ABSTRACT
Background
Huntington disease (HD) is an inherited neurodegenerative disorder characterized by motor, psychiatric, and cognitive symptoms. Little is known about the effects of environmental factors on HD symptom onset and severity.
Objective
To evaluate the relationship between education level and age of diagnosis, symptom onset, and symptom severity in HD.
Methods
This study evaluated 4537 adult‐onset, motor‐manifest HD participants from the Enroll‐HD global registry. Education level was assessed using International Standard Classification of Education categories, stratified into three education groups corresponding to pre‐secondary, secondary, and post‐secondary educational attainment. Motor and behavioral symptoms of HD, cognition, and functional capacity were measured using baseline Unified Huntington's Disease Rating Scale (UHDRS), Mini‐Mental State Exam (MMSE), Symbol Digit Modalities Test (SDMT), verbal fluency, and Stroop assessments.
Results
After adjusting for CAG repeats, higher level of education predicted lower age of onset of motor symptoms, depression, irritability, and cognitive impairment (all P‐values < 0.001). After adjusting for age of enrollment and CAG repeats, the highest education level predicted the lowest UHDRS motor scores, higher UHDRS total functional capacity and functional assessment scores, and higher SDMT, MMSE, verbal fluency, and Stroop assessment scores (all P‐values < 0.001).
Conclusions
HD participants with higher education levels have earlier age of diagnosis and age of symptom onset, but lower motor exam scores and higher functional assessment scores. Earlier recognition of symptoms in more highly educated participants may explain earlier symptom onset and diagnosis. Better performance on motor and functional assessments may be explained by higher cognitive reserve in those with greater education.
Keywords: Huntington disease, education, cognitive reserve, enroll‐HD
Huntington disease (HD) is an autosomal dominantly inherited neurodegenerative disorder characterized by a triad of characteristic motor, psychiatric, and cognitive symptoms. The critical determinant of age of onset of HD is CAG trinucleotide repeat length in the mutant HTT allele. 1 However, significant variability in disease presentation exists among individuals with the same number of CAG repeats, even within families. While genetic modifiers of disease onset have been identified, 2 in one genetically related HD population, 63% of the variance of age of onset was determined by environmental effects. 1 While much is known about the genetic factors that lead to HD onset, less is known about the effects of environmental factors on disease onset and severity.
Prior research has shown that enriched environments delay symptom onset, improve motor symptoms, and slow progression of HD pathology in mice. 3 , 4 , 5 Cognitive reserve, defined as the brain's ability to resist injury through flexible use of existing neural networks as well as reliance on surrounding structures, could explain this disease‐modifying effect, and cognitive reserve is known to be influenced by education. 6 , 7 , 8 , 9 The protective role of education on both symptom onset and disease manifestations in Alzheimer's disease is well‐established. 10 , 11 , 12 , 13 In HD, one study did not find a specific association between education level and age of onset of HD symptoms, 3 while another study found that higher education levels were associated with earlier age of onset of HD symptoms and less severe Unified Huntington's Disease Rating Scale (UHDRS) scores. 14 We sought to further evaluate the effect of education on symptom onset and severity in Huntington's disease. The primary objective of our study was to determine the effect of education on the age of onset of motor symptoms with the primary measure being motor scores on the UHDRS, and age of HD diagnosis. Secondary objectives included determining the effect of education on the age of onset of depression, irritability, and cognitive impairment; and determining the effect of education on functional status after HD diagnosis.
Methods
Study Population
Clinical data used in this study were obtained from Enroll‐HD. 15 Enroll‐HD is a global clinical research platform designed to facilitate clinical research in Huntington's disease. Core datasets are collected annually from all research participants as part of this multi‐center longitudinal observational study. Data are monitored for quality and accuracy using a risk‐based monitoring approach. All sites are required to obtain and maintain local institutional review board approval. De‐identified clinical, biological, and descriptive data from Enroll‐HD are available to qualified researchers. Participants in Enroll‐HD are categorized as pre‐manifest/pre‐motor manifest, genotype negative, manifest/motor manifest, or family controls. The HD study population includes participants 18 years old or older that are carriers of mutant HD gene expansions (regardless of disease expression) or less than 18 years old with clinically diagnosed HD. The overall study enrollment process includes a review of inclusion/exclusion criteria, as defined by the Enroll‐HD protocol. Each participant provided oral and written informed consent at their participating site. For this study Enroll‐HD Periodic Dataset #3 was accessed on April 18, 2017.
