Abstract
Background:
In the current study, we assessed the effect of vitamin D supplementation on improvement of symptoms in mild-to-moderate asthma patients with vitamin D insufficiency and deficiency.
Materials and Methods:
This randomized, controlled clinical trial included 132 mild-to-moderate asthma patients with vitamin D insufficiency (n=66) and those with vitamin D deficiency (n=66). They were assigned randomly to two groups of cases (with two subgroups) and controls (with two subgroups). In the case subgroups, for patients with vitamin D deficiency, a dose of 50,000 U vitamin D supplementation was administered orally on a weekly basis and for six weeks followed by a maintenance dose of 1000 U daily. For patients with vitamin D insufficiency, a dose of 1000 U vitamin D supplementation was prescribed daily. In control group, we administered placebo. The information including asthma symptoms, parameters measured by spirometer (Forced Vital Capacity-FVC, Forced Expiratory Volume in one second-FEV1) and 25-hydroxyvitamin D [25(OH)D] concentration was collected at baseline and three months later and analyzed using SPSS, Version 20.
Results:
Improvement of FEV1/FVC ratios were found in both groups but this improvement in both case subgroups of patients with vitamin D insufficiency and deficiency suggested more appropriate results compared to control group (P-value=0.022). Moreover, the correlation between changes in 25(OH)D level and changes in FEV1 was positive and significant in patients receiving vitamin D supplementation within a three-month follow up (r=0.202, P-value=0.042).
Conclusion:
According to the results, vitamin D supplementation can be associated with the improvement of asthma symptoms and lung function in mild-to-moderate asthma patients with vitamin D insufficiency and deficiency.
Keywords: Vitamin D supplementation, asthma, Lung function, FEV1
INTRODUCTION
Asthma is considered as a disease of conducting airways which contract spontaneously and severely in reaction to a wide range of internal and external stimuli. This high reactivity of the airways is accompanied by increased sensory excitability and thereby increased secretion of mucus (1). This disease represents one of the most common chronic diseases and a major problem in the field of public health throughout the world (2). According to previous studies, the average prevalence of asthma in Iran is 13.4% (3). Severe asthma is two to three times more common in women than men (4). Childhood asthma is more common in boys but during adolescence, is more common in girls and this pattern persists in adulthood (5).
In order to control the asthma most of the patients are persisted to long term use of medications (2). However, despite high dose treatment, in considerable proportion of patients, the asthma is not controlled optimally. Severe asthma patients are mostly exposed to the risk of asthma exacerbation and mortality while they need medication more than other asthma patients (6).
Vitamin D deficiency, meanwhile, has been increasingly developed in the word nowadays. It is more associated with diet, lifestyle and behavioral changes (7, 8). While its’ musculoskeletal consequences is well proven, new hypotheses consider the association between asthma and abnormal levels of vitamin D (8–10). In fact, several effects of vitamin D in innate and adaptive immune system, which may be involved in the primary prevention of asthma, protection against asthma or reducing the symptoms of asthma and asthma exacerbations’ adjustment have been reported (8, 11, 12).
The 25-hydroxyvitamin D [25(OH)D] is the most frequent circulating form of vitamin D and its serum concentration reflects the vitamin D status (1). In many studies, low 25(OH)D concentration has been identified as a potential risk factor for several chronic pulmonary diseases and accompanied by reduced lung function, reduced glucocorticoid response, frequent exacerbation of asthma and therefore increased use of steroids (1, 13–17). On the one hand, there is insufficient evidence for an association between vitamin D status and asthma and on the other hand, there are few studies on the effect of vitamin D supplementation on the improvement of symptoms in mild-to-moderate asthma patients with vitamin D insufficiency or deficiency. Therefore, this study aims to assess the effect of vitamin D supplementation on the improvement of symptoms in mild-to-moderate asthma patients with vitamin D insufficiency or deficiency.
MATERIALS AND METHODS
In this randomized, controlled clinical trial, 132 mild-to-moderate asthma patients with vitamin D insufficiency (n=66) and vitamin D deficiency (n=66), presented in the pulmonary clinic of Alzahra Hospital in Isfahan during a one-year period. The trial was registered in the Iranian Registry of Clinical Trials under the registration number of IRCT20200825048515N10. The severity of the asthma in these patients who had no acute exacerbation was measured by Forced Expiratory Volume in 1 second (FEV1); on this basis, they were considered as moderate (FEV1: 60–80% predicted) and mild (FEV1≥80% predicted).
