Abstract
Background:
Inflammatory mediators are an important component in the pathophysiology of the coronavirus disease 2019 (COVID-19). This study aimed to assess the effects of reducing inflammatory mediators using hemoperfusion (HP) and continuous renal replacement therapy (CRRT) on the mortality of patients with COVID-19.
Materials and Methods:
Twelve patients with confirmed diagnosis of COVID-19 were included. All patients had acute respiratory distress syndrome (ARDS). Patients were divided into three groups, namely, HP, CRRT and HP+CRRT. The primary outcome was mortality and the secondary outcomes were oxygenation and reduction in inflammatory mediators at the end of the study.
Results:
Patients were not different at baseline in demographics, inflammatory cytokine levels, and the level of acute phase reactants. Half of the patients (3 out of 6) in the HP+CRRT group survived along with the survival of one patient (1 out of 2) in the HP group. All four patients in the CRRT group died. Serum creatinine (SCr), Interleukin-1 (IL1), Interleukin-6 (IL6), Interleukin-8 (IL8), partial pressure of oxygen (PaO2), O2 saturation (O2 sat), and hemodynamic parameters improved over time in HP+CRRT and CRRT groups, but no significant difference was observed in the HP group (All Ps > 0.05).
Conclusion:
Combined HP and CRRT demonstrated the best result in terms of mortality, reduction of inflammatory mediators and oxygenation. Further investigations are needed to explore the role of HP+CRRT in COVID-19 patients.
Keywords: Coronavirus disease 2019, COVID-19, Acute respiratory distress syndrome, ARDS, Inflammatory marker, Hemoperfusion, Continuous renal replacement therapy, Cytokine, Oxygenation, Mortality
INTRODUCTION
Following the official recognition of the novel coronavirus disease 2019 (COVID-19), it rapidly turned into a pandemic overwhelming most of the healthcare systems. The clinical spectrum of COVID-19 pneumonia ranges from mild upper respiratory tract involvement to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and ultimately death. Critically ill patients with COVID-19 are more likely to develop acute respiratory distress syndrome (ARDS) related to systemic pro-inflammatory cytokine responses over a short period of time. ARDS is a dreaded complication of COVID-19 patients with high mortality (1–3).
In February 2020, Masih Daneshvari Hospital reported its first COVID-19 patient and became the referral center for COVID-19 cases in Iran. The pursuing high load of patients was inevitably associated with a significant mortality of patients in the intensive care unit (ICU) due to severe ARDS (4). Data suggests that increased mortality of COVID-19 patients is associated with a profound systemic inflammatory response (5).
Various cytokines are linked to COVID-19 and associated with COVID-19 induced lung injury and ARDS. The production of a wide array of proinflammatory cytokines is significantly elevated in patients with COVID-19 compared to healthy individuals (6). Hypercytokinemia, also known as a cytokine storm, is shown to be related to an alarming incidence of ARDS and critical illness requiring ICU admission. Plasma levels of several cytokines in COVID-19 patients could serve as potential biomarkers of disease severity in COVID-19 (7). These findings have demonstrated the immunopathological mechanisms of COVID-19 (8).
Various therapeutic techniques should be applied to control the inflammatory storm as confounding factors exist in patients with ARDS requiring invasive mechanical ventilation (IMV). Hemoperfusion (HP) and continuous renal replacement therapy (CRRT) can increase inflammatory cytokine elimination from the bloodstream and modulate immune function and, thus, improve patient prognosis (9).
In this study, we aimed to assess the effects of CRRT, HP, and their combination on mortality in COVID-19 patients undergoing IMV admitted to Masih Daneshvari Hospital in Tehran, Iran. Various clinical indices, including the time of treatment and biochemical parameters, were analyzed to assess the effects of HP and CRRT on the patient survival and to compare the effects of these techniques to remove plasma inflammatory factors.
