Abstract
Background:
Large scale United States (US) surveys guide efforts to maximize the health of its population. Cervical cancer screening is an effective preventive measure with a consistent question format among surveys. The aim of this study is to describe the predictors of cervical cancer screening in older women as reported by three national surveys.
Methods:
The Behavioral Risk Factor Surveillance System (BRFSS 2016), the Health Information National Trends Survey (HINTS 2017), and the Health Center Patient Survey (HCPS 2014) were analyzed with univariate and multivariate analyses. We defined the cohort as women, without hysterectomy, who were 45–65 years old. The primary outcome was cytology within the last 3 years.
Results:
Overall, Pap screening rates were 71% (BRFSS), 79% (HINTS) and 66% (HCPS), among 41,657, 740 and 1571 women, respectively. BRFSS showed that women 60–64 years old (aPR = 0.88, 95% CI: 0.85, 0.91), and in rural locations (aPR = 0.95, 95% CI: 0.92, 0.98) were significantly less likely to report cervical cancer screening than women 45–49-years old or in urban locations. Compared to less than high school, women with more education reported more screening (aPR = 1.20, 95% CI: 1.13, 1.28), and those with insurance had higher screening rates than the uninsured (aPR = 1.47, 95% CI: 1.33, 1.62). HINTS and HCPS also showed these trends.
Conclusions:
All three surveys show that cervical cancer screening rates in women 45–65 years are insufficient to reduce cervical cancer incidence. Insurance is the major positive predictor of screening, followed by younger age and more education. Race/ethnicity are variable predictors depending on the survey.
Keywords: Cervical cancer screening, Older women, Health Center Patient Survey, Health Information National Trends Survey, Behavioral Risk Factor Surveillance System, Disparities, Population screening effectiveness, Vulnerable populations
1. Introduction
National health goals for the United States (US) were established in 1979 through the Healthy People initiative (Healthy People, 2020a). Currently, the Healthy People 2020 (HP2020) goals are to decrease cervical cancer incidence to 7.3/100,000 women (Healthy People, 2020b). In order to do this, a minimum 70% population participation level in cervical cancer screening is needed (Quinn et al., 1999; IARC, 2005). The HP2020 goal is a 93% cervical cancer screening rate among women without hysterectomies (Healthy People, 2020b) with screening technique and interval informed by the United States Preventive Services Task Force (USPSTF) guidelines (US Preventive Services Task Force, 2018).
The age at which cervical cancer screening occurs has a significant impact on the likelihood of developing invasive cervical cancer (Sasieni et al., 2009). Younger women have significantly less impact on cancer incidence than do women screened at 45 years or older regardless of the screening interval or the testing technique (Sasieni et al., 2003; Zappa et al., 2004; Lonnberg et al., 2012). Screening under 30 years of age is considered the most expensive and least effective (Drain et al., 2002; Vijayaraghavan et al., 2009; Campos et al., 2017). Screening of women over 42 years provides up to an 80% protective effect (Sasieni et al., 2009; Lonnberg et al., 2012) against cervical cancer detection at the next screen. Hence, we focus our work on the self-reported screening rates of 45–65 years old women in the United States.
The large scale self-reported health surveys query the health of the US population at measured intervals to evaluate policy goals and to set future targets. Hamilton et al. (2015) led an effort to harmonize cancer screening questions, using similar constructs (question stems) but allowing correlate domains to be adaptable to each survey’s population and purpose. Over time, the cancer screening constructs around cervical cancer screening have converged to the same language and offered appropriate evidence-based options for a response around Pap testing, minimizing response variation due to construct variation (Gonzales et al., 2017).
Predictors of screening identified from other surveys include age, geolocation, insurance status, education and race (Seo and Langabeer II, 2018; Hirth et al., 2016; Zhao et al., 2018; Coughlin et al., 2008). The significance of the predictors varies depending both on the survey and the cycle of the survey. For instance, historically, the association of education and cervical cancer screening has varied. The 2013 Health Information National Trends Survey (HINTS) indicated education was not a predictor of cervical cancer screening uptake (Hirth et al., 2016); neither did the 2014 Behavioral Risk Factor Surveillance System (BRFSS) (Zhao et al., 2018). But, the earlier 2012 BRFSS data (Miles-Richardson et al., 2018) showed that increased education was associated with greater likelihood of screening. Compared to ‘high school or less’, some college was associated with a 38% higher likelihood of screening and a college graduate was 2.23 times as likely to be screened. While the constructs of how cervical cancer screening questions have been harmonized, the variation in response rates is still substantial among surveys. Discordant results may lead to confusion in policy recommendations leaving certain populations at risk and others over-screened. The Health Center Patient Survey (HCPS) focuses particularly on low income health recipients who represent many of the characteristics seen among women under-screened for cervical cancer. This unique database has recently been released for public analysis.
The primary aim of this study is to explore the recent self-reported cervical cancer screening rates of older women, 45–65 years, in two representative US databases (BRFSS, HINTS) and one new database (HCPS) to describe if the predictors of cervical cancer screening within the vulnerable HCPS population differ from those derived from the BRFSS or HINTS populations.
2. Methods
2.1. National health surveys
BRFSS (https://www.cdc.gov/brfss/data_documentation/index.htm, n.d.) is a Centers for Disease Control and Prevention (CDC)-developed health-related telephone survey that is designed to collect disease prevalence and state-level risk factor data on adults in all 50 states and District of Columbia in order to establish health related policies and priorities at the local level. Individual state health departments conduct landline and cell phone interviews with representative citizens to gather self-reported health risk behaviors, the receipt of clinical preventive services and access to health care. Weighting of the raw data is based on the number of phones in each household such that the BRFSS population is representative of gender, age, race, education, marital status, and home ownership, in state and sub-state regions. The most recent BRFSS dataset, including the health care access module, available for our analysis is 2016.
HINTS was created by the National Cancer Institute (NCI) Division of Cancer Control and Population Sciences (DCCPS) to monitor changes in the field of cancer-related health communications (Health Information National Trends Survey 5 (HINTS 5) Cycle 1 Methodology Report, 2017). It has been used to understand how adults use different communication channels to access information about cancer risk factors, screening, and care in the US. Questionnaires are mailed to home addresses including high density housing (e.g. public housing) which are oversampled by design. The questionnaire includes a $2 pre-paid incentive; reminder postcards are mailed up to three times. Weighting, calibration and adjustments are applied such that HINTS is representative of the US population by age, gender, education, marital status, race, ethnicity and census region. Each HINTS is administered in four cycles over four years; we used HINTS 5 cycle 1 (2017).
