γ-Glutamyl carboxylase (GGCX) is a vitamin K (VK)-dependent enzyme that catalyzes the γ-carboxylation of glutamic acid residues in VK-dependent proteins. The anticoagulant warfarin is known to reduce GGCX activity by inhibiting the VK cycle and was recently shown to disrupt spermatogenesis.
KEYWORDS: γ-glutamyl carboxylase, Sertoli cell, infertility, testis, knockout
ABSTRACT
γ-Glutamyl carboxylase (GGCX) is a vitamin K (VK)-dependent enzyme that catalyzes the γ-carboxylation of glutamic acid residues in VK-dependent proteins. The anticoagulant warfarin is known to reduce GGCX activity by inhibiting the VK cycle and was recently shown to disrupt spermatogenesis. To explore GGCX function in the testis, here, we generated Sertoli cell-specific Ggcx conditional knockout (Ggcx scKO) mice and investigated their testicular phenotype. Ggcx scKO mice exhibited late-onset male infertility. They possessed morphologically abnormal seminiferous tubules containing multinucleated and apoptotic germ cells, and their sperm concentration and motility were substantially reduced. The localization of connexin 43 (Cx43), a gap junction protein abundantly expressed in Sertoli cells and required for spermatogenesis, was distorted in Ggcx scKO testes, and Cx43 overexpression in Sertoli cells rescued the infertility of Ggcx scKO mice. These results highlight GGCX activity within Sertoli cells, which promotes spermatogenesis by regulating the intercellular connection between Sertoli cells and germ cells.
INTRODUCTION
Vitamin K (VK) is an essential nutrient that plays roles in blood coagulation and bone homeostasis. Natural VK forms are VK1 and VK2 (1), with the former being converted into the latter in vivo (2, 3). As one of the major functions of VK, it behaves as a cofactor for γ-glutamyl carboxylase (GGCX), which catalyzes a posttranslational modification of a glutamic acid (Glu) residue into a γ-carboxyglutamic acid (Gla) residue (4). This γ-carboxylation is a critical protein modification for VK-dependent proteins involved in physiological processes, including blood coagulation, fibrinolysis, and bone homeostasis (3, 5, 6).
In humans and mice, the GGCX protein is localized in the endoplasmic reticulum, with 3 potential Gla modification sites in its amino acid residues (5). Clinically, loss-of-function GGCX mutations exhibit the deficient activity of VK-dependent coagulation factors, leading to bleeding disorders (7, 8). In contrast, a gain-of-function GGCX polymorphism is associated with high bone mineral density in elderly women (9). Regarding the loss of function of GGCX in mice, conventional Ggcx knockout (KO) mice exhibited peripartum lethality (10). To dissect the tissue-specific activity of GGCX, we previously generated conditional Ggcx KO (cKO) mice by crossing mice harboring a conditional Ggcx allele by flanking exon 6 of the Ggcx gene with loxP sites (Ggcx floxed) with tissue-specific Cre recombinase-expressing mice (4). Liver-specific Ggcx-deficient mice exhibited a shorter life span due to bleeding diathesis, showing that GGCX is required for the activities of coagulation factors II and IX (4). In addition, osteoblast-specific Ggcx-deficient mice revealed that GGCX regulates bone mineralization and glucose metabolism (11–15).
As GGCX activity is dependent on VK levels, the anticoagulant warfarin reduces GGCX activity by blocking VK epoxide reductase (VKOR) complex subunit 1 (VKORC1) and inhibiting the VK cycle (16). In a study using rats with daily intake of a low dose of warfarin, endogenous substrates for GGCX accumulated in various tissues, including the testes, lungs, spleen, and liver (17). Warfarin also suppresses dithiothreitol-dependent VKOR activity in the testes, liver, and kidney (18). Recent studies have revealed that warfarin also exerts negative effects on spermatogenesis (19, 20). Based on these findings, we questioned whether testis-specific loss of function of GGCX directly causes disorders of spermatogenesis.
In the present study, we generated Sertoli cell-specific Ggcx cKO (scKO) mice by crossing transgenic mice expressing anti-Müllerian hormone (AMH) gene promoter-driven Cre recombinase (AMH-Cre) (21, 22) with Ggcx-floxed mice (4, 14, 15). Our study aimed to examine the physiological role of VK-dependent GGCX in Sertoli cells, which are essential for testis formation and spermatogenesis (23). Ggcx scKO male mice exhibited infertility with atrophy of seminiferous tubules and sperm cells. Notably, the expression of the gap junction protein connexin 43 (Cx43) was distorted in Sertoli cells of Ggcx scKO mice. Our results indicate that VK-dependent GGCX in Sertoli cells is a critical regulator of spermatogenesis.
RESULTS
Generation of Sertoli cell-specific Ggcx-deficient mice.
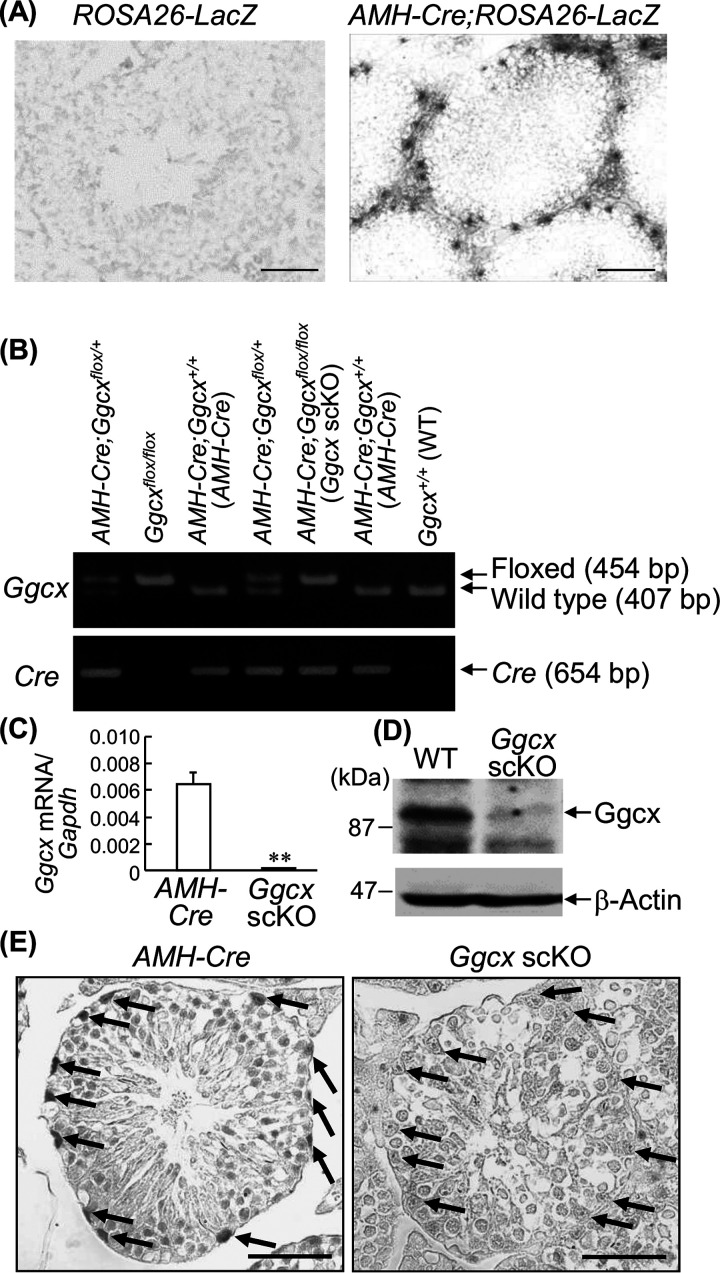
To examine the testis-specific activity of GGCX, we generated Sertoli cell-specific Ggcx cKO (scKO) mice by crossing AMH-Cre transgenic mice with Ggcx-floxed mice (4, 14, 15). In AMH-Cre mice, Sertoli cell-specific AMH-Cre expression was shown by crossing them with ROSA26-LacZ reporter mice (Fig. 1A). Male Ggcx scKO (AMH-Cre; Ggcxflox/flox) mice were generated by mating male and female AMH-Cre; Ggcxflox/+ mice. For genotyping, the Cre recombinase gene (654 bp) was amplified by the primers generated in it by PCR using genomic DNA prepared from the tail (4). The intact (wild-type [WT]) (407-bp) and loxP-containing (floxed) (454-bp) exons 5 of the Ggcx gene were also amplified for genotyping the Ggcx allele (4) (Fig. 1B). AMH-Cre; Ggcx+/+ (AMH-Cre) or Ggcx+/+ (WT) mice were used as controls in further experiments.
FIG 1.