MATERIALS AND METHODS
Study design and participants
This study was conducted at Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences after the approval of the study protocol by the Clinical Research Ethics Committee (IR.SBMU.NRITLD.REC.1399. 011). We included 12 adult patients who required mechanical ventilation and had a partial pressure of arterial oxygen/fraction of inspired oxygen ratio (PaO2/FiO2) under 150 with a confirmed diagnosis of COVID-19 using reverse transcription polymerase chain reaction RT-PCR. The initial blood sample was collected immediately after the patient was admitted to the ICU and prior to receiving any treatment.
Patients were divided into three groups (HP, CRRT, and HP+CRRT) based on their initial kidney function, glomerular filtration rate (GFR), and neutrophil gelatinase-associated lipocalin (NGAL) as a specific biomarker of acute kidney injury (AKI), diagnosed by an increase in the serum creatinine (SCr) level of at least 0.3 mg/dL within 48 hours. Estimated GFR (eGFR) in the HP group was > 60 mL/min. In the setting of AKI, CRRT was performed. Two patients were in the HP group, four patients were in the CRRT group and six patients were in the HP+CRRT group. All clinical data were recorded and standardized in a spreadsheet by two critical care physicians who were unaware of the purpose of this study.
Medical care in ICU
All patients received conventional ICU care including ventilator support, enteral or parenteral nutrition, correction of water-electrolyte imbalances, intravascular fluid replacement, and control of body temperature. Patients received hydroxychloroquine sulfate 400 mg twice-a-day on day 1 followed by 200 mg twice-a-day. To prevent untoward pulmonary injury patients were started on atazanavir and ritonavir once daily for 7–14 days. Vancomycin (25 mg/kg loading dose, 15 mg/kg twice-a-day), imipenem (500 mg every six hours) IV infusion combined with azithromycin 500 mg daily for three days were administered to treat the bacterial and atypical pneumonia. Norepinephrine was administered in the range of 0.5 to 2.5 mcg/kg/min IV infusion to improve blood perfusion and cardiac function.
The therapeutic effect was assessed via blood indices including arterial blood gas, PaO2 and O2 saturation, hemodynamic parameters (central venous oxygen saturation [ScvO2], oxygen delivery [DO2]), plasma levels of three cytokines (interleukin-1 [IL1], interleukin-6 [IL6], and interleukin-8 [IL8]), and kidney function (blood urea nitrogen [BUN] and SCr). Acceptable treatment outcome was defined as a marked improvement of ARDS severity demonstrated by increase in PaO2/FiO2 ratio, O2 saturation, DO2, ScvO2, improvement of renal function, and reduction of inflammatory cytokines, norepinephrine, and ultimately improved mortality. The survival time was calculated from the time of ICU admission to hospital discharge.
Blood purification
HP and/or CRRT therapies were initiated to improve O2 saturation and reduce proinflammatory cytokine levels (10,11).
Hemoperfusion (HP): Elevated IL-6 levels in ARDS patients with normal kidney function suggested its role in lethal lung injury. HP was carried out with a cutoff value of 100 pg/mL (12) for IL-6, through femoral venous catheters at a blood flow rate of 100–300 mL/min. HP was implemented over six hours per day and the number of HP treatments was determined by the plasma levels of the IL-1, IL-6 and IL-8 along with clinical assessment of the patient. A fully automated dialysis machine and a HA380 membrane filter (Jafron Biomedical Co) were used for the HP.
Continuous renal replacement therapy (CRRT): Among patients with direct kidney involvement and a high level of IL-6, the most important causative cytokine in ARDS, a recombinant humanized anti-IL-6 monoclonal antibody, tocilizumab (8 mg/kg, at a maximum dose of 800 mg per infusion, with an intravenous drip time longer than 1 h) was used to treat cytokine storm followed by continuous venovenous hemofiltration (CVVH), whereas in the setting of AKI, continuous venovenous hemodiafiltration (CVVHDF) was performed. CRRT was performed with an ultrafiltration rate of 50–200 cc/h for 24 h every other day. A dedicated machine (PrismaFlex system) and filter (ST150, Baxter) were used for the CRRT. The blood flow rate was set at 50–250 mL/min, and the dialysis rate was set at 25–35 mL/kg/h. Vascular access was obtained using arrow double lumen hemodialysis (12 Fr × 20 cm) and central venous catheters (CV-15122-F) in the femoral vein.