Standard Protocol Approvals, Registrations, and Patient Consents
All sites were required to obtain and maintain local ethics committee approvals. Participants must have signed informed consent forms for their data to be included in the datasets.
Data Availability
These results were generated using the Enroll‐HD database (enroll-hd.org), which is funded by CHDI, Inc. This dataset is made widely available to any interested researcher working at a recognized research institution through a straightforward approval process. Researchers requesting this database will be asked to sign the respective agreements governing access and use of these resources. Data not provided in the article because of space limitations may be shared at the request of any qualified investigator (defined as an investigator with granted access to the Enroll‐HD database) for purposes of replicating procedures and results.
Study Sample
Of the 8714 individuals included in Enroll‐HD dataset #3, we analyzed baseline data for all participants with a clinical diagnosis of adult onset motor‐manifest HD (n = 4537; mean age: 53.5 years (SD = 12.2 years); 50.6% female). Participants categorized as pre‐manifest/pre‐motor manifest, genotype negative, or family controls were excluded from the study. Of those with motor‐manifest HD, participants with age of motor symptom onset before 21 years were excluded out of concern that early disease onset would affect educational attainment. Lastly, we excluded participants with unreported baseline education levels (see Fig. 1).
FIG. 1.
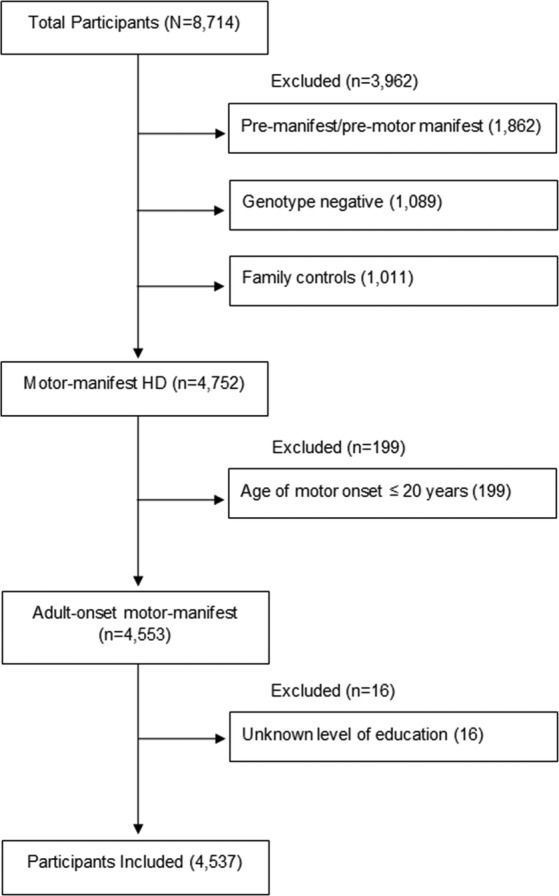
Flow diagram of enroll‐HD participants included in this study.
Clinical Characteristics and Outcomes
The following demographic and clinical characteristics were analyzed: age at baseline, sex, age at HD diagnosis, number of CAG repeats, education level, and participant‐reported age of onset of motor symptoms, depression, irritability, and cognitive impairment. Duration of disease was calculated as the time between clinician‐determined age at HD diagnosis and age at baseline visit. Education level was defined using the International Standard Classification of Education (ISCED) 1997 coding system, an internationally developed education classification system defining seven education levels ranging from ISCED levels 0 to 6. 16 The ISCED system definitions vary for each level by country. For analytical purposes, the ISCED levels were stratified into three education groups: ISCED 0–2, ISCED 3, and ISCED 4–6. In relation to US and Canadian ISCED coding definitions, these three groups correspond to primary and lower secondary education (ISCED 0–2), completion of secondary education (ISCED 3), and post‐secondary education (ISCED 4–6) (ISCED Level Mapping for the US and Canadian Education Systems). ISCED levels were stratified into these three groups for two reasons: (1) it avoided small group sizes and led to comparably sized groups and (2) the three groups corresponded to completion of lower secondary education or below, completion of upper secondary education, and completion of at least some post‐secondary education, thus making our results more easily interpretable.
Scores for the following clinical assessments were analyzed: the Unified Huntington's Disease Rating Scale (UHDRS), including the motor scale, total functional capacity, and functional assessment; Mini‐mental State Exam (MMSE); Symbol Digit Modalities Test (SDMT); verbal fluency; Stroop color naming; and Stroop word reading. Participant use of vesicular monoamine transporter 2 (VMAT2) inhibitors and antipsychotics at the baseline visit was also analyzed.
