Abstract
Rationale and Objectives
Despite all the benefits and effectiveness of the coronavirus disease 2019 (COVID-19) vaccines mentioned in recent clinical trials, some post-vaccination side effects such as lymphadenopathy (LAP) were observed. The present study reviewed all studies with imaging findings presentation of LAP after COVID-19 vaccination.
Materials and Methods
We conducted a literature search in online databases, including Scopus, Medline (PubMed), Web of Science, Embase (Elsevier), Cochrane library, and Google Scholar.
Results
A total of 19 studies (68 cases), including 60 (88.2%) females and eight (11.8%) males with a presentation of LAP after COVID-19 vaccination, were reviewed. LAP was identified after first or second dosages of three types of COVID-19 vaccines, including Pfizer-BioNTech (n = 30, 44.1%), Moderna (n = 17, 25%), and Oxford-AstraZeneca (n = 1, 1.5%). In 20 (29.4%) cases, vaccine type was not reported or only reported as mRNA COVID-19 vaccine. The median days of LAP presentation after the first and second dosages of COVID-19 vaccination, were 12 and 5 days, respectively. Most of the LAP imaging findings related to COVID-19 vaccination (n = 66, 97%) were seen from first day to 4 weeks after vaccination. However, LAP remained after 5 and 6 weeks of the first and second dosages of COVID-19 vaccination with decreased lymph nodes’ size and residual cortical thickening in two cases.
Conclusion
This review study of cases with LAP-associated COVID-19 vaccination guides radiologists and physicians to rely on patient's clinical context and updated resources to prevent potential disease upstaging and change in therapy.
Key Words: Coronavirus, SARS-CoV-2, Vaccination, Pfizer-BioNTech, Moderna, Oxford-AstraZeneca, Adenopathy, Radiology
Abbreviations: COVID-19, Coronavirus disease 2019; LAP, Lymphadenopathy; LN, Lymph node, BC, Breast cancer; EUA, Emergency use authorization; FDA, Food and Drug Administration; CDC, Centers for Disease Control and Prevention; BIRADS, Breast Imaging Reporting and Data System
INTRODUCTION
Since December 2019, coronavirus disease 2019 (COVID-19) has faced the world with a considerable challenge affected many other items besides health (1). According to the World Health Organization (WHO) statistics, as of March 27, 2021, more than 125 million people worldwide have been infected, and more than 2.700.000 have died (2). After implementing various methods to deal with the destructive effects of the virus, efforts to develop an effective vaccine as the final solution accelerated (3,4).
Since December 2020, various vaccines with mRNA, vector, and protein subunit mechanisms marketed over time. Pfizer-BioNTech and Moderna are among the first vaccines approved emergency use authorization (EUA) from the United States Food and Drug Administration (FDA) (5, 6, 7). Vaccination began immediately in the United States, and until March 27, 2021, more than 91 million (27.6%) of the USA population have received one or more doses (8). In the latest update, the FDA issued EUA for the Janssen vaccine on February 27, 2021 (9).
Despite all the vaccines' benefits and effectiveness, as mentioned previously, mild and negligible side effects have been observed. Some of them include local pain at the injection site, fatigue, headache, muscle or joint pain, fever, and chills. Furthermore, some severe adverse effects were noted in physical exams, including lymphadenopathy (LAP), which was reported in 0.3% and 1.1% of Pfizer-BioNTech and Moderna vaccines, respectively (10, 11, 12).
Over time, with increasing vaccination rates in the general population, regional adenopathy on the same side of vaccination was frequently reported as an incidental finding in different imaging modalities (13). Determining whether the adenopathy is benign or malignant has critical importance following detecting it in imaging examinations. It can affect some plans in the screening or follow-up of cancerous patients simultaneously (14). Comprehensive clinical practice and analysis have confirmed that COVID-19 is a heterogeneous multisystem disorder; consequently, it can have various imaging features. Chest images such as CXR and CT illustrate characteristics that display lower lobe and peripheral ground-glass opacities in the lungs. These characteristics for the gastrointestinal system in COVID-19 patients are enteritis or mesenteric ischemia. Findings on imaging of pediatric patients are relatively similar to adults, particularly in the respiratory system. The notable CT findings are feeding vessel sign, halo sign, and pleural thickening (15, 16, 17). Here, we reviewed the imaging findings of cases with the presentation of LAP after COVID-19 vaccination.
MATERIALS AND METHODS
Study Selection and Eligibility Criteria
We searched various online data sources, including Scopus, Medline (PubMed), Web of Science, The Cochrane Library, Embase (Elsevier), and Google Scholar from January 1, 2019, to February 28, 2021, and updated on March 25, 2021. All types of studies, including original research studies, clinical perspective, case series/reports, editorials, and commentaries were assessed. Studies on COVID-19 vaccinated individuals (with any type of COVID-19 vaccine with the United States FDA approval) presented with LAP by various imaging modalities such as sonography, mammography, MRI, PET/CT scan, and PET/MRI were included. Duplicates, studies reported other adverse events of COVID-19 vaccines rather than adenopathy, and studies without available full text were excluded. Keywords of literature search included “COVID-19,” “coronavirus disease,” “SARS-CoV-19,” “Vaccin*,” and “Vaccination,” “Immunization,” “side effect*,” “adenopath*,” and “Lymphadenopathy.” The details of the PubMed keywords search strategy are presented in appendix A.
Data Extraction
Two independent authors screened the reference lists of included studies to increase the sensitivity of our search process, and the corresponding author resolved any disagreements. The following data from each study were extracted: First author's name, country and region, study type, population characteristics, vaccine type, a dosage of vaccination, site and location of adenopathy, size of adenopathy, presence of cortical thickening and hilar fat, type of imaging modality, and imaging findings. Furthermore, we reported a series of imaging findings from two included studies after obtaining formal permissions (34,36) (Figs. 2–4).
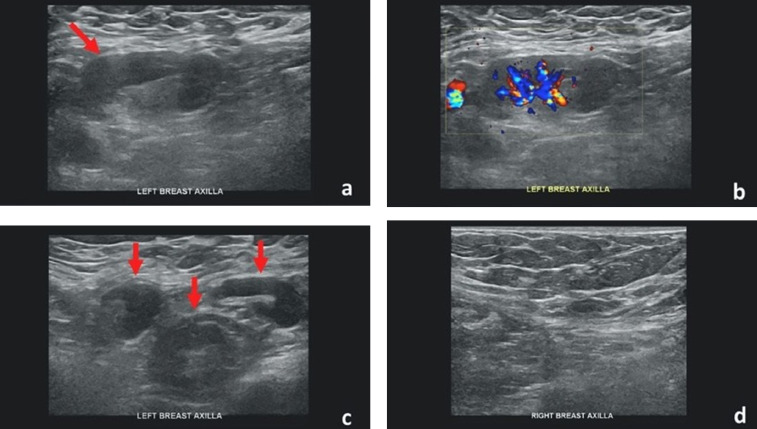
Figure 2.