Combined hemoperfusion and continuous renal replacement therapy (HP+CRRT): In this group, patients underwent CRRT plus HP using a HA380 membrane filter, which was changed routinely after 6 hours over the course of 24 hours of CCRT due to the saturation of the adsorptive sites in the aseptic technique step.
During each blood purifying technique, continuous infusion of heparin (ranging from 5 to 20 units/kg/h) was used to prevent clotting. Therapeutic level was defined as an activated partial thromboplastin time (aPTT) ranging between 15 and 20 times the normal.
Data collection
For every patient, sequential (sepsis-related) organ failure assessment (SOFA) score, and acute physiology and chronic health evaluation II (APACHE II) score were calculated on the day of ICU admission. Serum NGAL levels were measured using a commercially available ELISA kit (Antibody Shop, Gentofte, Denmark). Ethylene diamine tetra acetic acid (EDTA) blood samples were collected in sterile (endotoxin-free) tubes before and after the therapeutic techniques. Samples destined for cytokine detection were placed in ice and transported immediately to the laboratory. The plasma was separated by centrifugation (2000 ×g for 10 min) at 4 °C and stored in 300 μL aliquots at -70 °C until analysis. Serum concentrations of IL-1, IL-6, and IL-8 were measured by ELISA using Quantikine kits. The minimum detectable concentration of all cytokines with the R&D kits was less than 10 pg/mL. The operating procedure provided by the manufacturer was strictly followed. Pulse contour cardiac output (PiCCO) was used to detect ScvO2 and DO2 through a central venous catheter and an arterial line. Measuring DO2 is dependent upon hemoglobin/hematocrit, cardiac output, and saturation of the individual hemoglobin molecules. The obtained blood samples were analyzed with an automatic biochemical analyzer for the measurement of acute phase reactants (procalcitonin [PCT] and ferritin], kidney function (BUN, SCr), and blood gas factors (PaO2, O2 sat) at the same time as cytokine level determination.
Data analysis
Results were presented as mean ± standard deviation. A P < 0.05 was considered statistically significant. Categorical variables were analyzed using the chi-squared test. Other indices were analyzed using ANOVA. To assess the relationship between the therapeutic techniques and the mortality rate, multivariate Cox regression survival curves with the Cox proportional hazard model were generated. The primary dependent variable was mortality. The treatment outcome was recorded as categorical variables (1: Better; 0: Worse or death). Multivariate adjustment was made separately for age, sex, ICU length of stay, time of the first treatment and number of treatments, SOFA and APACHE II scores, ferritin, PCT, arterial blood gas indices, hemodynamic parameters, and renal function tests.
RESULTS
Table 1 summarizes demographics and baseline characteristics of the patients. At baseline, there was no significant difference (All Ps > 0.05) in age, sex, SOFA and APACHE II scores, ferritin, PCT, and time of the first treatment between the three groups. A significant difference was seen the mean SCr and BUN levels at baseline (Ps = 0.010 and 0.035, respectively). Changes in the biochemical and hemodynamic parameters, cytokine levels, and blood gas factors are summarized in table 2 showing before and after treatment measurements. With progression of hypoxemia, multi-organ damage became a serious concern. There were significant differences in the mean BUN, O2 sat (at a 0.10 level), and norepinephrine dosage (at a 0.05 level) among the three groups over the study period. The CRRT group had the best result in clearing BUN and the HP+CRRT group showed the best outcome on the improvement in O2 saturation and reduction of norepinephrine dosage.