Generation of Sertoli cell-specific Ggcx-deleted mice. (A) AMH gene expression in the Sertoli cells of male AMH-Cre; ROSA26-LacZ mice was visualized by β-galactosidase staining. In vivo recombination of the Rosa26-LacZ locus is mediated by the Cre transgene product. Bars, 20 μm. (B) Generation of AMH-Cre; Ggcxflox/flox (Ggcx scKO) mice. Ggcx-floxed and AMH-Cre mice were crossed to generate AMH-Cre; Ggcxflox/flox (Ggcx scKO) mice. Genotyping was performed using genomic DNA extracted from tails using primer pairs that were derived from the 5′- and 3′-flanking sequences of the loxP sequence (top) and Cre recombinase (bottom). AMH-Cre; Ggcx+/+ mice were used as control animals in the experiments. (C) Ablation of Ggcx mRNA expression in Ggcx scKO testes. Expression levels of Ggcx mRNA were quantified by qRT-PCR in testes from 2-month-old Ggcx scKO and AMH-Cre mice. Data are presented as means ± standard errors of the means (SEM) (n = 3). **, P < 0.01 (using Student’s t test). (D) Deletion of GGCX protein expression in Ggcx scKO testes. Western blot analysis of GGCX was performed in testes from 2-month-old Ggcx scKO and WT mice. β-Actin antibody was used as a loading control. (E) Sertoli cell-specific deletion of GGCX expression in Ggcx scKO mice. Immunohistochemical analyses of GGCX in testes were performed in 4-month-old Ggcx scKO and AMH-Cre mice. The arrows indicate Sertoli cells. Bars, 20 μm.
In the Ggcx scKO testes, quantitative reverse transcriptase PCR (qRT-PCR) showed that Ggcx mRNA expression was barely detectable at 2 months of age (Fig. 1C). GGCX protein levels were also substantially decreased in the Ggcx scKO testes compared to those of controls based on Western blot analysis (Fig. 1D) and immunohistochemical analysis (Fig. 1E).
Ggcx scKO mice exhibit late-onset male infertility.
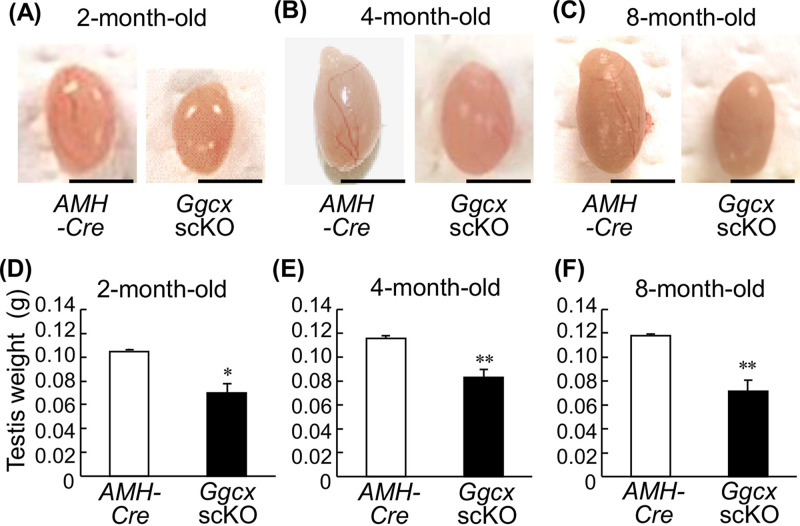
We found that Ggcx scKO male mice exhibit infertility during the generation of Ggcx scKO mice. To further monitor breeding capacity, Ggcx scKO male mice were mated with WT females (Table 1). Although 2-month-old Ggcx scKO male mice were fertile to produce pups, 3- and 6-month-old Ggcx scKO male mice did not exhibit reproductive abilities, while copulatory plugs were observed in the mated WT females. These results indicate that Ggcx depletion in Sertoli cells causes late-onset male infertility. In contrast, male Ggcxflox/flox mice (without Cre) and female Ggcx scKO mice had normal reproductive abilities. Notably, the Ggcx scKO male mice had apparently smaller testes than control mice at 2, 4, and 8 months of age (Fig. 2A to C). The difference in the testis weights was statistically significant between Ggcx scKO and control mice at the examined age (Fig. 2D to F). Serum testosterone levels, however, did not exhibit significant differences between Ggcx scKO male and AMH-Cre mice (Table 2).
TABLE 1.
Fertility evaluation of male Ggcx scKO micea
| Age of males (mo) | No. of mated pairs | No. of pregnancies | No. of litters | Avg litter size |
|---|---|---|---|---|
| 2 | 2 | 1 | 5 | 5 |
| 3 | 6 | 0 | 0 | NA |
| 6 | 6 | 0 | 0 | NA |
Individual male Ggcx scKO mice (n = 2 at 2 months of age, and n = 3 at 3 and 6 months of age) were mated with one or two WT (C57BL/6) females for 3 months. NA, not applicable.
FIG 2.
Decreased testis size in Ggcx scKO mice. The appearance (A to C) and weight (D to F) of testes were examined in Ggcx scKO and AMH-Cre mice at 2 months (A and D), 4 months (B and E), and 8 months (C and F) of age. Data are presented as means ± SEM (n = 3). *, P < 0.05; **, P < 0.01 (using Student’s t test). Bars, 5 mm.
TABLE 2.
Serum testosterone levels in male Ggcx scKO and control micea
| Age (mo) | Mean serum testosterone level (ng/ml) ± SEM |
|
|---|---|---|
| Control | Ggcx scKO | |
| 2 | 20.29 ± 8.46 | 9.75 ± 7.15 |
| 4 | 12.46 ± 6.70 | 18.88 ± 7.57 |
| 8 | 24.29 ± 2.43 | 22.00 ± 6.32 |
n = 3 mice/group. The P values were not significant.
Histological abnormality in Ggcx scKO testes.
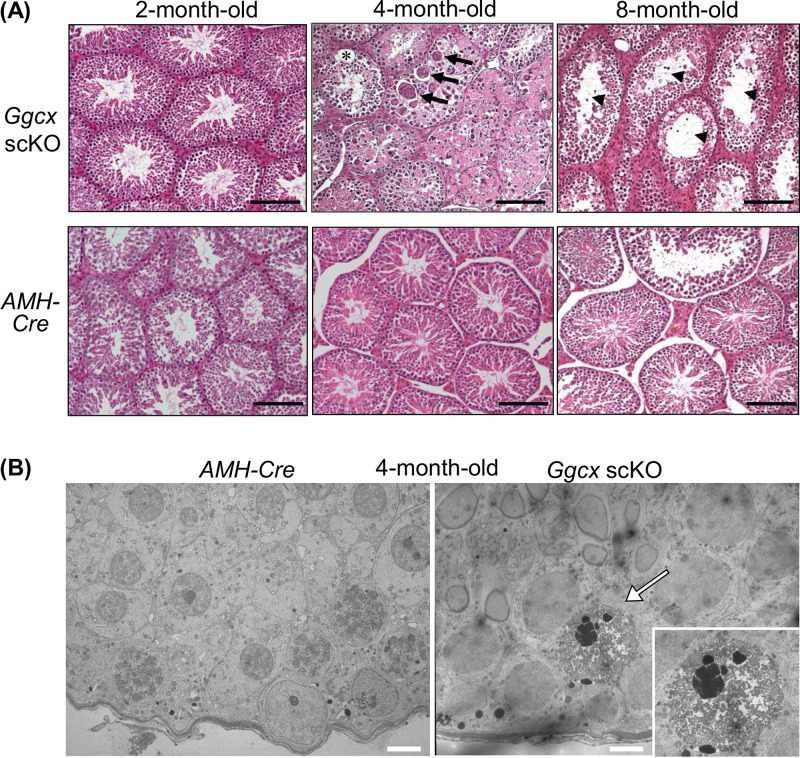
We next examined testicular histology by hematoxylin and eosin (HE) staining (Fig. 3A). Although the 2-month-old Ggcx scKO mice exhibited nearly normal testicular histology, their testis size was smaller than that of AMH-Cre mice. In contrast, drastic histological abnormality was detected in Ggcx scKO testes at both 4 and 8 months of age. The 4-month-old Ggcx scKO testes exhibited large multinuclear spermatids and intercellular space. The 8-month-old Ggcx scKO testes exhibited large clear lumen regions of the seminiferous tubules with severely decreased spermatids. Further analysis by transmission electron microscopy (TEM) revealed that the multinuclear spermatids possess chromatin aggregation, nuclear fragmentation, vacuoles, and destroyed organelles, implicating apoptotic features (Fig. 3B) (24, 25).
FIG 3.
Histological anomaly in Ggcx scKO testis. Hematoxylin and eosin (HE) staining (A) and transmission electron microscopic analysis (B) of testes were performed in Ggcx scKO and AMH-Cre mice. In panel A, arrows and arrowheads indicate large multinuclear spermatids and intercellular space in seminiferous tubules, respectively. Bars, 50 μm. In panel B, the arrow indicates apoptotic alteration of multinuclear spermatids. The inset shows a higher-magnification image. Bars, 5 μm.
