Abstract
Coronavirus disease 2019 (COVID-19) is caused by a novel form of the coronavirus that caused severe acute respiratory syndrome (SARS). SARS-CoV-2 raised in China and has broadcast to 261 countries globally. SARS-CoV-2 a member of β-coronavirus family and has an almost matching genome sequence to a bat coronavirus, pointing to the bat as the natural host before it was transmitted to humans. SARS-CoV-2 uses the same receptor, angiotensin-converting enzyme 2 (ACE2) as that used by SARS-CoV and principally infects the respiratory tract. The clinical symptoms of COVID-19 patients include fever, cough and fatigue whilst small populations of patients have gastrointestinal symptoms. The old people and people with underlying metabolic and cardiovascular diseases are more affected to infection and have worse outcomes. These may be associated with acute respiratory distress syndrome (ARDS) and a cytokine storm. In this review, we discuss the pathogenesis and clinical characteristics of disease and the pharmacologic approaches that may control COVID-19.
Keywords: SARS-CoV-2, Coronavirus disease 2019 (COVID-19), Pathogenesis, Diagnosis, Therapy
INTRODUCTION
Coronaviruses (CoVs) have been associated with a important epidemics in East Asia and most countries in the Middle East. Severe acute respiratory syndrome (SARS) and the Middle East respiratory syndromes (MERS) arose in 2002 and 2012, respectively. A new CoV appeared in 2019 (1), known as SARS-COV-2, whose genome showed 96.2% sequence sameness to the bat CoV RaTG13 (2). SARS-CoV-2 causes coronavirus disease 2019 (COVID-19) that originated in China before spreading all over the world. Due to the severity of this epidemic the WHO announced a “global health emergency” in on January 31st, 2020 (3).
At the moment no effective therapy approach or approved vaccines for COVID-19 are accessible. Most countries attempt to prevent further spreading of this lethal virus by implementing preventive plans and using control strategies (1). Aerosols inhalation of SARS-CoV-2 results in infection via angiotensin-converting enzyme 2 (ACE2)-bearing cells such as airway epithelial cells and alveolar type 2 cells (4). The virus decrease anti-viral IFN responses that results in uncontrolled viral replication and the subsequent infiltration of neutrophils and monocytes/macrophages cause excess pro-inflammatory cytokines secretion. Th1/Th17 cells may be activated and these help exacerbate inflammatory responses in severe patients (4).
The most common symptoms for COVID-19 infection are polypnea, fever, dry cough, expectoration and nausea/vomiting, fatigue/myalgia and headache with lymphopenia and acute respiratory distress syndrome (ARDS) with pulmonary ground-glass background occurring in severe patients (5). A reduction in the ability to smell distinct from anosmia is considered a major marker of SARS-CoV-2 infection (6).
Both septic shock and multi-organ dysfunction syndrome (MODS) have been defined as common complications in critically ill patients. Increased levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), procalcitonin, ALT and D-dimer are reported (5). The current review will focus on recent evidence regarding the pathogenesis and progress of disease and how knowledge of the structure of SARS-CoV-2 including viral structure is leading to new insights and pharmacological approaches towards protection and cure of COVID-19.
The SARS-CoV-2 structure and complexity
The viral structure was first reported by Chinese scientists on 7 January 2020 (2). SARS-CoV-2 was 100nm in size and belonged to the β-coronavirus family and caused the third severe zoonotic coronavirus disease after SARS and MERS (5, 7, 8). CoVs are also found in animals such as birds and wild mammals. Four genera of the virus have been characterized so far: α, β, γ and δ CoVs (9). Human CoVs (HCoVs) include 229E, OC43, HKU1, NL63, SARS-CoV, MERS-CoV (10). 229E and NL63 are two members of the CoVα family whilst the others are β family members (10). CoVs are RNA viruses with a polycistronic genome of ∼30kb in size that codes for multiple non-structural proteins such as ORF1a and ORF1b. They also encode some multiple structural proteins including spike (S), envelope (E), membrane (M) and nucleocapsid (N) proteins and lineage-specific accessory proteins including ORF3a, ORF3b, ORF6, ORF7a, ORF7b, ORF8a, ORF8b and ORF9b in SARS-CoV (3, 10). Main differences between SARS-COV-2 and other CoVs have been described in ORF3b and ORF8 (11). Both SARS-CoV and MERS-CoV are mainly pathogenic and cause acute respiratory distress syndrome (ARDS) whereas 229E, OC43, HKU1 and NL63 cause the common cold (10).
The S protein has two parts, S1 and S2, and is a essential immunodominant protein for coronaviruses (2). S1 is associated with virus-host range and cellular tropism as it possesses the receptor-binding domain (RBD) whilst S2 is responsible for virus-cell membrane fusion via heptad repeat (HR1 and HR2) domains.
RBD in SARS-CoV S1 recognizes angiotensin converting enzyme 2 (ACE2). The RBD continuously moves between a receptor binding stand-up position and a lie-down position for immune evasion (12) By masking the RBD domain from neutralizing antibodies, this bias towards the lying state can favor SARS-CoV-2 to immune evasion (13). S1 protein in corona viruses has two domain N-terminal (S1-NTD) and C-terminal (S1-CTD) domains. S1-NTDs are responsible for binding sugar and S1-CTDs recognize ACE2, APN, and DPP4 receptors (14).
The M protein is the viral protein that gives a shape to the viral envelope by binding to the N protein and acting as a central core for coronavirus assembly. M proteins are extremely diverse with respect to amino acid sequence across viruses but maintain structural similarity (2, 3). The E protein plays a key role in the pathogenesis, assembly, and release of the virus. It is a small integral membrane polypeptide that acts as ion-channel. The N protein, as described above, interacts with the M protein during virion assembly and also increases transcription efficiency of the virus. Besides, the most significant structural proteins, the SARS-CoV-2 genome contain 15 non-structural proteins (NSPs).
The above viral proteins have vital roles in viral replication (3). Mutations, recombination and deletion are important in host conversion of CoVs between SARS-CoV, SARS-CoV-2, and MERS-CoV. A mutation rate of 0.80–2.38×10−3 nucleotide substitutions per base each year has been reported for SARS-CoV. Thus, adjustment mutations in the S protein of SARS-CoV were documented the SARS outbreak that resulted in improved binding to the ACE2 receptor (15, 16). A single N501T mutation (corresponding to the S487T mutation in SARS-CoV) increases the binding affinity between the SARS-CoV-2 RBD and human ACE2 (17). All HCoVs are considered as zoonotic in origin, whereas bats are the important source for development and survival of 229E, NL63, SARS-CoV, MERS-CoV and SARS-CoV-2 strains (18). Intermediate and amplifying hosts of HCoVs have been identified in domestic and wild mammals (18). Strong evidence indicates the origin of MERS-related-CoV and SARS-related-CoV being in bats whereas civet cats and camels act as amplifier hosts for SARS and MERS respectively (3). The SARS-CoV-2 viral genome shows 96.2% similarity with the bat CoV RaTG13 (2).