Moreover, the exclusion criteria were considered as: diagnosis of Chronic Obstructive Pulmonary Disease (COPD), sarcoidosis, hyperthyroidism, kidney stone, active tuberculosis, vitamin D intolerance, liver failure, renal failure, lymphoma or other malignant tumors with no improvement for two years, intake of anticonvulsants, vitamin D supplementation, systemic corticosteroid therapy up to three months prior to or during the study, breastfeeding or pregnancy, baseline serum calcium level greater than 2.65 mmol/L, asthma exacerbation 3 months prior to or during the study, active or passive smoking, and incomplete use of asthma controller drugs during the study.
This study was approved by Local Ethics Committee (Isfahan University of Medical Sciences, Iran; Ethical code: 395544) and written informed consent was obtained from all patients. At baseline, 25(OH) D serum level was measured through radioimmunoassay for all patients. To evaluate lung function, we performed the spirometry and the parameters of FEV1 and Forced Vital Capacity (FVC) was measured and recorded as well. To evaluate and control asthma symptoms during the study, we used the Asthma Control Test (ACT score) which contains five questions. The score of 25 is indicative of the least symptoms of asthma, representing the asthma is under the control completely; the score of 20–24 indicates mild symptoms of asthma, representing incomplete control of asthma and finally the score of <20 indicates sever symptoms of asthma representing no control over asthma.
Sample assignment was performed as follows: we selected 3–4 patients daily through simple randomization from accessible patients with mild-to-moderate asthma. Then, we measured their vitamin D levels. So, patients with insufficiency (serum 25(OH)D level of 13–30 ng/mL) or deficiency (serum 25(OH)D level ≤12 ng/mL) in vitamin D were included in the study. We ceased sampling when a number of 66 patients were assigned to each group (patients with vitamin D insufficiency and patients with vitamin D deficiency). Next, we divided each of these two groups (n=66) into two subgroups (n=33) of case and control. Thus, we had four groups to assess as follows:
Asthma patients with vitamin D insufficiency (Case group; n=33)
Asthma patients with vitamin D deficiency (Case group; n=33)
Asthma patients with vitamin D insufficiency (Control group; n=33)
Asthma patients with vitamin D deficiency (Control group; n=33)
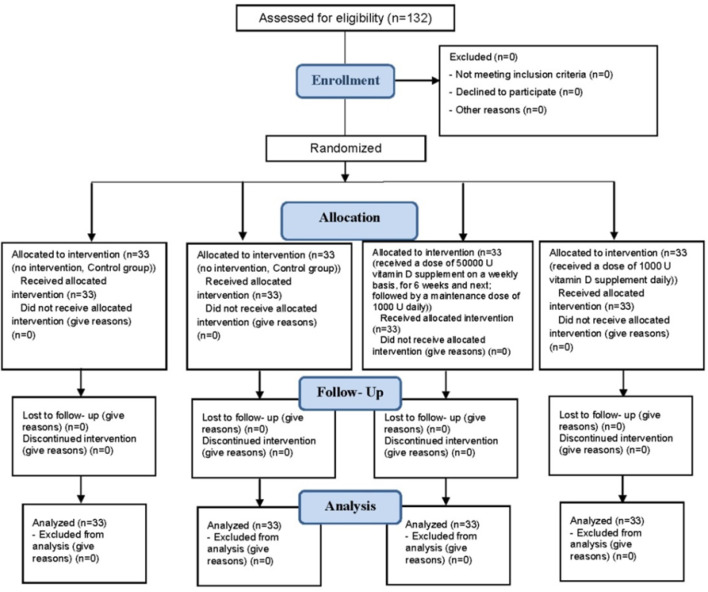
All groups of cases and controls received a similar asthma controller (Symbicort). Moreover, in patients with vitamin D deficiency in the case group, we administered a dose of 50,000 U vitamin D supplement on a weekly basis, for 6 weeks and next, to achieve serum 25(OH)D level >20 ng/ml; followed by a maintenance dose of 1000 U daily. In patients with vitamin D insufficiency we administered a dose of 1000 U vitamin D supplement daily. In control group in patients with vitamin D deficiency/insufficiency, we administered placebo (Figure 1).
Figure 1.
Consort flow diagram
After 3 months, the ACT score, FEV1, FVC, FEV1/FVC parameters and serum 25(OH) D level were re-measured and re-recorded for all patients.
