levels, which may contribute to further psychostimulant abuse. Despite the extensive evidence for a primary role for the dopaminergic system in the stimulus–reward properties of cocaine and amphetamines, clinical trials of agents that modify dopamine release or block its receptors have been disappointing.36 More recently, however, a critical role for selective dopamine receptor subtypes (e.g., D1 receptors) in the self-administration and conditioned responses to cocaine suggests that agents directed at this receptor may be effective.37,38
Opiates
Opiate drugs, such as morphine and codeine, are commonly used in the clinical setting for pain relief. However, this class of psychoactive agent can also elicit intense euphoric effects followed by feelings of well-being in the user when taken in high doses, which can lead to their abuse and ultimately may result in addiction. Animal research suggests that, as with the psychostimulants, opiates appear to mediate their reinforcing effects by modulating the activity of the mesolimbic pathway, although not directly.39 The opiates enhance NAcc dopamine release by increasing the activity of VTA dopamine neurons. It is postulated that this is achieved via activation of mu-opioid receptors located on GABA neurons within the VTA, which play an important role in regulating the activity of VTA dopamine neurons. Opiates also have dopamine-independent effects within the NAcc, which play an important role in opiate reward.40 Evidence supporting these proposed mechanisms of action includes the observation that morphine and heroin self-administration can be modified by specifically blocking the actions of GABA in the VTA and opioid receptors in the NAcc.
Functional neuroimaging in opiate addicts confirms that heroin administration and heroin-related cues activate the same neural systems identified in preclinical animal studies,41 providing evidence that these systems modulate the direct and conditioned pharmacological effects of the drug. The latter effects are an important element of drug craving. In addition, pharmacological challenges with drugs interacting with dopamine receptors have demonstrated that the activity of brain dopaminergic systems has been altered by long-term opiate use in heroin addicts,42 and these changes are proposed to be related to the neural processes leading to abuse and dependence. The treatment strategies for opiate dependence using pharmacological agents have involved 3 different approaches. The most common strategy employed is a harm reduction approach by substituting less harmful long-acting, orally active opioid agonists for the more harmful abused substance (e.g., methadone and buprenorphine maintenance programs).43,44 Another approach has been to alleviate symptoms resulting from acute withdrawal (e.g., using clonidine), which has proved successful for facilitating detoxification.45 A final treatment strategy has been to block the pharmacological consequences of further opiate use following detoxification by administering an opioid blocker (e.g., naltrexone). However, clinical trials suggest that this approach is mainly acceptable to relatively opiate-free addicts who are highly motivated for change.46
Conclusion
The identification of the underlying biological mechanisms of drug dependence has lead to targeted drug development. In the case of alcohol, both the opioid and NMDA systems have been targeted and have resulted in marketed products, namely, naltrexone and acamprosate respectively. On the other hand, the discovery that bupropion is effective in treating tobacco dependence was clinical serendipity. This will, however, probably lead to the recognition of a new contributing neurochemical basis for nicotine dependence. Perhaps it is most surprising that there are no effective pharmacological interventions for cocaine or stimulant abuse despite our extensive knowledge about how they exert their effects in the brain. This may be a reflection of the fact that these substances cause long-term and often permanent changes in the brain. Current research efforts are now also focusing on exploring the neurobiological factors that may underlie relapse into drug-taking behaviour. This is an important issue because a successful treatment strategy should be effective in preventing relapse, which occurs with high prevalence in most drug- and alcohol-abusing populations.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Reprint requests to: Dr. Denise M. Tomkins, Centre for Addiction and Mental Health, 33 Russell St., Toronto ON M5S 2S1; fax 416 595-6922; denise_tomkins@camh.net
References
- 1.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron 1998;21: 467-76. [DOI] [PubMed]
- 2.Corrigall WA. Understanding brain mechanisms in nicotine reinforcement. Br J Addict 1991;86:507-10. [DOI] [PubMed]
- 3.Picciotto MR. Common aspects of the action of nicotine and other drugs of abuse. Drug Alcohol Depend 1998;51:165-72. [DOI] [PubMed]
- 4.Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology 1997;130:28-40. [DOI] [PubMed]
- 5.Perkins KA, Sanders M, Fonte C, Wilson AS, White W, Stiller R, et al. Effects of central and peripheral nicotinic blockade on human nicotine discrimination. Psychopharmacology 1999;142:158-64. [DOI] [PubMed]
- 6.Brauer LH, Behm FM, Westman EC, Patel P, Rose JE. Naltrexone blockade of nicotine effects in cigarette smokers. Psychopharmacology 1999;143:339-46. [DOI] [PubMed]
- 7.Jorenby DE. New developments in approaches to smoking cessation. Curr Opin Pulm Med 1998;4:103-6. [DOI] [PubMed]