Increased apoptosis in Ggcx scKO testes.
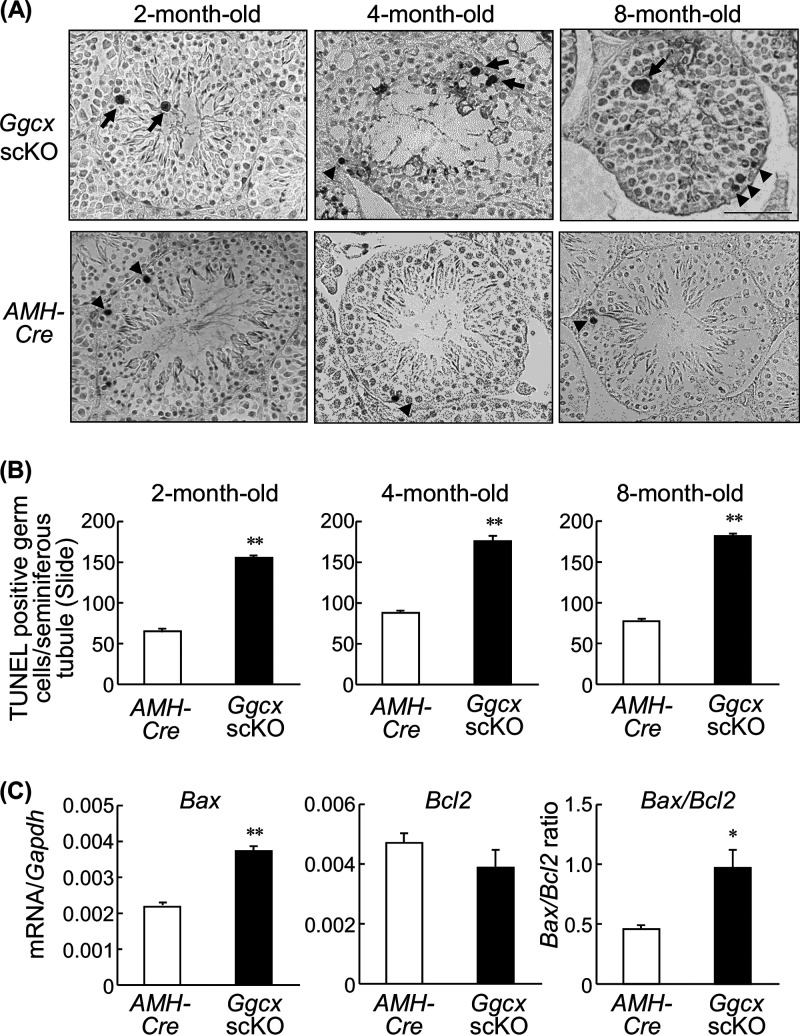
To assess whether apoptosis predominantly occurred in Ggcx scKO testes, a terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) assay was performed on testis sections of 2-, 4-, and 8-month-old mice (Fig. 4A). While TUNEL-labeled spermatogenic cells were observed in AMH-Cre mouse testes throughout the examined period, the counts of labeled cells were significantly increased in age-matched Ggcx scKO mouse testes (Fig. 4B). Large TUNEL-positive spermatocytes were especially observed in Ggcx scKO mouse testes (Fig. 4A). In addition, we examined the expression levels of proapoptotic Bax and antiapoptotic Bcl2 in 8-month-old AMH-Cre and Ggcx scKO testes (Fig. 4C). Bax expression and Bax/Bcl2 ratios were significantly elevated in Ggcx scKO compared with AMH-Cre mice.
FIG 4.
Increase of TUNEL-positive cells in Ggcx scKO testes. (A) TUNEL staining of the testis paraffin sections from 2-, 4-, and 8-month-old Ggcx cKO and AMH-Cre mice. Arrowheads indicate TUNEL-labeled spermatogenic cells observed in both AMH-Cre and Ggcx scKO mice. Arrows indicate large TUNEL-positive spermatocytes that are observed predominantly in Ggcx scKO testes. (B) Number of TUNEL-positive cells. (C) Expression levels of Bax and Bcl2 mRNAs were quantified by qRT-PCR in 8-month-old Ggcx cKO and AMH-Cre mouse testes. Data are presented as means ± SEM (n = 3). *, P < 0.05; **, P < 0.01 (using Student’s t test).
Abnormal spermatozoa in Ggcx scKO mouse testes.
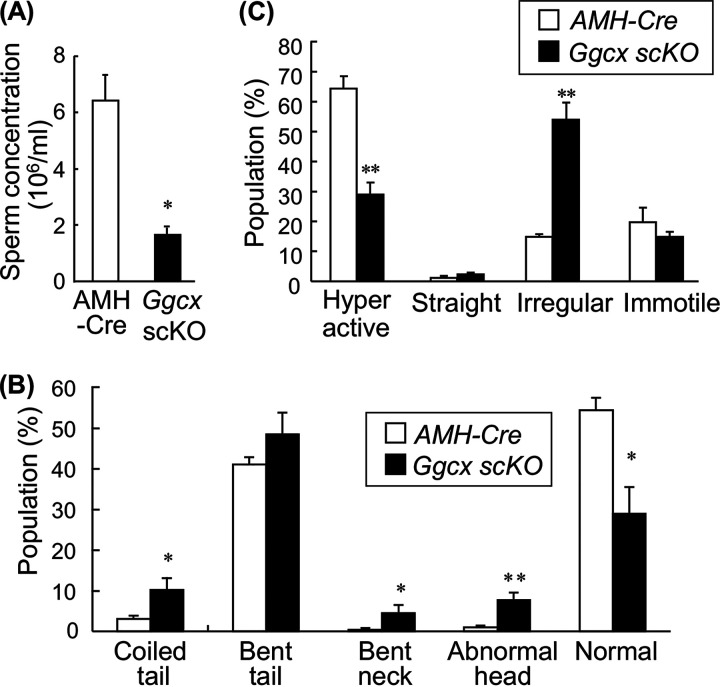
We then analyzed the phenotype of spermatozoa prepared from the epididymis. The sperm concentration was significantly decreased in the Ggcx scKO mice compared to AMH-Cre mice (Fig. 5A). In addition, the populations of morphologically abnormal spermatozoa were significantly increased in Ggcx scKO mice compared with control mice, exhibiting phenotypes with a disordered flagellum and sperm head, as exemplified by a coiled tail, bent neck, and abnormal head (Fig. 5B). In the sperm motility analysis, the percentage of normal hyperactive sperm cells of Ggcx scKO mouse testes was decreased to half of that of AMH-Cre mouse testes (Fig. 5C). In contrast, irregularly moving sperm cells were drastically increased in Ggcx scKO mouse testes compared to AMH-Cre mouse testes. However, a spontaneous acrosome reaction of spermatozoa was similarly found in AMH-Cre (2.38- ± 1.84-fold) and Ggcx scKO (2.42- ± 0.92-fold) mice during capacitation incubation, suggesting no apparent defect in sperm capacitation.
FIG 5.
Abnormal phenotype of spermatozoa in Ggcx scKO mice. (A) Sperm concentration from cauda epididymides in Ggcx scKO and control mice. Sperm cells were recovered from the cauda epididymides of 4-month-old Ggcx scKO (n = 3) and AMH-Cre (n = 3) mice. (B) Morphological analysis of sperm cells. Morphological abnormalities of sperm cells were classified into 4 categories: round tail, bent midpiece, bent head, and unclear head. At least 100 sperm cells per mouse were examined. (C) Sperm motility analysis. Sperm motility was categorized by either hyperactive, straight, irregular, or immotile sperm. At least 100 sperm cells per mouse were counted. Data are presented as means ± SEM (n = 3). *, P < 0.05, **, P < 0.01 (using Student’s t test).
Abnormal expression and localization of connexin 43 in Ggcx scKO Sertoli cells.