Statistical Analysis
Data Summarization
Categorical data were summarized by frequencies and percentages, while continuous and integer scaled data were summarized by the mean and standard deviation (SD) and by the median and interquartile range (IQR), respectively.
Education Level Bivariate Associations
Bivariate associations between categorical variables (eg sex) and education level were assessed via a global Pearson Chi‐square test that tested the null hypothesis that there is no bivariate association between the categories of the categorical variable (eg female, male) and the categories of education level (ie ISCED 0–2, ISCE 3, ISCED 4–6). Inter‐education level pairwise hypothesis testing was conducted via Pearson Chi‐square tests if the global Pearson Chi‐square hypothesis test was rejected at the P ≤ 0.05 significance level. Bivariate associations between continuous scaled variables (eg age) and education level were assessed via a global analysis of variance (ANOVA) F‐test that tested the null hypothesis that there is no bivariate association between mean of the continuous variable and the education level. Inter‐education level pairwise hypothesis testing was conducted via the t‐test if the global hypothesis test was rejected at the P ≤ 0.05 significance level. Bivariate associations between integer scale variables (eg UHDRS motor score) and education level, were assessed by way of a global Kruskal‐Wallis test that tested the null hypothesis that there is no bivariate association between the median of the integer variable and education level. Inter‐education level pairwise hypothesis testing was conducted via a Wilcoxon Rank Sum test if the global hypothesis test was rejected at the P ≤ 0.05 significance level.
Adjusted Education Level Bivariate Associations
Ordinary least squares (OLS) multivariate linear regression was utilized to examine CAG repeats adjusted bivariate associations between education level and the dependent variables of age of onset of motor symptoms, depression, irritability, and cognitive impairment and age at HD diagnosis. It is important to note that ages of onset of other psychiatric symptoms (eg psychosis, apathy) were not examined because these symptoms occurred in less than 60% of participants. OLS multivariate linear regression was additionally utilized to examine CAG repeats and age at enrollment adjusted bivariate associations between education level and the dependent variables of UHDRS scores, MMSE, SDMT, verbal fluency, and Stroop assessments. For the UHDRS motor exam score, we conducted an additional regression analysis that adjusted for VMAT2 inhibitor and/or antipsychotic use in addition to CAG repeats and age at enrollment. Given the regional heterogeneity and wide variation in age of our study population, cognitive test raw scores were used in these analyses.
For both of the aforementioned sets of OLS linear regression analyses, a Type III F‐test was conducted per regression analysis (ie per dependent variable) to test the null hypothesis that education level is not uniquely associated with the regression dependent variable (eg age of onset of motor symptoms) after removing for the variance in the “dependent variable” explained by the adjustment variable(s) (eg CAG repeats). If the Type II F‐test P‐value was less than or equal to 0.05, pairwise inter‐education level comparison hypothesis testing was conducted to compare the adjusted effect that each education level has on the dependent variable prediction. A P ≤ 0.05 decision rule was used as the null hypothesis rejection criterion for all pairwise inter‐education level comparisons. To correct for hypothesis testing across 14 different multiple linear regression models (Table 2), the Benjamini and Hochberg (BH) false positive discovery error procedure was applied to the complete set of linear regression model Type III F‐test P‐values. This restricted the probability of falsely rejecting the null hypothesis that there was no unique education level association to be no greater than 0.05.
TABLE 2.
