Abstract
Coronavirus disease-2019 (COVID-19), associated with the outbreak of deadly virus originating in Wuhan, China, is now a global health emergency and a matter of serious concern. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is rapidly spreading worldwide, and WHO declared the outbreak of this disease a pandemic on March 11, 2020. Though some of the countries have succeeded in slowing down the rate of the spread of this pandemic, most the countries across the globe are still continuing to experience an increasing trend in the growth and spread of this deadly disease. Hence, in the current scenario, is has now become essential to control and finally irradicate this deadly disease using an effective vaccine. One can expect the prominent role of already available antivirals, antibodies and anti-inflammatory drugs in the market, in this pandemic. Immunomodulatory and biological therapeutics are also in the high expectations to combat COVID-19. RNA based vaccines might be more advantageous over traditional vaccines, to deal with the pandemic threat. Aiming towards this direction, clinical trials for SARS-CoV-2 vaccine are currently underway all across the globe. Currently, about 150 health related organizations and research labs are in the progress for the evolution of COVID-19 vaccines, globally. The initial aim of these clinical trials is to assess vaccine’s safety, which is tested in Phase I/II/III studies where the primary outcomes typically examine the frequency of adverse effects. The vaccine is about to undergo phase III testing in several countries such as India, USA, South Africa, Brazil and England. US Government, under Operation Wrap Speed is even ready to sponsor three candidates, namely-The University of Oxford and AstraZeneca’s AZD1222; Moderna’s mRNA-1273; and Pfizer and BioNTech’s BNT162 for Phase III trials.
Keywords: COVID-19, Pandemic, Drug discovery, Vaccine, Clinical trial
Overview
Since earlier times, emergence or reemergence of numerous diseases, mainly viral diseases, have been adversely affecting the human life, tremendously increasing the mortality rate. Since twentieth century, numerous viruses have already emerged and have chances to emerge at an even greater rate in the future ahead. Viral infections such as HIV, influenza, hepatitis, chikungunya, Zika, Ebola etc. have always been a matter of great concern to the human health. Researchers, pharmaceutical companies and Medicare industry at large have always been addressing such problems by taking out suitable antiviral medicaments and vaccine that may be used for selective and sensitive detection of the viral loads [[1], [2], [3], [4]].
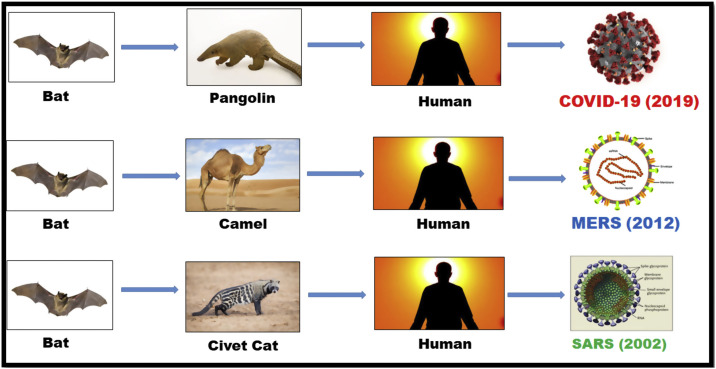
Coronaviruses (CoV), is responsible for the diseases like Severe Acute Respiratory Syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV). A novel coronavirus (nCoV) is said to be a “novel virus” as it has not been identified earlier in human beings [[5], [6], [7]]. Coronaviruses fall in the category of zoonotic, as the virus is transfused between animals and humans. Studies reveal that SARS-CoV and MERS-CoV were communicated from civet cats and dromedary camels to humans respectively. But the ultimate origin of the virus SARS-CoV-2, responsible for COVID-19, has been found to be bats with a possible unknown intervening host, may be pangolins, transferring the virus to humans. The fast spreading of COVID-19 worldwide, has demonstrated that this new disease has higher transmissibility compared with SARS-CoV and MERS-CoV. Though various types of coronaviruses are circulating in animals but have not yet infected the human beings [[8], [9], [10], [11]] (Fig. 1).
Fig. 1.
Transference of Human Coronaviruses (Intra- and Inter-organisms).
COVID-19 virus is very contagious and spreads from one infected person to another through respiratory droplets, commonly resulting from coughing, sneezing, and close personal contact etc. Respiratory symptoms, cough, fever, shortness of breath are the common symptoms of this infection. In extreme cases, the virus may lead to severe acute respiratory syndrome, pneumonia, kidney and lung failure or may even prove to be fatal.
Face masks and respirators are the first line of defence against SARS-CoV-2. Currently there are two good quality masks available in the market namely N-95 and N-99. Ideally these two categories of Masks are designed to protect from any particulate matter ≥2.5 μm. Only difference is that in case of N95 it should be capable of arresting 95% of the particulate matter while the N99 is designed to sieve out 99% of the particulate matter.
At this time of pandemic, safety measures are imperative and WHO has recommended several preventive measures like wearing facemask, washing hands regularly (at least 20 s each time), social distancing, hygiene, proper diet etc. It is also recommended to maintain a safe distance from a person displaying the symptoms of respiratory illness such as coughing and sneezing [[12], [13], [14]]. The doctors, medical and paramedical staff taking care of a corona patient is advised to take all precautionary measures and to wear Personal Protection Equipment (PPE), before coming in contact with such a patient.
This review paper aims to evaluate the requirements and desirable features of a successful Covid-19 vaccine candidate and therapies, as well as the methods surrounding its implementation all across the globe. The drugs, vaccines and herbal medicaments as antibodies and antivirals candidates for immunity boosters and viral inhibition are in continuous development and under investigation. Here, Table 1 has been given for the more understanding and elaboration of the literature.
Table 1.
Candidate vaccines against SARS-CoV-2 and their stages of development (adapted from WHO draft on vaccine candidates).