It should be noted that the person who was responsible for collecting data, was blinded to how groups were classified in this study.
Finally, the collected data were entered into SPSS software, Version 20 and analyzed using the tests including chi-square, independent t-test, paired t-test and Pearson correlation coefficient. For all analyses, we considered a significance level of 0.05.
RESULTS
The study included 132 asthmatics in which 66 patients underwent the treatment with Vitamin D supplement, including 38 (57.6%) men (mean age=44.03±12.35 years) and 28 (42.4%) women (mean age=41.04±12.86 years); and 66 patients in control group including 34 (51.5%) men (mean age=40.06±15.33 years) and 32 (48.5%) women (mean age=42±13.57 years) with no significant difference in age and sex (P value>0.05). Also, there was no significant difference in frequency distribution of sex, mean age of patients and the vitamin D deficiency and insufficiency in each group (P value>0.05) (Table 1).
Table 1.
Main characteristics of patients in the study
| Characteristics | Case | Control | P1 | P2 | P3 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
Total (n=66) |
Insufficient (n=33) |
Deficient (n=33) |
Total (n=66) |
Insufficient (n=33) |
Deficient (n=33) |
||||
| Sex | |||||||||
| Male | 38(57.6%) | 22(66.7%) | 16(48.5%) | 34(51.5) | 14(47.4%) | 20(60.6%) | 0.106 | 0.109 | 0.300 |
| Female | 28(42.4%) | 11(33.3%) | 17(51.5%) | 32(48.5%) | 19(57.6%) | 13(39.4%) | |||
| Age; year | 42.77±12.55 | 12.37±3.80 | 11.48±4.74 | 41.00±14.42 | 12.41±4.78 | 12.04±3.23 | 0.535 | 0.354 | 0.468 |
P1: The significant level of comparison two subgroups insufficient vit. D with deficient in Case group
P2: The significant level of comparison two subgroups insufficient vit. D with deficient in Control group
P3: The significant level of comparison two groups study (case group with control group)
On the other hand, evaluation of ACT score in patients revealed no significant difference between both groups of case and control at the baseline (P value=0.504), but three months after the treatment, the mean ACT score in the case group (undergoing the treatment with vitamin D supplement) was 13.30±4.51 and significantly higher than the control group (undergoing basic treatment without vitamin D supplement) with the mean of 11.45±3.63. (P value=0.011). Also at the baseline there was no statistically significant difference between these two groups in this regard (P value=0.862), while patients with vitamin D insufficiency and patient with vitamin D deficiency showed significant difference in 25 (OH)D level (P value<0.001). Also, over the course of time during a three-month follow up, the level of 25 (OH)D in case group showed a significant increase (P value<0.001) in which 25 (OH)D level reached sufficient level but in control group this level of vitamin D showed no significant change (Table 2).
Table 2.
Comparison of clinical factors in patients with asthma in two groups and in patients with vitamin D insufficiency and patients with vitamin D deficiency
| Variables | Case | Control | P1 | P2 | P3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=66) | Insufficient (n=33) | Deficient (n=33) | Total (n=66) | Insufficient (n=33) | Deficient (n=33) | ||||
| ACT score | |||||||||
| Before | 11.26±3.66 | 11.73±3.56 | 10.79±0.65 | 11.69±3.75 | 11.58±3.62 | 11.81±3.94 | 0.307 | 0.923 | 0.504 |
| 3 months later | 13.30±4.51 | 13.18±4.65 | 13.42±4.44 | 11.45±3.63 | 11.61±3.74 | 11.78±3.57 | 0.812 | 0.752 | 0.011 |
| P4 | <0.001 | 0.014 | 0.002 | 0.486 | 0.956 | 0.236 | |||
| 25(OH)D (ng/ml) | |||||||||
| Before | 17.04±6.42 | 23.42±2.64 | 11.29±0.79 | 17.17±6.59 | 23.64±3.26 | 11.36±0.75 | <0.001 | <0.001 | 0.862 |
| 3 months later | 34.88±8.04 | 36.36±11.33 | 33.54±2.68 | 17.34±6.17 | 23.43±2.96 | 11.88±0.71 | 0.184 | <0.001 | <0.001 |
| P4 | <0.001 | <0.001 | <0.001 | 0.102 | 0.167 | 0.054 | |||
| FVC (%) | |||||||||
| Before | 79.76±9.17 | 79.27±9.37 | 81.67±7.63 | 79.03±10.47 | 80.27±9.33 | 79.91±10.23 | 0.405 | 0.117 | 0.589 |
| 3 months later | 81.29±7.13 | 80.91±6.68 | 81.52±9.95 | 80.47±9.59 | 81.06±10.53 | 80.88±10.55 | 0.922 | 0.052 | 0.536 |