Forty-two-year-old female with unilateral left axillary adenopathy noted 5 days after receiving the second dose of the Pfizer-BioNTech COVID-19 vaccine in her left upper extremity. (a) Gray-scale and (b) color Doppler images of an enlarged left axillary lymph node with cortical thickening (arrow). (c) Multiple additional morphologically abnormal left axillary lymph nodes were also present (arrows). Unremarkable right axilla was documented (d). Images obtained from Mehta et al., (38) Clinical Imaging, 2021, Vol. 75:12-15, and permission to use granted by Elsevier and Copyright Clearance Center. (Color version of figure is available online.)
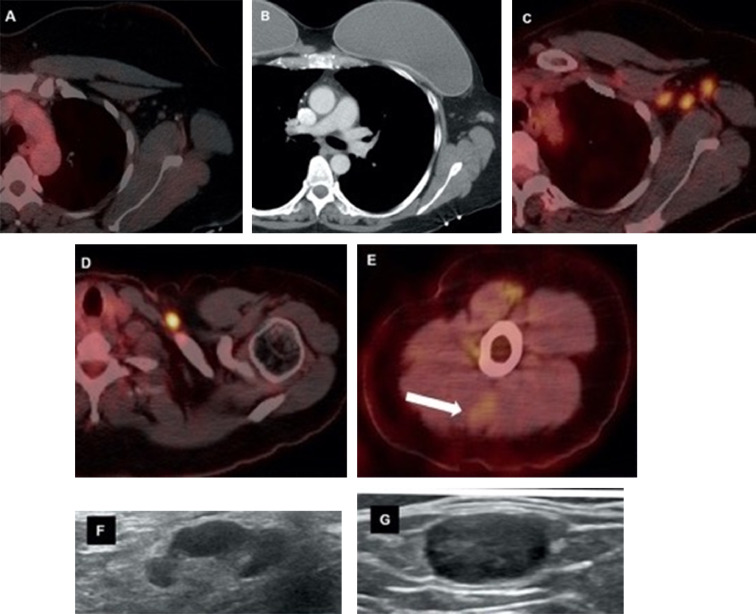
Figure 4.
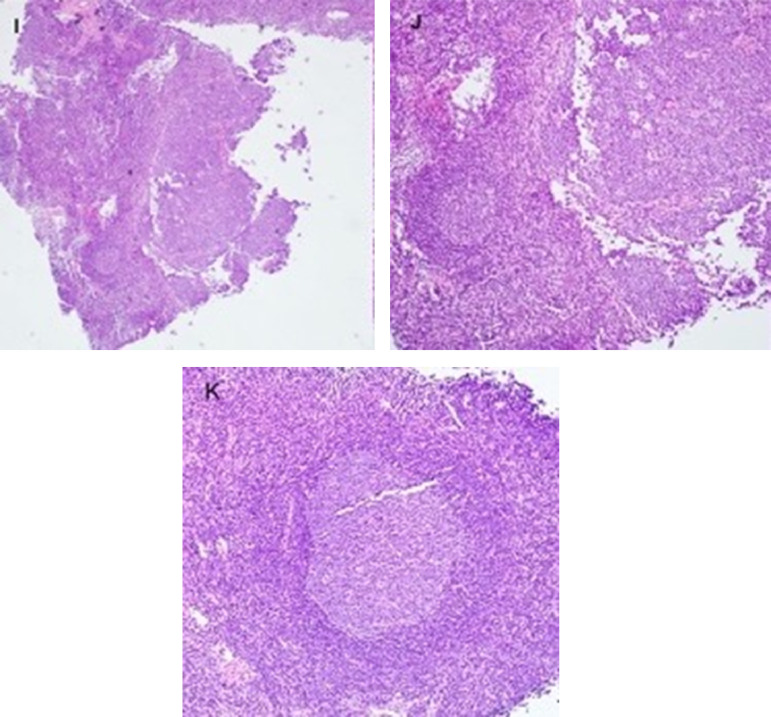
Forty-six-year-old female with triple negative left breast cancer, disease free for three years. A, Axial fused 18-FDG PET/CT three years earlier with no concerning lymph node in the left axilla. B, Surveillance contrast enhanced axial chest CT showed new left axillary lymphadenopathy with fat stranding 15 days after the first Covid-19 vaccine. Further evaluation with PET/CT six days after the 2nd dose of vaccine, demonstrated, C, multiple enlarged hypermetabolic left axillary lymph nodes and, D, a hypermetabolic round shaped left supraclavicular lymph node in axial fused 18-FDG PET/CT images. E, A subtle wedge-shaped intramuscular hypermetabolism (white arrow) was also noted in this case, similar to first and third cases. Ultrasonography guided core needle biopsy was performed. F, On ultrasonography, axillary lymph nodes had thickened cortex while the supraclavicular lymph node demonstrated, G, thickened cortex with loss of normal fatty hilum. I, Hematoxylin and eosin staining under 40x magnification shows enlarged germinal center with interfollicular expansion by small lymphocytes. 100x magnification images show, J, prominent germinal center with tingible body macrophage and, K, reactive germinal center with expansion of interfollicular regions by small lymphocytes and focally prominent endothelial cells. Images obtained from Özütemiz et al. (39) Radiology, published online: February 24, 2021, and permission to use granted by LaShundra Carson, Coordinator, Journal Business Publications, Radiological Society of North America (RSNA). (Color version of figure is available online.)
RESULTS
Overview of Included Study
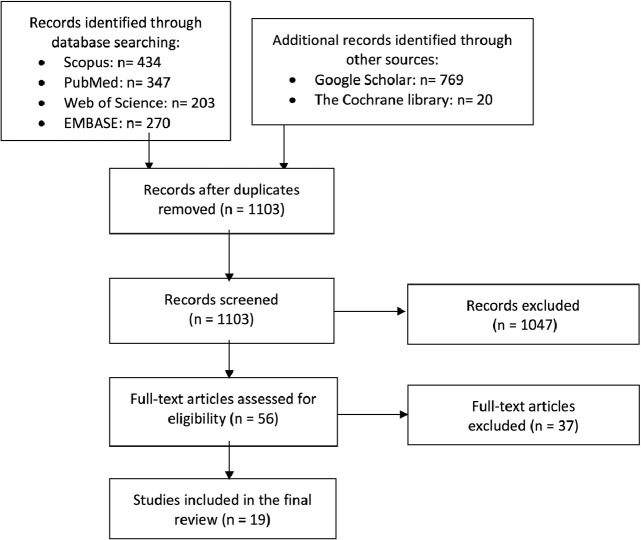
We identified a total of 2043 records through the initial search in databases. Following the removal of duplicates, 1103 studies remained for a title and abstract screening, 56 studies were selected as the candidates for assessment according to our eligibility criteria. Finally, nineteen studies were identified to be eligible for this study. Figure 1 reveals the flow diagram of the study selection process.