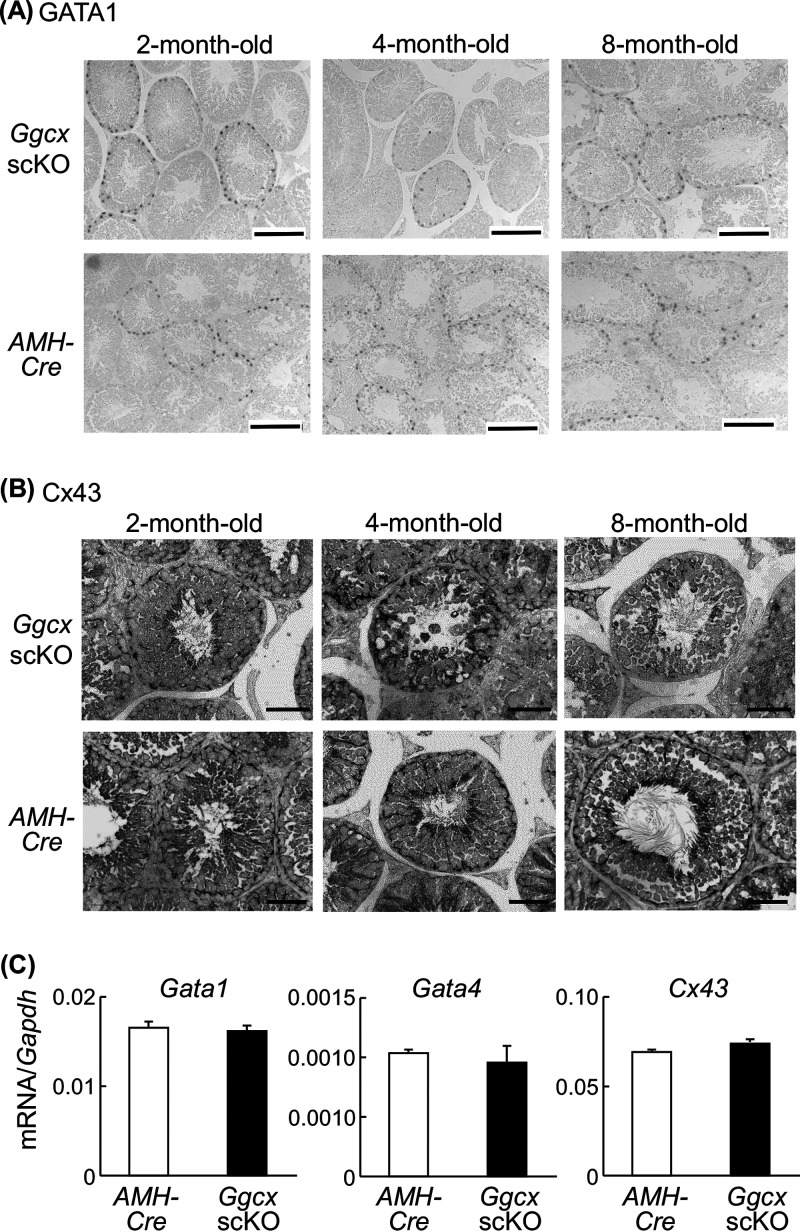
To further define the physiological characteristic of Sertoli cells in Ggcx scKO mouse testes, we performed immunohistochemical analysis for the transcriptional factor GATA1 and the gap junction protein connexin 43 (Cx43). GATA1 is known as a Sertoli cell-specific marker that functions as a developmental stage- and spermatogenic cycle-specific regulator of gene expression (26, 27). Cx43 is a predominant testicular gap junction protein and plays an essential role in spermatogenesis but not spermatogonial maintenance/proliferation in Sertoli cells (28). GATA1-positive signals in Ggcx scKO testes were basically equal to those in AMH-Cre testes (Fig. 6A). While Cx43-positive signals were substantially observed at the basal side of Sertoli cells in AMH-Cre testes, those in Ggcx scKO mice were distorted, particularly at 4 and 8 months of age (Fig. 6B). The signals were slightly moved to the inner side of the seminiferous tubules in 4-month-old Ggcx scKO mouse testes, and their intensity was substantially reduced in 8-month-old Ggcx scKO mouse testes. Taken together, the expression and localization of Cx43 were disordered in Sertoli cells of Ggcx scKO mouse testes. However, the mRNA levels of Gata1 and Cx43, in addition to Gata4, were not substantially different between AMH-Cre and Ggcx scKO mice (Fig. 6C), implying that the transcription of these genes is not primarily regulated by Ggcx-mediated signaling pathways.
FIG 6.
Expression of Sertoli cell-specific markers GATA1, GATA4, and connexin 43 (Cx43) in Ggcx scKO testes. (A and B) Immunohistochemistry of GATA1 (A) and Cx43 (B) was performed on testicular sections of Ggcx scKO and AMH-Cre mice. Bars, 100 μm for Gata1 and 50 μm for Cx43. (C) Expression levels of Gata1, Gata4, and Cx43 mRNAs were quantified by qRT-PCR in 8-month-old Ggcx cKO and AMH-Cre mouse testes. Data are presented as means ± SEM (n = 3).
Cx43 overexpression rescues the pathological testicular phenotype of Ggcx scKO mice.
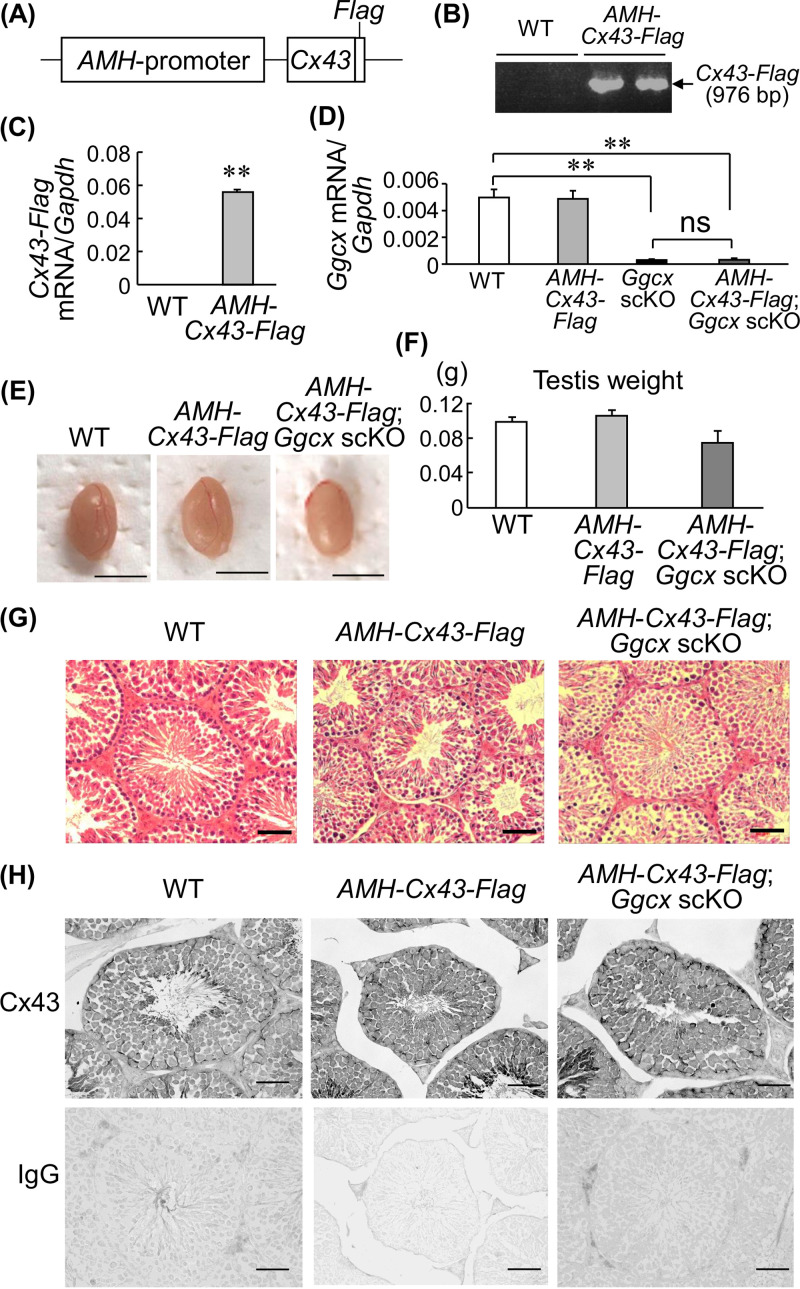
Because Cx43 plays a role in testicular germ cell development and differentiation (29), we further investigated whether Cx43 overexpression in Sertoli cells of Ggcx scKO mice rescues their infertility. We generated transgenic mice expressing C-terminally Flag-tagged Cx43 under the control of the AMH promoter (AMH-Cx43-Flag) (Fig. 7A and B). The AHM-Cx43-Flag mice expressed the Cx43-Flag transgene in the testes (Fig. 7C). AMH-Cx43-Flag mice were then crossed with Ggcx scKO mice to generate AMH-Cx43-Flag; Ggcx scKO mice. In AMH-Cx43-Flag; Ggcx scKO testis, the Ggcx level was similar to that in Ggcx scKO mice and significantly lower than those in WT and AMH-Cx43-Flag mice, indicating adequate testicular Ggcx ablation in crossing Ggcx scKO mice with AMH-Cx43-Flag mice (Fig. 7D). Fertility analysis revealed that male AMH-Cx43-Flag; Ggcx scKO mice have reproductive abilities, indicating that Cx43 overexpression in Sertoli cells recovers the sterility of Ggcx scKO mice (Table 3). The testis size and weight of AMH-Cx43-Flag; Ggcx scKO mice at 8 months of age were not significantly different from those of WT and AMH-Cx43-Flag mice (Fig. 7E and F). Moreover, no apparent abnormality was observed in the testicular histology of AMH-Cx43-Flag; Ggcx scKO mice at 8 months of age compared with the testicular histology of Ggcx scKO mice with multinuclear spermatids and large clear lumen regions (Fig. 7G). Moreover, immunohistochemical analysis demonstrated that the Cx43 protein distribution in Ggcx scKO testis rescued by Cx43 was recovered to a level similar to that in WT or AMH-Cx43-Flag mice (Fig. 7H).
