Abstract
Lynch syndrome is an autosomal dominant disorder leading to cancer predisposition caused by mutations in mismatch repair genes. There is minimal published experience treating glioblastoma in patients with Lynch syndrome. We report a patient with Lynch syndrome who was initially diagnosed with a left occipital isocitrate dehydrogenase (IDH) wild-type glioblastoma. After resection, she was treated with chemoradiation, followed by tumour treating fields. Three years after diagnosis, recurrence was resected. After refusing cytotoxic chemotherapy, decision was made to treat with off-label nivolumab concurrently with radiation. She has been maintained on nivolumab without recurrence of her glioblastoma now over 5 years out from her initial diagnosis. This case provides the first report of glioblastoma in a patient with Lynch syndrome responding to nivolumab and concurrent radiation. In patients with Lynch syndrome and glioblastoma, immunotherapy in the form of nivolumab may be an alternative option to standard cytotoxic chemotherapy.
Keywords: immunological products and vaccines, neurology (drugs and medicines), immunology, neurooncology, CNS cancer
Background
Despite advances, glioblastoma remains a challenging malignancy with a poor prognosis. Even with aggressive resection and chemoradiation, glioblastoma is a fatal disease with average survival only 15 months and 5-year survival rate less than 10%.1 There is an immediate need for novel therapies in glioblastoma.
While programmed cell death protein 1 (PD-1) inhibitors have shown great success with malignancies, including melanoma and non-small cell lung adenocarcinoma, the results in glioblastoma have been disappointing. Nivolumab is a human monoclonal immunoglobulin G4 antibody that inhibits PD-1 expressed on activated T cells, thereby promoting T cell proliferation and cytokine production. Checkmate 143 is a phase III clinical trial, which investigated nivolumab compared with bevacizumab in patients with glioblastoma at first recurrence. Median overall survival (mOS) was comparable between both treatment arms, resulting in the study not meeting the primary end point of improved OS with nivolumab versus bevacizumab.2
Lynch syndrome is an autosomal dominant disorder caused by mutations in mismatch repair (MMR) genes. MMR genes maintain genomic integrity, and when mutated lead to microsatellite instability and carcinogenesis. Patients with Lynch syndrome have a 30%–60% risk of colorectal carcinoma (greatest for males), 25%–50% risk of endometrial carcinoma (greatest risk for MSH6), 8%–20% risk of ovarian cancer, 2%–11% risk of gastric cancer, 1%–5% risk of small bowel cancer and pancreatic cancer, 1%–12% risk of urothelial cancer (greatest for MSH2) and 1%–6% risk of brain cancer (MSH2 at greatest risk).3
Pembrolizumab, a monoclonal antibody to the PD-1 receptor, is FDA approved for the treatment of unresectable or metastatic microsatellite instability high (MSI-H) or deficient MMR (d-MMR) solid tumours. The phase II Keynote-158 trial enrolled 233 patients representing 27 different tumour types. Of those 233 patients, 4 had brain metastases and 13 had primary brain tumours. Objective response rate (ORR) for the entire study was 34.3% and mOS was 23.5 months. For the primary brain tumours, ORR was 0.0 with an mOS of 5.6 months.4
The FDA granted accelerated approval of nivolumab for the treatment of metastatic colorectal carcinoma in tumours harbouring MSI-H or d-MMR after progressive on conventional chemotherapy based on the results of CheckMate 142. In this single arm phase II trial, 74 patients with metastatic colorectal carcinoma with tumours demonstrating d-MMR or MSI-H received nivolumab. At a median follow-up of 12 months, 31.1% of patients (23 patients of 74 patients) achieved an objective response and 69% (51 patients) demonstrated control of disease for at least 12 weeks.5 Here, we present the case of a Lynch syndrome patient with recurrent isocitrate dehydrogenase (IDH) wild-type glioblastoma with MSI-H who has had a response to nivolumab.
Case presentation
A 57-year-old woman presented with progressive headache and memory loss over several weeks. Patient had a history of a resected colon cancer 10 years prior and known Lynch syndrome. MRI of the brain demonstrated a large enhancing mass in the left occipital lobe with surrounding vasogenic oedema and mass effect (Figure 1A, B). She underwent a left occipital craniotomy (figure 1C, D) with pathology consistent with IDH wild-type glioblastoma, O6-methylguanine-DNA methyltransferase (MGMT) unmethylated.
Figure 1.