The Health Resources and Services Administration’s (HRSA) Bureau of Primary Health Care (BPHC) is responsible for the oversight of the Health Center Programs under Section 330 of the Public Health Services Act (Health Center Patient Survey 2014 Data, n.d.). Vulnerable populations cared for by Section 330 funding health care are 15% of the uninsured, 15% of Medicaid recipients and 7% of the entire US population (Russell, 2013). The HCPS is gathered every five years from an in-person, one-on-one interview with people who are nationally representative of the Health Center Program patient population. The data are used to improve health center performance/quality improvement and operational efficiencies; and to identify health conditions, behaviors, and trends of vulnerable populations over time by comparison to the US population as a whole. In addition, the HCPS queries patients’ access to and satisfaction with health care. The 2014 dataset is the first and most recent HCPS released to the public and was used in our work. The sampling frames of each survey are presented in Table 1 and the constructs of the surveys are in Supplemental Table 1.
Table 1.
Sampling frames of the three databases.
| BRFSS 2016 | HINTS (HINTS5 Cycle 1 2017) | HCPS 2014 | |
|---|---|---|---|
| Home | CDC; Population Health Surveillance Branch, under the Division of Population Health at the National Center for Chronic Disease Prevention and Health Promotion | NCI; Health Communication and Informatics Research Branch (HCIRB) of the Division of Cancer Control and Population Sciences (DCCPS) | Bureau of Primary Health Care; The Health Center Patient Survey (HCPS), sponsored by Health Resources and Services Administration HRSA |
| Mode of administration | Interviewer administered telephone surveys: landline and cellular phones | Survey was conducted exclusively by mail with a $2 pre-paid monetary incentive to encourage participation | Survey results come from in-person, one-on-one interviews with patients, and are nationally representative of the Health Center Program patient population. |
| Population | 18 yr and older, noninstitutionalized, residing in the US | 18 yr and older, noninstitutionalized, residing in the US | Eligible patients who had at least one visit in the past 12 months to an eligible health center site. Clients of funded HC located within the 50 U.S. states and the District of Columbia were included. |
| Sampling method | Disproportionate stratified sample as a probability sample of all households with telephones in the state | Two Strata: 1. Addresses in areas with high concentrations of minority population; and 2. Addresses in areas with low concentrations of minority population | All 169 Community Health Centers (CHC), Migrant Health Centers (MHC), Health Care for the Homeless (HCH), and Public Housing Primary Care (PHPC) |
| Overall sample size | 450,016 | 13,360 | 11,852 |
| Overall response rate | Landline 45.3% | High concentration of minority response rate 23.47% | 59% |
| Cell phone 44.5% | Low concentration of minority response rate 35.83% | ||
| Pertinent sample size for this work | 41,747 | 745 | 1,573 |
| Representative-ness | 50 states and DC, Guam, Puerto Rico; individual state health departments | All non-vacant residential addresses in the United States present | Participants in the HCPS are derived from vulnerable populations in the U.S: the poor, homeless, public housing residents, migrant and seasonal farm workers, at-risk women, minorities, persons with HIV/AIDS, uninsured and underinsured, and non-English speakers located within the 50 U.S. states and the District of Columbia. |
| Purpose | Establish state specific health related policies and priorities at the local level | Created to monitor changes in the rapidly evolving field of health communication. Survey researchers are using the data to understand how adults 18 years and older use different communication channels, including the Internet, to obtain vital health information for themselves and their loved ones. | Robust patient-level data to determine how well health centers funded under Section 330 of the Public Health Service Act provide access to primary and preventive health care. How well health centers meet the health care needs of the medically underserved; and How health center patients perceive the quality of their care. |
| Geocoding | Census region, census division, designated market area, rural urban continuum code | State, county, some metropolitan and micropolitan statistical areas (MMSA) | Urban vs Rural defined by BPHC’s Uniform Data System (UDS) |
| Website | https://www.cdc.gov/brfss/annual_data/2017/pdf/overview-2017-508.pdf | https://hints.cancer.gov/data/default.aspx | https://bphc.hrsa.gov/datareporting/research/hcpsurvey/index.html |
| https://www.cdc.gov/brfss/annual_data/2017/pdf/2017-sdqr-508.pdf | https://hints.cancer.gov/docs/methodologyreports/HINTS5_Cycle_1_Methodology_Rpt.pdf | https://bphc.hrsa.gov/datareporting/research/hcpsurvey/2014usermanual.pdf | |
| https://www.cdc.gov/brfss/annual_data/annual_2017.html | https://www.cms.gov/About-CMS/Agency-Information/OMH/resource-center/hcps-and-researchers/data-tools/sgm-clearinghouse/hcps.html | ||
2.2. Demographics and predictors of screening
In our study we included women aged 45–65 years with no known history of uterine or cervical cancer, and who had not had a hysterectomy. Unlike BRFSS and HINTS, HCPS did not ask for specific cancer history so all women in the specified age range were included. All surveys used a common categorization of race and ethnicity: Hispanic, non-Hispanic White (NHW), non-Hispanic Black (NHB), and other women of non-Hispanic (NH) ethnicity. Across all three surveys, educational status was categorized as less than high school, high school graduate, more than high school. Geographical identification was described differently across all three surveys. For BRFSS data we combined center of city metropolitan statistical area (MSA), same county as MSA center city and suburban county of MSA into “urban” and not in an MSA as “rural” as has been described by the National Center for Health Statistics Urban-Rural Classification Scheme (Lundeen et al., 2018; National Center for Health Statistics Urban-Rural Classification scheme for counties, n.d.). For HINTS data we combined large metro, large fringe metro and medium metro into “urban” and small metro, micropolitan and non-metro into “rural” using the same classification scheme (National Center for Health Statistics Urban-Rural Classification scheme for counties, n.d.). For HCPS data, urban and rural were the only categories defined. Insurance status (insured vs. uninsured) was used in the majority of analysis for all three surveys. BRFSS collected information on whether or not respondents had some form of health plan but did not identify the source; HINTS and HCPS further categorized insurance source by employer/private, publicly funded, or uninsured. Employer/private and publicly funded were combined to create an ‘insured’ category when necessary.
2.3. Cervical cancer screening
The primary technique for cervical cancer screening in this study was cytology. All three surveys queried about cytology screening: did the woman ever have a Pap; and when did the last Pap occur. A secondary indicator for cervical cancer screening was human papillomavirus (HPV) testing. Only BRFSS and HCPS queried about HPV screening: did the woman have an HPV test; and when was the last HPV test.