FIG 7.
Cx43 rescues the Ggcx scKO phenotype. (A) Schematic of the AMH promoter-driven C-terminally Flag-tagged Ggcx (AMH-Cx43-Flag) transgene. (B) Generation of AMH-Cx43-Flag mice. Genotyping was performed using genomic DNA extracted from tails, using primer pairs that were derived from the AMH promoter and Cx43 and Flag sequences. (C) Expression levels of the Cx43-Flag transgene were quantified by qRT-PCR in testes from 2-month-old AMH-Cx43-Flag and WT mice. (D) Ggcx levels in WT, AMH-Cx43-Flag, Ggcx scKO, and AMH-Cx43-Flag; Ggcx scKO mouse testes. Ggcx expression levels were quantified by qRT-PCR in the testes from 8-month-old mice. Data are presented as means ± SEM (n = 3). **, P < 0.01 (using Student’s t test). ns, not significant. (E) Representative testes of WT, AMH-Cx43-Flag, and AMH-Cx43-Flag; Ggcx scKO mice at 8 months of age. Bars, 5 mm. (F) Testis weights of WT, AMH-Cx43-Flag, and AMH-Cx43-Flag; Ggcx scKO mice at 8 months of age. Data are presented as means ± SEM (n = 3). (G and H) HE staining (G) and immunohistochemistry of Cx43 (H) of testes from 8-month-old WT, AMH-Cx43-Flag, and AMH-Cx43-Flag; Ggcx scKO mice. Immunohistochemistry using normal IgG instead of the antibody is also shown. Bars, 50 μm.
TABLE 3.
Fertility evaluation of male AMH-Cx43-Flag; Ggcx scKO micea
| Age of males (mo) | No. of mated pairs | No. of pregnancies | No. of litters | Avg litter size |
|---|---|---|---|---|
| 2 | 2 | 2 | 12 | 6.0 |
| 8 | 2 | 2 | 13 | 6.5 |
Individual male AMH-Cx43-Flag; Ggcx scKO mice (n = 2 at 2 and 8 months of age) were mated with one WT (C57BL/6) female for 3 months.
DISCUSSION
In the present study, we generated Sertoli cell-specific Ggcx knockout (Ggcx scKO) mice and analyzed the role of GGCX in spermatogenesis. The Ggcx scKO mice exhibited late-onset male infertility. Histologically, their testes showed substantial atrophy in seminiferous tubules and increased apoptotic degeneration of spermatids and spermatocytes. Decreased concentration and motility were observed in sperm cells prepared from the epididymis of Ggcx scKO mice. Our results reveal that GGCX in Sertoli cells plays a crucial role in spermatogenesis.
In seminiferous tubules, the interface between adjacent Sertoli cells near the basement membrane consists of the immunological barrier system called the blood-testis barrier (BTB) by specialized junctions, including desmosomes, gap junctions, and tight junctions (30). Sertoli cells exert essential functions in testicular differentiation and development in embryos by producing AMH, which causes developmental regression of Müllerian ducts (31). In adults, Sertoli cells function as supporting or nursing cells for testicular germ cells in spermatogenesis (32, 33). The actin-based anchoring cell-cell junction between Sertoli cells and germ cells at the stage of the seminiferous epithelium cycle is especially known as apical ectoplasmic specialization (ES) (30), which structurally provides a spermatogenic niche that regulates the migration and release of germ cells (33–35). The junctions between adjacent Sertoli cells also comprise the basal ES at the BTB, along with desmosomes, gap junctions, and tight junctions (30). Spermatocytes translocate to the adluminal compartment via the BTB at specific seminiferous epithelial cycle stages (36). Considering that Sertoli cells are the main component in the BTB and ES, we assume that GGCX and its substrates in Sertoli cells exert particular functions in spermatogenesis.
Cx43 is a major type of connexin in the testes and predominantly expressed in Sertoli cells (37). Cx43 comprises gap junctions that are located in the apical ES between Sertoli cells and germ cells, including spermatogonia and primary spermatocytes (38, 39). The loss of Sertoli cell-specific Cx43 in mice clarified that Cx43 is necessary for normal testicular development and spermatogenesis (28, 29, 40, 41). Sertoli cell-specific Cx43 KO mice exhibited smaller testicular sizes and weights, reductions of spermatogonia, and increases in apoptotic germ cells, leading to spermatogenic arrest. Because the testicular phenotype of Sertoli cell-specific Cx43 KO mice exhibits similarity compared with that of Ggcx scKO mice in our study, we assume that a loss of function of GGCX may impair the function of Cx43 in the gap junctions of Sertoli cells and germ cells or the BTB system.
Intriguingly, AMH-Cx43-Flag; Ggcs scKO mice in the present study exhibited a phenotype with normal histology of seminiferous tubules and male fertility, indicating that Cx43 overexpression in Sertoli cells can rescue the loss of function of GGCX in Ggcx KO mice. Our results indicated that the expression levels of Cx43 mRNA are not significantly changed between the testes of 8-month-old WT and Ggcx scKO mice. In addition, Ggcx scKO testes with Cx43 overexpression (AMH-Cx43-Flag; Ggcx scKO) exhibited normal testis morphology and Cx43 tissue distribution, suggesting that Cx43 overexpression would recover the physiological functions of Sertoli cells. Based on these results, we speculate that the aberrant distribution of Cx43 protein in Ggcx scKO testes originates from the abnormality of Sertoli cell morphology and functions due to the Ggcx ablation rather than from the undercarboxylation of Gla residues in the Cx43 protein.
On the other hand, elevated Bax expression levels and Bax/Bcl2 ratios were found in Ggcx scKO mouse testes compared with those in WT mice. It has been reported that the imbalance of the Bax/Bcl2 ratio due to the upregulation of Bax and the downregulation of Bcl2 was substantially associated with germ cell apoptosis mediated by oxidative stress in a study of male mice orally administered T-2 toxin (42). We speculate that the aberrant activation of the mitochondrial apoptosis pathway may be upregulated in spermatogenesis of Ggcx scKO mice.
In Ggcx scKO mice, severely impaired seminiferous tubules were observed only after 2 months of age, suggesting that the function of GGCX in Sertoli cells is particularly relevant to sex maturation. During puberty, the major androgen hormone testosterone in testis is produced by Leydig cells. Testosterone is considered to play critical roles in the spermatogenesis process, including in the maintenance of the BTB, Sertoli cell-spermatid adhesion, meiosis, and sperm release (43). Androgen receptor (AR) facilitates BTB remodeling, which is necessary to transmit germ cells from the basal compartment to the adluminal compartment to complete meiosis (44). Because germ cells do not express AR (45–48), it is likely that testosterone acts on AR-expressing cells in seminiferous tubules, such as Sertoli cells, in a paracrine fashion. Notably, Sertoli cell-specific AR knockout male mice exhibited reduced testis weight with no elongated spermatids and germ cell arrest during meiosis (49, 50). In AR hypomorphic mice with decreased AR activity, Sertoli cells could not maintain their adhesive connection to elongated spermatids, thereby inducing premature release and spermatid loss (51). As a recent study revealed that AR is a substrate for γ-carboxylation (52), GGCX may affect puberty by modulating androgen signaling.
As other known GGCX targets, Gas6 and protein S may also be involved in the development of germ cells because these Gla-modified proteins are produced by Leydig cells before sexual maturity and by both Leydig and Sertoli cells thereafter (53). The Tyro3, Axl, and Mer (TAM) receptor tyrosine kinases are receptors for Gas6 and protein S, and triple-knockout mice that lack all three TAM receptors (TAM TKO mice) demonstrate azoospermia and possess abnormal seminiferous tubules that are filled with apoptotic germ cells (53). All three TAM receptors and both of their ligands are expressed in Sertoli cells, and the conjunctive activation of TAM receptors is crucial for the phagocytosis of apoptotic germ cells during spermatogenesis. We assume that a prototypic Gla-containing protein, Gas6, could be a relevant substrate of GGCX in testis, as its function in Sertoli cells has been shown to remove apoptotic cells by binding to the Tyro3 subfamily of the receptor tyrosine kinases Tyro3 and Axl (54). We speculate that the enhancement of apoptosis in Ggcx scKO testis could be partly due to the increase in undercarboxylated Gla-modified Gas6, leading to the reduction of the elimination of apoptotic cells by Sertoli cells.