Results of linear regression models evaluating effect of education level on Huntington's disease characteristics and clinical features
| Adjusted effect size [95% CI] (P‐value) | ||||
|---|---|---|---|---|
| Model | ISCED 3 – ISCED 0–2 | ISCED 4–6 – ISCED 0–2 | ISCED 4–6 – ISCED 3 | ISCED partial association test P‐value* |
| Model 1: Age of onset of motor symptoms a |
−1.32 [−1.91, −0.073] (P < 0.001) |
−1.15 [−1.71, −0.53] (P < 0.001) |
0.18 [−0.33, 0.69] (P = 0.497) |
<0.001 † |
| Model 2: Age of onset of depression a |
−1.85 [−2.93, −0.77] (P = 0.001) |
−2.90 [−3.93, −1.86] (P < 0.001) |
−1.05 [−1.99, −0.11] (P = 0.029) |
<0.001 † |
| Model 3: Age of onset of cognitive impairment a |
−1.58 [−2.41, −0.74] (P < 0.001) |
−2.04 [−2.83, −1.25] (P < 0.001) |
−0.46 [−1.19, 0.26] (P = 0.211) |
<0.001 † |
| Model 4: Age of onset of irritability a |
−1.66 [−2.68, −0.64] (P = 0.002) |
−2.34 [−3.32, −1.36] (P < 0.001) |
−0.68 [−1.59, 0.23] (P = 0.145) |
<0.001 † |
| Model 5: Age of HD diagnosis a |
−1.62 [−2.24, −1.00] (P < 0.001) |
−1.56 [−2.16, −0.97] (P < 0.001) |
0.06 [−0.49, 0.60] (P = 0.839) |
<0.001 † |
| Model 6a: UHDRS motor score b |
−1.17 [−2.63, 0.29] (P = 0.118) |
−5.35 [−6.74, −3.96] (P < 0.001) |
−4.18 [−5.44, −2.91] (P < 0.001) |
<0.001 † |
| Model 6b: UHDRS motor score c |
−0.62 [−2.03, 0.78] (P = 0.385) |
−3.99 [−5.33, −2.65] (P < 0.001) |
−3.36 [−4.58, −2.14] (P < 0.001) |
<0.001 † |
| Model 7: UHDRS total functional capacity score b |
0.74 [0.48, 1.00] (P < 0.001) |
1.25 [1.00, 1.49] (P < 0.001) |
0.51 [0.28, 0.73] (P < 0.001) |
<0.001 † |
| Model 8: UHDRS functional assessment score b |
1.78 [1.29, 2.27] (P < 0.001) |
2.85 [2.39, 3.30] (P < 0.001) |
1.07 [0.65, 1.48] (P < 0.001) |
<0.001 † |
| Model 9: MMSE score b |
1.47 [1.05, 1.90] (P < 0.001) |
2.77 [2.37, 3.17] (P < 0.001) |
1.30 [0.94, 1.65] (P < 0.001) |
<0.001 † |
| Model 10: SDMT score b |
3.94 [3.02, 4.87] (P < 0.001) |
8.19 [7.32, 9.06] (P < 0.001) |
4.25 [3.46, 5.03] (P < 0.001) |
<0.001 † |
| Model 11: Verbal fluency test score b |
1.38 [0.96, 1.80] (P < 0.001) |
2.56 [2.17, 2.96] (P < 0.001) |
1.18 [0.82, 1.54] (P < 0.001) |
<0.001 † |
| Model 12: Stroop color naming test score b |
5.56 [4.70, 6.92] (P < 0.001) |
10.13 [8.84, 11.42] (P < 0.001) |
4.57 [3.40, 5.75] (P < 0.001) |
<0.001 † |
| Model 13: Stroop word reading test score b |
6.82 [5.12, 8.53] (P < 0.001) |
12.79 [11.18, 14.41] (P < 0.001) |
5.97 [4.50, 7.44] (P < 0.001) |
<0.001 † |
Adjusted by CAG repeats.
Adjusted by age of enrollment and CAG repeats.
Adjusted by age of enrollment, CAG repeats and VMAT2 inhibitor and/or antipsychotic use.
ISCED Partial Association Test P‐value is the P‐value for the null hypothesis test that after concomitant variable adjustment ISCED is not uniquely associated with the outcome variable.
Indicates that the P‐value for the global null hypothesis test that “education level” is not uniquely associated with the regression “dependent variable” after removing for the variance in the “dependent variable” explained by the adjustment variable(s) is less than the Benjamini and Hochberg false discovery error rate (0.05) P‐value threshold that if exceeded would indicate a false positive finding.
Abbreviations: SD, Standard deviation; HD, Huntington disease; ISCED, International standard classification of education; MMSE, Mini‐Mental State Exam; SDMT, Symbol Digit Modalities Test; UHDRS, Unified Huntington disease rating scale. ISCED 0–2 vs. ISCED 3, ISCED 3 ‐ ISCED 0–2; ISCED 0–2 vs. ISCED 4–6, ISCED 4–6 ‐ ISCED 0–2; ISCED 3 vs. ICED 4–6, ICED 4–6 ‐ ISCE 3.
Statistical Software
The statistical package Spot‐Fire S+ version 8.2 (TIBCO Inc., Palo Alto, CA) was used to conduct all of the statistical analyses.