| Therapeutic intervention | Description | Countries | Trial sponsor(s) | Outcomes | Clinical Trails Status & References |
|---|---|---|---|---|---|
| Inactivated SARS-CoV-2 vaccine | Inactivated + Alum Vaccine |
China | Sinovac Biotech and Butantan Institute | Positive results from its Phase I/II clinical trial of CoronaVac, its vaccine against the viral load of SARS-CoV-2. Now procede for phase III. | Phase-III NCT04456595 |
| ChAdOx1 nCoV-19 | Adenovirus vaccine vector | UK, USA | University of Oxford, university’s Jenner Institute | Considered as the world’s most advanced candidate against COVID-19. | Phase-III ISRCTN89951424 |
| Recombinant Novel Coronavirus (2019-nCOV) Vaccine | Non-Replicating Viral Vector, Adenovirus Type 5 Vector |
China | CanSino Biological Inc./Beijing Institute of Biotechnology |
Revolutionized to Phase II/III studies. | Phase-III NCT04526990 |
| mRNA-1273 | mRNA vaccine | US | Moderna in collaboration with investigators from Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID), a part of the NIH. | mRNA vaccine against COVID-19 encoding for a prefusion stabilized form of the Spike (S) protein. Positive results in II/III phase of trails. | Phase-III NCT04583995 |
| Recombinant SARS CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | Protein Subunit | US, Sweden | Novavax | Positive and encouraging results after trials and successfully proceed further. | Phase-III NCT04611802 |
| INO-4800 | DNA plasmid vaccine with electroporation |
US | There was a togetherness of Inovio Pharmaceuticals and Beijing Advaccine Biotechnology Company in the work. | Inovio had stated that the preliminary Phase I results showed INO-4800 to be safe and tolerable and that 94% of the subjects demonstrated overall immunological response rates. | Phase-II/III NCT04642638 |
| Virus-Like Particle (CoVLP) | Plant-derived VLP adjuvanted with GSK Vaccine | Quebec City, Canada | Medicago, Biopharmaceutical company, Quebec City, Canada | After the first phase clinical trials to obtaining safety and immunogenicity results. | Phase-I NCT04450004 |
| mRNA vaccine BNT162 | mRNA based Vaccine | Global, US, Germany | Pfizer and BioNTech | Clinical programme development and fast track trails approval by FDA. | Phase-II/III NCT04368728 |
| COVAXIN (BBV152) | Whole-Virion Inactivated | India | Bharat Biotech | Positive outcome and fast track trails conducted. | Phase-III CTRI/2020/11/028976 & NCT04641481 |
| AG0301-COVID19 | DNA plasmid vaccine + Adjuvant |
Japan | Osaka University/ AnGes/ Takara Bio |
Positive results in phase ½ and Process for another phase. | Phase-II/III NCT04655625 |
| Remdesivir | Antiviral representative; counterpart of adenosine nucleotide obstructing RNA synthesis in SARS-CoV-2 | Global, Europe, USA, China, Japan | Gilead, WHO, INSERM, NIAID | Effective for Covid-19 can used in emergency situation. | Phase-III NCT04292730 |
| Favipiravir | Antiviral against influenza | China, Thailand | Fujifilm Holdings Corporation | Approved coronavirus drug in China | Phase-II NCT04358549 |
| Ribavirin | Antiviral & RNA polymerase inhibitors | China, Hong Kong | Manufactured by multiple companies | Triple Combination with ribavirin, lopinavir–ritonavir and interferon beta-1b for treating COVID infected patients | Phase-II NCT04276688 |
| (Arbidol) Umifenovir | Antiviral medication with binding capacity to hemagglutinin protein | China, Russia, India | Multiple Institute of China & Russia, Central Drug Research Institute (CDRI), India | An earlier study of hospitalized patients, not admitted to ICU (n = 81) took place in China and did not show a betterment in the health conditions of the patients. | Phase-II GDCT0379047, GDCT0379500, NCT04260594, NCT04255017 |
| Lopinavir and ritonavir | Antiviral, immune suppression, Protease Inhibitors | Global Solidarity and Discovery Trails, multiple countries | CEPI, WHO, UK Government, University of Oxford, INSERM | Recovery trial in finds no benefit with lopinavir-ritonavir in Covid-19. | DOI: 10.1056/NEJMc2008043 |
| Hydroxychloroquine, chloroquine | Antimalaria drugs | Global Solidarity | CEPI, WHO, INSERM | Not continued by WHO and also not possessing any clinical benefits in the patients hospitalized due to COVID-19. | Phase-III NCT04342221 and NCT04315948 |
| Corticosteroids | Anti-inflammatory drugs | China, UK, Italy | European Society of Intensive Care Medicine | In contradiction with the safety concerns in extreme virus and infection cases. | NCT04273321 |
| Adenovirus-based NasoVAX expressing SARS2-CoV spike protein | Non-Replicating Viral Vector | Birmingham (UAB) | Altimmune, biopharmaceutical company has collaborated with the University of Alabama | Altimmune shows positive preclinical results. | |
| CD24Fc | Biological immunomodulatory | Multiple sites in the United States | OncoImmune, Inc. | Interim efficacy and adverse events data from the phase III SAC-COVID trial in COVID-2019 infections released by OncoImmune | Phase-III NCT04317040 |
| Tocilizumab | Antibody | Global Solidarity | Roche Holding AG, Switzerland | Tocilizumab, an immunomodulatory drug that modifies the immune system or its for “restricted emergency use” on hospitalised COVID-19. | Phase-III NCT04356937 NCT04372186 |
| Ascorbic acid (Vitamin C) | Antioxidants and Protective Agents | China, Global solidarity | ZhiYong Peng, Zhongnan Hospital | Positive response and accepted results of trails will come. | Phase-II NCT04264533, NCT04363216 |
| JAK inhibitors (ruxolitinib, baricitinib) |
Cell Therapy | China, Italy, Canada | Fabrizio Cantini, Hospital of Prato | Mild to moderate COVID-19 infection receiving baricitinib combined with antiviral therapy. | Phase-II/III NCT04320277 DOI: 10.1183/13993003.01919-2020 |
| Convalescent plasma | Antibody therapy | India, China, Italy | Institute of Liver and Biliary Sciences, India and multiple hospital | Encouraging results were seen in phase I/II trails. | Phase-III NCT04425915, NCT04372979 |
History of treatments for coronavirus outbreaks
Middle east respiratory syndrome (MERS) and Severe Acute respiratory syndrome (SARS) are the two historical outbreaks of this coronavirus in the past two decades, and now the world is facing this novel coronavirus disease known as COVID-19 [15,16].
SARS was the first CoV outbreak that appeared between November 2002–July 2003 all over the world. It had a mortality rate of 9.6% and 8098 infected cases, 774 deaths were reported in 29 countries [17]. The disease was cured using broad-spectrum antibiotics, as no specific and effective antiviral medication was known [18], though a purine nucleoside analogue, Ribavirin, was tried as a broad-spectrum antiviral agent. Antiviral medicament such as lopinavir/ritonavir combination used as Human Immunodeficiency Virus (HIV) protease inhibitor was also tried to treat SARS-CoV as it was reported to have in-vitro antiviral activity [19]. Therapies included traditional Chinese medicine (TCM), interferons and immunomodulators like pentaglobulin, corticosteroid, convalescent plasma etc. were also found to be useful to treat SARS [20,21], though no efficient vaccine was developed. Even after the end of SARS in 2004, various suspected cases have been observed from time to time.
The MERS was another CoV outbreak that appeared between September 2012 and January 2020 all over the globe. It had a mortality rate of 34.4% and 2519 affected authenticated cases, 858 reported death cases in 29 countries globally [22]. Still, no prominent vaccine or treatment for MERS have been developed even after the investigation of several antiviral medicaments [23,24]. The phenomenal trial to treat MERS-CoV was initiated in July 2016, in which 194 patients participated. In this trial a mixture of interferon-β1b and Lopinavir/ritonavir was used on random basis to test the potency and efficiency of the drug combination on the patients under trial [25]. The results are not at all encouraging, as in the first phase of trial only three efficient vaccine candidates to combat MERS-CoV have been identified [26]. Moreover, it is very likely that even in near future, no MERS vaccine will be available in the market.
The COVID-19 pandemic outbreak was first recognized at Wuhan, China in December, 2019. It affected both health and economy of the entire world in a very short span of time [27]. Currently, there are more than 96.2 million substantiate COVID-19 cases with about 2.06 million death reported so far across the world, with a reported the recovered patients 69.8 million from COVID-19 [28]. The data reported is in approximation as there was not efficient testing to report the exact morbidity levels. Moreover, the treatments and therapies to combat the virus, were neither in the order of expectations, nor clinically proven to fight against the viral loads of SARS-CoV-2, making the treatments supportive only. Further, the WHO has not released the treatment guidelines to win over the virus. Several COVID-19 drugs and medications have been started to develop but everlasting result for the COVID‐19 affected patients continue to remain a hope.
Investigative COVID-19 drugs
For the deterrence and handling of COVID-19, life-saving vaccine or other effective medication has still not been introduced in the market but various health sector agencies like US Food and Drug Administration (FDA), World Health Organization (WHO), Chinese drug manufacturers and European Medicines Agency (EMA) are in coordination with researchers and medical industries for fast development of vaccines. Various vaccines, antiviral agents, antibodies and immunotherapies are under continuous investigation as capable therapy for COVID-19 infection [[29], [30], [31], [32]].
Investigating antiviral drugs
Remdesivir (GS-5734), an antiviral drug for ‘EBOLA’, has been initially declared to treat COVID-19 patients “under emergency use” by Emergency Use Authorization (EUA) of Food and Drug Administration (FDA), United State of America. Remdesivir has demonstrated mechanism to overcome genetic mutations and drug resistance in coronavirus. It imitates as a fragment of the RNA of the virus and gets added into the strand of RNA during the replication of the virus. Once this happens, further replication of the virus does not take place, leaving the RNA strand incomplete, further making the generation of critical parts of viral RNA impossible [33,34]. Beigel et al. [35] though have reported the results of the drug trial on Covid-19 patients, highlighting the decrease in the mortality rate and the squatter recovery time but fail to give ant statistical significance.