| P4 | 0.098 | <0.001 | 0.125 | 0.166 | 0.107 | 0.506 | |||
| FEV1 (%) | |||||||||
| Before | 71.76±7.70 | 73.52±8.01 | 70.00±7.07 | 72.12±8.04 | 71.12±7.25 | 73.12±8.76 | 0.069 | 0.300 | 0.791 |
| 3 months later | 74.17±10.16 | 72.85±15.77 | 75.48±9.98 | 72.55±8.82 | 73.09±9.60 | 72.00±8.08 | 0.343 | 0.694 | 0.041 |
| P4 | 0.130 | 0.796 | 0.003 | 0.658 | 0.172 | 0.376 | |||
| FEV 1 / FVC (%) | |||||||||
| Before | 74.89±5.99 | 75.58±5.17 | 74.21±6.73 | 73.95±6.54 | 73.97±6.93 | 73.94±6.22 | 0.381 | 0.984 | 0.391 |
| 3 months later | 80.08±11.13 | 80.15±10.54 | 80.00±11.85 | 75.37±12.09 | 77.44±10.83 | 73.36±6.43 | 0.958 | 0.160 | 0.022 |
| P4 | <0.001 | 0.006 | 0.008 | 0.447 | 0.322 | 0.531 | |||
Abbreviate: ACT score: Asthma Control Test score; FEV1: Forced Expiratory Volume in one second; FVC: Forced Vital Capacity
P1: The significant level of comparison two subgroups insufficient vitamin D with deficient in Case group
P2: The significant level of comparison two subgroups insufficient vitamin D with deficient in Control group
P3: The significant level of comparison two groups study (Case group with Control group)
P4: The significant level of comparison between before and 3 months later in each groups and subgroups
The mean of FEV1 (%) in all patients of case group had an insignificant increase slightly in the course of time (P value>0.05), but in patients with vitamin D deficiency undergoing the treatment, FEV1 (%) within 3 months with the mean of 70.00±7.07% was significantly higher compared to FEV1 (%) at baseline with the mean of 75.48±9.98 % (P value=0.003). At baseline, we observed no significant change in FEV1/FVC ratio either in case and control groups or in patients with vitamin D insufficiency and patients with vitamin D deficiency (in both groups) (P value>0.05). But three month later, in case group the mean of FEV1/FVC ratio showed significant increase compared to control group (P value=0.022) and the role of vitamin D supplement was significant in increased mean of FEV1/FVC ratio (P value: in case group: total<0.001, Insufficient=0.006, Deficient=0.008) (Table 2).
Finally, the correlation between variations of 25(OH) D level and FEV1 within a three-month follow up of both groups indicated that in case group as 25(OH)D level increased due to receiving vitamin D supplement, FEV(%) increased as well (Correlation=0.202, P value=0.042), but in control group, the variations of these two factors showed no significant correlation (Correlation =−0.011, P value=0.936). Also, the correlation between variations of 25(OH)D level and ACT score showed a positive but not significant correlation within a three-month follow up (in Case group: Correlation=0.113, P value=0.411 and in Control group: Correlation =0.023, P value=0.889).
DISCUSSION
The results indicated that the levels of vitamin D in both groups of case and control were the same (no significant difference) at baseline but after receiving vitamin D supplement, in case group the level of vitamin D showed a significant increase. Thus, it was higher compared to control group (P value<0.001).
Moreover, the current study has considered the treatment with vitamin D as an adjunct therapy for asthma; the results suggested that although reduced asthma symptoms were observed in both groups, three months later this improvement in patients receiving vitamin D supplement statistically led to better results compared to control group.
In accordance with the current study, there are many clinical and epidemiologic surveys that have evaluated the effect of vitamin D on the treatment of asthma; however, more trials seem to be required. Yadav and Mittal revealed that a dose of 60,000 U of vitamin D monthly for six months as an adjunct treatment for asthma, could lead to reduced asthma severity, number of exacerbations, corticosteroid intake, emergency department visits, and peak expiratory flow rate (18). Clifford and Knox found different roles of vitamin D in the treatment of asthma and relevant mechanisms in this respect including variable adaptive and innate mechanisms (19).