Results
Clinical and demographic information for all participants included in this study are summarized in Table 1. There were 13 participants in ISCED level 0, 210 participants in ISCED level 1, 892 participants in ISCED level 2, 1491 participants in ISCED level 3, 765 participants in ISCED level 4, 1079 participants in ISCED level 5, and 87 participants in ISCED level 6. Based on the bivariate association hypothesis tests, significant associations were observed between education level and age at HD diagnosis, age of onset of depression, irritability, and cognitive impairment, UHDRS scales, cognitive assessments, and VMAT2 inhibitor and antipsychotic use at the baseline visit.
TABLE 1.
Clinical and demographic characteristics of Huntington disease participants by education
| ISCED 0–2 | ISCED 3 | ISCED 4–6 | Global | |
|---|---|---|---|---|
| (N = 1115) | (N = 1491) | (N = 1931) | P‐value* | |
| Men/Women, n (%)/n (%) | 536 (48.1)A/579 (51.9) | 719 (48.2)A/772 (51.8) | 987 (51.1)A/944 (48.9) | 0.144 |
| Region, n (%) | ||||
| Europe | 1051 (94.3)A | 979 (65.7)B | 889 (46.0)C | |
|
North America Australasia Latin America |
42 (3.8)A 14 (1.3)A 8 (0.7)A |
455 (30.5)B 49 (3.3)B 8 (0.5)A |
954 (49.4)C 77 (4.0)B 11 (0.6)A |
<0.001 |
| Age at baseline visit, years (SD) | 54.7A (12.9) | 52.5B (12.0) | 53.6C (11.8) | 0.156 |
| CAG repeat number, number (SD) | 43.9AB (3.6) | 44.0A (3.4) | 43.6B (3.2) | 0.185 |
|
Age at HD diagnosis, years (SD) [sample size] |
50.0A (12.7) [n = 1097] |
48.0B (12.1) [n = 1459] |
49.0C (11.7) [n = 1880] |
0.048 |
| Duration of disease, years (SD) a | 4.9A (4.3) | 4.5B (4.3) | 4.7AB (4.4) | 0.006 |
| Age of onset of motor symptoms, years (SD) | 47.0A (12.1) | 45.4A (11.5) | 46.4A (11.1) | 0.167 |
|
Age of onset of depression, years (SD) [sample size] |
44.6A (13.5) [n = 734] |
42.8B (13.2) [n = 1023] |
42.5B (13.5) [n = 1284] |
0.011 |
|
Age of onset of irritability, years (SD) [sample size] |
46.9A (13.4) [n = 726] |
44.7B (13.1) [n = 921] |
45.0B (12.6) [n = 1147] |
0.001 |
|
Age of onset of cognitive impairment, years (SD) [sample size] |
49.0A (13.2) [n = 649] |
47.4B (11.7) [n = 866] |
47.9AB (11.4) [n = 1144] |
0.009 |
|
UHDRS motor score, median [IQR] [sample size] |
40.0A [26, 58] [n = 1105] |
37.0B [24.0, 54.0] [n = 1486] |
33.0C [20.0, 48.0] [n = 1919] |
<0.001 |
|
UHDRS total functional capacity score, median [IQR] [sample size] |
7.0A [4.0, 10.0] [n = 1065] |
8.0B [5.0, 11.0] [n = 1490] |
9.0C [6.0, 11.0] [n = 1928] |
<0.001 |
|
UHDRS function assessment score, median [IQR] [sample size] |
16.0A [10.0, 21.0] [n = 1065] |
20.0B [14.0, 23.0] [n = 1443] |
21.0C [16.0, 24.0] [n = 1906] |
<0.001 |
|
MMSE score, median [IQR] [sample size] |
24.0A [20.0, 27.0] [n = 618] |
26.0B [23.0, 28.0] [n = 917] |
27.0C [25.0, 29.0] [n = 1197] |
<0.001 |
|
SDMT, median raw score [IQR] [sample size] |
16.0A [8.0, 23.0] [n = 1002] |
21.0B [13.0, 30.0] [n = 1380] |
25.0C [18.0, 34.0] [n = 1823] |
<0.001 |
|
Verbal fluency test, median raw score [IQR] [sample size] |
9.0A [6.0, 13.0] [n = 1072] |
11.0B [8.0, 15.0] [n = 1453] |
12.0C [9.0, 17.0] [n = 1891] |
<0.001 |
|
Stroop color naming test score, median raw score [IQR] [sample size] |
33.0A [22.0, 44.0] [n = 1056] |
40.0B [29.0, 52.0] [n = 1430] |
45.0C [33.0, 56.0] [n = 1871] |
<0.001 |
|
Stroop word reading test, median raw score [IQR] [sample size] |
45.0A [29.0, 60.0] [n = 1046] |
53.0B [38.0, 69.0] [n = 1426] |
59.0C [45.0, 75.0] [n = 1869] |
<0.001 |
| Antipsychotic medication use, n (%) b | 394A (35.3) | 432B (29.0) | 427C (22.1) | 0.001 |
| Vesicular Monoamine Transporter 2 Inhibitor use, n (%) | 204A (18.3) | 241A(16.2) | 261B (13.5) | 0.002 |
| Antipsychotic or VMAT2 Inhibitor use, n (%) | 532A (47.7) | 604B(40.5) | 641C (33.2) | 0.001 |
Duration of disease = age at study baseline – age of HD diagnosis.