2.4. Statistics
Within each dataset, predictors of screening were summarized using proportions, unweighted and weighted with a 95% confidence interval. Cervical cancer screening rates were calculated by determining whether individuals had received a Pap test within the last 3 years. For BRFSS, we summarized reported HPV screening rates within the last 5 years as a secondary guideline directed screening method. Pap test and HPV screening rates were compared in the BRFSS data using McNemar’s test. We performed pairwise comparisons of screening rates using post-hoc marginal probability estimates obtained from logistic regression models for each predictor. Adjustments were made to p-values using the Bonferroni correction to account for multiple testing. Interaction terms between age with race, education and geolocation were tested with no significant findings, thus non-age stratified rates are presented. Prevalence ratios comparing 5-year HPV screening rates between different groups were estimated using binomial regression models with a log link. Adjusted prevalence ratios for Pap testing were estimated using Poisson models. Covariates included age, race, insurance status, geolocation and education. All datasets were analyzed using weighted sampling methods to ensure valid inferences from the responding sample to the population, correcting for non-response and non-coverage biases (StataCorp, 2017). Weighting methods were specific to each survey (https://www.cdc.gov/brfss/annual_data/2017/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2017–508.pdf, n.d.; https://hints.cancer.gov/docs/methodologyreports/HINTS5_Cycle_1_Methodology_Rpt.pdf, n.d.; https://bphc.hrsa.gov/datareporting/research/hcpsurvey/2014usermanual.pdf, n.d.).
3. Results
Age groups were categorized by five-year intervals for BRFSS and HINTS with intervals of interest starting at 45–49 years old; HCPS uses 10-year intervals (Table 2). All three surveys reported similarly aging cohorts; but HINTS and HCPS reported lower enrollment of those over 55 years. Rural and urban distributions were represented differently. BRFSS respondents were more urban than rural (35% vs 7%), but the majority failed to report any location (58%). HINTS was more urban than rural (79% vs. 21%) and HCPS was predominantly rural (60%). While the HCPS population was more equally distributed across levels of educational attainment, both the BRFSS and HINTS populations had “more than a high school” education compared to “less than a high school” education (62% vs 13% and 70% vs 7%, respectively). Racial and ethnic representation was similar across all surveys, with the majority being NHW. Both HINTS (26%) and HCPS (30%) have a higher combined proportion of NHB and Hispanic respondents than BRFSS (19%). Insurance coverage varied across surveys as well; 90% of BRFSS respondents reported some form of insurance without identifying private vs public sources. HINTS reported more employer/private insurance than public or uninsured (67% vs 32%) and HCPS reported more Medicaid/Medicare and uninsured populations than private (88% vs 12%).
Table 2.
National health survey database demographic correlates.
| BRFSS 2016 |
HINTS 2017 |
HCPS 2014 |
||||
|---|---|---|---|---|---|---|
| Unweighted %, (N) N = 77,143 | Weighted % (95% CI) | Unweighted %, (N) N = 761 | Weighted % (95% CI) | Unweighted %, (N) N = 1580 | Weighted % (95% CI) | |
| Age (years) | ||||||
| 45 to 49 | 19.7% (15,207) | 24.2% (23.5, 24.8) | 21.3% (162) | 31.8% (27.4, 36.6) | 51.7% (817) | 58.2% (52.0, 64.1) |
| 50 to 54 | 23.8% (18,362) | 28.2% (27.5, 28.9) | 21.9% (167) | 29.0% (24.9, 33.4) | ||
| 55 to 59 | 27.3% (21,079) | 24.2% (23.6, 24.8) | 25.4% (193) | 20.6% (18.6, 22.7) | 48.3% (763) | 41.8% (35.9, 48.0) |
| 60 to 64 | 29.2% (22,495) | 23.4% (22.8, 24.0) | 31.4% (239) | 18.6% (17.2, 20.2) | ||
| Geolocation | ||||||
| Rural | 16.9% (13,064) | 6.8% (6.5, 7.0) | 21.3% (162) | 23.2% (18.9, 28.0) | 28.2% (446) | 59.5% (48.0, 70.0) |
| Urban | 38.0% (29,322) | 35.2% (34.5, 35.8) | 78.7% (599) | 76.8% (72.0, 81.1) | 71.8% (1134) | 40.5% (30.0, 52.0) |
| Unknown/missing | 45.1% (34,757) | 58.1% (57.4, 58.8) | ||||
| Education | ||||||
| Less than high school | 6.2% (4791) | 12.6% (12.0, 13.2) | 4.5% (34) | 6.9% (3.9, 11.9) | 45.0% (711) | 32.9% (25.6, 41.1) |
| High school | 24.7% (19,076) | 25.4% (24.7, 26.0) | 20.1% (153) | 22.4% (18.1, 27.4) | 24.6% (388) | 33.8% (25.8, 42.9) |
| More than high school | 68.9% (53,129) | 61.8% (61.0, 62.5) | 74.8% (569) | 70.3% (65.2, 75.0) | 30.0% (474) | 33.1% (27.0, 29.7) |
| Unknown/missing | 0.2% (147) | 0.2% (0.2, 0.4) | 0.7% (5) | 0.4% (0.1, 1.4) | 0.4% (7) | 0.2% (0.1, 0.6) |
| Race | ||||||
| Non-Hispanic White | 76.5% (59,017) | 64.9% (64.1, 65.6) | 59.8% (455) | 65.8% (61.3, 70.1) | 24.1% (381) | 56.6% (48.3, 64.5) |
| Non-Hispanic Black | 8.9% (6869) | 11.3% (10.9, 11.8) | 19.6% (149) | 15.8% (11.9, 20.5) | 25.6% (404) | 19.6% (15.1, 25.0) |
| Hispanic | 7.5% (5787) | 7.4% (6.8, 8.0) | 10.6% (81) | 10.6% (7.8, 14.2) | 33.7% (533) | 16.4% (11.9, 22.4) |
| Women of other non-Hispanic ethnicitya | 5.8% (4451) | 14.8% (14.1, 15.4) | 8.2% (62) | 6.6% (4.5, 9.6) | 16.6% (262) | 7.4% (4.6, 11.6) |
| Unknown/missing | 1.3% (1019) | 1.7% (1.5, 2.0) | 1.8% (14) | 1.3% (0.6, 2.6) | 0 | 0 |
| Insurance | ||||||
| Employer/private | 92.4% (71,254) | 89.9% (89.3, 90.4) | 64.4% (490) | 66.8% (61.4, 71.9) | 10.4% (164) | 11.5% (8.1, 16.0) |
| Publicly fundedb | 28.7% (218) | 25.7% (21.0, 31.1) | 64.2% (1014) | 52.7% (43.2, 62.0) | ||
| Uninsured | 7.4% (5704) | 9.8% (9.3, 10.3) | 6.4% (56) | 6.6% (4.3, 10.0) | 25.3% (400) | 35.8% (26.9, 45.7) |
| Missing | 0.2% (185) | 0.4% (0.2, 0.5) | 0.7% (6) | 0.9% (0.3, 2.9) | 0.1% (2) | 0% (<0.01, 0.2) |
Women of Other Non-Hispanic ethnicity means a weighted distribution of:
BRFSS: 13% American Indian, 65% Asian, 15% multiracial, 3% Native Hawaiian, 4% other.
HCPS: 40% Non-Hispanic Asian and 60% other.