Contrary to this study, Ginde et al. suggested that within the first year of life, intake of vitamin D supplement may lead to increased risk of asthma; there is not enough evidence for different roles of vitamin D in this regard, therefore more trials seem to be required (20).
Furthermore, the effect of vitamin D supplement on lung function revealed that at baseline, FVC, FEV1 and FEV1/FVD ratios had no significant difference in both groups of case and control and also 3 month later these factors showed no significant improvement in control group, but a significant increase was observed in FEV1 (%) and FEV1/FVC ratios in patients undergoing the treatment with vitamin D compared to control group. There is no significant difference in the increase of these factors in patients with vitamin D insufficiency and patients with vitamin D deficiency; thereby, the treatment with vitamin D can affect these factors.
Three months after the treatment, in case group, variation of FEV1 in patients with vitamin D in sufficiency was not significant and considerable but in patients with vitamin D deficiency it showed a significant increase. Overall FEV1/FVC ratio (%) in this group (case) had a significant increase. However, there was no difference between patients with vitamin D insufficiency and patients with vitamin D deficiency in the FEV1/FVC ratio (%). Moreover, a positive and significant correlation was found between changes in vitamin D (ΔVitamin D) and FEV1 (ΔFEV1) after 3 months (r=0.202, P value=0.042) (the positive and significant correlation of Δ vitamin D and Δ FEV1 only after 24 weeks). Due to this correlation and higher increase in 25(OH)D concentration of patients with vitamin D deficiency compared to patients with vitamin D insufficiency (in case group), it could be noted that treatment with vitamin D supplement may be more effective in individuals with vitamin D deficiency as against individuals with vitamin D insufficiency.
In agreement with the current study, Li et al. suggested that there is a significant association between low serum vitamin D levels and airway obstruction as evaluated by FEV1 and FEV 1 /FVC ratio, while they found that this association was independent of the confounders including smoking, Body Mass Index (BMI), age and sex (1).
However, in the current study also no smoker, whether active or inactive one, was found in patients and there was no difference between two groups in age, sex and BMI. So, confounders could not affect the results.
Another study on the effects of vitamin D supplement on airways function in asthmatics found that after 16 weeks, vitamin D supplement led to a significant improvement in intervention group compared to control group and after 24 weeks FEV1 showed more appropriate results in intervention group compared to control group as well (21). Moreover, the results of many studies indicating a positive correlation between vitamin D status and lung function in asthmatics, showed that high level of vitamin D was associated with improvement in FEV1 and FVC results (13, 21–23).
In fact, the correlation between 25(OH)D levels and asthma severity or disease control demonstrates that hormonal effects can affect asthmatic inflammation or vice versa. Regardless of mechanisms underlying the association between vitamin D deficiency and asthma, several evidences suggest the major role of vitamin D in the incidence of asthma and presence of negative correlation between vitamin D status and disease severity or its control. High levels of the enzymes in airway epithelia convert circulating 25-OH-vitamin D3 into its active form (1,25-OH-vitamin D3). The vitamin D active form in response to respiratory infections may result in local effects, thereby it can prevent from the inflammation caused by these infections (24).
In addition, the study on bronchial biopsies suggested an inverse association between vitamin D concentrations and airway smooth muscle mass (25). In vitro, vitamin D can affect remodeling of airway smooth muscle through an inhibitory effect on the growth and contractility of airway smooth muscle (26).
Therefore, vitamin D can be involved in different mechanisms of asthma pathogenesis. It has been demonstrated that vitamin D can affect immune cells as well as the function and proliferation of smooth muscle which can have effects on lung function in asthmatics as well as airway remodeling (26). One of the main characteristics of asthma is airway remodeling which is correlated with airflow limitation (27). Vitamin D can affect airway remodeling through the effects on muscle cell growth, movement and contractility as well as inhibiting growth factor β, matrix metalloproteinase and fibroblast proliferation (26, 28). Moreover, it has been suggested that vitamin D in animal models can influence lung development, maturation in utero and in the period of postpartum (29, 30).