- 8.Hughes JR, Goldstein MG, Hurt RD, Shiffman S. Recent advances in the pharmacotherapy of smoking. JAMA 1999;281:72-6. [DOI] [PubMed]
- 9.Koob GF, Weiss F. Neuropharmacology of cocaine and ethanol dependence. In: Galanter M, editor. Recent developments in alcoholism. vol 10. New York: Plenum Press; 1992. p. 201-33. [DOI] [PubMed]
- 10.Li TK. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J Stud Alcohol 2000;61:5-12. [DOI] [PubMed]
- 11.Grant KA. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav Pharmacol 1994;5:383-404. [DOI] [PubMed]
- 12.Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. Prog Neuropsychopharmacol Biol Psychiatry 1999;23:1171-212. [DOI] [PubMed]
- 13.Repo E, Kuikka JT, Bergstron KA, Karhu J, Hiltunen J, Tiihonen J. Dopamine transporter and D2-receptor density in late-onset alcoholism. Psychopharmacology 1999;147:314-8. [DOI] [PubMed]
- 14.Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 1996;20:1594-8. [DOI] [PubMed]
- 15.Tupala E, Hall H, Sarkioja T, Rasanen P, Tiihonen J. Dopamine-transporter denisty in nucleus accumbens of type-1 alcoholics. Lancet 2000;355:380. [DOI] [PubMed]
- 16.Guardia J, Catafau AM, Battle F, Martin JC, Segura L, Gonzalvo B, et al. Striatal dopaminergic D(2) receptor density measured by [(123)I]iodobenzamide SPECT in the prediction of treatment outcome of alcohol-dependent patients. Am J Psychiatry 2000;157:127-9. [DOI] [PubMed]
- 17.Gatch MB, Lal H. Pharmacological treatment of alcoholism. Prog Neuropsychopharmacol Biol Psychiatry 1998;22:917-44. [DOI] [PubMed]
- 18.Volpicelli JR, Alterman AI, Hayasgida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry 1992;49:876-80. [DOI] [PubMed]
- 19.O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. Arch Gen Psychiatry 1992;49:881-7. [DOI] [PubMed]
- 20.Swift RM. Drug therapy for alcohol dependence. N Engl J Med 1999;340: 1482-90. [DOI] [PubMed]
- 21.Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust, et al. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcohol 1990;25:613-22. [DOI] [PubMed]
- 22.Whitworth AB, Fisher F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, et al. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet 1996;347:1438-42. [DOI] [PubMed]
- 23.Pelc I, Verbanck P, Le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiatry 1997; 171:73-7. [DOI] [PubMed]
- 24.Poldrugo F. Acamprosate treatment in a long term community-based alcohol rehabilitation program. Addiction 1997;92:1537-46. [PubMed]
- 25.Geerlings PJ, Ansoms C, van den Brink W. Acamprosate and prevention of relapse in alcoholics. Eur Addict Res 1997;3:129-37.
- 26.Johnson BA, Ait-Daoud N. Neuropharmalogical treatments for alcoholism: scientific basis and clinical findings. Psychopharmacology 2000;149:327-44. [DOI] [PubMed]
- 27.Johnson BA, Ait-Daoud N, Prihoda TJ. Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypothesis to preliminary clinical evidence. Alcohol Clin Exp Res 2000;24:737-42. [PubMed]
- 28.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996;379:606-12. [DOI] [PubMed]
- 29.Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Barrio RA, Engel J, et al. Localization of neurochemical effects of cocaine and other stimulants in the human brain. J Clin Psychiatry 1988;49:23-6. [PubMed]
- 30.Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci 1999; 877:523-47. [DOI] [PubMed]
- 31.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999;156:11-8. [DOI] [PMC free article] [PubMed]
- 32.Volkow ND, Wang GS, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 1999;291:409-15. [PubMed]
- 33.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol 1999; 13:337-45. [DOI] [PubMed]
- 34.Little KY, Carroll FI, Butts JD. Striatal [125I]RTI-55 binding sites in cocaine-abusing humans. Prog Neuropsychopharmacol Biol Psychiatry 1998;22:455-66. [DOI] [PubMed]
- 35.Little KY, McLaughlin DP, Zhang L, McFinton PR, Dalack GW, Cook EH, et al. Brain dopamine transporter messenger RNA and binding sites in cocaine users: a postmortem study. Arch Gen Psychiatry 1998;55:793-9. [DOI] [PubMed]
- 36.Klein M. Research issues related to development of medications for treatment of cocaine addiction. Ann N Y Acad Sci 1998;844:75-91. [PubMed]
- 37.Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology 1999;143:102-10. [DOI] [PubMed]
- 38.Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, et al. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam. Arch Gen Psychiatry 1999;56:1101-6. [DOI] [PubMed]
- 39.Shippenberg TS, Elmer GI. The neurobiology of opiate reinforcement. Crit Rev Neurobiol 1998;12:267-303. [DOI] [PubMed]
- 40.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science 1988;242:715-23. [DOI] [PubMed]
- 41.Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. Eur J Neurosci 1999;11:1042-8. [DOI] [PubMed]
- 42.Casas M, Guardia J, Prat G, Trujols J. The apomorphine test in heroin addicts. Addiction 1995;90:831-5. [DOI] [PubMed]
- 43.Warner EA, Kosten TR, O'Connor PG. Pharmacotherapy for opioid and cocaine abuse. Med Clin North Am 1997;81:909-25. [DOI] [PubMed]
- 44.Effective medical treatment of opiate addiction. National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction. JAMA 1998;280:1936-43. [PubMed]
- 45.Gerra G, Marcato A, Caccavari R, Fontanesi B, Delsignore R, Fertonani G, et al. Clonidine and opiate receptor antagonists in the treatment of heroin addiction. J Subst Abuse Treat 1995;12:35-41. [PubMed]
- 46.Clinical evaluation of naltrexone treatment of opiate-dependent individuals. Report of the National Research Council Committee on Clinical Evaluation of Narcotic Antagonists. Arch Gen Psychiatry 1978;35:335-40. [PubMed]