Table 1.
Demographic and baseline characteristics of the studies patients.
| HP (n=2) | CRRT (n=4) | HP+CRRT (n=6) | P value | |
|---|---|---|---|---|
| Age, years | 54.5 ± 23.3 | 48.0 ± 4.8 | 54.2 ± 12.4 | 0.721 |
| Sex | 0.509 | |||
| Female | 0 (0%) | 2 (50%) | 1 (16%) | |
| Male | 2 (100%) | 2 (50%) | 5 (84%) | |
| SOFA score | 13.0 ± 0.0 | 13.3 ± 1.0 | 12.8 ± 1.0 | 0.786 |
| APACHE II score | 35.0 ± 0.0 | 33.3 ± 2.2 | 32.5 ± 2.5 | 0.434 |
| Serum ferritin, μg/L | 725.0 ± 106.1 | 1112.5 ± 278.0 | 960.8 ± 218.2 | 0.207 |
| Procalcitonin, ng/mL | 0.4 ± 0.0 | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.470 |
| ICU length of stay, days | 7.5 ± 2.1 | 7.0 ± 1.4 | 8.0 ± 2.2 | 0.737 |
| Time to first treatment, hours | 32.5 ± 12.0 | 33.8 ± 6.8 | 29.0 ± 11.9 | 0.774 |
| Number of treatments | 1.5 ± 0.7 | 2.0 ± 0.0 | 3.0 ± 1.1 | 0.097 |
| Survival, n | 1 out of 2 | 0 out of 4 | 3 out of 6 | 0.269 |
Continuous variables are presented as means ± standard deviation. HP, hemoperfusion; CRRT, continuous renal replacement therapy; SOFA, sequential organ failure assessment; APACHE II, acute physiology and chronic health evaluation II; ICU, intensive care unit.
Table 2.
Laboratory findings before and after treatment.
| HP (n=2) | CRRT (n=4) | HP+CRRT (n=6) | P value | |
|---|---|---|---|---|
| Blood Urea Nitrogen, mg/dl | 0.062 | |||
| Before | 70.0 ± 7.1 | 131.3 ± 33.3 | 97.0 ± 18.4 | |
| After | 45.0 ± 21.2 | 76.0 ± 13.4 | 82.2 ± 4.3 | |
| Creatinine, mg/dl | 0.384 | |||
| Before | 1.6 ± 0.1 | 2.7 ± 0.5 | 2.9 ± 0.3 | |
| After | 1.1 ± 0.1 | 1.7 ± 0.2 | 2.1 ± 0.6 | |
| IL-1, pg/ml | 0.194 | |||
| Before | 75.0 ± 14.1 | 70.0 ± 7.6 | 58.3 ± 9.3 | |
| After | 40.5 ± 6.4 | 44.5 ± 5.6 | 35.8 ± 4.2 | |
| IL-6, pg/ml | 0.527 | |||
| Before | 245.0 ± 91.9 | 228.8 ± 23.9 | 248.0 ± 57.8 | |
| After | 160.0 ± 70.7 | 164.0 ± 29.5 | 153.8 ± 31.4 | |
| IL-8, pg/Ml | 0.741 | |||
| Before | 742.5 ± 10.6 | 830.0 ± 125.7 | 805.2 ± 72.3 | |
| After | 383.0 ± 60.8 | 489.0 ± 57.0 | 431.0 ± 55.9 | |
| Partial pressure of oxygen, mmHg | 0.516 | |||
| Before | 46.5 ± 2.1 | 44.5 ± 3.3 | 53.0 ± 10.9 | |
| After | 71.5 ± 4.9 | 66.3 ± 10.3 | 86.0 ± 20.5 | |
| O2 saturation (%) | 0.073 | |||
| Before | 78.0 ± 5.7 | 80.0 ± 3.9 | 77.0 ± 5.4 | |
| After | 91.0 ± 2.8 | 92.3 ± 2.7 | 94.2 ± 5.0 | |
| Oxygen delivery (mL/min) | 0.485 | |||
| Before | 425.0 ± 35.4 | 500.0 ± 40.8 | 500.0 ± 54.8 | |
| After | 825.0 ± 176.8 | 750.0 ± 40.8 | 841.7 ± 128.1 | |
| Central venous oxygen saturation (%) | 0.369 | |||
| Before | 45.0 ± 7.1 | 46.3 ± 2.5 | 47.5 ± 4.2 | |
| After | 62.5 ± 10.6 | 61.3 ± 2.5 | 69.2 ± 7.4 | |
| Norepinephrine (mcg/kg/min) | 0.022 | |||
| Before | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.0 | |
| After | 1.5 ± 1.5 | 2.6 ± 0.3 | 1.0 ± 0.8 | |
Continuous variables are presented as means ± standard deviation. HP, hemoperfusion; CRRT, continuous renal replacement therapy; IL, interleukin.