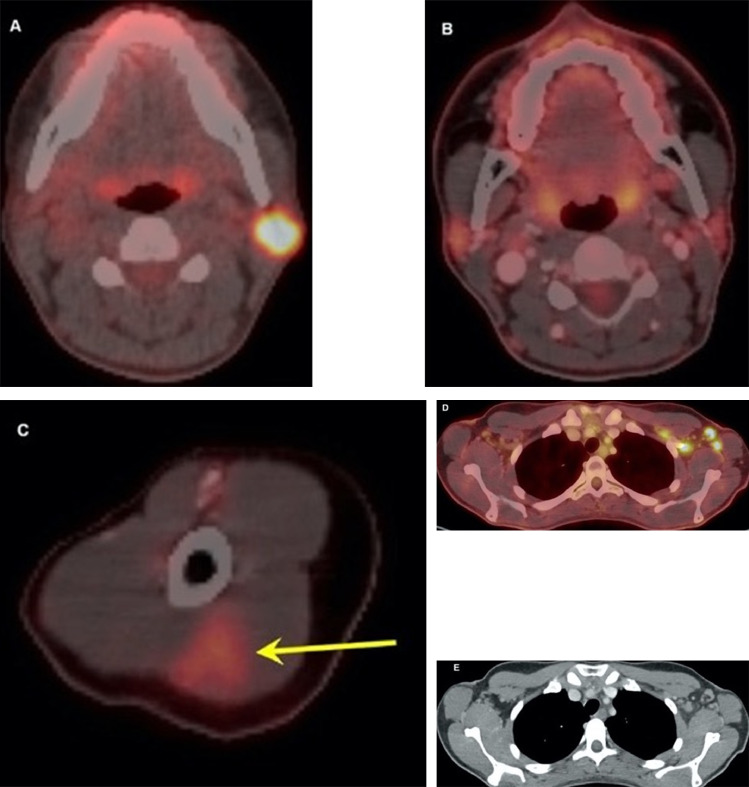
Figure 3.
Thirty-two-year-old female. A, Axial fused 18-FDG PET/CT showed hypermetabolic biopsy proven intraparotid lymph node with metastatic malignant melanoma. B, Three-month follow-up axial fused 18-FDG PET/CT shows complete resolution of the neck mass following chemotherapy, C, while left arm shows hypermetabolic triangular shaped inflammation (arrow) at the COVID vaccine injection site. D, Axial fused images at the axilla level shows multiple new hypermetabolic lymph nodes. E, Axial contrast enhanced CT demonstrates mild fat stranding surrounding the ovoid lymph nodes with preserved fatty hilum. Images obtained from Özütemiz et al., (39) Radiology, published online: February 24, 2021, and permission to use granted by LaShundra Carson, Coordinator, Journal Business Publications, Radiological Society of North America (RSNA). (Color version of figure is available online.)
Figure 1.
Flow diagram of the study selection process.
Patients Characteristics
The present study reviewed 68 cases, including 60 (88.2%) females and 8 (11.8%) males with the age range of 32–76 years old. The majority of cases (n = 56, 82.3%) had previous or active history of malignancy, including breast cancer (BC; n = 40; such as triple-negative, HER2 positive, invasive ductal, BRCA mutation carrier, breast focal lesion or had positive family history of BC), smoldering myeloma (n = 1), malignant melanoma (n = 3), oligometastatic myxoid liposarcoma (n = 1), oligosecretory myeloma (n = 1), mantle cell lymphoma (n = 1), lung cancer (n = 4, including, two squamous cell, one solitary pulmonary nodule, and one another type), cervical cancer (n = 1), diffuse large B-cell lymphoma (n = 1), cutaneous melanoma (n = 1), parotid malignancy (secretory carcinoma; n =1), and oral cavity squamous cell carcinoma (n = 1). Five (7.4%) cases presented with left palpable painful/painless axillary or supraclavicular LAP. In seven (10.3%) cases, the accurate characteristics of cases were not reported (Table 1 ).
Table 1.
Characteristics of Cases with LAP Following COVID-19 Vaccination
| First Author (Ref) | Region | Study design (N) | Population Characteristics (age) | Vaccine Type (dosage) | Location of LAP |
|---|---|---|---|---|---|
| Becker et al. (22) | USA | Special Report (n=2) |
1) Female with breast focal lesion (41y/o) 2) Male with smoldering myeloma (60y/o) |
1) NR† 2) NR |
1) Lt. axillary, CT‡ 2) Lt. axillary |
| Mehta et al. (38) | USA | Case Series (n=4) | 1) Female with palpable lump* (59y/o) 2) Female, routine breast screening* (42y/o) 3) Female with suspicious benign breast masses (42y/o) 4) Female, routine breast screening (57y/o) |
1) Pfizer-BioNTech (1st) 2) Pfizer-BioNTech (2nd) 3) Moderna (1st) 4) Pfizer-BioNTech (1st) |
1) Lt. axillary, CT 2) Multiple Lt. axillary, CT 3) Lt. axillary, CT 4) Lt. axillary, CT |
| Washington et al. (40) | USA | Editorial: Images in Radiology (n=1) | 1) Female with palpable Lt. supraclavicular LAP (37y/o) | 1) Moderna (1st) | 1) Lt. axillary and supraclavicular, CT |
| Mortazavi et al. (18) | USA | Short report: Retrospective HIPAA††Compliant Study (n=23) | Twenty-three females, including BIRADS-2§ (n=1), BIRADS-3 (n=21), BIRADS-4 (n=1) (49±21y/o) | Moderna (n=5) Pfizer-BioNTech (n=12) NR (n=6) |
Axillary regions |
| Edmonds et al. (37) | USA | Clinical Perspective (n=2) |
1) Female with BRCA1 mutation carrier* (48y/o) 2) Female, Diagnostic follow-up breast MRI* (33y/o) |
NR | 1) Lt. axillary, CT 2) Lt. axillary |
| Özütemiz et al. (39) | USA | Case series: Retrospective HIPAA-Compliant Study (n=5) |
1) Female with Lt. intraparotid mass (metastatic lymph node with malignant melanoma) (32y/o) 2) Female* (57y/o) 3) Male with oligometastatic myxoid liposarcoma of the Lt. thigh (41y/o) 4) Female with Lt. triple-negative BC$ (46y/o) 5) Female with Lt. axillary pain*(38y/o) |
1) Pfizer-BioNTech (2nd) 2) Pfizer-BioNTech (2nd) 3) Pfizer-BioNTech (2nd) 4) Pfizer-BioNTech (2nd) 5) Pfizer-BioNTech (1st) |
1) Multiple Lt. axillary 2) Lt. axillary, CT 3) Lt. axillary, CT 4) Multiple Lt. axillary, Lt. supraclavicular 5) Lt. axillary, CT |
| Hiller et al. (19) | Israel | Case Report (n=3) | 1) Female presented with painless Lt. infraclavicular lump (47y/o) 2) Female presented with painful Lt. supraclavicular and axillary lump (46y/o) 3) Female with BRCA mutation carrier (42y/o) |
1) Pfizer-BioNTech (1st) 2) Pfizer-BioNTech (1st) 3) Pfizer-BioNTech (1st) |
1) Lt. infraclavicular 2) Multiple Lt. axillary, supraclavicular, and low lateral neck 3) Lt. axillary |
| Ahn et al. (20) | USA | Editorial: Images in Cardiothoracic imaging (n=3) | 1) Male (32y/o) 2) Female (34y/o) 3) Female (39y/o) |
1) mRNA COVID-19 (2nd) 2) mRNA COVID-19 (1st) 3) mRNA COVID-19(NR) |
1) Lt. axillary 2) Lt. axillary, CT 3) Lt. axillary |