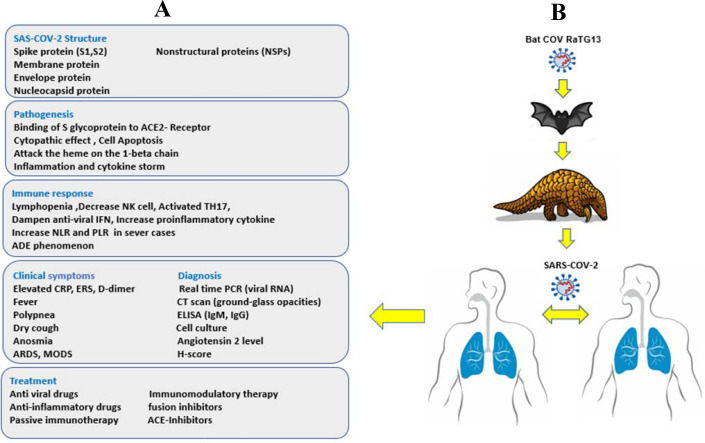
Bats are adapted to CoVs due to their high level of reactive oxygen species (ROS) and continue expression of interferon-stimulated genes which suppress CoV replication. In addition, they have an attenuated NF-κB and NLRP3 inflammasome response that also leads to decreased viral virulence (10). Thus, infected bats generally have no symptom or have moderate symptoms. The high levels of ROS in bats is also mutagenic which affects the proofreading capability of CoV polymerase and this effect is compounded by the long life span of bats (>25 years) (10). Recent evidence suggests that pangolins may be the intermediary species between bats and human for SARS-CoV-2 cross-species transmission (19). Blast analysis on proteins from 2,845 CoV reference genomes, including RaTG13, SARS-CoV-2s, and other recognized CoVs, 22 contigs were similar to SARS-CoV-2s (95.41% amino acid similarity) and that 12 contigs similar to bat SARS-CoV-like CoV (97.48% amino acid similarity). These results show that the Malayan pangolin might reservoir of a new CoV that is similar to SARS-CoV-2 (19) (Figure 1).
Figure 1.
Summary of COVID-19 infection and transmission
A) Panel summarizing the viral character, pathogenesis and host immune response.
B) Indicating the possible source of SARS-CoV-2 and transmission between host animals and finally human to human as end transmission.
Abbreviations: NLR: neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio ADE: Antibody dependent enhancement; ARDS: Acute respiratory distress syndrome; MODS: Multi organs dysfunction syndrome
Mechanisms of SARS-CoV-2-mediated infection and inflammation
SARS-CoV-2 spreads through the respiratory system by droplets, respiratory secretions, and by direct contact (2). The existence of SARS-CoV-2 in fecal swabs and in blood indicates that additional routes of transmission might be existed. The incubation period for the development of COVID-19 is between 1–14 days but mostly between 3–7 days, and it is very contagious during this latency period (2).
The S-glycoprotein on the surface of CoVs enables binding to ACE2 on the surface of human cells such as lung alveolar epithelial cells and enterocytes of the small intestine (2). Furthermore, Cleavage of the viral spike glycoproteins by serine protease causes their activation and facilitates virus-cell membrane fusions leading to host cell entry, replication, and spread (20). The transmembrane protease serine 2 (TMPRSS2) is generally required for plasma membrane fusion of SARS-CoV-2 (21).
SARS-CoV-2 has a high binding affinity between the HR1 and HR2 domains that induce simple viral membrane fusion and augment viral infectivity or transmissibility. Furthermore, the binding affinity between ACE2 and RBD in S protein of SARS-CoV-2 is almost 10-fold higher than that of SARS-CoV which also enhances infectivity and transmissibility (21).
Following membrane fusion with host cells, SARS-CoV-2 releases its RNA into the cell cytoplasm enabling the translation of two polyproteins (pp1a and pp1ab) by host ribosomes. These code non-structural proteins and produce a replication-transcription complex (RTC) within the virus-containing double-membrane vesicle. The newly formed RNA, nucleocapsid proteins and envelope glycoproteins assemble inducing viral particle buds. Finally, the vesicles that carry viral particle fuse with the plasma membrane and release (2, 10).
Toll-like receptor (TLR) 3, -7, -8 and -9 recognize viral RNA and DNA in the endosome. The viral RNA receptor retinoic-acid inducible gene I (RIG-I), the cytosolic receptor melanoma differentiation-associated gene 5 (MDA5) and nucleotidyl transferase cyclic GMP-AMP synthase (cGAS) recognize viral RNA and DNA in the cytoplasm (2). Engagement and activation of these receptor pathways activates the transcription factors NF-κB and IRF3, secretion of type I Interferons (IFN-α/β) and a gamut of pro-inflammatory cytokines (2). In ARDS mouse models induced by multiple noxae, including SARS-CoV, inhibition of the TLR-4 gene, but not TLR-3 or 9 genes alleviate acute lung injury. Although TLR-4 responds to bacteria, one hypothesis is that oxidized phospholipids can be activate TLR-4 by SARS-COV-2 and onset of ARDS. TLR-7 agonists may inhibit severe COVID-19 and reveal synergic activity with active anti-viral therapy (22). IL-6 and TNF-α are important cytokines in sever COVID-19 and secretion after TLR4 activation. A remarkable binding is between the viral S protein and TLR1, TLR4, and TLR6 and TLR4 has the highest binding energy.. Studies indicate that assessing the role of TLR4 signaling in the heart may valuable to predict cardiovascular disease outcomes in elderly COVID-19 patients (23).
Cytokine storm and pyroptosis are detected in highly pathogenic HCoVs. Cell death programmes including apoptosis and necrosis are also observed in the pathogenesis of COVID-19 (10). Pyroptosis is a programmed cell death with high inflammation and higher IL-1β, considered as an important cytokine released within pyroptosis, is reported to be up-regulated in SARS-CoV-2 infection (24).
LDH is a cytosolic enzyme that after membrane damage it release from the cells. In fact, LDH is a marker of pyroptosis monitoring. Also, IL-1β and IL-1Rα increased in the serum of COVID-19 patients upon inflammasome activation. The inhibition of inflammasome-mediated pyroptosis in macrophages might alleviate blood clotting by inhibition the release of tissue factor. Moreover, NLRP3 inhibition is beneficial to decreasing hypertension, chronic obstructive pulmonary disease, type 2 diabetes, and cardiovascular disease that associated with COVID19 (25).