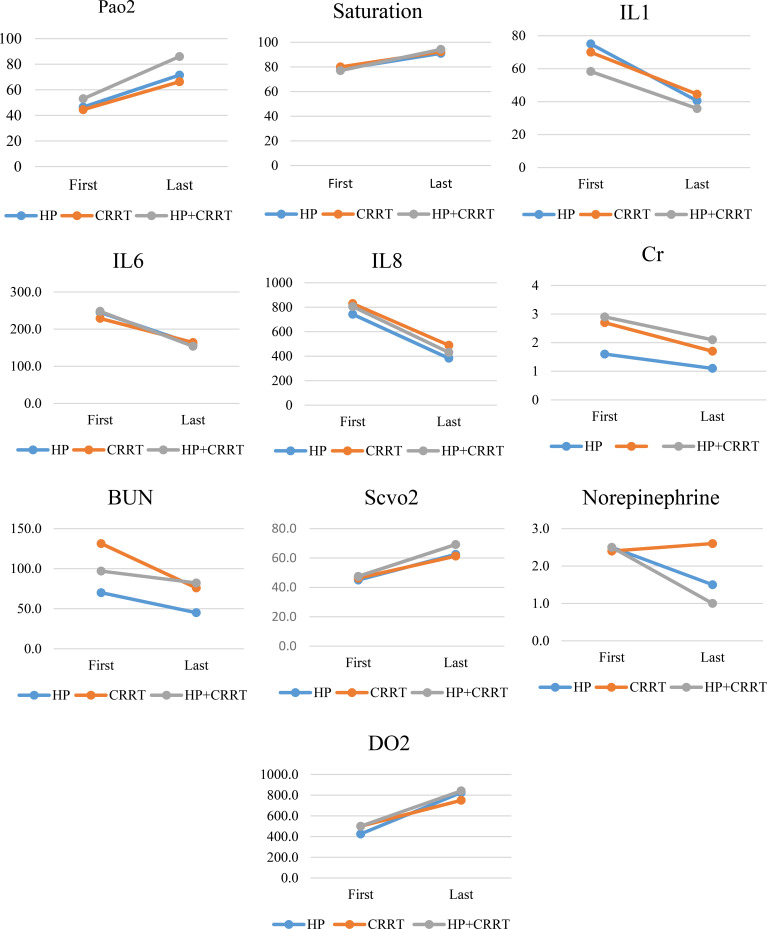
The median ICU length of stay was not different (P=0.737) between the three groups (7.5±2.1, 7.0±1.4, and 8.0±2.2 days for HP, CRRT, and HP+CRRT groups, respectively). The three groups were also similar in the number of interventions they received (1.5 ± 0.7, 2.0 ± 0.0, and 3.0 ± 1.1 for HP, CRRT, and HP+CRRT groups, respectively). As shown in figure 1, CRRT and HP+CRRT groups were superior to HP in improving patients’ PaO2, O2 sat, ScvO2, and DO2. Patients in the HP+CRRT group demonstrated a marked improvement in IL-6 and IL-8 levels.