Interestingly, these degenerative phenotypes of TAM TKO mice were found after 5 weeks of age ∼1 week after the onset of active sperm production (53). Thus, it is implied that GGCX regulates spermatogenesis cooperatively with androgen signaling. TAM receptor tyrosine kinases also play important roles in the phagocytosis process of apoptotic cells in adult testes (55). Consistent with our findings, the GGCX-Gas6/protein S-TAM axis contributes to phagocytosis in Sertoli cells.
In spermiogenesis, GGCX and its known substrate matrix Gla protein (MGP) colocalized in vesicular structures of the epithelial cell cytoplasm and spermatozoon surface in the epididymal lumen, as analyzed in a warfarin-treated rat study (19). Carboxylated MGP reduces the calcium concentration in epididymal fluid, which is critical for normal spermiogenesis. Thus, the intercellular communication network constructed by vesicle-mediated transport and membrane trafficking may play an important role in spermiogenesis in the epididymis. Although MGP expression is relatively low in the testis, MGP might also play a role in Sertoli cells.
MATERIALS AND METHODS
Generation of AMH-Cre and AMH-Cx43-Flag mice and genotyping.
All animal experiments were approved by the Animal Care and Use Committee of the Saitama Medical University. Mice were maintained in a temperature-controlled room (23°C) with a 12-h-light/dark schedule and fed a standard diet (CE2; Clea Japan, Tokyo, Japan) with free access to water. The LPANH3 plasmid that contains the human AMH gene promoter was kindly provided by Jean-Yves Picard (21). The AMH promoter region (3.6 kb) was inserted into pxCANCre by exchanging the CAG (cytomegalovirus enhancer, chicken β-actin promoter, and rabbit β-globin splice acceptor site) promoter (56). The resulting AMH-Cre plasmid was linearized by restriction enzyme digestion and microinjected into the pronuclei of fertilized eggs from C57BL/6 mice, as previously described (57). AMH-Cre transgenic mice were identified by a PCR assay of the genomic DNA using primers for Cre (4). In addition, the same AMH promoter region and C-terminally Flag-tagged mouse Cx43 cDNA were inserted into pcDNA3 (Invitrogen, Carlsbad, CA, USA) by exchanging the cytomegalovirus (CMV) promoter. The resulting AMH-Cx43-Flag plasmid was used to generate AMH-Cx43-Flag transgenic mice. The AMH-Cx43-Flag transgenic mice were identified by a PCR assay of the genomic DNA using primers that are specific to the AMH promoter and Cx43 cDNA (5′-AGAGATGGCTGTACCTTGGAGAT-3′ and 5′-CAATCCCATACTTGAACTTCTTGAT-3′, respectively).
Generation of Sertoli cell-specific Ggcx-deficient (AMH-Cre; Ggcxflox/flox scKO) mice and genotyping.
Ggcx-floxed mice and their genotyping were previously described (4, 14, 15). AMH-Cre mice were mated with Ggcxflox/flox mice, and their offspring were subsequently intercrossed to generate Sertoli cell-specific Ggcx-deficient (Ggcxflox/flox; AMH-Cre scKO) mice.
Fertility assay.
Individual male Ggcx scKO (n = 2 at 2 months of age, and n = 3 at 3 and 6 months of age) and AMH-Cx43; Ggcx scKO (n = 2 at 2 and 8 months of age) mice were mated with one or two WT (C57BL/6) females for 3 months. C57BL/6 mice were purchased from Clea Japan.
β-Galactosidase staining.
ROSA26-LacZ reporter mice were obtained from the Jackson Laboratory (4). Sertoli cell-specific expression of Cre recombinase was confirmed by mating AMH-Cre mice with ROSA26-LacZ mice. β-Galactosidase staining of the testes was performed, as previously described (22). Briefly, frozen sections of the testes were incubated in phosphate-buffered saline (PBS) containing 2 mM MgCl2, 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, and 20 mM 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-gal) at 37°C for 20 h. The sections were counterstained with orange G.
Quantitative reverse transcriptase PCR.
Total RNAs were extracted from the testes using Isogen reagent (Nippon Gene, Tokyo, Japan). To investigate the gene expression of Ggcx, Cx43, Bax, and Bcl2, quantitative reverse transcriptase PCR (qRT-PCR) was performed as previously described (58). First-strand cDNA generated from the total RNA was subjected to qRT-PCR using the SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and the ABI Prism 7000 system (Applied Biosystems). The sequences of PCR primers are as follows: 5′-TTCATCGCGGGTGTGAAGA-3′ (forward) and 5′-CTCCGACAACACCAGCTTGAA-3′ (reverse) for Ggcx, 5′-GAACTCCAGCCCTTAGCTATCGT-3′ (Cx43 forward) and 5′-TTATTTGTCATCATCATCGTCCTTATAGTC-3′ (Flag reverse) for C-terminally Flag-tagged Cx43, 5′-CTCACCTATGTCTCCTCCTGGG-3′ (forward) and 5′-GGGAGTTGGAGATGGTGCTTC-3′ (reverse) for Cx43, 5′-GCTGCAGACATGCTGTGGATC-3′ (forward) and 5′-TCACAGCCAGGAGAATCGCAC-3′ (reverse) for Bax, 5′-ACCGTCGTGACTTCGCAGAG-3′ (forward) and 5′-GGTGTGCAGATGCCGGTTCA-3′ (reverse) for Bcl2, 5′-GTCAGAACCGGCCTCTCATC-3′ (forward) and 5′-GGTGCCTGCCCGTTTG-3′ (reverse) for Gata1, 5′-CCTGGAAGACACCCCAATCTC-3′ (forward) and 5′-GCCCCACAATTGACACACTCT-3′ (reverse) for Gata4, and 5′-GCATGGCCTTCCGTGTTC-3′ (forward) and 5′-TGTCATCATACTTGGCAGGTTTCT-3′ (reverse) for the glyceraldehyde-3-phosphate dehydrogenase gene (Gapdh). A comparative analysis of the PCR product amounts was conducted by the comparative cycle threshold (CT) method using Gapdh as a control.
Western blot analysis.
The cytosolic fraction was prepared from the 8-month-old Ggcx scKO and WT testes by homogenization using radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.0], and 1% Triton X-100) and centrifugation at 19,100 × g for 20 min at 4°C. These samples were incubated with a sample buffer (100 mM Tris-HCl [pH 6.5], 4% SDS, 20% glycerol, 0.2% bromophenol blue, and 4% 2-mercaptoethanol) at 37°C for 5 min, resolved using 8% SDS-PAGE, and finally electrophoretically transferred onto polyvinylidene difluoride membranes (Merck Millipore, Billerica, MA, USA). The membranes were probed with diluted anti-human GGCX antiserum at a ratio of 1:10,000 (14) and diluted anti-β-actin antibodies at a ratio of 1:10,000 (AC-74; Sigma-Aldrich, St. Louis, MO, USA). Binding of the primary antibodies was detected by diluted horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse immunoglobulin G (IgG) antibodies at a ratio of 1:4,000 (GE Healthcare, Menlo Park, CA). Immunoreactive proteins were visualized using enhanced chemiluminescence (Pierce ECL Plus Western blotting substrate; Thermo Fisher Scientific, Somerset, NJ, USA).
Measurement of serum testosterone levels.
Serum testosterone levels of male 2-, 4-, and 8-month-old Ggcx scKO and control mice were measured using the rodent testosterone enzyme-linked immunosorbent assay (ELISA) kit (Endocrine Technologies, Newark, CA, USA) according to the manufacturer’s instructions.
Hematoxylin and eosin staining.
Testes were fixed in 4% paraformaldehyde (PFA) in PBS at 4°C overnight, embedded in paraffin, cut into 5-μm-thick sections, and placed onto glass slides that were coated with 3-aminopropyltriethoxysilane. Using a standard protocol, the slides were deparaffinized, rehydrated, and stained with hematoxylin and eosin (HE).
Transmission electron microscopy.
Mice were perfused with a 4% paraformaldehyde solution, and the testes were then resected for subsequent fixation in half-Karnovsky solution (2% paraformaldehyde and 2.5% glutaraldehyde diluted in a 0.067 M cacodylate buffer [pH 7.4]). The specimens were postfixed with OsO4 and dehydrated with ascending concentrations of acetone prior to epoxy resin embedding as previously described (15). Ultrathin sections of the testes were placed onto grids and stained with uranyl acetate and lead citrate for transmission electron microscopy (TEM) observation as previously described (59).
Immunohistochemistry.
Immunohistochemical analysis was performed as previously described (14, 60). The sections were reacted with anti-GGCX rabbit antibody (10 μg/ml) (14), anti-GATA1 rat antibody (10 μg/ml) (catalog number sc-265; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-Cx43 rabbit antibody (0.32 μg/ml) (catalog number C6219; Sigma-Aldrich) for 3 h at room temperature and subsequently incubated for 1 h with HRP-labeled anti-rabbit or -rat IgG at a dilution ratio of 1:200. The sites of HRP were visualized after incubation with a solution that contains diaminobenzidine, H2O2, CoCl2-6H2O, and NiSO4-6H2O. As a control, sections were reacted with normal rabbit or rat IgG rather than the primary antibodies.