Favilavir, an oral anti-viral drug has been approved as a treatment for coronavirus by The National Medical Products Administration of China. The drug was originally manufactured by Fujifilm Toyama Chemical Ltd., Japan, for the treatment of influenza. Studies have revealed that favilavir gets activated on getting inside the cell and then finally get incorporated into the RNA of the virus, as the novel coronavirus is an RNA virus. The multiplication of the virus is restricted inside the body of the host, resulting in the reducing viral load inside the host cell [36]. In collaboration with the University of Massachusetts Medical School and Massachusetts General Hospital (Brigham and Women’s Hospital), the United States’ second phase trial will take up about fifty patients of COVID-19. Experimental data given by Qingxian Cai et al., [37] for COVID treatment with Favipiravir has not been substantiated as a game changers but is defiantly helpful in diminishing the load of the virus.
Ribavirin, an antiviral drug, is frequently used in amalgamation with other drugs for curing the viral hemorrhagic fevers and chronic hepatitis C viral infection (HCV), with the approval of FDA. It is a prodrug which is metabolized into nucleoside analogs which block the viral RNA synthesis and viral mRNA capping [38]. Currently, Ivan Fan-Ngai Hung et al. [39] have published trial reports that the combination of ribavirin with interferon and/or ritonavir/lopinavi, as a triple combination, is having a potential to combat COVID-19 virus.
Umifenovir (Arbidol), a small potent compound has been governed for decades in China and Russia, without any major untoward effects. The drug is reported to inhibit virus-associated cytokine dysregulation and pneumonia. This medication has a potential to act therapeutically against various viral and infectious diseases due to its broad-spectrum antiviral characteristics. Arbidol hinders viral entry into a cell of host’s body and stimulates enduring response. Moreover, it could suppress fusion between the cell membrane of the cell, which has been targeted and the viral capsid, thus averting the entry of the virus [40,41]. Umifenovir is used alone or in combination with other antiviral medication, in a few COVID-19 clinical studies. Recently, Zhen Zhu et al. reported that Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19 [42]. Recently, preliminary reports given by Jin-nong Zheng [43], China, testified that it unveiled compelling anti-COVID-19 characteristics in contrast to the mechanism with IC50 from 10 to 30 μM. And this is how, arbidol has been appraised for the therapeutic usage in COVID-19 cases. Central Drug Research Institute (CDRI), India, has secured approval for carrying out Phase III trial for the use of Umifenovir in the treatment of Covid-19.
Merimepodib is an Oral antiviral, developed by ViralClear Pharmaceuticals and is in second phase of trial together with remdesivir (ClinicalTrials.gov Identifier: NCT04410354). It’s usage to cure COVID-19 patients has even been cleared for trails by US FDA, for the patients who have been hospitalized with COVID-19 and require supplemental oxygen or are on non-invasive ventilation/high flow oxygen devices. Merimepodib acts by controlling the function of inosine-5′-monophosphate dehydrogenase (IMPDH), resulting in attenuation of guanosine that is used at the time of replication by the viral polymerase [44].
Nitazoxanide Nitazoxanide tablets (NT-300; Romark Laboratories) amalgamated with other antiviral medications obstruct the imitation of a wide range of viruses related to respiratory tracts in cell culture, including SARS-CoV-2 [45]. Two third phase trials for deterrence of COVID-19 are being introduced in high-risk populations, including healthcare workers and elderly residents enduring care facilities. Along with this step, a third trial towards the early medicaments of COVID-19 is intended (ClinicalTrials.gov Identifier: NCT04343248).
Ivermectin The recent report by Caly et al., [46] approved by FDA, describing the antiviral potential of ivermectin against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a potential candidates for COVID-19 treatment. According to the studies by Carlos Chaccour et al., [47] ivermectin considerably lower down the mortality rates in the patients receiving the drug with intense care.
Niclosamide, an anthelmintic agent, approved by FDA, is capable of showing the antiviral properties [48]. A trademarked formulation that decreases prolonged transmission and infection by targeting the viral pool in the gut has been synthesized. Commencement of a phase 2a or 2b research and study has been scheduled for the mid-2020 (ClinicalTrials.gov Identifier: NCT04399356).
Rintatolimod is a broad-spectrum antiviral agent. Roswell Park Cancer Institution in collaboration with AIM ImmunoTech Inc., US. examined Toll-like receptor 3 (TLR-3) agonist as a potential therapy for COVID-19 (ClinicalTrials.gov Identifier: NCT04379518).
Beta-d-N4-hydroxycytidine, a broad-spectrum antiviral drug [49] is bioavailable for oral intakes. It has been examined on SARS-CoV infected mice by both therapeutic and prophylactic administration, and has ended up to show reduced viral titer, a better pulmonary function and body weight loss. Very soon clinical trials on human body will be announced.
Bemcentinib is a selective oral AXL kinase impediment, which has shown to unveil potent antiviral activity against several cloaked viruses, including Zika virus and Ebola virus. A second phase of research and trials of the drug, in hospitalized COVID-19 patients, is premeditated as a part of the UK’s “ACCORD” i.e. Accelerating COVID-19 Research and Development inventiveness [50]. Recently, preclinical data suggest that bemcentinib is potentially useful for the treatment of early SARS-CoV-2 infection.
Plitidepsin (Aplidin) is effective to the nanomolar order [51], a fact revealed by the in-vitro studies of plitidepsin (Aplidin) on HCoV-229E. PharmaMar (MSE:PHM) reported in the research, at the Spanish National Research Council’s (CSIC) ‘Centro Nacional de Biotecnología’ (National Biotechnology Centre), that the results of this medication on human coronavirus HCoV-229E is very similar in propagation and multiplication mechanism to that of COVID-19 virus.
VIR-2703 (ALN-COV), identified as RNAi (RNA interference) curative development candidate, is used for the COVID-19 treatments [52], by Alnylam Pharmaceuticals, Inc. and Vir Biotechnology, Inc. RNA interference (RNAi) is a natural cellular procedure of gene silencing and the drug attacks small interfering RNA (siRNA), as shown by the in-vitro studies. The siRNA facilitate RNAi function by silencing mRNA i.e. messenger RNA which encodes or encrypts for disease-causing proteins. Advancements in the drug development as an inhalational drug formulation is in the future planning of most of the companies.
N4-Hydroxycytidine’s isopropyl ester prodrug is also known as EIDD-2801. This drug is an investigational, orally-bioavailable form of a potent ribonucleoside analog which inhibits the replication of multiple RNA viruses including SARS-CoV-2, the causative agent of COVID-19. Ridgeback has begun the enrolment for phase 2 Covid-19 trial of EIDD-2801 to check its Safety, Tolerability and Efficacy, in both inpatient and outpatient settings. (ClinicalTrials.gov Identifier: NCT04405570, NCT04405739)
Emetine hydrochloride In order to treat amebiasis, potent ingredient of ipecac syrup, which is given orally to prompt emesis, has come into existence as an injection. Clinical trials were conducted for varicella-zoster virus infection and viral hepatitis. It has been shown to have potency against RNA and DNA replicating viruses, including Ebola, Zika, Rabies Lyssavirus, HIV, CMV, echovirus, influenza A, HSV2 and metapneumovirus. Multiple genetically distinguished coronaviruses can also be inhibited by this latent drug usage. There are plannings to examine the antiviral activity and safety of emetine with blinded, randomized phase 2/3 and placebo-controlled multicenter tests in COVID-19 patients, with high-risk adults showing symptoms (Source: Acer Therapeutics Inc.).
AT-527 is an oral purine nucleotide prodrug, designed for inhibition of RNA polymerase enzyme. It has revealed both, in-vivo and in-vitro studies. Its antiviral activity and characteristics against numerous encased single-stranded RNA viruses, including coronaviruses and human flaviviruses is well established. IND for second phase study for patients admitted in hospitals with moderate COVID-19 symptoms has been accepted by FDA (ClinicalTrials.gov Identifier: NCT04396106).