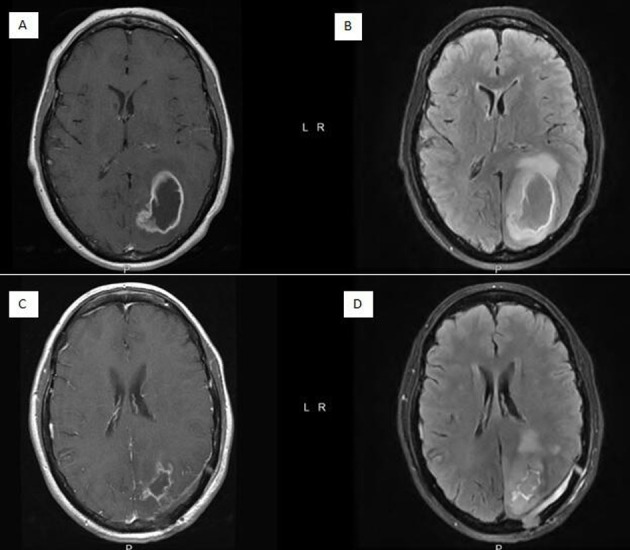
(A) Preoperative axial T1 post-contrast MRI demonstrating ring enhancing mass with central necrosis. (B) Preoperative axial T2 flair MRI demonstrating mass effect and cerebral oedema. (C) Postoperative axial T1 post-contrast MRI. (D) Postoperative axial T2 flair MRI.
Treatment
Patient went on to receive 6000 cGy of radiation therapy with concomitant temozolomide. Temozolomide was held for the last 3 weeks of radiation due to grade 4 thrombocytopenia. After platelet recovery, cycle 1 of adjuvant temozolomide was attempted at a 50% dose reduction. Patient was unable to receive further temozolomide due to prolonged pancytopenia. Patient was subsequently started on monotherapy with tumour treating fields. After 14 months of therapy with tumour treating fields without tumour recurrence, patient elected to discontinue tumour treating fields due to quality of life.
MRI performed 18 months after stopping tumour treating fields, over 3 years since her initial glioblastoma diagnosis, showed non-enhancing tumour recurrence in the right frontal lobe (figure 2A). She underwent a second craniotomy, this time for resection of the frontal lobe recurrence (figure 2B) with pathology consistent with IDH wild-type high-grade glioma, not classifiable as glioblastoma though concern that tumour was transitioning to glioblastoma, MGMT unmethylated. Genomic sequencing with germline comparison revealed the tumour to be microsatellite unstable with a high tumour mutational burden.
Figure 2.
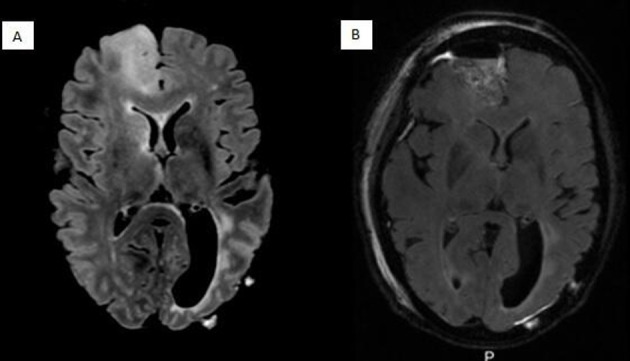
(A) Preoperative axial T2 flair MRI showing non-enhancing disease recurrence in right frontal lobe. (B) Postoperative axial T2 flair MRI showing interval resection.
She refused any further cytotoxic chemotherapy. She was started on off label nivolumab 240 mg intravenously every 2 weeks. She received nivolumab, while she underwent radiation to the right frontal lobe, receiving 4500 cGy in 25 fractions. She continued on nivolumab after completion of radiation therapy. Nivolumab had to be periodically delayed a week due to grade 2 neutropenia, but, however, she tolerated treatment very well without other adverse effect.
Outcome and follow-up
Neurologically, she continued to perform well with mild short-term memory deficit. As of 5 years out from her initial diagnosis of IDH wild-type glioblastoma, she is without evidence of further intracranial or systemic recurrence of neoplasm and remains on nivolumab monotherapy.
Discussion
While the association is known between Lynch syndrome and glioma, there is very little known in regards to the clinical course and outcome of a glioblastoma in a patient with Lynch syndrome. Universally, glioblastoma is incurable with a prognosis of 15 months on average. Depending on which MMR mutation is harboured by a patient with Lynch syndrome, studies site a 1%–6% lifetime risk to those aged 70 years of brain neoplasm in patients with Lynch syndrome.3
The role of MMR genes is to maintain genomic integrity. Mutated MMR genes lead to highly mutated repetitive DNA sequences. These in turn lead to microsatellite instability and ultimately formation of neoplasms. The neopeptides and neoantigens resulting from the microsatellite instability are immunogenic.6 These can be recognised by CD8+ lymphocytes, a process which is enhanced by immune checkpoint blockade. And while the results to date have been disappointing in regards to the efficacy of immune checkpoint inhibitors in recurrent glioblastoma, whether a glioblastoma might respond in a patient with Lynch syndrome to immune checkpoint blockade has not been extensively studied.