| Nawwar et al. (41) | UK | Images of the month (n=1) | 1) Female with oligosecretory myeloma (76y/o) | 1) Oxford-AstraZeneca (NR) | 1) Lt. axillary |
| Mitchell et al. (42) | UK | Short Communication (n = 2) |
1) Female (47y/o) 2) Female (55y/o) |
NR | 1) Lt. supraclavicular 2) Lt. supraclavicular |
| Eifer et al. (43) | Israel | Images in Radiology (n = 1) | 1) Female with BC (HER2 positive) (72y/o) | 1) Pfizer-BioNTech (NR) | 1) Rt. axillary |
| Hanneman et al. (21) | Canada | Images in Radiology (n = 1) | 1) Female (56y/o) | 1) Pfizer-BioNTech (2nd) | 1) Lt. axillary |
| Xu et al. (44) | USA | Interesting Image (n = 1) | 1) Male with mantle cell lymphoma (72y/o) | 1) mRNA COVID-19(NR) | 1) Lt. axillary |
| Cellina et al. (36) | Italy | Letter to the editor (n = 2) | 1) Female with painful, enlarged LAP (60y/o) 2) Female with headache and painful LAP (45y/o) |
1) Pfizer-BioNTech (1st) 2) Pfizer-BioNTech (2nd) |
1) Lt. axillary, CT 2) Rt. axillary, CT |
| Mclntosh et al. (26) | USA | Clinical Perspective (n = 6) | 1) Female with BC (40y/o) 2) Female with BC (72y/o) 3) Female with solitary pulmonary nodule (72y/o) 4) Female (40 y/o) 5) Male with lung cancer (squamous cell) (59y/o) 6) Female with treated cervical cancer (68y/o) |
1) Moderna (NR) 2) Pfizer-BioNTech (1st) 3) NR (2nd) 4) Moderna (NR) 5) NR 6) Moderna (NR) |
1) Lt. axillary, supraclavicular, and cervical 2) Rt. axillary 3) Rt. axillary, CT 4) Lt. axillary 5) Lt. axillary, supraclavicular, and cervical 6) Lt. axillary, CT |
| Lehman et al. (45) | USA | Original Research (n = 7) | 1) Female with invasive ductal BC (BIRADS II) (52y/o) 2) Female, routine breast screening* (BIRADS II) (33y/o) 3) Male with lung cancer (64y/o) (Lung-RADS II) 4) Female with diffuse large B-cell lymphoma (70y/o) 5) Female with cutaneous melanoma (51y/o) 6) Female with metastatic squamous cell lung cancer (stage IV) (59y/o) 7) Female with suspicious mammography* (42y/o) |
1) Moderna (2nd) 2) Moderna (2nd) 3) Moderna (1st) 4) Moderna (2nd) 5) Moderna (1st) 6) Pfizer-BioNTech (2nd) 7) Moderna (1st) |
1) Lt. axillary 2) Lt. axillary 3) Lt. axillary 4) Lt. axillary 5) Lt. axillary and subpectoral 6) Lt. axillary 7) Lt. axillary, CT |
| Avner et al. (46) | Israel | Images of the month (n=1) | 1) Male with BRAF-mutant melanoma of right thigh with multiple metastases organs (57y/o) | 1) Pfizer-BioNTech (2nd) | 1) Lt. axillary |
| Moghimi et al. (47) | Canada | Interesting Image (n = 1) | 1) Male with melanoma of right arm (71y/o) | NR | 1) Lt. axillary |
| Johnson et al. (48) | USA | Letter to the editor (n = 2) | 1) Female with left side parotid malignancy (secretory carcinoma) 2) Female with oral cavity SCC$$ |
1) Moderna (1st) 2) NR (1st) |
1) Lt. axillary, supraclavicular 2) Lt. axillary, supraclavicular |
Noted - Lt.: Left, Rt.: Right.
Not reported.
Cortical thickness.
The patient has a positive family history of breast cancer.
Breast Imaging Reporting and Data System.
Breast cancer.
Health Insurance Portability and Accountability Act.
Squamous Cell Carcinoma.
Imaging Findings after COVID-19 Vaccination
Among 68 cases, the most applied radiologic modality for evaluating the suspicious LNs was the United States, which revealed LNs enlargement with diffuse or focal cortical thickening in 29 (42.6%) cases, preserved nodal hilar fat reported in one case. Six (8.8%) cases were screened only by mammography (18). Additionally, LNs demonstrated a necrotic pattern evidenced by homogenous hypo-echogenicity and absence of internal vascular flow in a single case (19). In two (2.9%) cases, abnormal LNs were detected in the chest CT scan performed due to other reasons (13, 20).
Abnormal axillary adenopathy was detected in 12 (17.6%) cases who underwent MR imaging for other reasons (such as BC screening or follow-up). Except for the LNs enlargement, the feature reported was nodal cortical thickening ranging from 3 to 7 mm in five cases, irregular nodal cortex in one case, and preserved hilar fat reported in two cases. Otherwise, no discriminative characteristic of LNs involved by the COVID-19 infection was detected in this modality.
Another modality was 18F-FDG-PET/CT that demonstrated abnormal LNs in 18 (26.4%) cases as increased FDG uptake with the mean SUV max of 6.8 ± 3.4 g/ml (range, 1.8–13). Moreover, associated increased FDG uptake at the injection site and in ipsilateral superficial soft tissue was detected in three cases with a mean SUV max of 7.6 ± 3.3 g/ml, and preserved nodal hilar fat was reported in one case. An incidental note was taken of abnormal FDG uptake in axillary nodes in the performed cardiac PET/MRI in one (1.5%) case (21). The mean of maximum LNs dimension reported in all modalities was 20.9 ± 5.8 mm (range, 13–30), with a mean short axis of 12 ± 2.9 mm, and cortical thickness of 6.2 ± 0.8 mm. A summary of the imaging findings is provided in Table 2 .
Table 2.