Trabedersen is an antisense oligonucleotide that hinders transforming growth factor (TGF)-beta2 expression. The role of the drug is to facilitate the cell cycle arrest required for the viral replication. IND for second phase controlled, randomised, multicenter tests and trials has been acquiesced to FDA. (Source: Mateon Therapeutics, Inc.).
Stannous protoporphyrin is an effective antiviral agent. Renibus Therapeutics, Inc. has begun the phase 2 trial of stannous protoporphyrin and it’s effective results in the therapeutic role for the critical COVID-19 patients with either comorbidities (e.g., cardiovascular or kidney disease) or in old-age, has been demonstrated (ClinicalTrials.gov Identifier: NCT04364763).
Antroquinonol is an anti-inflammatory/antiviral medication which decreasers the viral protein synthesis and viral nucleic acid replication in both animal and cell experiments. It has also been demonstrated, during the treatment of mice with high inflammation, that this drug prevents tissue and organ damage. The antroquinonol drug from Golden Biotech, has been approved by FDA, for a second phase clinical trial on mild-to-moderate COVID-19 pneumoniatic patients (Source: Golden Biotech).
Protease inhibitors
In 2000, Ritonavir and Lopinavir, were approved by FDA. Both belong to a class of drugs and medicines called protease inhibitors, also known to target 3CLpro, a SARS-CoV nonstructural protein. During the SARS-CoV epidemic, the combination of these medications with ribavirin was dealing with the reduction of viral load and adversarial clinical death outcomes or ARDS when compared with controlled cases from past [19,39]. Currently, WHO reject the further recovery trial and Cao et al., have [53] reported that the lopinavir/ritonavir (n = 1596) intakes by hospitalized COVID-19 patients are not ending up with any kind of beneficial effects on comparison with the patients who received usual care (n = 3376).
Antimalaria drugs
Chloroquine, a quinoline derivative, was introduced into the field of drug and medicine in 1940s. It is an antimalarial agent [54,55]. It avoids virus-cell fusion by intruding with glycosylation of ACE2 receptor and on merging with spike protein it can impede the entry of nCoV-2. This mechanism of chloroquine suggests its efficiency as a therapeutic usage in initial stage of infection only. Hence, COVID-19 virus failed to get any chance to decrease the ACE2 activity and expression. But Borba et al. [56] have also highlighted the side effects of the drug. According to the authors, high intakes of chloroquine i.e. total dose of 12 g or 600 mg twice daily for 10 days, may lead to cardiac infarction and should not be acclaimed for handling COVID-19. Therefore, there are some safety and efficiency issues of these medications in preventing the virus of COVID-19.
Hydroxychloroquine (HCQ) falls into the class of medication of DMARD i.e. Disease-Modifying Anti-Rheumatic Drug. The drug is helpful in diminishing the pain and swelling in patients suffering from arthritis [57]. It was initially synthesized in India for the treatment of malaria. A latest study by Cochrane et al. [58] highlighted that this drug failed to give higher negative conversion rates, but its anti-inflammatory properties had decreased the symptoms and had helped in recovery of lymphopenia. However, UK Recovery trial and WHO Solidarity trial discontinued the usage of this drug in the month of June due to its failure in showing any clinical benefits in the COVID-19 patients [59,60].
Immunology frontiers
LEAPS COVID-19 immunotherapy, known to diminish consequential lung damage and COVID-19 viral load, is directed by Cellular Immunology CEL-SCI Corporation using Ligand Epitope Antigen Presentation System (LEAPS) peptide technology (Source: CEL-SCI Corporation).
Allogeneic Natural Killer (NK) cells - For the medicaments of adults with COVID-19, in April 2020, an investigational New Drug application (NDA) was approved by FDA. This drug exhibits a series of biological actions of NK cells, which includes activating receptors like DNAM-1, NKG2D and the natural cytotoxicity receptors like NKp46, NKp44, and NKp30, which bind to viral antigens and stress ligands of infected cells (ClinicalTrails.Gov: phase-I NCT04280224).
Bucillamine donates thiols for antioxidant revitalization and attenuate the cellular glutathione’s action which results in diminishing the clinical symptoms in the infected humans beings. A third phase trial, in the late April 2020, got the approval from FDA for the medicaments of COVID-19 patients showing mild-to-moderate symptoms (Source: Revive Therapeutics Ltd.).
Intravenous Immunoglobulin IV This immunotherapy got an acceptance from FDA in order to examine the safety in patients with the symptoms of COVID-19 and also to check its efficiency in the third phase trials. (Contemporary trials emphasizing on the complementary effects of low dose IVIG (0.5 g/kg for 5 days, NCT04261426).
Investigational antibody therapies
Antibody-based therapy is one viable treatment option targeting the immune system. Here, for the therapeutic and prophylactic treatment of COVID-19, the active strategies have been discussed in an approach to develop neutralizing antibodies for the inhibition of SARS-CoV-2 [61].
Anti-SARS-CoV-2 polyclonal H-IG (hyperimmune globulin): Japanese pharmaceutical company, “Takeda” is working on a plasma-derived therapy to treat high-risk patients of Covid-19. Alwis et al., [62] explain the impact of immune enhancement on Covid-19. Its therapeutic action works by concentrating on virus-specific plasma antibodies collected from cured COVID-19 patients.
Convalescent Plasma therapy: Convalescent Plasma therapy (CPT) is a process which entails the conglomeration of the blood plasma from a treated patient into COVID-19 affected patient and boost their fight against the virus. Binding of the conglomerated antibodies to the pathogen, leads to phagocytosis, cellular cytotoxicity, or direct counteraction of the pathogen [63,64]. This therapy is purposeful for the people suffering from a cytokine storm or critical patients with meagre oxygen saturation levels. According to the ICMR protocol, people with severe risk of cytokine storm, pneumonitis in extreme case along with breathlessness are on priority for the therapy. It is an already used procedure for numerous diseases but the potency for SARS-CoV-2 is still not known and is under investigation [65]. (ClinicalTrials.gov Identifier: NCT04425915 and NCT04372979).
Tocilizumab, also known as atlizumab, is an immunosuppressive drug, mainly for the treatment of rheumatoid arthritis (RA) and systemic juvenile idiopathic arthritis which is now beneficial in combating the COVID-19 virus also. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R) calming that ‘Cytokine Strom’ may lower the risk of COVID-19 death [66,67].
Covi-shield: Neutralizing Antibody Cocktail is developed by Sorrento, in order to get merged with different epitopes on Spike protein (SARS-CoV-2). The antibody cocktail efficiently develops a high barrier to emanation of resistant modifications in perfectly cured individuals. Covi-shield mAbs Fc areas are devised to exclude the interactions with host Fc receptors, and hence minimizing risk of Antibody Dependent Enhancement of SARS-CoV-2 viral load. (Source: Sorrento Inc.).
VIR-7831 & VIR-7832 bind to an epitope on SARS-CoV-2 that is in collaboration with SARS-CoV-1, and factualize that the epitope is strongly scrimped and not easy to modify. They are developed to act potentially as a T-cell vaccine with extended half-life (Source: Vir Biotechnology collaborating with Biogen and Generations Bio).
TZLS-501 For the handling and combating of COVID-19 virus, Tiziana Life Sciences is in process of developing a monoclonal antibody known by the name of “TZLS-501”. It helps in preventing increased levels of IL-6 and lung damage, thereby is also known as human anti-interleukin-6 receptor (IL-6R). The drug is beneficial in diminishing chronic lung inflammation by binding to IL-6R and deteriorating the quantity of IL-6 in the body (Source: UK-based biotechnology firm Tiziana Life Sciences).