Other structural compounds of the virus contribute towards COVID-19 pathophysiology. For example, ORF8 and surface glycoprotein bind to porphyrin to inhibit haem metabolism whilst ORF1ab, ORF10, and ORF3a proteins attack the haem β1 chain in haemoglobin to separate the bound iron (26). The overall result being reduced haemoglobin and oxygen transport and an activation of the coagulation cascade.
SARS-CoV-2 genomes analysis shows two types of SARS-CoV-2: an L type (∼70%) and an S type (∼30%) (27). The S type of virus is an ancestral version and less aggressive whilst the L type was more common in the early phase of the outbreak in Wuhan but its frequency decreased early in 2020 (27). A missense mutation in SARS-CoV-2 has significant association with a difference in asymptomatic and symptomatic patients (28). A missense variation in the ORF1ab gene (11083G>T), located in the NSP6 protein of virus, may affect NSPs interactions and the formation of double membrane vesicles is found patients with less severe symptoms (28). Furthermore, a spike protein mutation (D614G) induces two variants of SARS-CoV-2 virus including G614 and D614 (29). G614 is dominant worldwide and positive samples have higher yield of RNA, however, it does not appear to affect disease severity (29). The D614G mutation plays an essential role in viral entry and immune responses (29).
Three stages of the disease have been defined: Stage 1, asymptomatic state which takes1-2 days and in during this stage the virus binds to epithelial cells of the nasal cavity and starts genome replicating. Stage 2, follows after a few days and is defined by the involvement of upper airway symptoms. The virus in stage 2 spread and migrates to the respiratory tract onward the conducting airways and begins to activate the innate immune system. CXCL10 serum levels may be predictive of disease progression (30, 31). In final stage or stage 3, hypoxia and ‘ground glass’ infiltration of the lung as seen by computed tomography develops which may lead to the induction of ARDS (31). In stage 3 of the disease, the virus spreads to the alveoli and infects alveolar type II cells, large numbers of virion are released which induces the apoptosis and necrosis. Aberrant wound healing may lead to more severe scaring and fibrosis than other forms of ARDS (10, 31). Lung biopsy samples from COVID-19 patients showed propagate alveolar damage, cellular fibromyxoid exudates, hyaline membrane production, and desquamation of pneumocytes, characteristic of ARDS (3).
COVID-19 patients have perivascular involvement following viral-induced severe endothelial injury and disrupted endothelial cell membranes. In addition, COVID-19 triggered lungs vascular thrombosis with microangiopathy leading to the occlusion of alveolar capillaries (32).
The lung injury is more obvious in sever patients, which is correlation with a cytokine storm. The most patients who died had comorbidities such as hypertension, diabetes or cardiovascular disease that compromised their immune system. SARS-CoV-2 causes severe health problems among the elderly probably due to their aging immune system (5). It has been proposed that treatment with ACE inhibitors and angiotensin receptor blockers (ARBs) could elevated the expression of ACE2 and thereby might be associated with more severe infection during COVID-19 infection (33). Moreover, ACE inhibitors may play a role in the anti-inflammatory action of ACE2 and contributed to increase survival rates of COVID-19 patients (34). However, two large population-based studies showed no evidence that ACE inhibitors or ARBs affected the risk of COVID-19 (34, 35). Cardiovascular disease is independently associated with a higher risk of in-hospital death (34). In addition, ACE2 polymorphisms have been correlated to diabetes mellitus, cerebral stroke, and hypertension, particularly in Asian populations (33). Amino acids N90 and T92 within ACE2 confer protection and are CoV host modifiers. Furthermore, the ACE2 variants K31R, E35K, E37K, D38V, N33I, H34R, Q388L and Y83H are predicted to decrease ACE2 binding to the SARS-CoV-2 S-protein and protect these individuals (36).
COPD patients and smokers are more susceptible to severe COVID-19 infection (37). Smoking increases ACE2 in human lungs and smoking cessation causes down regulation in lung ACE2 levels (38). The impact of asthma on COVID-19 severity is unclear. A study looking at COVID-19 death across 17m patients in the UK indicated that severe asthma was a strong risk factor (39). In addition, reports differ regarding gene expression for ACE2 and TMPRSS2 between asthma and health and across asthma severity with some suggesting that both Th2 cytokines and inhaled corticosteroid use suppresses expression and others showing the converse (40–43). Finally, some reports suggest that severe asthma is much more common in children and adults with mild to severe COVID-19 (44).
There is a difference in COVID-19 fatality rate between males (2.8%) and females (1.7%). As ACE2 is located on the X chromosome, there may be alleles that give resistance to COVID-19 in females. Furthermore, estrogen and testosterone could affect ACE2 expression and immune responses in COVID-19 patients (24, 34). TMPRSS2, a coreceptor for SARS-COV-2, is highly expressed in human prostate epithelial cells but no significant difference in TMPRSS2 expression is seen in the lungs of females or males (45).
Children show rather milder clinical symptoms in comparison with adults (46). An age stratification of disease severity exists, where young children and infants (<5 years) had a higher disease severity compared to children over the age of 5 years (46). The reason for the low severity of infection in children is still unclear. However, different hypotheses have been proposed including that children have a different immune response to the SARS-CoV-2 virus than the adults (47, 48) and that common viruses within the lung and airway mucosa of young children may limit the growth of SARS-CoV-2 by direct virus-to-virus interactions (49). This latter scenario is supported by the association between the number of viral particle numbers and COVID-19 severity (50). This may also accounts for the deaths of healthcare workers who have been exposed to high dose of SARS-CoV-2 (50).
As mentioned above treatment with ACE inhibitors or angiotensin receptor blockers may be trigger expression of ACE2 receptor (47). Both treatments approaches are general in adults with hypertension, but much less useful in children (47). Interestingly, children are not less prone to severe ARDS during respiratory tract infections than adults (51).
The gut microbiota features also represents a risk factor for susceptibility to COVID-19 due to their correlation with proinflammatory cytokine expression (52). Microbiota play an essential role in pulmonary immune development. Alterations in gut microbiota (dysbiosis) by antibiotic use or nutrition style can increase respiratory diseases risk (53). Dysbiosis can trigger inflammatory bowel disease (IBD) and neurodegenerative diseases (54).The systemic inflammation associated with COVID-19 causes the death of neuronal cells. Interestingly, both the neurons and glial cells within the brain express ACE2 receptors. Also, proinflammatory mediators in the GI tract can reach to the brain via vessels and compromises the blood brain barrier (BBB) whilst SARS-CoV-2 can enter to the brain by the gut-brain axis (54, 55). Finally, the patient develops either acute encephalitis. Acute encephalitis causing spectrum of symptoms ranging from headache to seizure (55).