Figure 1.
Laboratory parameters at baseline and after treatment in each study group. There was no difference (All Ps > 0.05) in the HP group. In the CRRT group, only norepinephrine dose did not show a significant difference (Ps > 0.05). In the HP+CRRT group, all parameters, except BUN, showed a significant increase after treatment (All Ps < 0.05).
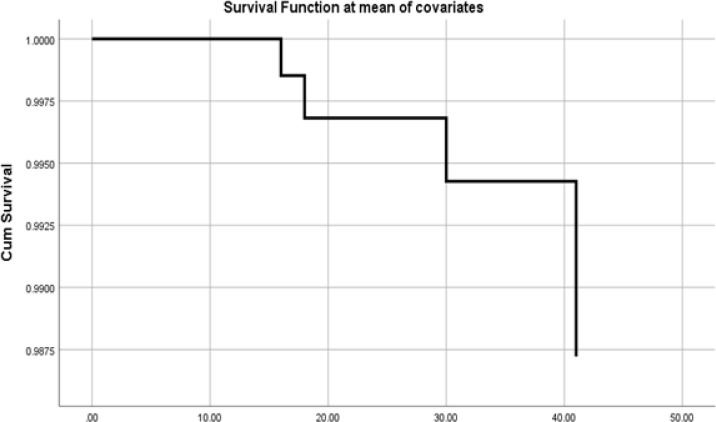
The overall mortality was 66.6% (8 out of 12 patients). Mortality rates were 50% in the HP group (n=2), 100% in the CRRT group (n=4), and 50% in the HP+CRRT group (n=6). The HP+CRRT group had the best overall primary and secondary outcomes. The Cox proportional hazards model for overall survival is shown in figure 2.
Figure 2.
Overall survival using Cox proportional model.
DISCUSSION
Acute lung injury and ARDS in COVID-19 patients have a higher mortality rate than what is observed in other diseases. To date, the management of ARDS in COVID-19 patient remains supportive, while no effective therapeutics, including antivirals or vaccines, is shown to improve mortality. In previous studies, COVID-19 patients have received antimicrobial agents, antiviral, antimalarial, and corticosteroid therapy with variable dosage depending on disease severity, however, no effective outcomes is observed (13–15). Hence, our study was designed to evaluate the blood purification therapies using HP, CRRT, and their combination on inflammatory mediator elimination from plasma and improvement of survival. Significant elevation in plasma inflammatory cytokines and chemokines was seen in severe COVID-19 patients, associated with higher mortality rates in moderate-to-severe ARDS. HP, CRRT, and HP+CRRT techniques were designed to remove cytokines and other circulating inflammatory mediators from the bloodstream.
An earlier study has demonstrated that increased plasma level of cytokines was associated with extensive lung inflammation in COVID-19 pneumonia (16). IL-1 is the most important cytokine in the early stage of ARDS, associated with increasing pro-inflammatory chemokines leading to edema; and the therapeutic target could be achieved by removing it (17). Also, higher plasma levels of IL-6 and IL-8 in bronchoalveolar lavage fluid are associated with a higher mortality rate (18). We noted that patients with ARDS had higher levels of IL1, IL6, and IL8, probably leading to an activated systemic inflammatory response. Moreover, patients requiring ICU admission had higher concentrations of proinflammatory mediators than those not requiring ICU admission, suggesting that the cytokine storm might play a crucial role in the severity of the disease (19).
The initial concentration of IL1, IL6, and IL8 was elevated and could be used as an index for assessing the blood purifying techniques and hypoxemia prognosis. Therefore, in this study, we divided patients into three groups (HP, CRRT, and HP+CRRT), and the measured cytokines were evaluated to compare the efficacy of each techniques. Data from Italy and China showed extracorporeal organ support therapies, including hemoperfusion, could remove plasma inflammatory cytokines, broadly used in clinical settings to support hemodynamic and organ function recovery (11,19). HP and CRRT achieved high solute clearance rates across a semi-permeable membrane and they could eliminate inflammatory mediators from the bloodstream (9).