HINTS: 33% multi-racial, 17% Chinese, 8% Vietnamese, 13% Filipino, 22% Asian Indian, 5% American Indian, 1% Korean, and 1% Japanese.
Medicaid, Medicare, Tricare, Veteran’s Affairs, Tribal, and other related publicly sponsored programs.
Table 3 shows cervical cancer screening rates among women without a hysterectomy. All survey constructs used the “within the last 3 years” for current Pap testing, which corresponds to guidelines for cytology screening alone in women 21–65 years (US Preventive Services Task Force, 2018). Survey constructs for HPV testing were aligned with current guidelines for BRFSS, with the option of “within last 5 years” for women older than 30 years.
Table 3.
Cervical cancer screening rates.
| BRFSS 2016 |
HINTS 2017 |
HCPS 2014 |
||||
|---|---|---|---|---|---|---|
| Unweighted %, (N) N = 77,143 | Weighted % (95% CI) | Unweighted %, (N) N = 1580 | Weighted % (95% CI) | Unweighted %, (N) N = 761 | Weighted % (95% CI) | |
| Pap test within last 3 years | ||||||
| Yes | 71.7% (55,304) | 71.3% (70.6, 72.0) | 79.8% (607) | 79.1% (74.4, 83.2) | 73.3% (1158) | 66.0% (60.0, 71.5) |
| Yes, but not within the last 3 years | 17.7% (13,677) | 15.6% (15.1, 16.1) | 16.6% (126) | 15.1% (11.9, 18.9) | 19.9% (315) | 29.7% (24.0, 36.0) |
| No | 2.3% (1751) | 2.8% (2.5, 3.1) | 1.1% (8) | 1.2% (0.5, 2.8) | 5.9% (93) | 3.8% (1.9, 7.4) |
| Don’t know/unsure/missing | 8.3% (6411) | 10.3 (9.8, 10.8) | 2.6% (20) | 4.6% (2.0, 10.1) | 0.9% (14) | 0.5% (0.2, 1.2) |
| HPV test within last 3 years | ||||||
| Yes | 18.4% (14,156) | 19.9% (19.3, 20.6) | 15.0% (237) | 9.8% (7.0, 13.6) | ||
| Yes, but not within the last 3 years | 7.1% (5469) | 6.8% (6.4, 7.1) | 4.3% (68) | 5.9% (0.3, 11.2) | ||
| No | 40.7% (31,377) | 39.0% (38.2, 39.7) | 73.2% (1156) | 76.1% (69.5, 81.7) | ||
| Don’t know/unsure/missing | 33.9% (26,141) | 34.3% (33.6, 35.1) | 7.5% (119) | 8.2% (5.3, 12.6) | ||
| HPV test within last 5 years | ||||||
| Yes | 20.7% (15,985) | 22.3% (21.7, 22.9) | ||||
| Yes, but not within the last 5 years | 4.7% (3640) | 4.4% (4.1, 4.7) | ||||
| No | 40.7% (31,377) | 39.0% (38.2, 39.7) | ||||
| Don’t know/unsure/missing | 33.9% (26,141) | 34.3% (33.6, 35.1) | ||||
HINTS does not query HPV testing and HCPS queries HPV testing >3 years ago without including a 5 year limit.
Overall estimates of screening rates within the past 3 years were 71% (BRFSS), 79% (HINTS) and 66% (HCPS). Among those women who participated in Pap testing but at a longer interval than guideline directed, HCPS reported twice the rate of longer interval participation than did HINTS or BRFSS (30% vs. 15%/16%, respectively). In BRFSS, HPV testing within the last 5 years significantly lagged Pap testing within the last 3 years by 49% (22% vs 71%, 95% CI of the difference: 48.2–49.8).
3.1. Univariate analyses
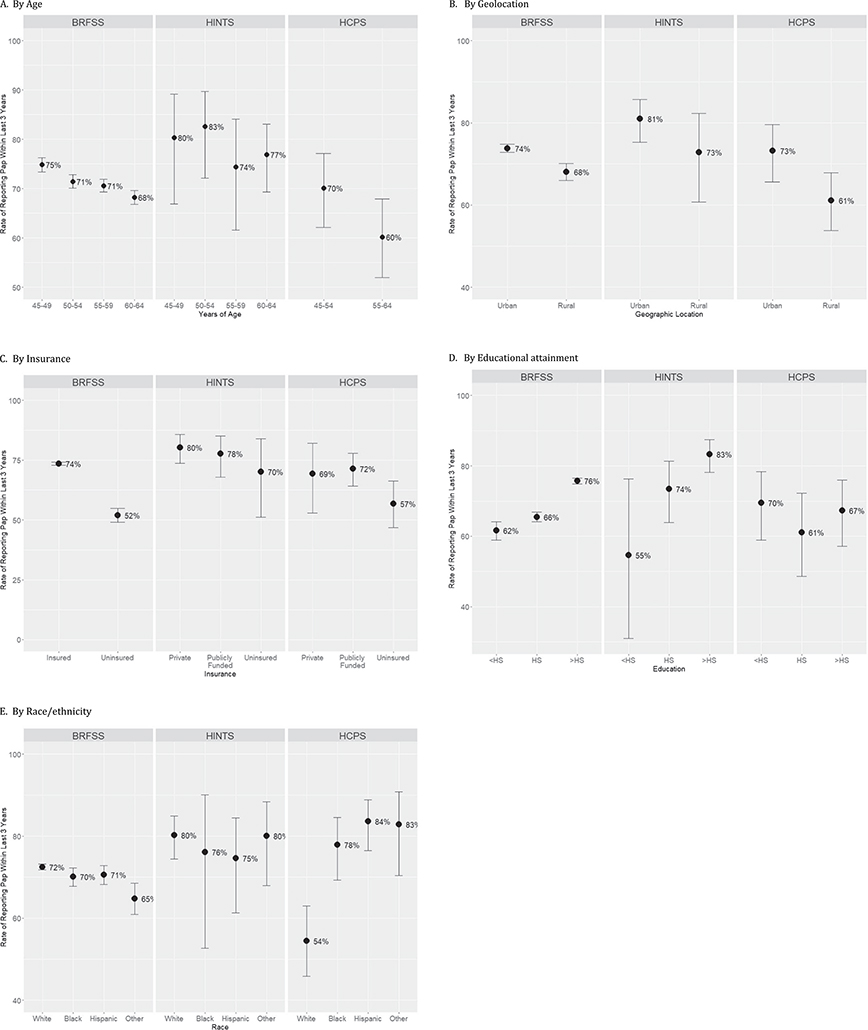
Fig. 1 stratifies Pap screening within the last 3 years by five groups: age (A), geolocation (B), insurance (C) and educational attainment (D) and race/ethnicity (E). As age increases, Pap screening rates decreased across all three surveys. Only the BRFSS data showed consistent and statistically significant decreases by age group from 75% for the youngest group to 68% for the oldest. While HINTS showed point estimates of screening rates above the 70% threshold and HCPS point estimates were at or below this 70% threshold, both showed trends for decreased screening as age increases.