Radiological Findings of Cases with LAP Following COVID-19 Vaccination
| First Author (Ref) | Imaging Findings |
|---|---|
| Becker et al. (22) | 1) Follow-up breast MRI: asymmetric Lt. sided axillary LN† enlargement (30×17 mm) with irregular cortex and preserved hilar fat (within 5 days of vaccination), Follow-up US: The decreasing size of adenopathy (22×11 mm) with a residual cortical thickness (within 42 days of vaccination) 2) 18F-FDG-PET/CT: FDG uptake in the injection site, Lt. deltoid muscle (SUV max 5.9 g/ml), and the draining Lt. sided axillary LNs (SUV max 9.6 g/ml) |
| Mehta et al. (38) | 1) Targeted axillary US: Lt. axillary LN enlargement (26×15×16 mm) with diffuse 0.7 cm cortical thickening (within 9 days of vaccination) 2) Screening breast US: Multiple enlarged Lt. axillary LNs with diffuse cortical thickening, largest measuring 27×12×10 mm (within 5 days of vaccination) 3) Follow-up breast US: Diffuse cortical thickening in a single Lt. axillary LN (within 13 days of vaccination) 4) Screening breast US: Single enlarged LN in Lt. axilla (10 mm in short axis) with diffuse cortical thickening (within 8 days of vaccination) |
| Washington et al. (40) | 1) Diagnostic Mammogram and confirmatory US: Enlargement of Lt. sided intramammary, axillary and supraclavicular LNs (level I) associated with cortical thickening (within 12 days of vaccination), Follow-up US: No significant changes in LAP‡ (within 26 days of vaccination) |
| Mortazavi et al. (18) | Total of 23 patients, 5 Mammography, 12 in US, 4 in Mammography and US, 2 in MRI: Abnormal LNs (in Mammograms: with size, density or shape disproportionate to other nodes, in US: focal or diffuse cortical thickening more than 3 mm, in MRI: nodes asymmetric in size and/or number compared to the contralateral side) ipsilateral to the injection site (median interval between vaccination and imaging findings: 9.5 days) |
| Edmonds et al. (37) | 1) Screening Baseline breast MRI in a high-risk patient: Several enlarged Lt. sided axillary LAP (level I), with cortical thickening up to 6 mm and preserved hilar fat (within 13 days of vaccination) 2) Diagnostic follow up breast MRI: Lt. sided axillary LAP (levels I and II) (within 16 days of vaccination) |
| Özütemiz et al. (39) | 1) Follow-up 18F-FDG-PET/CT: Multiple enlarged Lt. axillary LNs with associated surrounding fat stranding with largest measuring 14×10 mm and SUV max of 7.7 g/ml. Also, triangular intramuscular uptake was detected at the site of injection (within 6 days of vaccination) 2) Screening baseline breast MRI in a high-risk patient: Several enlarged Lt. axillary LNs with 5mm cortical thickness and longest nodes measuring 21 mm (within 5 days of vaccination) 3) Whole-body MRI: Enlarged Lt. axillary LNs largest measuring 20 mm with 7mm cortical thickness (within 4 days of vaccination) 4) Follow-up chest CT scan and subsequent 18F-FDG-PET/CT: Multiple enlarged Lt. axillary LNs with increased uptake, the largest measuring 20×12 mm and SUV max of 9 g/ml, also in Lt. supraclavicular region measuring 138 mm with SUV max of 13.4 g/ml (within 7 days of vaccination) 5) Follow-up breast US: Single enlarged LN in Lt. axilla with diffuse cortical thickening 6 mm (within 8 days of vaccination) |
| Hiller et al. (19) | 1) Diagnostic axillary US: Enlarged benign looking LNs with preserved vascular architecture, in Lt. infraclavicular region on the side of injection (within 15 days of vaccination) 2) Diagnostic axillary US: Multiple enlarged LNs in Lt. sided axillary, supraclavicular, and low lateral neck regions. LNs were homogeneously hypoechoic and with no identifiable blood flow consistent with necrosis (within 5 days of vaccination) 3) Screening Baseline breast MRI and subsequent US: Enlarged benign-looking Lt. axillary LNs up to 20 mm in diameter with normal architecture and vascular flow (within 18 days of vaccination) |
| Ahn et al. (20) | 1) CT angiography of chest: Unilateral Lt. sided axillary LN enlargement with the maximum short axis of 15 mm (within 7 days of vaccination) 2) Screening baseline breast MRI and US in a high-risk patient: Single Lt. axillary LN (20 mm) with diffuse cortical thickening (within 13 days of vaccination) 3) Screening baseline breast MRI and US: Lt. axillary LAP up to 14 mm in short axis (within 8 days of vaccination) |
| Nawwar et al. (41) | 1) Follow-up 18F-FDG-PET/CT: Low-grade uptake in the subcutaneous region of the Lt. arm and a single axillary LN (level I) (within 14 days of vaccination) |
| Mitchell et al. (42) | 1) US of Lt. supraclavicular region: Benign looking reactive LNs (within 3 days of vaccination) 2) US of Lt. supraclavicular region: Benign looking reactive LNs (within 3 days of vaccination) |
| Eifer et al. (43) | 1) Follow-up 18F-FDG-PET/CT in a proven BC$ case in her Lt. breast: Mild focal increased uptake in Rt. deltoid muscle and moderate increased uptake in two ipsilateral axillary LNs of normal size |
| Hanneman et al. (21) | 1) Research Cardiac 18FDG PET/MRI: Lt. sided enlarged axillary LNs with moderate increased uptake (SUV max of 5.6 g/ml and maximum short axis of 13 mm) (1 day after vaccination), Follow-up 18FDG PET/MRI: Mild decreasing size of Lt. sided axillary LNs without FDG uptake (within 35 days of vaccination) |
| Xu et al. (44) | 1) Follow-up 18F-FDG-PET/CT in a case of mantle cell lymphoma: Multiple sub centimeter Lt. axillary LNs with avid uptake (SUV max of 1.8-2.7 g/ml) associated with focal uptake in the ipsilateral superficial soft tissue of the arm, the injection site demonstrating SUV max of 3.4 g/ml (within 2 days of vaccination) |
| Cellina et al. (36) | 1) Diagnostic axillary US: Multiple enlarged Lt. axillary LNs with diffuse cortical thickening (within 14 days of vaccination) 2) Diagnostic axillary US: Single enlarged Rt. Axillary LN measuring 17.3×10.3 mm with significant cortical thickening and preserved hilar fat (within 3 days of vaccination) |
| Mclntosh et al. (26) | 1) Diagnostic 18F-FDG-PET/CT in a case of BC: Moderate uptake in Lt. axillary region at I, II and III levels, also in supraclavicular and lower neck nodes (within 2 days of vaccination) 2) Diagnostic 18F-FDG-PET/CT in a case of BC in Lt. breast: Barely perceptible uptake in normal appearing Rt. axillary LNs (within 11 days of vaccination) 3) Surveillance 18F-FDG-PET/CT in a case with solitary pulmonary nodule: Moderate to avid uptake in mildly prominent Rt. axillary LNs in levels I and II, associated diffuse cortical thickening with preserved hilar fat is demonstrated (within 4 days of vaccination) 4) 18F-FDG-PET/CT: Mildly enlarged Lt. axillary LN with avid uptake, which demonstrates rounded morphology and no visible hilar fat (within 3 days of vaccination) 5) Diagnostic 18F-FDG-PET/CT in a case of lung cancer: Avid uptake in Lt. sided levels I and II axillary, supraclavicular and lower neck LNs (within 14 days of vaccination) 6) Diagnostic 18F-FDG-PET/CT in a case of cervical cancer: Mild FDG uptake in Lt. axillary levels I and II associated with mild cortical thickening (within 9 days of vaccination) |
| Lehman et al. (45) | 1) Screening mammogram: Single enlarged LN of Lt. axilla (within 22 days of vaccination) 2) Follow-up breast MRI in a high-risk patient: Multiple enlarged Lt. axillary LNs (levels I and II) (within a day of vaccination) 3) Follow-up chest CT in a case of lung cancer: Multiple mild enlargements of Lt. axillary LNs (within 10 days of vaccination) 4) Surveillance 18FDG PET/CT in a known case of diffuse large B-cell lymphoma: Mild enlargement of Lt. axillary LNs with the maximum short axis of 7 mm associated with intense FDG uptake (within 3 days of vaccination) 5) Surveillance contrast-enhanced chest CT scan in a case with the history of cutaneous melanoma: Mild enlargement of Lt. axillary and subpectoral LNs with the maximum short axis of 13 mm (within 3 days of vaccination) 6) Surveillance 18FDG PET/CT in a case of metastatic squamous cell lung cancer: Lt. axillary LN enlargement associated with moderate to intense FDG uptake (within 5 days of vaccination) 7) Diagnostic MRI and subsequent US in a case with suspicious mammography finding: Levels I and II Lt. axillary LNs enlargement and cortical thickening up to 6 mm (within 12 days of vaccination) |
| Avner et al. (46) | 1) Surveillance 18FDG PET/CT in a case of metastatic melanoma: Lt. axillary and subpectoral LNs show enlargement and increased uptake with SUV max of 9.4. (within 6 days of vaccination) |
| Moghimi et al. (47) | 1) 18FDG PET/CT for staging in a case of melanoma: Lt. axillary and base of neck LAP with increased uptake (SUV: 4.9), Lt. deltoid muscle hematoma at the injection site, associated with increased uptake (SUV: 9.9). (within 6 days of vaccination) |
| Johnson et al. (48) | 1) 18FDG PET/CT for work-up in a case of Lt. sided parotid malignancy: increased uptake in Lt. sided axillary and single supraclavicular LNs with SUV max of 4.5. (within 10 days of vaccination) 2) Surveillance 18FDG PET scan in a case of oropharyngeal SCC:$$ increased uptake in Lt. axillary and supraclavicular LNs with SUV max of 5.1. (within 14 days of vaccination) |
Note - Lt., Left; Rt., Right.