Excludes use of quetiapine, clozapine, and pimavanserin.
Global P‐value is the P‐value for the tests for no association between the variable of interest and the ISCED category (eg Sex vs. ISCED). Variable of interest, pairwise hypothesis tests between the three different ISCED groups are summarized symbolically in the form of matched superscripts. ISCED groups which have any superscripts in common (eg A vs. A or A vs. AB) indicate that the pairwise hypothesis test was non‐significant at the P ≤ 0.05 significance level.
Abbreviations: HD, Huntington disease; IQR, interquartile range; ISCED, International Standard Classification of Education; MMSE, Mini‐Mental State Exam; SD, standard deviation; SDMT, Symbol Digit Modalities Test; UHDRS, Unified Huntington Disease Rating Scale.
Multivariate linear regression analyses findings are summarized in Table 2. Higher levels of education beyond ISCED 0–2 predicted earlier onset of motor symptoms, depression, irritability, and cognitive impairment (Models 1–5, Table 2). Higher education level was also associated with lower UHDRS motor scores, higher UHDRS total functional capacity and functional assessment scores (Models 6–8, Table 2). Higher education level was also associated with higher SDMT, MMSE, verbal fluency, and Stroop scores (Models 9–13, Table 2). Application of the BH false discovery procedure found that for all models, education level was unique predictor of outcome <0.05. When adjusted for use of medications that reduce chorea, that is VMAT2 inhibitors and/or antipsychotics, participants in the highest education group had a 3.99 unit (95% CI: [2.65, 5.33unit]) reduction in UHDRS motor score compared to the lowest education group (P < 0.001), but the reduction in the UHDRS motor score for the middle education group; compare to the lowest education group, was only 0.62 units (95% CI: [−0.78, 2.03 units], P = 0.385).
There was an association between age of motor symptom onset and education level when comparing those with motor symptom onset at ages 21–25 and >25 (P = 0.022). There was no association between age of motor symptom onset and education level when comparing those with motor symptom onset at ages 25–30 and >30 (P = 0.235). Therefore, we repeated the linear regression analyses in those with onset of motor symptoms >25 and found no differences in our results.
Discussion
Our results show that HD participants with higher education level report earlier onset of participant‐reported HD associated symptoms and have earlier age of HD diagnosis. A prior study evaluating the effects of education on HD found that motor symptom onset occurred earlier in HD patients with greater than 10 years of education. 14 Our results extend these findings, as we showed that those with greater education not only have earlier age of onset of motor symptoms, but also of depression, irritability, and subjective cognitive impairment. We suspect that these findings may be explained by increased self‐awareness and/or insight and thus earlier recognition of mood, motor symptoms, irritability, and cognitive symptoms in more highly educated individuals. In the same way, earlier age of diagnosis in HD participants with higher education levels may arise from earlier recognition of symptoms and earlier evaluation by health care providers.
After adjusting for age and number of CAG repeats, we found that HD participants with higher education levels had lower motor exam scores and higher scores on functional assessments. This finding is consistent with a prior study which suggested a protective effect of education on the manifestations of HD. 14 Greater education was associated with less disease‐related motor impairment as measured by the UHDRS, even when controlling for use of medications that can suppress chorea. We expected that individuals with higher education would have greater access to medical care and therefore be more likely to be receive medications for their symptoms. Unfortunately, socioeconomic status (SES) data is not available within the Enroll‐HD registry and this is a limitation of our study. As a proxy for SES, differences in pharmacological treatment of chorea was compared across the three education groups under the assumption that participants in higher education levels would have better healthcare access and would subsequently be more likely to be treated pharmacologically for their chorea. Our results show that participants with lower education experience a higher severity of motor symptoms at baseline and are more likely to receive treatments that suppress chorea compared to participants with higher levels of education. This could suggest that healthcare access is not a significant factor influencing baseline motor symptoms, although this cannot be concluded with confidence given that this is an indirect measure of healthcare access.