Fig. 1.
Reported Pap screening rates within the last 3 years.
A. By age.
B. By geolocation.
C. By insurance.
Publicly funded means Medicaid, Medicare, Tricare, Tribal, Veterans’ Affairs, and others.
D. By educational attainment.
<HS means less than high school graduation; HS means high school graduation; >HS means more than high school education.
E. By race/ethnicity.
Race: Non-Hispanic White (NHW), Non-Hispanic Black (NHB), Hispanic; and, ‘Other’ means a weighted distribution:
BRFSS: 13% American Indian, 65% Asian, 15% multiracial, 3% Native Hawaiian, 4% other.
HCPS: 40% Non-Hispanic Asian and 60% other.
HINTS: 33% multi-racial, 17% Chinese, 8% Vietnamese, 13% Filipino, 22% Asian Indian, 5% American Indian, 1% Korean, and 1% Japanese.
The three surveys similarly reported differences in screening rates by geolocation. Urban respondents, regardless of survey, had rates higher than the 70% threshold. Rural respondents from HCPS reported the lowest screening rate or the surveys at 61%.
All three surveys showed that screening rates tended to be higher among insured woman compared to the uninsured women, with BRFSS reporting the largest disparity (74% vs 52%, 95% CI of difference: 18.6–24.4) closely followed by the HCPS rates (71% insured vs 57% uninsured, 95% CI of difference: 1.7–26.9). While the overall screening rates differed between HINTS (~80%) and HCPS (~70%), within each survey, similar screening rates were found for public and privately insured women.
Increasing educational attainment led to significantly higher screening rates reported in BRFSS (62% [less than high school] vs. 66% [high school graduation] vs. 76% [more than high school], overall p < 0.001). In HINTS, while the screening rates similarly trended upward, from 55% to 83%, for higher educational attainment, the trend was not significant. The HCPS data, on the other hand, showed relatively uniform screening rates by educational attainment predominantly at or under the 70% threshold.
Pap screening rates were most strikingly different across surveys by race/ethnicity. In BRFSS, NHW, NHB and Hispanic women reported screening at very similar rates, around 70%; while the women of other NH ethnicities reported significantly lower screening rates (72% (NHW) vs. 65% (women of other NH ethnicities), 95% CI of difference: 2.5–13.0). In HINTS, all racial/ethnic groups reported very similar rates above the 70% threshold, but far from the 93% HP2020 goal.
By contrast, HCPS data showed that NHW women reported significantly lower screening rates than each of the race/ethnicity categories by pairwise comparisons. All women who receive health care from Section 330 Public Health funding have the option to go elsewhere for Pap screening. Among the subset who reported having received a recent Pap screening and having it performed at a Health Center, 82% were within the 3-year guideline; whereas, if the Pap screening was done elsewhere, 54% were within the 3-year guideline (95% CI of difference: 15.6–42.0). Of those screened at the HCPS health center within the past 3 years, the screening rate for Hispanic women was 92% (95% CI: 83–97), for NHB women 93% (95% CI: 86–97) and for women of other NH races 95% (95% CI: (86–98).
Within BRFSS, of the 22% who reported HPV testing within the last 5 years, the adjusted prevalence rates in Table 4 showed similar trends to Pap screening: lower rates were more likely among older women, women living in rural areas, women with less education and uninsured women. HPV testing among women of different races/ethnicities differed from Pap testing with NHB and Hispanic women have a higher likelihood of screening than NHW women.
Table 4.
BRFSS HPV Screening Rate in the last 5 years.
| HPV screening rate in last 5 yearsa % (95% CI) | PR (95% CI)b | aPR (95% CI)c | |
|---|---|---|---|
| Age, yrs | |||
| 45 to 49 | 28.8 (27.3, 30.3) | Reference | Reference |
| 50 to 54 | 23.8 (22.6, 25.1) | 0.83 (0.77, 0.89) | 0.88 (0.80, 0.98) |
| 55 to 59 | 19.7 (18.6, 20.9) | 0.68 (0.63, 0.74) | 0.73 (0.65, 0.81) |
| 60 to 64 | 16.5 (15.4, 17.7) | 0.57 (0.53, 0.63) | 0.59 (0.52, 0.67) |
| Geolocation | |||
| Urban | 22.2 (21.2, 23.2) | Reference | Reference |
| Rural | 15.3 (14.0, 16.7) | 0.69 (0.63, 0.76) | 0.75 (0.68, 0.83) |
| Education | |||
| Less than high school | 15.9 (14.1, 17.8) | Reference | Reference |
| High school/GED | 20.2 (19.0, 21.5) | 1.27 (1.12, 1.45) | 1.49 (1.19, 1.87) |
| More than high school | 24.5 (23.7, 25.3) | 1.55 (1.37, 1.74) | 1.86 (1.50, 2.30) |
| Race/ethnicity | |||
| Non-Hispanic White | 21.7 (21.0, 22.3) | Reference | Reference |
| Non-Hispanic Black | 26.3 (24.3, 28.4) | 1.21 (1.11, 1.32) | 1.30 (1.17, 1.45) |
| Other non-Hispanic races | 18.3 (15.3, 21.9) | 0.85 (0.71, 1.02) | 0.71 (0.57, 0.88) |
| Hispanic | 24.0 (22.0, 26.1) | 1.11 (1.01, 1.21) | 1.50 (1.28, 1.76) |
| Insurance status | |||
| Uninsured | 16.3 (14.5, 18.4) | Reference | |
| Insured | 23.0 (22.3, 23.7) | 1.41 (1.24, 1.60) | 1.52 (1.19, 1.94) |
Weighted rates of receiving HPV screening within the past 5 years.
Unadjusted prevalence ratios resulting from binomial models with log link; screening as outcome and each variable as individual predictor.
Adjusted prevalence ratio results from a binomial model with log link; screening as outcome and all listed variables as predictors (n = 41,657).
3.2. Multivariate modeling
We calculated the adjusted prevalence ratios for Pap testing for each survey (Table 5, raw counts are in Supplemental Table 2). BRFSS reported the greatest number of significant predictors of cervical cancer screening. Women 60–64 years old were 12% less likely to report screening than the 45–49-year-olds (aPR 0.88, 95% CI: 0.85–0.91). Likewise, women living in rural areas were 5% less likely to screen than urban dwellers (aPR 0.95, 95% CI: 0.92–0.98). Women with more than a high school education were 20% more likely to screen than women with less than a high school education (aPR 1.20, 95% CI: 1.13–1.28). Compared to NHW women, NHB women were equally likely to screen, but women of other NH races were 10% less likely to report screening (aPR 0.90, 95% CI: 0.83–0.98), and Hispanic women were 11% more likely to report screening (aPR 1.11, 95% CI: 1.06–1.17). Insured women were 47% more likely to report screening than uninsured (aPR 1.47, 95% CI: 1.33–1.62).