Mucosal inflammation in IBD may increase expression of ACE2 and patients with IBD might be particularly have higher risk for COVID-19. However, there is no evidence that patients with IBD are highly susceptible to COVID-19. Interestingly, the TNF-α convertase ADAM17 is a protease increase in patients with active IBD that cleavage membrane ACE2. The soluble form of ACE2 acts as a competitive binding receptor for viruses and may decrease susceptibility to infection (56).
Pregnancy is a significant risk factor for COVID-19 infection (3). Infection with SARS-CoV-2 may induce adverse effects on the fetus, including slow growth, abortion, preterm delivery and perinatal death. In spite intrauterine maternal-fetal transmission of CoVs is low, and it is not known in either SARS or MERS (3).
Immune response against SARS-CoV-2
After viral infection, pattern recognition receptors (PRRs) including TLRs, RIG-I and NOD-like receptor (NLR) detect the viral nucleic acid and induce the synthesis of type I interferons (IFNs) (57). The N-protein of SARS-CoV plays as an antagonist of the immune escape protein and even interferon response (57). IFN-I levels associated with severity of disease and COVID-19 is able to block the release of IFN pathways and activates other pathways (58). Subsequently, neutrophils and monocytes/macrophages are triggered which induces hyperproduction of pro-inflammatory cytokines (4). Specific Th1 and Th17 cells are also initiated and these further contribute to the exaggerated inflammatory response (4).
In severe disease, or when the viral load is high, the host immune system attempts to kill the virus. This may result in the release of many inflammatory mediators and produce a severe cytokine storm (59). The cytokine storm can induce organ damage and subsequently edema, ARDS, acute lung injury (ALI), acute cardiac injury and secondary infection, leading to death (60). ACE2 receptors are abundantly expressed in the cardiovascular system, liver, digestive organs and kidneys. In addition, almost all endothelial cells and smooth muscle cells across organs express ACE2, enabling viral circulation and spread (61). In the early phase of CoV infection, dendritic cells and epithelial cells release pro-inflammatory cytokines and chemokines such as IL-1β, IL-2, IL-6, IL-8, both IFN-α/β, tumor necrosis factor (TNF), C-C motif chemokine 3 (CCL3), CCL5, CCL2 and IP-10 (60). Furthermore, there is a systemic lymphopenia, especially of NK cells, and atrophy of the spleen and lymph nodes. Infiltration of activated monocytes, macrophages and lymphocytes into the lung tissues and vascular system induces lesions in these organs (62–64).
There is a heightened increase of IL-6 in COVID-19 subjects with fatal disease compared to infected patients who die (65–69). enhancement of IL-6 level in COVID-19 patients is associated with high levels IL-2, IL-7, IFN-ɣ and GM-CSF which elevate in secondary hemophagocytic lymphohistiocytosis (sHLH) (58). The systemic cytokine release syndrome (CRS) and sHLH-like inflammatory response can causes neutrophilic NETosis and microthrombosis which is seen with severe COVID-19 infection (58).
In COVID-19 infection, CD4+ and CD8+ cells and CD4+CCR4+CCR6+Th17 cells express high level of HLA-DR (70). In some severe cases, NK cells were very low or even undetectable (71). Moreover, memory helper T cells, regulatory T cells and γδ-T cell numbers decrease in severe cases (58, 71). In addition, total T lymphocytes, particularly CD4+ T cells, CD8+ T cells, and IFNγ-expressing CD4+ T cells considerably decrease in severe conditions (72). Vγ9Vδ2 T cells are the dominant γδ T-cell subset in adults and with age the numbers are more variable. Old people with decrease numbers of Vγ9Vδ2 T cells are susceptible to the SARS-CoV-2 Infections (73). Overall, the degree of cytokine expression and systemic T cell lymphopenia correlates with pulmonary damage and unfavorable outcome (72).
In COVID-19 patients, the expression of NKG2A on NK and CTLs cells is upregulated. These cells are unable to produce sufficient levels of CD107a, IFN-γ, IL-2, granzyme B, and TNF-α (74). Interestingly, the percentage of NKG2A+ cytotoxic lymphocytes was decline in recovered patients which emphasizes the potential the role of NKG2A expression in cytotoxic lymphocyte exhaustion and disease severity (74). Despite lower numbers, T cells in severe COVID-19 are highly more activated and exhibit a tendency to exhaustion with the expression of PD-1 and TIM-3 markers. Conversely, recovering patients were shown to have an increase in follicular helper CD4 T cells (TFH) and decreased levels of inhibitory markers with increased levels of granzyme and perforin (58).
Decreased frequencies of mucosal-associated invariant T (MAIT) cells and invariant natural killer T (iNKT) cells occur in the peripheral blood of severe COVID-19 patients, which may account for the induction of ARDS. In early COVID-19 infection, the level of MAIT and iNKT cells in peripheral blood is correlated with preserved lung oxygenation (75).
Almost all of the infiltrating cells in the lungs are monocytes and macrophages, with moderate numbers of multi nucleated giant cells but few lymphocytes. Most of the infiltrating lymphocytes were CD4 positive T cells (62). Peripheral blood of a patient with severe COVID-19 showed extremely high numbers of CCR6+TH17 cells providing further evidence for a role for TH17 cells in the cytokine storm (76). TH17 cells produce IL22 that upregulates antimicrobial peptides mucins and fibrinogen. Therefore, IL-22 may drive the l dangerous edema with abundant of mucins and fibrin which is seen in SARS-CoV-2 and SARS-CoV patients (76). It would be interesting to see the effects of an IL-22 antibody such as fezakinumab in these patients.
Upregulation of monocyte subsets was seen in COVID-19 patients including CD14+CD16+ macrophages bearing CD68+CD80+CD163+CD206+ surface markers (77). Analysis of autopsy samples from COVID-19 patients showed that monocytes from infected patients that bear ACE2 receptors were associated with a delayed type I INF response (77).
There is an increase in the neutrophil-to-lymphocyte ratio (NLR) in severe COVID-19 patients than mild cases (71). NLR is a indicator of systemic inflammation and infection and a key predictor of bacterial infection. Increased NLR in infected patients associated with disease severity and unfavorable consequence, addressing a possible role for hyper-inflammatory responses in COVID-19 (71). The platelet-to-lymphocyte ratio (PLR) is another response of the body to severe disease. Patients with highly increased platelets are hospitalized for longer (78) and acts as a monitor of the intensity of the cytokine storm (78). Increase in thymosin could regulate immune responses to elevate lymphocytes and develop the situation and prevent the development to a severe disease condition (78).