Although it is still controversial whether HP or CRRT is beneficial in viral pneumonia, CRRT is reported to have a positive effect on the reduction of the mortality risk in COVID-19 patients requiring IMV, (10) while other studies found that CRRT is a risk factor for mortality in COVID-19 (20). To compare the efficacy of HP and CRRT, we set up groups based on the GFR cutoff, and further divided patients into three groups for comparing plasma cytokine levels, blood gas factors, hemodynamic parameters, BUN, SCr, and the mortality rate. There was no significant differences in the overall survival during ICU stay among the three groups (P > 0.05).
Our study has several limitations. First, the sample size of this study was not large. Second, this was a single-center study. Although potentially more patients could be involved in such a study, resource limitation is the main factor limiting this investigation to only one center. Third, patient data was only available until time of discharge which was included in the analysis. This study lacked long-term data.
CONCLUSION
The best clinical outcome in COVID-19 patients was observed in the HP+CRRT group which not only had a comparatively better survival rate but also demonstrated better improvement in plasma cytokine levels, blood gas factors, and hemodynamic parameters. Role of HP+CRRT needs to be investigated in larger and preferably randomized studies.
Ethical Approval and Consent to participate
Clinical Research Ethics Committee approval was IR.SBMU.NRITLD.REC.1399.011. Patients and/or next of kin provided consent for participation.
Consent for publication
Patients and/or next of kin provided consent for participation and publication of clinical data.
All authors approved the final manuscript and consented for publication.
Availability of supporting data
All the data provided in the manuscript no additional supporting data.
Acknowledgements
We wish to thank Jafron Biomedical Co. for providing all the required equipment for HP and CRRT free of charge to COVID-19 patients.
Footnotes
Competing interests
Not applicable.
Funding
Not applicable.
REFERENCES
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020;8(5):506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet 2020;395(10224):e35–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamaati H, Dastan F, Tabarsi P, Marjani M, Saffaei A, Hashemian SM. A Fourteen-day Experience with Coronavirus Disease 2019 (COVID-19) Induced Acute Respiratory Distress Syndrome (ARDS): An Iranian Treatment Protocol. Iran J Pharm Res 2020;19(1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gralinski LE, Baric RS. Molecular pathology of emerging coronavirus infections. J Pathol 2015;235(2):185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Zhang C, Huang F, Yang Y, Wang F, Yuan J, et al. Elevated plasma level of selective cytokines in COVID-19 patients reflect viral load and lung injury. National Science Review 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondonnet R, Constantin JM, Sapin V, Jabaudon M. A Pathophysiologic Approach to Biomarkers in Acute Respiratory Distress Syndrome. Dis Markers 2016; 2016: 3501373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han F, Sun R, Ni Y, Hu X, Chen X, Jiang L, et al. Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. Am J Med Sci 2015;349(3):199–205. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Shi J, Ge S, Guo S, Xing X, Wang Y, et al. Effect of continuous renal replacement therapy on all-cause mortality in COVID-19 patients undergoing invasive mechanical ventilation: a retrospective cohort study. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med 2020;8(3):240–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong J, Dong H, Xia SQ, Huang YZ, Wang D, Zhao Y, et al. Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia. MedRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 2016;14(8):523–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect 2020;50(4):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004;136(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashemian SM, Mortaz E, Tabarsi P, Jamaati H, Maghsoomi Z, Khosravi A, et al. Elevated CXCL-8 expression in bronchoalveolar lavage correlates with disease severity in patients with acute respiratory distress syndrome resulting from tuberculosis. J Inflamm (Lond) 2014;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, et al. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet 1993;341(8846):643–7. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]