Table 5.
Adjusted prevalence ratios for Pap testing.b
| BRFSS 2016 | HINTS 2017 | HCPS 2014 | |
|---|---|---|---|
| aPR (95% CI)a N = 41,657 | aPR (95% CI)a N = 740 | aPR (95% CI)a N = 1571 | |
| Age | |||
| 45 to 49 | Reference | Reference | Reference |
| 50 to 54 | 0.96 (0.93, 0.99) | 1.03 (0.86, 1.23) | |
| 55 to 59 | 0.91 (0.88, 0.94) | 0.94 (0.78, 1.15) | 0.85 (0.72, 1.01) |
| 60 to 64 | 0.88 (0.85, 0.91) | 0.96 (0.80, 1.15) | |
| Geolocation | |||
| Urban | Reference | Reference | Reference |
| Rural | 0.95 (0.92, 0.98) | 0.92 (0.78, 1.08) | 0.96 (0.84, 1.10) |
| Education | |||
| Less than high school | Reference | Reference | Reference |
| High school | 1.05 (0.97, 1.12) | 1.32 (0.78, 2.22) | 1.02 (0.81, 1.28) |
| More than high school | 1.20 (1.13, 1.28) | 1.50 (0.92, 2.46) | 1.08 (0.87, 1.33) |
| Race/ethnicity | |||
| Non-Hispanic White | Reference | Reference | Reference |
| Non-Hispanic Black | 0.99 (0.95, 1.03) | 0.94 (0.71, 1.23) | 1.35 (1.09, 1.68) |
| Other non-Hispanic races | 0.90 (0.83, 0.98) | 0.98 (0.85, 1.12) | 1.47 (1.16, 1.86) |
| Hispanic | 1.11 (1.06, 1.17) | 0.96 (0.82, 1.13) | 1.48 (1.25, 1.76) |
| Insurance status | |||
| Uninsured | Reference | Reference | Reference |
| Insured | 1.47 (1.33, 1.62) | 1.06 (0.82, 1.37) | 1.20 (0.96, 1.51) |
Adjusted for all other variables in table.
The count numbers for the modeling are provided in Supplemental Table 2.
HINTS only provided trends, not any significant predictors, of cervical cancer screening. Likewise, HCPS, representing the most vulnerable US population, mirrored the BRFSS trends, but without significance, for women of older age, uninsured and in rural locations having less likelihood of screening. Contrary to BRFSS, though, HCPS showed that NHB were 35% more likely (95% CI: 9–68%), Hispanic 48% more likely (95% CI: 25–76%) and women of other NH races 47% more likely (95% CI: 16–86%) compared to NHW women to be screened.
4. Discussion
Our work is the first to describe and contrast responses from three large US national health surveys about self-reported cervical cancer screening rates in women 45–65 years old; and, the first to report the predictors of screening across these three national surveys. This work also includes the first analysis of HCPS data for cervical cancer screening. Most importantly, regardless of which US health survey we analyzed, we have shown that as women age, they are less likely to participate in screening. This is of concern for several reasons. Older women now comprise nearly half of the women diagnosed with cervical cancer in the US (Quinn et al., 2018). In addition, there is greater benefit in decreasing the incidence of cervical cancer when women 45–65 years old, compared to younger women, participate in screening (Sasieni et al., 2003). While screening will benefit an individual woman, a population-based screening rate of 70% is the minimum screening threshold required to reduce the national cervical cancer incidence (Quinn et al., 1999; IARC, 2005). Our work shows that the most vulnerable populations, as reported by HCPS data, are reporting screening at or below the 70% minimal threshold.
The national health surveys are sufficiently different from each other in population composition and sampling frame that differences in predictors of cervical cancer screening by survey were expected. Where we see significant predictors of cervical cancer screening in BRFSS, the relationships are only trends in HCPS and HINTS. All three surveys, though, were consistent in the direction and magnitude of older age, rural location, no insurance and less education predicting less screening.
The most noticeable difference in survey prediction of cervical cancer screening was for HCPS, though. Despite being the survey reporting the lowest overall cervical cancer screening rate (66%), the Hispanic, NHB and other NH ethnicities self-reported over 90% screening rates when screening occurred at the Health Center, nearly approaching the HP2020 goal. Compared to NHW women, these women self-reported a significantly greater 35–48% likelihood of screening. While this seems very encouraging that targeted programs successfully reach underserved women, validation studies show that the actual rates of screening are between a quarter to a third less than self-reported in community health centers and county hospitals (McPhee et al., 2002; Pizarro et al., 2002; Ferrante et al., 2008; Lofters et al., 2015). Reasons for over-reporting include behaviors that favor social desirability, and acquiescence with expected behaviors, especially among NHB and Hispanic women (Burgess et al., 2009; Rauscher et al., 2008). All surveys, though, have an element of over-reporting, even among a managed care population (Caplan et al., 2003), suggesting that trends among surveys are more important than the magnitude of the predictors found.
The sampling frames of the surveys contributed to the population differences of the women participating in the survey. The sampling frame for BRFSS includes a telephone interview that results in a near 45% response rate in each of the 50 states and District of Columbia. Nonetheless, this response rate resulted in at least tenfold greater absolute magnitude than is produced by the other sampling frames. This large sample size allows very small differences to be statistically significant, whereas, other surveys can only show predictive trends.
The sampling frame for HINTS had a 25–33% survey response rate, despite providing a $2 incentive in the mailed interview; and yet, HINTS showed the highest self-reported cervical cancer screening rate of all surveys. We expect this as HINTS reflects the primarily employed and privately insured whose health plans directly incentivize providers to prioritize cervical cancer screening (https://www.ncqa.org/hedis/measures/cervical-cancer-screening/, n.d.). Insurance effectively removes economic barriers to accessing screening, especially when combined with the Affordable Care Act (ACA) which required all health plans, including Section 330 funding, to cover cervical cancer screening with no co-pays for the woman when screening was performed by a network provider (https://www.hrsa.gov/womens-guidelines/index.html, n.d.). Even though the ACA removed out of pocket copays, the screening rates did not increase. In fact, Steenland et al. (2019) show continuing downward cervical cancer screening participation despite no insurance co-pay after the ACA implementation. Moreover, Watson et al., (2017, 2018) followed cervical cancer screening rates in the US between 2000 and 2014 (pre-, post-ACA), and also showed progressively declining screening rates, particularly in older women. Our analysis updates these reports and continues to show the downward trend. Other social determinants of health might explain what barriers remain for the remaining 20%–50% who are not participating in screening (Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health, 2019).