TUNEL staining.
TUNEL staining was performed according to a previously described protocol (60). The sections were deparaffinized, rehydrated, and treated with proteinase K (10 μg/ml) for 15 min at 37°C. After rinsing, the sections were preincubated with terminal deoxynucleotidyltransferase (TdT) buffer (Roche Diagnostics, Penzberg, Germany) for 30 min at room temperature and then reacted with 800 U/ml TdT in TdT buffer that was supplemented with 0.1 mM dithiothreitol, 1.5 mM cobalt chloride, 20 μM dATP, and 1 μM biotin-16-dUTP for 90 min at 37°C. Next, the reaction mixture was washed with 50 mM Tris-HCl buffer (pH 7.5), and the reaction was effectively terminated. Endogenous peroxidase was inactivated by immersing the sections in 0.3% H2O2 and methanol for 15 min. The signals were detected immunohistochemically with HRP-conjugated goat antibiotin antibody (catalog number SP-3010; Vector Laboratories, Burlingame, CA, USA).
Analysis of spermatozoa.
Sperm samples were collected from the cauda epididymides of 4-month-old Ggcx scKO (n = 3) and control (n = 3) mice and transferred to 400 μl of Toyoda Yokoyama Hoshi (TYH) medium (LSI Medience, Tokyo, Japan) covered with paraffin oil. After incubation at 35°C for 2 h, sperm concentration and morphology were measured using a hemocytometer. The morphological abnormalities of the sperm cells were classified into the following 4 categories: (i) coiled tail, (ii) bent tail, (iii) bent neck, and (iv) abnormal head. Sperm motility was categorized as either hyperactive, straight, irregular, or immotile. At least 100 sperm cells per mouse were counted. Spontaneous acrosome reactions of spermatozoa from WT and Ggcx scKO mice were compared by Coomassie staining before and after capacitation incubation.
Statistical analysis.
Differences between two groups were analyzed for statistical significance using unpaired Student’s t test.
Data availability.
The data that support the findings of this study are fully available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
We thank T. Tsukui and Y. Takahashi for their kind support in the generation of transgenic animals and in the analysis of spermatozoa. We also thank S. Kondo, M. Fujitani, T. Suzuki, N. Sasaki, and W. Sato for their technical assistance.
This work was partially supported by a grant of the Support Project of the Strategic Research Center in Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to Satoshi Inoue); by grants from the Japan Society for the Promotion of Science, Japan (16K09809 [to Kazuhiro Ikeda], 20H03734 [to Kuniko Horie-Inoue], 17H04205 [to Kuniko Horie-Inoue], and 19K07404 [to Sachiko Shiba]); and by the Takeda Science Foundation (to Satoshi Inoue).
We declare no conflicts of interest.
Satoshi Inoue and Sachiko Shiba, conception and design; Sachiko Shiba, Kazuhiro Ikeda, Toshihiko Takeiwa, and Yasuaki Shibata, performance of experiments and development of methodology; Tomoka Hasegawa and Kotaro Azuma, acquisition of data; Norio Amizuka, Tomoaki Tanaka, Kuniko Horie-Inoue, and Takehiko Koji, analysis and interpretation of data; Sachiko Shiba, Kazuhiro Ikeda, and Kuniko Horie-Inoue, writing the original draft of the manuscript; Norio Amizuka, Tomoaki Tanaka, Takehiko Koji, and Satoshi Inoue, reviewing and editing the manuscript.
REFERENCES
- 1.Azuma K, Ouchi Y, Inoue S. 2014. Vitamin K: novel molecular mechanisms of action and its roles in osteoporosis. Geriatr Gerontol Int 14:1–7. doi: 10.1111/ggi.12060. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. 2010. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 468:117–121. doi: 10.1038/nature09464. [DOI] [PubMed] [Google Scholar]
- 3.Azuma K, Inoue S. 2019. Multiple modes of vitamin K actions in aging-related musculoskeletal disorders. Int J Mol Sci 20:2844. doi: 10.3390/ijms20112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azuma K, Tsukui T, Ikeda K, Shiba S, Nakagawa K, Okano T, Urano T, Horie-Inoue K, Ouchi Y, Ikawa M, Inoue S. 2014. Liver-specific γ-glutamyl carboxylase-deficient mice display bleeding diathesis and short life span. PLoS One 9:e88643. doi: 10.1371/journal.pone.0088643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkner KL, Pudota BN. 1998. Vitamin K-dependent carboxylation of the carboxylase. Proc Natl Acad Sci U S A 95:466–471. doi: 10.1073/pnas.95.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker CH, Morgan CR, Rand KD, Engen JR, Jorgenson JW, Stafford DW. 2014. A conformational investigation of propeptide binding to the integral membrane protein γ-glutamyl carboxylase using nanodisc hydrogen exchange mass spectrometry. Biochemistry 53:1511–1520. doi: 10.1021/bi401536m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B, Sánchez-Vega B, Wu SM, Lanir N, Stafford DW, Solera J. 1998. A missense mutation in gamma-glutamyl carboxylase gene causes combined deficiency of all vitamin K-dependent blood coagulation factors. Blood 92:4554–4559. doi: 10.1182/blood.V92.12.4554. [DOI] [PubMed] [Google Scholar]
- 8.Spronk HM, Farah RA, Buchanan GR, Vermeer C, Soute BA. 2000. Novel mutation in the gamma-glutamyl carboxylase gene resulting in congenital combined deficiency of all vitamin K-dependent blood coagulation factors. Blood 96:3650–3652. doi: 10.1182/blood.V96.10.3650. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita H, Nakagawa K, Narusawa K, Goseki-Sone M, Fukushi-Irie M, Mizoi L, Yoshida H, Okano T, Nakamura T, Suzuki T, Inoue S, Orimo H, Ouchi Y, Hosoi T. 2007. A functional single nucleotide polymorphism in the vitamin-K-dependent gamma-glutamyl carboxylase gene (Arg325Gln) is associated with bone mineral density in elderly Japanese women. Bone 40:451–456. doi: 10.1016/j.bone.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Zhu A, Sun H, Raymond RM, Furie BC, Furie B, Bronstein M, Kaufman RJ, Westrick R, Ginsburg D. 2007. Fatal hemorrhage in mice lacking gamma-glutamyl carboxylase. Blood 109:5270–5275. doi: 10.1182/blood-2006-12-064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A 105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. 2010. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiba S, Ikeda K, Azuma K, Hasegawa T, Amizuka N, Horie-Inoue K, Inoue S. 2014. γ-Glutamyl carboxylase in osteoblasts regulates glucose metabolism in mice. Biochem Biophys Res Commun 453:350–355. doi: 10.1016/j.bbrc.2014.09.091. [DOI] [PubMed] [Google Scholar]
- 15.Azuma K, Shiba S, Hasegawa T, Ikeda K, Urano T, Horie-Inoue K, Ouchi Y, Amizuka N, Inoue S. 2015. Osteoblast-specific γ-glutamyl carboxylase-deficient mice display enhanced bone formation with aberrant mineralization. J Bone Miner Res 30:1245–1254. doi: 10.1002/jbmr.2463. [DOI] [PubMed] [Google Scholar]
- 16.Garcia AA, Reitsma PH. 2008. VKORC1 and the vitamin K cycle. Vitam Horm 78:23–33. doi: 10.1016/S0083-6729(07)00002-7. [DOI] [PubMed] [Google Scholar]
- 17.Roncaglioni MC, Soute BA, de Boer-Van den Berg MA, Vermeer C. 1983. Warfarin-induced accumulation of vitamin K-dependent proteins. Comparison between hepatic and non-hepatic tissues. Biochem Biophys Res Commun 114:991–997. doi: 10.1016/0006-291x(83)90658-7. [DOI] [PubMed] [Google Scholar]
- 18.Thijssen HH, Janssen CA, Drittij-Reijnders MJ. 1986. The effect of S-warfarin administration on vitamin K 2,3-epoxide reductase activity in liver, kidney and testis of the rat. Biochem Pharmacol 35:3277–3282. doi: 10.1016/0006-2952(86)90424-7. [DOI] [PubMed] [Google Scholar]
- 19.Ma H, Zhang BL, Liu BY, Shi S, Gao DY, Zhang TC, Shi HJ, Li Z, Shum WW. 2019. Vitamin K2-dependent GGCX and MGP are required for homeostatic calcium regulation of sperm maturation. iScience 14:210–225. doi: 10.1016/j.isci.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyaolu AO, Oremosu AA, Osinubi AA, Vermeer C, Daramola AO. 2019. Warfarin-induced vitamin K deficiency affects spermatogenesis in Sprague-Dawley rats. Andrologia 51:e13416. doi: 10.1111/and.13416. [DOI] [PubMed] [Google Scholar]
- 21.Dutertre M, Rey R, Porteu A, Josso N, Picard JY. 1997. A mouse Sertoli cell line expressing anti-Müllerian hormone and its type II receptor. Mol Cell Endocrinol 136:57–65. doi: 10.1016/s0303-7207(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 22.Lécureuil C, Fontaine I, Crepieux P, Guillou F. 2002. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis 33:114–118. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- 23.Griswold MD. 1998. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 24.Wang RA, Nakane PK, Koji T. 1998. Autonomous cell death of mouse male germ cells during fetal and postnatal period. Biol Reprod 58:1250–1256. doi: 10.1095/biolreprod58.5.1250. [DOI] [PubMed] [Google Scholar]