Lymph node.
Lymphadenopathy.
Breast cancer.
Squamous Cell Carcinoma.
Vaccination-associated LAP
LAP was reported after first or second dosages of three types of COVID-19 vaccines, including Pfizer-BioNTech (n = 30, 44.1%), Moderna (n = 17, 25%), and Oxford-AstraZeneca (n = 1, 1.5%). In 20 (29.4%) cases, vaccine type was not reported or only reported as mRNA COVID-19 vaccine. Location of LAP was reviewed, including axillary 82.3% (65/79), supraclavicular 11.4% (9/79), infraclavicular 1.2% (1/79), and subpectoral or neck regions 5.1% (4/79; Table 3 ).
Table 3.
Frequency of Location of LAP Based on the Type of Vaccine
| Vaccine Type | Location of LAP | Number (%) |
|---|---|---|
| Pfizer-BioNTech | Axillary | 29 (36.7) |
| Supraclavicular | 2 (2.5) | |
| Infraclavicular | 1 (1.2) | |
| Lateral neck | 1 (1.2) | |
| Moderna | Axillary | 17 (21.5) |
| Supraclavicular | 2 (2.5) | |
| Lateral neck | 1 (1.2) | |
| Subpectoral | 1 (1.2) | |
| Oxford-AstraZeneca | Axillary | 1 (1.2) |
| Total† | Axillary | 65 (82.3) |
| Supraclavicular | 9 (11.4) | |
| Infraclavicular | 1 (1.2) | |
| Neck and subpectoral | 4 (5.1) |
We included the LAP location of 20 cases who vaccine type was not reported or only reported as mRNA COVID-19 vaccine in the total section.
LAP was observed in imaging examinations after the first and second dosages of Pfizer-BioNTech vaccine with median days of 10.5 (range, 5–18 days) and 5 (range, 1–7 days), respectively. About the Moderna vaccine, LAP was observed with median days of 11 (range, 3–26 days), 3 (range, 1–22 days), respectively. Overall, the median days of LAP presentation after the first and second dosages of COVID-19 vaccines were observed 12 (range, 3–26 days) and 5 (range, 1–22 days), respectively.
DISCUSSION
This study is a first review study of LAP presentation followed by COVID-19 vaccination. Several radiologic findings are consistent with a reactive LAP, such as diffuse or focal cortical thickening and preserved hilar fat in the imaging modalities, reported in some cases of present study. Moreover, it was found that 97% of imaging findings of LAP after COVID-19 vaccination were seen from the first day to four weeks after vaccination. Although, LAP remained after 5 and 6 weeks of the first and second dosages of COVID-19 vaccination with decreased LNs size and residual cortical thickening in two cases (21,22). Fernandes et al. reported LAP in 20 cases after injection of both Pfizer-BioNTech and Moderna vaccines and observed the resolution of LAP after five days to more than 4 weeks (23). Thereby, most studies recommended routine imaging screening before or at least 4–6 weeks after the second dose of COVID-19 vaccines to prevent potential disease upstaging and change in therapy.
Centers for Disease Control and Prevention (CDC) reported the average duration presentation of LAP after COVID-19 vaccines to 2–4 days after either dosage of Pfizer-BioNTech and Moderna vaccines, and around 10 and 1–2 days, in Pfizer-BioNTech and Moderna vaccines, respectively (11,12). Our results revealed that this duration was more than the CDC average, and in the recent studies, LAP was observed with the median days of 12 and 5 days after the first and second dosages of these COVID-19 vaccines, respectively.
More than a year after the inception of the COVID-19 pandemic, various vaccines have been developed to boost immunity and human resistance to the virus with different mechanisms (24). Global immunization has been started, with the first FDA- approved vaccines (Pfizer and Moderna) for EUA. It continues with other vaccines such as Sputnik V, Oxford-AstraZeneca, and Sinopharm in different countries (25). LAP is one of the rare complications of immunization before COVID-19 vaccination. Thereby, new image interpretation conflicts appear due to LAP-related vaccination (6,7,26).
Based on former experiences, H1N1 Influenza, smallpox, measles, Bacille Calmette-Guerin, and human papillomavirus (HPV) vaccines can cause infrequent axillary LAP (27, 28, 29, 30, 31). Several studies showed increased axillary nodal FDG uptakes following H1N1 influenza vaccination in the FDG PET/CT imaging. Most of them recommended interpreting radiologic findings in the setting of the location and time since vaccination. H1N1 virus vaccination can induce increased FDG uptake at the needle site and in ipsilateral axillary LNs if the PET/CT is conducted within 14 days after vaccination. Also, in vital situations of evaluation of same regions LNs, a cautious interval of 30–50 days from vaccination to PET scan is considered (27,32,33). Although, we reviewed two recent studies with persistent LAP even after five and six weeks of COVID-19 vaccination (21,22).
According to the safety clinical trials of Moderna and Pfizer-BioNTech vaccines, outcomes exhibited that 10.2% of cases manifested localized axillary swelling or tenderness on the same side to the vaccination arm in seven days of the first dose (vs. 4.8% of controls) and 14.2% of cases among seven days of the second vaccine dose (vs. 3.9% of controls) of Moderna vaccine. This rate is lower with the Pfizer-BioNTech vaccine considering severe adverse reactions, including LAP, which was observed in 0.3% or 64 of participants occurred up to 4.6% of partners and were more frequent after the second dose and adults less than 55 years of age (6,7,34).
Different factors come to the radiologists' and physicians' aid to distinguish a malignant versus benign focal LAP in selected patients. Such factors include both the clinical and radiological findings. For instance, in physical examination, palpable focal axillary LAP ipsilateral to the injection site in a BC patient (of the contralateral side of vaccination) is more likely to be reactive in nature. However, since one case of necrotic LAP was reported in post-COVID19 vaccination (19), differentiation of the adenopathy caused solely by this feature should be cautioned. The other reported radiologic characteristic of abnormal LNs was cortical thickening, defined as more than 3 mm thickness of the affected node (35). Although this feature does not help distinguish a reactive process from a neoplastic one, both diffuse and focal cortical thickenings were reported in approximately 25% of the present study. Furthermore, regarding the cases who underwent nuclear imaging, normal-sized LNs with normal cortical thickness also could be involved by the associated pathologic process (26).