Professional guideline changes in cervical cancer screening techniques and intervals may have also contributed to the lack of understanding of timely screening. Similarly, the electronic medical record ordering systems for cervical cancer screening have also provided multiple techniques and intervals from which to choose leading to order confusion. These changes started in 2003 with the approval of HPV testing in combination with cytology (co-testing) to screen women 30 and older, in addition to the option of cytology alone (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P890064], n.d.). In 2004 (Smith et al., 2004) the guidelines changed from annually to every two years with liquid based Pap tests starting no later than 21 years or three years after the woman begins having vaginal intercourse. At 30 years, if the woman has had three normal consecutive tests, screening may extend to every 2–3 years using liquid Pap tests or every 3 years with HPV-cytology co-testing. By 2012, guidelines evolved to every 3 years with cytology for 21–29-year olds and a preferred recommendation of every 5-year co-testing for women 30–65 years (Saslow et al., 2012). By 2018 co-testing was no longer favored (Schiffman et al., 2018), and now the USPSTF recommends primary HPV testing every five years (US Preventive Services Task Force, 2018). The many changes in techniques and intervals could have led to misunderstandings on what screening was recommended and what screening was implemented.
4.1. Study limitations and strengths
As we have discussed above, the limitations and consequences therefrom include different years of survey implementation, responses that are self-reported, different sampling frames, different survey sample sizes and changing professional guidelines. Despite these limitations, the trends and impact of predictors are still evaluable, and valuable to future strategies for cervical cancer elimination. Strengths include the description of the results from three large-scale national surveys routinely used to guide US health policy, including the first analysis of cervical cancer screening data from HCPS, a Section 330 funding program serving the vulnerable population.
5. Conclusions
The three nationally representative large-scale surveys reinforce that current cervical cancer screening for women 45–65 years old is near or below the 70% minimum threshold needed to reduce cervical cancer incidence across the population. While having insurance is the strongest predictor of screening participation, and US health care law now requires no copay for cervical cancer screening, the screening rate of women 45–65 years remains low. Specifically, older age, not having insurance, not finishing high school, and living in a rural area are indicators of less cervical cancer screening.
Supplementary Material
Funding status
This work was supported by the Michigan Institute for Clinical and Health Research UL1TR002240 and by the The University of Michigan Rogel Cancer Center P30CA046592 grants.
Abbreviations:
- BRFSS
Behavioral Risk Factor Surveillance System
- HCPS
Health Center Patient Survey
- HINTS
Health Information National Trends Survey
- HP2020
- USPSTF
United States Preventive Services Task Force
- CDC
Centers for Disease Control and Prevention
- HRSA
Health Resources and Services Administration
- BPHC
Bureau of Primary Health Care
- FQHC
Federally Qualified Health Center
- NCI
National Cancer Institute
- DCCPS
Division of Cancer Control and Population Sciences
- MSA
metropolitan statistical area
- HPV
human papillomavirus
- SEER
Surveillance, Epidemiology, and End Results
- US
United States
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2019.105880.
References
- Burgess DJ, Powell AA, Griffin JM, Partin MR, 2009. February. Race and the validity of self-reported cancer screening behaviors: development of a conceptual model. Prev. Med. 48 (2), 99–107. 10.1016/j.ypmed.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Campos NG, Jeronimo J, Tsu V, Castle PE, Mvundura M, Kim JJ, 2017. October. The cost-effectiveness of visual triage of human papillomavirus-positive women in three low- and middle-income countries. Cancer Epidemiol. Biomark. Prev. 26 (10), 1500–1510. 10.1158/1055-9965.EPI-16-0787. [DOI] [PubMed] [Google Scholar]
- Caplan LS, McQueen DV, Qualters JR, Leff M, Garrett C, Calonge N, 2003. November. Validity of women’s self-reports of cancer screening test utilization in a managed care population. Cancer Epidemiol. Biomark. Prev. 12 (11 Pt 1), 1182–1187. [PubMed] [Google Scholar]
- Coughlin SS, Leadbetter S, Richards T, Sabatino SA, 2008. January. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc. Sci. Med. 66 (2), 260–275 (Epub 2007 Nov 19). [DOI] [PubMed] [Google Scholar]
- Drain PK, Holmes KK, Hughes JP, Koutsky LA, 2002. July 10. Determinants of cervical cancer rates in developing countries. Int. J. Cancer 100 (2), 199–205. 10.1002/ijc.10453. [DOI] [PubMed] [Google Scholar]
- Ferrante JM, Ohman-Strickland P, Hahn KA, Hudson SV, Shaw EK, Crosson JC, Crabtree BF, 2008. November. Self-report versus medical records for assessing cancer-preventive services delivery. Cancer Epidemiol. Biomark. Prev. 17 (11), 2987–2994. 10.1158/1055-9965.EPI-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales FA, Willis GB, Breen N, Yan T, Cronin KA, Taplin SH, Yu M, 2017. An exploration of changes in the measurement of mammography in the national health interview survey. Cancer Epidemiol. Biomarkers Prev. 26 (11), 1611–1618. 10.1158/1055-9965.EPI-17-0213. [DOI] [PubMed] [Google Scholar]
- Hamilton JG, Breen N, Klabunde CN, Moser RP, Leyva B, Breslau ES, Kobrin SC, 2015. January. Opportunities and challenges for the use of large-scale surveys in public health research: a comparison of the assessment of cancer screening behaviors. Cancer Epidemiol. Biomark. Prev. 24 (1), 3–14. 10.1158/1055-9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Center Patient Survey 2014 Data. US Department of Health and Human Services’ Office of the Assistant Secretary for Planning and Evaluation (ASPE). Accessed on August 15, 2019 at: https://bphc.hrsa.gov/datareporting/research/hcpsurvey/index.html
- Health Information National Trends Survey 5 (HINTS 5) Cycle 1 Methodology Report. Westat. Accessed on August 15, 2019 at: https://hints.cancer.gov/docs/methodologyreports/HINTS5_Cycle_1_Methodology_Rpt.pdf.
- Healthy People, 2020a. Accessed on August 15, 2019 at. https://www.healthypeople.gov/2020/About-Healthy-People/History-Development-Healthy-People-2020.
- Healthy People 2020b. Washington, DC: U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. [Google Scholar]
- Healthy People 2020. Objectives C-10, C-4 and C-15. CDC. Accessed on August 15, 2019 at https://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives [Google Scholar]
- Hirth JM, Laz TH, Rahman M, Berenson AB, 2016. April. Racial/ethnic differences affecting adherence to cancer screening guidelines among women. J. Women’s Health (Larchmt) 25 (4), 371–380. 10.1089/jwh.2015.5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HCPS weighting. Accessed on August 15, 2019 at: https://bphc.hrsa.gov/datareporting/research/hcpsurvey/2014usermanual.pdf.