Investigational vaccines for combating COVID-19
After the publication of the genetic sequencing of COVID-19 virus (SARS-CoV-2) on Jan 11, 2020, there was a rapid popping up of various studies and research, among the scientists with collaboration of researchers from biopharmaceutical industries, towards the production, confirmed tests and trials of phase II/III active COVID vaccine candidates. There are numerous methods which are used for the development of vaccine. Following are the few illustrations of the development process of vaccines globally.
ChAdOx1 nCoV-19 vaccine The Jenner’s institute of Oxford’s University produced an adenovirus candidate of vaccine, ChAdOx1 nCoV-19. Its clinical trial is scheduled to be performed in the Thames Valley Region. It is used to prevent replication and consists of a replication-deficient chimpanzee adenovirus vector (ChAdOx1), which delivers the S-protein. According to the results recently obtained by Oxford’s University research group [68,69], it is safe and successfully triggers an immune response against the coronavirus in human. The study showed that close to 90% of people developed neutralizing antibodies after consuming only one dose of the vaccine. There were no adverse side-effects of the vaccine. However, 70% of people on the trails developed either fever or headache, which could be managed with paracetamol. Vaccine is considered as the world’s most advanced candidate against COVID-19 in terms of development.
mRNA-1273: This mRNA vaccine encodes for a Spike (S) protein in a stabilized form inside the body of the target to generate an immune reaction. This was developed by Moderna in collaboration with investigators from Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID), a part of the NIH. This mechanism is expected to offer long lasting protection by affecting the binding of S-proteins of the coronavirus to prevent infection [70]. A preliminary report submitted by Jackson et al. [71] concluded that anti–SARS-CoV-2 immunity boosters were noted, using this vaccine, in all the targeted bodies and the identifications of concerns related to the trial’s safety were not studied and reported. This concluding statement further supports the vaccine’s development in future ahead. (Sponsored by the National Institute of Allergy and Infectious Diseases, NIAID and others; mRNA-1273 ClinicalTrials.gov identifier, NCT04405076 & NCT04283461).
mRNA vaccine BNT162 This vaccine is a nu-modmRNA i.e., nucleoside-modified messenger RNA and developed by BioNTech, which encodes for an enhanced SARS-CoV-2 receptor-binding domain (RBD) antigen. The trials of vaccine on humans were started in May 2020. Primary results [72] marked that the dose of this vaccine can be completely tolerated when given to the target body and according to the administered dosages, an immunogenicity can be developed inside the host body, quantified by the RBD-binding IgG dosage concentrations and antibody titers for neutralizing SARS-CoV-2. BNT162b1 is a mRNA vaccine which is clinically examined. (ClinicalTrials.gov Identifier: NCT04368728).
Covaxin Covaxin is developed by Bharat Biotech in association with NIV (National Institute of Virology) at ICMR, Indian Council of Medical Research. This is India’s native vaccine for combating COVID-19. Covaxin, an immobilize vaccine candidate, has been developed using SARS-CoV-2 particles that were eliminated and makes it unable to multiply in the people having the dosages of vaccine and hence avoid the chances of infection in them. The administration of vaccine doses also help in enhancing immunity by allowing the target body to generate antibodies against the killed virus. DCGI also approved the vaccine for Phase I & II Human Clinical Tests and Trials, results are very positive and encouraging after the trials. (Phase-I/II CTRI/2020/07/026300 and NCT04471519).
INO-4800: Inovio INO-4800 utilises innovate DNA Vaccine technology composed of modified plasmids to produce an immune response targeting the S-protein. The vaccine combat COVID-19 virus by triggering the immune response. The vaccine is being developed by the joint venture of The Inovio Pharmaceuticals and Beijing Advaccine Biotechnology Company. Moreover, the companies claimed that INO-4800 is effective in 94% of persons who ended up the “first” phase clinical trial voluntarily. Now, Inovio is thinking for a beginning of a Phase II/III trial to examine the potency of the vaccine in the coming summer days [73].
Nanoparticle SARS-CoV-2 vaccine Novel nanoparticle vaccine technology based on a self-assembling nanoparticle could prove to be a game changer in the development of a COVID-19 vaccine. The development of this vaccine involves the use of self-assembling protein nanoparticle (1c-SapNP) vaccine technology. The nanoparticle vaccine would induce the immune system to rapidly generate antibodies to neutralize and deactivate the coronavirus, offering a recipient protection against the real SARS-CoV-2 virus [74].
Inactivated SARS-CoV-2 vaccine: Chinese biopharmaceutical products company Sinovac Biotech developed the inactivated vaccine against Covid-19. This was a purified but not activated virus vaccine candidate for SARS-CoV-2 virus named by “PiCoVacc”, produced by Qiang Gao and his team [75,76] from China, which generated antibodies specific to the virus neutralization in non-human primates, rats and mice. These antibodies have a wide range of neutralizing activities against the COVID-19 virus globally, as this vaccine has efficiently diminishes 10 prototypical SARS-CoV-2 virus and strains. Two different dosages, 3 μg or 6 μg per dose were administered in macaques for combating the virus challenge and it leads to the conclusion that there was enhancement in immune responses with two distinct doses as partially or completely respectively and no amplification of infection have been reported. Regular examination of PiCoVacc via monitoring clinical manifestations, histophathological analys and hematological and biochemical index, in macaques indicates that it is secure. This report and conclusions support the speedy clinical blooming of SARS-CoV-2 vaccines for humans. (ClinicalTrials.gov Identifier: NCT04456595).
Recombinant Novel Coronavirus (2019-nCOV) Vaccine: To assess efficacy and safety of the recombinant adenovirus type-5-vectored COVID-19 vaccine manufactured by Beijing Institute of Biotechnology/CanSino Biological Inc., health care professionals were involved [77]. Positive Encouraging results have been obtained from its Phase I/II clinical trial against the SARS-CoV-2 virus, responsible for Covid-19. The work is in process for putting it for phase III trial, (ClinicalTrials.gov Identifier: NCT04341389 and ChiCTR2000031781).
Coronavirus Virus-Like Particle (CoVLP) vaccine: Medicago, Biopharmaceutical company, Quebec City, Canada, is manufacturing VLP (Virus-Like Particles), responsible for COVID-19 and are synthesizing drug candidates against the disease, after the first phase clinical trials in order to obtain safety and immunogenicity results. (ClinicalTrails.Gov: NCT04450004).
Adenovirus-based NasoVAX expressing SARS2-CoV spike protein: Altimmune, clinical-stage Biopharmaceutical Company, in association with the University of Alabama at Birmingham (UAB) synthesized AdCOVID, a single dosage of intranasal vaccine to cure COVID-19. The pre-clinical trial results reported the powerful serum countervailing action and efficient mucosal immunization in respiratory tract. The administration of IgA antibody in the target body is useful to resist both transmission and infection of the virus proliferating the COVID-19. (Source: Altimmune).
Immunomodulators experimental therapies
There are methodologies used for reprocessing the already existing medications and drugs. This technique of remodulating is responsible for the immunomodulators which are being successfully tested, in order to combat hyperinflammation caused by the release of cytokine. Janus kinase inhibitors, interferons and Interleukin (IL) inhibitors are few examples of the drugs that are under test and examination which might be useful in neutralizing the COVID-19 virus.
Interleukin-6 inhibitors The purpose of interleukins, which fall in the category of cytokines, is hindered by IL–inhibitors. Macrophages, monocytes, lymphocytes, and few other cells are playing a major role in the synthesis of interleukin and are known as immunoregulators. IL inhibitors are immunosuppressant. Interleukin (IL) inhibitors such as tocilizumab [67], sarilumab [78], siltuximab [79] have been reported to improve the conditions of damaged lung tissues, caused by the deliverance of cytokine, in the COVID-19 infected patients.
Janus-associated kinase (JAK) and numb-associated kinase (NAK) inhibitors: A class of proteins, cytokine, is involved in generating the inflammatory responses which intervene with the immune system of the infected patient. Drugs such as Baricitinib, Ruxolitinib with kinases AAK1, and BIKE in complex that target JAK and NAK may attenuate the usual and alveolar inflammation in such pneumoniatic patients by inhibiting the actual process of the cytokines [[80], [81], [82]].