- 25.Koji T, Hishikawa Y. 2003. Germ cell apoptosis and its molecular trigger in mouse testes. Arch Histol Cytol 66:1–16. doi: 10.1679/aohc.66.1. [DOI] [PubMed] [Google Scholar]
- 26.Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, Yamamoto M. 1994. Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Development 120:1759–1766. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis S, Elliott DJ, Morgan D, Winston R, Readhead C. 2005. Molecular markers for the assessment of postnatal male germ cell development in the mouse. Hum Reprod 20:108–116. doi: 10.1093/humrep/deh565. [DOI] [PubMed] [Google Scholar]
- 28.Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. 2007. Proliferation of adult Sertoli cells following conditional knockout of the gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod 76:804–812. doi: 10.1095/biolreprod.106.059212. [DOI] [PubMed] [Google Scholar]
- 29.Rode K, Weider K, Damm OS, Wistuba J, Langeheine M, Brehm R. 2018. Loss of connexin 43 in Sertoli cells provokes postnatal spermatogonial arrest, reduced germ cell numbers and impaired spermatogenesis. Reprod Biol 18:456–466. doi: 10.1016/j.repbio.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CY, Mruk DD. 2010. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picard J-Y, Josso N. 2019. Persistent Müllerian duct syndrome: an update. Reprod Fertil Dev 31:1240–1245. doi: 10.1071/RD17501. [DOI] [PubMed] [Google Scholar]
- 32.Berndtson WE, Thompson TL. 1990. Changing relationships between testis size, Sertoli cell number and spermatogenesis in Sprague-Dawley rats. J Androl 11:429–435. [PubMed] [Google Scholar]
- 33.Ni F-D, Hao S-L, Yang W-X. 2019. Multiple signaling pathways in Sertoli cells: recent findings in spermatogenesis. Cell Death Dis 10:541. doi: 10.1038/s41419-019-1782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong C-H, Xia W, Lee NPY, Mruk DD, Lee WM, Cheng CY. 2005. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion-associated proteins in testosterone-suppressed rat testes. Endocrinology 146:1192–1204. doi: 10.1210/en.2004-1275. [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, Yu J, Guo Y, Gao T, Shen C, Zhang X, Li H, Huang X. 2018. Cellular nucleic acid-binding protein is vital to testis development and spermatogenesis in mice. Reproduction 156:59–69. doi: 10.1530/REP-17-0666. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed N, Yang P, Chen H, Ujjan IA, Haseeb A, Wang L, Soomro F, Faraz S, Sahito B, Ali W, Chen Q. 2018. Characterization of inter-Sertoli cell tight and gap junctions in the testis of turtle: protect the developing germ cells from an immune response. Microb Pathog 123:60–67. doi: 10.1016/j.micpath.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Risley MS, Tan IP, Roy C, Sáez JC. 1992. Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J Cell Sci 103:81–96. [DOI] [PubMed] [Google Scholar]
- 38.Matsuo Y, Nomata K, Eguchi J, Aoki D, Hayashi T, Hishikawa Y, Kanetake H, Shibata Y, Koji T. 2007. Immunohistochemical analysis of connexin43 expression in infertile human testes. Acta Histochem Cytochem 40:69–75. doi: 10.1267/ahc.07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebourcet D, O’Shaughnessy PJ, Monteiro A, Milne L, Cruickshanks L, Jeffrey N, Guillou F, Freeman TC, Mitchell RT, Smith LB. 2014. Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PLoS One 9:e105687. doi: 10.1371/journal.pone.0105687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brehm R, Zeiler M, Rüttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lécureuil C, Steger K, Konrad L, Biermann K, Failing K, Bergmann M. 2007. A Sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol 171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giese S, Hossain H, Markmann M, Chakraborty T, Tchatalbachev S, Guillou F, Bergmann M, Failing K, Weider K, Brehm R. 2012. Sertoli-cell-specific knockout of connexin 43 leads to multiple alterations in testicular gene expression in prepubertal mice. Dis Model Mech 5:895–913. doi: 10.1242/dmm.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Zhang X, Zhang J, Ji Q, Huang W, Zhang X, Li Y. 2019. Spermatogenesis disorder caused by T-2 toxin is associated with germ cell apoptosis mediated by oxidative stress. Environ Pollut 251:372–379. doi: 10.1016/j.envpol.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Smith LB, Walker WH. 2014. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelletier R-M. 2011. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem 46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Lyon MF, Glenister PH, Lamoreux ML. 1975. Normal spermatozoa from androgen-resistant germ cells of chimaeric mice and the role of androgen in spermatogenesis. Nature 258:620–622. doi: 10.1038/258620a0. [DOI] [PubMed] [Google Scholar]
- 46.Johnston DS, Russell LD, Friel PJ, Griswold MD. 2001. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology 142:2405–2408. doi: 10.1210/endo.142.6.8317. [DOI] [PubMed] [Google Scholar]
- 47.Tsai M-Y, Yeh S-D, Wang R-S, Yeh S, Zhang C, Lin H-Y, Tzeng C-R, Chang C. 2006. Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells. Proc Natl Acad Sci U S A 103:18975–18980. doi: 10.1073/pnas.0608565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang R-S, Yeh S, Tzeng C-R, Chang C. 2009. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 30:119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Gendt K, Swinnen JV, Saunders PTK, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willems A, Roesl C, Mitchell RT, Milne L, Jeffery N, Smith S, Verhoeven G, Brown P, Smith LB. 2015. Sertoli cell androgen receptor signalling in adulthood is essential for post-meiotic germ cell development. Mol Reprod Dev 82:626–627. doi: 10.1002/mrd.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holdcraft RW, Braun RE. 2004. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 52.Licciardello MP, Ringler A, Markt P, Klepsch F, Lardeau C-H, Sdelci S, Schirghuber E, Müller AC, Caldera M, Wagner A, Herzog R, Penz T, Schuster M, Boidol B, Dürnberger G, Folkvaljon Y, Stattin P, Ivanov V, Colinge J, Bock C, Kratochwill K, Menche J, Bennett KL, Kubicek S. 2017. A combinatorial screen of the CLOUD uncovers a synergy targeting the androgen receptor. Nat Chem Biol 13:771–778. doi: 10.1038/nchembio.2382. [DOI] [PubMed] [Google Scholar]
- 53.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. 1999. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 54.Xiong W, Chen Y, Wang H, Wang H, Wu H, Lu Q, Han D. 2008. Gas6 and the Tyro 3 receptor tyrosine kinase subfamily regulate the phagocytic function of Sertoli cells. Reproduction 135:77–87. doi: 10.1530/REP-07-0287. [DOI] [PubMed] [Google Scholar]
- 55.Lemke G, Burstyn-Cohen T. 2010. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci 1209:23–29. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I. 1995. Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res 23:3816–3821. doi: 10.1093/nar/23.19.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeda K, Tsukui T, Imazawa Y, Horie-Inoue K, Inoue S. 2012. Conditional expression of constitutively active estrogen receptor α in chondrocytes impairs longitudinal bone growth in mice. Biochem Biophys Res Commun 425:912–917. doi: 10.1016/j.bbrc.2012.07.170. [DOI] [PubMed] [Google Scholar]
- 58.Horie-Inoue K, Takayama K, Bono HU, Ouchi Y, Okazaki Y, Inoue S. 2006. Identification of novel steroid target genes through the combination of bioinformatics and functional analysis of hormone response elements. Biochem Biophys Res Commun 339:99–106. doi: 10.1016/j.bbrc.2005.10.188. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa T, Yamamoto T, Sakai S, Miyamoto Y, Hongo H, Qiu Z, Abe M, Takeda S, Oda K, de Freitas PHL, Li M, Endo K, Amizuka N. 2019. Histological effects of the combined administration of eldecalcitol and a parathyroid hormone in the metaphyseal trabeculae of ovariectomized rats. J Histochem Cytochem 67:169–184. doi: 10.1369/0022155418806865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koji T, Kondo S, Hishikawa Y, An S, Sato Y. 2008. In situ detection of methylated DNA by histo endonuclease-linked detection of methylated DNA sites: a new principle of analysis of DNA methylation. Histochem Cell Biol 130:917–925. doi: 10.1007/s00418-008-0487-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are fully available from the corresponding author upon reasonable request.