Some patients have high level of anti-phospholipid antibodies, including anti-cardiolipin antibodies and anti-β2 glycoprotein antibodies that result in severe thrombosis. The underlying mechanism of vascular damage may be due to the direct damage of endothelial cells by the virus, result in disseminated intravascular coagulation (DIC), anti-phospholipid syndrome (APS) and following vasculitis (62, 79).
In a cohort of 452 patients with COVID-19 in Wuhan, China, immunoglobulins (IgA, IgG and IgM) and complement proteins (C3 and C4) were in the normal level (71). In addition, no considerable differences in the levels of IgA, IgG, and complement proteins C3 or C4 between the mild and severe groups was found although IgM levels were a little decreased in severe cases (71). However, two studies, based on the analysis of 222 and 173 patients with COVID-19, showed that most of the patients with severe disease had an increase in IgG production and a higher concentration of total antibodies. Moreover, this effects was associated with adverse outcome which may suggestive of possible antibody- dependent enhancement (ADE) during SARS-CoV-2 infection (80). ADE occurs when antiviral neutralizing antibodies (NAb) cannot absolutely neutralize the virus. The virus-NAb complex binds to the Fc receptor (FcR), and infects the cells. Virus-NAb complex binding to FcR can also activate proinflammatory responses and switches macrophages towards the M1 proinflammatory phenotype (81).
In another study, the N-proteins of SARS-CoV-2 were bound to mannose-binding protein-associated serine protease2 (MASP-2), a key serine protease in the lectin pathway of complements activation, able to aberrant complement activation and exacerbated inflammatory lung injury. Complement hyperactivation was also reported in COVID-19 patients, and an improvement in patients was observed when the deteriorating patients were treated with an anti-C5a antibody (82).
Laboratory diagnosis
Diagnosis of SARS-CoV-2 (COVID-19) is on the basis of RNA tests using real-time RT-PCR or next-generation sequencing (3). SARS-CoV-2 RNA is detectable in throat swabs, nasal swabs, sputum, BALF, stool, and blood. Sampling of the lower respiratory tract by BALF aspirate is recommended due to its higher positivity rate and higher sensitivity but is more invasive and not amenable to population screening (3). The patient is considered recovered when two consecutive oral swabs results are negative by RT-PCR (3). However, an initial screen with RT-PCR may give negative results and so both repeated swab tests using RT-PCR and, if available, CT scans are necessary to decrease false-negative results (3). During the COVID-19 widespread, 1-step quantitative RT-PCR assays have been expanded that detect the ORF1b and N regions of the SARS-CoV-2 genome (83, 84).
Seroconversion usually occurs in COVID-19 patients between 7 and 14 days after early symptoms (58). Serological analysis such as enzyme linked immunosorbent assay (ELISA) and lateral flow assay (LFA) for COVID-19 IgM and IgG antibodies assay have been developed (85). However, the total antibody is more sensitive than IgM and IgG for detecting SARS-CoV-2 infection (86). The sensitivity of the various diagnostic tests, available for use following the onset of symptoms are summarized in Table 1 (3, 86).
Table 1.
Diagnostic test sensitivity in the days after symptom onset (87).
| Days after symptom onset | |||
|---|---|---|---|
| SARS-COV-2 Test | 1–7 | 8–14 | 15–39 |
| RNA by RT-PCR | 67% | 54% | 45% |
| Total Ab | 38% | 90% | 100% |
| IgM | 29% | 73% | 94% |
| IgG | 19% | 54% | 80% |
Isolation of SARS-CoV-2 and culturing of virus and infected cells are other ways to confirm the infection. In this manner, a human airway epithelial cell culture was effective for SARS-CoV-2 isolation (3). Although cell culture is not recommended for diagnostic purposes (87).
Several other biomarkers have been proposed as being useful for the detection of infection and these relate to data showing correlations with SARS-CoV-2 infection. For example, plasma angiotensin 2 level correlated with the viral load and lung injury (88) and all patients with severe COVID-19 should be screened for hyperinflammation assay tests including ferritin, ESR and the hemophagocytosis or H-score (89).
There are similarities between COVID19 and primary hemophagocytic lymphohistiocytosis (HLH). The H-score makes a prospect for the existence of secondary HLH. H-scores more than 169 are 93% sensitive and 86% specific for HLH (89). For example primary HLH characterizes by hyper inflammatory immunodeficiency states that often associate with deficiency of for the lysis viral infected cells (90).
Pharmacological approaches for controlling of disease
Symptomatic patients with respiratory complications require ventilatory support. High-flow oxygen therapy, intubation, interferon-β and antiviral treatments are recently used. Intravenous glucocorticoid and immunoglobulin therapies, arbidol, fluid therapy and antibiotic therapy to manage secondary bacterial infections, and even muscle relaxation medicine to prevent ventilator-related lung damage are indicated (3, 5).
Antiviral drugs
Anti-viral medications have been used to treat COVID-19 and these include Lopinavir/Ritonavir (protease inhibitors), Remdesivir (Nucleotide analogue prodrug), Ribavirin (Synthetic guanosine nucleoside), Oseltamivir (Neuraminidase inhibitor), Penciclovir/ Acyclovir (Nucleoside analog), Ganciclovir (Nucleoside analog), Favipiravir (Nucleoside analog: Viral RNA polymerase inhibitor) and Nitazoxanide (Antiprotozoal agent) (2, 3). Ivermectin, an anti-parasitic drug that gives FDA-approved, and have broad spectrum anti-viral effect in vitro and suppress SARS-CoV-2 infection of Vero-hSLAM cells (91). Since SARS-CoV-2 attacks the haem β1 chain of haemoglobin (see above), Favipiravir that inhibits the envelope protein and ORF7a protein bind to porphyrin and thereby inhibits v penetration of virus to host cells has also been tested (26).
Anti-inflammatory drugs
Anti-inflammatory drugs are used to suppress inflammation and include glucocorticoids, chloroquine/hydroxychloroquine, inflammatory cytokine antagonists such as IL6R monoclonal antibodies (Tocilizumab), TNF inhibitors, IL-1 antagonist (Anakinra), janus kinase (JAK) inhibitors and intravenous immunoglobulin (IVIG) (92, 93). Corticosteroids may hinder the inhibition of virus replication and increase the risk of secondary infection, particularly in immunocompromised individuals (62). Although many COVID-19 patients treatment approach not suggested the use of glucocorticoids, a recent study shows usage of dexamethasone at a dose of 6 mg once daily for up to 10 days caused lower 28-day mortality among the patients under respiratory support (94).