Immune checkpoint inhibition during radiation was initially of concern, with hypotheses that it could potentially amplify toxicity and oedema from concurrent brain irradiation. However, studies have since shown that not only is it safe to give concurrently, but there is rationale for an increased benefit when doing so. A retrospective study of patients with metastatic melanoma to the brain treated with concurrent pembrolizumab and radiation therapy demonstrated both safety and efficacy of the combined treatment.7 Aside from the direct cytotoxicity of radiation therapy to tumour cells, radiation therapy provokes a systemic immune response, a phenomenon known as the abscopal effect involving the ‘trafficking of lymphocytes into the tumour microenvironment, enhanced tumour recognition and killing via up-regulation of tumour antigens and antigen presenting machinery and induction of positive immunomodulatory pathways’.8 Glioblastoma induces a systemic immunosuppressive state through multiple mechanisms, including hypoxia in the tumour microenvironment, cytokine dysregulation and lymphocyte dysfunction: ‘the ability to cause severe, systemic T-cell deficits is one of the most prominent and earliest reported immune-related effects of HGGs [high grade gliomas]’.9 The combination of immune checkpoint inhibition and radiation therapy may have a synergistic modulatory effect both locally and systemically attempting to overcome glioblastoma-induced immunosuppression and take advantage of the radiation-induced antigen production.
This patient had known Lynch syndrome at the time of her initial diagnosis of glioblastoma. Genomic sequencing of her tumour revealed MSI-H with a high tumour mutational burden, both findings associated with a response to checkpoint inhibition in other solid tumours. She has exhibited a durable response to nivolumab administered concurrently with radiation and continued as monotherapy afterwards, without evidence of disease progression after over 20 months of treatment with nivolumab. Importantly, not only is there stability at the frontal site of recurrence, but also there has not been recurrence at the original site of disease at 5 years. While there are rare glioblastoma patients with extended survival, in this patient, the fact that she developed a second glioma as well as the abnormalities harboured, which are associated with a response to nivolumab, indicate a potential therapeutic effect of nivolumab in her case. While previous work in glioblastoma did not find a significant response in patients treated with nivolumab, most glioblastomas do not harbour microsatellite instability, d-MMR or high tumour mutational burden, which are associated with response to immune checkpoint inhibition in other solid tumours. This prior study did not select patients based off of these indications.
This report suggests tumour mutational burden and microsatellite instability should be evaluated at the time of diagnosis of glioblastoma and might lead one to consider the possibility of immune checkpoint inhibition, particularly in the setting of Lynch syndrome where this treatment has demonstrated success in other hypermutated malignancies associated with the syndrome. This is the first case report of a patient with Lynch syndrome and glioblastoma treated with radiation therapy with concurrent checkpoint inhibition. This warrants further study.
Learning points.
There may be a role for immunotherapy in tumours associated with Lynch syndrome.
In glioblastoma, mismatch repair deficiency and microsatellite instability should be evaluated to determine if there is a role for immunotherapy.
There is a rationale for synergism between radiation therapy and immunotherapy.
Footnotes
Contributors: WJS and TWV both contributed to the acquisition, analysis and interpretation of this work. WJS drafted the work and TWV revised it along with WJS. Both gave the final approval for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 2018;54:7–13. 10.1016/j.jocn.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol 2020;6:1003-1010. 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow E, Hill J, Evans DG. Cancer risk in Lynch syndrome. Fam Cancer 2013;12:229–40. 10.1007/s10689-013-9615-1 [DOI] [PubMed] [Google Scholar]
- 4.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with Noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeterdal I, Bjørheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci U S A 2001;98:13255–60. 10.1073/pnas.231326898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson ES, Postow MA, Wolchok JD, et al. Melanoma brain metastases treated with stereotactic radiosurgery and concurrent pembrolizumab display marked regression; efficacy and safety of combined treatment. J Immunother Cancer 2017;5:76. 10.1186/s40425-017-0282-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park B, Yee C, Lee K-M. The effect of radiation on the immune response to cancers. Int J Mol Sci 2014;15:927–43. 10.3390/ijms15010927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabowski MM, Sankey EW, Ryan KJ, et al. Immune suppression in gliomas. J Neurooncol 2021;151:3–12. 10.1007/s11060-020-03483-y [DOI] [PMC free article] [PubMed] [Google Scholar]