Statins: It has been reported by the scientists, Bifulco [83] and Vincenzo Castiglione [84] that HMG-CoA reductase inhibitors (statins), are benignant in diminishing the COVID-19 caused inflammations, due to their ability to lower down the inflammation caused by atherosclerosis [101], along with their cholesterol-lowering activity.
Corticosteroids: Corticosteroids find place in major recommendations of the European Society of Intensive Care Medicine. They are the inflammation-inhibiting drugs, with a contrasting safety profile in extreme infections caused by viral loads [85]. Corticosteroids are in strong recommendation for the patients on ventilation suffering with Acute Respiratory Distress Syndrome (ARDS), with Cytokine Storm Syndromes (CSS) and with the hemophagocytic lymphohistiocytosis syndromes (HLH)(ClinicalTrials.gov Identifier: NCT04273321).
CD24Fc: CD24Fc is a biological immunomodulatory response to tissue injuries which is believed to be involved in autoimmune disease, cancer, graft-versus-host disease (GvHD) and metabolic syndromes [86]. US FDA approves IND application for CD24Fc in COVID-2019 infection. Mechanism data from the phase III SAC-COVID trial in COVID-2019 infections has been released by OncoImmune, National Institute of Neurological Disorders and Stroke; Ohio State University; OncoImmune; University of Michigan Comprehensive Cancer Center. (ClinicalTrials.gov Identifier: NCT04317040).
Dexamethasone: Dexamethasone is a generic steroid widely used in other diseases to reduce inflammation. As per the studies and reports of the drug, it may also lower down the inflammation caused, when immune system of the infected patient combat with the COVID-19 infection inside him [87,88]. It is a type of corticosteroids, which imitates cortisol to some extent. Cortisol is a hormone secreted by adrenal glands in human body. In the preliminary report [89] that is now also available in a pre-print server (Horby et al., medRxiv, June 22nd) and the UK-based RECOVERY,it is mentioned that the drug has minimized the death rates in the ventilated patients (up to by a third patient) and in the oxygen supported patients (up to by a fifth patients). Some harmful effects of the drug has also been studied.
Adjunctive therapy
Multiple ongoing clinical studies are evaluating the use of vitamin and mineral supplements for both the treatment and prevention of SARS-CoV-2 infection [90,91]. Grant et al. [92] published a review on evidence that vitamin ‘D’ supplements are required for proper functioning of the immune system and reduces the threat of influenza and COVID-19. Vitamin C shields the cells and tissues of a human body from dysfunction and oxidative damage, its ability to scavenge the impaired reactive oxygen species make it known to be the best antioxidant for the human body [93,94]. Recently, China has registered a new clinical trial (clincialtrials.gov Identifier: NCT04264533 & NCT04363216) to assess the action of vitamin C against a potential viral load of COVID-19. Zinc is a producer of WBC in the infected body and also known as an immune-booster. Due to its capability to minimize the replication of the virus, zinc is currently being examined for prevention and handling of COVID-19 cases [95,96].
Ayurveda treatment
Ayurveda, being the science of life, propagates the gifts of nature in maintaining healthy and happy living by improving the quality of standard care. Till now, there is no highly potential drug or medication in the market which can unroot the cause of the COVID-19 disease completely. In this critical times, it will be better to take ayurvedic medicines and preventive measures to boost immunity to stop the coronavirus from entering your body [[97], [98], [99]]. As per the report of WHO, around 80% population of the country is utilizing the herbal plants as the immunity-booster and to cure number of diseases. Various natural products produce important and useful ingredients like fennel, oregano oil, garlic, Echinacea, peppermint, sambucus, astragalus, licorice, ginger, turmeric, tulsi (ocimum tenuiflorum), etc. which contain different polyphenols, alkaloids, flavones, etc. These substances get involved into combating various viral loads and diseases as they generate interferons and antibodies in the infected or targeted person. It increases the amount of phagocytosis, protecting agent from foreign unfavorable particles and also resists ACE2 to protect us from CoV-19 [[100], [101], [102], [103], [104]].
Mechanism of action of SARS-CoV2 [105,106]
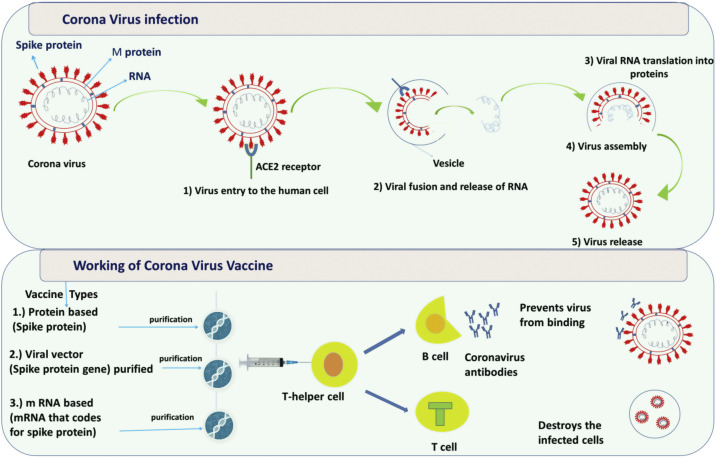
The mechanisms of infection by the SARS-CoV2 are not clear yet, however, it is genetically similar to SARS-CoV and other coronaviruses [107]. Hence the common mode of pathogenicity is being explored for targets of treatment. The therapeutic targets depending on the common mode of pathogenicity can be classifed as virus-related targets and host-related targets. The mechanism of coronavirus entry and the working of vaccines against Covid-19 is shown in Fig. 2 . The basic structure of coronavirus comprises of structural proteins- Spike protein and M (membrane) protein and a single stranded RNA. The coronavirus enters the human body with the spike (S) protein which attaches to angiotensin converting enzyme 2 (ACE2) receptors on the surface of human cells. Once it enters the cell, it fuses with the vesicle and then the viral genetic material, RNA, is fully released into the cytoplasm. Following, viral RNA genome is translated to generate replicase proteins. In cytoplasm, encapsidation of these replicase proteins result in the self-assembly of new virions. Finally, virions are released from the infected cells.
Fig. 2.
Overview of the reprocessed medicated drugs examined in clinical trial against COVID-19 taking into consideration the host pathways and mechanism of virus replication.
How coronavirus vaccines work
Scientists have taken genes from different viruses or viral parts (spike protein, RNA) and put them into harmless virus to make a vaccine. Broadly, these vaccines can be categorized into three types such as protein-based, viral vector and mRNA-based vaccines. Protein-based vaccines: Virus’s spike protein is purified and directly injected into body. Viral vector vaccines: A virus such as measles or adenovirus is genetically engineered and injected. mRNA-based vaccines: mRNA that codes for spike protein is purified and injected.
When the vaccine is injected into the body, it provokes the immune system to produce antibodies and activate T-helper cells. T-helper cells enable other immune responses namely B cells and T cells. B cells are responsible for making antibodies that can block the coronavirus from binding to the cells or for marking the virus for destruction. The function of T cells is to identify and destroy coronavirus-infected cells. If the patient encounters coronavirus again, these long-lived ‘memory’ B and T cells are triggered to fight the virus, thus providing immunity.