Chloroquine (CQ) is used for treating malarial and autoimmune disease whilst hydroxychloroquine (HCQ) is a disease-modifying anti-rheumatic drug (DMARDs). Both can inhibit ACE2 glycosylation and decrease the effectiveness of binding between ACE2 and the virus S-protein and prevent viral replication by increasing both endosomal and lysosomal pH (95). HCQ also enters APCs and inhibits antigen processing and MHC class II-mediated autoantigen presentation to T cells. After activation of T cells and expression of CD154, cytokines level suppressed. Finally, HCQ disrupts the interaction of DNA/RNA with TLRs and the nucleic acid sensor cGAS and therefore suppresses the expression of pro-inflammatory genes (95). Chloroquine may also inhibit the ability of ORF1ab, ORF3a and ORF10 to target haem and, as described above, relieve the symptoms of respiratory distress (26).
AP2-associated protein kinase 1 (AAK1) is an endocytosis regulator and AAK1 inhibitors can attenuate viral infections and possibly that of SARS-CoV-2. Baricitinib, a pan JAK1 and JAK2 inhibitor and an AAK1 inhibitor, is a candidate COVID-19 treatment as it is relative safety and has a high affinity. Therapeutic dosing with either 2mg or 4mg once daily gave a plasma concentration sufficient for target inhibition (96). However, JAK1 inhibitors block IFN-α production and may not, therefore, be suitable for the treating the cytokine storm (62, 76).
Fedratinib is a JAK2 inhibitor can inhibit secretion of Th17 cytokines and probably IL-6 effects on other cell types, and may prevent the TH17-associated cytokine storm (76). JAK2 inhibitors can also be used with other anti-viral treatments. As JAK2 inhibition is reversible, acute treatment before the disease transition from serious to critical or during the critical phase would not be expected to affect TH17 activation, which is necessary to maintain innate immunity and response to extracellular pathogens (76).
A multicenter, randomized controlled Clinical trials on tocilizumab (TCZ, an IL-6 receptor blocker approved for cytokine release syndrome), has been approved in patients with COVID-19 (89). In one study, intravenous TCZ (400mg) given to 20 patients together with the basic anti-viral treatment rapidly improved the fever and other symptoms. 75% of patients had improved oxygen saturation and their lungs CT scan were improved in 90% of patients. Moreover, lymphocyte count back to normal range in 50% of patients. The investigation indicates that TCZ can be an efficient treatment in severe COVID-19 patients (62). A clinical trial using certolizumab pegol (a TNF blocker) along with other anti-virus therapies showed it may also effective on COVID-19 patients (60).
Studies of IL-1 blockade (anakinra) in sepsis, showed significant survival benefits in patients with excessive inflammation is beneficial, without increased adverse effects (89). This suggests that targeting IL-1 may be of benefit in COVID-19. IL-1β maturation occurs following through proteolytic cleavage of pro-IL-1β by caspase 1, for the formation of inflammasome complex. MCC950 and INF58 are small-molecule which able to inhibits NLRP3 inflammasomes signaling and may considered as potential target in the application of SARS-COV-2 infection (10). Tranilast is a tryptophan analogue and can inhibit NLRP3. Disulfiram is a potent inhibitor of pyroptosis cytokines and approved for COVID-19 therapy. Furthermore, quercetin is a natural antioxidative flavonoid in a lot of plants, has inhibitory effects on inflammasomes, including NLRP3. Nonsteroidal drugs anti-inflammatory drugs (NSAIDs) can also be used repurposed to inhibition of NLRP3 (25).
IVIG has shown efficiency as an immunomodulatory treatment for inflammatory and auto-immune diseases and demonstrated clinical benefits with good tolerance in SARS (97). In one small study, three patients with severe COVID-19 were treated using high-doses IVIG (0.3–0.5g/Kg per day for five days) had a rapid clinical improvement with their temperature normalizing within 1–2 days and breathing difficulties alleviating in 3–5 days. The authors emphasis the importance of the therapeutic intervention timing as “Patients might not receive much benefit when overall systemic damage has already taken place” (97). IVIG can prevent ADE and decrease the levels of antiviral NAb (81).
Passive immunotherapy
Convalescent plasma is another approach that can be used for treating COVID-19 patients. The SARS-CoV specific human monoclonal antibody CR3022 also binds the SARS-CoV-2 RBD, could be consider as a drug for COVID-19 treatment (3). Furthermore, potential therapeutic and prophylactic monoclonal antibodies have been isolated from eight COVID-19 patients. The isolated antibodies inhibited RBD-ACE2 interaction and have high binding affinity and neutralizing activity against SARS-CoV-2. Plasma from these patients was not cross-reactive against SARS-CoV or MERS-CoV, with isolated antibodies targeting SARS-CoV-2 only. However, there was no clear link between antibody response and disease status and further studies need to be conducted (98).
Immunomodulatory therapy
Melatonin is an immunomodulatory agent and has some efficacy in sever patients by decreasing vessel permeability, anxiety, sedation, and improving sleeping quality (60). Melatonin has both anti-inflammatory and anti-oxidative effects and potentiates the immune response and, therefore, has indirect anti-viral effects (60). Sirtuin-1 (SIRT1) mediates the anti-inflammatory actions of melatonin and reduces melatonin-induced skewing of macrophages to the pro-inflammatory phenotype (99). Furthermore, melatonin down-regulates NF-κB activation in T cells and in lung tissue (100, 101).
Melatonin reduces TNF-α, IL-1β, IL-6 and IL-8 expression and enhances the levels of IL-10 in serum (102, 103). It also improved the immune response by increasing the proliferation and maturation of NK cells, T and B cells, granulocytes and monocytes in both bone marrow and other tissues (104). Although there is no report related to the use of melatonin in COVID-19 patients, melatonin has shown some benefit in other inflammatory diseases. Short-term melatonin use is safe, even at high doses, and the reported adverse effects are limited (60).
Umbilical cord mesenchymal stem cells (UC-MSCs) have immunomodulatory ability (105). They may have beneficial effects for suppression the cytokine storm by producing anti-inflammatory factors. A study on seven patients with COVID-19 in Beijing shows that SARS-CoV-2 did not infect the injected UC-MSCs and these cells activated phagocytes and the secretion of antimicrobial peptides and proteins (AMPs) and by reducing the expression of indoleamine 2,3-dioxygenase (IDO) and IL-17 (61). Among all the stem cell types, UC-MScs may be the best candidate for preventing COVID-19 infection (61).
Fingolimod (FTY720) is an immunomodulator that down regulates sphingosine-1-phosphate receptor activity and inhibits T cell activation (24, 106) and is currently being assessed in a clinical trial for COVID-19 (clinical trial no: NCT04280588).