Recommendations for Clinical and Imaging Centers
Among the modalities in use for abnormal LN detection, the FDG PET/CT could be one of the most confusing. We reviewed fourteen cases with known underlying malignancies presented with increased uptake in focal LNs draining the axillary territory. Therefore, for the accurate image interpretation by the radiologists and to avoid false-positive results in such patients, the date and the side of the vaccination were recommended to be mentioned on patients’ history records (36). Also, the radiologist should add the post-vaccination reactive changes to the top of their list of differential diagnoses in patients with relevant vaccination history.
Meanwhile, several recommendations have been suggested to lower the burden of diagnostic mistakes. The vaccine should be injected in the arm contralateral to the primary or suspected malignancy side, specifically in the BC case, with both dosages administered in the same arm (26). Recent studies suggested routine imaging screening for all individuals who presented LAP followed by COVID-19 vaccination before or at least 4–6 weeks after the second dose of COVID-19 vaccination (18,22). In the case of screening for BC, it is recommended to assign the patients with reactive LNs changes as BIRADS 0. After ipsilateral breast diagnostic workup, the BIRADS score upgrades to III which mandates follow-up US. The follow-up schedule is controversial from 4–8 weeks (36,37) to 4–12 weeks (18,38) after the second dose of the COVID-19 vaccine.
Study Limitations
At first, the number of reported cases after COVID-19 vaccination was limited due to the recent emergency use authorization from the United States FDA-approved vaccination. Second, few studies published cases with the presentation of LAP-associated COVID-19 vaccination till now. Additionally, most of them reported the observational data of a small number of cases, and there is not any control group in these studies to be able to accurate comments on the incidence of LAP-associated COVID-19 vaccination. Third, the results and recommendations of recent related studies were varied in terms of population characteristics, follow-up days after the first or second dosages of vaccination, type of vaccine, rate of LAP, size and number of affected LNs, and image modalities that they used. A standard guideline regarding the report of suspicious LNs should be introduced. Thereby, all the imaging reports may contain LN description items such as short or long axis dimensions, presence or absence of hilar fat, and the pattern of cortical thickening.
Conclusion
This review study of cases with LAP-associated COVID-19 vaccination guides radiologists and physicians to rely on patient's clinical context and updated resources to prevent potential disease upstaging, start or change in therapy such as chemotherapy and radiotherapy with more invasive interventions, particularly in patients with underlying disease.
Acknowledgments
Funding information
The authors state that this work has not received any funding.
Author contributions
Guarantors of the integrity of the entire study, P.K.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of the final version of the submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, P.K., F.Y., F.R.; and manuscript editing, all authors.
Informed consent
Informed consent was not required because it was a literature study.
Ethical approval
Institutional Review Board approval was not required because it was a literature study.
Appendix
Search Strategy
PubMed Keywords
("coronavirus 2" [Title/Abstract] OR "coronavirus 2"[Mesh] OR "coronavirus infections"[Title/Abstract] OR "coronavirus infections"[Mesh] OR "COVID-19"[Title/Abstract] OR "COVID-19"[Mesh] OR "coronavirus"[Title/Abstract] OR "coronavirus"[Mesh] OR "2019-nCoV"[Title/Abstract] OR "COVID-2019"[Title/Abstract] OR "COVID19"[Title/Abstract] OR "nCoV"[Title/Abstract] OR "coronavirus disease 2019"[Title/Abstract] OR "2019 novel coronavirus"[Title/Abstract] OR "severe acute respiratory syndrome"[Title/Abstract] OR "SARS-CoV-19"[Title/Abstract] OR "SARS-CoV-2"[Title/Abstract] OR "2019-CoV-19"[Title/Abstract] OR "SARS-CoV"[Title/Abstract] OR "2019nCoV"[Title/Abstract] OR "coronavirinae"[Title/Abstract] OR "2019 Novel Coronavirus Infection"[Title/Abstract] OR "2019 nCoV Infection"[Title/Abstract] OR "Bat coronavirus"[Title/Abstract] OR "betacoronavirus*"[Title/Abstract] OR "coronavirus Infection Disease 2019"[Title/Abstract] OR "covid*"[Title/Abstract] OR "Novel Coronavirus Pneumonia"[Title/Abstract] OR "Wuhan virus"[Title/Abstract]) AND ("Vaccination"[Title/Abstract] OR "Vaccination"[Mesh] OR "Vaccines"[Title/Abstract] OR "Vaccines"[Mesh] OR "Mass Vaccination"[Title/Abstract] OR "Mass Vaccination"[Mesh] OR "Immunization"[Title/Abstract] OR "Immunization"[Mesh] OR "Immunization Programs"[Title/Abstract] OR "Immunization Programs"[Mesh] OR "Vaccination*"[Title/Abstract] OR "Vaccin*"[Title/Abstract] OR "Vaccination program"[Title/Abstract] OR "Vaccine"[Title/Abstract] OR "Vaccine-mediated protection"[Title/Abstract] OR "Post-vaccine"[Title/Abstract] OR "Post vaccine"[Title/Abstract] OR "Vaccination-induced"[Title/Abstract] OR "Post-vaccination"[Title/Abstract] OR "Post vaccination"[Title/Abstract] OR "Immunisation*"[Title/Abstract] OR "Immunization*"[Title/Abstract]) AND ("Side effect*"[Title/Abstract] OR "Side-effect*"[Title/Abstract] OR "adverse effect*"[Title/Abstract] OR "adverse-effect*"[Title/Abstract] OR "adverse event*"[Title/Abstract] OR "adverse-event*"[Title/Abstract] OR "adverse reaction*"[Title/Abstract] OR "adverse-reaction*"[Title/Abstract] OR "Vaccine Adverse Event Reporting System"[Title/Abstract] OR "negative effect*"[Title/Abstract] OR "negative consequence*"[Title/Abstract] OR "negative-effect*"[Title/Abstract] OR "negative-consequence*"[Title/Abstract] OR "negative outcome*"[Title/Abstract] OR "Lymphadenopathy"[Title/Abstract] OR "Lymphadenopathy"[Mesh] OR "Lymphadenopath*"[Title/Abstract] OR "Adenopath*"[Title/Abstract] OR "Adenopathy"[Title/Abstract] OR "Axillary adenopathy"[Title/Abstract] OR "Axillary lymphadenopathy"[Title/Abstract] OR "Supraclavicular adenopathy"[Title/Abstract] OR "Supraclavicular lymphadenopathy"[Title/Abstract] OR "Enlarged lymph node*"[Title/Abstract] OR "Lymph node enlargement"[Title/Abstract] OR "LAP"[Title/Abstract])
Time: Jan, 01, 2019 – Feb 28, 2021
References
- 1.Hsiang S, Allen D, Annan-Phan S. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature. 2020;584(7820):262–267. doi: 10.1038/s41586-020-2404-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Website. WHO coronavirus (COVID-19) dashboard. Accessed March 8, 2021. Available from: https://covid19.who.int/?gclid=Cj0KCQiAvvKBBhCXARIsACTePW9nQttX871YsapnSDYfdD04KCtYI2SY6NJyGKG6VpC58lgtzm4ZJlEaApW4EALw_wcB.