Interestingly, and somewhat counter‐intuitively, lower motor scores and better functional status were present in those with a higher level of education despite an earlier age of onset of motor, cognitive, and mood symptoms. One explanation for these findings is that earlier recognition and diagnosis in HD patients with higher level of education is associated with being less symptomatic at the time of evaluation compared to the lower education group. Another explanation is that educational attainment exerts a protective effect on HD progression. Even after adjusting for age and CAG repeats, greater educational attainment was associated with milder motor symptoms and overall better functional status.
Better performance on measures of functional capacity in HD patients with higher level of education may be explained by greater cognitive reserve. 6 , 7 , 8 , 9 Greater cognitive reserve in those with greater education is supported by this group having better performance on all cognitive tasks independent of age of enrollment and CAG repeats. A neuroprotective effect from education in HD is supported by a previous study which found that lower educational attainment was associated with increased risk of manifesting HD in those with intermediate CAG repeat alleles (36–39). 17 We cannot exclude the possibility that factors other than cognitive reserve could be contributing to the lower UHDRS motor scores and better functional status in participants with higher education. Educational attainment correlates with overall health, 18 and this may influence motor symptoms, cognition, and functional status.
One strength of this study is the inclusion of a very large and multinational cohort of manifesting HD patients. Our finding that participants in the lowest education group had more severe motor symptoms, despite being more likely to be treated with medications that can suppress chorea, further supports the idea that greater cognitive reserve is associated with less motor impairment in HD. Compared to the prior study, 14 our study included neurocognitive assessments that further support superior cognitive abilities in more highly educated individuals. In our study, we also addressed the possibility of reverse causality, that is, a greater number of CAG repeats in the mutated HTT allele may influence educational attainment. In support of this, we found a difference in educational attainment in those with motor symptom onset between 21–25 compared to those with motor symptom onset >25, but we did not find a difference in educational attainment in those with motor symptom onset between 25 and 30 compared to those with motor symptom onset >30. Considering that premotor manifestations of HD may have affected educational attainment in those with motor symptom onset between 21 and 25, we repeated analyses in those with motor symptom onset >25 and our findings were the same. A limitation of this study is the way in which educational level was defined. Educational systems vary by country and are therefore difficult to compare. ISCED coding is one way to standardize educational attainment, but it is not perfect given the heterogeneity of educational systems worldwide. The Enroll‐HD database ascertains individuals from multiple sites internationally, although sites are predominantly located in North America and Europe. Another limitation of the study is that it is cross‐sectional and relies on participant‐reported outcomes. As the Enroll‐HD study matures, it will offer the opportunity to expand on the current findings by allowing longitudinal analyses in premanifest HD participants.
In conclusion, HD participants with higher education levels have earlier age of diagnosis and age of symptom onset, but they have lower motor exam scores and higher scores on functional assessments. Earlier recognition of symptom onset in more highly educated populations may explain earlier symptom onset and diagnosis, while better performance on motor and functional assessments may be explained by higher cognitive reserve in those with greater education. Treatment implications of this study include supporting pursuit of education as a way to reduce severity of motor and functional impairment associated with HD as has been proposed for Alzheimer's disease. 10 , 11 Our study also supports using educational attainment as a key factor in interpreting outcomes of clinical trials, specifically age at diagnosis, motor impairment as measured by UHDRS, and functional assessments. Future research should evaluate how educational attainment affects progression of disease‐relevant outcomes over time.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
K.K.C.: 1A, 1B, 1C, 2B, 3A
J.L.F.: 1C, 2C, 3B
W.A.D.: 1C, 2C, 3B
J.P.: 2A, 2B, 2C, 3B
M.B.H.: 1A, 2C, 3B
M.J.B.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Ethical Compliance Statement
All sites were required to obtain and maintain local ethics committee approvals. Participants must have signed informed consent forms for their data to be included in the datasets. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest
Dr. Cain, Mr. Flanigan, Dr. Dalrymple, Mr. Patrie, Dr. Harrison, and Dr. Barrett report no funding sources or conflicts of interest.
Financial Disclosures for the Previous 12 Months
Dr. Cain, Mr. Flanigan, Dr. Dalrymple, and Mr. Patrie report no disclosures. Dr. Harrison received grant support from the Huntington's Disease Society of America, the American Parkinson Disease Association and is site investigator for clinical trials funded by CHDI and Neurocrine. Dr. Barrett served on advisory boards for Amneal and Acorda.