- HINTS weighting. Accessed on August 15, 2019 at: https://hints.cancer.gov/docs/methodologyreports/HINTS5_Cycle_1_Methodology_Rpt.pdf.
- HPV testing approved by FDA. Accessed on August 15, 2019 at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P890064].
- BRFSS weighting. Accessed on August 15, 2019 at: https://www.cdc.gov/brfss/annual_data/2017/pdf/Complex-Smple-Weights-Prep-Module-Data-Analysis-2017-508.pdf.
- The BRFSS data user guide. Accessed on August 15, 2019 at: https://www.cdc.gov/brfss/data_documentation/index.htm.
- National Center for Health Statistics Urban-Rural Classification scheme for counties. Accessed on August 15, 2019 at: https://www.cdc.gov/nchs/data_access/urban_rural.htm#2013_Urban-Rural_Classification_Scheme_for_Counties.
- ACA coverage of cervical cancer screening. Accessed on August 15, 2019 at: https://www.hrsa.gov/womens-guidelines/index.html.
- HEDIS measures. Accessed on August 15, 2019 at: https://www.ncqa.org/hedis/measures/cervical-cancer-screening/.
- IARC. IARC Handbooks of Cancer Prevention Volume 10: Cervix Cancer Screening. Chapter 5. Effectiveness of Screening in Populations. IARC Press. 2005. ISBN-13 PDF: 978–92-832–3010-6. Accessed August 15, 2019 at http://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Cervix-Cancer-Screening-2005. [Google Scholar]
- Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. NAM Accessed on September 26, 2019. 10.17226/25467. [DOI] [PubMed]
- Lofters A, Vahabi M, Glazier RH, 2015. January 29. The validity of self-reported cancer screening history and the role of social disadvantage in Ontario, Canada. BMC Public Health 15, 28. 10.1186/s12889-015-1441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnberg S, Anttila A, Luostarinen T, et al. , 2012. Age-specific effectiveness of the Finnish cervical cancer screening programme. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology 21, 1354–1361. 10.1158/1055-9965.EPI-12-0162. [DOI] [PubMed] [Google Scholar]
- Lundeen EA, Park S, Pan L, O’Toole T, Matthews K, Blanck HM, 2018. June 15. Obesity prevalence among adults living in metropolitan and nonmetropolitan counties - United States, 2016. MMWR Morb. Mortal. Wkly Rep. 67 (23), 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee SJ, Nguyen TT, Shema SJ, Nguyen B, Somkin C, Vo P, Pasick R, 2002. November. Validation of recall of breast and cervical cancer screening by women in an ethnically diverse population. Prev. Med. 35 (5), 463–473. 10.1006/pmed.2002.1096. [DOI] [PubMed] [Google Scholar]
- Miles-Richardson S, Allen S, Claridy MD, Booker EA, Gerbi G, 2018. Factors associated with self-reported cervical cancer screening among women aged 18 years and older in the United States. J. Community Health 42, 72–77. 10.1007/s10900-016-0231-5. [DOI] [PubMed] [Google Scholar]
- Pizarro J, Schneider TR, Salovey P, 2002. A source of error in self-reports of Pap test utilization. J. Community Health 27, 351–356. [DOI] [PubMed] [Google Scholar]
- Quinn M, Babb P, Jones J, Allen E, 1999. April 3. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ 318 (7188), 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2018. October 22. pii: S1538-4721(18)30453-7. doi: 10.1016/j.brachy.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher GH, Johnson TP, Cho YI, Walk JA, 2008. April. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol. Biomark. Prev. 17 (4), 748–757. 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Russell L, July 22, 2013. Federally Qualified Health Centers: An Overview. Center for Health and Research Transformation Accessed on August 15, 2019 at: https://www.chrt.org/publication/federally-qualified-health-centers-overview/.
- Sasieni P, Adams J, Cuzick J, 2003. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br. J. Cancer 89, 88–93. 10.1038/sj.bjc.6600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasieni P, Castanon A, Cuzick J, 2009. Effectiveness of cervical screening with age: population-based case-control study of prospectively recorded data. BMJ 339, b2968. 10.1136/bmj.b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS Jr., Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER, ACS-ASCCP-ASCP Cervical Cancer Guideline Committee, 2012. May-Jun. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 62 (3), 147–172. 10.3322/caac.21139. (Epub 2012 Mar 14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M, Kinney WK, Cheung LC, Gage JC, Fetterman B, Poitras NE, Lorey TS, Wentzensen N, Befano B, Schussler J, Katki HA, Castle PE, 2018. May 1. Relative performance of HPV and cytology components of cotesting in cervical screening. J. Natl. Cancer Inst. 110 (5), 501–508. 10.1093/jnci/djx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Langabeer II JR, 2018. Determinants of potentially unnecessary cervical cancer screenings in American women. J. Prev. Med. Public Health 51, 181–187. 10.3961/jpmph.18.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Eyre HJ, American Cancer Society, 2004. January-Feb. American Cancer Society guidelines for the early detection of cancer, 2004. CA Cancer J. Clin. 54 (1), 41–52. [DOI] [PubMed] [Google Scholar]
- StataCorp, 2017. Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX. [Google Scholar]
- Steenland M, Sinaiko A, Glynn A, Fitzgerald T, Cohen J. The effect of the Affordable Care Act on patient out-of-pocket cost and use of preventive cancer screenings in Massachusetts. Prev Med Rep. 2019. June 21; 15: 100924. doi: 10.1016/j.pmedr.2019.100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force, 2018. Screening for cervical cancer US Preventive Services Task Force recommendation statement. JAMA. 320 (7), 674–686. 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan A, Efrusy M, Lindeque G, Dreyer G, Santas C, 2009. February. Cost effectiveness of high-risk HPV DNA testing for cervical cancer screening in South Africa. Gynecol. Oncol. 112 (2), 377–383. 10.1016/j.ygyno.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Watson M, Benard V, King J, Crawford A, Saraiya M, 2017. July. National assessment of HPV and Pap tests: changes in cervical cancer screening, National Health Interview Survey. Prev. Med. 100, 243–247. 10.1016/j.ypmed.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M, Benard V, Flagg EW, 2018. February 2. Assessment of trends in cervical cancer screening rates using healthcare claims data: United States, 2003–2014. Prev. Med. Rep. 9, 124–130 doi: 10.1016/j.pmedr.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa M, Visioli CB, Ciatto S, et al. , 2004. Lower protection of cytological screening for adenocarcinomas and shorter protection for younger women: the results of a case-control study in Florence. Br. J. Cancer 90, 1784–1786. 10.1038/sj.bjc.6601754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Okoro CA, Li J, Town M, 2018. January. Health insurance status and clinical cancer screenings among U.S. adults. Am. J. Prev. Med. 54 (1), e11–e19. 10.1016/j.amepre.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.