- 3.Yazdanpanah F, Hamblin MR, Rezaei N. The immune system and COVID-19: Friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vabret N, Britton GJ, Gruber C. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Different COVID-19 vaccines. Accessed March 3, 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html.
- 6.U.S. Food and Drug Administration. Moderna COVID-19 Vvaccine. Accessed March 3, 2021. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine
- 7.U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Accessed March 3, 2021. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- 8.Centers for Disease Control and Prevention. COVID data tracker. Accessed March 8, 2021. Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations
- 9.U.S. Food and Drug Administration. Janssen COVID-19 vaccine. Accessed March 3, 2021. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/janssen-covid-19-vaccine.
- 10.Polack FP, Thomas SJ, Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Local reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention; December 20, 2020. Accessed March 3, 2021. Available from: www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
- 12.Local reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. Centers for Disease Control and Prevention, Centers for Disease Control and Prevention. December 13, 2020. Accessed March 3, 2021. Available from: www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
- 13.Lehman CD, Lamb LR, D'Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25688. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Gaddey HL, Riegel AM. Unexplained lymphadenopathy: evaluation and differential diagnosis. Am Fam Physician. 2016;94(11):896–903. [PubMed] [Google Scholar]
- 15.Keshavarz P, Rafiee F, Kavandi H. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imaging. 2020;73:86–95. doi: 10.1016/j.clinimag.2020.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayramoglu Z, Canıpek E, Comert RG. Imaging features of pediatric COVID-19 on chest radiography and chest CT: a retrospective, single-center study. Academic Radiol. 2021;28(1):18–27. doi: 10.1016/j.acra.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capaccione K, Yang H, West E. Pathophysiology and imaging findings of COVID-19 infection: An organ-system based review. Academic Radiol. 2021 doi: 10.1016/j.acra.2021.01.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortazavi S. Coronavirus disease (COVID-19) vaccination associated axillary adenopathy: Imaging findings and follow-up recommendations in 23 women. Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25651. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Hiller N, Goldberg SN, Cohen-Cymberknoh M. Lymphadenopathy associated with the COVID-19 vaccine. Cureus. 2021;13(2):e13524. doi: 10.7759/cureus.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn RW, Mootz AR, Brewington CC. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3(1) doi: 10.1148/ryct.2021210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanneman K, Iwanochko RM, Thavendiranathan P. Evolution of lymphadenopathy at PET/MRI after COVID-19 vaccination. Radiology. 2021 doi: 10.1148/radiol.2021210386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker AS, Perez-Johnston R, Chikarmane SA. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: Radiology scientific expert panel. Radiology. 2021 doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Prada M, Rivero-Calle I, Calvache-González A. Acute onset supraclavicular lymphadenopathy coinciding with intramuscular mRNA vaccination against COVID-19 may be related to vaccine injection technique, Spain, January and February 2021. Euro Surveill. 2021;26(10) doi: 10.2807/1560-7917.ES.2021.26.10.2100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeyanathan M, Afkhami S, Smaill F. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannah Ritchie EOO, Diana Beltekian, Edouard Mathieu, et al. Coronavirus (COVID-19) vaccinations. Accessed March 3. Available from: https://ourworldindata.org/covid-vaccinations
- 26.McIntosh LJ, Bankier AA, Vijayaraghavan GR. COVID-19 vaccination-related uptake on FDG PET/CT: An emerging dilemma and suggestions for management. Am J Roentgenol. 2021 doi: 10.2214/AJR.21.25728. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Burger IA, Husmann L, Hany TF. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Cl Nucl Med. 2011;36(10):848–853. doi: 10.1097/RLU.0b013e3182177322. [DOI] [PubMed] [Google Scholar]
- 28.Studdiford J, Lamb K, Horvath K. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy: Pharmacology. 2008;28(9):1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 29.Casey CG, Iskander JK, Roper MH. Adverse events associated with smallpox vaccination in the United States, January-October 2003. JAMA. 2005;294(21):2734–2743. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- 30.Marais BJ, Wright CA, Schaaf HS. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis-endemic area. Pediatr Infect Dis J. 2006;25(2):142–146. doi: 10.1097/01.inf.0000199259.04970.d1. [DOI] [PubMed] [Google Scholar]
- 31.Dorfman RF, Herweg JC. Live, attenuated measles virus vaccine: inguinal lymphadenopathy complicating administration. JAMA. 1966;198(3):320–321. doi: 10.1001/jama.1966.03110160148051. [DOI] [PubMed] [Google Scholar]
- 32.Shirone N, Shinkai T, Yamane T. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 33.Thomassen A, Nielsen AL, Gerke O. Duration of 18 F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38(5):894–898. doi: 10.1007/s00259-011-1729-9. [DOI] [PubMed] [Google Scholar]
- 34.Group CS. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin K, Weaver O, Wei W. Sonographic features of benign and malignant axillary nodes post-neoadjuvant chemotherapy. Breast J. 2020;26(2):182–187. doi: 10.1111/tbj.13488. [DOI] [PubMed] [Google Scholar]
- 36.Cellina M, Irmici G, Carrafiello G. Unilateral axillary lymphadenopathy after coronavirus disease (COVID-19) vaccination. Am J of Roentgenol. 2021 doi: 10.2214/ajr.21.25683. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. Am J of Roentgenol. 2021 Feb 5 doi: 10.2214/AJR.21.25604. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Mehta N, Sales RM, Babagbemi K. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Özütemiz C, Krystosek LA, Church AL. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021 doi: 10.1148/radiol.2021210275. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Washington T., Bryan R., Clemow C. Adenopathy Following COVID-19 Vaccination. Radiology. 2021 Feb 24 doi: 10.1148/radiol.2021210236. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nawwar AA, Searle J, Hagan I. COVID-19 vaccination induced axillary nodal uptake on [18F] FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021:1–2. doi: 10.1007/s00259-021-05274-7. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell OR, Dave R, Bekker J, Brennan PA. Supraclavicular lymphadenopathy following COVID-19 vaccination–an increasing presentation to the 2 week wait neck lump clinic? Br J Oral Maxillofac Surg. 2021 doi: 10.1016/j.bjoms.2021.02.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eifer M, Eshet Y. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology. 2021;28 doi: 10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu G, Lu Y. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med. 2021;46(4):353–354. doi: 10.1097/rlu.0000000000003597. [DOI] [PubMed] [Google Scholar]
- 45.Lehman CD, D'Alessandro HA, Mendoza DP. Unilateral lymphadenopathy post COVID-19 vaccination: A practical management plan for radiologists across specialties. J Am Coll Radiol. Mar 4. Epub ahead of print. https://doi:10.1016/j.jacr.2021.03.001. [DOI] [PMC free article] [PubMed]
- 46.Avner M, Orevi M, Caplan N. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur J Nucl Med Mol Imaging. 2021:1–2. doi: 10.1007/s00259-021-05278-3. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moghimi S., Wilson D., Martineau P. FDG PET findings post-COVID vaccinations: signs of the times? Clin Nucl Med. 2021 doi: 10.1097/RLU.0000000000003636. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Johnson BJ, Van Abel K, Ma D. FDG avid axillary lymph nodes after COVID-19 vaccination. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262108. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]