Conclusion
Due to the persisting global Covid-19 pandemic, researchers worldwide are working rapidly to identify and develop a viable vaccine candidate. At present, more than 200 prophylactic vaccines are in development against Covid-19. A Covid-19 vaccine candidate would first need to demonstrate a more than adequate safety profile. Vaccines for “COVID-19” responsible virus, SARS-CoV-2, are in the development process and also in the process of different clinical examinations and trials by various labs and health institutions on a global basis. It is also concluded that the reports and studies of the antivirals like lopinavir-ritonavir and remdesivir, which are already in the picture, are not that much effective and promising against SARS-CoV-2. A mild respiratory disease in the majority of cases have been marked in the case of COVID-19, but the studies of some cases have demonstrated that cytokine activation leads to sepsis and Acute Respiratory Distress Syndrome (ARDS) in the host cell, which may result to mortality and morbidity. An active exploration of biologics and treatments on the basis of immunomodulation, for the therapeutic usage in the COVID-19 cases has also been concluded here. Moreover, the severe effects by steroidal and nonsteroidal anti-inflammatory drugs (NSAIDs) has also been noticed and hence been discouraged to get into the use. It is in great hope that vaccines and specific medicaments targeting SARSCoV-2 will be in the world’s health market in near future.
Funding
No funding sources.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Olival K.J., Hosseini P.R., Zambrana-Torrelio C., Ross N., Bogich T.L., Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A., Kumar S., Kumar R., Choudhary A.K., Kumari K., Singh P., et al. COVID-19: emergence of infectious diseases, nanotechnology aspects, challenges, and future perspectives. ChemistrySelect. 2020;5:7521–7533. doi: 10.1002/slct.202001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao M., Zhang H., Liu K., Gao G.F., Liu W.J. Human T-cell immunity against the emerging and re-emerging viruses. Sci China Life Sci. 2017;60:1307–1316. doi: 10.1007/s11427-017-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabaan A.A., Al-Ahmed S.H., Haque S., Sah R., Tiwari R., Malik Y.S., et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28:174–184. [PubMed] [Google Scholar]
- 6.Hon K.L., Leung K.K.Y., Leung A.K.C., Sridhar S., Qian S., Lee S.L., et al. Overview: the history and pediatric perspectives of severe acute respiratory syndromes: novel or just like SARS. Pediatr Pulmonol. 2020;55:1584–1591. doi: 10.1002/ppul.24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halaji M., Farahani A., Ranjbar R., Heiat M., Dehkordi F.S. Emerging coronaviruses: first SARS, second MERS and third SARS-COV-2. Epidemiological updates of COVID-19. Infez Med. 2020;28:6–17. [PubMed] [Google Scholar]
- 8.Zhang N., Wang L., Deng X., Liang R., Su M., He C., et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L.F., Anderson D.E. Viruses in bats and potential spillover to animals and humans. Curr Opin Virol. 2019;34:79–89. doi: 10.1016/j.coviro.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contini C., Di Nuzzo M., Barp N., Bonazza A., de Giorgio R., Tognon M., et al. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Dev Ctries. 2020;14:254–264. doi: 10.3855/jidc.12671. [DOI] [PubMed] [Google Scholar]
- 12.Nelson P.P., Rath B.A., Fragkou P.C., Antalis E., Tsiodras S., Skevaki C. Current and future point-of-care tests for emerging and new respiratory viruses and future perspectives. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati A., Pomeranz C., Qamar Z., Thomas S., Frisch D., George G., et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic. Am J Med Sci. 2020;360:5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarner J. Three emerging coronaviruses in two decades: the story of SARS, MERS, and now COVID-19. Am J Clin Pathol. 2020;153:420–421. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meo S.A., Alhowikan A.M., Khlaiwi T.A.L., Meo I.M., Halepoto D.M., Iqbal M., et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . 2003. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003.https://www.who.int/csr/sars/country/table2004_04_21/en/ [Google Scholar]
- 18.Cheng V.C.C., Chan J.F.W., To K.K.W., Yuen K.Y. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100:407–419. doi: 10.1016/j.antiviral.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng V.C.C., Tang B.S.F., Wu A.K.L., Chu C.M., Yuen K.Y. Medical treatment of viral pneumonia including SARS in immunocompetent adult. J Infect. 2004;49:262–273. doi: 10.1016/j.jinf.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arthur Chun-Wing L.A.U., Loretta Yin-Chun Y.A.M., Loletta Kit-Ying S.O. Management of critically ill patients with severe acute respiratory syndrome (SARS) Int J Med Sci. 2012;1:1–10. doi: 10.7150/ijms.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization . 2020. MERS situation update, January 2020.http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html [Google Scholar]
- 23.Zumla A., Hui D.S., Perlman S. Middle east respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Momattin H., Al-Ali A.Y., Al-Tawfiq J.A. A systematic review of therapeutic agents for the treatment of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Travel Med Infect Dis. 2019;30:9–18. doi: 10.1016/j.tmaid.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arabi Y.M., Asiri A.Y., Assiri A.M., Aziz Jokhdar H.A., Alothman A., Balkhy H.H., et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yong C.Y., Ong H.K., Yeap S.K., Ho K.L., Tan W.S. Recent advances in the vaccine development against middle east respiratory syndrome-coronavirus. Front Microbiol. 2019;10:1–18. doi: 10.3389/fmicb.2019.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization . 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 29.Hon K.L., Leung K.K.Y., Leung A.K., Qian S.Y., Chan V.P., Ip P., et al. Coronavirus disease 2019 (COVID-19): latest developments in potential treatments. Drugs Context. 2020;9:1–14. doi: 10.7573/dic.2020-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saxena A. Drug targets for COVID-19 therapeutics: ongoing global efforts. J Biosci. 2020;45 doi: 10.1007/s12038-020-00067-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus department of pharmacy services. Int J Antimicrob Agents. 2020:1–25. doi: 10.20944/preprints202003.0378.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu Y.F., Chien C.S., Yarmishyn A.A., Lin Y.Y., Luo Y.H., Lin Y.T., et al. A review of sars-cov-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21:2657–2675. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9:1–15. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. 2020:1–12. doi: 10.1056/nejmoa2007764. [DOI] [PubMed] [Google Scholar]
- 36.Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020;0:1–6. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 37.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. 2020:5–11. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crotty S., Maag D., Arnold J.J., Zhong W., Lau J.Y.N., Hong Z., et al. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 39.Hung I.F.N., Lung K.C., Tso E.Y.K., Liu R., Chung T.W.H., Chu M.Y., et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boriskin Y., Leneva I., Pecheur E.-I., Polyak S. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 41.Yang C., Ke C., Yue D., Li W., Hu Z., Liu W., et al. Effectiveness of arbidol for COVID-19 prevention in health professionals. Front Public Health. 2020;8:1–6. doi: 10.3389/fpubh.2020.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81:21–23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nong Zhang J., Jing Wang W., Peng B., Peng W., Sheng Zhang Y., Ling Wang Y., et al. Potential of arbidol for post-exposure prophylaxis of COVID-19 transmission—a preliminary report of a retrospective cohort study. Curr Med Sci. 2020;40:1–6. doi: 10.1007/s11596-020-2203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bukreyeva N., Mantlo E.K., Sattler R.A., Huang C., Paessler S., Zeldis J. The IMPDH inhibitor merimepodib suppresses SARS-CoV-2 replication in vitro. bioRxiv. 2020;0:1–10. doi: 10.1101/2020.04.07.028589. In press. [DOI] [Google Scholar]
- 45.Kelleni M.T. Nitazoxanide/azithromycin combination for COVID-19: a suggested new protocol for early management. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:3–6. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaccour C., Hammann F., Ramón-García S., Rabinovich N.R. Ivermectin and COVID-19: keeping rigor in times of urgency. Am J Trop Med Hyg. 2020;102:1156–1157. doi: 10.4269/ajtmh.20-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J., Shi P.Y., Li H., Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 2020;6:909–915. doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheahan T.P., Sims A.C., Zhou S., Hill C., Leist S.R., Schaefer A., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus. Science (80) 2020;5883 doi: 10.1101/2020.03.19.997890. 2020.03.19.997890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Encinar J.A., Menendez J.A. Potential drugs targeting early innate immune evasion of SARS-coronavirus 2 via 2’-O-methylation of viral RNA. Viruses. 2020;12 doi: 10.3390/v12050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delgado-Calle J., Kurihara N., Atkinson E.G., Nelson J., Miyagawa K., Galmarini C.M., et al. Aplidin (plitidepsin) is a novel anti-myeloma agent with potent anti-resorptive activity mediated by direct effects on osteoclasts. Oncotarget. 2019;10:2709–2721. doi: 10.18632/oncotarget.26831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pharmaceuticals A., Biotechnology A.V. 2020. Vir and alnylam identify RNAi therapeutic development candidate, VIR-2703 (ALN-COV), targeting SARS-CoV-2 for the treatment of COVID-19, 2703. [Google Scholar]