The current study had some limitations. Firstly, small sample size of patients in this study who were referred from an asthma center, thereby it is likely that they could not be representative of asthmatics population and it cannot reflect their living conditions such as their social, economic and occupational status and lifestyle. Thus, it is recommended that future studies consider the effect of vitamin D supplement in different groups of asthmatics such as obese asthmatics, those with occupational asthma which aggravate asthma, rural asthmatics versus urban asthmatics and so on. Although there cases were not taken into account in the current study, it has been tried the two groups in this study to be similar in major factors including age, sex, BMI and living place. But in this study it was likely that a few participants use Over-the-Counter (OTC) anti-inflammatory medication. However, considering there are a few participants in this regard, its effect on the association between vitamin D and lung function could be ignorable.
In summary, the current study suggested the role of vitamin D in the improvement of asthma symptoms. Finally it was clarified that the lung function (measured by factors including FEV1 and FEV1/FVC ratio) in patients with asthma receiving vitamin D supplement had a significant improvement, while the improvement in patients with vitamin D deficiency led to more appropriate results compared to patients with vitamin D insufficiency.
REFERENCES
- 1.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, et al. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration 2011;81(6):469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman ED, Global Initative For Asthma (GINA) Reports . Global Strategy for Asthma Management and Prevention (2015 update).
- 3.Heidarnia MA, Entezari AB, Moein M, Mehrabi Y, Pourpak Z. Prevalence of asthma symptom in Iran: a meta-analysis. Research in Medicine 2007;31(3):217–25. [Google Scholar]
- 4.Boskabady MH, Kolahdoz GH. Prevalence of asthma symptoms among the adult population in the city of Mashhad (north-east of Iran). Respirology 2002;7(3):267–72. [DOI] [PubMed] [Google Scholar]
- 5.Wright AL, Stern DA, Kauffmann F, Martinez FD. Factors influencing gender differences in the diagnosis and treatment of asthma in childhood: the Tucson Children’s Respiratory Study. Pediatr Pulmonol 2006;41(4):318–25. [DOI] [PubMed] [Google Scholar]
- 6.Korn S, Hübner M, Jung M, Blettner M, Buhl R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir Res 2013;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 8.Paul G, Brehm JM, Alcorn JF, Holguín F, Aujla SJ, Celedón JC. Vitamin D and asthma. Am J Respir Crit Care Med 2012;185(2):124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85(3):788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 2007;120(5):1031–5. [DOI] [PubMed] [Google Scholar]
- 11.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J Allergy Clin Immunol 2010;126(1):52–8.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol 2012;129(5):1243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med 2010;181(7):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu AC, Tantisira K, Li L, Fuhlbrigge AL, Weiss ST, Litonjua A, Childhood Asthma Management Program Research Group . Effect of vitamin D and inhaled corticosteroid treatment on lung function in children. Am J Respir Crit Care Med 2012;186(6):508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brehm JM, Celedón JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 2009;179(9):765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brehm JM, Acosta-Pérez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 2012;186(2):140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr 2011;158(3):437–41. [DOI] [PubMed] [Google Scholar]
- 18.Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J Pediatr 2014;81(7):650–4. [DOI] [PubMed] [Google Scholar]
- 19.Clifford RL, Knox AJ. Vitamin D - a new treatment for airway remodelling in asthma? Br J Pharmacol 2009;158(6):1426–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginde AA, Mansbach JM, Camargo CA., Jr.Vitamin D respiratory infections, and asthma. Curr Allergy Asthma Rep 2009;9(1):81–7. [DOI] [PubMed] [Google Scholar]
- 21.Arshi S, Fallahpour M, Nabavi M, Bemanian MH, Javad-Mousavi SA, Nojomi M, et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Ann Allergy Asthma Immunol 2014;113(4):404–9. [DOI] [PubMed] [Google Scholar]
- 22.Sharif MR, Tabatabaei F, Madani M. The relationship between serum vitamin D levels and asthma in children referred to the pediatric clinics in Isfahan during 2012–2013. KAUMS Journal (FEYZ) 2014;18(5):462–8. [Google Scholar]
- 23.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 2005;128(6):3792–8. [DOI] [PubMed] [Google Scholar]
- 24.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 2008;181(10):7090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med 2011;184(12):1342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, et al. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol 2009;158(6):1429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. J Allergy Clin Immunol 1999;104(3 Pt 1):509–16. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee A, Damera G, Bhandare R, Gu S, Lopez-Boado Y, Panettieri R, Jr, et al. Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Br J Pharmacol 2008;155(1):84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen M, Trubert CL, Rizk-Rabin M, Rehan VK, Besançon F, Cayre YE, et al. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol 2004;89–90(1–5):93–7. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, et al. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol 2009;297(3):L496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]