Fusion inhibitors
Fusion inhibitors can target different receptors on the virus or host such as the HR1 domain of S protein, the furin-like cleavage site in the S protein, the host actin protein, the S protein/ACE2 interaction and TMPRSS2 to prevent viral fusion and entry into the host cell. There is a high similarity between the SARS-CoV-2 and SARS-like CoVs genomic sequences. Comparative analysis identified a furin-like cleavage site in the SARS-CoV-2 S-protein that is absent in other SARS-like CoVs. The furin-like cleavage site seem to has a role in the viral life cycle, disease pathogenicity and might use as target for furin inhibitors in therapeutic approaches (3).
Actin protein is the host factor that is involved in cell entry and pathogenesis of SARS-CoV-2. Therefore, drugs effect on actin activity, such as ibuprofen, might have some therapeutic effects on the disease (3).
Arbidol (Umifenovir) is a small indole-derivative molecule, and involves inhibition of virus-mediated fusion by inhibiting the S-protein/ACE2 interaction with resultant blocking of viral entry into target cells. In one study, Arbidol gave a tendency to improve the discharge rate and reduce mortality in COVID-19 patients (107, 108).
TMPRSS2 inhibitors can be divided into two groups. The first include drugs already approved by the FDA or other organizations for treatment of different diseases, including: Camostat, aprotinin and rimantadine (20). Camostat mesylate, an approved agent in Japan for the treatment of pancreatitis, inhibits SARS-COV-2 lung cell entry by inhibiting TMPRSS2 (108). Nafamostat mesylate is another TMPRSS2 inhibitor that is approved in several countries (24). The second group includes potential drugs not yet approved for the human use, including plasminogen activator inhibitor type 1 (PAI-1). Finally, derivatives of sulfonylated 3- amindinophenylalanylamide were found to inhibit TMPRSS2 with a high efficiently block the influenza virus transmition in human cells (20). ACE2 blockade might be used in treating patients with COVID-19. Soluble recombinant human ACE2 (hrsACE2) reduced SARS-CoV-2 recovery in vitro. This agent now is undergoing Phase 1 clinical trial in healthy subjects and Phase 2 clinical trial in patients with ARDS due to COVID-19 (109).
CONCLUSION
The mechanism underlying the cytokine storm induced by COVID-19 is unclear and may be under genetic control. The reason for the comparative resistance of young children to COVID-19 is unanswered but may reflect an under-developed immune reaction compared to that seen in adults.
Underlying disorders such as diabetes and hypertension are associated with an increased susceptibility to develop more serious illness requiring hospital admission and invasive ventilation.
Furthermore, COVID-19 patients with comorbidities such as previous cardiovascular and/or metabolic diseases are as high risk for the developing severe form of disease.
In addition there is a genetic link between COVID-19 and ACE2 polymorphisms in disorders such as diabetic mellitus, cardiac diseases in Asian populations (110).
In summary, the immune response by the host is the critical factor in driving COVID-19. Analysis of this response may provide a clearer picture of the mechanisms driving disease severity and why most infected people only show no, or only mild, symptoms. Recent investigation by single cell sequencing shows some promising features. In this line a variation in IFN-stimulated responses and HLA class II down regulation has been shown.
Thus, the study of the host immune response against SARS-CoV 2 in COVID-19 patients may shed light on the immunopathogenesis of disease. Understanding the molecular pathways for providing any medical intervention by which we may enable protection and long-term immune memory and enable the design of prophylactic and therapeutic measures to overcome future outbreaks similar to coronaviruses.
REFERENCES
- 1.Rodriguez-Morales AJ, Bonilla-Aldana DK, Balbin-Ramon GJ, Rabaan AA, Sah R, Paniz-Mondolfi A, Pagliano P, et al. History is repeating itself: Probable zoonotic spillover as the cause of the 2019 novel Coronavirus Epidemic. Infez Med 2020;28(1):3–5. [PubMed] [Google Scholar]
- 2.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 2020;7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. Coronavirus disease 2019–COVID-19. Clinical microbiology reviews 2020;33(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 2020;38(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5.Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr 2020;16(3):251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol 2020;10(8):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395(10224):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife 2020;9:e57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo PC, Lau SK, Lam CS, Lau CC, Tsang AK, Lau JH, et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol 2012;86(7):3995–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect 2020;9(1):558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 2020;9(1):221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 2020;117(21):11727–11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taefehshokr N, Taefehshokr S, Hemmat N, Heit B. Covid-19: Perspectives on Innate Immune Evasion. Front Immunol 2020;11:580641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol 2016;3(1):237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z, Li H, Wu X, Zhong Y, Zhang K, Zhang YP, et al. Moderate mutation rate in the SARS coronavirus genome and its implications. BMC Evol Biol 2004;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang S, Du L, Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg Microbes Infect 2020;9(1):275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol 2020;94(7):e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijgen L, Keyaerts E, Moës E, Thoelen I, Wollants E, Lemey P, et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol 2005;79(3):1595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr Biol 2020;30(7):1346–1351.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankun J. COVID-19 pandemic; transmembrane protease serine 2 (TMPRSS2) inhibitors as potential drugs. Translation: The University of Toledo Journal of Medical Sciences 2020;7:1–5. [Google Scholar]
- 21.Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res 2020;30(4):343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onofrio L, Caraglia M, Facchini G, Margherita V, Placido S, Buonerba C. Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA 2020;6(8):FSO605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandão SCS, Ramos JOX, Dompieri LT, Godoi ETAM, Figueiredo JL, Sarinho ESC, et al. Is Toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev 2020:S1359–6101(20)30205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap JKY, Moriyama M, Iwasaki A. Inflammasomes and Pyroptosis as Therapeutic Targets for COVID-19. J Immunol 2020;205(2):307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenzhong L, Hualan L. COVID-19: Attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv 2020. Preprint. 10.26434/chemrxiv. 2020;11938173:v6. [DOI]
- 27.Tang X, Wu C, Li X, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-2. National Science Review 2020;7(6):1012–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Rincon A, Tonda A, Mendoza-Maldonado L, Claassen E, Garssen J, Kraneveld AD. A Missense Mutation in SARS-CoV-2 Differentiates Between Asymptomatic and Symptomatic Cases. [Preprint]. Bull World Health Organ. E-pub: 9 April 2020. doi:http://dx.doi.org/10.2471/BLT.20.258889 [Google Scholar]
- 29.Grubaugh ND, Hanage WP, Rasmussen AL. Making Sense of Mutation: What D614G Means for the COVID-19 Pandemic Remains Unclear. Cell 2020;182(4):794–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 2020;53:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020;55(4):2000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020;383(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med 2020;382(25):e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med 2020;382(25):2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stawiski EW, Diwanji D, Suryamohan K, Gupta R, Fellouse FA, Sathirapongsasuti F, et al. Human ACE2 receptor polymorphisms predict SARS-CoV-2 susceptibility. BioRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS One 2020;15(5):e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith JC, Sheltzer JM. Cigarette smoke triggers the expansion of a subpopulation of respiratory epithelial cells that express the SARS-CoV-2 receptor ACE2. BioRxiv 2020. [Google Scholar]