- 53.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdulkadir A. The benefits of chloroquine multi-mechanisms of action on the nervous system. Theranostics Brain Spine Neural Disord. 2019;3:2018–2020. doi: 10.19080/JOJS.2019.03.555606. [DOI] [Google Scholar]
- 56.Borba M.G.S., Almeida Val F.F., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection A randomized clinical trial. JAMA Netw Open. 2020;3(4):e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 57.Pal A., Pawar A., Goswami K., Sharma P., Prasad R. Hydroxychloroquine and Covid-19: a cellular and molecular biology based update. Indian J Clin Biochem. 2020;35(3):274–284. doi: 10.1007/s12291-020-00900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cochrane Targeted update: safety and efficacy of hydroxychloroquine or chloroquine for treatment of COVID-19. Cochrane Database Syst Rev. 2020;0:1–18. In preparation. [Google Scholar]
- 59.Li X., Wang Y., Agostinis P., Rabson A., Melino G., Carafoli E., et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11:512. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shahani S.R., Shahani L.R. Status of chloroquine and hydroxychloroquine in COVID-19 infection. Int J Basic Clin Pharmacol. 2020;9:1301–1309. [Google Scholar]
- 61.Ku Z., Ye X., Salazar G.T., Zhang N., An Z. Antibody therapies for the treatment of COVID-19. Antibody Ther. 2020;3:101–108. doi: 10.1093/abt/tbaa007. [DOI] [Google Scholar]
- 62.de Alwis R., Chen S., Gan E.S., Ooi E.E. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020;55:102768. doi: 10.1016/j.ebiom.2020.102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown B.L., McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus Apher Sci. 2020;59 doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L., Pang R., Xue X., Bao J., Ye S., Dai Y., et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (Albany NY) 2020;12:6536–6542. doi: 10.18632/AGING.103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A., et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;9913:1–11. doi: 10.1016/s2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-rammerstorfer S., et al. Articles Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020:1–13. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020 doi: 10.1101/2020.05.13.093195. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 70.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARScov-2 vaccine development. Med Sci Monit. 2020;26:1–8. doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., mRNA-1273 Study Group, et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lockhart S., Neuzil K., Raabe V., Bailey R., Kena A. 2020. Judith Absalon; pp. 1–16. [Google Scholar]
- 73.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J., et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:1–13. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiss C., Carriere M., Fusco L., Fusco L., Capua I., Regla-Nava J.A., et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 75.Gao A.Q., Bao L., Mao H., Wang L., Xu K., Yang M. Rapid development of an inactivated vaccine for SARS-CoV-2. Sci. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;81 doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;6736 doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torre E.D., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an openlabel cohort study. Epidemiology. 2020:1–9. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palanques-Pastor T., López-Briz E., Poveda Andrés J.L. Involvement of interleukin 6 in SARS-CoV-2 infection: siltuximab as a therapeutic option against COVID-19. Eur J Hosp Pharm. 2020;27(5):297–298. doi: 10.1136/ejhpharm-2020-002322. ejhpharm-2020-002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehta P., Ciurtin C., Scully M., Levi M., Chambers R.C. JAK inhibitors in COVID-19: need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919. doi: 10.1183/13993003.01919-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., et al. JAK inhibition as a new treatment strategy for patients with COVID-19. Int Arch Allergy Immunol. 2020;181:467–475. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sorrell F.J., Szklarz M., Abdul Azeez K.R., Elkins J.M., Knapp S. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24:401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bifulco M., Gazzerro P. Statin therapy in COVID-19 infection: much more than a single pathway. Eur Hear J Cardiovasc Pharmacother. 2020:3–4. doi: 10.1093/ehjcvp/pvaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur Heart J Cardiovasc Pharmacother. 2020;6(4):258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye Z., Wang Y., Colunga-Lozano L.E., Tangamornsuksan W., Rochwerg B., Yao L., et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. Can Med Assoc J. 2020;192 doi: 10.1503/cmaj. 200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian R.R., Zhang M.X., Liu M., Fang X., Li D., Zhang L., et al. CD24Fc protects against viral pneumonia in simian immunodeficiency virus-infected Chinese rhesus monkeys. Cell Mol Immunol. 2020:4–5. doi: 10.1038/s41423-020-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giles A.J., Hutchinson M.K.N.D., Sonnemann H.M., Jung J., Fecci P.E., Ratnam N.M., et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. 2018;6:1–13. doi: 10.1186/s40425-018-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson R.M., Vinetz J.M. Dexamethasone in the management of covid-19. BMJ. 2020;370:m2648. doi: 10.1136/bmj.m2648. [DOI] [PubMed] [Google Scholar]
- 89.Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020 doi: 10.1101/2020.06.22.20137273. 2020.06.22.20137273. [DOI] [Google Scholar]
- 90.Bauer S.R., June P. What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19? Cleveland Clinic J Med. 2020:3–5. doi: 10.3949/ccjm.87a.ccc046. [DOI] [PubMed] [Google Scholar]
- 91.Zabetakis I., Lordan R., Norton C., Tsoupras A. Covid-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:1–28. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grant W.B., Lahore H., Mcdonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 1980:1–19. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carr A.C. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:1–2. doi: 10.1186/s13054-020-02851-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Carr A.C. Vitamin C administration in the critically ill: a summary of recent meta-analyses. Crit Care. 2019;23:1–3. doi: 10.1186/s13054-019-2538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.te Velthuis A.J.W., van den Worml S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar D., Kumari K., Vishvakarma V.K., Jayaraj A., Kumar D., Ramappa V.K., et al. Promising inhibitors of main protease of novel corona virus to prevent the spread of COVID-19 using docking and molecular dynamics simulation. J Biomol Struct Dyn. 2020;0:1–15. doi: 10.1080/07391102.2020.1779131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of ayurveda and yoga for COVID-19 prophylaxis. J Altern Complement Med. 2020;26:360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- 98.Mirzaie A., Halaji M., Dehkordi F.S., Ranjbar R., Noorbazargan H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19) Complement Ther Clin Pract. 2020;40:101214. doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ang L., Lee H.W., Choi J.Y., Zhang J., Lee M.S. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res. 2020;9:100407. doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo L., Jiang J., Wang C., Fitzgerald M., Hu W., Zhou Y., et al. Analysis on herbal medicines utilized for treatment of COVID-19. Acta Pharm Sin B. 2020;10(7):1192–1204. doi: 10.1016/j.apsb.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thuy B.T.P., My T.T.A., Hai N.T.T., Hieu L.T., Hoa T.T., Thi Phuong Loan H., et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega. 2020;5:8312–8320. doi: 10.1021/acsomega.0c00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Golechha M. Time to realise the true potential of Ayurveda against COVID-19. Brain Behav Immun. 2020;87:130–131. doi: 10.1016/j.bbi.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rammanohar P., Kataria S., Unnikrishnan P.M., Prasad M., Nampoothiri V., Sharma P., et al. Ayurvedic clinical profile of COVID-19 – a preliminary report. J Ayurveda Integr Med. 2020:1–14. doi: 10.1016/j.jaim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 Coronavirus structure, mechanism of action, antiviral drug promises and rule out against it’s treatment. J Biomol Struct Dyn. 2020;38:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh T.U., Parida S., Lingraju M.C., Kesavan M., Kumar D., Singh R.K. Durg repurposing approach to fight covid-19. Pharmacol Rep. 2020;72:1479–1508. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identifca-tion of potential vaccine targets for the COVID-19 Coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):E254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]