Acknowledgments
Data used in this work were generously provided by the participants in the Enroll‐HD study and made available by CHDI Foundation, Inc. Enroll‐HD is a clinical research platform and longitudinal observational study for Huntington's disease families intended to accelerate progress towards therapeutics; it is sponsored by CHDI Foundation, a nonprofit biomedical research organization exclusively dedicated to collaboratively developing therapeutics for HD. Enroll‐HD would not be possible without the vital contribution of the research participants and their families. We thank the CHDI Foundation, Inc., for supporting Enroll‐HD and granting access to the Enroll‐HD periodic datasets. We thank all sites and investigators who contributed to collection of data and we also thank patients and their families for participating in the study. All individuals who have contributed to the collection of the Enroll‐HD database can be found here: https://www.enroll-hd.org/acknowledgments/.
Statistical analysis conducted by Kristina Cain, Matthew Barrett, and James Patrie.
References
- 1. Wexler NS, Lorimer J, Porter J, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc Natl Acad Sci U S A 2004;101:3498–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GeM‐HD Consortium . Identification of genetic factors that modify clinical onset of Huntington's disease. Cell 2015;162:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trembath MK, Horton ZA, Tippett L, et al. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord 2010;25:1444–1450. [DOI] [PubMed] [Google Scholar]
- 4. Schilling G, Savonenko AV, Coonfield ML, et al. Environmental, pharmacological, and genetic modulation of the HD phenotype in transgenic mice. Exp Neurol 2004;187:137–149. [DOI] [PubMed] [Google Scholar]
- 5. Hockly E, Cordery PM, Woodman B, et al. Environmental enrichment slows disease progression in R6/2 Huntington's disease mice. Ann Neurol 2002;51:235–242. [DOI] [PubMed] [Google Scholar]
- 6. Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn Sci 2013;17:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia‐Gorro C, Garau‐Rolandi M, Escrichs A, et al. An active cognitive lifestyle as a potential neuroprotective factor in Huntington's disease. Neuropsychologia 2019;122:116–124. [DOI] [PubMed] [Google Scholar]
- 8. Soloveva MV, Jamadar SD, Poudel G, Georgiou‐Karistianis N. A critical review of brain and cognitive reserve in Huntington's disease. Neurosci Biobehav Rev 2018;88:155–169. [DOI] [PubMed] [Google Scholar]
- 9. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–460. [PubMed] [Google Scholar]
- 10. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: An analysis of population‐based data. Lancet Neurol 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 11. Lo RY, Jagust WJ. Effect of cognitive reserve markers on Alzheimer pathologic progression. Alzheimer Dis Assoc Disord 2013;27:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jankowsky JL, Melnikova T, Fadale DJ, et al. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci 2005;25:5217–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology 1993;43:13–20. [DOI] [PubMed] [Google Scholar]
- 14. López‐Sendón JL, Royuela A, Trigo P, et al. What is the impact of education on Huntington's disease? Mov Disord 2011;26:1489–1495. [DOI] [PubMed] [Google Scholar]
- 15. Landwehrmeyer GB, Fitzer‐Attas CJ, Giuliano JD, et al. Data analytics from enroll‐HD, a global clinical research platform for Huntington's disease. Mov Disord Clin Pract 2017;4:212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. UNESCO . International Standard Classification of Education: ISCED 1997 (Re‐Edition). UNESCO Institute for Statistics: Montreal; 2006. [Google Scholar]
- 17. Panegyres PK, Shu C‐C, Chen H‐Y, Paulsen JS. Factors influencing the clinical expression of intermediate CAG repeat length mutations of the Huntington's disease gene. J Neurol 2015;262:277–284. [DOI] [PubMed] [Google Scholar]
- 18. Cutler D, Lleras‐Muney A. Education and Health: Evaluating Theories and Evidence. Making Americans Healthier: Social and Economic Policy as HealthPolicy. New York: Russell Sage Foundation; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These results were generated using the Enroll‐HD database (enroll-hd.org), which is funded by CHDI, Inc. This dataset is made widely available to any interested researcher working at a recognized research institution through a straightforward approval process. Researchers requesting this database will be asked to sign the respective agreements governing access and use of these resources. Data not provided in the article because of space limitations may be shared at the request of any qualified investigator (defined as an investigator with granted access to the Enroll‐HD database) for purposes of replicating procedures and results.