- 39.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584(7821):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Jr, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol 2020;146(2):327–329.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradding P, Richardson M, Hinks TSC, Howarth PH, Choy DF, Arron JR, et al. ACE2, TMPRSS2, and furin gene expression in the airways of people with asthma-implications for COVID-19. J Allergy Clin Immunol 2020;146(1):208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura H, Francisco D, Conway M, Martinez FD, Vercelli D, Polverino F, et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol 2020;146(1):80–88.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters MC, Sajuthi S, Deford P, Christenson S, Rios CL, Montgomery MT, et al. COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids. Am J Respir Crit Care Med 2020;202(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morais-Almeida M, Pité H, Aguiar R, Ansotegui I, Bousquet J. Asthma and the Coronavirus Disease 2019 Pandemic: A Literature Review. Int Arch Allergy Immunol 2020;181(9):680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song H, Seddighzadeh B, Cooperberg MR, Huang FW. Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in Prostate Epithelial Cells. Eur Urol 2020;78(2):296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong Y, Mo XI, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020;145(6):e20200702.32179660 [Google Scholar]
- 47.Brodin P. Why is COVID-19 so mild in children? Acta Paediatr 2020;109(6):1082–1083. [DOI] [PubMed] [Google Scholar]
- 48.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 2015;282(1821):20143085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nickbakhsh S, Mair C, Matthews L, Reeve R, Johnson PCD, Thorburn F, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A 2019;116(52):27142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Yan LM, Wan L, Xiang TX, Le A, Liu JM, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis 2020;20(6):656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nye S, Whitley RJ, Kong M. Viral Infection in the Development and Progression of Pediatric Acute Respiratory Distress Syndrome. Front Pediatr 2016;4:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gou W, Fu Y, Yue L, Chen GD, Cai X, Shuai M, et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. MedRxiv 2020. [Google Scholar]
- 53.Ubags NDJ, Marsland BJ. Mechanistic insight into the function of the microbiome in lung diseases. Eur Respir J 2017;50(3):1602467. [DOI] [PubMed] [Google Scholar]
- 54.Bostancıklıoğlu M. Temporal Correlation Between Neurological and Gastrointestinal Symptoms of SARS-CoV-2. Inflamm Bowel Dis 2020;26(8):e89–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheraton M, Deo N, Kashyap R, Surani S. A Review of Neurological Complications of COVID-19. Cureus 2020;12(5):e8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neurath MF. COVID-19 and immunomodulation in IBD. Gut 2020;69(7):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, et al. Coronavirus infections and immune responses. J Med Virol 2020;92(4):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, et al. Immunology of COVID-19: Current State of the Science. Immunity 2020;52(6):910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect 2020;80(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci 2020;250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 62.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 2020,;34(2):327–331. [DOI] [PubMed] [Google Scholar]
- 65.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruan Q, Yang K, Wang W, Jiang L, Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46(6):1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 2020;180(7):934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). MedRxiv 2020. [Google Scholar]
- 70.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8(4):420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest 2020;130(5):2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rijkers G, Vervenne T, van der Pol P. More bricks in the wall against SARS-CoV-2 infection: involvement of γ9δ2 T cells. Cell Mol Immunol 2020;17(7):771–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020;17(5):533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jouan Y, Guillon A, Gonzalez L, Perez Y, Ehrmann S, Ferreira M, et al. Functional alteration of innate T cells in critically ill Covid-19 patients. Medrxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 2020;53(3):368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang D, Guo R, Lei L, Liu H, Wang Y, Wang Y, et al. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J Leukoc Biol 2021;109(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qu R, Ling Y, Zhang YH, Wei LY, Chen X, Li XM, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol 2020;92(9):1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 2020;20(5):269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin 2020;35(3):266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao T, Hu M, Zhang X, Li H, Zhu L, Liu H, et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. MedRxiv 2020. [Google Scholar]
- 83.Zhang W, Du RH, Li B, Zheng XS, Yang XL, Hu B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020;9(1):386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem 2020;66(4):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Elslande J, Houben E, Depypere M, Brackenier A, Desmet S, André E, et al. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect 2020;26(8):1082–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin Infect Dis 2020;71(16):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol 2020;58(6):e00512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63(3):364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev 2020;19(6):102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020;178:104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant 2020;39(5):405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen C, Zhang XR, Ju ZY, He WF. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua shao shang za zhi= Zhonghua shaoshang zazhi= Chinese journal of burns 2020;36:E005–. [DOI] [PubMed] [Google Scholar]
- 94.RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19—preliminary report. New England Journal of Medicine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother 2020;75(7):1667–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020;395(10223):e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect Dis 2020;7(3):ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ju B, Zhang Q, Ge J, Wang R, Sun J, Ge X, et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020;584(7819):115–9. [DOI] [PubMed] [Google Scholar]
- 99.Hardeland R. Melatonin and inflammation-Story of a double-edged blade. J Pineal Res 2018;65(4):e12525. [DOI] [PubMed] [Google Scholar]
- 100.Pedrosa AM, Weinlich R, Mognol GP, Robbs BK, Viola JP, Campa A, et al. Melatonin protects CD4+ T cells from activation-induced cell death by blocking NFAT-mediated CD95 ligand upregulation. J Immunol 2010;184(7):3487–94. [DOI] [PubMed] [Google Scholar]
- 101.Shang Y, Xu SP, Wu Y, Jiang YX, Wu ZY, Yuan SY, et al. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J (Engl) 2009;122(12):1388–93. [PubMed] [Google Scholar]
- 102.Habtemariam S, Daglia M, Sureda A, Selamoglu Z, Gulhan MF, Nabavi SM. Melatonin and Respiratory Diseases: A Review. Curr Top Med Chem 2017;17(4):467–488. [DOI] [PubMed] [Google Scholar]
- 103.Hardeland R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int J Mol Sci 2019;20(5):1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller SC, Pandi-Perumal SR, Esquifino AI, Cardinali DP, Maestroni GJ. The role of melatonin in immuno-enhancement: potential application in cancer. Int J Exp Pathol 2006;87(2):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int 2016;2016:6901286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baer A, Colon-Moran W, Bhattarai N. Characterization of the effects of immunomodulatory drug fingolimod (FTY720) on human T cell receptor signaling pathways. Sci Rep 2018;8(1):10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis 2020;71(15):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020;323(18):1824–1836. [DOI] [PubMed] [Google Scholar]
- 109.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020;181(4):905–913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]